Abstract
In 2012, the Strategic Advisory Group of Experts on Immunization (SAGE) recommended introduction of at least one inactivated poliovirus vaccine (IPV) dose in essential immunization programs. We evaluated systemic humoral and intestinal mucosal immunity of a sequential IPV-bivalent oral poliovirus vaccine (bOPV) schedule compared with a co-administration IPV + bOPV schedule in an open-label, randomized, controlled, non-inferiority, inequality trial in Dhaka, Bangladesh. Healthy infants aged 6 weeks were randomized to either: (A) IPV and bOPV at 6 and bOPV at 10 and 14 weeks (IPV + bOPV-bOPV-bOPV); or (B) IPV at 6 and bOPV at 10 and 14 weeks (IPV-bOPV-bOPV). Of 456 participants enrolled and randomly assigned during May–August 2015, 428 (94%) were included in the modified intention-to-treat analysis (arm A: 211, arm B: 217). Humoral immune responses did not differ at 18 weeks between study arms: type 1 (98% versus 96%; p = 0.42), type 2 (37% versus 39%; p = 0.77), and type 3 (97% versus 93%; p = 0.07). Virus shedding one week after the bOPV challenge dose in arm B was non-inferior to arm A (type 1 difference = −3% [90% confidence interval: −6 – 0.4%]; type 3 difference: −3% [−6 to −0.2%]). Twenty-six adverse events including seven serious adverse events were reported among 25 participants including one death; none were attributed to study vaccines.
An IPV-bOPV-bOPV sequential schedule induced comparable systemic humoral immunity to all poliovirus types and types 1 and 3 intestinal mucosal immunity as an IPV + bOPV-bOPV-bOPV co-administration schedule.
Keywords: Bivalent oral poliovirus vaccine, Inactivated poliovirus vaccine, Immunogenicity, Bangladesh
1. Introduction
The oral poliovirus vaccine (OPV) and inactivated poliovirus vaccine (IPV) are used globally in essential childhood immunization programs. While both lead to the development of systemic humoral immunity to prevent paralysis, OPV has numerous advantages over inactivated IPV including development of primary intestinal mucosal immunity to prevent fecal-oral transmission, low cost of vaccination, ease of administration, and indirect immunization of susceptible children [1]. Yet one important drawback of OPV is that in rare instances, the live-attenuated Sabin poliovirus strain can revert to a neurovirulent form during replication causing vaccine-associated paralytic poliomyelitis (VAPP) in the vaccine recipient (recipient VAPP), or a susceptible close contact (contact VAPP), that is indistinguishable from wild poliovirus (WPV) disease. The global risk for VAPP was estimated to range 2.4–9.7 cases per million births in the 125 countries that exclusively used OPV in 2012 [2]. The risk is generally highest with the first OPV dose, declining with subsequent OPV administrations except in countries with lower effectiveness [2]. Type 3 is more often associated with VAPP in infants receiving the vaccine whereas type 2 is often observed in contacts of immunized infants. VAPP risk is substantially reduced, though not eliminated, when IPV is administered before OPV because humoral immunity induced by IPV protects against paralysis from all three types. An important disadvantage of IPV used alone is that it provides little to no intestinal mucosal immunity in OPV-naïve children and is therefore limited in its impact in preventing fecal-oral transmission [3].
In countries with high poliovirus vaccination coverage (≥90%), low risk of poliovirus importation, and concern for VAPP, WHO has recommended since 2010 a sequential IPV-OPV schedule (also known as VAPP-protective schedule) in essential immunization programs beginning at 2 months of age [4,5]. In 2012, the Strategic Advisory Group of Experts on Immunization (SAGE) recommended that all countries only using OPV introduce at least one IPV dose in essential immunization programs in preparation for the 2016 synchronized global withdrawal of type 2 from OPV (i.e., switch from trivalent OPV (tOPV) containing types 1, 2, 3 to bivalent OPV (bOPV) containing types 1 and 3) [6]. The recommended schedule was three doses of bOPV with one IPV dose administered at ≥14 weeks of age; evidence indicated this schedule minimized maternal antibody interference and therefore provided a relatively higher percentage of type 2 immunity with a single IPV dose compared with an earlier administered dose, and closed the gap in types 1 and 3 immunity [5,7]. In countries that use an essential childhood immunization schedule of 6–10-14 weeks of age, this means IPV is given with the third bOPV dose. For countries already using IPV, SAGE recommendations were consistent with the continued use of IPV-bOPV sequential schedules. The Technical Advisory Group (TAG) on Vaccine-preventable Diseases of the Pan American Health Organization (PAHO) recommended in 2014 that countries in the region implement a three-dose primary sequential schedule (IPV-IPV-bOPV or IPV-bOPV-bOPV) followed by two bOPV booster doses to reduce the risk of VAPP because the risk of poliovirus importation was deemed low and vaccination coverage was relatively high [8]. The first IPV dose was recommended to be administered with the first diphtheria, tetanus, and pertussis (DTP) dose, generally at 2 months of age in PAHO countries.
Another advantage of administering IPV first (as sequential or co-administered with bOPV) is the increased number and percentage of infants vaccinated against all poliovirus types [9]. In countries that use a polio vaccination schedule of 6–10-14 weeks, the first dose of the DTP (DTP1) and the third DTP dose (DTP3) coincide with the first bOPV and last bOPV/first IPV doses and can be used to estimate the number and percent of IPV drop-out (i.e., no subsequent vaccination). According to the 2021 WHO/UNICEF Immunization Coverage (WUENIC) estimates, global DTP1 coverage was 86% and DTP3 coverage was 81%, representing approximately 6.76 million eligible infants who did not receive their single 14-week IPV dose [10].
IPV-OPV sequential schedules will continue to be an important option for essential immunization programs as the global community moves closer to WPV type 1 (WPV1) eradication and the eventual cessation of all OPVs from immunization programs. However, there are limited data on the systemic humoral and intestinal mucosal immunity induced by a first IPV dose administered as part of a sequential or co-administered schedule with bOPV, including the optimal number of bOPV doses. Earlier studies in the US and UK suggested that two doses of tOPV given with one or two doses of IPV induced protective levels of humoral and intestinal immunity comparable to three doses of tOPV [11–13]. Administration of two doses was supported by studies in Chile and China that found IPV-bOPV-bOPV arms had high type 1 (≥98%) and type 3 (≥98%) immune responses and type 2 responses ranging 56–78%; no assessment of types 1 and 3 intestinal immunity were conducted [14–16]. A study in Bangladesh found no difference in humoral immunity among infants who received a three-dose bOPV schedule in comparison with a fractional-IPV (fIPV)-bOPV-fIPV schedule for type 1 (99% vs 96%) or type 3 (94% vs 94%) [17]. The study found significant differences in vaccine virus shedding after a bOPV challenge dose among infants in the three-dose bOPV-only arm compared to infants in the fIPV-bOPV-fIPV arm for type 1 (3.6% vs 13.3%) and type 3 (6.1% vs 13.7%); a second dose of bOPV instead of fIPV might have led to no differences in virus shedding.
We conducted a clinical trial in Bangladesh among OPV-naïve infants to investigate systemic humoral and intestinal mucosal immunity of a three-dose IPV-bOPV sequential schedule (VAPP-protective) compared with a four-dose IPV + bOPV co-administration schedule. We evaluated differences in humoral immunity to all poliovirus types after completion of vaccinations at 18 weeks of age. We also indirectly assessed the non-inferiority of intestinal mucosal immunity for types 1 and 3 one-week after a bOPV challenge dose at 18 weeks of age, as well as differences in intestinal immunity two-weeks after the bOPV challenge dose.
2. Methods
2.1. Study design and participants
We conducted a randomized, controlled, open-label, parallel, non-inferiority, inequality, phase IV clinical trial in Mirpur, a suburb of Dhaka, Bangladesh, in 2015 when tOPV was still used. Ethical approval for the study protocol and amendments were obtained from the icddr,b Institutional Review Board and the Human Subjects Research Office of the Center for Global health, US Centers for Disease Control and Prevention (CDC). CDC staff had no interaction with participants nor access to any personally identifiable information.
Field workers within assigned communities identified expectant mothers and arranged clinic visits for interested parents. Full-term (≥37 weeks), singleton, healthy infants 6 weeks of age (42–48 days) were eligible for the study if families planned to remain in the area for the study duration (15 weeks). Infants were ineligible if they had already received polio vaccination, had a diagnosis or suspicion of a health condition that would contraindicate conducting study procedures (e.g., bleeding disorders, immunodeficiency (including immediate family), acute illness that required hospitalization, vomiting or liquid intolerance 24 h prior to the enrollment visit, and known allergy or sensitivity to polio vaccines or content). Parents could withdraw consent for the study at any time. Participants were discontinued if parents withdrew consent; participant was diagnosed/suspected with a medical condition that posed a health risk, received immunosuppressive medications, received polio vaccination outside the study, or had an allergic reaction to study vaccine; or if blood could not be collected at enrollment.
2.2. Randomization and masking
Participants were randomly allocated (1:1) to one of two study arms: arm A received IPV and bOPV at 6 weeks of age and bOPV at 10 and 14 weeks of age (IPV + bOPV co-administration; “IPV + 3bOPV”); and arm B received IPV only at 6 weeks of age and bOPV at 10 and 14 weeks of age (IPV-bOPV sequential; “IPV + 2bOPV”). Block randomization of 76 blocks with a block size of six was used. Investigators not involved in data collection generated the randomization sequencing using R (R foundation, version 3.2.1) and provided sequentially numbered, sealed, opaque envelopes to medical officers who enrolled and assigned participants. Study staff and parents were masked to arm assignment until envelopes were opened; laboratory staff remained blinded throughout the study.
2.3. Procedures
Once enrolled, staff obtained participant clinical history (i.e., breast feeding, vaccination history, presence of diarrhea), conducted a physical examination (i.e., temperature, length, weight), collected a blood sample, administered poliovirus (study) vaccines, and observed for 30 min for any adverse events. Length and weight were measured twice and the mean was used to assess the presence of stunting and wasting respectively per WHO’s Multicenter Growth Study’s child-growth standard curves. [18] Stunting or wasting was present if measurements were more than two SD below the reference population mean. Three additional clinic visits were conducted at 10, 14, and 18 weeks of age where study staff again collected clinical information, obtained blood samples (18 weeks only), administered study vaccines, and monitored for 30 min for any adverse events. Four home visits were also conducted: the first was 24–48 h after the 6-week clinic visit at which all participants had received IPV. Staff documented any systemic or injection site adverse events not observed while at the study clinic. Home visits were conducted for participants in all arms at 18, 19, and 20 weeks of age to deliver and collect stool collection kits; kits were delivered one day prior to scheduled collection date. Mothers were instructed to collect the first specimen of the day, place the container in a cool place, and to immediately notify study staff. Study staff collected specimens within two hours of notification and placed containers in the study refrigerator at 2–8 °C within 30 min of pick-up.
IPV was manufactured by Bilthoven Biologicals and each single dose vial (0·5 mL) contained serotype 1 (40 D-antigen units of Mahoney strain), serotype 2 (8 D-antigen units of MEF1 strain) and serotype 3 (32 D-antigen units of Saukett strain). IPV was administered intramuscularly on the outer, upper right thigh. bOPV was in 20-dose vials manufactured by Sanofi Pasteur; only one vial was used per participant per visit. Each 0.1 mL dose (two drops) contained ≥106 median cell culture infective dose (CCID50) of Sabin serotype 1 and ≥ 105.8 median CCID50 of Sabin serotype 3. Participants also received the pentavalent (diphtheria, pertussis, tetanus, Hepatitis B, Haemophilus influenzae type b) vaccine and pneumococcal conjugate vaccine (PCV) as recommended by the Bangladesh Ministry of Health and Family Welfare’s Expanded Programme on Immunization (EPI) at 6, 10, and 14 weeks. Upon completion of study activities at 20 weeks of age, all infants received one IPV dose and the first of three doses of tOPV given four weeks apart to ensure adherence to Bangladesh’s EPI schedule for polio.
Blood samples (1 mL) were collected by venipuncture at 6 and 18 weeks of age from all participants, before study vaccines were administered. Stool samples (~8 g) were collected from all participants prior to the 18-week clinic visit when the bOPV challenge dose was administered, then one- and two- weeks after the challenge vaccination. Blood and stool specimens were sent to icddr,b by the end of the day. Blood samples were centrifuged within 24 h of collection and sera aliquoted for testing (−20 °C) and storage (−70 °C). Stool samples were also separated for testing and storage (−20 °C). Sera and stool samples were shipped to the CDC laboratory in Atlanta, GA, USA, for testing upon completion of all study activities. Backup stool samples stored at icddr,b were shipped to the CDC laboratory for confirmatory testing of initial results. The polio microneutralization assay was used to measure serum antibody titers to poliovirus serotypes 1, 2, and 3; the lower and upper limit of detection were a reciprocal antibody titer of ≤5.66 and ≥ 1448, respectively [19]. The standard WHO protocol for culture and characterization by real-time reverse-transcription polymerase chain reaction was performed yielding a qualitative “yes/no” result for the presence/absence of type 1 and 3 vaccine virus [20].
2.4. Outcomes
The two primary outcomes of the study were 1) immune response to poliovirus types 1, 2, and 3 at 18 weeks of age, four weeks after the last study vaccination. Immune response was defined as seroconversion from seronegative (<1:8) at baseline (i.e., 6 weeks of age) to seropositive (≥1:8) at 18 weeks of age, or a four-fold rise in antibody titers between baseline and 18 weeks of age among those seropositive at 6 weeks, adjusted for the exponential decay of maternal antibodies assuming a half-life of 28 days; and 2) presence of poliovirus type 1 and 3 vaccine virus in stool specimens collected one-week after the bOPV challenge dose at 18 weeks of age. There were also two secondary outcomes: 1) reciprocal antibody titers measured at 18 weeks of age to all types among immune responders; and 2) presence of poliovirus type 1 and 3 vaccine virus in stool specimens two-weeks after the 18-week bOPV challenge dose.
Systemic and injection site adverse events were monitored during the study. Adverse events were defined as any illness experienced by a participant during the study period. Serious adverse events were defined as anaphylaxis, hospitalization, paralysis or severe disability/in-capacity, and death. During clinic visits, parents were asked about any illness since the last clinic visit and participants were monitored for adverse events for 30 min after each study vaccination. Monitoring was also performed between clinic visits: at the home visit 24–48 h after first IPV administration, and at 18, 19, and 20 weeks when stool specimens were collected. Parents were instructed at enrollment and all visits to seek medical care immediately and notify study staff of any illnesses. The Principal Investigator reviewed all adverse event reports; reports of all serious adverse event were shared with the icddr,b Institutional Review Board, the Data Safety and Monitoring Board, and CDC within 24 h.
2.5. Sample size and statistical analysis
The sample size was calculated to address the primary objectives and was driven by the non-inferiority comparisons for type 1 and 3 vaccine virus shedding at 19 weeks of age. Based on a study in Bangladesh that found type 1 vaccine virus shedding in 3.6% of infants who had received three doses of bOPV and 13.3% of infants who had received one dose of bOPV plus two doses of fractional IPV, we conservatively estimated that 10% of participants who had received two doses of bOPV would shed type 1 vaccine virus one week after the bOPV challenge dose [17]. A sample size of 203 per arm provided at least 85% power and a one-sided alpha of 0.05 for evaluating non-inferiority defined as at least an 8% difference in virus shedding assessed under an alternative hypothesis of 10% shedding in each arm. To account for 10% attrition and the block randomization scheme, the final enrollment target was 228 participants per arm for a total of 456 participants.
Fisher’s Exact test was used to assess any differences in the proportion of type-specific immune response and type-specific vaccine virus shedding between arms. Non-inferiority was assessed by comparing the lower bound of the 90% Wald confidence interval to the non-inferiority margin of −8%. Differences in antibody titer distributions between arms was assessed using the Kruskal-Wallis test. Adjustment for multiple comparisons was not conducted as all hypotheses were identified a-priori at varied outcome endpoints. Post-hoc analyses were conducted to assess influence of maternal antibodies and correlation of type-specific immune response with vaccine virus shedding. Reverse cumulative distribution function curves were used to visualize reciprocal antibody titer distributions among responders with the y-axis representing the proportion of participants with antibody titers at the corresponding x-axis and greater. Descriptive analyses (percentages, medians) were conducted for baseline characteristics, adverse events, influence of maternal antibodies, and correlation of type-specific immune response with vaccine virus shedding after challenge. Antibody titers at baseline (i.e., 6 weeks of age) were assumed to be maternally derived and titers ≥64 were classified as high while those <64 were classified as low/undetectable. The primary analytic approach was modified intention-to-treat which included participants who received study vaccinations per group assignment and had poliovirus antibody titer results available at 6 and 18 weeks of age as well as vaccine virus detection results available for stool specimens collected at 18, 19, and 20 weeks of age. The secondary analytical approach was per-protocol and included the additional requirement that all clinic visits (i.e., blood collection, vaccination) and home visits (stool collection) were conducted within 3 days of the originally scheduled date. Findings from both analyses were similar; results from the modified intention-to-treat analyses are presented. Data were analyzed in SAS (version 9.4) and R (version 4.1.3). This trial is registered at ClinicalTrials.gov (NCT02412514).
2.6. Role of the funding source
The sponsor of the study participated in the study design, protocol development, data analysis, data interpretation, and manuscript development but did not participate in data collection. The corresponding author had full access to all study data, except personally identifiable information, and had final responsibility for the decision to submit for publication.
3. Results
A total of 720 parents were approached from whom 456 participants were enrolled from 26 May to 26 August 2015 (Fig. 1) and all follow-up activities were completed by 25 December 2015. The modified intention-to-treat population consisted of 428 participants (94%); baseline characteristics are summarized in Table 1.
Fig. 1.
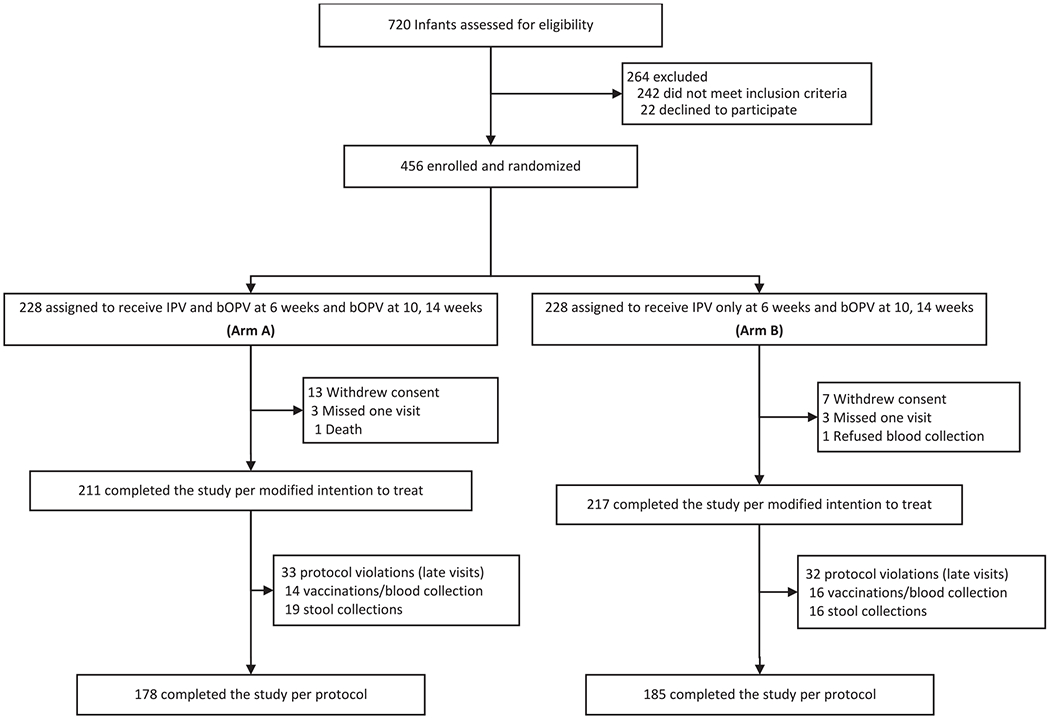
Trial profile, Bangladesh, 2015.
IPV = inactivated poliovirus vaccine. bOPV = bivalent oral poliovirus vaccine.
Table 1.
Baseline characteristics of the intention-to-treat population
| Baseline Characteristics | Arm A |
Arm B |
||
|---|---|---|---|---|
| IPV + 3 bOPV |
IPV + 2bOPV |
|||
| (n = 211) | (n = 217) | |||
| Age (days) | 44 (42–49) | 44 (42–48) | ||
| Male | 103 | 49% | 101 | 46% |
| Mother’s education | ||||
| No formal school | 33 | 15.6% | 39 | 18.0% |
| Primary | 74 | 35.1% | 105 | 48.4% |
| Middle | 52 | 24.6% | 41 | 18.9% |
| High | 41 | 19.4% | 26 | 12.0% |
| Graduate | 11 | 5.2% | 6 | 2.7% |
| Exclusive breastfeeding | 133 | 63.0% | 136 | 62.7% |
| Wasting present | 19 | 9.0% | 27 | 12.4% |
| Stunting present | 29 | 13.7% | 21 | 9.7% |
| Type 1 poliovirus | ||||
| Seropositive | 138 | 65.4% | 133 | 61.3% |
| Reciprocal titers | 28 | (14–114) | 36 | (14–91) |
| Type 2 poliovirus | ||||
| Seropositive | 145 | 68.7% | 140 | 64.5% |
| Reciprocal titers | 23 | (14–72) | 36 | (18–114) |
| Type 3 poliovirus | ||||
| Seropositive | 72 | 34.1% | 69 | 31.8% |
| Reciprocal titers | 28 | (11–93) | 28 | (14–144) |
Data are n (%), median (range) for age in days, or median (interquartile range) for reciprocal antibody titers among seropositive participants. Participant baseline measurements were obtained at 6 weeks of age.
There were no differences in immune responses for types 1, 2, and 3 between the arms although antibody titer distributions differed (Table 2). Among the 211 participants who received IPV + 3bOPV doses (arm A), 206 (98%, [95% confidence interval: 95–99%]) had a type 1 immune response compared with 208 of 217 participants (96% [92–98%]) who received IPV + 2bOPV doses (arm B) (p = 0.42). And while type 1 median reciprocal antibody titers in both arms reached the upper limit of detection (≥1448), antibody titer distributions were higher (p = 0.002) among those who received the additional bOPV dose [arm A: interquartile range (IQR): ≥1448 – ≥1448] vs arm B: [1152 – ≥1448]) (Fig. 2). Although types 2 and 3 immune responses were similar across arms, antibody titer distributions were higher among participants who received IPV + 2bOPV doses (arm B) compared with IPV + 3bOPV doses (arm A). Seventy-eight participants (37% [31–44%]) in arm A had a type 2 immune response compared with 84 participants (39% [33–45%] in arm B (p = 0.77). Type 2 antibody titer distributions were significantly higher (p = 0.02) among participants in arm B (51 [18–724]) compared with arm A (20 [11–144]). Type 3 immune responses were similarly high in both arms (arm A: 97% [94–99%]; arm B: 93% [89–96%]) and not significantly different (p = 0.07). Median type 3 reciprocal antibody titers in both arms reached the upper limit of detection (≥1448) but distributions were significantly higher (p = 0.004) among participants in arm B [≥1448 – ≥1448] compared with arm A [1152 – ≥1448].
Table 2.
Summary of poliovirus types 1, 2, and 3 immune response and reciprocal antibody titers at 18 weeks and types 1 and 3 vaccine virus shedding at 18–19-20 weeks, by study arms.
| Arm A |
Arm B |
Fisher’s Exact Test/ Kruskal-Wallis Test | |||
|---|---|---|---|---|---|
| IPV + 3 bOPV |
IPV + 2bOPV |
||||
| (n = 211) | (n = 217) | ||||
| Type 1 | |||||
| Immune response at 18 weeks | 206 | 97.6% (94.6–99.0%) | 208 | 95.9% (92.3–97.8%) | p = 0.42 |
| Median reciprocal antibody titers | ≥1448 | (≥1448- ≥ 1448) | ≥1448 | (1152- ≥ 1448) | p = 0.002 |
| Vaccine virus shedding at 18 weeks | 22/211 | 10.4% (7.0–15.3%) | 13/217 | 6.0% (3.5–10.0%) | |
| Vaccine virus shedding at 19 weeks | 10/189 | 5.3% (2.9–9.5%) | 5/204 | 2.5% (1.1–5.6%) | |
| Vaccine virus shedding at 20 weeks | 8/189 | 4.2% (2.2–8.1%) | 14/204 | 6.9% (4.1–11.2%) | p = 0.28 |
| Type 2 | |||||
| Immune response at 18 weeks | 78 | 37.0% (30.7–43.7%) | 84 | 38.7% (32.5–45.3%) | p = 0.77 |
| Median reciprocal antibody titers | 20 | (11–144) | 51 | (18–724) | p = 0.02 |
| Type 3 | |||||
| Immune response at 18 weeks | 205 | 97.2% (94.0–98.7%) | 202 | 93.1% (88.9–95.8%) | p = 0.07 |
| Median reciprocal antibody titers | ≥1448 | (1152- ≥ 1448) | ≥1448 | (≥1448- ≥ 1448) | p = 0.004 |
| Vaccine virus shedding at 18 weeks | 19/211 | 9.0% (5.8–13.6%) | 25/217 | 11.5% (7.9–16.5%) | |
| Vaccine virus shedding at 19 weeks | 9/192 | 4.7% (2.5–8.7%) | 3/192 | 1.6% (0.5–4.5%) | |
| Vaccine virus shedding at 20 weeks | 11/192 | 5.7% (3.2–10.0%) | 15/192 | 7.8% (4.8–12.5%) | p = 0.54 |
Data are the percentage of participants with immune response expressed as n/N and percentage including 95% confidence interval (CI). Immune response defined as seroconversion from seronegative (<1:8) to seropositive (≥1:8) after vaccination, or a four-fold rise in antibody titers among those seropositive after vaccination adjusted maternal antibody decay. Reciprocal antibody titers are presented as the median (interquartile range) among immune responders.
IPV = inactivated poliovirus vaccine; bOPV = bivalent oral poliovirus vaccine.
Fisher’s Exact test was used to test for inequality of proportions between study arms. Kruskal-Wallis Test was used to test for inequality of antibody titer distributions between study arms.
Fig. 2.
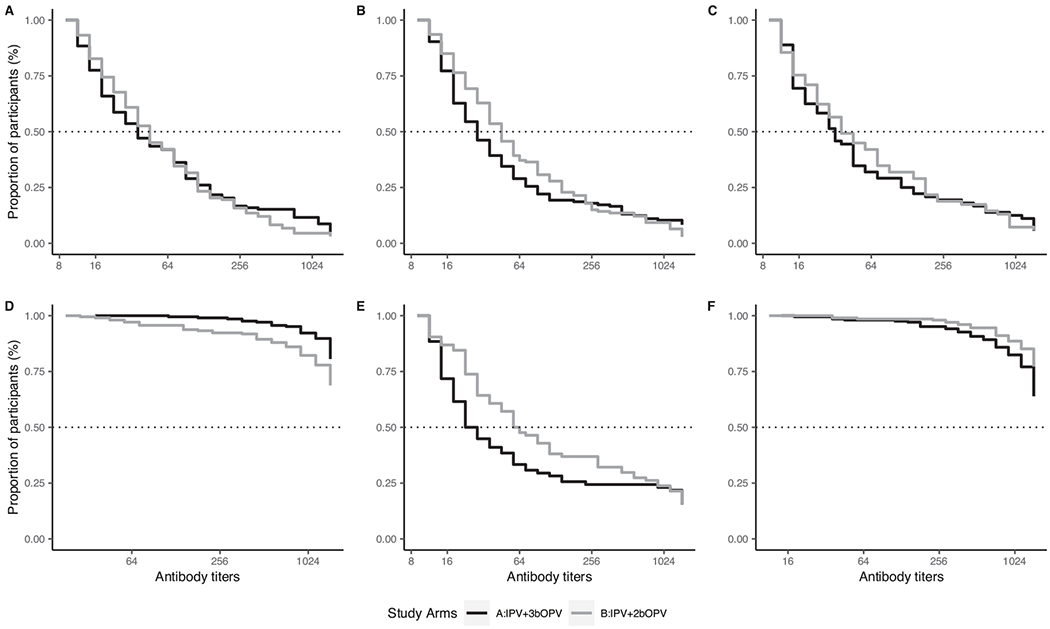
Reverse cumulative distribution function curves of reciprocal antibody titers to poliovirus types 1, 2 and 3 by study arm.
(A-C) Percentage of participants (y-axis) with measured reciprocal antibody titers and all greater titers (x-axis) among those seropositive at 6 weeks before vaccination for types 1 (A), 2 (B), and 3 (C). (D—F) percentage of participants with measured or greater reciprocal antibody titers among those with an immune response at 18 weeks for types 1 (D), 2 (E), and 3 (F). IPV = inactivated poliovirus vaccine. bOPV = bivalent oral poliovirus vaccine.
Type-specific vaccine virus shedding one- and two- weeks after the bOPV challenge dose at 18 weeks were non-inferior among participants who received IPV + 2bOPV (arm B) compared with those who received IPV + 3bOPV (arm A) (Table 2, Fig. 3). Vaccine virus shedding one week after the challenge dose among participants in arm B was non-inferior for both types 1 and 3 compared with arm A; both lower limits of the confidence intervals were – 6% and higher than the non-inferiority margin of −8%. Two-weeks after the challenge dose, no difference in virus shedding for types 1 (p = 0.28) and 3 (p = 0.54) were observed. An incidental finding was detection of an adenovirus outbreak at the time of the study.
Fig. 3.
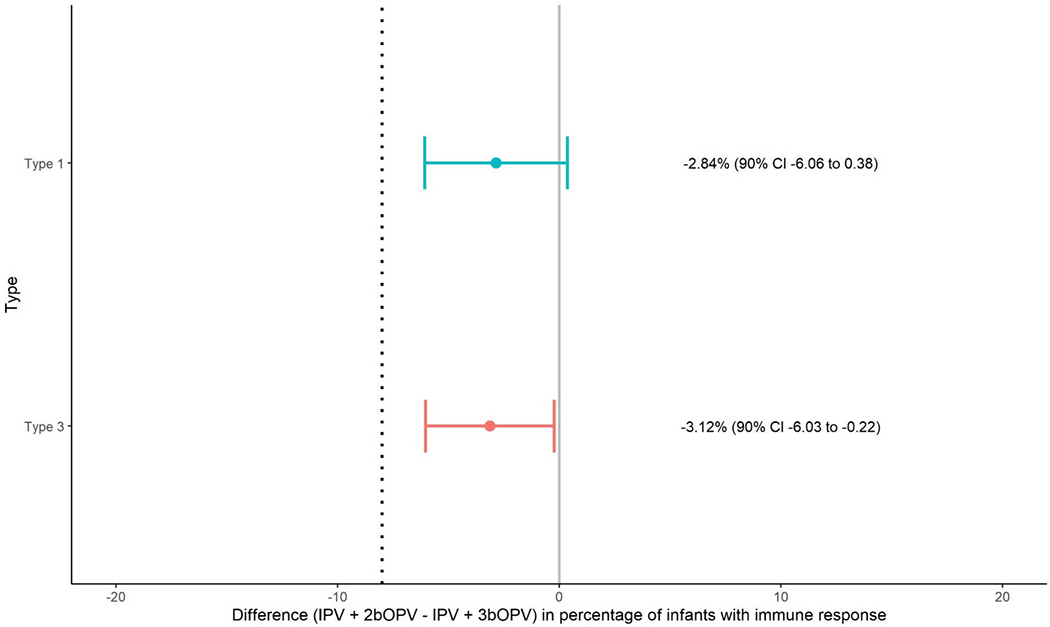
Non-inferiority assessment of poliovirus types 1 and 3 vaccine virus shedding at 19 weeks of age (one week after bOPV challenge dose).
Differences in immune response between study arm B (IPV + 2bOPV) and A (IPV + 3bOPV) for type 1 and type 3 are presented with 90%CI around the estimated difference. The hashed gray line represents the non-inferiority margin of −8%. Non-inferiority is concluded if the lower bound of the 90%CI falls to the right of the non-inferiority margin. IPV = inactivated poliovirus vaccine. bOPV = bivalent oral poliovirus vaccine.
We could not assess the association of type 1 or type 3 immune response at 18 weeks with vaccine virus shedding one- or two-weeks after the 18-week bOPV challenge dose due to the low percentage of virus shedders.
The correlation of high maternal antibodies at 6 weeks with type-specific immunity at 18 weeks of age varied between the two arms. There was interference for types 1 and 3 but only for participants in arm A who received IPV + 3bOPV doses: 46 of 50 participants (92% [81–97%]) had type 1 immune response among those classified as having high maternal antibodies compared with 160/161 (99% [97–100%]) participants with type 1 immune response among those classified as having low/undetectable maternal antibodies (p = 0.018); and 18 of 21 participants (86% [65–95%]) had type 3 immune response among those classified as having high maternal antibodies compared with 187 of 190 (98% [95–99%]) had type 3 immune response among those classified as having low/undetectable maternal antibodies (p = 0.01). There were no differences observed for type 2 immune response between the two arms, or for any poliovirus type among participants in arm B who received IPV + 2bOPV doses.
Twenty-six adverse events were reported among 25 participants during the study period, of which seven were serious adverse events including one death. The most commonly reported adverse events were common cold (5), scabies (4), and two each: conjunctivitis, candidiasis (oral), gastrointestinal illness, and tinea capitis. None of the adverse events were attributed to the study vaccines. No adverse events were detected during the 30-min post-vaccination observation period. Twenty (4%) of 456 participants had <1 cm swelling at the injection site during the 24–48-h post IPV vaccination home visit; no redness was observed.
4. Discussion
Our findings indicate no difference in systemic humoral immunity against all poliovirus types and non-inferiority of types 1 and 3 intestinal mucosal immunity in a three-dose IPV-bOPV sequential schedule (IPV + 2bOPV) compared with a four-dose IPV + bOPV co-administration schedule (IPV + 3bOPV). Humoral immunity was proportionately high in both arms after the full series for types 1 (≥95%) and 3 (≥93%) and shedding at 19 weeks and 20 weeks was low in both arms (<8%) with only 2–3% difference between arms suggesting high levels of intestinal mucosal immunity. Although there were differences in reciprocal antibody titer distributions for all types (higher for type 1 in the IPV + 3bOPV arm yet lower for types 2 and 3 compared with the IPV + 2bOPV arm), median titers for both arms for types 1 and 3 reached the upper limit of detection (≥1448). This suggests some inducement of a greater type 1 immune response when IPV is co-administered with bOPV that leads to relatively lower titer levels to types 2 and 3 but does not affect general development of an immune response. Overall, our findings are consistent with other studies that demonstrated proportionately high type 1 (≥98%) and type 3 (≥98%) humoral immunity from a single IPV dose followed by two bOPV doses [14–16]. In addition, the percentage of type 1 and 3 vaccine virus shedding in both arms in our study were similarly low as a prior study’s three-dose bOPV-only arm (4% and 6%, respectively), and much lower than type 1 and 3 vaccine virus shedding (13% and 14%, respectively) in the fIPV-bOPV-fIPV arm [17]. These results highlight the importance of administering at least two doses of bOPV to induce high levels of intestinal mucosal immunity to interrupt poliovirus transmission [17].
Our study and another study [15] were conducted at a time when tOPV was used in essential immunization programs and while both studies took steps to limit background type 2 exposure (i.e., no sibling scheduled to receive tOPV), inadvertent exposure might still have occurred. Type 2 immune response in both arms was ~37% and was comparable to our other study (34%) in the study area around the same time (A Anand, unpublished data) but lower than the 56–78% reported in other studies which administered the first IPV dose at 2 months (8 weeks) instead of 6 weeks [14–16]. The two-week difference in IPV administration alone cannot explain the difference, and we did not observe maternal antibody interference with type 2 response.
There were several limitations to the study. Our study used a 6–10-14 weeks of age vaccination schedule which differed from WHO’s recommended IPV-OPV sequential schedule of 2–4-6 months of age. The improved humoral and intestinal mucosal responses reported with a first dose administered at 2 months of age suggests that our results are more conservative, and we would anticipate higher humoral and intestinal mucosal immunity had we used the WHO recommended scheduled. The ~37% immune response for type 2 may be an overestimate or underestimate of the humoral immunity developed from a single IPV dose. As previously mentioned, the study was conducted at a time in which tOPV was used and so the 37–39% may be higher due to background exposure. A previous study in the same area reported 14% type 2 response in a bOPV-only arm which suggests some level of background exposure [17]. In addition, we did not measure priming in the study and therefore the 37% is likely to underestimate the number of infants who developed humoral immunity with a single IPV dose [21]. Background exposure to types 1 and 3 may have occurred among participants despite efforts to limit household exposure. Secondary exposure has been an advantage of OPV; our study did not assess the effect a reduction from three to two bOPV doses would have on population immunity. Although we adhered to randomization, more mothers in Arm B reported no formal or primary school education than mothers in Arm A; this difference was due to chance and does not affect our findings. Our study assessed immunity of a VAPP-protective schedule but we did not directly assess the risk of VAPP. Finally, we extrapolated our findings on the development of types 1 and 3 intestinal immunity from bOPV to predict protection against WPV1 and cVDPV1 although the infectious doses differ.
Our findings support WHO’s recommendation for an IPV-bOPV sequential schedule for countries that want to minimize VAPP risk and provides evidence for removing one dose of bOPV while still maintaining high levels of systemic humoral and intestinal mucosal immunity. This schedule also has the added benefit of reaching more infants with a single IPV dose at 6-weeks of age during their first essential immunization visit than at 14 weeks of age. WHO recently updated its polio vaccination recommendation to include a second dose of IPV administered ≥4 months after the first 14-week dose [22], and this should offset the lower type 2 immunity induced from IPV administration before 14 weeks. The recent detection of type 2 circulating vaccine-derived poliovirus in the Ukraine, United Kingdom, and United States [23–25] highlights that no country is at zero risk for a poliovirus outbreak and therefore poliovirus vaccination is an essential immunization component and must remain a priority for all countries if eradication is to be achieved.
Acknowledgements
icddr,b acknowledges with gratitude the commitment of CDC to its research efforts. Icddr,b is also grateful to the Governments of Bangladesh, Canada, Sweden and UK for providing core/unrestricted support. We thank the study staff at the Mirpur site in Dhaka, Bangladesh; Kathryn Jones, Demetrius Mathis, Sharla McDonald, Deborah Moore, Mario Nicolas, and Yiting Zhang for processing and testing the samples at the Polio and Picornavirus Laboratory Branch in the Centers for Disease Control and Prevention; Howard E Gary Jr. (design and statistical support during protocol development) and Qian An (statistical review) with the Global Immunization Division in the Centers for Disease Control and Prevention; and all parents and infants who participated in this study.
Funding statement
This study was funded by the Global Immunization Division of the U.S. Centers for Disease Control and Prevention.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention.
Footnotes
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Khalequ Zaman, Asma Binte Aziz, Mohammad Yunus, Warda Haque b reports financial support was provided by Centers for Disease Control and Prevention. Cynthia J Snider’s spouse previously owned stock in Sanofi Pasteur
Data sharing
The study is registered on the clinicaltrials.gov website (NCT02412514) and aggregated data from Table 2 will added to the registration with publication.
CRediT authorship contribution statement
Cynthia J. Snider: Conceptualization, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. Khalequ Zaman: Conceptualization, Data curation, Investigation, Methodology, Project administration, Supervision, Writing – review & editing. Concepcion F. Estivariz: Conceptualization, Methodology, Validation, Writing – review & editing. Asma Binte Aziz: Data curation, Investigation, Project administration, Writing – review & editing. Mohammad Yunus: Conceptualization, Project administration, Validation, Writing – review & editing. Warda Haque: Conceptualization, Data curation, Investigation, Project administration, Writing – review & editing. William C. Weldon: Conceptualization, Data curation, Validation, Writing – review & editing. M. Steven Oberste: Conceptualization, Data curation, Validation, Writing – review & editing. Mark A. Pallansch: Conceptualization, Formal analysis, Methodology, Writing – review & editing. Steven G.F. Wassilak: Conceptualization, Validation, Writing – review & editing. Abhijeet Anand: Conceptualization, Formal analysis, Funding acquisition, Methodology, Validation, Writing – review & editing.
Data availability
Aggregated data from Table 2 in the manuscript will be added to the Clinicaltrials.gov registration webpage with publication
References
- [1].Sutter RW, Kew OM, Cochi SL, Aylward RB. Poliovirus vaccine - live. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 6th ed. Elsevier; 2013. p. 598–645. [Google Scholar]
- [2].Platt LR, Estivariz CF, Sutter RW. Vaccine-associated paralytic poliomyelitis: a review of the epidemiology and estimation of the global burden. J Infect Dis 2014;210(Suppl. 1):S380–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hird TR, Grassly NC. Systematic review of mucosal immunity induced by oral and inactivated poliovirus vaccines against virus shedding following oral poliovirus challenge. PLoS Pathog 2012;8:e1002599. 10.1371/journal.ppat.1002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].World Health Organization. Polio vaccines and polio immunization in the preeradication era: WHO position paper. Wkly Epidemiol Rec 2010;85:213–28. [PubMed] [Google Scholar]
- [5].World Health Organization. Polio vaccines: WHO position paper – March, 2016. Wkly Epidemiol Rec 2016;91:145–68. https://apps.who.int/iris/handle/10665/357168.27039410 [Google Scholar]
- [6].World Health Organization. Meeting of the strategic advisory group of experts on immunization, November 2012 — conclusions and recommendations. Wkly Epidemiol Rec 2013;88:1–16. https://apps.who.int/iris/bitstream/handle/10665/242011/WER8801_1-6.PDF?sequence=1&isAllowed=y. [PubMed] [Google Scholar]
- [7].World Health Organization. Meeting of the strategic advisory group of experts on immunization, November 2013 – conclusions and recommendations. Wkly Epidemiol Rec 2014;89:1–19. https://www.who.int/publications/i/item/WER8901. [PubMed] [Google Scholar]
- [8].Pan American Health Organization. Practical guide: Inactivated poliovirus vaccine (IPV) introduction. Washington, DC. https://www.paho.org/hq/dmdocuments/2014/Polio-ipv-2014-eng.pdf; 2014. [Google Scholar]
- [9].Anand A, Pallansch MA, Estivariz CF, Gary H, Wassilak SG. Estimating the likely coverage of inactivated poliovirus vaccine in routine immunization: evidence from demographic and health surveys. J Infect Dis 2014;210(Suppl. 1):S465–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].World Health Organization. WHO/UNICEF Immunization Coverage: Immunization data dashboard, global. Diphtheria tetanus toxoid and pertussis (DTP) vaccination coverage. Available at, https://immunizationdata.who.int/index.html. [Accessed 22 August 2022].
- [11].Faden H, Modlin JF, Thoms ML, McBean AM, Ferdon MB, Ogra PL. Comparative evaluation of immunization with live attenuated and enhanced-potency inactivated trivalent poliovirus vaccines in childhood: systemic and local immune responses. J Infect Dis 1990;162:1291–7. [DOI] [PubMed] [Google Scholar]
- [12].Modlin JF, Halsey NA, Thoms ML, Meschievitz CK, Patriarca PA. Humoral and mucosal immunity in infants induced by three sequential inactivated poliovirus vaccine-live attenuated oral poliovirus vaccine immunization schedules. Baltimore area polio vaccine study group. J Infect Dis 1997;175(Suppl. 1):S228–34. [DOI] [PubMed] [Google Scholar]
- [13].Ramsay ME, Begg NT, Gandhi J, Brown D. Antibody response and viral excretion after live polio vaccine or a combined schedule of live and inactivated polio vaccines. Pediatr Infect Dis J 1994;13:1117–21. [DOI] [PubMed] [Google Scholar]
- [14].Hu Y, Xu K, Han W, Chu K, Jiang D, Wang J, et al. Safety and Immunogenicity of Sabin Strain Inactivated Poliovirus Vaccine Compared With Salk Strain Inactivated Poliovirus Vaccine, in Different Sequential Schedules With Bivalent Oral Poliovirus Vaccine: Randomized Controlled Noninferiority Clinical Trials in China. Open Forum Infect Dis 2019;6:ofz380. 10.1093/ofid/ofz380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].O’Ryan M, Bandyopadhyay AS, Villena R, Espinoza M, Novoa J, Weldon WC, et al. Inactivated poliovirus vaccine given alone or in a sequential schedule with bivalent oral poliovirus vaccine in Chilean infants: a randomised, controlled, open-label, phase 4, non-inferiority study. Lancet Infect Dis 2015;15:1273–82. [DOI] [PubMed] [Google Scholar]
- [16].Qiu J, Yang Y, Huang L, Wang L, Jiang Z, Gong J, et al. Immunogenicity and safety evaluation of bivalent types 1 and 3 oral poliovirus vaccine by comparing different poliomyelitis vaccination schedules in China: a randomized controlled non-inferiority clinical trial. Hum Vaccin Immunother 2017;13:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Anand A, Zaman K, Estivariz CF, Yunus M, Gary HE, Weldon WC, et al. Early priming with inactivated poliovirus vaccine (IPV) and intradermal fractional dose IPV administered by a microneedle device: a randomized controlled trial. Vaccine 2015;33:6816–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].WHO Multicentre Growth Reference Study Group. WHO child growth standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: Methods and development. Geneva: World Health Organization; 2006. Available at, https://www.who.int/tools/child-growth-standards/who-multicentre-growth-reference-study. [Accessed 15 September 2021]. [Google Scholar]
- [19].Weldon WC, Oberste MS, Pallansch MA. Standardized methods for detection of poliovirus antibodies. Methods Mol Biol 2016;1387:145–76. 10.1007/978-1-4939-3292-4_8. [DOI] [PubMed] [Google Scholar]
- [20].World Health Organization. Polio laboratory manual. In: Immunization V, and Biologicals, editor. 4th ed. Geneva; 2004. Available at, https://polioeradication.org/wp-content/uploads/2017/05/Polio_Lab_Manual04.pdf. [Accessed 15 September 2021]. [Google Scholar]
- [21].Resik S, Tejeda A, Sutter RW, Diaz M, Sarmiento L, Alemani N, et al. Priming after a fractional dose of inactivated poliovirus vaccine. N Engl J Med 2013;368:416–24. [DOI] [PubMed] [Google Scholar]
- [22].World Health Organization. Polio vaccines: WHO position paper – June 2022. Wkly Epidemiol Rec 2022;97:277–300. https://apps.who.int/iris/handle/10665/357168. [Google Scholar]
- [23].UK Health Security Agency. Poliovirus detected in sewage from North and East London. Investigation underway to protect public, who are urged to ensure polio vaccines are up to date, especially parents of young children who may have missed an immunisation opportunity. UK Health Security Agency press office; 2022. Available at, https://www.gov.uk/government/news/poliovirus-detected-in-sewage-from-north-and-east-london. [Accessed 9 February 2023]. [Google Scholar]
- [24].Link-Gelles R, Lutterloh E, Schnabel Ruppert P, Backenson PB, St George K, Rosenberg ES, et al. Public health response to a case of paralytic poliomyelitis in an unvaccinated person and detection of poliovirus in wastewater - New York, June-August 2022. MMWR Morb Mortal Wkly Rep 2022;71:1065–8. https://www.ncbi.nlm.nih.gov/pubmed/35980868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].World Health Organization. Circulating vaccine-derived poliovirus type 2 (cVDPV2) – Ukraine. Available at, https://www.who.int/emergencies/disease-outbreak-news/item/circulating-vaccine-derived-poliovirus-type-2-(cvdpv2)-ukraine. [Accessed 22 October 2021].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Aggregated data from Table 2 in the manuscript will be added to the Clinicaltrials.gov registration webpage with publication


