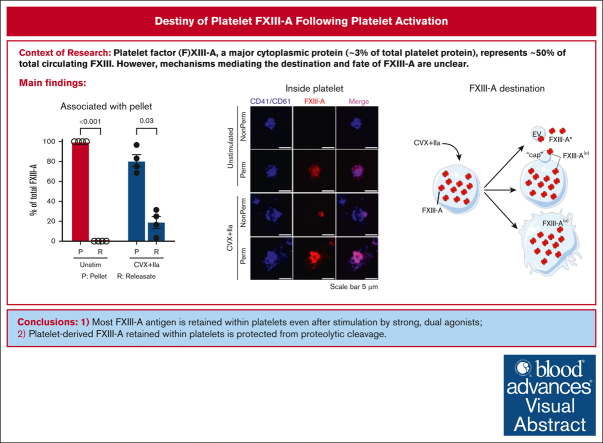
Key Points
-
•
Most FXIII-A antigen is retained within platelets even after stimulation by strong, dual agonists.
-
•
Platelet-derived FXIII-A retained within platelets is protected from proteolytic cleavage by thrombin and plasmin.
Visual Abstract
Abstract
Platelet factor XIII-A (FXIII-A) is a major cytoplasmic protein (∼3% of total), representing ∼50% of total circulating FXIII. However, mobilization of FXIII-A during platelet activation is not well defined. To determine mechanisms mediating the retention vs release of platelet FXIII-A, platelets from healthy humans and mice (F13a1−/−, Fga−/−, Plg−/−, Stim1fl/flPf4-Cre, and respective controls) were stimulated with thrombin, convulxin plus thrombin, or calcium ionophore (A23187), in the absence or presence of inhibitors of transglutaminase activity, messenger RNA (mRNA) translation, microtubule rearrangement, calpain, and Rho GTPase. Platelet releasates and pellets were separated by (ultra)centrifugation. FXIII-A was detected by immunoblotting and immunofluorescence microscopy. Even after strong dual agonist (convulxin plus thrombin) stimulation of human platelets, >80% platelet FXIII-A remained associated with the platelet pellet. In contrast, essentially all tissue factor pathway inhibitor, another cytoplasmic protein in platelets, was released to the supernatant. Pellet-associated FXIII-A was not due to de novo synthesis via platelet F13A1 mRNA. The proportion of platelet FXIII-A retained by vs released from activated platelets was partly dependent on STIM1 signaling, microtubule rearrangement, calpain, and RhoA activation but did not depend on the presence of fibrinogen or plasminogen. Immunofluorescence microscopy confirmed the presence of considerable FXIII-A within the activated platelets. Although released FXIII-A was cleaved to FXIII-A∗ and could be degraded by plasmin, platelet-associated FXIII-A remained uncleaved. Retention of substantial platelet-derived FXIII-A by activated platelets and its reduced susceptibility to thrombin- and plasmin-mediated proteolysis suggest platelet FXIII-A is a protected pool with biological role(s) that differs from plasma FXIII.
Introduction
The protransglutaminase factor XIII (FXIII) is found in plasma and cellular compartments. Plasma FXIII is a heterotetramer (FXIII-A2B2), whereas cellular FXIII is a homodimer (FXIII-A2) in hematopoietic-derived cells, including monocytes, macrophages, and platelets.1 Platelet FXIII-A represents ∼3% of the human platelet proteome and ∼50% of total circulating FXIII-A.2
Although platelets can take up small amounts of plasma FXIII-A2B2, most platelet FXIII-A is synthesized and packaged by megakaryocytes.1 Although most soluble coagulation proteins in platelets are stored in α-granules, FXIII-A resides in the cytoplasm and lacks a conventional secretion mechanism.1 Platelets also contain transcripts encoding FXIII-A (F13A1), and ribosome occupancy of platelet F13A1 is increased after thrombin stimulation, suggesting these cells are capable of de novo FXIII-A synthesis.3 Both plasma and platelet FXIII can be activated by thrombin-mediated cleavage of activation peptides from the A-subunits to generate active FXIIIa (FXIII-A∗), or nonproteolytically by a conformational change in response to elevated Ca2+ (to FXIII-A°).4, 5, 6 Both FXIII-A∗ and FXIII-A° can catalyze the formation of ε(γ-glutamyl)lysyl isopeptide bonds and stabilize clots against biochemical and mechanical disruption.4,5 However, accumulating data from clinical and experimental studies show plasma FXIII-A2B2 is sufficient to promote hemostasis.7,8 Thus, the biological role of platelet FXIII-A is not well understood. Notably, the temporal and spatial transformation of platelet FXIII-A to its activated forms and their subsequent biological roles are unclear, in part, because mechanisms mediating FXIII-A mobilization, exposure, and fate are poorly defined.
Prior studies showed that platelet FXIII-A can be released from activated platelets. Single agonists (eg, thrombin) induce very little FXIII-A release.9,10 However, after stimulation with dual agonists (eg, collagen/convulxin plus thrombin) to activate both glycoprotein VI (GPVI) and protease-activated receptors (PARs), FXIII-A can be detected on the platelet surface and in platelet-derived extracellular vesicles (EVs).11,12 Signaling events downstream of GPVI and/or PARs, including Ca2+-independent signaling through RhoA, have been implicated in FXIII-A exposure after platelet activation11; however, potential contributions of other events downstream of GPVI and PARs, and the extent to which these events lead to FXIII externalization, are not well understand. Moreover, FXIII-A antigen that is retained or released from activated platelets may have distinct properties,4,5,13 with significant implications for its biological role(s) in health and disease.
Here, we studied human and mouse platelets to characterize mechanisms mediating the retention/release and fate of FXIII-A after platelet activation. Our data suggest platelet FXIII-A is mobilized by cytoskeletal changes induced by Ca2+-dependent and -independent signaling that lead to the release of only a small amount of FXIII-A to the surrounding milieu. Notably, most platelet FXIII-A cargo is retained within the platelet and protected from proteolytic activation and degradation. These observations suggest platelet FXIII-A is reserved for a unique function that is distinct from the hemostatic role of plasma FXIII-A2B2.
Materials and methods
Approvals for work with humans and mice
Methods involving human participants were approved by the University of North Carolina at Chapel Hill Institutional Review Board (protocol number 01-1274). Mouse studies were approved by the University of North Carolina at Chapel Hill Institution of Animal Care and Use Committee (protocols numbers 22-019 and 23-017).
Preparation and stimulation of washed platelets
Fresh blood from humans and mice was drawn into acid-citrate-dextrose buffer, and washed platelets were prepared by centrifugation. Platelets were stimulated with thrombin (5 U/mL), convulxin plus thrombin (100 ng/mL and 5 U/mL, respectively), or calcium ionophore (A23187; 10 μM) and then quenched with 20-mM EDTA and 100 U/mL hirudin. Platelet releasates and pellets were separated by (ultra)centrifugation and analyzed by immunoblotting and immunofluorescence microscopy.
In selected experiments, inhibitors of transglutaminase (T101, 1, 3, 4, 5-Tetramethyl-2-[(2-oxopropyl)thio] imidazolium chloride, 50 μM final), protein synthesis (cycloheximide, 50 μg/mL final), microtubule polymerization (colchicine, 1 mg/mL final), calpain (calpeptin, 80 μg/mL final), or RhoA (rhosin, 40 μg/mL final) were preincubated with platelets for 5 minutes at 37°C before activation. Plasmin (50 nM final) was added at the same time as the agonists, as indicated.
To characterize the effect of platelets on exogenous FXIII, 20 μg/mL mouse FXIII-A2B2 or 10 μg/mL recombinant human FXIII-A2 was added to FXIII-A-deficient mouse platelets and then platelets were stimulated with 100 ng/mL convulxin plus 5 U/mL thrombin for 30 minutes. Reactions were quenched and centrifuged (1500g for 15 minutes) to separate the pellet and releasate and remove unbound mouse or human FXIII(a).
Detailed sources of materials and methods for isolating and activating washed platelets, immunoblotting, flow cytometry, nanoparticle tracking analysis, and immunofluorescence are described in the supplemental Materials.
Statistical methods
Statistical analysis was performed using GraphPad Prism v10.1.1. Data normality was assessed by Shapiro-Wilk tests. Normally distributed data were analyzed by ordinary 1-way analysis of variance, with Šídák multiple comparisons test for between-group comparisons. Nonnormally distributed data were analyzed by Friedman test, with Dunn multiple comparisons test for between-group comparisons. P value < .05 was considered significant.
Results
Most platelet FXIII-A remains associated with the platelet pellet even after dual agonist (convulxin plus thrombin) stimulation
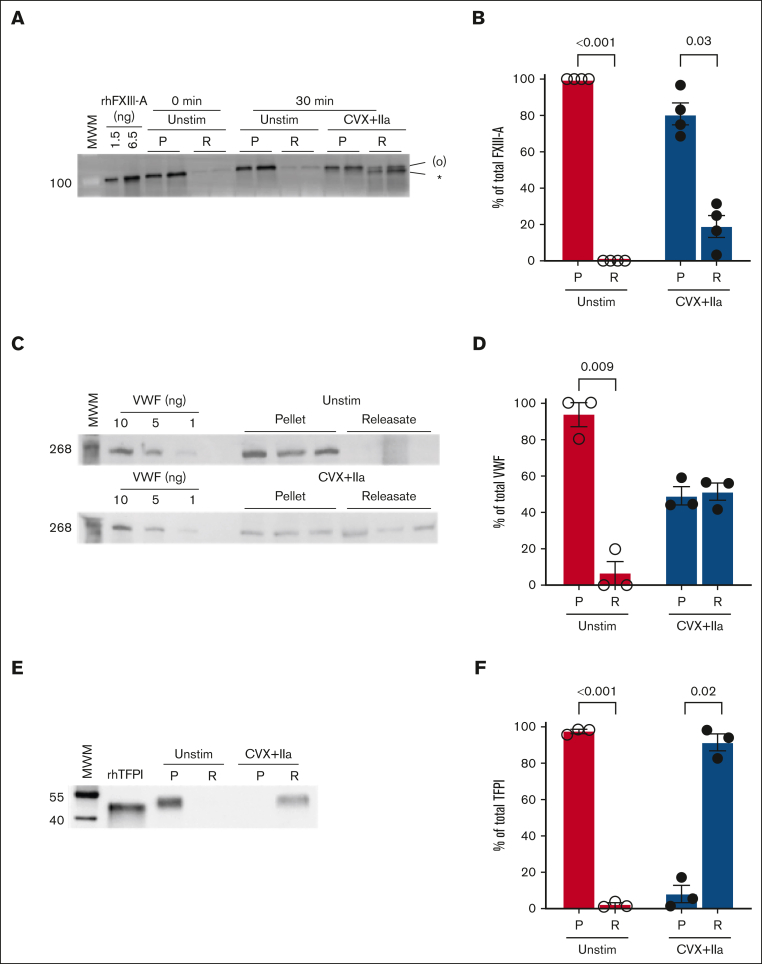
To characterize FXIII-A release from activated platelets, we first stimulated human platelets with convulxin plus thrombin for 30 minutes and quantified the relative amount of FXIII-A antigen in the platelet pellet and releasate by immunoblotting and densitometry (illustrated in supplemental Figure 1). Unexpectedly, most (81% ± 6%) FXIII-A remained associated with the platelet pellet (Figure 1A-B). FXIII-A retention was not decreased in the presence of the transglutaminase inhibitor T101 (supplemental Figure 2), suggesting the retention mechanism was independent of transglutaminase activity. Agglutination, promoted by centrifugation, slightly enhanced FXIII-A release; however, most (∼69%) FXIII-A remained associated with the platelet pellet (supplemental Figure 3). To contextualize these results, we compared FXIII-A retention with 2 other platelet proteins. von Willebrand factor is stored in platelet α-granules and released during platelet activation when it can bind to platelet GPIb-IX-V and αIIbβ3.14,15 In contrast to FXIII-A, only ∼50% of platelet von Willebrand factor was retained after convulxin plus thrombin stimulation (Figure 1C-D). Similar to FXIII-A, tissue factor pathway inhibitor (TFPI) also resides in the platelet cytoplasm, and stimulation with thrombin plus A23187 promotes TFPI translocation to the platelet surface and release in EVs and/or as soluble protein.16 Again in contrast to FXIII-A, essentially no TFPI was retained (ie, all platelet TFPI was released to the supernatant) after convulxin plus thrombin stimulation (Figure 1E-F).
Figure 1.
Most platelet FXIII-A is retained with the platelet after dual agonist stimulation. Washed human platelets were unstimulated (Unstim) or stimulated with convulxin (CVX) plus thrombin (IIa) for 30 minutes. The platelet pellet (P) and releasate (R) were separated by centrifugation. FXIII-A, TFPI, and von Willebrand factor (VWF) were visualized by immunoblotting and quantified by densitometry. For analysis of pellets, proteins from 1 × 105 and 2 × 105 platelets were loaded in the first and second lanes of each pairing, respectively. For analysis of releasates, supernatants from 0.5 × 106 and 1 × 106 platelets were loaded for the first and second lanes of each pairing, respectively. (A) Representative immunoblot for FXIII-A. Recombinant human FXIII-A loading control is labeled rhFXIII-A. FXIII-A species are labeled on the right of the immunoblot as (O) (representing zymogen FXIII-A or nonproteolytically activated FXIII-A°) and ∗ (representing FXIII-A∗). (B) Quantification of FXIII-A. (C) Representative immunoblot of VWF; each lane represents a separate donor. (D) Quantitation of VWF. (E) Representative immunoblot of TFPI (recombinant human TFPI [rhTFPI] loading control). (F) Quantification of TFPI. For all immunoblots, molecular weight marker (MWM) is indicated on the left. For the bar graphs, the data show mean ± standard error of the mean (SEM); each dot represents a separate donor.
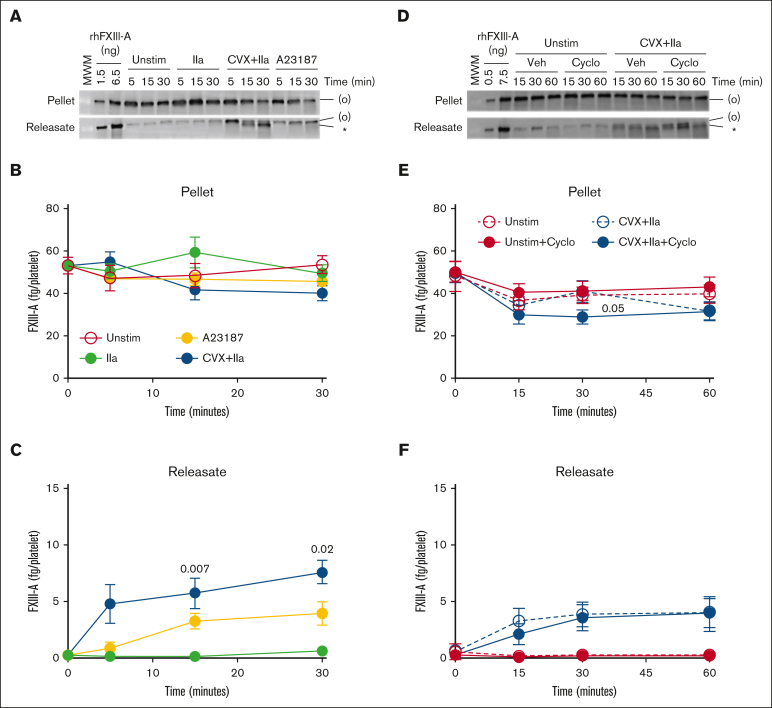
To further characterize FXIII-A mobilization after platelet activation, we stimulated human platelets with single (thrombin) or dual (convulxin plus thrombin) agonists or calcium ionophore (A23187) and compared the kinetics of FXIII-A2 release. As expected,9,10 thrombin-stimulated platelets released little to no FXIII-A (Figure 2A-C). Convulxin plus thrombin–stimulated human platelets released a small fraction of their FXIII-A over time; by 30 minutes, only ∼8 ± 1 fg per platelet (16% ± 2%) and 4 ± 1 fg per platelet (8% ± 2%) was released by convulxin plus thrombin– and A23187-stimulated human platelets, respectively (Figure 2A-C). However, most FXIII-A remained associated with the platelet pellet.
Figure 2.
Platelet FXIII-A is not synthesized during activation of human platelets. (A-C) Washed human platelets were unstimulated (Unstim) or stimulated with thrombin (IIa), CVX plus IIa, or A23187. The platelet pellet (P) and releasate (R) were separated by centrifugation. FXIII-A was visualized by immunoblotting and quantified by densitometry. (A) Representative immunoblots of FXIII-A from the pellet (1.5 × 105 platelets) or releasate (7.5 × 105 platelets). (B-C) Quantification of total FXIII-A in the pellet and releasate, respectively (mean ± SEM; n = 5 separate donors; same symbols in both panels; P values vs unstimulated platelets at the same time point are indicated). (D-F) Washed human platelets were unstimulated (Unstim) or stimulated with CVX plus IIa in the presence of cycloheximide (cyclo) or vehicle (Veh [ethanol]). Samples were processed as those in panels A-C. (D) Representative immunoblots of FXIII-A. For analysis of the pellet, proteins from ∼2 × 105 platelets were loaded. For analysis of the releasate, supernatant from 1 × 106 platelets were loaded. (E-F) Quantification of total FXIII-A in the pellet and releasate, respectively (mean ± SEM; n = 6 separate donors; same symbols in both panels; P = .05 vs convulxin plus thrombin in the absence of cycloheximide at the same time point; the slight increase in FXIII-A in the pellet in the absence of cycloheximide at 30 minutes is likely an artifact because this is too short of a time frame for significant protein synthesis). For both immunoblots, rhFXIII-A is recombinant human FXIII-A loading control, and the band in the MWM lane indicates 100 kDa. FXIII-A species are labeled on the right of the immunoblot as (O) (representing zymogen FXIII-A or nonproteolytically activated FXIII-A°) and ∗ (representing FXIII-A∗).
To determine whether new FXIII-A synthesis from F13A1 transcripts accounts for the high amount of FXIII-A associated with the activated platelet pellet, we pretreated human platelets with the protein synthesis inhibitor cycloheximide before activating platelets with convulxin plus thrombin. Immunoblots of the platelet pellet and releasate indicated that FXIII-A antigen was relatively stable through 60 minutes (Figure 2D-F). Moreover, there was little-to-no change in FXIII-A content in either fraction even 7 hours after platelet activation (supplemental Figure 4). These data indicate that convulxin plus thrombin stimulation does not promote F13A1 translation in washed platelets, and the high amount of FXIII-A detected in the platelet pellet represents preexisting protein that is selectively retained during platelet activation.
Platelet FXIII-A mobilization is partly regulated by Ca2+-dependent and -independent signaling and cytoskeletal rearrangements
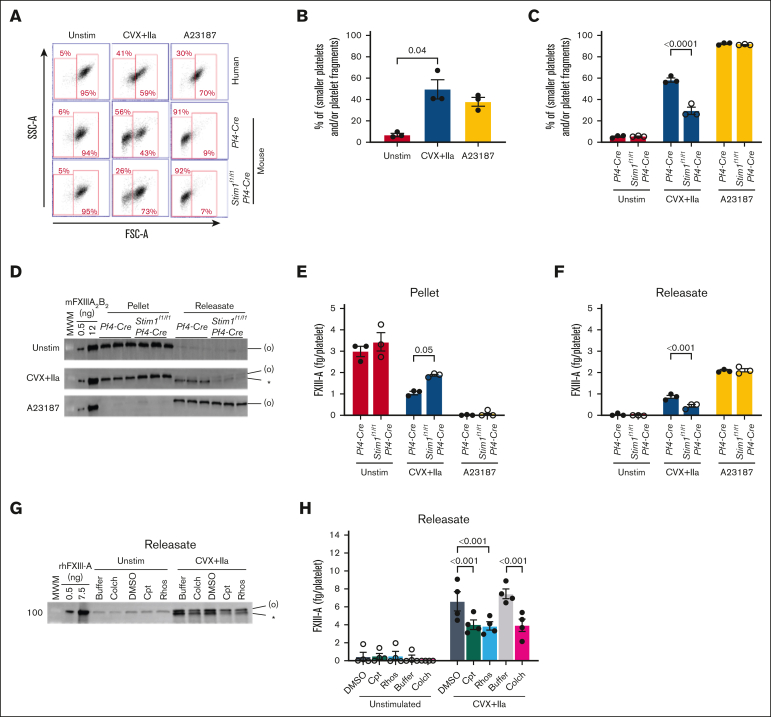
Stimulation of platelet GPVI and PARs induces signals through inostitol-1,4,5-triphosphate–mediated efflux of intracellular stores of Ca2+ to the cytosol and subsequent stromal interaction molecule 1 (STIM1)/calcium release-activated calcium modulator 1 (ORAI1)–mediated influx of extracellular Ca2+.17 In contrast, A23187 causes nonspecific entry of extracellular Ca2+ via A23187-Ca2+ complexes.18,19 To characterize the relative contribution of STIM1-mediated mobilization of Ca2+ stores vs extracellular Ca2+ influx to FXIII-A retention or release, we studied platelets from STIM1-deficient (Stim1fl/fl Pf4-Cre) and control (Pf4-Cre) mice. Compared with human platelets (43 ± 8 fg/platelet), mouse platelets contained less total FXIII-A. However, Pf4-Cre and Stim1fl/fl Pf4-Cre mouse platelets contained similar total FXIII-A (3.0 ± 0.2 and 3.4 ± 0.4 fg/platelet, respectively, representing ∼0.3%-0.5% of total platelet protein). Similar to human platelets, convulxin plus thrombin–stimulated Pf4-Cre mouse platelets formed a population of smaller platelets and/or platelet fragments (Figure 3A-C). However, compared with stimulated human platelets, stimulated Pf4-Cre mouse platelets retained less FXIII-A (∼80% [Figure 1., Figure 2. and 2] vs ∼55% [Figure 3D-F] for human and mouse, respectively). Compared with Pf4-Cre mouse platelets, activated Stim1fl/fl Pf4-Cre mouse platelets had fewer small platelets/fragments (Figure 3A,C), likely due to reduced vesiculation, and retained more FXIII-A (1.0 ± 0.1 vs 1.9 ± 0.1 fg/platelet; Figure 3D-F). These data suggest FXIII-A translocation is partially mediated by STIM1-dependent Ca2+ signaling in activated platelets. As expected, A23187 stimulation of human platelets for 30 minutes induced ∼100% phosphatidylserine-positive platelets (data not shown) and produced a population of smaller platelet per fragments (∼38%). Similar to a previous report,11 A23187 released little (∼10%) FXIII-A from human platelets (Figure 2A-C). In contrast, A23187 stimulation of mouse platelets induced nearly complete formation of small platelets per fragments (Figure 3A-C) and released essentially all FXIII-A to the supernatant (Figure 3D-F).
Figure 3.
FXIII-A translocation is partly dependent on STIM1 signaling and cytoskeletal rearrangements. (A-F) Washed human or mouse platelets were unstimulated (Unstim) or stimulated with CVX plus thrombin (IIa) or A23187 for 30 minutes. (A-C) Five-thousand events per sample were analyzed by flow cytometry. (A) Cytograms of human and mouse platelets. Two gates (smaller platelets and/or platelet fragments [left]; intact platelets [right]) were set based on unstimulated platelets. (B) Percentage of smaller platelets and/or platelet fragments for human platelets (n = 3; each dot represents a separate donor). (C) Percentage of smaller platelets and/or platelet fragments for mouse platelets (n = 3; each dot represents a separate mouse). (D-F) Mouse platelet pellet and releasate were separated by centrifugation at 1500g. FXIII-A was visualized by immunoblotting and quantified by densitometry. (D-F) Representative immunoblots (D) and quantitation (E-F) of FXIII-A in the mouse platelet pellet and releasate from 1.5 × 106 platelets. Each lane and dot represents a separate mouse. (G-H) Washed human platelets were unstimulated or stimulated with CVX plus IIa in the absence (buffer, DMSO [vehicle]) or presence of calpeptin (Cpt), rhosin (Rhos), or colchicine (Colch). The platelet pellet and releasate were separated by centrifugation. FXIII-A was quantified by densitometry. (G-H) Representative immunoblot and quantitation of FXIII-A in the human platelet releasate from 1 × 106 platelets (mean ± SEM of 4 separate donors). FXIII-A species are labeled on the right of the immunoblot as (O) (representing zymogen FXIII-A or nonproteolytically activated FXIII-A°) and ∗ (representing FXIII-A∗). Bars show mean ± SEM. DMSO, dimethyl sulfoxide. Statistical comparison in (H) are with appropriate controls (DMSO or buffer [diluted Tyrodes]).
Stimulation of platelet GPVI and PARs also activates proteases20 that may facilitate FXIII-A release from platelets. Inhibition of the Ca2+-dependent enzyme calpain by calpeptin partly reduced FXIII-A release from human platelets (from 6.6 to 4.1 fg/platelet; P < .001; Figure 3G-H). We also confirmed that inhibition of Ca2+-independent RhoA activation with the RhoA inhibitor rhosin also reduced FXIII-A release from human platelets (from 6.6 to 3.9 fg/platelet; P < .001; Figure 3G-H and Somodi et al11). Platelet activation through both Ca2+-dependent and -independent signaling promotes cytoskeletal rearrangements, and FXIII-A colocalizes with cytoskeletal proteins.21,22 Accordingly, pretreatment of platelets with colchicine to inhibit microtubule rearrangements reduced FXIII-A release (from 7.5 to 4.0 fg/platelet; P < .001; Figure 3G-H). Collectively, these data implicate both Ca2+-independent and -dependent signaling and cytoskeletal rearrangements in FXIII-A mobilization in convulxin plus thrombin–stimulated platelets.
Most released FXIII-A is present as free protein
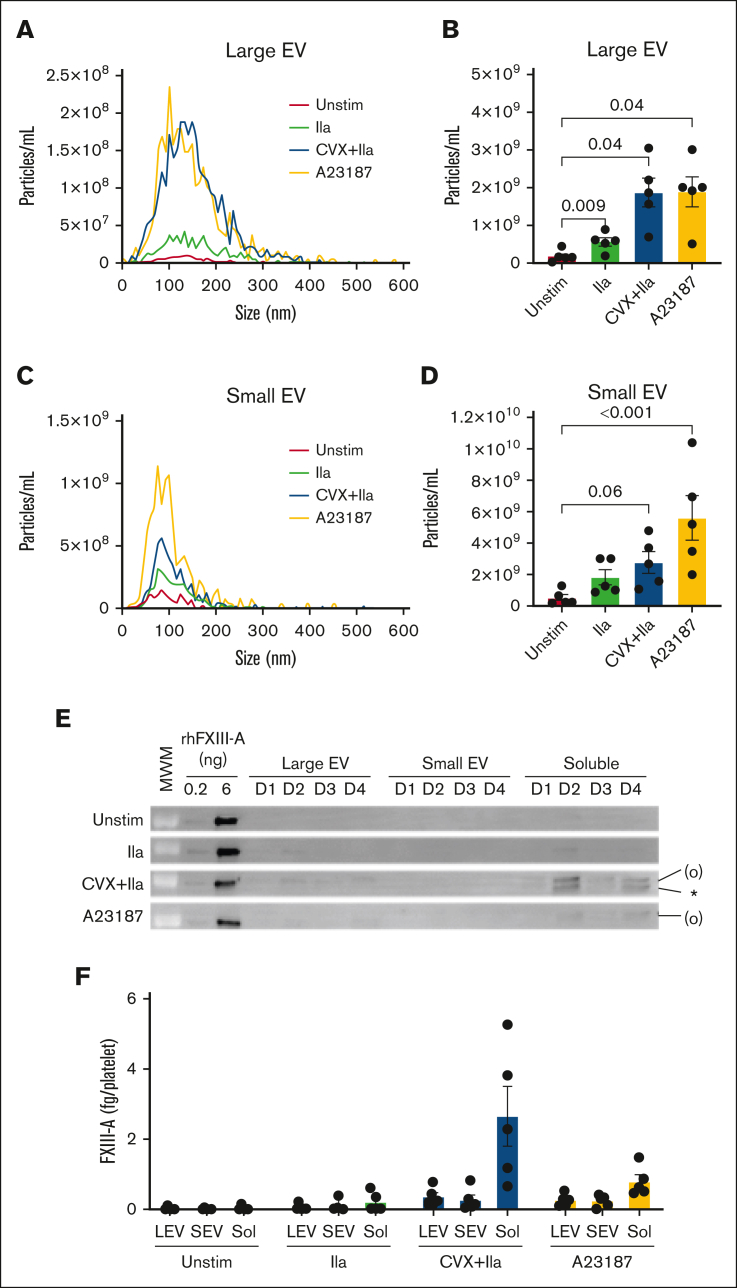
The minor fraction of total FXIII-A that is released may be associated with EVs11,23 and/or present as free protein. To determine the distribution of EV-associated vs free FXIII-A in the platelet releasate, we used differential centrifugation (as described in Sachetto et al24 and Krishnamachary et al24, 25 and illustrated in supplemental Figure 5) and nanoparticle tracking analysis to separate and quantify large EVs, small EVs, and soluble proteins generated by activated platelets. In parallel, we used immunoblotting to quantify FXIII-A in these fractions. Compared to unstimulated platelets, stimulation with thrombin, convulxin plus thrombin, and A23187 resulted in ∼3.1-, 10.6-, and 10.6-fold more large EVs (Figure 4A-B) and 3.5-, 6.5-, and 10.7-fold more small EVs (Figure 4C-D), respectively. However, ∼75% ± 5% of the minor fraction of FXIII-A released from convulxin plus thrombin–stimulated platelets remained in the supernatant even after centrifugation at 100 000g (considered the non-EV, “free” fraction; Figure 4E-F). Accordingly, the inhibition of calpain by calpeptin only slightly (nonsignificantly) decreased EVs but significantly reduced FXIII-A release from collagen plus thrombin–stimulated platelets (supplemental Figure 6). Thus, although platelets can release both EVs and FXIII-A after strong stimulation, these events are at least partly separable, suggesting these processes are mediated by independent pathways.
Figure 4.
Released FXIII-A is mainly free protein. Washed human platelets were unstimulated (Unstim) or stimulated with thrombin (IIa), CVX plus thrombin (IIa), or A23187 for 30 minutes at 37°C. The reaction mixture was then quenched and centrifuged serially to separate the pellet and releasate and then large EVs, small EVs, and remaining soluble fraction (free proteins), as described in the “Methods” and illustrated in supplemental Figure 5. (A-D) Nanoparticle tracking analysis of size distribution and enumeration of large EVs (A-B) and small EVs (C-D). (E) Representative immunoblots of FXIII-A in large EVs (pellet of 20 000g, 15 minutes), small EVs (pellet of 100 000g, 70 minutes), and soluble fraction (supernatant of 100 000g, 70 minutes) from 4 of the donors (D) studied; rhFXIII-A is recombinant human FXIII-A loading control; the band in the MWM lane indicates 100 kDa. FXIII-A species are labeled on the right of the immunoblot as (O) (representing zymogen FXIII-A or nonproteolytically activated FXIII-A°) and ∗ (representing FXIII-A∗). (F) Quantification of FXIII-A in large EVs (LEVs), small EVs (SEVs), and soluble proteins (Sol). The data show mean ± SEM of 5 separate donors; each dot represents a separate donor.
Retention of platelet pellet–associated FXIII-A does not require fibrinogen or plasminogen
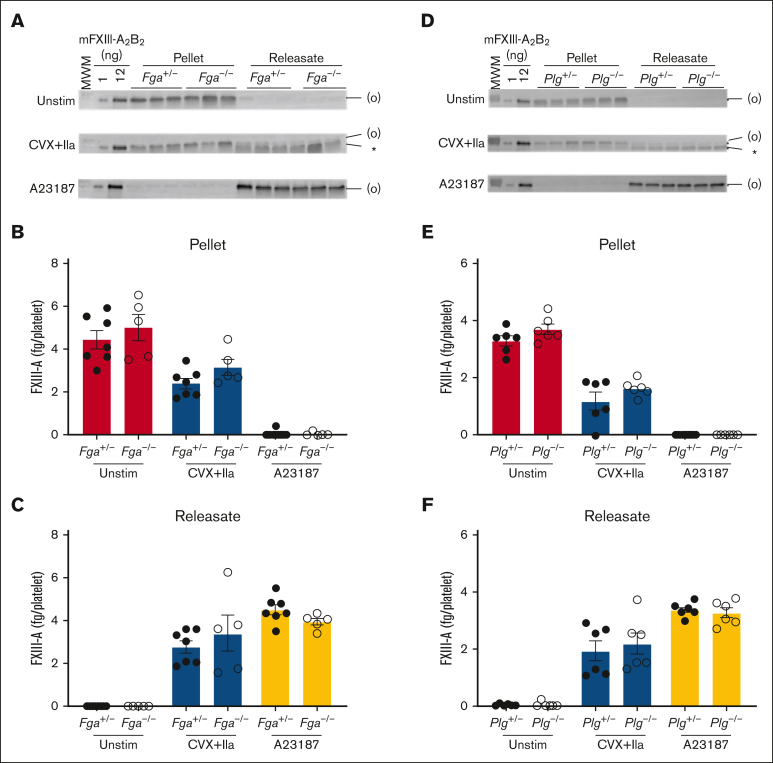
Both fibrin(ogen) and plasmin(ogen) can be detected in a focal region on the surface of convulxin plus thrombin–stimulated platelets, and FXIII can interact with each of these proteins.12,13,26,27 Moreover, plasma FXIII binds platelets via surface-bound fibrin(ogen).28 To determine whether fibrin(ogen) or plasmin(ogen) mediate retention of platelet FXIII-A, we activated platelets from fibrinogen- or plasminogen-deficient (Fga−/− or Plg−/−) and -expressing (Fga+/− or Plg+/−) mice with convulxin plus thrombin or A23187 and measured FXIII-A by immunoblotting. As seen in Pf4-Cre and Stim1fl/fl Pf4-Cre mouse platelets, A23187 stimulation released essentially all FXIII-A to the supernatant. Interestingly, however, similar amounts of FXIII-A were detected in the pellets and releasates of convulxin plus thrombin–stimulated Fga−/− and Plg−/− platelets compared with their respective controls (Figure 5A-F), indicating neither of these proteins is required to retain platelet FXIII-A in the pellet.
Figure 5.
Retention and release of FXIII-A from convulxin plus thrombin–stimulated platelets do not depend on endogenous fibrinogen or plasminogen. Washed platelets from fibrinogen-deficient (Fga−/−), plasminogen- deficient (Plg−/−), or littermate control (Fga+/− and Plg+/−, respectively) mice were unstimulated (Unstim) or stimulated with CVX plus thrombin (IIa) or A23187 for 30 minutes. The platelet pellets and releasate were separated by centrifugation. FXIII-A was visualized by immunoblotting and quantified by densitometry. (A-C) Studies with fibrinogen-sufficient and -deficient platelets. (D-F) Studies with plasminogen-sufficient and -deficient platelets. (A,D) Immunoblots of FXIII-A in the pellet or releasate from 1.5 × 106 mouse platelets (mouse [m]FXIII-A2B2 loading control). The band in the MWM lane indicates 100 kDa. FXIII-A species are labeled on the right of the immunoblot as (O) (representing zymogen FXIII-A or nonproteolytically activated FXIII-A°) and ∗ (representing FXIII-A∗). (B,E) Quantification of total FXIII-A in the pellet. (C,F) Quantification of total FXIII-A in the releasate. The data show mean ± SEM; each dot is a separate mouse.
Platelet-associated FXIII-A is protected from thrombin cleavage
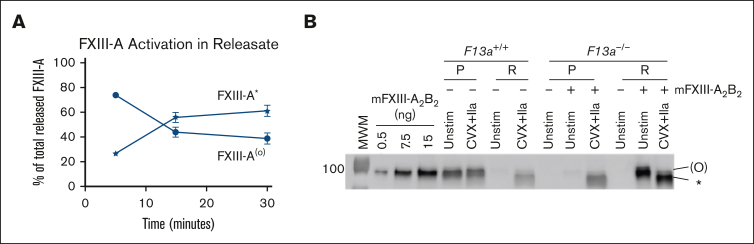
Throughout our experiments, we consistently detected 2 FXIII-A bands in the releasate of platelets stimulated with convulxin plus thrombin: an upper band labeled FXIII-A(°) that represents zymogen FXIII-A or nonproteolytically activated FXIII-A° (because these cannot be distinguished by immunoblot); and a lower band representing cleaved FXIII-A∗ that appears over time (Figure 1., Figure 2., Figure 3., Figure 4., Figure 5.). Quantification of data in Figure 2A revealed that by 30 minutes, ∼61% ± 4% of convulxin plus thrombin–released FXIII-A was present as FXIII-A∗ (Figure 6A). Although inhibitors of calpain, RhoA, and microtubule rearrangement reduced the total FXIII-A released to the supernatant (Figure 3G-H), these inhibitors did not significantly alter the ratio of FXIII-A∗ to FXIII(°) present in the releasate (supplemental Figure 7). Notably, however, pellet-associated FXIII-A remained uncleaved in even convulxin plus thrombin–stimulated platelets (Figure 1., Figure 2., Figure 3., Figure 4., Figure 5.). The only exception was in long experiments (7 hours), in which a subset of retained FXIII-A appeared as FXIII-A∗ (supplemental Figure 4). These data suggested released FXIII-A is cleaved by thrombin, but platelet-associated FXIII-A is largely protected from proteolytic activation.
Figure 6.
Released FXIII-A is cleaved by thrombin, whereas platelet-derived FXIII-A that remains associated with the platelet is protected from thrombin cleavage. (A) Quantification of relative FXIII-A(°) and FXIII-A∗ in the releasate of CVX plus thrombin (IIa)–stimulated platelets as in Figure 2A-C. The data show mean ± SEM of 5 separate donors. (B) Representative immunoblot of FXIII-A in pellets (P) and releasate (R) from experiments with washed platelets from F13a1+/+ or F13a1−/− mice unstimulated or stimulated with CVX plus IIa in the absence (for F13a1+/+ platelets) or presence (for F13a1−/− platelets) of exogenous mouse FXIII (mFXIII-A2B2) for 30 minutes (n = 3). Proteins from ∼1.5 × 106 platelets were loaded. FXIII-A species are labeled on the right of the immunoblot as (O) (representing zymogen FXIII-A or nonproteolytically activated FXIII-A°) and ∗ (representing FXIII-A∗).
To determine whether platelets can protect exogenous (nonplatelet-derived) FXIII-A from thrombin-mediated proteolysis, we used platelets from F13a1+/+ and F13a1−/− mice.29 As expected, FXIII-A was not detected in F13a1−/− platelets (Figure 6B). As in experiments with human platelets, FXIII-A retained in the pellets from F13a1+/+ mice remained uncleaved, whereas released FXIII-A was cleaved to FXIII-A∗ (Figure 6B). We then added mouse FXIII-A2B2 to F13a1−/− platelets, stimulated platelets with convulxin plus thrombin, and detected FXIII-A by immunoblotting. Consistent with previous findings,10,30,31 convulxin plus thrombin–stimulated, but not unstimulated, F13a1−/− platelets bound exogenous FXIII only as FXIII-A∗ (Figure 6B). We also saw similar findings in experiments testing recombinant human FXIII-A2 binding to mouse platelets even in the absence of FXIII-B (data not shown). Together with studies indicating activated, but not unactivated, platelets provide binding sites for FXIII-A∗ but not FXIII-A(°),10,30,31 these data suggest platelets can protect platelet-derived and sequestered, but not exogenous (plasma or released), FXIII-A from proteolysis.
Activated platelets retain most of their FXIII-A inside the cell
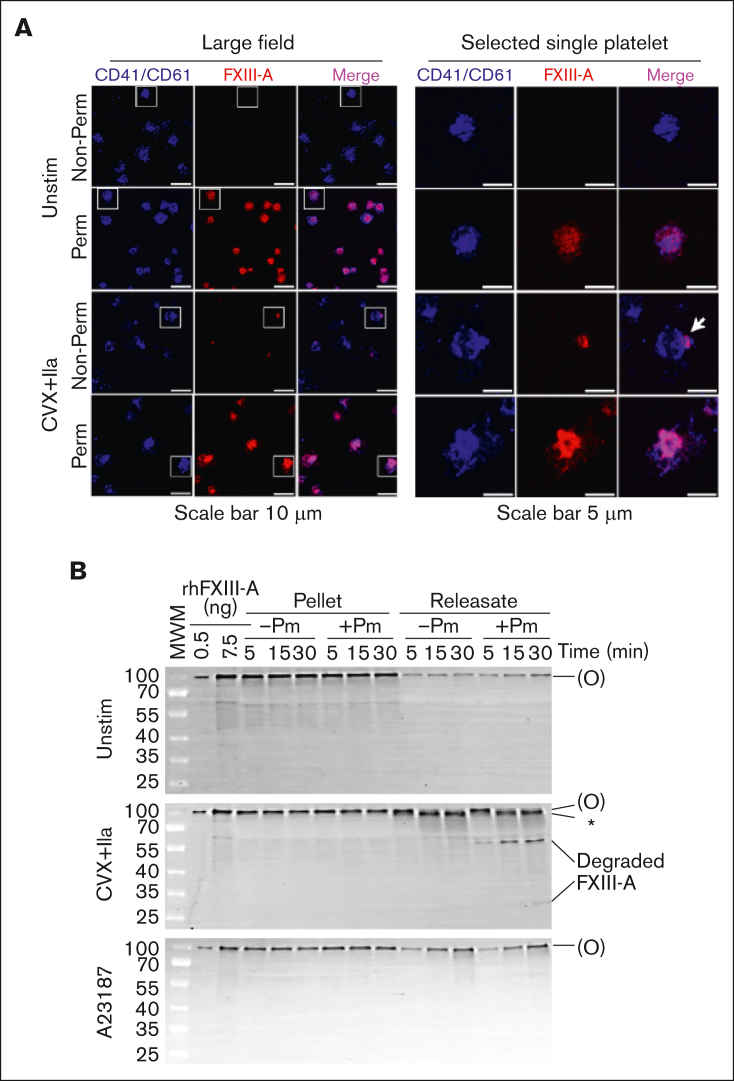
In resting platelets, FXIII-A can be detected in the cytoplasm and is associated with internal membrane. In convulxin (or collagen) plus thrombin–stimulated platelets, FXIII-A is detected in a focal region termed the platelet “cap.”11,12 Given our observation that platelet-associated FXIII-A is not cleaved by thrombin, we used confocal microscopy to directly visualize FXIII-A in unstimulated and convulxin plus thrombin–stimulated platelets and test the hypothesis that this pool was present inside the activated platelet. As expected, unstimulated, nonpermeabilized platelets showed little to no FXIII-A staining, whereas unstimulated, permeabilized platelets stained strongly for FXIII-A (Figure 7A). In accordance with published data,11,12,28 a subpopulation (47% ± 2.4% of platelets; n = 3 separate donors) of convulxin plus thrombin–stimulated, nonpermeabilized platelets showed a small amount of FXIII-A present on a cap-like structure on the platelet surface. Notably, however, stimulated, permeabilized platelets stained intensely for FXIII-A throughout the platelet interior (Figure 7A), suggesting most platelet-associated FXIII-A is retained intracellularly.
Figure 7.
Activated platelets retain most of their FXIII-A inside the cell, where it is protected from proteolytic degradation. (A) Washed human platelets were unstimulated (Unstim) or stimulated with CVX plus thrombin (IIa) for 30 minutes. Intact (nonpermeabilized [Non-Perm]) or permeabilized (Perm) platelets were labeled with antibodies against CD41/CD61 (surface marker, blue) and FXIII-A (red). A merge of CD41/CD61 and FXIII-A is shown in pink. Platelets were imaged on a Zeiss LSM 900 confocal microscope (UNC Microscopy Service Laboratory) through a Plan-Apochromat 63×/1.40 oil immersion objective. For optimal display, the brightness/contrast was adjusted separately; gray scale images with equivalent settings are shown in supplemental Figure 8. Representative images of experiments performed on platelets from 3 different donors are shown. The white boxes (left images) indicate the selected individual platelets shown on the right images. White arrow points to FXIII-A exposed on the cap-like structure on the platelet surface. (B) Representative immunoblots of FXIII-A from washed human platelets (n = 5 separate donors) that were unstimulated (Unstim) or stimulated with CVX plus thrombin (IIa) in the absence or presence of plasmin (Pm) for up to 30 minutes. The pellet and releasate were separated by centrifugation. For analysis of the pellet, proteins from 2 × 105 platelets were loaded. For analysis of the releasate, supernatant from 1 × 106 platelets were loaded. FXIII-A species are labeled on the right of the immunoblot as (O) (representing zymogen FXIII-A or nonproteolytically activated FXIII-A°) and ∗ (representing FXIII-A∗).
FXIII-A retained inside the platelet is protected from proteolytic degradation by plasmin
Our data showed that released, but not platelet-associated, FXIII-A can be cleaved to FXIII-A∗. Because plasmin can degrade FXIII-A∗ but not FXIII-A or FXIII-A°,13 we then determined the fate of these FXIII-A compartments by incubating activated platelets with exogenous plasmin. FXIII-A∗ in the releasate of convulxin plus thrombin–stimulated platelets underwent time- and plasmin-dependent cleavage to degradation products of ∼55 kDa (and ∼25 kDa visible in 1 donor; Figure 7B). In contrast, no FXIII-A degradation products were detected in the pellet of convulxin plus thrombin– or A23187-stimulated platelets up to 30 minutes after activation (Figure 7B).
Collectively, these data suggest even strong platelet agonists (eg, convulxin plus thrombin) induce the release and cleavage of only a small portion of platelet FXIII-A, and most platelet FXIII-A is selectively retained within and protected by the platelet.
Discussion
Despite its abundance, the function of platelet FXIII-A has been unclear, partly because the mechanisms determining its externalization and fate have not been defined. Our findings from human and mouse experimental systems reveal new aspects of platelet FXIII biology and biochemistry. First, most of the platelet FXIII-A cargo is retained within the platelet body even after stimulation with strong, dual agonists. Second, the release of the minor fraction of platelet FXIII-A after platelet activation involves both Ca2+-dependent and -independent signaling and cytoskeletal rearrangements. Third, although released FXIII-A is susceptible to cleavage by thrombin and plasmin, FXIII-A retained within the platelet is protected from proteolysis. Collectively, these findings reveal limited accessibility of most platelet-derived FXIII-A even after strong platelet activation and suggest the role(s) of platelet FXIII-A is distinct from those of plasma FXIII-A2B2 during and after hemostatic events.
Although both FXIII-A and TFPI are found in the platelet cytoplasm,1,16 the retention of most platelet FXIII-A contrasts with TFPI and suggests these proteins undergo differential trafficking and/or that specific mechanisms mediate FXIII-A interactions with platelets. Plasma FXIII(a) has been reported to engage several potential surface receptors, including αIIbβ3, αVβ3, and αIIbβ3-bound fibrinogen10,28,31,32; however, FXIIIa can bind to platelets from patients with Glanzmann thrombasthenia,30 and we found neither fibrin(ogen) nor plasmin(ogen) is needed to retain platelet FXIII-A after activation. Notably, our data suggest most platelet FXIII-A remains inside the platelet body after platelet activation. This premise is supported by several observations: (1) uncleaved exogenous FXIII-A cannot bind to platelets30; (2) platelet FXIII-A colocalizes with multiple regulatory and cytoskeletal proteins21,22; and (3) microscopy of permeabilized, collagen plus thrombin–stimulated platelets reveals substantial intracellular FXIII-A (Figure 7A) that may be partially colocalized with F-actin,33 and platelet-associated, but not released, FXIII-A is protected from proteolysis.
It is difficult to disentangle contributions of platelet activation pathways from mechanisms specifically required for FXIII-A mobilization from the cytoplasm to the platelet surface and/or surrounding milieu. Both convulxin plus thrombin and A23187/ionomycin induce sustained Ca2+ elevation, generation of phosphatidylserine-positive procoagulant platelets, and microvesiculation.34 However, convulxin plus thrombin promotes greater FXIII-A mobilization, release in platelet-derived EVs, and crosslinking activity on the surface of platelets and platelet-derived EVs, indicating receptor-mediated activation and signaling is required for maximal FXIII-A translocation.11,35 After convulxin plus thrombin stimulation, the cytosolic Ca2+ concentration increases from nM to μM.17 Genetic loss of STIM1 or pharmacologic inhibition of calpain or RhoA only partly impairs platelet FXIII-A release, suggesting both Ca2+-dependent and -independent pathways contribute to FXIII-A mobilization. These mechanisms are currently unknown but may include loss of mitochondrial potential and metabolic adenosine triphosphate depletion, which influence EV release and/or platelet fragmentation.36,37 FXIII-A has been detected in EVs,11 and our differential centrifugation experiments suggested a substantial fraction of released FXIII-A is also present in the free/soluble fraction. Thus, the extent to which mechanisms mediating EV formation and FXIII-A release overlap is currently unclear and will be the subject of future studies.
Increasing evidence suggests plasma and platelet FXIII(a) function in different capacities. IV infusion of plasma-derived FXIII-A2B2 or recombinant FXIII-A2 to reconstitute FXIII levels in plasma restores hemostasis in FXIII-A–deficient patients.7,8 Moreover, fibrin crosslinking by plasma, but not platelet, FXIII(a) promotes red blood cell retention in contracted clots and enhances thrombus mass.38, 39, 40 FXIII-A° has lower conformational flexibility and reduced affinity for glutamine substrates than FXIII-A∗.4 Although some studies reported that platelet FXIII-A is required for contractile events,41 others have been unable to reproduce this finding.38,40,42,43 Thus, despite platelet FXIII-A accounting for ∼50% of the circulating pool, plasma FXIIIA2B2 is sufficient to carry out most hemostatic functions. The sequestration and protection of FXIII-A antigen within platelets may impart this pool with different characteristics than the more accessible FXIII-A2B2 in plasma. The FXIII-A subunits in plasma and platelets are synthesized from the same gene (F13A1) and are biochemically capable of catalyzing the same crosslinking events.4,5,12 However, the FXIII-A activation mechanisms in these 2 compartments differ. Thrombin-mediated activation of plasma FXIII-A2B2 occurs rapidly and almost simultaneously with thrombin-mediated conversion of fibrinogen to fibrin. The temporal association of these events is facilitated by FXIII-A2B2 binding to fibrinogen residues γ390-396 near the thrombin binding site on polymerizing fibrin (central E-region interaction with D regions and extended γʹ sequence).38,44 That the fibrin crosslinking residues (γQ398, γQ399, and γK406) are also located within this region enables plasma FXIII-A∗ to rapidly crosslink fibrin and antifibrinolytic proteins to promote fibrin stiffness and biochemical stability needed for clot contraction38, 39, 40 and protection from fibrinolysis.45 In contrast, only a small amount of FXIII-A is released from activated platelets and only during strong (dual-agonist) stimulation that likely occurs in vivo only when coagulation activation is accompanied by local vascular damage.46,47 Even once exposed, it is not clear whether or how platelet FXIII-A(°/∗) would be spatially directed to accruing fibrin to perform its crosslinking function in a short time frame. Accordingly, platelet FXIII-A(°/∗) antifibrinolytic activity is detectable in platelet lysates only in the presence of low (<20%) plasma FXIII.12 Notably, however, plasma FXIII-A∗ is susceptible to inactivation by oxidation-induced conformational changes and/or thrombin- or plasmin-mediated proteolytic degradation,13,48,49 which may limit its role to the acute setting. A source of FXIII-A that is slowly and nonproteolytically activated and protected from inactivation may be a reservoir for transglutaminase activity needed for specific biological mechanisms and/or events that occur at more delayed time scales (eg, crosslinking cytoskeletal proteins, regulating platelet spreading, and/or facilitating platelet-dependent immune functions or endothelial cell migration and proliferation).21,22,33,50,51 This protected FXIII-A pool may contribute to wound healing or pregnancy, both of which are impaired in FXIII-deficient humans and mice.1,29,52,53 Platelet F13A1 transcripts are lower in atrial fibrillation patients who have embolized thrombi vs patients without emboli,54 implicating platelet F13A1 and specifically its translated product in thrombus stabilization. Although thrombin can induce translation of platelet messages,55, 56, 57, 58 we did not detect F13A1 translation in washed platelets. In each of these situations, FXIII-A retained within or synthesized by trapped platelets may be exposed by exocytosis or passively released as these cells disintegrate.37 Delayed release of FXIII-A from disintegrating cells to the local milieu could circumvent the lack of a FXIII-A secretory peptide and enable platelet FXIII-A to serve extracellular functions in specific settings. These possibilities warrant future studies to determine whether platelet FXIII-A is merely vestigial or to identify biological function(s) for this pool.
This study identified new features mediating platelet FXIII-A dynamics during and after platelet activation but has potential limitations. First, although stimulation with soluble agonists is an established method for studying platelet activation in vitro, interactions with insoluble agonists (eg, fibrin and fibrillar collagen) may alter processes that determine platelet shape change, EV formation, and/or exposure/expulsion of cytoplasmic contents. Second, because we tested relatively high concentrations of GPVI and PAR agonists in which essentially all platelets were P-selectin positive and almost half were phosphatidylserine-positive (data not shown), our data likely overestimate FXIII-A release from platelets in most situations. Third, our findings revealed differences in FXIII-A content and liberation between human and mouse platelets that may have been driven by unique evolutionary pressures on these species. Ultimately, humanized mice may be needed to determine whether these differences alter platelet functions in various settings. Finally, although our experimental strategy (immunoblotting) aptly identified FXIII-A∗, we were not able to differentiate zymogen FXIII-A vs nonproteolytically activated FXIII-A° (these run together on the blot), so the functional state of platelet-associated FXIII-A is unclear.
Collectively, our data show Ca2+-dependent and -independent signaling mediate only limited release of platelet FXIII-A during platelet activation. Although released FXIII-A is subject to thrombin-mediated activation and plasmin-mediated degradation, FXIII-A that remains associated with the platelet is protected from proteolysis. The specific retention and protection of platelet FXIII-A suggests this compartment represents a unique pool of circulating FXIII with as-yet undefined roles in intracellular mechanisms, wound healing, and other settings.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Acknowledgments
The authors thank László Muszbek for the polyclonal anti-FXIII-A antibody, Abigail R. Ballard for technical support, Pablo Ariel for help with the immunofluorescence imaging and analysis, Marina Sokolsky for help with nanoparticle tracking analysis, and Nigel Mackman and Kadri Kangro for reading the manuscript.
The Microscopy Services Laboratory, Department of Pathology and Laboratory Medicine, is supported in part by a Cancer Center Core Support Grant to the UNC Lineberger Comprehensive Cancer Center (P30 CA016086). This study was supported by funding from the American Heart Association (23POST1017878; Y.S.), the OTKA Bridging Fund from the University of Debrecen (É.K.), and the National Institutes of Health, National Heart, Lung, and Blood Institute (R01HL126974 and R01HL147894 [A.S.W.], R01HL168009 [M.J.F.], and R35HL144976 [W.B.]).
Authorship
Contribution: Y.S. performed experiments, analyzed data, and wrote the manuscript; R.H.L. performed experiments and provided advice on experimental design and interpretation; A.L. performed experiments; É.K. provided essential reagents; C.S.W., N.L.S., M.J.F., and N.J.M. interpreted data and edited the manuscript; A.E.M. provided essential reagents, interpreted data, and edited the manuscript; W.B. provided advice on experimental design and interpretation, and edited the manuscript; A.S.W. conceived of the study, interpreted data, and wrote the manuscript; and all authors reviewed, edited, and approved the final version of the manuscript.
Footnotes
Original data are available upon reasonable request from the corresponding author, Alisa S. Wolberg (alisa_wolberg@med.unc.edu).
The full-text version of this article contains a data supplement.
Supplementary Material
References
- 1.Muszbek L, Bereczky Z, Bagoly Z, Komaromi I, Katona E. Factor XIII: a coagulation factor with multiple plasmatic and cellular functions. Physiol Rev. 2011;91(3):931–972. doi: 10.1152/physrev.00016.2010. [DOI] [PubMed] [Google Scholar]
- 2.Katona E E, Ajzner E, Toth K, Karpati L, Muszbek L. Enzyme-linked immunosorbent assay for the determination of blood coagulation factor XIII A-subunit in plasma and in cell lysates. J Immunol Methods. 2001;258(1-2):127–135. doi: 10.1016/s0022-1759(01)00479-3. [DOI] [PubMed] [Google Scholar]
- 3.Mills EW, Green R, Ingolia NT. Slowed decay of mRNAs enhances platelet specific translation. Blood. 2017;129(17):e38–e48. doi: 10.1182/blood-2016-08-736108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohammed RDS, Ablan FDO, McCann NM, Hindi MM, Maurer MC. Transglutaminase activities of blood coagulant factor XIII are dependent on the activation pathways and on the substrates. Thromb Haemost. 2023;123(4):380–392. doi: 10.1055/a-1993-4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anokhin BA, Dean WL, Smith KA, et al. Proteolytic and nonproteolytic activation mechanisms result in conformationally and functionally different forms of coagulation factor XIII A. FEBS J. 2020;287(3):452–464. doi: 10.1111/febs.15040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muszbek L, Polgar J, Boda Z. Platelet factor XIII becomes active without the release of activation peptide during platelet activation. Thromb Haemost. 1993;69(3):282–285. [PubMed] [Google Scholar]
- 7.Abdel-Samad N. Treatment with recombinant factor XIII (Tretten) in a pregnant woman with factor XIII deficiency. Am J Case Rep. 2017;18:436–439. doi: 10.12659/AJCR.901502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beckman JD, Kasthuri RS, Wolberg AS, Ma AD. Challenges in diagnosis and management of acquired factor XIII (FXIII) inhibitors. Haemophilia. 2018;24(6):e417–e420. doi: 10.1111/hae.13603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joist JH, Niewiarowski S. Retention of platelet fibrin stabilizing factor during the platelet release reaction and clot retraction. Thromb Diath Haemorrh. 1973;29(3):679–683. [PubMed] [Google Scholar]
- 10.Nagy B, Jr., Simon Z, Bagoly Z, Muszbek L, Kappelmayer J. Binding of plasma factor XIII to thrombin-receptor activated human platelets. Thromb Haemost. 2009;102(1):83–89. doi: 10.1160/TH09-01-0054. [DOI] [PubMed] [Google Scholar]
- 11.Somodi L, Beke Debreceni I, Kis G, et al. Activation mechanism dependent surface exposure of cellular factor XIII on activated platelets and platelet microparticles. J Thromb Haemost. 2022;20(5):1223–1235. doi: 10.1111/jth.15668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitchell JL, Lionikiene AS, Fraser SR, Whyte CS, Booth NA, Mutch NJ. Functional factor XIII-A is exposed on the stimulated platelet surface. Blood. 2014;124(26):3982–3990. doi: 10.1182/blood-2014-06-583070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hur WS, Mazinani N, Lu XJ, et al. Coagulation factor XIIIa is inactivated by plasmin. Blood. 2015;126(20):2329–2337. doi: 10.1182/blood-2015-07-650713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jonnalagadda D, Izu LT, Whiteheart SW. Platelet secretion is kinetically heterogeneous in an agonist-responsive manner. Blood. 2012;120(26):5209–5216. doi: 10.1182/blood-2012-07-445080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bryckaert M, Rosa JP, Denis CV, Lenting PJ. Of von Willebrand factor and platelets. Cell Mol Life Sci. 2015;72(2):307–326. doi: 10.1007/s00018-014-1743-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maroney SA, Haberichter SL, Friese P, et al. Active tissue factor pathway inhibitor is expressed on the surface of coated platelets. Blood. 2007;109(5):1931–1937. doi: 10.1182/blood-2006-07-037283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilio K, van Kruchten R, Braun A, et al. Roles of platelet STIM1 and Orai1 in glycoprotein VI- and thrombin-dependent procoagulant activity and thrombus formation. J Biol Chem. 2010;285(31):23629–23638. doi: 10.1074/jbc.M110.108696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pasquet JM, Dachary-Prigent J, Nurden AT. Calcium influx is a determining factor of calpain activation and microparticle formation in platelets. Eur J Biochem. 1996;239(3):647–654. doi: 10.1111/j.1432-1033.1996.0647u.x. [DOI] [PubMed] [Google Scholar]
- 19.Bergmeier W, Weidinger C, Zee I, Feske S. Emerging roles of store-operated Ca(2)(+) entry through STIM and ORAI proteins in immunity, hemostasis and cancer. Channels. 2013;7(5):379–391. doi: 10.4161/chan.24302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee RH, Stefanini L, Bergmeier W. In: Platelets. Michelson AD, editor. Academic Press; 2019. Platelet signal transduction; pp. 329–348. [Google Scholar]
- 21.Serrano K, Devine DV. Intracellular factor XIII crosslinks platelet cytoskeletal elements upon platelet activation. Thromb Haemost. 2002;88(2):315–320. [PubMed] [Google Scholar]
- 22.Rex S, Beaulieu LM, Perlman DH, et al. Immune versus thrombotic stimulation of platelets differentially regulates signalling pathways, intracellular protein-protein interactions, and alpha-granule release. Thromb Haemost. 2009;102(1):97–110. doi: 10.1160/TH08-08-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holme PA, Brosstad F, Solum NO. The difference between platelet and plasma FXIII used to study the mechanism of platelet microvesicle formation. Thromb Haemost. 1993;70(4):681–686. [PubMed] [Google Scholar]
- 24.Sachetto ATA, Archibald SJ, Hisada Y, et al. Tissue factor activity of small and large extracellular vesicles in different diseases. Res Pract Thromb Haemost. 2023;7(3) doi: 10.1016/j.rpth.2023.100124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krishnamachary B, Cook C, Kumar A, Spikes L, Chalise P, Dhillon NK. Extracellular vesicle-mediated endothelial apoptosis and EV-associated proteins correlate with COVID-19 disease severity. J Extracell Vesicles. 2021;10(9) doi: 10.1002/jev2.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whyte CS, Swieringa F, Mastenbroek TG, et al. Plasminogen associates with phosphatidylserine-exposing platelets and contributes to thrombus lysis under flow. Blood. 2015;125(16):2568–2578. doi: 10.1182/blood-2014-09-599480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whyte CS, Morrow GB, Baik N, et al. Exposure of plasminogen and a novel plasminogen receptor, Plg-RKT, on activated human and murine platelets. Blood. 2021;137(2):248–257. doi: 10.1182/blood.2020007263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kotova YN, Podoplelova NA, Obydennyy SI, et al. Binding of coagulation factor XIII zymogen to activated platelet subpopulations: roles of integrin alphaIIbbeta3 and fibrinogen. Thromb Haemost. 2019;119(6):906–915. doi: 10.1055/s-0039-1683912. [DOI] [PubMed] [Google Scholar]
- 29.Souri M, Koseki-Kuno S, Takeda N, Degen JL, Ichinose A. Administration of factor XIII B subunit increased plasma factor XIII A subunit levels in factor XIII B subunit knock-out mice. Int J Hematol. 2008;87(1):60–68. doi: 10.1007/s12185-007-0005-z. [DOI] [PubMed] [Google Scholar]
- 30.Greenberg CS, Shuman MA. Specific binding of blood coagulation factor XIIIa to thrombin-stimulated platelets. J Biol Chem. 1984;259(23):14721–14727. [PubMed] [Google Scholar]
- 31.Cox AD, Devine DV. Factor XIIIa binding to activated platelets is mediated through activation of glycoprotein IIb-IIIa. Blood. 1994;83(4):1006–1016. [PubMed] [Google Scholar]
- 32.Magwenzi SG, Ajjan RA, Standeven KF, Parapia LA, Naseem KM. Factor XIII supports platelet activation and enhances thrombus formation by matrix proteins under flow conditions. J Thromb Haemost. 2011;9(4):820–833. doi: 10.1111/j.1538-7836.2011.04234.x. [DOI] [PubMed] [Google Scholar]
- 33.Mitchell JL, Little G, Bye AP, et al. Platelet factor XIII-A regulates platelet function and promotes clot retraction and stability. Res Pract Thromb Haemost. 2023;7(5) doi: 10.1016/j.rpth.2023.100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chu Y, Guo H, Zhang Y, Qiao R. Procoagulant platelets: generation, characteristics, and therapeutic target. J Clin Lab Anal. 2021;35(5) doi: 10.1002/jcla.23750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mattheij NJ, Swieringa F, Mastenbroek TG, et al. Coated platelets function in platelet-dependent fibrin formation via integrin alphaIIbbeta3 and transglutaminase factor XIII. Haematologica. 2016;101(4):427–436. doi: 10.3324/haematol.2015.131441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gasecka A, Nieuwland R, Siljander PRM. In: Platelets. Michelson AD, editor. Academic Press; 2019. Platelet-derived extracellular vesicles; pp. 401–416. [Google Scholar]
- 37.Kim OV, Nevzorova TA, Mordakhanova ER, et al. Fatal dysfunction and disintegration of thrombin-stimulated platelets. Haematologica. 2019;104(9):1866–1878. doi: 10.3324/haematol.2018.202309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aleman MM, Byrnes JR, Wang JG, et al. Factor XIII activity mediates red blood cell retention in venous thrombi. J Clin Invest. 2014;124(8):3590–3600. doi: 10.1172/JCI75386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Byrnes JR, Duval C, Wang Y, et al. Factor XIIIa-dependent retention of red blood cells in clots is mediated by fibrin alpha-chain crosslinking. Blood. 2015;126(16):1940–1948. doi: 10.1182/blood-2015-06-652263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kattula S, Byrnes JR, Martin SM, et al. Factor XIII in plasma, but not in platelets, mediates red blood cell retention in clots and venous thrombus size in mice. Blood Adv. 2018;2(1):25–35. doi: 10.1182/bloodadvances.2017011890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kasahara K, Souri M, Kaneda M, Miki T, Yamamoto N, Ichinose A. Impaired clot retraction in factor XIII A subunit-deficient mice. Blood. 2010;115(6):1277–1279. doi: 10.1182/blood-2009-06-227645. [DOI] [PubMed] [Google Scholar]
- 42.Rao KM, Newcomb TF. Clot retraction in a factor XIII free system. Scand J Haematol. 1980;24(2):142–148. doi: 10.1111/j.1600-0609.1980.tb02358.x. [DOI] [PubMed] [Google Scholar]
- 43.Jelenska M, Kopec M, Breddin K. On the retraction of collagen and fibrin induced by normal, defective and modified platelets. Haemostasis. 1985;15(3):169–175. doi: 10.1159/000215140. [DOI] [PubMed] [Google Scholar]
- 44.Byrnes JR, Wilson C, Boutelle AM, et al. The interaction between fibrinogen and zymogen FXIII-A2B2 is mediated by fibrinogen residues gamma390-396 and the FXIII-B subunits. Blood. 2016;128(15):1969–1978. doi: 10.1182/blood-2016-04-712323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alshehri FSM, Whyte CS, Mutch NJ. Factor XIII-A: an indispensable "factor" in haemostasis and wound healing. Int J Mol Sci. 2021;22(6):3055. doi: 10.3390/ijms22063055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carminita E, Tourn J, Crescence L, et al. A thrombus is formed by a gradient of platelet activation and procoagulant endothelium. Res Pract Thromb Haemost. 2023;7(7) doi: 10.1016/j.rpth.2023.102209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stalker TJ, Traxler EA, Wu J, et al. Hierarchical organization in the hemostatic response and its relationship to the platelet-signaling network. Blood. 2013;121(10):1875–1885. doi: 10.1182/blood-2012-09-457739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robinson BR, Houng AK, Reed GL. Catalytic life of activated factor XIII in thrombi. Implications for fibrinolytic resistance and thrombus aging. Circulation. 2000;102(10):1151–1157. doi: 10.1161/01.cir.102.10.1151. [DOI] [PubMed] [Google Scholar]
- 49.Takahashi N, Takahashi Y, Putnam FW. Primary structure of blood coagulation factor XIIIa (fibrinoligase, transglutaminase) from human placenta. Proc Natl Acad Sci U S A. 1986;83(21):8019–8023. doi: 10.1073/pnas.83.21.8019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dardik R, Solomon A, Loscalzo J, et al. Novel proangiogenic effect of factor XIII associated with suppression of thrombospondin 1 expression. Arterioscler Thromb Vasc Biol. 2003;23(8):1472–1477. doi: 10.1161/01.ATV.0000081636.25235.C6. [DOI] [PubMed] [Google Scholar]
- 51.Herter JM, Rossaint J, Zarbock A. Platelets in inflammation and immunity. J Thromb Haemost. 2014;12(11):1764–1775. doi: 10.1111/jth.12730. [DOI] [PubMed] [Google Scholar]
- 52.Inbal A, Lubetsky A, Krapp T, et al. Impaired wound healing in factor XIII deficient mice. Thromb Haemost. 2005;94(2):432–437. doi: 10.1160/TH05-04-0291. [DOI] [PubMed] [Google Scholar]
- 53.Kleber C, Sablotzki A, Casu S, et al. The impact of acquired coagulation factor XIII deficiency in traumatic bleeding and wound healing. Crit Care. 2022;26(1):69. doi: 10.1186/s13054-022-03940-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gosk-Bierska I, McBane RD, Wu Y, et al. Platelet factor XIII gene expression and embolic propensity in atrial fibrillation. Thromb Haemost. 2011;106(1):75–82. doi: 10.1160/TH10-11-0765. [DOI] [PubMed] [Google Scholar]
- 55.Weyrich AS, Dixon DA, Pabla R, et al. Signal-dependent translation of a regulatory protein, Bcl-3, in activated human platelets. Proc Natl Acad Sci U S A. 1998;95(10):5556–5561. doi: 10.1073/pnas.95.10.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pabla R, Weyrich AS, Dixon DA, et al. Integrin-dependent control of translation: engagement of integrin alphaIIbbeta3 regulates synthesis of proteins in activated human platelets. J Cell Biol. 1999;144(1):175–184. doi: 10.1083/jcb.144.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weyrich AS, Denis MM, Schwertz H, et al. mTOR-dependent synthesis of Bcl-3 controls the retraction of fibrin clots by activated human platelets. Blood. 2007;109(5):1975–1983. doi: 10.1182/blood-2006-08-042192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wurtzel JGT, Lazar S, Askari S, et al. Plasma growth factors maintain constitutive translation in platelets to regulate reactivity and thrombotic potential. Blood Adv. 2024;8(6):1550–1566. doi: 10.1182/bloodadvances.2023011734. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.