Abstract
Background
Pre-cluster symptoms (PCSs) are symptoms preceding cluster bouts and might have implications for the treatment of cluster headache (CH). This study investigated the prevalence of PCSs, and their utility in predicting upcoming bouts as well as the associations with therapeutic efficacy.
Methods
We prospectively collected data from patients with CH. Each patient received a structured interview and completed questionnaire surveys during CH bouts. In sub-study 1, we cross-sectionally analyzed the prevalence, symptomatology, and predictability of upcoming bouts. Overall, 34 PCSs, divided into seven categories, were queried, including head and neck pain, cranial autonomic symptoms, restlessness, fatigue or mood changes, sleep alterations, constitutional symptoms, and generalized pain. In sub-study 2, we recorded the weekly frequency of CH attacks after the initiation of verapamil concurrently with a 14-day transitional therapy based on the patients’ headache diary. A responder to verapamil was defined as a patient who have a reduction from baseline of at least 50% in the weekly frequency of CH attacks 4 weeks after the initiation of verapamil.
Results
A total of 168 CH patients (women/men: 39/129) completed the study. In sub-study 1, we found 149 (88.7%) experienced PCSs, with a median of 24 (IQR 18 to 72) hours before the bouts. Up to 57.7% of patients with PCS reported that they could predict upcoming bouts. Among the seven categories of PCSs, head and neck pain was the most common (81.0%) and was associated with a higher predictability of upcoming bouts (odds ratio [OR] = 4.0; 95% confidence interval [CI] 1.7–9.6). In sub-study 2, we found two categories of PCSs were associated with the response to verapamil: sleep alteration (OR = 2.5 [95% CI = 1.3–4.8], p = 0.004) and ≥ 1 cranial autonomic symptoms (OR = 2.7 [95% CI = 1.4–5.1], p = 0.003).
Conclusion
PCSs were very common in CH and could be used to predict upcoming bouts. Different symptom categories of PCSs may have different clinical implications.
Keywords: Cluster headache, Pre-cluster symptoms, Verapamil, Prodromal symptoms
Introduction
Cluster headache (CH) is one of the most severe and disabling pain disorders [1], characterized as strictly unilateral severe or very severe pain in the periorbital or temporal regions that is accompanied by ipsilateral cranial autonomic symptoms or restlessness [2, 3]. Additionally, CH is linked to various comorbidities, such as sleep disturbances, depressive states, and a heightened risk of suicidality [4–6]. One important characteristic of CH is its temporal profile of attacks; patients usually experience frequent headache attacks during ‘cluster bouts’, which last several weeks or months, separated by pain-free remission periods [3, 7]. Recently, there has been increasing recognition of the symptoms within one week preceding the upcoming bouts, namely, pre-cluster symptoms (PCSs) [8]. Also, another group of symptoms that preceding each CH attacks, which called pre-attack symptoms (usually lasting 10 min according to previous studies), has also received increased attention [9, 10]. The prevalence of PCSs varies among different cohorts [8, 11–13]. For example, a Danish study revealed that 86% of CH patients had PCSs, and 57% of patients could predict upcoming cluster bouts [8]. On the other hand, a recent Korean study revealed that only 35.3% of CH patients could predict upcoming bouts based on their PCSs [11]. The discrepancy in study findings suggests that PCSs might differ among CH patients from various regions or different methodology of studies. Hence, further research into their prevalence, symptomatology, and temporal profiles in Asian CH patients is needed.
Currently, there are practice gaps in the treatments for CH. First, CH patients usually seek medical treatment after unbearable CH bouts begin. The recognition of PCSs may help predict upcoming cluster bouts and provide patients with a therapeutic time window to obtain treatment earlier [8]. As recent studies demonstrated that ubrogepant, when taken during the prodrome phase of migraine, was effective to reduce the occurrence of moderate to severe headache attacks, similar early treatment opportunities could be explored for CH with more recognition of PCS [14]. Second, there are limited clinical predictors of response to preventive therapies for CH [15, 16]. Hence, there is a need to identify clinical features, such as PCSs, that might help to distinguish patients who are more likely to respond to one preventive therapy than to others. In this study, we aimed to investigate the following: (1) the prevalence and symptomatology of PCSs and their predictability of upcoming cluster bouts; and (2) the association between PCSs and treatment efficacy.
Methods
Study population
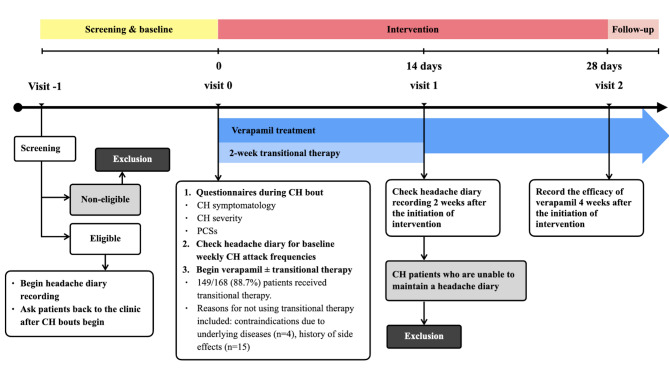
We prospectively recruited consecutive patients with CH from the Headache Clinic of the Taipei Veterans General Hospital between December 2021 and December 2023. All CH patients were diagnosed according to the International Classification of Headache Disorders, 3rd edition (ICHD-3) [17] by neurologists with an interest in headache medicine. All patients completed the questionnaire survey during cluster bouts, which included questions about CH symptomatology, severity, and PCSs (Fig. 1). In addition, we obtained information on the efficacy of verapamil as a preventive treatment based on record of the frequency and duration of CH attacks during the follow-up visits.
Fig. 1.
The study design and the recording of treatment efficacy
Inclusion and exclusion criteria for CH patients
The inclusion criteria for CH patients were as follows: (1) patients were diagnosed to have episodic CH (3.1.1) or chronic CH (3.1.2) according to the ICHD-3 diagnostic criteria; (2) patients were between 20 and 65 years of age; (3) patients had experienced ≥ 3 cluster bouts that received treatment in Headache Clinic of the Taipei Veterans General Hospital, and the most recent CH bout onset time was less than one month prior to the questionnaire survey; and (4) the therapeutic efficacy of verapamil was recorded during follow-up appointments. Criterion 3 was to ensure the validity of PCS symptomatology and minimize recall bias. In this study, the exclusion criteria for the CH patients were as follows: (1) patients who were unable to complete the questionnaire survey. (2) patients who could not distinguish CH features from other primary or secondary headache disorders according to the ICHD-3 criteria. In fact, patients with other primary headache disorders, such as migraine, could be included in the study if they were able to distinguish CH attacks, CH bouts, and symptoms that preceded CH bouts from other headache disorders. (3) Patients with chronic CH without remission were excluded from this study, as it would be impossible to identify PCSs. On the other hand, the diagnosis of chronic CH in ICHD-3 also includes patients with remissions lasting < 3 months for at least 1 year. Hence, chronic CH patients who had a remission ≥ 1 month, and who could clearly distinguish between remission and bout periods, were allowed to enter this study.
Sub-study 1: questionnaire
In this study, all patients completed questionnaire surveys and received a structured interview during CH bouts by neurologists specializing in CH (JWW and SJW). The questionnaire was designed by a panel of headache specialists (JWW, SJW, CCC) and a psychologist (SYT), and items on the survey included questions on CH symptomatology, CH severity, and PCSs. The Cluster Headache Severity Scale (CHSS) was used to assess CH severity, including three parameters: CH attack duration, number of CH attacks per day, and the bout duration [18]. The scale ranges from 3 to 12, and CHSS score ≥ 9 indicates severe CH [18]. In the questionnaire, we also recorded the presence of circadian rhythmicity of CH attacks (the CH attacks always at the same specific circadian times by 1-hour blocks) and seasonal rhythmicity of CH bouts (the CH bouts always occur at the same season(s) of the year) [19, 20].
To clarify the presence of PCS, we first asked patients, ‘Have you experienced symptoms before the onset of a CH bout?’ If the answer was ‘yes,’ we further asked, ‘Did you experience the symptoms just before the CH bouts, not before each CH attack’ To distinguish PCS from pre-attack symptoms, only patients who answered ‘yes’ to both questions were recorded as having PCS [9, 10]. For PCSs, we incorporated 34 symptoms and divided them into seven categories that were adopted from previous studies, which is helpful for comparison [8, 11]. The seven PCS categories were as follows: 1). head and neck (localized) pain symptoms, 2). cranial autonomic symptoms, 3). a sense of restlessness, 4). fatigue and mood symptoms, 5). sleep alterations, 6). constitutional symptoms, and 7). generalized pain symptoms [21, 22].
Sub-study 2: response to preventive treatment (verapamil with short-term transitional therapy)
In this study, we recorded the response to verapamil (120–720 mg/day) because it is the first-line preventive treatment according to Taiwan’s treatment guideline for CH, and it is the most commonly used preventives in other studies [10, 16, 23, 24]. During the treatment period, no concomitant preventive medications for CH were permitted, except the transitional therapy as an add-on to verapamil [16, 25]. Transitional therapy with short-term steroids (oral prednisolone) was initiated concurrently with verapamil. According to Taiwan’s guideline for CH, the transitional therapy included prednisone at 5 mg/kg/day orally for 7 days, administered in the morning along with famotidine to prevent gastric side effects [16]. This was followed by a tapering dose, reducing by 10 mg every 2 days for an additional 7 days [16, 24].
Also, we asked the patient to keep a headache diary throughout the verapamil treatment period during CH bouts, and we recorded the weekly frequency of CH attacks without preventive treatment as the baseline [26]. During the follow-up visits, a neurologist (J. W. Wu) recorded the efficacy of verapamil as a preventive treatment by cross-referencing both clinical records and headache diaries. A responder to verapamil was defined as a patient who has a reduction from baseline of at least 50% in the weekly frequency of CH attacks 4 weeks after the initiation of verapamil [26]. The timeline for recording treatment response is illustrated in Fig. 1.
Statistical analysis
SPSS version 22.0 (SPSS Inc., Chicago, Illinois, USA) was used for the data analyses. The demographic and clinical profile data of the CH patients were presented as the means and standard deviations (SDs), and the non-normally distributed continuous variables are expressed as medians and interquartile ranges (IQRs). The associations between the demographic and clinical profile data and the ability to predict upcoming bouts were analyzed by using t tests and chi-square tests, as appropriate, and p < 0.05 was considered statistically significant. Also, Mann‒Whitney U test was used to compare nonnormally distributed continuous variables, and p < 0.05 was considered statistically significant.
In this study, the associations between PCS categories and the predictability of upcoming bouts were analyzed by the chi-square test, Bonferroni’s corrections for multiple comparisons were applied, and p < 0.0071 was considered significant (corrected for seven pairwise comparisons: p = 0.05/7 tests = 0.0071). Regarding the response to verapamil treatment, the associations between PCS categories and the response to verapamil treatment were analyzed by the chi-square test, Bonferroni correction for multiple comparisons was applied, and p < 0.0071 was considered significant (corrected for seven pairwise comparisons: p = 0.05/7 tests = 0.0071). In the exploratory analyses of this study, we further investigated the associations between each PCS and the predictability of upcoming bouts, and p < 0.05 was considered statistically significant.
Standard protocol approvals, registrations, and patient consent
The protocol of this study was approved by the Institutional Review Board of the Taipei Veterans General Hospital (2021-04-008CC). All the participants provided written consent before the study.
Results
Patients
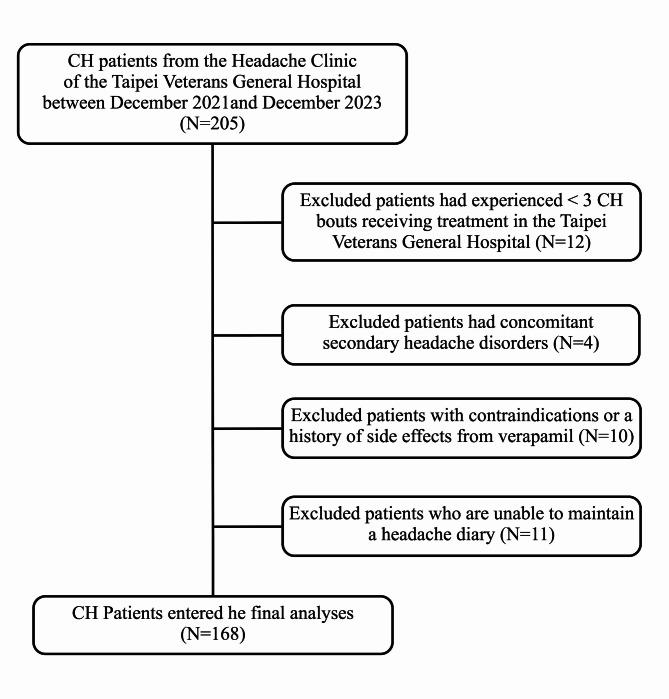
During the study period, a total of 205 CH patients were invited to participate this study. After exclusion, 168 consecutive CH patients (women/men: 39/129) completed the study (Fig. 2) (Table 1). The mean (SD) age of the CH patients was 38.5 (11.0) years with a male predominance (76.8%). Most of the CH patients had episodic CH (n = 164, 97.6%), and only four patients had chronic CH (2.4%). Thirty-four CH patients (20.2%) had comorbid migraine. The median (IQR) disease duration was 10 (5–17) years. Patients experienced a median (IQR) of 8.5 (5–14) CH bouts, and 131 (78.0%) patients had ≥ 5 CH bouts. Additionally, the median (IQR) duration of CH bouts was 1.5 (1.0–2.0) months. The mean (SD) Cluster Headache Severity Scale (CHSS) was 6.0 (1.3). In our CH population, 62.5% of patients had a circadian rhythmicity of CH attacks, and 75.6% of patients had a seasonal rhythmicity of their CH bouts.
Fig. 2.
Study schematic flow chart
Table 1.
The demographics and clinical profile data of all CH patients
| Total N = 168 |
Ability to predict the upcoming CH bouts | p value* | ||
|---|---|---|---|---|
| Yes N = 86 (51.2%) |
No N = 82 (48.8%) |
|||
| Female sex, N (%) | 39 (23.2%) | 19 (22.1%) | 20 (24.4%) | 0.724 # |
| Age, mean (SD), years | 38.5 (11.0) | 37.9 (10.1) | 39.1 (11.9) | 0.492§ |
| Disease duration, median (IQR), years | 10 (5.0–17.0) | 12 (6.8–18) | 10 (4.0-15.3) | 0.073### |
| Total experienced CH bouts, median (IQR) | 8.5 (5.0–14.0) | 8.5 (6.0–14.0) | 8.5 (3.0–14.0) | 0.287### |
| Duration of bout, median (IQR), months | 1.5 (1.0–2.0) | 1.8 (1.0-2.1) | 1.5 (1.0–2.0) | 0.078### |
| Attacks per day in bout, mean (SD) | 2.1 (1.5) | 2.0 (1.2) | 2.1 (1.7) | 0.504§ |
| Attack duration without treatment, mean (SD), mins | 118.0 (160.5) | 112.2 (157.5) | 124.2 (164.4) | 0.632§ |
| CHSS score, mean (SD) | 5.96 (1.32) | 5.92 (1.27) | 6.00 (1.37) | 0.690§ |
| Circadian rhythmicity of CH attacks, % | 105 (62.5%) | 62 (72.1%) | 43 (53.4%) | 0.009# * |
| Seasonal rhythmicity of CH bouts, % | 127 (75.6%) | 64 (74.4%) | 63 (76.8%) | 0.716# |
| Comorbid migraine, n (%) | 34 (20.2%) | 21 (24.4%) | 13 (15.9%) | 0.167# |
| Out-of-bout shadow attacks, n (%) | 58 (34.5%) | 36 (41.9%) | 22 (26.8%) | 0.041 #* |
CHSS: Cluster Headache Severity Scale
* p < 0.05 was considered to indicate statistical significance
# The differences in these categorical variables between patients who were able and unable to predict upcoming bouts were analyzed by the chi-square test
## The differences in these categorical variables between patients who were able and unable to predict upcoming bouts were
analyzed by Fisher’s exact test
### The Mann‒Whitney U test was used to compare nonnormally distributed continuous variables, and p < 0.05 was
considered to indicate statistical significance
§ The differences in these continuous variables between patients who were able and unable to predict upcoming bouts were
analyzed by t tests
Sub-study 1
Prevalence, temporal profile, and predictability of upcoming bouts by PCSs
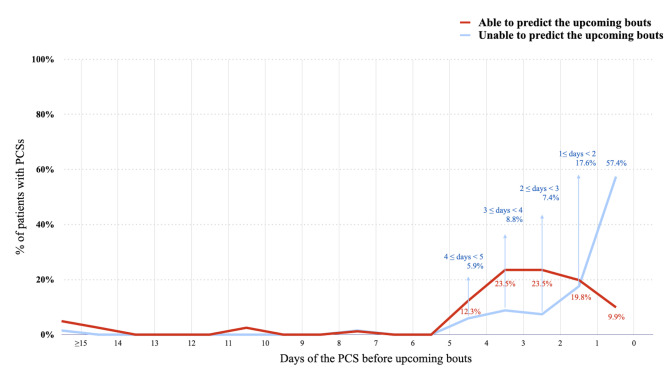
Among all CH patients, 149 (88.7%) experienced PCS before the upcoming bouts. Among patients with PCS (n = 149), 86 (57.7%) were able to predict upcoming bouts. The median duration of PCSs was 24 (IQR 18 to 72) hours (range 12 h-20 days) before CH bouts, and 50 (33.6%) patients had duration of PCS ≥ 3 days. Among chronic CH patients (n = 4), the duration of remission ranged from 1.5 to 2.5 months, and only 1 had PCSs with a duration of 2 days before the CH bouts. Also, patients who could predict CH bouts tended to have a longer duration of PCSs (able vs. unable to predict: 48 [24–72] vs. 18 [12-42] hours, p < 0.001; Mann‒Whitney U test). The temporal distribution of the duration of PCSs is shown in Fig. 3. Moreover, patients who were able to predict the upcoming bout were more likely to have a circadian rhythmicity of CH attacks (able vs. unable to predict: 72.1% vs. 53.4%, p = 0.009). Sex, age, presence of comorbid migraine, disease duration, number of experienced bouts, seasonal rhythmicity, or CHSS score were not associated with the predictability of upcoming CH bouts (Table 1).
Fig. 3.
The duration of PCSs and the predictability of CH bouts
Symptomatology of PCSs and the predictability of upcoming bouts
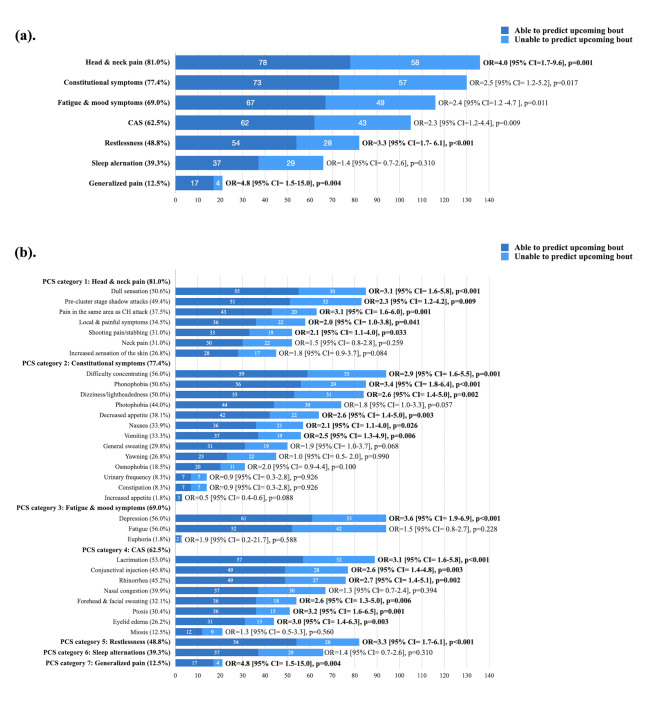
Among the seven PCS categories, head and neck pain symptoms were most common (81.0%), followed by constitutional symptoms (77.4%), fatigue or mood changes (69.0%), cranial autonomic symptoms (62.5%), sense of restlessness (48.8%), sleep alterations (39.3%), and generalized pain symptoms (12.5%) (Fig. 4A). Head and neck pain symptoms (odds ratio [OR] = 4.0 [95% CI = 1.7–9.6], p = 0.001), a sense of restlessness (OR = 3.3 [95% CI = 1.7–6.1], p < 0.001), and generalized pain (OR = 4.8 [95% CI = 1.5–15.0], p = 0.004) were associated with the predictability of upcoming bouts (Fig. 4A).
Fig. 4.
The predictability of upcoming bouts of CH by PCSs: (a). by seven categories of PCSs and (b). by each PCS
The associations between 34 individual PCSs and predictability were also explored. Among the category of head and neck pain symptoms, dull sensation (OR = 3.1 [95% CI = 1.6–5.8], p < 0.001) and pain in the same area as subsequent attacks (OR = 3.1 [95% CI = 1.6-6.0], p = 0.001) were more likely to predict upcoming bouts (Fig. 4B). Six out of eight cranial autonomic symptoms, namely, conjunctival injection (OR = 2.6 [95% CI = 1.4–4.8], p = 0.003), lacrimation (OR = 3.1 [95% CI = 1.6–5.8], p < 0.001), rhinorrhea (OR = 2.7 [95% CI = 1.4–5.1], p = 0.002), forehead and facial sweating (OR = 2.6 [95% CI = 1.3–5.5], p = 0.006), ptosis (OR = 3.2 [95% CI = 1.6–6.5], p = 0.001) and eyelid edema (OR = 3.0 [95% CI = 1.4–6.3], p = 0.003), were more likely to predict upcoming bouts (Fig. 4B). The associations between each PCS and the predictability of upcoming bouts are shown in Table 2; Fig. 4B.
Table 2.
The prevalence of each PCS and its ability to predict upcoming CH bouts
| Symptoms | Total (n = 168) |
Ability to predict the upcoming CH bouts | p value# | |
|---|---|---|---|---|
| Yes N = 86 (51.2%) |
No N = 82 (48.8%) |
|||
| Head and neck pain symptoms | ||||
| Localized and painful symptoms, n (%) | 58 (34.5%) | 36 (41.9%) | 22 (26.8%) | 0.041* |
| Pain in the same area as the subsequent attack, n (%) | 63 (37.5%) | 43 (50.0%) | 20 (24.4%) | 0.001* |
| Pre-cluster stage shadow attacks, n (%) | 83 (49.4%) | 51 (59.3%) | 32 (39.0%) | 0.009* |
| Shooting pain/stabbing, n (%) | 52 (31.0%) | 33 (38.4%) | 19 (23.2%) | 0.033* |
| Dull sensation, n (%) | 85 (50.6%) | 55 (64.0%) | 30 (36.6%) | < 0.001* |
| Neck pain, n (%) | 52 (31.0%) | 30 (34.9%) | 22 (26.8%) | 0.259 |
| Increased sensation of the skin, n (%) | 45 (26.8%) | 28 (32.6%) | 17 (20.7%) | 0.084 |
| Cranial autonomic symptoms | ||||
| Conjunctival injection, n (%) | 77 (45.8%) | 49 (57.0%) | 28 (34.1%) | 0.003* |
| Lacrimation, n (%) | 89 (53.0%) | 57 (66.3%) | 32 (39.0%) | < 0.001* |
| Rhinorrhea, n (%) | 76 (45.2%) | 49 (57.0%) | 27 (32.9%) | 0.002* |
| Nasal congestion, n (%) | 67 (39.9%) | 37 (43.0%) | 30 (36.6%) | 0.394 |
| Forehead and facial sweating, n (%) | 54 (32.1%) | 36 (41.9%) | 18 (22.0%) | 0.006* |
| Ptosis, n (%) | 51 (30.4%) | 36 (41.9%) | 15 (18.3%) | 0.001* |
| Miosis, n (%) | 21 (12.5%) | 12 (14.0%) | 9 (11.0%) | 0.560 |
| Eyelid edema, n (%) | 44 (26.2%) | 31 (36.0%) | 13 (15.9%) | 0.003* |
| Restlessness, n (%) | 82 (48.8%) | 54 (62.8%) | 28 (34.1%) | < 0.001* |
| Fatigue and mood symptoms | ||||
| Fatigue, n (%) | 94 (56.0%) | 52 (60.5%) | 42 (51.2%) | 0.228 |
| Euphoria, n (%) | 3 (1.8%) | 2 (2.3%) | 1 (1.2%) | 0.588 |
| Depression, n (%) | 94 (56.0%) | 61 (70.9%) | 33 (40.2%) | < 0.001* |
| Sleep alteration | ||||
| Sleep issues, n (%) | 66 (39.3%) | 37 (43.0%) | 29 (35.4%) | 0.310 |
| Constitutional symptoms | ||||
| Yawning, n (%) | 45 (26.8%) | 23 (26.7%) | 22 (26.8%) | 0.990 |
| Difficulty concentrating, n (%) | 94 (56.0%) | 59 (68.6%) | 35 (42.7%) | 0.001* |
| Increased appetite, n (%) | 3 (1.8%) | 3 (3.5%) | 0 (0.0%) | 0.088 |
| Decreased appetite, n (%) | 64 (38.1%) | 42 (48.8%) | 22 (26.8%) | 0.003* |
| Urinary frequency, n (%) | 14 (8.3%) | 7 (8.1%) | 7 (8.5%) | 0.926 |
| Constipation, n (%) | 14 (8.3%) | 7 (8.1%) | 7 (8.5%) | 0.926 |
| Nausea, n (%) | 57 (33.9%) | 36 (41.9%) | 21 (25.6%) | 0.026* |
| Vomiting, n (%) | 56 (33.3%) | 37 (43.0%) | 19 (23.2%) | 0.006* |
| Photophobia, n (%) | 74 (44.0%) | 44 (51.2%) | 30 (36.6%) | 0.057 |
| Phonophobia, n (%) | 85 (50.6%) | 56 (65.1%) | 29 (35.4%) | < 0.001* |
| Osmophobia, n (%) | 31 (18.5%) | 20 (23.3%) | 11 (13.4%) | 0.100 |
| Dizziness or lightheadedness, n (%) | 84 (50.0%) | 53 (61.6%) | 31 (37.8%) | 0.002* |
| General sweating, n (%) | 50 (29.8%) | 31 (36.0%) | 19 (23.2%) | 0.068 |
| Generalized pain symptoms, n (%) | 21 (12.5%) | 17 (19.8%) | 4 (4.9%) | 0.004* |
* The analyses of the associations between each PCS symptom and the predictability of upcoming bouts were exploratory, and p < 0.05 was considered to indicate statistical significance
# The differences in these categorical variables between patients who were able to predict upcoming bouts and those who were unable to predict upcoming bouts were analyzed by the chi-square test
Sub-study 2: PCSs and response to verapamil (with short-term transitional therapy)
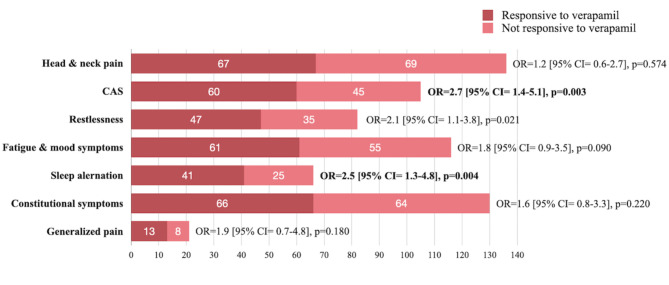
In this study, 81 (48.2%) patients were responders to verapamil (patients with a reduction from baseline of ≥ 50% in the weekly frequency of CH attacks 4 weeks after the initiation of verapamil). Among all CH patients, 149 (88.7%) patients received transitional therapy with short-term oral prednisolone. Reasons for not using transitional therapy (oral prednisolone) included contraindications due to underlying diseases (n = 4) and a history of side effects (n = 15). However, there were no significant differences in the use of transitional therapy between verapamil responders and non-responders (88.9% vs. 88.5%, p = 0.938). In all participants (n = 168), CH patients with PCS categories of sleep alteration (OR = 2.5 [95% CI = 1.3–4.8], p = 0.004) and ≥ 1 cranial autonomic symptom (OR = 2.7 [95% CI = 1.4–5.1], p = 0.003) were associated with response to verapamil (Fig. 5). After excluded patients not received transitional therapy, CH patients (n = 149) with PCS categories of sleep alteration (OR = 2.6 [95% CI = 1.3–5.1], p = 0.005) and ≥ 1 cranial autonomic symptom (OR = 2.8 [95% CI = 1.4–5.5], p = 0.004) were still associated with response to verapamil.
Fig. 5.
The associations between PCSs and the response to verapamil treatment
Discussion
In this study, we found that 88.7% (149/168) of CH patients reported having PCS. Among them, 57.7% (86/149) of patients could predict upcoming bouts. Head and neck pain was the most common category of PCS and was associated with the highest predictability of CH bouts. The median duration of PCSs was one day before the cluster bout, and about one-third of patients had a duration of PCSs ≥ 3 days. Moreover, we found that the presence of sleep alterations or ≥ 1 cranial autonomic symptom during the pre-cluster stage was associated with positive treatment response to verapamil. As demonstrated by the PRODROME study, recognizing the prodrome symptoms of migraine has led to a significant shift in treatment strategies, moving the timing of acute intervention from the onset of the headache to the prodrome phase [14]. Our study highlights the importance of identifying PCS, as they are predictive of impending cluster bouts and are associated with treatment response to verapamil.
The present study had two strengths. First, we studied PCSs in a large and consecutive cohort of CH patients in Taiwan, and this would be helpful to compare the clinical characteristics of CH between different populations [27]. Second, our inclusion criteria were stricter to minimize recall bias: patients (1) had experienced ≥ 3 CH bouts and (2) had a short time interval (< 1 month) between the onset of CH bouts and the completion of the questionnaire survey. Our study revealed that PCSs are common in CH patients in Taiwan, as also shown in a recently published Danish study (88.7% and 86.0%, respectively), but this percentage is much higher than that reported in one earlier study in the United Kingdom (9.8%) [8, 13]. On the other hand, the prevalence of PCSs was 35.5% in one recent Korean study [11], but the definition of PCSs in that study required a prerequisite that patients were able to predict the CH bouts based on the symptom(s), which is different from the Danish and the present studies [8, 11]. The reason for separating the concepts of ‘presence of PCSs’ and ‘ability to predict the upcoming bout’ in the present study is that some patients might have various symptoms before the CH bouts but could not accurately predict the upcoming bout. Another Chinese study (Li et al.) with a large sample size (n = 327) reported a lower prevalence of PCSs (20.8%) [12]. This discrepancy in the prevalence of PCSs may also be attributed to differences in methodology. In Li et al., PCSs were analyzed using open-ended questions, with symptoms described by the patients and recorded by the doctors [12]. In contrast, the present study utilized questionnaire surveys with closed-ended questions to assess symptoms. Despite the different methodologies and definitions for analyzing PCSs, almost all studies have shown a high prevalence of head and neck pain-related symptoms [8, 11, 12]. However, there were also differences in symptomatology and predictability between studies on PCSs. For example, dull sensation and shadow attacks were common (62% and 60%, respectively) in the Danish study but were slightly less common in the present study (50.6% and 49.4%, respectively) [8]. Conversely, the present study revealed more constitutional symptoms, fatigue or mood changes, and cranial autonomic symptoms than did the Danish study [8]. Contrary to the present study and the Danish study, the Korean study showed a lower predictability of cluster bouts (35.3%) [8, 11]. The differences in the predictability among studies might be related to the disease duration and the number of experienced CH bouts [11]. For example, both the Danish and Korean studies found that patients who experienced more CH bouts were more capable of predicting upcoming bouts [8, 11]. In the present study, the reason for the greater predictability than in the Korean study might be that our study was based on a group of patients who experienced more CH bouts (≥ 5 CH bouts: present study 78.0% vs. Koran study 60.9%) and thus were more able to predict upcoming bouts [8]. Other factors contributing to these differences include ethnicity and climate. For instance, the climates of Denmark and Korea are temperate, which is different from the subtropical low-altitude weather of Taiwan [27]. These factors might have substantial impacts on the clinical features of CH, a pain disorder with strong chronobiological features [27].
Our study on the clinical profiles of PCSs sheds light on their pathophysiology and the potential for early intervention before CH bouts. Approximately one-third of patients had at least a three-day duration of PCSs, which provides a suitable time window for future preemptive treatment strategies. Additionally, as in previous studies, we found that head and neck pain symptoms (i.e., dullness and pain in the same area as the subsequent attack) were the most common symptoms and the best predictor of cluster bouts [8, 11]. These localized pain symptoms are less intense than CH attacks; thus, patients could easily differentiate these pain-related PCSs from CH attacks [8]. These localized sensitization or pain symptoms might be explained by the ‘threshold hypothesis’ [28, 29]. According to this hypothesis, patients with CH have fluctuating rhythmicity with active and inactive periods [28]. The onset of CH bouts is determined by disease activity exceeding the threshold, which is modulated by several environmental as well as physical or hormonal factors [28]. Therefore, the pathophysiology underlying these localized pain symptoms preceding the upcoming bouts is the transitional state from below to above this threshold [28]. In addition, our study revealed that constitutional symptoms, mood changes, and fatigue, which are commonly reported during the premonitory phase of migraine, are also common in patients with PCSs [22, 30, 31]. Thus, the hypothalamus, limbic system, and trigeminocervical complex might be the neurological substrates for PCSs [22, 30]. This hypothesis is in line with several neuroimaging studies with findings suggesting the importance of altered hypothalamic function in the development of CH bouts [32, 33].
Moreover, our study revealed novel findings regarding the association between the symptomatology of PCSs and treatment response. We found that sleep alterations in patients with PCSs or the presence of ≥ 1 cranial autonomic symptom were associated with greater response rates to verapamil. The mechanisms underlying this finding are unknown. We propose the following hypotheses. First, the association between the presence of cranial autonomic symptoms before the upcoming bouts and response to verapamil may be linked to the inhibitory effect of calcium channel blockers on calcitonin gene-related peptide (CGRP) release from trigeminal presynaptic terminals [34]. However, this peripheral mechanism cannot explain the association of response to verapamil to the central symptoms of PCSs [22]. Second, the association between sleep alterations before CH bouts and response to verapamil might be due to the chronobiological modulatory property of verapamil, which has been demonstrated in an animal study [35]. Verapamil is an L-type calcium channel blocker that has additional effects on T- and P-type calcium channels [36, 37]. The L-type calcium channel Cav1.2 is associated with the modulation of the circadian rhythm at night [34, 38]. In CH, one study revealed that patients taking verapamil could delay nocturnal attacks, suggesting a role of verapamil in modulating the temporal pattern of CH attacks [39]. Patients with sleep alterations during the pre-cluster stage might have more profound chronobiological dysregulation [30], and this subgroup of CH patients might be more responsive to verapamil.
Our study has some limitations. First, the predictability of upcoming bouts was based on patients’ self-reports rather than prospective observation. To ensure the reliability of patients-reported predictability, only patients who experienced ≥ 3 CH bouts that received treatment in our institution were eligible to participate in this study. This allowed us to cross-reference past medical records to verify the patients’ self-reported ability to forecast impending bouts. To minimize recall bias, we conducted our survey during the CH bout, and the time interval between the onset of CH bouts and the survey was less than one month. Second, responder status was determined by clinical history records with reference to headache diary data. Unlike a typical clinical trial, we did not include a placebo arm in our study; therefore, placebo effects may have confounded treatment response data [40, 41]. Third, the present study did not exclude the usage of transitional therapy along with verapamil. However, there were no significant differences in the percentage of patients using transitional therapy between verapamil responders and non-responders (88.9% vs. 88.5%, p = 0.938), indicating that the use of transitional therapy is not likely to affect the categorization of CH patients as verapamil responders or non-responders. Additionally, observational studies analyzing the efficacy of CH preventive treatments typically allow the concomitant use of transitional and preventive therapies [42–44]. Considering the unbearable CH attacks, our research protocol followed Taiwan’s treatment guideline, providing prevention verapamil with add-on transitional therapy with a standardized dosing schedule for patients [16]. Fourth, we did not analyze the association between PCSs and preventive treatments other than verapamil in this study. The reason for not exploring other preventive options is that patients in our institution usually begin other medications (e.g., topiramate, lithium, or galcanezumab) after failing or being intolerant to verapamil [16]. Hence, based on our study population and the response rate data, patients receiving preventive treatments other than verapamil might represent a subgroup of patients more refractory to treatment. Therefore, further studies are needed to analyze the associations between PCSs and other preventive treatment options. Lastly, we included only Taiwanese CH patients in our study, which may limit the generalizability of our results to patients from other ethnic backgrounds.
Conclusions
PCSs are common and might be used to predict impending CH bouts. Our findings on the use of certain PCSs for treatment response prediction suggest the existence of distinct CH phenotypes, which may correspond to different underlying pathophysiologies.
Acknowledgements
We thank the patients who participated in this cohort, and we wish to thank the participants of the 2023 Annual Meeting of the American Academy of Neurology and the 2023 International Headache Congress for inspiring discussions during the meeting.
Abbreviations
- PCSs
Pre-cluster symptoms
- CH
Cluster headache
- CHSS
Cluster Headache Severity Scale
- ICHD-3
International Classification of Headache Disorders, 3rd edition
- CGRP
Calcitonin gene-related peptide
Author contributions
SJW organized the Cluster Headache Research Group. JWW, STC, YSK, CCC, and SJW conceived and designed the study, sought and achieved study funding, and developed clinical data collection workflow. JWW, SYT, CCC, and SJW designed the questionnaires of this study. JWW, YFW, SPC, WTC, YSK, and SJW helped to recruit patients. JWW and STC contributed to the interpretation, statistics, and analysis of data. JWW, STC, and SJW contributed to drafting the text or preparing the figures. All authors reviewed the manuscript.
Funding
This analysis was supported by grants from the National Science and Technology Council of Taiwan (NSTC 111-2314-B-075-064-MY2 and NSTC 110-2314-B-075-081 [to JWW]; NSTC 113-2314-B-075-004, 110-2314-B-075-035-MY2 and NSTC 109-2314-B-075-002 [to STC]; and 111-2321-B-A49-004, 111-2314-B-075-086-MY3, 111-2321-B-A49-011, and 112-2321-B-075-007 [to SJW]]; and Taipei Veterans General Hospital (V112C-129 and V113C-097 [to JWW]). This work was also supported by the Brain Research Center, National Yang Ming Chiao Tung University from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval
The protocol of this study was approved by the Institutional Review Board of the Taipei Veterans General Hospital (2021-04-008CC). All the participants provided written consent before the study.
Consent for publication
All authors authorize the publication.
Competing interests
JWW served as the Chair of Communication Committee of International Headache Society and has received travel reimbursement and honoraria from the American Academy of Neurology, International Headache Society, and Taiwan Headache Society. He has received honoraria (as a speaker) from Biogen-Idec, Eli Lilly, and Hoan Pharmaceuticals. Additionally, JWW and STC received research grants from the Taiwan National Science and Technology Council and Taipei Veterans General Hospital. CCC has served as a consultant for Satsuma and eNeura. She receives a research grant from the American Heart Association with funds paid to her institution. Shuu-Jiun Wang has received honoraria as a moderator from AbbVie, Biogen, Eli-Lilly, Hava Biopharma, and Pfizer; has received consulting fees from AbbVie, Eli-Lilly Taiwan, Percept Co., and Pfizer Taiwan; and has been the principal investigator in trials sponsored by Eli-Lilly, Lundbeck, and Novartis. He has received research grants from the Taiwan branches of Eli Lilly, Novartis, and Orient Europharma.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jr-Wei Wu and Shu-Ting Chen contributed equally to this work.
References
- 1.Petersen AS et al (2022) The economic and personal burden of cluster headache: a controlled cross-sectional study. J Headache Pain 23(1):58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diana YW, Modar K, Peter JG (2019) Managing cluster headache. Pract Neurol 19(6):521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.May A et al (2018) Cluster headache. Nat Rev Dis Primers 4:18006 [DOI] [PubMed] [Google Scholar]
- 4.de Coo IF et al (2019) Chronobiology and Sleep in Cluster Headache. Headache 59(7):1032–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ji Lee M et al (2019) Increased suicidality in patients with cluster headache. Cephalalgia 39(10):1249–1256 [DOI] [PubMed] [Google Scholar]
- 6.Liang JF et al (2013) Cluster headache is associated with an increased risk of depression: a nationwide population-based cohort study. Cephalalgia 33(3):182–189 [DOI] [PubMed] [Google Scholar]
- 7.Hoffmann J, May A (2018) Diagnosis, pathophysiology, and management of cluster headache. Lancet Neurol 17(1):75–83 [DOI] [PubMed] [Google Scholar]
- 8.Pedersen AS et al (2021) Prevalence of pre-cluster symptoms in episodic cluster headache: is it possible to predict an upcoming bout? Cephalalgia 41(7):799–809 [DOI] [PubMed] [Google Scholar]
- 9.Snoer A et al (2018) Pre-attack signs and symptoms in cluster headache: characteristics and time profile. Cephalalgia 38(6):1128–1137 [DOI] [PubMed] [Google Scholar]
- 10.Snoer A et al (2018) Cluster headache beyond the pain phase: a prospective study of 500 attacks. Neurology 91(9):e822–e831 [DOI] [PubMed] [Google Scholar]
- 11.Cho S et al (2022) Characteristics of pre-cluster symptoms in cluster headache: a cross-sectional multicentre study. Cephalalgia 42(7):570–578 [DOI] [PubMed] [Google Scholar]
- 12.Li K et al (2022) Pre-attack and pre-episode symptoms in cluster headache: a multicenter cross-sectional study of 327 Chinese patients. J Headache Pain 23(1):92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blau JN, Engel HO (1998) Premonitory and prodromal symptoms in cluster headache. Cephalalgia, 18(2): p. 91 – 3; discussion 71 – 2. [DOI] [PubMed]
- 14.Dodick DW et al (2023) Ubrogepant for the treatment of migraine attacks during the prodrome: a phase 3, multicentre, randomised, double-blind, placebo-controlled, crossover trial in the USA. Lancet 402(10419):2307–2316 [DOI] [PubMed] [Google Scholar]
- 15.Tso AR et al (2021) Machine phenotyping of cluster headache and its response to verapamil. Brain 144(2):655–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang F-C, Tsai C-L, Lin G-Y; Treatment Guidelines Subcommittee of the Taiwan Headache Society (2022) 2022 Taiwan Guidelines for Acute and Preventive Treatment of Cluster Headaches. Acta Neurol Taiwan 31(4):254–273 [PubMed] [Google Scholar]
- 17.Headache Classification Committee of the International Headache Society (2018) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 38(1):1–211 [DOI] [PubMed]
- 18.Steinberg A et al (2017) Cluster headache – clinical pattern and a new severity scale in a Swedish cohort. Cephalalgia 38(7):1286–1295 [DOI] [PubMed] [Google Scholar]
- 19.Burish MJ, Chen Z, Yoo SH (2018) Cluster headache is in Part a disorder of the Circadian System. JAMA Neurol 75(7):783–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benkli B et al (2023) Circadian features of Cluster Headache and Migraine: a systematic review, Meta-analysis, and genetic analysis. Neurology 100(22):e2224–e2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maniyar FH et al (2014) Brain activations in the premonitory phase of nitroglycerin-triggered migraine attacks. Brain 137(Pt 1):232–241 [DOI] [PubMed] [Google Scholar]
- 22.Karsan N, Goadsby PJ (2018) Biological insights from the premonitory symptoms of migraine. Nat Rev Neurol 14(12):699–710 [DOI] [PubMed] [Google Scholar]
- 23.Søborg M-LK et al (2023) Transition of cluster headache phenotype: an interview-based study. Cephalalgia 43(1):03331024221128287 [DOI] [PubMed] [Google Scholar]
- 24.Peng KP, Burish MJ (2023) Management of cluster headache: treatments and their mechanisms. Cephalalgia 43(8):3331024231196808 [DOI] [PubMed] [Google Scholar]
- 25.Obermann M et al (2021) Safety and efficacy of prednisone versus placebo in short-term prevention of episodic cluster headache: a multicentre, double-blind, randomised controlled trial. Lancet Neurol 20(1):29–37 [DOI] [PubMed] [Google Scholar]
- 26.Schoenen J et al (2022) Guidelines of the International Headache Society for Controlled Clinical Trials in Cluster Headache. Cephalalgia 42(14):1450–1466 [DOI] [PubMed] [Google Scholar]
- 27.Peng KP, Takizawa T, Lee MJ (2020) Cluster headache in Asian populations: similarities, disparities, and a narrative review of the mechanisms of the chronic subtype. Cephalalgia 40(10):1104–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naber WC et al (2019) The biological clock in cluster headache: a review and hypothesis. Cephalalgia 39(14):1855–1866 [DOI] [PubMed] [Google Scholar]
- 29.Gil-Martínez A et al (2019) Hyperalgesia and Central Sensitization Signs in patients with cluster headache: a cross-sectional study. Pain Med 20(12):2562–2570 [DOI] [PubMed] [Google Scholar]
- 30.Al-Karagholi MA-M et al (2022) Debate: are cluster headache and migraine distinct headache disorders? J Headache Pain 23(1):151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eigenbrodt AK et al (2022) Premonitory symptoms in migraine: a systematic review and meta-analysis of observational studies reporting prevalence or relative frequency. J Headache Pain 23(1):140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.May A, Burstein R (2019) Hypothalamic regulation of headache and migraine. Cephalalgia 39(13):1710–1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peter J Goadsby, Arne May (1999) PET demonstration of hypothalamic activation in cluster headache. Neurology. 52(7): p. 1522 [DOI] [PubMed]
- 34.Petersen AS et al (2019) Verapamil and Cluster Headache: Still a Mystery. A Narrative Review of Efficacy, Mechanisms and Perspectives. Headache: The Journal of Head and Face Pain, 59(8): pp. 1198–1211 [DOI] [PubMed]
- 35.Burish MJ et al (2021) The first-line cluster headache medication verapamil alters the circadian period and elicits sex-specific sleep changes in mice. Chronobiol Int 38(6):839–850 [DOI] [PubMed] [Google Scholar]
- 36.Tfelt-Hansen P, Tfelt-Hansen J (2009) Verapamil for cluster headache. Clinical pharmacology and possible mode of action. Headache 49(1):117–125 [DOI] [PubMed] [Google Scholar]
- 37.Bergson P et al (2011) Verapamil block of T-type calcium channels. Mol Pharmacol 79(3):411–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cipriani A et al (2016) A systematic review of calcium channel antagonists in bipolar disorder and some considerations for their future development. Mol Psychiatry 21(10):1324–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barloese M et al (2018) Chronorisk in Cluster headache: a tool for individualised therapy? Cephalalgia 38(14):2058–2067 [DOI] [PubMed] [Google Scholar]
- 40.Nilsson Remahl AI et al (2003) Placebo response in cluster headache trials: a review. Cephalalgia 23(7):504–510 [DOI] [PubMed] [Google Scholar]
- 41.Basedau H et al (2023) Placebo and nocebo in the treatment of migraine: how much does real world effectiveness depend on contextual effects? Cephalalgia 43(12):03331024231218392 [DOI] [PubMed] [Google Scholar]
- 42.Lamas Pérez R, Millán-Vázquez M, González-Oria C (2024) Efficacy and safety of galcanezumab as chronic cluster headache preventive treatment under real world conditions: observational prospective study. Cephalalgia 44(3):03331024231226181 [DOI] [PubMed] [Google Scholar]
- 43.Membrilla JA et al (2022) Efficacy and safety of galcanezumab as a treatment of refractory episodic and chronic cluster headache: Case series and narrative review. Headache: J Head Face Pain 62(10):1395–1405 [DOI] [PubMed] [Google Scholar]
- 44.Hong Y et al (2023) Preventive therapy with galcanezumab for two consecutive cluster bouts in patients with episodic cluster headache: an observational multicenter study. J Headache Pain 24(1):136 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.