ABSTRACT
Antimicrobial resistance is extremely common in Mycoplasma genitalium, a frequent cause of urethritis in men and cervicitis, vaginitis, and pelvic inflammatory disease in women. Treatment of M. genitalium infections is difficult due to intrinsic and acquired resistance to many antibiotic classes. We undertook a program to identify novel antimicrobials with activity against M. genitalium from fungal natural products. Extracts of Ramularia coccinea contained a molecule with potent activity that was subsequently identified as fusidic acid, a fusidane-type antibiotic that has been in clinical use for decades outside the United States. We found that minimum inhibitory concentrations of fusidic acid ranged from 0.31 to 4 µg/mL among 17 M. genitalium strains including laboratory-passaged and low-passage clinical isolates. Time-kill data indicate that bactericidal killing occurs when M. genitalium is exposed to ≥10 µg/mL for 48 h, comparing favorably to serum concentrations obtained from typical loading dose regimens. Resistance to fusidic acid was associated with mutations in fusA consistent with the known mechanism of action in which fusidic acid inhibits protein synthesis by binding to elongation factor G. Interestingly, no mutants resistant to >10 µg/mL fusidic acid were obtained and a resistant strain containing a F435Y mutation in FusA was impaired for growth in vitro. These data suggest that fusidic acid may be a promising option for the treatment of M. genitalium infections.
KEYWORDS: Mycoplasma genitalium, fusidic acid, antimicrobial resistance, fungal natural products
INTRODUCTION
Mycoplasma genitalium is a slow-growing, atypical bacterium associated with reproductive tract disease including urethritis in men and vaginitis, cervicitis, and pelvic inflammatory disease in women (1). Recent surveillance data estimated that the overall prevalence of M. genitalium was 16.6% among individuals seeking care at sexual health clinics in six US cities (2).
Treatment of M. genitalium infections is becoming increasingly difficult. As M. genitalium lacks the targets of commonly used antimicrobials (e.g., peptidoglycan, outer membrane/LPS, and folic acid synthesis pathways), it is intrinsically resistant to these agents. An rpoB mutation common to all Mollicutes confers resistance to rifampin (3). Furthermore, the poor efficacy of doxycycline (30–40% effective) and increasing acquired resistance to macrolides (>60% of US strains) and fluoroquinolones (>10%) have resulted in the appearance of multidrug-resistant strains (4). Treatment of strains resistant to both azithromycin and moxifloxacin is challenging as few drugs with proven efficacy are available in the United States. For these reasons, M. genitalium was placed on the CDC Watch List of Antibiotic Resistance Threats in 2019.
To address the acute need for new treatments, we embarked on a collaborative effort to identify molecules with activity against M. genitalium within libraries of fungal natural products. Here, we report the identification of fusidic acid produced by Ramularia coccinea, its in vitro activity against multiple strains of M. genitalium, killing kinetics and mechanism of resistance. These data suggest that fusidic acid, an antibiotic used safely for decades outside the United States for other indications, may represent a promising option to treat drug-resistant M. genitalium infections.
RESULTS
Identification of fusidic acid in fungal extracts
We screened approximately 4,200 extracts prepared from fungi that are part of the Natural Products Discovery Group library housed at the University of Oklahoma, a collection that contains fungi derived from diverse ecological niches across the United States. Initial library screening demonstrated that a fungus identified by ITS sequencing as R. coccinea produced a substance with activity against M. genitalium. The minimum inhibitory concentration (MIC) of the crude extract was 31 µg/mL as determined in microbroth dilution assays. Cytotoxicity assays were performed against Vero cells to assess selectivity for M. genitalium. Vero cells were exposed to crude extracts for 48 h then cytotoxicity was measured using an Alamar blue reduction assay. We detected no cytotoxicity with crude R. coccinea extract as high as 775 µg/mL (25× MIC). Fresh crude extracts from scale-up cultures were also active against M. genitalium (MIC 2 µg/mL) thus confirming that the active molecule is consistently produced by this fungus. Through a process of bioassay-guided fractionation (Fig. 1), we identified a fraction containing a single purified compound. Analysis of spectroscopic data determined that the compound was fusidic acid (Fig. S1 and S2), a fusidane triterpene-based antibiotic first identified in the 1960s as a natural product produced by Fusarium coccineum (5). Fusidic acid is approved for clinical use outside the United States (>20 countries) in oral, intravenous, or topical forms to treat various types of Staphylococcus aureus infections (e.g., wound infections, pneumonia, osteomyelitis, and septicemia) (6). Helvolic acid—another fusidane-type molecule obtained from our NPDG pure compound library—was also active against M. genitalium with complete inhibition of growth by ≤50 µM. As helvolic acid is not used clinically, its activity against M. genitalium was not investigated further.
Fig 1.
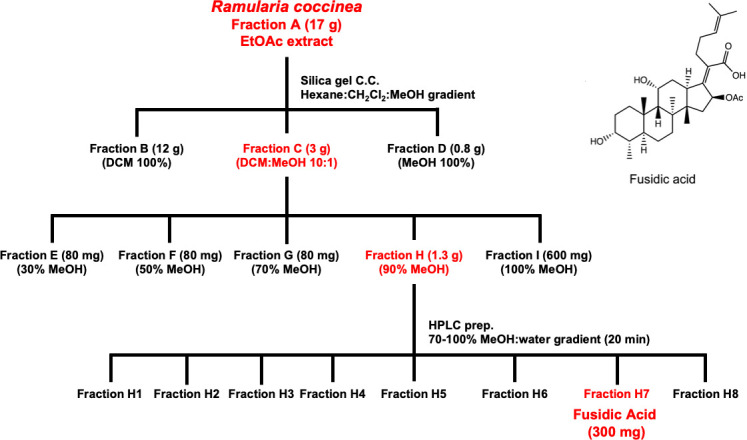
Bioassay guided fractionation of Ramularia coccinea extract to yield purified fusidic acid. The fractions with the highest activity are shown in red.
Susceptibility of M. genitalium strains to fusidic acid
The fusidic acid MIC was determined for eight M. genitalium strains, including the G37 type strain and other laboratory passaged, broth-adapted strains using commercially available fusidic acid. MICs, determined by assessing color change in microbroth dilution assays, ranged from 0.63 to 2.5 µg/mL MICs (Table 1). To precisely measure inhibition, and confirm dose response, we quantified the growth of three strains of M. genitalium in these microbroth dilution assays by qPCR. The IC50 for these strains was similar: 0.59 ± 0.17 for G37, 0.20 ± 0.08 for Sea-1, and 0.55 ± 0.07 for Sea-2 (Fig. 2).
TABLE 1.
In vitro susceptibility of M. genitalium strains to fusidic acidf
| Strain designation | Strain typea, year of isolation, location | MIC (µg/mL) | |||
|---|---|---|---|---|---|
| Fusidic acid | Doxycycline | Moxifloxacin | Azithromycin | ||
| Microbroth dilutionb | |||||
| G37 | J-1, 1980, United Kingdom | 1.25 | 0.25 | 0.125 | 0.002 |
| Sea-1 | J-39, 1998, Seattle, WA, USA | 0.63 | 0.004c | ND | 0.002 |
| Sea-2 | J-6, 1998, Seattle, USA | 2.5 | 0.004 | ND | 0.002 |
| M30 | J-2, 1980, United Kingdom | 2.5 | 0.5 | 0.125 | 0.008 |
| TW60 | ND, 2000, San Antonio TX, USA | 1.25 | ND | ND | ND |
| M2282 | J-5, 1991, Denmark | 0.31 | 0.5 | 0.25 | <0.002 |
| M2300 | J-20, 1991, Denmark | 1.25 | 0.125 | 0.125 | <0.002 |
| M2341 | J-2, 1991, Denmark | 0.63 | 0.04 | ND | ND |
| Vero cell coculturec | |||||
| MEGA 216 | J-39, 2008, Seattle, WA, USA | <2 | 2 | ND | >8 A2058Cd |
| MEGA 552 | J-6, 2008, Seattle, WA, USA | <4 | 1 | ND | >8 A2058G |
| MEGA 601 | J-2, 2008, Seattle, WA, USA | 2 | 0.25 | ND | 0.004 |
| MEGA 1082 | GB-6, 2009, Seattle, WA, USA | 4 | 0.25 | >1 G248T (Ser83I)e |
>8 A2058G |
| MEGA 1202 | 43ND, 2009, Seattle, WA, USA | 4 | 0.5 | ND | >8 A2059G |
| MEGA 1256 | GB-2, 2009, Seattle, WA, USA | <2 | 0.25 | ND | >8 A2058G |
| MEGA 1272 | J-51, 2009, Seattle, WA, USA | <2 | 0.25 | ND | >8 A2059G |
| MEGA 1568 | ND, 2010, Seattle, WA, USA | <2 | 0.5 | ND | <0.001 |
| MEGA 1606 | ND, 2010, Seattle, WA, USA | 4 | 1 | ND | 0.002 |
MIC defined as lowest concentration with no color change.
MIC is the concentration inhibiting growth by ≥99% as compared to untreated M. genitalium determined by qPCR.
Macrolide resistance mutation in 23S rRNA gene (7).
Quinolone resistance mutation in the parC gene (amino acid change).
ND, not determined.
Fig 2.
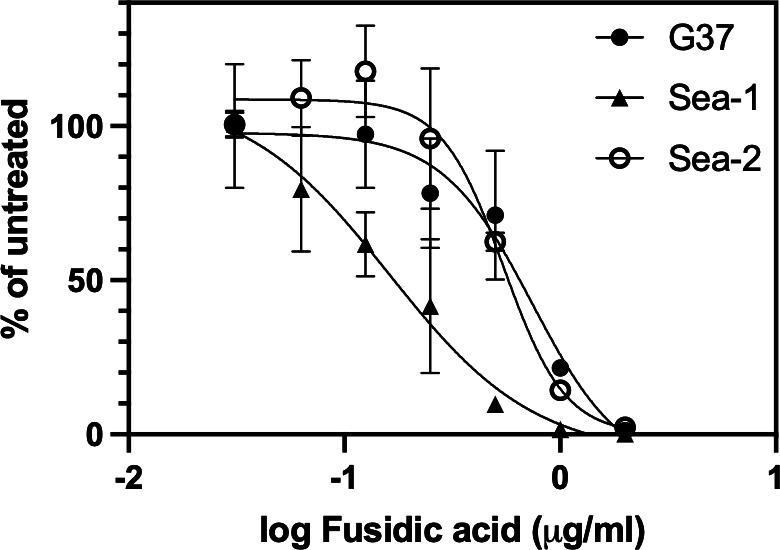
Growth inhibition dose response of M. genitalium strains G37, Sea-1, and Sea-2 to fusidic acid. The y-axis shows the mean number genomes detected in triplicate qPCR measurements as a percentage of untreated M. genitalium. Errors bars show standard deviation of triplicate drug-treated wells. The experiment was repeated two times with similar results.
To determine if fusidic acid susceptibility is a general characteristic of M. genitalium strains, we determined MICs for nine low-passage clinical isolates that have not been adapted to axenic culture and are dependent on Vero coculture for growth. These strains were isolated from men with non-gonococcal urethritis who were enrolled in a trial comparing azithromycin and doxycycline for treatment of M. genitalium infection conducted from 2007 to 2011 (7), and represent a variety of mgpB strain types and azithromycin and moxifloxacin resistance mutations (9). Fusidic acid MICs for these clinical isolates ranged from <2 to 4 µg/mL. Considering all M. genitalium strains tested the MIC50 was 2 µg/mL and the MIC90 was 4 µg/mL.
Bactericidal activity of fusidic acid
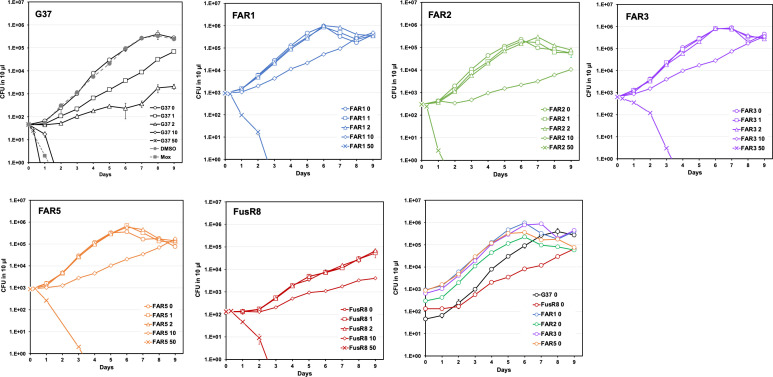
Time-killing kinetics were investigated by exposing M. genitalium strain G37 to fusidic acid concentrations ranging from 0 to 50 µg/mL in SP-4 broth cultures. Aliquots were removed at intervals (0 and 8 h, then daily for 9 days) and then dilution plated on SP-4 agar plates in triplicate. As shown in Fig. 3 (upper left), fusidic acid at 1 and 2 µg/mL inhibited the growth of wild-type strain G37 by 90–99% as compared to untreated or solvent control-treated cultures. Fusidic acid at 10 and 50 µg/mL was bactericidal and killed >99.9% of M. genitalium in 48 or 24 h, respectively.
Fig 3.
Time-kill experiments. Wild-type and fusidic acid-resistant M. genitalium were treated with various concentrations of fusidic acid over time. Curves show the average colony-forming units per 10 µL aliquot spotted in triplicate on SP-4 agar plates. Error bars indicate standard deviation. Dimethyl sulfoxide (DMSO, solvent control) was added at 0.5% corresponding to the highest drug concentration. Moxifloxacin (Mox, positive control) was used at 0.25 µg/mL. The last panel shows the growth of each strain in the absence of fusidic acid. Results of a typical experiment repeated two times are shown.
Resistance to fusidic acid
To investigate fusidic acid resistance potential in M. genitalium, we determined the resistance rate by plating M. genitalium strain G37 onto SP-4 agar plates containing fusidic acid at 10, 25, or 50 µg/mL or on SP-4 agar without fusidic acid to quantify the inoculum. Colonies were visible after 2 weeks of incubation on 10 µg/mL fusidic acid, but no colonies appeared on 25 or 50 µg/mL fusidic acid. When compared to the inoculum, the resistance rate was calculated as ~5 × 10−7 on 10 µg/mL and <3 × 10−7 on 25 and 50 µg/mL. The colonies growing on 10 µg/mL had an atypical morphology (flat colonies rather than the “fried egg” morphology characteristic of M. genitalium). All eight colonies grew when subcultured to plain SP-4 broth, but fewer than half grew in 10 µg/mL fusidic acid suggesting that not all clones were truly resistant. Four clones (named FAR1, FAR2, FAR3, and FAR5) that grew at 10 µg/mL were chosen for further analysis. In a complementary strategy, we isolated fusidic acid-resistant mutants by serial passage of strain G37 in increasing concentrations of fusidic acid. This approach yielded a culture that grew slowly in 3 µg/mL fusidic acid. We obtained single colonies after filtering through 0.45 µm and then characterized the resulting clone, FusR8, as described below.
Characterization of fusidic acid-resistant M. genitalium
Using microbroth dilution assays, we determined that the MIC for each of the five fusidic acid-resistant mutants was 6.3 µg/mL, fivefold higher than wild-type strain G37. Growth curve and time-kill experiments were performed with each resistant mutant (Fig. 3). As expected, growth of these resistant strains was unaffected by fusidic acid at 1 and 2 µg/mL. Growth was inhibited 94–99% by 10 µg/mL and >99.9% killing was observed after 48–96 h in 50 µg/mL. Interestingly, growth of one mutant was slower than the parent strain in plain SP-4 with doubling times of 13.1 ± 1.82 and 27.8 ± 5.47 h for G37 and FusR8, respectively (P = 0.006, Student’s one-tailed t test for independent samples). The growth rate of fusidic acid-resistant mutants FAR1, FAR2, FAR3, and FAR5 did not differ significantly from wild-type G37 with doubling times ranging from 11.5 to 12.5 h.
We tested whether fusidic acid resistance affected the susceptibility of M. genitalium to doxycycline and moxifloxacin in the resistant mutants. All of the fusidic acid-resistant mutants had MICs for doxycycline and moxifloxacin identical to the parent G37 strain (0.25 and 0.125 µg/mL, respectively). Furthermore, clinical isolates resistant to azithromycin and/or moxifloxacin had low fusidic acid MICs that were similar to strains that are susceptible to these antibiotics (Table 1) thus supporting that resistance to macrolides and fluoroquinolones does not affect susceptibility to fusidic acid.
Identification of fusidic acid resistance-associated mutations
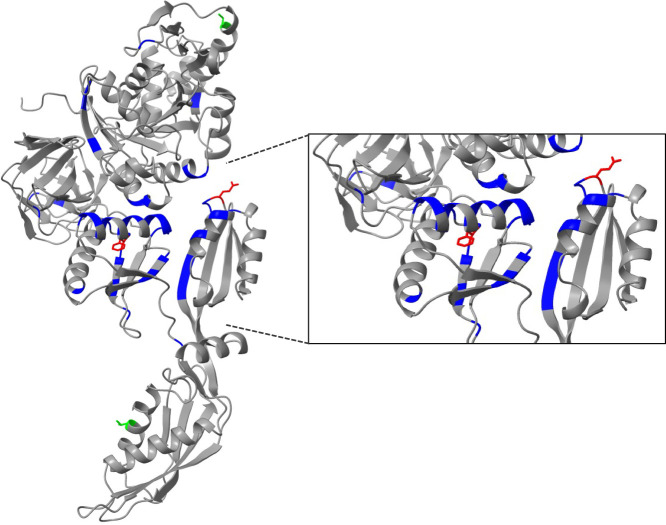
Whole-genome sequencing was performed on all five fusidic acid-resistant clones to identify resistance associated mutations and infer the target of fusidic acid in M. genitalium. We identified a single base change in the MG_089 gene, encoding FusA, also known as elongation factor G (EF-G), in all five fusidic acid-resistant mutants that were not present in the wild-type G37 parent strain maintained in our laboratory [sequenced previously (10)]. Mutants FAR1, FAR3, and FAR5 each acquired an A to G point mutation at base pair 116,786 (bp 1,979 of fusA) predicting a Q660R mutation in FusA. Mutant FAR2 contained a C to A mutation at bp 116,785 (bp 1,978 of fusA) encoding a Q660K mutation. A single T to A point mutation at base pair 116,111 (bp 1,304 of fusA) occurred in the FusR8 mutant predicting an F435Y mutation in FusA. The absence of other mutations in FusR8 suggested that the F435Y mutation also affected the growth of this strain. The presence of mutations in fusA is consistent with the known mechanism in which fusidic acid binds to FusA to inhibit translation. The S. aureus FusA has been co-crystallized with fusidic acid (11). Given the similarity of the structure of M. genitalium FusA predicted by AlphaFold (PDB P47335) to S. aureus FusA, the F435Y mutation is likely to affect the fusidic acid binding pocket, whereas Q660L/R may affect the interaction of EF-G with the ribosome (11). Figure 4 shows the location of the M. genitalium and S. aureus resistance-associated mutations (6, 11) (in red and blue, respectively) mapped onto the predicted M. genitalium FusA structure. These data provide further evidence that fusidic acid prevents the growth of M. genitalium by binding to FusA and inhibiting protein synthesis (12).
Fig 4.
Structure of M. genitalium FusA predicted by AlphaFold (P47335). Close-up shows fusidic acid binding pocket. The locations of amino acids associated with fusidic acid resistance are shown in blue (S. aureus) and red (M. genitalium). Two variant residues identified among M. genitalium strains that are unlikely to affect fusidic acid susceptibility are shown in lime green.
Fusidic acid resistance-associated mutations in Mycoplasma spp.
To determine if fusidic acid resistance mutations are present among M. genitalium strains, we aligned the G37 FusA protein sequence with that of 23 fully genome-sequenced strains (13). FusA is highly conserved among these strains with variability at only two residues: V or A at amino acid 204 and T or A at amino acid 540. These amino acids are distant from the fusidic acid binding pocket (Fig. 4, lime green) and neither of these residues is implicated in fusidic acid resistance in S. aureus (6, 11). Furthermore, no M. genitalium strains had mutations in F435 or Q660. Taken together, these results suggest that most M. genitalium strains express a fusidic acid susceptible FusA. In addition, F435 and Q660 are conserved in FusA among more than 20 Mycoplasma species. F435 is conserved among four M. fermentans sequenced strains (PG18, JER, MF-I1, and M64), but Q660 is conserved in only one strain (PG18) and three strains (JER, MF-I1, and M64) instead have A660. Interestingly, M. fermentans fusidic acid MICs ranged from 2.5 to 25 µg/mL with an MIC50 of 10 µg/mL and MIC90 of 25 µg/mL suggesting that most strains are resistant (14). It is tempting to speculate that the Q660A mutation confers fusidic acid resistance in these M. fermentans strains.
DISCUSSION
Antimicrobial resistance complicates the treatment of most M. genitalium infections. This unique bacterium lacks a cell wall and outer membrane rendering antibiotics targeting these structures, such as b-lactams and colistins, respectively, entirely ineffective. Additionally, a mutation in rpoB common to all Mollicutes imparts resistance to rifampins, and the absence of folic acid synthesis pathways in these organisms makes treatment with sulfonamides and trimethoprim futile. Natural product libraries are invaluable for identifying agents effective against M. genitalium, including novel molecules like xanthoquinodins, N-hydroxypyridones, and tetramic acids [(15, 16) and Peramuna et al., submitted]. Additionally, they have helped uncover new activities in previously known agents, including nitroimidazoles (10) and fusidic acid, as outlined herein. We demonstrated that fusidic acid has potent activity (MIC90 = 4 µg/mL) against a variety of laboratory-passaged and low-passage clinical isolates of M. genitalium. Bactericidal killing was observed when M. genitalium was exposed to ≥10 µg/mL of fusidic acid, well below plasma concentrations resulting from typical treatment regimens (12). Mutations in M. genitalium fusA confer resistance to fusidic acid consistent with direct interaction of this drug with EF-G and its known mechanism of action. Although we obtained resistant M. genitalium in vitro, at least one strain had a reduced growth rate suggesting that some mutations could confer a competitive disadvantage. Fusidic acid may prove useful as an alternative treatment for multidrug-resistant M. genitalium or in individuals for whom current front-line agents are contraindicated.
Fusidic acid, a fusidane triterpene-based antibiotic, was first identified in the 1960s as a natural product produced by Fusarium coccineum that inhibits primarily Gram-positive organisms. Approved for clinical use outside the United States (>20 countries) for decades, fusidic acid is available in oral, intravenous, or topical forms for indications such as methicillin-resistant S. aureus infection. Fusidic acid binds to EF-G, a component of the ribosome that catalyzes the translocation of the growing peptide from the A to P site. When fusidic acid binds EF-G, the complex is trapped in the A site and protein synthesis is stalled. Dozens of fusidic acid analogs have been tested including synthetic (5, 17) and naturally occurring (cephalosporin P and helvolic acid) molecules. None are more potent than fusidic acid against susceptible Gram-positive organisms, but one analog has improved activity against fusidic acid-resistant S. aureus both in vitro and in a mouse thigh infection model (18).
The mechanisms of fusidic acid resistance have been extensively studied, particularly in S. aureus as fusidic acid is indicated for the treatment of local and systemic infections (12). In staphylococci, fusidic acid resistance (MIC >1 µg/mL as defined by the European Committee on Antimicrobial Susceptibility Testing) arises from spontaneous mutations in fusA or rplF with different mutations conferring different levels of resistance. For example, S. aureus FusA P406L mutants are resistant to 8 µg/mL, H457Y to 64 µg/mL, and L461K to >256 µg/mL as compared to 0.032 µg/mL for the parent susceptible strain (19). Some mutations conferring high-level resistance (e.g., S. aureus FusA F88L MIC >64 µg/mL) also affect growth rate (20), similar to the M. genitalium FusA F435Y mutation identified in this study. However, secondary mutations (e.g., M16I) can restore fitness in S. aureus without reducing MIC. Because of the high rate of spontaneous resistance, a second antibiotic is recommended (e.g., rifampin) to reduce selection of resistant S. aureus and improve treatment outcomes (12). However, recent data suggest that co-administration of rifampin lowers plasma concentrations of fusidic acid potentially reducing clinical efficacy and increasing the opportunity for resistance development (21). As noted above, all Mollicutes are resistant to rifampin so this strategy would be ineffective in reducing fusidic acid resistance development in M. genitalium.
In S. aureus, the frequency of spontaneous resistance in vitro decreases with higher fusidic acid concentrations: 10−6 at 2× MIC versus 10−8 at 16× MIC (12). We observed a similar phenomenon, where fewer resistant M. genitalium colonies emerged at higher fusidic acid concentrations, and no clones capable of consistent growth in concentrations greater than 25 µg/mL were obtained. Future experiments will assess whether high-level resistance can be selected during long-term passage in low concentrations of fusidic acid, and whether second-site mutations can restore normal growth in the F435Y mutant.
Although more than 30 resistance mutations in fusA have been described in S. aureus during in vitro selection and in clinical isolates, fusidic acid resistance in staphylococci more commonly develops via horizontal acquisition of the fusB, fusC, fusD, or fusF genes encoding EF-G protection proteins (6, 22). These small proteins, each under 25 kDa, interact with EF-G when fusidic acid is bound inducing a conformational shift, which releases EF-G from the stalled ribosome complex allowing translation to resume. Acquisition of resistance genes via horizontal transfer has not been demonstrated in M. genitalium clinical isolates although a mechanism for low-frequency horizontal gene transfer in vitro has been described (23). The non-canonical genetic code used by M. genitalium in which the typical TGA stop codon encodes tryptophan (24) may hinder gene acquisition from other bacterial species.
Safety and pharmacokinetics of fusidic acid have been well documented. Single-dose fusidic acid results in high plasma concentrations ranging from 33 µg/mL for 550 mg to 93 µg/mL for 1650 mg (25). When a loading dose regimen is used (e.g., 1650 mg bid, then 825 mg bid) mean trough plasma concentrations reach 146 µg/mL at 24 h rising to 204 µg/mL after 8 days. Importantly, these high doses were well tolerated and effective in a US phase 2 trial for acute bacterial skin and skin structure infections (25, 26). Compared to our in vitro killing data, these pharmacokinetic data suggest that cure of both fusidic acid susceptible and resistant strains of M. genitalium may be achieved with high dose, short duration treatment.
Fusidic acid activity is affected by pH which may be relevant to treatment of M. genitalium infections. Acidic growth conditions (pH 5–5.5) reduce fusidic acid MICs for S. aureus, and enhance the accumulation of the drug within the bacterial cell approximately fourfold as compared to pH 7 (27). Fusidic acid is highly protein bound in neutral pH (>95%); however, in acid pH protein binding is reduced thereby increasing the proportion of free drug and reducing the MIC (28). Fusidic acid accumulates in macrophages in neutral pH where it can kill intracellular bacteria, and intracellular concentrations are further increased in low pH (27, 28), an ability that may enhance clearance of intracellular M. genitalium (29–31). These phenomena may suggest that the low pH of the vagina, or inflamed microenvironments in other tissues, would increase fusidic acid potency against M. genitalium. Fitzgerald et al. found that a strain of Enterococcus faecalis resistant to fusidic acid due to a FusA C316A mutation developed compensatory mutations in fusA during in vitro passage in low pH (4.8) medium. Interestingly, the second-site mutations selected in low pH also restored fusidic acid susceptibility (32). The authors suggest that the growth in low pH could select against certain fusidic acid resistance alleles.
Other properties of fusidic acid may enhance its activity in vivo. Fusidic acid has anti-inflammatory activity as demonstrated in a mouse ear edema model (33), which may improve symptoms of infection, similar to azithromycin (34). The lipophilicity and large size of fusidic acid impede its passage through the Gram-negative outer membrane, rendering fusidic acid ineffective against Enterobacterales. This suggests that fusidic acid may have a lesser effect on the microbiome compared to other broad-spectrum antibiotics (6). Finally, the chemical scaffold and mechanism of action of fusidic acid differ from other antimicrobials, so cross-resistance between fusidic acid and other antibiotics does not occur (12).
Importantly, fusidic acid has in vitro activity against Neisseria gonorrhoeae and Chlamydia trachomatis (35), sexually transmitted bacterial pathogens with similar symptomology. In addition, 10–25% of patients with M. genitalium infections are also co-infected with one or both of these pathogens (1). A drug that treats all three pathogens would be invaluable, especially in resource poor settings where sexually transmitted infections are managed syndromically.
MATERIALS AND METHODS
Strains, media, and antibiotics
M. genitalium strains used in this study comprised strains capable of axenic growth including the G37 type strain (36), M30, M2282, M2300, and M2341 (37), and Sea-1 and Sea-2 (38). In addition, nine low-passage clinical strains cultured from men with urethritis were chosen as representatives of a variety of strain types with known resistance profiles to azithromycin, doxycycline, and moxifloxacin (7). Axenic strains were grown in SP-4 (39) and clinical isolates were grown in Vero cell co-cultures in EMEM (Corning Life Sciences) supplemented with 10% fetal bovine serum (FBS; RND Systems), 6% yeast dialysate, and 25 mM HEPES, pH 7.2 as previously described (7). Antibiotics for susceptibility testing were purchased from Sigma and dissolved in water (moxifloxacin, doxycycline), DMSO (fusidic acid), or 95% ethanol (azithromycin) and stored in aliquots at −20°C. Helvolic acid was obtained from the NPDG collection maintained at the University of Oklahoma.
Fungal isolates and fermentation
The Ramularia sp. isolate (TX10278 TV8-5) was obtained from soil sample collected from a garden near Texarkana, TX, USA. The fungus was identified by collecting mycelium and subjecting the samples to homogenization in TE buffer (10 mM EDTA HCl, 0.1 mM EDTA, pH 8.0) with zirconium oxide beads in a Bullet Blender (MidSci #BBY24M). The DNA was subsequently collected, and the ribosomal internal transcribed spacer region and the 5.8S rRNA genes were amplified by PCR for sequencing. The resulting sequence data were compared to fungal sequences contained in GenBank, which led to 100% identity matches to isolates described as R. coccinea (isolate from TX, USA).
To prepare the isolates for chemical studies, fungi were recovered from cryogenic storage (stored in a vial at −80°C as mycelium with 20% aqueous glycerol). Following recovery on Czapek agar plates (30 g sucrose, 2 g NaNO3, 1 g K2HPO4, 0.5 g MgSO4⋅7 H2O, 0.5 g KCl, 0.01 g FeSO4⋅7 H2O, 0.05 g chloramphenicol, and 1 L DI H2O), lawns of fungal mycelium were aseptically cut into small pieces (~1 cm2) for use as the scale-up culture inoculum. Scale-up cultures were carried out by charging mycobags (Unicorn Bags, Plano, TX, USA) with monolayers of Cheerios breakfast cereal supplemented with a 0.3% sucrose solution and 0.005% chloramphenicol. The pieces of mycelium were aseptically added to three mycobags and the cultures were grown at room temperature for 4 weeks.
Extraction, purification, and identification of fusidic acid
R. coccinea cultured on Cheerios cereal in the three mycobags was extracted with 2 L ethyl acetate (×3) at room temperature, the organic solvent layers were recovered, and the solvent was removed under vacuum. The crude EtOAc (fraction A, 17 g) was subjected to silica gel vacuum column chromatography with elution performed using dichloromethane (fraction B), dichloromethane-MeOH (10:1) (fraction C), and MeOH (fraction D). Fraction C (3 g) was also further fractionated by HP20ss gel vacuum column chromatography into five samples: fractions E (30% MeOH), F (50% MeOH), G (70% MeOH), H (90% MeOH), and I (100% MeOH). Fraction H (1.3 g) was further subjected to preparative HPLC (C18, gradient elution with 70–100% MeOH in H2O over 20 min using a 10 mL/min flow rate) to afford eight subfractions (H1–H8). Among these subfractions, H-7 was identified as fusidic acid (300 mg) by comparing the physicochemical and spectroscopic data with published values (Supporting Information Fig. S1 and S2) (40).
Microbroth dilution assays
Minimum inhibitory concentrations of axenic strains were determined in microbroth dilution assays as previously described (10). Briefly, M. genitalium cultures were grown to late log phase, scraped, passed through a 0.45-µm filter to remove aggregates, then diluted to 105 colony-forming units per mL. Dilutions of fusidic acid or doxycycline (comparator) were prepared in 0.1 mL in 96-well plates then 0.1 mL of the inoculum was added to each well. Plates were incubated at 37°C with 5% CO2 in a humidified atmosphere until wells containing no drug turned from red to yellow (indicating fermentation of glucose and late log phase growth). The MIC was identified as the lowest concentration of drug-inhibiting growth (no color change). As growth rates varied between axenic strains, incubation times ranged from 6 to 14 days.
Time-kill experiments
Time-kill experiments were performed as previously described (10, 41). Adherent, log phase M. genitalium strain G37 was scraped off plastic petri dishes into the culture supernatant, filtered through 0.45 µm, and then diluted to 104–105 CFU per mL in 3 mL SP-4 broth containing DMSO (0.5%, solvent control corresponding to the highest drug concentration), or 1, 2, 10, or 50 µg/mL of fusidic acid. Immediately after inoculation and at intervals during 7–10 days incubation, the tubes were vortexed, 10-fold serial dilutions were prepared, and 10 µL aliquots were spotted onto SP-4 agar plates in triplicate. Colonies were counted under 40× magnification after 2–3 weeks of incubation. Control cultures treated 1, 0.5, 0.25, or 0.125 µg/mL of moxifloxacin have been previously reported (10). Doubling times were calculated using an online tool (https://www.omnicalculator.com/biology/bacteria-growth).
Antibiotic susceptibility testing of Vero cell-dependent clinical isolates
To determine the MIC for M. genitalium clinical isolates, we based our protocol on the methods of Hamasuna et al. (7, 37). Vero cells (1 × 105 cells) were cultured for 1 day in 25 cm2 tissue culture flasks with Eagles minimal essential medium (EMEM; Corning) supplemented with 10% FBS (RND Systems), 25 mM HEPES, and penicillin (100 U/mL). Fresh media (4 mL, EMEM supplemented with 10% FBS, 25 mM HEPES, 100 U/mL penicillin, 30 µg/mL colistin, and 6% yeast dialysate) was added containing serial twofold dilutions of fusidic acid or control antibiotics. The flasks were incubated for 28 days at 37°C in 5% CO2 and aliquots of culture supernatants were collected weekly to detect growth by M. genitalium-specific quantitative PCR. Each aliquot was quantified in triplicate qPCR reactions to verify growth (>100-fold increase in genomes/mL), identify the time point representing late log phase growth (generally 21 or 28 days of incubation), and determine MIC. MICs were defined as the minimum concentration of antibiotic that inhibited growth by ≥99% compared to the growth of each strain in control flasks containing no antibiotic.
Quantification of M. genitalium growth by qPCR
To obtain precise measurements of growth inhibition, we used qPCR to quantify M. genitalium genomes in microbroth dilution assays. After assessing MIC endpoints by color change, we added 1/10 vol (20 µL) of Triton lysis solution (10% Triton X-100, 100 mM Tris HCl pH 8, 10 mM EDTA) to the wells and incubated the plates at 95°C for 15–30 min. Lysates were mixed by pipetting, diluted 1:10 in water, and then used directly for qPCR using a TaqMan assay that detects a portion of the 5′ region of mgpB (8, 42). Each PCR was performed in triplicate reactions, and then compared to a standard curve of known M. genitalium genomes prepared in quadruplicate. The drug concentration resulting in a 50% reduction (IC50) in M. genitalium genomes relative to untreated wells was calculated using a four-parameter logistic regression model. Genome quantities were determined in Vero cell cocultures using a similar method except that lysates were prepared using 45 µL of culture supernatant and 5 µL of Triton lysis solution.
Whole-genome sequencing
Fusidic acid-resistant mutants were sequenced to identify the location of resistance-associated mutations. Resistant mutants were grown in 60 mm petri dishes in 4–5 mL SP-4 broth containing fusidic acid. When late log growth was observed the culture supernatant was discarded and the adherent cells were washed and scraped into phosphate-buffered saline (PBS). The cell suspension was centrifuged at 21,000 × g for 10 min and the cell pellet was resuspended in 150 µL of PBS. Total DNA was isolated utilizing the MasterPure Complete DNA and RNA purification kit (Lucigen, Middleton, WI), following the manufacturer’s instructions, with the exception that tubes were gently mixed by inversion instead of vertexing, to prevent DNA shearing and preserve high molecular weight DNA. Isolated DNA was suspended in nuclease-free water, quantified by Nanodrop, and prepared for sequencing using the Rapid Barcoding kit (Kit 14 chemistry, Oxford Nanopore Technologies, Oxford Science Park, UK). Libraries were sequenced using an R10.4.1 flow cell on the Mk1B MinION sequencing device according to the manufacturer’s instructions. Sequencing produced the following number of classified reads (N50 4.0 kb): FAR1 328,234; FAR2 231,260; FAR3 346,834; FAR5 22,748; and FusR8 9,098. Read files were processed using SAMtools (43), aligned to the G37 reference genome using GraphMap (44), and then manually examined for mutations across the genome using the Integrative Genomics Viewer (45).
ACKNOWLEDGMENTS
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number R21AI153863. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contributor Information
Gwendolyn E. Wood, Email: gwenwood@uw.edu.
Andreas H. Groll, University Children's Hospital Münster, Münster, Germany
DATA AVAILABILITY
Sequence data for the Ramularia sp. isolate were deposited in GenBank under accession no. PP476214.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/aac.01006-24.
NMR spectra.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Wood GE, Bradshaw CS, Manhart LE. 2023. Update in epidemiology and management of Mycoplasma genitalium Infections. Infect Dis Clin North Am 37:311–333. doi: 10.1016/j.idc.2023.02.009 [DOI] [PubMed] [Google Scholar]
- 2. Manhart LE, Leipertz G, Soge OO, Jordan SJ, McNeil C, Pathela P, Reno H, Wendel K, Parker A, Geisler WM, Getman D, Golden MR, MyGeniUS Study Team . 2023. Mycoplasma genitalium in the US (MyGeniUS): surveillance data from sexual health clinics in 4 US regions. Clin Infect Dis 77:1449–1459. doi: 10.1093/cid/ciad405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gaurivaud P, Laigret F, Bove JM. 1996. Insusceptibility of members of the class Mollicutes to rifampin: studies of the Spiroplasma citri RNA polymerase beta-subunit gene. Antimicrob Agents Chemother 40:858–862. doi: 10.1128/AAC.40.4.858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Machalek DA, Tao Y, Shilling H, Jensen JS, Unemo M, Murray G, Chow EPF, Low N, Garland SM, Vodstrcil LA, Fairley CK, Hocking JS, Zhang L, Bradshaw CS. 2020. Prevalence of mutations associated with resistance to macrolides and fluoroquinolones in Mycoplasma genitalium: a systematic review and meta-analysis. Lancet Infect Dis 20:1302–1314. doi: 10.1016/S1473-3099(20)30154-7 [DOI] [PubMed] [Google Scholar]
- 5. Long J, Ji W, Zhang D, Zhu Y, Bi Y. 2021. Bioactivities and structure-activity relationships of fusidic acid derivatives: a review. Front Pharmacol 12:759220. doi: 10.3389/fphar.2021.759220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Farrell DJ, Castanheira M, Chopra I. 2011. Characterization of global patterns and the genetics of fusidic acid resistance. Clin Infect Dis 52 Suppl 7:S487–S492. doi: 10.1093/cid/cir164 [DOI] [PubMed] [Google Scholar]
- 7. Wood GE, Jensen NL, Astete S, Jensen JS, Kenny GE, Khosropour CM, Gillespie CW, Manhart LE, Totten PA. 2021. Azithromycin and doxycycline resistance profiles of U.S. Mycoplasma genitalium strains and their association with treatment outcomes. J Clin Microbiol 59:e0081921. doi: 10.1128/JCM.00819-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jensen JS, Björnelius E, Dohn B, Lidbrink P. 2004. Use of TaqMan 5’ nuclease real-time PCR for quantitative detection of Mycoplasma genitalium DNA in males with and without urethritis who were attendees at a sexually transmitted disease clinic. J Clin Microbiol 42:683–692. doi: 10.1128/JCM.42.2.683-692.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hjorth SV, Björnelius E, Lidbrink P, Falk L, Dohn B, Berthelsen L, Ma L, Martin DH, Jensen JS. 2006. Sequence-based typing of Mycoplasma genitalium reveals sexual transmission. J Clin Microbiol 44:2078–2083. doi: 10.1128/JCM.00003-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wood GE, Kim CM, Aguila LKT, Cichewicz RH. 2023. In vitro susceptibility and resistance of Mycoplasma genitalium to nitroimidazoles. Antimicrob Agents Chemother 67:e0000623. doi: 10.1128/aac.00006-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen Y, Koripella RK, Sanyal S, Selmer M. 2010. Staphylococcus aureus elongation factor G--structure and analysis of a target for fusidic acid. FEBS J 277:3789–3803. doi: 10.1111/j.1742-4658.2010.07780.x [DOI] [PubMed] [Google Scholar]
- 12. Fernandes P. 2016. Fusidic acid: a bacterial elongation factor inhibitor for the oral treatment of acute and chronic staphylococcal infections. Cold Spring Harb Perspect Med 6:a025437. doi: 10.1101/cshperspect.a025437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fookes MC, Hadfield J, Harris S, Parmar S, Unemo M, Jensen JS, Thomson NR. 2017. Mycoplasma genitalium: whole genome sequence analysis, recombination and population structure. BMC Genomics 18:993. doi: 10.1186/s12864-017-4399-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hannan PC. 1995. Antibiotic susceptibility of Mycoplasma fermentans strains from various sources and the development of resistance to aminoglycosides in vitro. J Med Microbiol 42:421–428. doi: 10.1099/00222615-42-6-421 [DOI] [PubMed] [Google Scholar]
- 15. Lee JW, Collins JE, Hulverson MA, Aguila LKT, Kim CM, Wendt KL, Chakrabarti D, Ojo KK, Wood GE, Van Voorhis WC, Cichewicz RH. 2023. Appraisal of fungus-derived xanthoquinodins as broad-spectrum anti-infectives targeting phylogenetically diverse human pathogens. J Nat Prod 86:1596–1605. doi: 10.1021/acs.jnatprod.3c00283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Peramuna T, Kim CM, Aguila LKT, Wendt KL, Wood GE, Cichewicz RH. 2024. Iron(III) binding properties of PF1140, a fungal N-hydroxypyridone, and activity against Mycoplasma genitalium. J Nat Prod 87:1746–1753. doi: 10.1021/acs.jnatprod.4c00209 [DOI] [PubMed] [Google Scholar]
- 17. Salimova EV, Mozgovoj OS, Efimova SS, Ostroumova OS, Parfenova LV. 2023. 3-amino-substituted analogues of fusidic acid as membrane-active antibacterial compounds. Membranes (Basel) 13:309–414. doi: 10.3390/membranes13030309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Garcia Chavez M, Garcia A, Lee HY, Lau GW, Parker EN, Komnick KE, Hergenrother PJ. 2021. Synthesis of fusidic acid derivatives yields a potent antibiotic with an improved resistance profile. ACS Infect Dis 7:493–505. doi: 10.1021/acsinfecdis.0c00869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Besier S, Ludwig A, Brade V, Wichelhaus TA. 2003. Molecular analysis of fusidic acid resistance in Staphylococcus aureus. Mol Microbiol 47:463–469. doi: 10.1046/j.1365-2958.2003.03307.x [DOI] [PubMed] [Google Scholar]
- 20. Nagaev I, Björkman J, Andersson DI, Hughes D. 2001. Biological cost and compensatory evolution in fusidic acid-resistant Staphylococcus aureus. Mol Microbiol 40:433–439. doi: 10.1046/j.1365-2958.2001.02389.x [DOI] [PubMed] [Google Scholar]
- 21. Pushkin R, Iglesias-Ussel MD, Keedy K, MacLauchlin C, Mould DR, Berkowitz R, Kreuzer S, Darouiche R, Oldach D, Fernandes P. 2016. A randomized study evaluating oral fusidic acid (CEM-102) in combination with oral rifampin compared with standard-of-care antibiotics for treatment of prosthetic joint infections: a newly identified drug–drug interaction. Clin Infect Dis 63:1599–1604. doi: 10.1093/cid/ciw665 [DOI] [PubMed] [Google Scholar]
- 22. Chen H-J, Hung W-C, Lin Y-T, Tsai J-C, Chiu H-C, Hsueh P-R, Teng L-J. 2015. A novel fusidic acid resistance determinant, fusF, in Staphylococcus cohnii. J Antimicrob Chemother 70:416–419. doi: 10.1093/jac/dku408 [DOI] [PubMed] [Google Scholar]
- 23. Torres-Puig S, Martínez-Torró C, Granero-Moya I, Querol E, Piñol J, Pich OQ. 2018. Activation of σ20-dependent recombination and horizontal gene transfer in Mycoplasma genitalium. DNA Res 25:383–393. doi: 10.1093/dnares/dsy011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yamao F, Muto A, Kawauchi Y, Iwami M, Iwagami S, Azumi Y, Osawa S. 1985. UGA is read as tryptophan in Mycoplasma capricolum. Proc Natl Acad Sci U S A 82:2306–2309. doi: 10.1073/pnas.82.8.2306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Still JG, Clark K, Degenhardt TP, Scott D, Fernandes P, Gutierrez MJ. 2011. Pharmacokinetics and safety of single, multiple, and loading doses of fusidic acid in healthy subjects. Clin Infect Dis 52 Suppl 7:S504–S512. doi: 10.1093/cid/cir174 [DOI] [PubMed] [Google Scholar]
- 26. Craft JC, Moriarty SR, Clark K, Scott D, Degenhardt TP, Still JG, Corey GR, Das A, Fernandes P. 2011. A randomized, double-blind phase 2 study comparing the efficacy and safety of an oral fusidic acid loading-dose regimen to oral linezolid for the treatment of acute bacterial skin and skin structure infections. Clin Infect Dis 52 Suppl 7:S520–S526. doi: 10.1093/cid/cir167 [DOI] [PubMed] [Google Scholar]
- 27. Lemaire S, Van Bambeke F, Pierard D, Appelbaum PC, Tulkens PM. 2011. Activity of fusidic acid against extracellular and intracellular Staphylococcus aureus: influence of pH and comparison with linezolid and clindamycin. Clin Infect Dis 52 Suppl 7:S493–S503. doi: 10.1093/cid/cir165 [DOI] [PubMed] [Google Scholar]
- 28. Biedenbach DJ, Rhomberg PR, Mendes RE, Jones RN. 2010. Spectrum of activity, mutation rates, synergistic interactions, and the effects of pH and serum proteins for fusidic acid (CEM-102). Diagn Microbiol Infect Dis 66:301–307. doi: 10.1016/j.diagmicrobio.2009.10.014 [DOI] [PubMed] [Google Scholar]
- 29. Ueno PM, Timenetsky J, Centonze VE, Wewer JJ, Cagle M, Stein MA, Krishnan M, Baseman JB. 2008. Interaction of Mycoplasma genitalium with host cells: evidence for nuclear localization. Microbiol (Read) 154:3033–3041. doi: 10.1099/mic.0.2008/020735-0 [DOI] [PubMed] [Google Scholar]
- 30. McGowin CL, Popov VL, Pyles RB. 2009. Intracellular Mycoplasma genitalium infection of human vaginal and cervical epithelial cells elicits distinct patterns of inflammatory cytokine secretion and provides a possible survival niche against macrophage-mediated killing. BMC Microbiol 9:139. doi: 10.1186/1471-2180-9-139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jensen JS, Blom J, Lind K. 1994. Intracellular location of Mycoplasma genitalium in cultured Vero cells as demonstrated by electron microscopy. Int J Exp Pathol 75:91–98. [PMC free article] [PubMed] [Google Scholar]
- 32. Fitzgerald BA, Wadud A, Slimak Z, Slonczewski JL. 2023. Enterococcus faecalis OG1RF evolution at low pH selects fusidate-sensitive mutants in elongation factor G and at high pH selects defects in phosphate transport. Appl Environ Microbiol 89:e0046623. doi: 10.1128/aem.00466-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wu P-P, He H, Hong WD, Wu T-R, Huang G-Y, Zhong Y-Y, Tu B-R, Gao M, Zhou J, Zhao S-Q, Li D-L, Xu X-T, Sheng Z-J, Ward SA, O’Neill PM, Zhang K. 2018. The biological evaluation of fusidic acid and its hydrogenation derivative as antimicrobial and anti-inflammatory agents. Infect Drug Resist 11:1945–1957. doi: 10.2147/IDR.S176390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kricker JA, Page CP, Gardarsson FR, Baldursson O, Gudjonsson T, Parnham MJ. 2021. Nonantimicrobial actions of macrolides: overview and perspectives for future development. Pharmacol Rev 73:233–262. doi: 10.1124/pharmrev.121.000300 [DOI] [PubMed] [Google Scholar]
- 35. Jones RN, Biedenbach DJ, Roblin PM, Kohlhoff SA, Hammerschlag MR. 2010. Update on fusidic acid (CEM-102) tested against Neisseria gonorrhoeae and Chlamydia trachomatis. Antimicrob Agents Chemother 54:4518–4519. doi: 10.1128/AAC.00235-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Iverson-Cabral SL, Astete SG, Cohen CR, Totten PA. 2007. mgpB and mgpC sequence diversity in Mycoplasma genitalium is generated by segmental reciprocal recombination with repetitive chromosomal sequences. Mol Microbiol 66:55–73. doi: 10.1111/j.1365-2958.2007.05898.x [DOI] [PubMed] [Google Scholar]
- 37. Hamasuna R, Osada Y, Jensen JS. 2005. Antibiotic susceptibility testing of Mycoplasma genitalium by TaqMan 5’ nuclease real-time PCR. Antimicrob Agents Chemother 49:4993–4998. doi: 10.1128/AAC.49.12.4993-4998.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Iverson-Cabral SL, Wood GE, Totten PA. 2015. Analysis of the Mycoplasma genitalium MgpB adhesin to predict membrane topology, investigate antibody accessibility, characterize amino acid diversity, and identify functional and immunogenic epitopes. PLoS One 10:e0138244. doi: 10.1371/journal.pone.0138244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tully JG, Whitcomb RF, Clark HF, Williamson DL. 1977. Pathogenic mycoplasmas: cultivation and vertebrate pathogenicity of a new spiroplasma. Science 195:892–894. doi: 10.1126/science.841314 [DOI] [PubMed] [Google Scholar]
- 40. Rastrup-Anderson N, Duvold T. 2002. Reassignment of the 1H NMR spectrum of fusidic acid and total assignment of 1H and 13C NMR spectra of some selected fusidane derivatives. Magn Reson Chem 40:471–473. doi: 10.1002/mrc.1038 [DOI] [Google Scholar]
- 41. Waites KB, Crabb DM, Bing X, Duffy LB. 2003. In vitro susceptibilities to and bactericidal activities of garenoxacin (BMS-284756) and other antimicrobial agents against human mycoplasmas and ureaplasmas. Antimicrob Agents Chemother 47:161–165. doi: 10.1128/AAC.47.1.161-165.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wood GE, Patton DL, Cummings PK, Iverson-Cabral SL, Totten PA. 2017. Experimental infection of pig-tailed macaques (Macaca nemestrina) with Mycoplasma genitalium. Infect Immun 85:e00738-16. doi: 10.1128/IAI.00738-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, 1000 Genome Project Data Processing Subgroup . 2009. The sequence alignment/map format and SAMtools. Bioinformatics 25:2078–2079. doi: 10.1093/bioinformatics/btp352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sović I, Šikić M, Wilm A, Fenlon SN, Chen S, Nagarajan N. 2016. Fast and sensitive mapping of nanopore sequencing reads with GraphMap. Nat Commun 7:11307. doi: 10.1038/ncomms11307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Robinson JT, Thorvaldsdóttir H, Winckler W, Guttman M, Lander ES, Getz G, Mesirov JP. 2011. Integrative genomics viewer. Nat Biotechnol 29:24–26. doi: 10.1038/nbt.1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
NMR spectra.
Data Availability Statement
Sequence data for the Ramularia sp. isolate were deposited in GenBank under accession no. PP476214.