Abstract
Background
Obesity is a chronic disease characterized by excess body fat and is a risk factor for other chronic non-communicable diseases. Its multifactorial and complex nature makes its management a challenge for health services. This manuscript presents an investigation protocol that aims to analyze the effectiveness of collective nutritional interventions for obesity management applicable to primary health care.
Methods
Randomized Controlled Community Trial (RCCT) in a representative sample of users of the Programa Academia de Saúde (PAS), in Belo Horizonte, Minas Gerais, Brazil, with obesity. The research consists of four phases: (1) Screening to identify the participants eligible for the nutritional interventions (individuals with obesity, readiness for change to lose body weight, and willingness and interest to participate in a group activity for six months or more); (2) Baseline to characterize the participants; (3) Implementation of collective nutritional interventions; (4) Reassessment of the participants. Participants in the control group (CG) will receive the usual health service care, and participants in the intervention group (IG) will participate in collective nutritional interventions based on Therapeutic Group 1 (TG1) or Therapeutic Group 2 (TG2) of the “Instructive of Collective Approach for the obesity management in SUS”.
Discussion
The strengths of the study include its robust RCCT design, which allows for longitudinal analyses and is suitable for investigating causal hypotheses and applying strategies to improve adherence to interventions. Furthermore, the study included a representative sample of a public health service and aims to evaluate therapeutic proposals from the Brazilian Ministry of Health, which can contribute to implementation and extension in the national territory.
Trial registration
RBR-3vzsyqq and RBR-6pg682m.
Keywords: Longitudinal studies, Primary health care, Obesity management, Transtheoretical model, Randomized controlled trials
Background
Obesity is a chronic disease characterized by excess body fat and is a risk factor for other chronic non-communicable diseases (NCDs) [1]. It is a global epidemic responsible for 5 million deaths worldwide, including 178,000 in Brazil alone [2]. It is estimated that 26.8% [3] of the Brazilian adult population has obesity, which has an impact on individuals, families, communities, and the health care system [4].
The health care of individuals with obesity should include the promotion of healthy eating patterns, encouraging physical activity, and adopting a healthy lifestyle; and in severe cases, pharmacological and/or surgical interventions [5]. The 5–10% reduction in body weight presents health benefits [6, 7], however, most interventions aimed at obesity management demonstrated limited results [8].
The development of effective interventions is urgent. However, the multifactorial and complex nature of obesity makes its management a challenge for health services. International organizations have proposed the strengthening of Primary Health Care (PHC) as a viable to obesity management due to its attributes, proximity, and knowledge of the health needs of people and communities [9].
In Brazil, the systematization of obesity management within PHC faces challenges related to the physical and structural resources available; the lack of consensus on effective approaches, the standardization of protocols, and the qualification of health professionals [8, 10]. In this sense, the Brazilian Ministry of Health, in collaboration with researchers, has developed materials to guide the management of obesity in PHC [7, 11, 12]. Examples include the “Instruction for Collective Approach to Obesity Management in the SUS” (“Instrutivo de Abordagem Coletiva para Manejo da Obesidade no SUS”, in Portuguese) [7], which supports health professionals in the management of obesity using theoretical and methodological tools based on scientific evidence and in the autonomy of individuals in their self-care. To this end, it relies on behavioral change theories, such as the Transtheoretical Model and Cognitive Behavioural Therapy (CBT), anchored in the critical-reflective approach proposed by Paulo Freire.
The Transtheoretical Model has been utilized in multicomponent obesity interventions because it requires that individuals do not make changes abruptly, but in stages [13]. And, CBT considers that individuals develop and maintain beliefs throughout their lives, the basis on which they formulate their vision of themselves, the world, and the future [14], demanding the adopting strategies to act in the environment to promote change [7]. It is believed that, when used together, these theories can improve the care offered to individuals with obesity.
This manuscript presents the first methodological protocol of a study aimed at analyzing the effectiveness of collective nutritional interventions for obesity management applicable to PHC. To this end, the research employs a Randomized Controlled Community Trial (RCCT) design to evaluate the effectiveness of a collective nutritional interventions for individuals with and without an indication of bariatric surgery (Intervention Group) compared to usual care (Control Group) in a real context of care for individuals with obesity in a Brazilian PHC health promotion service.
Methods
Setting and study step
The study was conducted at the Health Academy Programme (Programa Academia de Saúde, PAS, in Portuguese) in Belo Horizonte, the capital of Minas Gerais, Brazil. The municipality is the sixth largest in the country, with an estimated population of 2,315,560 people distributed across nine administrative regions [15]. It has a high Human Development Index (0.797) and a well-established PHC, which includes 152 health centers and 81 PAS units [16].
Since 2011, PAS has been a Brazilian PHC service that aims to promote equity in access to health promotion and care interventions, particularly for NCDs. To achieve this, it provides body practices and physical activities; promotion of care and healthy lifestyles; promotion of healthy eating; integrative and complementary practices; artistic and cultural practices; health education; and community mobilization [17].
In Belo Horizonte, PAS was implemented in 2006 and mainly offers supervised physical exercise and health education activities. Participants are predominantly women (88.1%), with an average age of 55 years old and a high prevalence of obesity (32.0%) [10].
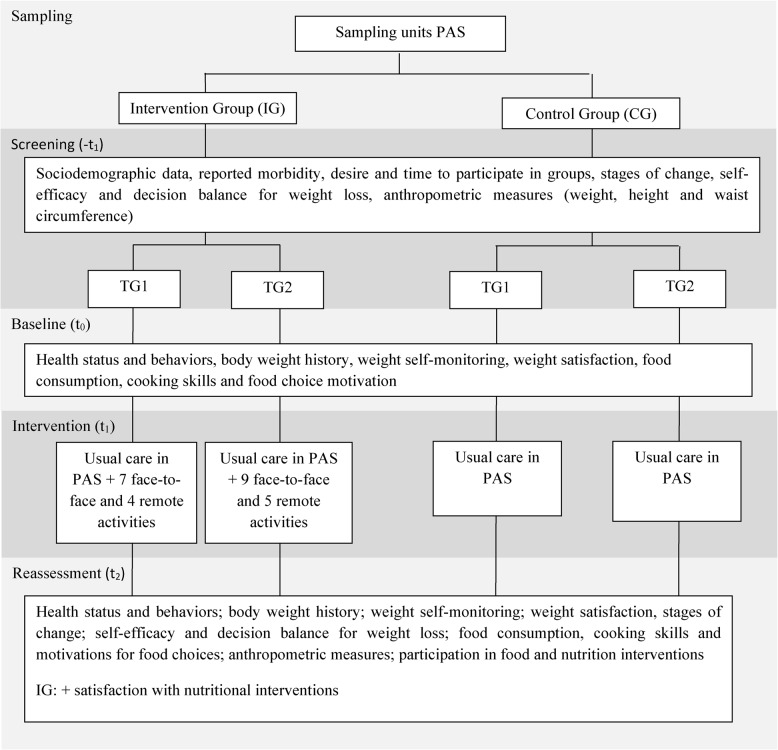
The ECCR presented in this manuscript will be developed in four phases: (1) Screening to identify the participants eligible for the nutritional interventions (-t1); (2) Baseline to characterize the participants (t0); (3) Implementation of the collective nutritional interventions (t1); (4) Reassessment of the participants (t2) (Fig. 1).
Fig. 1.
Description of the schedule of enrollment, interventions and assessments. Note: -t1=screening; t0=baseline; t1=intervention; t2=reassessment; TG: terapeutic group 1; PAS: Programa Academia da Saúde
Study sample
The sampling process will be conducted by a statistician hired for this purpose, and the researchers will have no involvement in the selection of PAS units or the allocation of the intervention and control groups. Simple random sampling will be executed, stratified by nine administrative regions of the municipality. To calculate the sample the PAS units in operation at the time of the sampling will be considered, excluding those that do not operate in the morning (the predominant shift of the health service in the municipality and in which the nutritional interventions will be developed) and that have participated in food and nutrition actions in the last 24 months, in consultation with PAS professionals. The parameters used to calculate the sample include a 5% difference between the groups in terms of body weight reduction, sampling power of 80%, a 35% increase in possible losses, and a 20% prevalence of obesity in the service.
To carry out the sampling process, a table of random numbers will be utilized in two stages. In the first stage, two units will be selected per administrative region. In the second stage, the units will be randomly drawn to determine the allocation group in the RCCT: CG and IG. In the RCCT design, each PAS unit represents a conglomerate, so a certain number of units must be selected for each regional stratum until the size of the conglomerate is complete.
For sample the calculation of samples, group comparison (proportion hypothesis tests) and bilateral test were considered, using the expression:
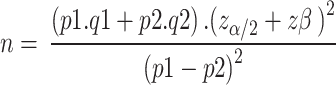 |
n = sample size (for each administrative region of the municipality);
z_(α⁄2) = value of the standardized normal distribution for the error α, assumed to be: 1.96 (5%);
zβ = value of the standardized normal distribution for the error β, assumed to be: 0.84 (20%);
p1 and p2 – proportion of participants with obesity for GC and GI of each region, respectively;
q1 and q2 – proportion of participants without obesity for GC and GI of each region, respectively.
Within each PAS unit, all participants aged 20 years old or older and participating in health service activities (attending at least one physical exercise class in the month before data collection) will be interviewed at the Screening stage. Exclusion criteria will be: being pregnant or having any cognitive difficulty that makes it impossible to answer the questionnaire, as reported by the health service professionals.
Data collection
The research phases (Screening, Baseline, Interventions, and Reassessment) will be preceded by a presentation and discussion of the Project and field logistics with the technical references of the Municipal Health Department and the local coordinators of the PAS units, to jointly draft up the research plan.
Data collection will take place in four panels, each panel consisting of one IG unit and one CG unit from each nine administrative regions of the municipally, according to the order in which the sample process was drawn and agreed upon with the local coordinators. The local PAS coordinators are informed by e-mail 72 h before the start of data collection.
In the PAS units, before data collection begins, the fieldwork supervisor will introduce individuals to the research and the importance of their participation. They will then be invited to participate in the research by the inclusion and exclusion criteria.
The data collection will be carried out during the opening hours of the PAS, and the teams will be made up of health professionals and undergraduates, divided into the roles of field supervisors and interviewers (an average of five), accompanied by the general field supervisor and the research coordinators. The interviewers will be responsible for clarifying doubts, obtaining informed consent, and conducting face-to-face interviews at all stages of the research. The field supervisors will be responsible for contacting the PAS units to start data collection; ensuring consistency of the questionnaires; anthropometric assessment of the participants; scheduling and coordinating the flow of interviews; recording absences, refusals, and exclusions; and sending a weekly field report.
The purpose of the field report will be to monitor data collection. It should include information identifying the unit and the responsible for the PAS; the number of classes and participants in the service; the team’s work schedule; the number of questionnaires used, refusals and exclusions; and any problems encountered in the field. Each report will be analyzed by the general data collection supervisor, who will also be responsible for organizing the weekly data collection work schedule, dealing with conflicts and challenges in the field, and training the team, with the support of an experienced field supervisor. All these activities will be monitored and discussed with the research coordinators.
All teams will be trained and the equipment calibrated before data collection begins. Data collection manuals and field logistics will be made available to clarify doubts and support the teams in the field. Field supervisors will have a specific manual and field logistics. Training and equipment calibration will take place every six months or when specific needs are identified.
Electronic tablets will be used for the interviews, with the questionnaires available on the Epicollet5 Data Collection app (Oxford University, CGPS, England). Each day after data collection, the field supervisor will be responsible for synchronizing the application data and automatically generating the consolidated database. If the tablets are unstable, the information will be collected on paper and typed by trained typists using the same app. The entire data processing phase will be supervised by the general data collection supervisor.
All the questionnaires used in the research have been developed jointly by the researchers, and their questions are based on national [3, 18] and international surveys [19, 20], and the previous experience of the research group [7, 21] and other research groups [22–25].
Screening
The Screening questionnaire will include information on the usual care provided in the PAS (days and times) and the date of admission to the PAS. The date of entry into the PAS will be obtained by the health service coordinators from the available records. The participant’s date of entry will be subtracted from the date of the Screening interview to obtain the duration of participation in the health service. In addition, the following variables will be collected: (1) Sociodemographic (sex, age, years of schooling, marital status, occupation, number of individuals living in the household, and family income); (2) Reported morbidities (cardiovascular disease, hypercholesterolemia, sleep apnea, joint diseases, diabetes mellitus, hypertension, history of bariatric surgery, and smoking); (3) Willingness and time to take part of groups activities lasting 6 months or more; (4) Stage of change, self-efficacy and balance of decisions to body weight loss, according to the Transtheoretical Model; (5) Anthropometry measurements (weight, height and waist circumference).
Participants will be invited to complete the Screening questionnaire during the time they are exercising at the PAS or on a day and time of their choosing. If they do not have obesity, the data collection will be stopped before the Screening interview is completed and they will be informed of their ineligibility. For individuals with obesity, the indication for bariatric surgery and the availability and desire to take part in group activities will be assessed. Then, the stage of change, self-efficacy and balance of decisions were assessed. If the participant were no eligible to interview (see Details of intervention and control group activities) will be ended and the interviewee informed of their ineligibility.
Respondents who are eligible to take part in the nutritional interventions (see Details of intervention and control group activities) will be referred to the Baseline interview, which can take place in the sequence or by appointment. After three unsuccessful attempts to an appointment or three consecutive absences from the Screening or Baseline interviews, the participant will be considered lost to the research.
The algorithm proposed by the Ministry of Health [26], adapted from Hawkins, Hornsby, and Schorting (2001) [27] and Chang (2007) [28], will be used to examine the stage of change to body weight loss. This algorithm is based on the individual’s perception of their body weight loss. Respondents are asked if they have a desire to lose weight, and those who answer “yes”, will be asked when they intend to do so. Those who have already started to make changes in their lifestyle to lose weight will be asked how long ago, more or less than 6 months. Based on their answers, individuals will be classified into one of five stages of change: pre-contemplation (not intending to lose weight in the next 6 months), contemplation (intending to lose weight but not in the next month), preparation (intending to lose weight and will do so in the next month), action (already started making lifestyle changes to lose weight, less than six months ago) or maintenance (already made lifestyle changes and this process has lasted more than six months) [26].
Self-efficacy for body weight loss will be assessed using a validated scale consisting of three statements, in which the respondents indicate their level of confidence in their weight loss [29]. Self-efficacy will be classified as high if the participant responds that they are very confident or completely confident in at least two of the three statements; or low self-efficacy, if they are very confident or completely confident in one or none of the statements. The balance of decisions toward weight loss will be assessed using a validated scale that assesses the importance the individual attaches to four positive situations related to weight loss, and the concern associated with another four negative situations [29].
Calibrated equipment approved by technical certification will be used for anthropometric assessment. Body weight (kg) will be measured using a digital scale with a maximum capacity of 200 kg. Height (cm) will be assessed using a portable stadiometer with a range of 0.35 m to 2.13 m. Waist circumference (WC) will be measured using an inelastic tape measure with a scale of 0 to 200 cm, with measurements taken in triplicate for later calculation of the arithmetic mean.
The Body Mass Index (BMI) (weight/height2) will be used to define the diagnosis of obesity, classified as normal (BMI ≥ 18.5 and < 25.0 kg/m2), overweight (BMI ≥ 25 and < 30 kg/m2), obesity class I (BMI ≥ 30 and < 35 kg/m2); obesity class II (BMI ≥ 35 and < 40 kg/m2) and obesity class III (BMI ≥ 40 kg/m2) [30]. WC will be used as an indicator of metabolic risk [31] and will be classified differently for women (high risk: ≥ 80.0 and < 88.0 cm; very high risk: e ≥ 88.0 cm) and men (high risk: ≥ 94.0 and < 102.0 cm; very high risk: ≥ 102.0 cm).
Health literacy was assessed with the following question: “Do you have difficulty understanding instructions from doctors or other health professionals?“. The health profile analysis will include the following self-reported questions: the presence of nonalcoholic fatty liver disease; treatment for depression, anxiety, or mental illness; health perception [3]; physical exercise practices [18]; history of body weight; previous attempts to lose weight [19, 20] and weight stigma (analyzed by the participant’s agreement with the following sentence: “Because of my body, I have been suffered bullying or prejudiced at different times in my life”.
To assess food consumption, the participants will answer about the frequency of weekly consumption of in natura or minimally processed foods (fruit, vegetables, beans, red meat, chicken, fish, milk) and ultra-processed foods (nectar or powdered soft drinks, soft drinks, biscuits and sweets, chocolate drinks or flavored yogurt, sausages and processed bread) [3]; monthly consumption of salt, fat, and sugar in the household [21]. For fruit and vegetables, the portions consumed will also be examined.
Cooking skills will be assessed using a validated scale with statements about cooking confidence and will be analyzed according to a three-point Likert scale [22]. Food choice motivation will be assessed using a five-point Likert scale indicating the frequency of food choice according to the following motivations: preference, habit, need and hunger, health, convenience, pleasure, traditional food, natural issues, socialization, price, visual attraction, weight control, emotional control, social norms, and social image [23].
To assess dietary patterns, the “How is your diet?” will be used. The “How is your diet?” test is a scale for measuring healthy eating practices measurement according to the recommendations of the Dietary Guidelines for the Brazilian Population recommendations. It consists of 24 statements related to the choice and combination of food, the importance of cooking, and commensality. The individual answers the frequency of each habit presented on a four-point Likert scale [25].
Reassessment of participants
All baseline participants will be invited after nutrition interventions to take part in the reassessment phase (t2). The questionnaire will cover the issues investigated in the Screening and Baseline phases, as well as questions about remaining in the PAS and participating in food and nutrition interventions outside the PAS setting. Specifically for IG participants, satisfaction with nutrition interventions will also be assessed.
Participants who are absent from the PAS unit at the time of the reassessment will be contacted by the research team by telephone and invited to answer the interview face-to-face at the PAS. If the interviewee is unable to attend, a telephone interview will be conducted with a questionnaire containing information on self-reported weight, food consumption, PAS activities, and strategies used to control body weight.
Details of intervention and control group activities
In the Screening phase (-t1), participants to be followed up in the study will be selected based on the following criteria: individuals with obesity (BMI ≥ 30 kg/m2), willing and interested in participating in a group activity for six months or more, and with readiness for change to body weight loss (stages of change: “preparation with high self-efficacy”, “action” or “maintenance”).
Participants selected from the PAS units assigned to the CG will receive the usual health service care, i.e., supervised group physical exercise three times a week, for one hour. In addition, IG participants will take part in a collective nutritional intervention based on Therapeutic Group 1 (TG1) or Therapeutic Group 2 (TG2) of the “Instructive of Collective Approach for the obesity management in SUS” [7]; and the accompanying Notebook of Educational Activities [32].
The TG1 will aim to reduce body weight by 3% and will include individuals who do not indicate bariatric surgery [7]. TG2 will aim to reduce body weight by 5% and will include individuals who indicate bariatric surgery [7]. To investigate the indication for bariatric surgery, participants will be asked if they are waiting to undergo surgery or if they have already undergone surgery; if the answer is “yes”, they will be asked how long ago they underwent the surgery. If the answer is “no”, the presence of comorbidities will be assessed by self-report. Individuals with diabetes mellitus or at least two comorbidities will be considered to indicate bariatric surgery, as recommended by Decree No. 424 of 19 March 2013 [33].
Collective nutritional interventions will only be developed in PAS units assigned to the IG that have a minimum of eight individuals eligible for TG1 or TG2. The units that do not reach the minimum participants requirement will remain IG units and will undergo data processing in the analyses, as recommended by CONSORT 2010 [34], and in agreement with the health service, aiming to maintain the group framework.
The collective nutritional interventions will start up to one month after the end of the Baseline phase. They will consist of closed regressive groups that will last for six months, to increase interaction and bonding among those participants, strengthening mutual trust, and sharing experiences (Fig. 1) [35]. Meetings will preferably take place before or after physical exercise at the PAS, depending on the availability of participants and groups in the unit. Each group will have a maximum of 20 individuals [7] to ensure everyone’s participation. If this limit is exceeded, the groups will be repeated as many times as necessary to include all eligible participants.
The TG1 consists of seven group face-to-face activities and four remote activities, and TG2 consists of nine face-to-face meetings and five remote activities. The participants were encouraged to self-monitoring weight and maintain regular physical exercise in the PAS. The face-to-face meetings will include workshops and actions in the environment; and the remote activities will involve sending postcards with motivational messages and use of Information and Communication Technologies (ICT), including messaging via an app or phone calls.
The face-to-face activities aim to increase the effectiveness of the actions; and the remote activities aim to motivate participation, improve adherence, and encourage reflection on the topics covered in the meetings. In these activities, participants receive guidance on controlling the size of food portions; the need to prioritize the consumption of fresh and minimally processed foods, and to reduce ultra-processed foods, as recommended by the Dietary Guidelines for the Brazilian population [12]. This is expected to reduce excessive consumption of carbohydrates and lipids and promote a calorie reduction of 500 to 1,000 kcal/day [5].
To encourage adherence to nutritional interventions, telephone calls will be made 48 h before the face-to-face meetings. Attendance lists will be used to monitor the presence/absence of participants at face-to-face meetings and to record participation in remote activities. All food and nutrition-related activities carried out during the research in the PAS units of the CG and IG will also be monitored and recorded every month, to control the results of the study. In addition, individuals’ participation in nutritional interventions outside the PAS will be examined during the Reassessment.
Given the characteristics of the nutritional interventions being developed, it will not be possible to blind the participants or the researchers. However, it has been decided to consider the intervention allocation group as the PAS unit to avoid contamination of the CG participants.
The nutritional interventions will be developed by the research group that participated in the development of the Instructive [7], which will strengthen its reliability. Three group teams will be formed to implement the interventions, including a supervisor, an assistant, and a supporter, all health professionals and academics, accompanied by the research coordinators. Systematic training, preparation of manuals and records of the logistics of the nutritional interventions, and supervision of the teams by a psychologist with experience in groups and the management of obesity will be procedures adopted to ensure the alignment of the team, as well as the correct posture, communication skills and empathy of the team.
Variables assessed
Primary outcomes
To assess the effectiveness of the nutritional interventions, the following primary outcomes will be analyzed: “change in body weight”, “rate of satisfactory weight change” and “change in WC” of the participants.
Changes in body weight will be assessed by calculating the delta [delta weight = weight at Screening - weight at Reassessment] and the percentage of weight change {% weight change = [(Weight at Screening - weight at Reassessment)/weight at Screening] x 100}. The rate of satisfactory weight change will be calculated by the number of participants achieving the target body weight reduction for their group (TG1: >3% weight loss; TG2: >5% weight loss).
The change in WC will also be assessed in the form of delta (delta WC = WC at Screening – WC at Reassessment) and the percentage of WC change {% WC change = [(WC at Screening - WC at Reassessment) / WC at Screening] x 100}.
Secondary outcomes
The following secondary outcomes will be assessed: evolution of the stages of change, self-efficacy, and balance of decisions to reduce body weight according to the Transtheoretical Model; variation in food consumption; culinary skills; self-monitoring of weight; and adherence to collective nutritional interventions.
Adherence to nutritional interventions will be calculated for total adherence, adherence to face-to-face activities, and adherence to remote activities. Total adherence will be calculated based on the proportion of individuals who participated in the face-to-face and remote activities offered relative to the total number of activities offered [% adherence = (number of face-to-face and remote activities the individual participated in/ total number of face-to-face and remote activities offered) x 100]. Similar procedures will be used to specifically assess adherence to face-to-face and remote activities.
Covariates
Socio-demographic data, such as age, sex, monthly per capita income, education, marital status, professional occupation, and participation in other nutritional interventions during the study period will be examined as potential confounders. Health conditions and behaviors such as smoking, self-reported morbidity, physical exercise, body weight at baseline, satisfaction with current weight, and frequency of weight self-monitoring will also be assessed.
Data management and analysis
A dictionary of variables will be created, including the name and label of the variable, and response codes. Once the data have been entered and the variable dictionary created, the database will be analyzed for consistency. This will involve a descriptive analysis of the data and identification of outliers and, where possible, the physical questionnaire will be consulted to check for inconsistencies.
Multiple imputation techniques will be used for missing data identified in the primary and secondary outcomes.
Statistical analyses of sample power and the primary and secondary outcomes will be performed using Stata software version 14.0. A 5% significance level (p < 0.05) will be adopted for all analyses.
In the case of participants moving from TG1 to TG2 because the minimum number of participants to form the group (n = 8) was not reached, the information from the displaced participants will be compared with the information from the original groups.
For the analyses of the outcomes and other variables, between-group comparisons will be made to identify the differences between the CG and IG participants. The Student’s t-test will be used to compare the means of independent sample means, the Mann-Whitney test to compare medians, and the Chi-square or Fisher’s exact test to compare categorical variables.
To analyze the effectiveness of nutritional interventions, primary and secondary outcomes will be compared using random effects models, adjusting for socio-demographic variables and time of participation in the PAS. Stratified analyses by adherence to nutritional interventions and health status may be added. All models will be checked for multicollinearity of predictors and appropriate assumptions. In the case of PAS units that implemented obesity-related interventions during the study period, descriptive analyses of the main outcomes will be performed to decide whether to exclude or keep the data, with subsequent data processing to control for potential bias.
The study procedures and collective nutritional interventions will be carried out independently of funders and competing interests. Therefore, the establishment of a Data Monitoring Committee will not be necessary. However, it should be noted that the overall conduct of the RCCT will be monitored by the principal investigator and three researchers.
Ethics and dissemination
This study received ethical approval from the Institutional Review Boards of the University (36395320.7.0000.5149; 42654421.1.0000.5149) and the City of Belo Horizonte (36395320.7.3001.5140; 42654421.1.3001.5140), and will be carried out according to the guidelines from the Declaration of Helsinki. The informed consent will be obtained from all participants.
The study was registered in Brazilian Clinical Trials Registry (RBR-3vzsyqq; Efeito de Grupos para o tratamento da Obesidade and RBR-6pg682m; Avaliação da efetividade de intervenção coletiva usuários da Atenção Primária com indicação de cirurgia bariátrica). The funders had no role in the design of the study, collection, analysis, and interpretation of data, writing of the report, and the decision to submit the article for publication.
Discussion
This article describes an RCCT protocol developed to analyze the effectiveness of collective nutritional interventions for the management of obesity in Brazilian PHC. The strengths of the study include its robust RCCT design, which allows longitudinal analyses and is suitable for investigating causal hypotheses, minimizing the influence of potential confounding variables on the results [36]. In addition, the study aims to address important gaps in the literature, such as the effectiveness of collective nutritional interventions to manage obesity in a representative sample of PHC health services [8]. More specifically, it also aims to evaluate the collective approaches proposed by the Brazilian Ministry of Health and contribute to their implementation and national expansion. And, the use of ICT in coordination with face-to-face activities has promising potential to contribute to the greater effectiveness of nutritional interventions [37].
Despite the various procedures presented to ensure the methodological quality of the RCCT, some challenges may remain. Noteworthy is the possible attrition of participants in PAS units [10], due to everyday situations such as change of address, illness, work, etc., which could have a negative impact on adherence to nutritional interventions and reassessment of participants. Additionally, individual aspects of the participants, such as social support, community norms, comparisons, or even their behavior, may affect the level of adherence to nutritional interventions, factors that can only be minimized but not controlled [38].
Another limitation is the impossibility of blinding participants and researchers. Given the nature of the interventions, which involve changing behavior related to eating, the participant agent makes blinding infeasible [39]. Finally, its external validity is highlighted, as the RCCT will be conducted with participants of PHC promotion service, who have specific characteristics and are more likely to adopt healthy eating behaviors when compared to the general population [40, 41]. Therefore, caution should be exercised in extrapolating the results of nutritional interventions to other contexts requires caution. However, it should be noted that 60% of the Brazilian population uses PHC services [42], including people with obesity, which added up to more than 4 million in 2021 [43].
This research will be used as RCCT to test the effectiveness of collective nutritional interventions proposed by the Brazilian Ministry of Health’s Instruction for collective management of obesity in PHC. To our knowledge, no other study has evaluated these nutritional interventions. In this sense, this study will contribute to providing evidence for their implementation in PHC and will encourage future research with the same objective, as well as providing relevant information for the design of effective collective nutritional interventions applicable to PHC.
Acknowledgements
Many thanks to the team of the Research Group on Nutrition Interventions (GIN/UFMG) at the Universidade Federal de Minas Gerais who will be data collection.
Abbreviations
- BMI
Body Mass Index
- CBT
Cognitive Behavioural Therapy
- CG
Control Group
- ICT
Information and Communication Technologies
- IG
Intervention Group
- NCDs
Chronic Non-Communicable Diseases
- PAS
Programa Academia de Saúde
- PHC
Primary Health Care
- RCCT
Randomized Controlled Community Trial
- TG1
Therapeutic Group 1
- TG2
Therapeutic Group 2
- WC
Waist Circumference
Authors’ contributions
PP FREITAS: Conception; methodology; data collection supervision; data curation; project management; drafting of manuscript; proofreading and editing.MS LOPES: Conception; methodology; project management; drafting of manuscript.JR ARAUJO: Data collection supervision; research; drafting of manuscript.RB CUNHA: Data collection supervision; research; drafting of manuscript.CK DUARTE: Conception; methodology; project management; drafting of manuscript.ACS LOPES: Conception; methodology; data collection supervision; data curation; project management; acquisition of funding; drafting of manuscript; proofreading and editing.
Funding
This study is funded to Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) [APQ 00303 − 22]; Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) [442877/2020-0; 302451/2022-6 ACSL productivity grant]; Brazilian Ministery of Health (Ministério da Saúde, MS) [TED 063/2019]; Pró-Reitoria de Pesquisa (PRPq/UFMG) and in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)—Finance code 001. The financial support agencies had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study received ethical approval from the Institutional Review Boards of the University (36395320.7.0000.5149; 42654421.1.0000.5149)) and the City of Belo Horizonte (36395320.7.3001.5140; 42654421.1.3001.5140), and will be carried out according to the guidelines from the Declaration of Helsinki. The informed consent will be obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Obesity Federation, World Obesity Atlas. 2023. https://data.worldobesity.org/publications/?cat=19. Accessed 18 Jan 2024.
- 2.Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2019. (GBD 2019) Results. Seattle, United States: Institute for Health Metrics and Evaluation (IHME). 2020. https://vizhub.healthdata.org/gbd-results/. Accessed 18 Jan 2024.
- 3.Instituto Brasileiro de Geografia e Estatística. Pesquisa nacional de saúde: 2019: percepção do estado de saúde, estilos de vida, doenças crônicas e saúde bucal: Brasil e grandes regiões. 2020. http://6a25a69bd2bb7bdcdabd528a5bfb5f7d.pdf (ibge.gov.br). Accessed 18 Jan 2024.
- 4.Velry-Jr E, Machado IE, Nilson EAF, Editorial. Economic and health impacts of dietary interventions. Front Nutr. 2023. 10.3389/fnut.2023.1283108. [DOI] [PMC free article] [PubMed]
- 5.Brasil. Ministério da Saúde. Portaria SCTIE/MS nº 53, de 11 de Novembro de 2020. 2020. https://bvsms.saude.gov.br/bvs/saudelegis/sctie/2020/prt0053_13_11_2020.html. Accessed 18 Jan 2024.
- 6.Wing RR, Look AHEAD, Research Group. Does Lifestyle Intervention Improve Health of Adults with Overweight/Obesity and type 2 diabetes? Findings from the look AHEAD randomized Trial. Obes (Silver Spring). 2021. 10.1002/oby.23158. [DOI] [PubMed]
- 7.Brasil. Ministério Da Saúde. Instrutivo De Abordagem Coletiva para manejo da obesidade no SUS / Ministério Da Saúde, Universidade Federal De Minas Gerais. Brasília: Ministério da Saúde; 2021. [Google Scholar]
- 8.Menezes MC, Duarte CK, Costa DVP, Lopes MS, de Freitas PP, Campos SF, et al. A systematic review of effects, potentialities, and limitations of nutritional interventions aimed at managing obesity in primary and secondary health care. Nutrition. 2020. 10.1016/j.nut.2020.110784. [DOI] [PubMed]
- 9.Kraef C, Wood B, von Philipsborn P, Singh S, Peterson SS, Kallestrup P. Primary health care and nutrition. Bull World Health Organ. 2020. 10.2471/BLT.20.251413. [DOI] [PMC free article] [PubMed]
- 10.Lopes ACS, Lopes MS, Duarte CK, de Freitas PP. Longitudinal effect of nutritional intervention on body weight: a randomized controlled trial. Nutrition. 2022. 10.1016/j.nut.2021.111436. [DOI] [PubMed]
- 11.Brasil. Ministério Da Saúde. Política Nacional De Alimentação E Nutrição. Brasília: Ministério da Saúde; 2012. [Google Scholar]
- 12.Brasil. Ministério Da Saúde. Secretaria De Atenção à Saúde. Departamento De Atenção Básica. Guia alimentar para a população brasileira. 2 ed. Brasília: Ministério da Saúde; 2014. [Google Scholar]
- 13.Prochaska JO, Di Clemente CC, Norcross JC. Search of how people change: applications to addictive behaviors. Am Psychol. 1992. 10.1037/0003-066X.47.9.1102. [DOI] [PubMed]
- 14.Beck JS. Terapia cognitivo-comportamental: teoria e prática. 2 ed. – Porto Alegre: Artmed; 2014. pp. 22–4. [Google Scholar]
- 15.Instituto Brasileiro de Geografia e Estatística (IBGE). Censo 2022: População e Domicílios - Primeiros Resultados. 2022. https://www.ibge.gov.br/cidades-e-estados/mg/belo-horizonte.html. Accessed 09 Nov 2023.
- 16.Prefeitura de Belo Horizonte. Atenção Primária à Saúde. 2022. https://prefeitura.pbh.gov.br/saude/informacoes/atencao-a-saude/atencao-primaria. Acessed 09 Nov 2023.
- 17.Brasil. Ministério Da Saúde. Secretaria De Atenção à Saúde. Departamento De Atenção Básica. Programa Academia Da Saúde: caderno técnico de apoio a implantação e implementação [recurso eletrônico]. Brasília: Ministério da Saúde; 2018. p. 220. [Google Scholar]
- 18.Brasil. Ministério da Saúde. Vigitel Brasil 2019: vigilância de fatores de risco e proteção para doenças crônicas por inquérito telefônico: estimativas sobre frequência e distribuição sociodemográfica de fatores de risco e proteção para doenças crônicas nas capitais dos 26 estados brasileiros e no Distrito Federal em 2019. Brasília: Ministério da Saúde; 2020. p. 137.
- 19.National Center for Health Statistics. NHANES 2019–2020. Questionnaire Instruments. https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/questionnaires.aspx?BeginYear=2019#print. Accessed 09 de Nov 2023.
- 20.Kaplan LM, Golden A, Jinnett K, Kolotkin RL, Kyle TK, Look M, et al. Perceptions of barriers to effective obesity care: results from the national ACTION study. Obes (Silver Spring). 2018. 10.1002/oby.22054. [DOI] [PubMed]
- 21.Menezes MC, de Lima Costa BV, Ferreira NL, de Freitas PP, de Deus Mendonça R, Lopes MS, et al. Methodological course of a community controlled trial in health care services: a translational epidemiological research on Nutrition. Demetra. 2017. 10.1017/S1368980021004341.
- 22.Martins CA, Baraldi LG, Scagliusi FB, Villar BS, Monteiro CA. Cooking skills index: development and reliability assessment. Rev Nutr. 2019. 10.1590/1678-9865201932e180124.
- 23.Moraes JMM, Alvarenga MS. Cross-cultural adaptation and apparent and content validity of the short version of the eating motivation survey (TEMS) in Brazilian Portuguese. Cad Saúde Pública. 2017. 10.1590/0102-311X00010317. [DOI] [PubMed]
- 24.Sproesser G, Moraes JMM, Renner B, Alvarenga MS. The Eating Motivation Survey in Brazil: results from a sample of the General Adult Population. Front Psychol. 2019. 10.3389/fpsyg.2019.02334. [DOI] [PMC free article] [PubMed]
- 25.Gabe KT, Jaime PC. Development and testing of a scale to evaluate diet according to the recommendations of the Dietary guidelines for the Brazilian Population. Public Health Nutr. 2019. 10.1017/S1368980018004123. [DOI] [PMC free article] [PubMed]
- 26.Brasil. Ministério Da Saúde. Caderno De Atenção Básica. Estratégias para o cuidado da pessoa com doença crônica: obesidade. Brasília: Ministério da Saúde; 2014. [Google Scholar]
- 27.Hawkins DS, Hornsby PP, Schorling JB. Stages of change and weight loss among rural African American women. Obes Res Silver Spring. 2001. 10.1038/oby.2001.8. [DOI] [PubMed]
- 28.Chang CT. Applicability of the stages of change and weight efficacy Lifestyle Questionnaire with natives of Sarawak, Malaysia. Rural Remote Health. 2007. 10.22605/RRH864. [PubMed]
- 29.Santos TSS, Carvalho MCR, Duarte K, Jaime PC, Lopes ACS. Medida do equilíbrio de decisões para redução do peso corporal entre pessoas com sobrepeso ou obesidade: uma revisão sistemática. DEMETRA: Alimentação Nutrição Saúde. 2023. 10.12957/demetra.2023.65401. [Google Scholar]
- 30.World health organization. Obesity: preventing and managing the global epidemic. Obesity Technical Report Series no. 894. Geneva: WHO. 2000. 253 p. [PubMed]
- 31.Brasil. Ministério da Saúde. Secretaria De Atenção à Saúde. Departamento De Atenção Básica. Orientações para a coleta e análise de dados antropométricos em serviços de saúde. Norma Técnica do Sistema De Vigilância Alimentar E Nutricional - SISVAN / Ministério Da Saúde, Secretaria De Atenção à Saúde, Departamento De Atenção Básica. – Brasília: Ministério da Saúde; 2011. p. 76.
- 32.Brasil. Ministério Da Saúde. Instrutivo para manejo da obesidade no Sistema Único De Saúde. Brasília: Ministério da Saúde; 2021. [Google Scholar]
- 33.Brasil. Ministério Da Saúde. Portaria nº 424, de 19 de março de 2013. Brasília: Ministério da Saúde; 2013. [Google Scholar]
- 34.Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010. 10.7326/0003-4819-152-11-201006010-00232. [DOI] [PubMed]
- 35.Vincha KRR, Santiago DA, de Campos EAL, Pellegrinelli MLP et al. Prática educativa em grupo: respondendo a inquietações. In: RW Diez-Garcia, AM Cervato-Mancuso. Mudanças alimentares e educação alimentar e nutricional. 2nd ed. Rio de Janeiro: Guanabara Koogan; 2017.
- 36.Hulley SB, Cummings SR, Browner WS, Grady DG, Newman TB. Designing clinical research – 4th ed. Philaelphia: L W W; 2013.
- 37.Oliveira LMR, Vergara CMAC, Sampaio HA, de C. Vasconcelos Filho JE De. Tecnologia mHealth na prevenção e no controle de obesidade na perspectiva do letramento em saúde. Lisa Obesidade. 2018. 10.1590/0103-1104201811814.
- 38.Smith NR, Zivich PN, Frerichs L. Social influences on obesity: current knowledge, emerging methods, and directions for future research and practice. Curr Nutr Rep. 2020. 10.1007/s13668-020-00302-8. [DOI] [PMC free article] [PubMed]
- 39.Staudacher HM, Irving PM, Lomer MCE, Whelan K. The challenges of control groups, placebos and blinding in clinical trials of dietary interventions. Proc Nutr Soc. 2017. 10.1017/s0029665117000350. [DOI] [PubMed]
- 40.Campos SF, Dos Santos LC, Lopes MS, de Freitas PP, Lopes AC. Consumption of ultra-processed foods and nutritional profile in a health promotion service of primary care. Public Health Nutr. 2021. 10.1017/S1368980021002202. [DOI] [PMC free article] [PubMed]
- 41.Campos SF, Lopes MS, Santos LCD, Freitas PP, Lopes ACS. Evaluation of nutrient consumption for the Prevention of Chronic Diseases in Health Promotion Services: a controlled and Randomized Community Trial to promote fruits and vegetables. Int J Environ Res Public Health. 2023. 10.3390/ijerph20136267. [DOI] [PMC free article] [PubMed]
- 42.Giovanella L, Bousquat A, Schenkman S, Almeida PF, Sardinha LMV, Vieira MLFP. Cobertura Da Estratégia Saúde Da Família no Brasil: o que nos mostram as Pesquisas Nacionais De Saúde 2013 e 2019. Ciênc Saúde Coletiva. 2021. 10.1590/1413-81232021266.1.43952020. [DOI] [PubMed]
- 43.Brasil. Ministério da Saúde. O impacto da obesidade. 2022. https://www.gov.br/saude/pt-br/assuntos/saude-brasil/eu-quero-ter-peso-saudavel/noticias/2022/o-impacto-da-obesidade. Accessed 18 Jan 2024.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available but are available from the corresponding author on reasonable request.