Summary
Objective
Digital pathology is an opportunity to revise the routine and old artisanal workflow, moving to standard operating procedures, quality control and reproducibility. Here the results of a survey promoted by the Coordinamento della Medicina di Laboratorio (CRC Med Lab) of the Lombardy region in Italy are reported to shed light on the current situation of digital adoption in the country.
Methods
The survey composed of 58 questions was sent to 60 pathology laboratories. The results were collected and most significant answers were reported and discussed.
Results
Answers were received from 57 (95%) laboratories, a minority organized in spoke-hub networks (16%) with a centralized processing phase (11%). Hybrid manual/computer-assisted traceability was prevalent (36%), with QR/barcode labeling starting within the pathology lab (23%). Different laboratory information systems (LIS) were employed, mostly with alert functions and/or multimedial file attachments (56% and 46%, respectively). The majority opted for a semi-automated tracking management (44, 77%) and 18 centers (32%) were partly digitizing the routine (¾ scanning < 25% of slides). Whole slide images were retained for 3.7 years in average; in-house blocks/slides archiving was still preferred (30, 53%), with 1838 (±1551) and 1798 (±1950) days (5 years) internal permanence for blocks and slides that are stored in out-source (mean turnaround time for return on-demand 3.7±2.1, range 1-10 days).
Conclusions
The advantages of digital pathology must be balanced against the challenges faced in the structural revision of the pathology workflow. This regional scouting can represent the foundation to build an efficient and connected digital pathology system in the territory.
Key words: digital pathology, whole slide images, archives, pre-analytic, traceability
Introduction
The digital pathology transition is not limited to the adoption of whole slide images (WSI) for the primary diagnosis 1, but represents a great opportunity to revise the old artisanal concept of the routine workflow, moving to standard operating procedures (SOP), quality control (QC) and reproducibility. The diagnostic workflow traceability typically encompasses several key steps to ensure accuracy and efficiency. Upgrades to the lab may involve specimen collection, labeling, transport to the pathology department with appropriate storage media, accessioning and subsequent processing phases. Moreover, new multitasking laboratory information systems (LIS) for tracking purposes are required. Throughout the process, QC measures ensure reliability, efficiency, and final accurate results. At the end, archives should be fully controlled and storage parameters maintained for molecular pathology tests or second opinions and (legal) follow-up 2. Based on the recent updated guidelines for labs certification (DGR XI/7044, 26/09/2022)3, an Italian working group of the Lombardy region organized a survey focused on the evaluation of pre-analytical, analytical and post-analytical variables in the current routine practice of pathology labs. The final aim was to give an overview on criticalities of the real-world activity and propose possible solutions to ensure the quality of pathology services, eventually implementing new processes. The present work summarizes the results of the survey, focusing on the most relevant findings, discussing possible and necessary corrective actions for specific levels of criticality highlighted.
Materials and methods
SURVEY
The survey questions were designed and piloted by the Centro di Coordinamento della Medicina di Laboratorio (CRC Med Lab) working group, organized by the Lombardy region in Italy, which included senior clinical scientists from different areas (see Acknowledgments). All the labs involved were part of the “Anagrafica Regionale delle Strutture Sanitarie (ASAN)”, that ensures the guidelines for the reclassification of laboratory macro-activities pursuant to DGR n. XI/7044 dated 26/9/2022 3. The survey was composed of closed answer questions, ensuring the strict control of the answers to obtain unbiased and representative data on the adoption of digital pathology. Moreover, once the first draft of the survey was obtained, it was circulated within the members of the working group for further refinements and to have a double check of the correctness and questions and possible answers. The method used was the “consensus opinion/consensus conference” 4. The closed online survey was then sent to up to 60 laboratories. Return of a single consensus response representing each laboratory was requested in June 2023 (after 40 days and 4 reminders). The survey was composed of 58 questions divided into 3 main topics: pre-analytical, analytical, post-analytical steps. The complete list of the questions is reported in Supplementary Table I.
Table I.
Most relevant questions of the survey per single workflow phase. LIS, Laboratory Information System.
| Pre-analytical | Analytical | Post-analytical |
|---|---|---|
|
Site Indicate whether your lab is structured as a multi-site service. How is the activity organized in the case of multi-site? How are the samples handled and transferred? |
LIS Who is your commercial provider for the LIS? |
Archives Where are the physical slides and blocks stored (in-house vs out-sourcing)? |
|
Labeling Indicate when the sample is labeled with the identification code. How is the label generated? |
Tracking If present, is computerized traceability applied to the process? Is molecular biology tracked? |
Storage Are the storage conditions guaranteed and monitored in the archive? |
|
Traceability What methods are adopted to guarantee the traceability of the samples in the pre-analytical phase? |
Slides In what percentage are the slides digitized? Indicate the average monthly number of slides prepared in 2022. What part of the routine was digitized (histology, cytology, immunohistochemistry)? Are the digital images stored? |
Out-source services In the case of an out-source service, what is the average storage time of the blocks in the laboratory, before they are sent to the external archive (in days)? What is the Turnaround Time (TaT) for block/slides retrieval on demand? |
DATA EXTRACTION
Based on the results extracted from the original list, questions were aggregated for homogeneity and relevance and the most useful were grouped for detailed findings (Tab. I). Obtained results were structured and organized following the same structure, in detail:
-
pre-analytical phase
site
labels
traceability
-
analytical phase
Laboratory Information System (LIS)
tracking
slides
-
Post-analytics:
archives
storage
out-source service
STATISTICS
For normally distributed and non-normally distributed continuous variables, mean ± standard deviation (SD) or median and interquartile range (IQR) were calculated, as appropriate. Qualitative variables were reported as count and frequency. Data analysis, tables and graphics were obtained with Google Sheets (Google, Mountain View, CA, USA).
Results
PRE-ANALYTICAL PHASE
Answers were received from 57 out of 60 laboratories (95%). As per site characteristics, 16 out of 57 laboratories (28%) were multi-site facilities and in almost all (15/16, 94%) there was a computer network connection among the spokes, the majority (10, 62%) also having a centralized processing phase. Overall, in 42 (74%) and 8 (14%) labs the biological samples were transferred using either an internal or external (e.g. private carriers) logistics service, respectively. Restricting the analysis to multisite centers, internal/external services for sample transfer were adopted in 14 (87%) and 2 (13%) labs, respectively. Regarding the labeling procedures, in 46 (81%) centers the sample identifier (through QR or barcode) was attributed at its arrival in the lab and only in 23 (40%) centers a unique institutional order entry was provided before. Finally, for traceability purposes, the majority used hybrid manual/computer-assisted approaches (35, 61%), followed by manual-only (14, 25%) and fully-automated systems (8, 14%).
ANALYTICAL PHASE
A wide variety of LIS was used by participating laboratories (Fig. 1, Tab. II). In 35 (61%) centers the LIS supported alert functionality (e.g. urgent cases and dynamic TATs) and in 45 (80%) the system allowed multimedial files attachment (e.g.. jpg,. tif,. pdf). The different phases of the workflow were tracked most frequently by a partially automated system (29, 50%, Tab. III and Fig. 2 for details). WSIs were introduced only in 18 laboratories (32%); 13 centers scanned less than 5% of the routine slides with the vast majority of the labs (75%) scanning less than 25% of cases (Fig. 3). WSIs were stored for 3.7 years on average (±2.8, range 1-10 years). The average number of slides per month produced was 18,515 (±11,643, ref. 2022). In 16 centers (88%), cases scanned belonged to the histology routine, along with corresponding ancillary tests (e.g. immunohistochemistry, IHC), while cytology was subjected to digitization in only 8 (44%) centers. The aims of digitization were mainly for research or training; only occasionally did centers scan slides for routine practice or in the field of ultra specialization (i.e. renal pathology).
Figure 1.
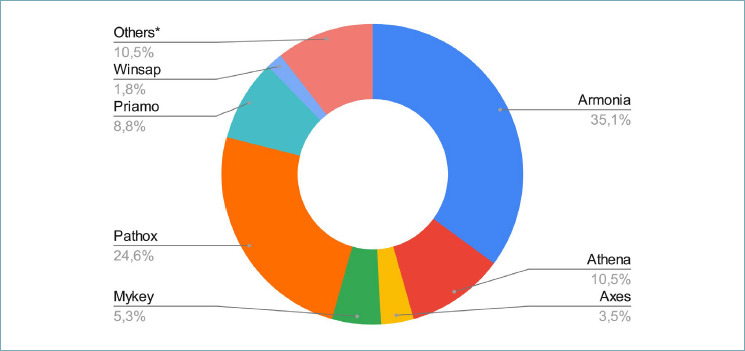
Laboratory Information System (LIS) software houses used in pathology laboratories as the Lombardy region survey with relative usage %. *property software (e.g. FileMaker, MadLad, Archigest, KAIROS, Xpath).
Table II.
Summary of the features of the different LIS reported by the participants to the survey as present (green) or absent (red).
| Traceability | Network integration | Case assignment | Alert functions | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LIS type | # of lab | Barcode | QR code | Worklists | Automated phases | Paper-based | Electronic | Automatic | Semiautomatic | Manual | TATs | Urgent cases alert | Attachments | Signature/access privileges |
| Armonia | 20 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Pathox | 14 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Athena | 6 | Y | Y | Y | Y | Y | Y | n | Y | Y | Y | Y | Y | Y |
| Priamo | 5 | Y | Y | Y | Y | Y | Y | n | N | Y | Y | Y | Y | Y |
| Mykey | 3 | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y |
| Axes | 2 | Y | N | Y | Y | Y | N | Y | N | Y | N | Y | Y | Y |
| FileMaker | 2 | Y | N | Y | Y | N | Y | Y | N | N | Y | Y | Y | Y |
| Winsap | 1 | N | N | Y | N | N | N | N | Y | N | N | N | N | Y |
| XPat | 1 | Y | N | Y | Y | Y | Y | N | N | N | Y | N | N | N |
| MadLan | 1 | Y | N | N | Y | N | N | N | N | N | N | N | N | N |
| KAIROS | 1 | Y | N | Y | Y | Y | N | N | N | N | N | N | Y | Y |
| Archigest | 1 | N | Y | Y | Y | Y | N | N | Y | N | Y | N | Y | Y |
Table III.
Laboratories with manual or partially/completely automated tracking systems in each workflow phase (n, %). IHC, immunohistochemistry.
| Manual | Automated | |||
|---|---|---|---|---|
| Partially | Completely | Total* | ||
| Accessioning | 6 (12%) | 35 (71%) | 8 (16%) | 49 |
| Sampling | 7 (14%) | 35 (70%) | 8 (16%) | 50 |
| Processing | 5 (11%) | 35 (74%) | 7 (15%) | 47 |
| Cutting | 6 (12%) | 36 (72%) | 8 (16%) | 50 |
| Staining | 5 (11%) | 32 (73%) | 7 (16%) | 44 |
| IHC | 7 (14%) | 36 (71%) | 8 (16%) | 51 |
| Histochemistry | 5 (14%) | 25 (71%) | 5 (14%) | 35 |
| Assignment | 5 (13%) | 31 (78%) | 4 (10%) | 40 |
| Reporting | 5 (9%) | 44 (77%) | 8 (14%) | 57 |
| Archiving | 1 (7%) | 13 (87%) | 1 (7%) | 15 |
| Residue disposal | 0 | 6 (86%) | 1 (14%) | 7 |
| Molecular tests | 3 (10%) | 24 (77%) | 4 (13%) | 31 |
| *data reported are related to the number of centers that answered those specific questions. | ||||
Figure 2.
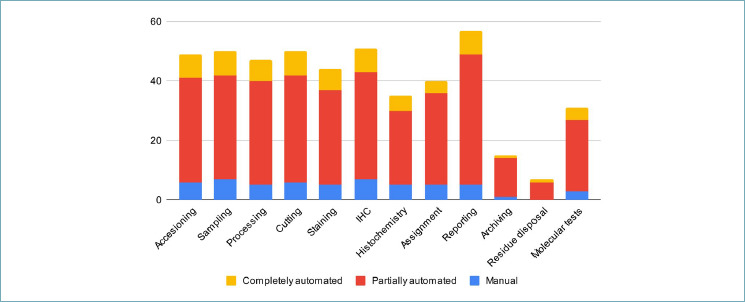
Distribution of the different tracking systems used (manual, partially or completely automated) for single workflow steps in the different laboratories that participated in the survey. IHC, immunohistochemistry.
Figure 3.
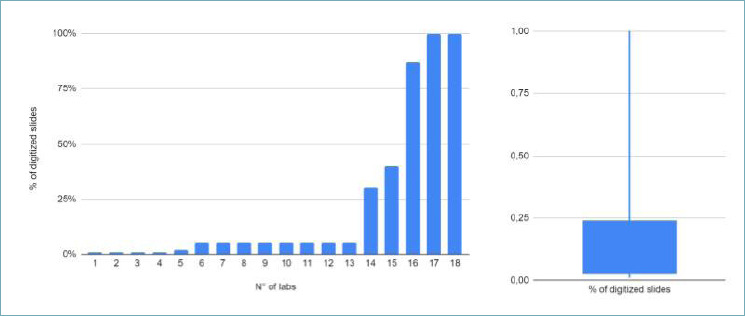
Distribution of the digitized slide percentage among laboratories. The majority declared to scan ≤ 5% of the routine (about 13 centers, as reported in the curve on the left), with three/fourth of the responding centers scanning < 25% of slides (see box plot on the right).
POST- ANALYTICAL PHASE
Regarding archives, the majority of the labs (30, 53%) stored glass slides and blocks in-house, followed by those using hybrid (23, 40%) and out-sourcing approaches (4, 7%). Storage conditions were certified in 28 (49%) internal archives and 8 (14%) out-source services. In this last case, the average in-house storage time for blocks and slides before the external archiving was 1838 (±1551) and 1798 days (±1950), respectively. The average TaT for sample return on-demand was 3.7 days (±2.1, range 1-10).
Discussion
Digital pathology, which involves the use of computer technology to acquire, manage, share, and interpret pathology information in a digital environment, offers several advantages. Digital images can be of higher quality and resolution compared to traditional microscope slides, as demonstrated by previous studies showing non-inferiority of WSI vs traditional microscope 5 This allows for more accurate and detailed examination of specimens. They can be easily shared with other pathologists anywhere in the world, facilitating remote consultations and second opinions. Files can be systematically stored and easily retrieved, which is more efficient compared to physical storage. Digital pathology platforms often include tools for image analysis that can assist pathologists in more precise and quantitative ways, like measuring cell count or tumor size, and identifying patterns that might be missed by the human eye. Systems can be integrated with other electronic health records, allowing for a more comprehensive view of patient history and diagnostic information. Although the initial setup cost for digital pathology systems can be high, they can be more cost-effective in the long run 6. Finally, the use of artificial intelligence (AI) and machine learning algorithms can help in automated detection and diagnosis, which can assist pathologists in handling large volumes of cases more efficiently. These advantages make digital pathology a rapidly evolving field, increasingly adopted in clinical and research settings for improved healthcare delivery. While these progresses are boosting the adoption of a digital workflow, the current real situation of many pathology labs is still characterized by procedural weaknesses, both in terms of QC and traceability, which may require a significant upgrading. The pathology diagnostic process can encounter various problems, including specimen identification errors (mislabeling or mix-ups of patient samples), transportation issues (delays or improper handling), pre-analytical errors (issues in specimen collection, fixation or processing), instrumentation failures (malfunctioning or poorly calibrated equipment), data management challenges (inaccurate or inefficient information systems), communication breakdowns (poor communication between laboratory staff and clinicians), regulatory compliance (failure to meet regulatory standards) 7. Addressing these challenges is essential for accurate diagnoses and effective patient care 8. The CRC Med Lab center in Lombardy focuses on improving and monitoring the quality of laboratory medicine services. This includes implementing quality programs, proposing evaluations and regulatory impacts, revising internal QC methods, assisting in inspection verifications for authorization and accreditation, and managing data collection related to laboratory structures and their outputs. The center also serves as an observatory on laboratory service activities, particularly in cases of significant clinical and epidemiological relevance. Based on the recently updated guidelines for lab certification (DGR XI/7044, 26/09/20223), the CRC Med Lab working group organized a regional survey focused on the evaluation of pre-analytical, analytical and post-analytical variables in the current routine practice to give an overview on criticalities of the real-world pathology labs and propose possible solutions to ensure the quality of pathology services or implement processes (Fig. 4). The preanalytical phase in histology involves critical steps before the slide analysis, including specimen collection, labeling, transport, and proper storage. Each specimen must be accurately labeled with patient information to ensure traceability and its transport must preserve integrity throughout all the subsequent phases. Proper storage conditions are crucial to maintain tissue quality for histological examination. This phase is vital for accurate diagnosis, as errors during pre-analytical handling can lead to compromised results and misdiagnoses 9,10.
Figure 4.

Possible operative solutions to face the criticalities unveiled by the survey.
From the survey, most laboratories applied a unique code to the sample only at the pathology lab arrival time point. The preferred and desirable possibility of a central order entry, which can allow a direct bedside labeling for both surgical and outservice samples, was present in 23 (40%) labs. The future creation of a regional order entry can significantly improve the smoothness of the accessioning phase, promoting a paperless and less error prone approach to this delicate phase, as already promoted by the current guidelines1, with preliminary successful experiences of central accessioning already described at European level 11. In detail, the previous experiences reported both in the North 12 and the South13 of Italy already demonstrated the successfulness of a hub-spoke network approach with centralized order entry, allowing a reduction in accessioning errors from 6.3% to less than 0.5% 14,15.
Currently, the vast majority of labs (35, 61%) use a hybrid traceability method, with more complete and secure automation still lagging back. This is crucial in multi-site facilities to guarantee complete monitoring even during the sample transfer phases among spokes. In the case studies it emerged that almost all the multi-site facilities were equipped with a connection and in 12/16 (70%) of these facilities samples are accessioned at its arrival in the laboratory. A modern and multitasking LIS is the fundamental requirement for good sample management 16,17, as evident by the standard qualifications/prerequisites outlined by the Lombardy region in its recent market study. Compelling features for a modern LIS are the ability to manage the case using unique ID code, specimen tracking with QR or barcode, allowance of data exchange process and coordination between pathologists, synoptic reporting and automatic coding, integration with ancillary tests (e.g. IHC and molecular pathology), QC systems with checkpoints and dashboards, image upload and analysis with digital pathology integration. In the Lombardy region, 12 different softwares were used, only 61% of them supporting alert functions (e.g. urgent cases or lapsing TaTs), the majority (80%) with multi-file attachment capabilities. A modern LIS should also allow the traceability of the sample throughout all the steps, with check list options, as reported by the CAP guidelines for the traceability of cyto-histological samples (8 out of 57 lab, 14%). This has been already previously demonstrated by other groups (e.g. in Portugal), where the adequate implementation of automated tracking systems and integrated information within the LIS allowed a rapid digitization of up to 70% of the pathology routine, the chance to easily integrate exams from the same patients in different timepoints, ultimately impacting positively on the turn-around time and a reduction of costs of up to 25% due to the paper printing reduction 18.
Regarding the scanning habits, only a minority of laboratories (32%) digitize glass slides in routine practice, with 13 centers scanning < 5% of slides and three-fourth of the answering centers scanning < 25% of the routine. In most of the cases (50%) the slides scanned were from routine histology while digitizing was used for cytology in about 33% of cases, even if sporadically. The average number of glass slides produced by those laboratories in a month in 2022 was 18,515, consisting of about 935 WSI with a scanning rate of 5%, corresponding to almost 11,200 in the year, with consequent issues in terms of digital storage. According to CAP, WIS for primary diagnosis need to be stored for 10 years if original glass slides are not available 19. From this experience, the average time for digital images storage was 3.7 years. Adequate validation of WSI for primary diagnosis should be performed before the progressive digitization in routine. This is of paramount importance with the progressive implementation of technical improvements of the pathologist diagnostic cockpit, as with the introduction of next generation professional grade or medical monitors, that can potentially improve the interpretation of histology, leading to an overall increase in diagnostic accuracy 20,21. In this setting, providing the most appropriate storage solution (physical/local, cloud or hybrid) is mandatory to ensure the best standard of patient care, and these storage solutions should fit the needs of each department. Here the concurrent experiences of Italian regions progressing in the digital transformation of pathology laboratories can be of help 12, showing the benefits of a cloud-based archiving of digital images and data in central/federated vendor-neutral archives, allowing easy and fast case identification and retrieval, planning for a long-term central archive after initial local management at the laboratory (short-term local archive). The purpose of this distinction is to streamline the management of the digital archive through centralization. It should be possible to automatically recover the slides of each patient every time there is an examination or letting the local pathologist decide which cases to keep in the short-term archive. Collection and storage times of the diagnostic material is another pivotal step especially in the molecular pathology section, which is gaining progressively more importance in the diagnostic process of pathology laboratories. According to the guidelines 22, the “warm” (peri-surgical) and “cold” (from collection to fixation) ischemia times should be reported for their impact on antigens/nucleic acids preservation. However, only a minority of centers (42%) is tracking molecular analysis steps 22. In the post-analytical phase, the proper storage of paraffin blocks is essential for tissue preservation and long-term integrity in view of possible future investigations i.e. next generation sequencing) 2, as in the case of glass slides as well (e.g. consultation) 23. In this direction, desirable archival conditions (e.g. constant temperature/humidity, ventilation systems, no direct light exposure, QR/barcode-based storing/retrieval systems, regular maintenance) are essential to preserve blocks for > 10 years, as indicated by CAP guidelines 19.
From the survey, the majority of the laboratories (53%) proceed with in-house archiving, followed by hybrid or purely out-source services (40% and 7%, respectively), the latter options still imposing a temporary in-house storing of material for about 10 and 5 years for slides and blocks, respectively, before moving to the external archives. Moreover, the average return time for samples on-demand ranges from 1 to 10 days, with traceability conditions not fully guaranteed in about 37% of cases. The definition of the most adequate archival/storage solution for slides/blocks should be customized based on the need of each department, always ensuring the safe/tracked transfer of samples with appropriate modulation of return times based on the urgency.
Based on the results obtained in this real-world survey on the adoption of digital pathology and automated workflow in the pathology labs in the Lombardy region, we tried to extract useful insights in the form of actionable points that can help to boost the progressive adoption of a fully tracked and integrated pathology workflow within the territory (Fig. 4 and Tab. IV). The efficacy and usefulness of these proposed practical insights is further unveiled by the already established experiences described both in Italy (e.g. Sicily 13 and Veneto 12 regions) and in Europe (e.g. Porto) 18, paving the way for the implementation of digital pathology in similar ecosystems and providing the appropriate use cases to start the progressive transformation of our laboratories.
Table IV.
Actionable insights to overcome challenges and hurdles in the adoption of digital pathology workflow. For each phase (pre-analytical, analytical and post-analytical) possible solutions or recommendations stemming from the real-world data obtained in the present survey are proposed to improve traceability and integrate digital systems more efficiently.
| Pre-analytical | Analytical | Post-analytical |
|---|---|---|
| Site Centralization of the accessioning in the context of hub-spoke network (centralized Order Entry and barcode). |
LIS Employment of softwares with minimum required technical features (e.g. alerts capabilities and inclusion of attachments). |
Archives Adoption of a safe, secure and environmentally stable archiving system, especially for paraffin block storage. |
| Labeling Regional-level order entry to ensure interoperability and interchangeability of the information among laboratories within the same territory. |
Tracking Adoption of tracking systems to ensure the traceability of histological, cytological and molecular samples within different labs in the same network. |
Storage Reduction of sample manipulation and handling, acquiring fast and safe path for specimens archival/retrieval. |
| Traceability Fully automated tracking systems shared among the centers belonging to the same network. |
Slides Strict validation of the WSI for primary diagnosis and re-arrangement of the long term storage in an automated and tracked way. |
Out-source services Specimens archival that ensure adequate TaTs with specific reference to the on-demand option of sample retrieval. |
Conclusions
Digital pathology has a great potential fto change the routine practice of pathology labs, although it is a complex endeavor which requires careful planning. Based on the current survey, the landscape currently reflects a situation in which only few facilities use digital pathology in routine practice. This may be due to the lack of traceability in many steps of the workflow (pre-analytical, analytical and post-analytical phase), being a great limitation. It will be important to assemble a team consisting of all the key players involved in the areas affected by digital pathology (including clinicians, lab technicians, researchers) to finally obtain this transition.
ACKNOWLEDGMENTS
The authors acknowledge all the center participants in the survey.
The survey was answered by the labs of the following Institutions: Fondazione IRCCS Istituto Nazionale dei Tumori Milano; Consulenze Diagnostiche cito-istologiche, Seregno; MEDIPAT SRLS; Clinica Polispecialistica San Carlo Srl, Paderno Dugnano; Istituto Europeo di Oncologia S.r.l., Milano, TOMA Advanced Biomedical Assays S.p.A. Busto Arsizio,; Istituto Auxologico Italiano Cusano Milanino; Istituti Ospedalieri Bergamaschi S.r.l. - Policlinico San Pietro Ponte San Pietro; Casa di Cura Privata - Policlinico di Monza S.p.A Monza, MB; MAD Analisi S.r.l. Voghera; Casa di Cura Multimedica S.p.A. Milano; C.D.I. Centro Diagnostico Italiano S.p.A. Milano, MI; NEXT LAB ITALY SRL Busto Arsizio, VA; Casa di Cura Igea S.p.A. Milano; Synlab Italia S.r.l. - Brescia; Centro Polispecialistico Beccaria S.r.l. Varese; Bianalisi SpA - (Carate Brianza); Studio Consulenza Citologica S.a.s. Gambolò; ASST della Brianza; ASST Valle Olona; ASST Centro Specialistico Ortopedico Traumatologico Gaetano Pini/Cto; ASST Lariana; ASST della Franciacorta; ASST Fatebenefratelli Sacco; Humanitas Mirasole S.p.A. Rozzano; ASST Santi Paolo e Carlo; ASST di Mantova; Fondazione IRCCS San Gerardo dei Tintori di Monza Monza; ASST di Bergamo Ovest; ASST Rhodense; Ospedale San Raffaele S.r.l. Milano; ASST Papa Giovanni XXIII; ASST Fatebenefratelli Sacco; Fondazione IRCCS Policlinico San Matteo Pavia; ASST di Crema; ASST di Lodi; ASST di Lecco; ASST Ovest Milanese; ASST Sette Laghi; Istituti Ospedalieri Bresciani S.p.A, Fondazione Poliambulanza;ASST di Pavia Voghera; ASST Grande Ospedale Metropolitano Niguarda, Milano; ASST degli Spedali Civili di Brescia; Fondazione IRCCS Ca Granda - Osp. Maggiore Policlinico,(Milano); ASST di Cremona; ASST della Valcamonica; ISTITUTI CLINICI SCIENTIFICI MAUGERI SPA SB Pavia; ASST Melegnano e della Martesana; ASST di Bergamo Est; Congregazione Suore Infermiere dell’Addolorata - Ospedale Valduce Como; Ospedale Galeazzi S.p.A., Milano; Fondazione IRCCS Istituto Nazionale Neurologico Carlo Besta Milano; Fondazione IRCCS Ca Granda - Osp. Maggiore Policlinico Milano; Cerba HC Italia s.r.l. Milano.
In memory of Giulio Rossi, MD.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
FUNDING
This research received no external funding.
AUTHORS’ CONTRIBUTIONS
FaPag, SB, EmBo and FaPas defined the original survey; GB, MC, FMC, ED, ADG, MF, AG, UG, PG, MM, MN, MP, and AP were part of the digital pathology working group of Regione Lombardia; ElBe, FaPag and FaPas extracted the results of the survey and processed the data; ElBe and FaPas drafted the original manuscript; VL cured the table and figures of the manuscript; FaPag, EmBo and SB critically revised the initial draft; all authors gave final approval of the submitted and published versions.
ETHICAL CONSIDERATION
The research was conducted ethically, with all study procedures being performed in accordance with the requirements of the World Medical Association’s Declaration of Helsinki.
History
Received: April 2, 2024
Accepted: June 27, 2024
Figures and tables
References
- 1.Fraggetta F, L’Imperio V, Ameisen D, et al. Best Practice Recommendations for the Implementation of a Digital Pathology Workflow in the Anatomic Pathology Laboratory by the European Society of Digital and Integrative Pathology (ESDIP). Diagnostics (Basel). 2021;11(11). https://https://doi.org/10.3390/diagnostics11112167 10.3390/diagnostics11112167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eccher A, Dei Tos AP, Scarpa A, et al. Cost analysis of archives in the pathology laboratories: from safety to management. J Clin Pathol. 2023;76(10):659-663. https://https://doi.org/10.1136/jcp-2023-209035. 10.1136/jcp-2023-209035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Determinazioni in merito all’organizzazione dei servizi di medicina di laboratorio e relativo aggiornamento dei requisiti specifici autorizzativi e di accreditamento. Accessed January 16, 2024. http://www.qualitalaboratorilombardia.it:8080/front/public/1671723526DGR_7044_DEL_26_SETTEMBRE_2022.pdf
- 4.Burns PB, Rohrich RJ, Chung KC. The levels of evidence and their role in evidence-based medicine. Plast Reconstr Surg. 2011;128(1):305-310. https://doi.org/10.1097/PRS.0b013e318219c171 10.1097/PRS.0b013e318219c171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mukhopadhyay S, Feldman MD, Abels E, et al. Whole Slide Imaging Versus Microscopy for Primary Diagnosis in Surgical Pathology: A Multicenter Blinded Randomized Noninferiority Study of 1992 Cases (Pivotal Study). Am J Surg Pathol. 2018;42(1):39-52. https://doi.org/10.1097/PAS.0000000000000948 10.1097/PAS.0000000000000948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams BJ, Bottoms D, Clark D, Treanor D. Future-proofing pathology part 2: building a business case for digital pathology. J Clin Pathol. 2019;72(3):198-205. Huang S, Patel V, Lee JB. Errors encountered in the diagnostic pathway: A prospective single-institution study. J Cutan Pathol. 2023;50(9):828-834. https://doi.org/10.1136/jclinpath-2017-204926 10.1136/jclinpath-2017-204926 [DOI] [PubMed] [Google Scholar]
- 7.Sirota RL. Error and error reduction in pathology. Arch Pathol Lab Med. 2005;129(10):1228-1233. https://doi.org/10.5858/2005-129-1228-EAERIP 10.5858/2005-129-1228-EAERIP [DOI] [PubMed] [Google Scholar]
- 8.Bussolati G, Annaratone L, Maletta F. The pre-analytical phase in surgical pathology. Recent Results Cancer Res. 2015;199:1-13. https://doi.org/10.1007/978-3-319-13957-9_1 10.1007/978-3-319-13957-9_1 [DOI] [PubMed] [Google Scholar]
- 9.Sotoudeh Anvari M, Gharib A, Abolhasani M, et al. Pre-analytical Practices in the Molecular Diagnostic Tests, A Concise Review. Iran J Pathol. 2021;16(1):1-19. https://doi.org/10.30699/ijp.2020.124315.2357 10.30699/ijp.2020.124315.2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Temprana-Salvador J, López-García P, Castellví Vives J, et al. DigiPatICS: Digital Pathology Transformation of the Catalan Health Institute Network of 8 Hospitals-Planification, Implementation, and Preliminary Results. Diagnostics (Basel). 2022;12(4). https://https://doi.org/10.3390/diagnostics12040852 10.3390/diagnostics12040852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eccher A, Marletta S, Sbaraglia M, et al. Digital pathology structure and deployment in Veneto: a proof-of-concept study. Virchows Arch. Published online May 14, 2024. https://https://doi.org/10.1007/s00428-024-03823-7 10.1007/s00428-024-03823-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fraggetta F, Caputo A, Guglielmino R, et al. A Survival Guide for the Rapid Transition to a Fully Digital Workflow: The “Caltagirone Example.” Diagnostics (Basel). 2021;11(10). https://https://doi.org/10.3390/diagnostics11101916 10.3390/diagnostics11101916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakhleh RE, Zarbo RJ. Surgical pathology specimen identification and accessioning: A College of American Pathologists Q-Probes Study of 1 004 115 cases from 417 institutions. Arch Pathol Lab Med. 1996;120(3):227-233. [PubMed] [Google Scholar]
- 14.Smith ML, Wilkerson T, Grzybicki DM, Raab SS. The effect of a Lean quality improvement implementation program on surgical pathology specimen accessioning and gross preparation error frequency. Am J Clin Pathol. 2012;138(3):367-373. https://doi.org/10.1309/AJCP3YXID2UHZPHT 10.1309/AJCP3YXID2UHZPHT [DOI] [PubMed] [Google Scholar]
- 15.Hanna MG, Pantanowitz L. Bar Coding and Tracking in Pathology. Surg Pathol Clin. 2015;8(2):123-135. Workflow Organization in Pathology. Clin Lab Med. 2012;32(4):601-622. https://doi.org/10.1016/j.path.2015.02.017 10.1016/j.path.2015.02.017 [DOI] [PubMed] [Google Scholar]
- 16.Eloy C, Vale J, Curado M, et al. Digital Pathology Workflow Implementation at IPATIMUP. Diagnostics (Basel). 2021;11(11). https://https://doi.org/10.3390/diagnostics11112111 10.3390/diagnostics11112111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.CAP Laboratory Accreditation Manual. Accessed January 16, 2024. https://documents-cloud.cap.org/pdf/laboratory-accreditation-manual-new.pdf
- 18.Cazzaniga G, Mascadri F, Marletta S, et al. Benchmarking digital displays (monitors) for histological diagnoses: the nephropathology use case. J Clin Pathol. Published online March 27, 2024. https://https://doi.org/10.1136/jcp-2024-209418 10.1136/jcp-2024-209418 [DOI] [PubMed] [Google Scholar]
- 19.Baxi V, Edwards R, Montalto M, Saha S. Digital pathology and artificial intelligence in translational medicine and clinical practice. Mod Pathol. 2022;35(1):23-32. https://doi.org/10.1038/s41379-021-00919-2 10.1038/s41379-021-00919-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ministero della Salute: Tracciabilità, Raccolta, Trasporto, Conservazione e Archiviazione di cellule e tessuti per indagini diagnostiche di ANATOMIA PATOLOGICA. Accessed January 16, 2024. https://www.salute.gov.it/imgs/C_17_pubblicazioni_2369_allegato.pdf
- 21.Kapila SN, Boaz K, Natarajan S. The post-analytical phase of histopathology practice: Storage, retention and use of human tissue specimens. Int J Appl Basic Med Res. 2016;6(1):3-7. https://doi.org/10.4103/2229-516X.173982 10.4103/2229-516X.173982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torresani E, Gentilini MA, Grassi S, et al. 2023. Diagnostic Concordance between Traditional and Digital Workflows. A Study on 1427 Prostate Biopsies. Pathologica 2023;115(4):221-26. https://https://doi.org/10.32074/1591-951X-896 10.32074/1591-951X-896 [DOI] [PMC free article] [PubMed] [Google Scholar]


