Abstract
Background
Understanding the level of exposure to Lassa virus (LASV) in at-risk communities allows for the administration of effective preventive interventions to mitigate epidemics of Lassa fever. We assessed the seroprevalence of LASV antibodies in rural and semiurban communities of two cosmopolitan cities in Nigeria with poorly understood Lassa epidemiology.
Methods
A cross-sectional study was conducted in ten communities located in the Abuja Municipal Area Council (AMAC), Abuja, and Ikorodu Local Government Area (LGA), Lagos, from February 2nd to July 5th, 2022. Serum samples collected from participants were analyzed for IgG and IgM antibodies using a ReLASV® Pan-Lassa NP IgG/IgM enzyme-linked immunosorbent assay (ELISA) kit. A questionnaire administered to participants collected self-reported sociodemographic and LASV exposure information. Seroprevalence of LASV IgG/IgM was estimated overall, and by study site. Univariate and multivariate log-binomial models estimated unadjusted and adjusted prevalence ratios (aPRs) and 95% confidence intervals (CI) for site-specific risk factors for LASV seropositivity. Grouped Least Absolute Shrinkage and Selection Operator (LASSO) was used for variable selection for multivariate analysis.
Results
A total of 628 participants with serum samples were included in the study. Most participants were female (434, 69%), married (459, 73%), and had a median age of 38 years (interquartile range 28–50). The overall seroprevalence was 27% (171/628), with a prevalence of 33% (126/376) in Abuja and 18% (45/252) in Lagos. Based on site-specific grouped LASSO selection, enrollment in the dry season (vs. wet; aPR, 95% CI: 1.73, 1.33–2.24), reported inconsistent washing of fruits and vegetables (aPR, 95% CI: 1.45, 1.10–1.92), and a positive malaria rapid test (aPR, 95% CI: 1.48, 1.09-2.00) were independently associated with LASV seropositivity in Abuja, whereas, only a self-reported history of rhinorrhea (PR, 95% CI: 2.21, 1.31–3.72) was independently associated with Lassa seropositivity in Lagos.
Conclusions
The LASV seroprevalence was comparable to that in other areas in Nigeria. Our findings corroborate those from other studies on the importance of limiting human exposure to rodents and focusing on behavioral factors such as poor hygiene practices to reduce exposure to LASV.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-024-09954-1.
Keywords: Epidemiology, Lassa virus, Seroprevalence, Community-based study, Emerging infectious disease, Nigeria
Background
Lassa virus (LASV) causes Lassa fever (LF), an acute viral illness belonging to the group of viral hemorrhagic fevers (VHFs), including Dengue, Ebola, and Marburg fevers [1]. Lassa virus is a single-stranded ribonucleic acid virus (RNA) belonging to the Arenaviridae family and is considered a zoonotic virus [2]. It was first identified in the town of Lassa in North-East Nigeria and surmised, by molecular dating, to have originated in Nigeria more than a thousand years ago and spread to neighboring West African countries including Guinea, Liberia, and Sierra Leone where it is now endemic [3–6]. Although dengue fever is the most common VHF, LF ranks second in global burden [7]. An estimated three million LASV infections and up to 67,000 deaths occur annually in endemic regions [8]. Despite the high burden, it was long considered a neglected tropical disease until a record 633 confirmed cases reported in 2018, marked it as the largest outbreak to have occurred in Nigeria. This led to the declaration of a public health emergency both nationally and by the World Health Organization (WHO) [9, 10]. Due to its epidemic potential and limited medical countermeasures, in 2021, LASV was listed among the top ten priority pathogens on the WHO’s research and development blueprint for a roadmap to outbreak response [11].
While LF cases are reported virtually all year, outbreaks peak annually in Nigeria during the dry season from December through April [4, 12]. Zoonotic transmission of LASV, primarily from Mastomys rodents, is the predominant mode of human infection. Transmission can occur via direct contact with infected animals, contact with contaminated household items or food, or inhalation of aerosolized viral particles from rodent droppings. However, person-to-person transmission also has been documented in situations with inadequate infection control practices [13, 14]. The incubation period for LF ranges from two to twenty one days [15]. Most infections are asymptomatic with approximately 20% of infected persons experiencing nonspecific symptoms such as fever, headache, sore throat, myalgia, and gastrointestinal symptoms also common to other VHFs and infectious diseases such as malaria and typhoid fever [16, 17]. Pregnant women are particularly vulnerable, with a high risk of maternal death and fetal loss in late pregnancy [18]. Although there are several candidate vaccines, there are currently no approved vaccines or immunotherapies to prevent or treat this illness [19]. Several laboratory tests like reverse transcriptase polymerase chain reaction (RT-PCR), antibody enzyme-linked immunosorbent assay (ELISA), antigen detection assays, and viral isolation in cell culture, can be used to definitively diagnose LF infection [15]. Unlike antibody tests, these methods allow for early detection of acute LF during the first week of symptoms by detecting the virus itself rather than the body’s immune response [20, 21]. In West Africa, where LASV exposure is common, LASV-specific immunoglobulin M (IgM) antibodies without detectable viremia cannot be used for definitive diagnosis of acute LF [22]. IgM antibodies have been shown to persist for 532 to more than 800 days after initial LASV infection [22, 23].
Although the epidemiology of LF and exposure characteristics have been reported for several areas in Nigeria, there is little to no knowledge among healthy adult human populations in Abuja, the nation’s capital, and Lagos, a major economic hub, despite the high volume of people moving in and out of these major cities [24]. National surveillance data suggests Lassa fever appears to be less prevalent in Lagos and Abuja compared to other parts of Nigeria [25]. The objectives of this study were to determine the seroprevalence of LASV infection and associated risk factors and co-infections in the Abuja Municipal Area Council (AMAC) and Ikorodu Local Government Area (LGA). The findings from this study provide useful information for future LASV vaccine development and implementation efforts.
Methods
Study area and population
We conducted a community-based cross-sectional study in rural and semiurban communities in AMAC, Abuja, the Federal Capital Territory (FCT), in North-Central Nigeria, and the Ikorodu LGA in Lagos State, South-West Nigeria (Fig. 1). Study sites in AMAC and Ikorodu LGA are hereon referred to as Abuja and Lagos, respectively. Nigeria is divided into six geopolitical regions (North-East, North-Central, North-West, South-East, South-South and South-West) with 36 states and a Federal Capital Territory, which are further divided into 774 LGAs and Area Councils, respectively, for ease of administration.
Fig. 1.
Geographic map of Nigeria, with emphasis on Abuja Federal Capital Territory (FCT) and Lagos state, where recruitment communities and primary health care centers for the study were situated
Sample size and recruitment
Enrollment for the study took place between February 2nd, 2022, and July 5th, 2022, at primary healthcare centers (PHCs) in Abuja and Lagos; an additional participant was enrolled on November 13th, 2022, to replace one who did not meet the screening criteria. The inclusion criteria were age ≥ 18 years, ability to provide written consent, willingness to provide location and contact information, and willingness to participate in study procedures.
The target sample size was achieved through a multi-stage process. In the first stage, we purposively selected the two LGAs due to their high population density, presence of a mix of urban and semi-urban communities, existing infrastructure, and established collaborations. In the second stage, following onsite assessments of PHCs for criteria such as rural/semi-urban location, functionality, community reach, collaboration, and safety, we employed random sampling to select ten PHCs from Abuja (n = 6) and Lagos (n = 4). Participants were recruited from communities surrounding selected PHCs and asked to meet at PHCs for study procedures. Study enrollment at each site was preceded by community engagement activities, including inaugural stakeholder meetings, advocacy visits to community heads and gatekeepers, the formation of community advisory boards, and community sensitization visits. A total of 1,271 adults in the communities were briefed about the study during the recruitment phase. In addition, individuals who routinely sought care at any of the selected PHCs were also engaged by the study staff and invited to participate. Enrollment in the study at each PHC proceeded on a sequential basis using a first-in, first-served approach. To detect city-to-city variations in Lassa seroprevalence exceeding 5% in Nigeria from a previously estimated national prevalence of 21.3%, a sample size of 500 was needed to achieve 77% power [26]. To compensate for potential attrition of 20% due to missing data or sample loss, a target sample size of 630 was sought for an enrollment allocation of 63 (630/10) per PHC. The study involved two visits. The first visit determined eligibility and enrolled participants. The second follow-up visit provided participants with their research laboratory results and an opportunity to discuss the results with the research team. Participants were provided with compensation for their time and travel.
Ethics approval
The study was approved as minimal risk human research by the Walter Reed Army Institute of Research (study # 2760) Institutional Review Board in the United States of America (USA) and the National Health Research Ethics Committee in Nigeria. Permission was obtained from FCT/AMAC and Lagos State/Ikorodu Primary Healthcare Boards and community stakeholders to visit the communities and PHCs and perform the study procedures. Participants provided written informed consent before any study procedures were conducted. The informed consent form was reviewed with participants in detail by trained and delegated study staff before written consent was obtained.
Specimen collection and laboratory procedures
Participants were screened for potential co-infections including human immunodeficiency virus (HIV), malaria, hepatitis B, hepatitis C, and relevant conditions like pregnancy. Blood (venous and capillary) and urine specimens were collected from each participant. Venous blood and urine specimens were labelled with unique identifiers and transported under appropriate temperature conditions to the Clinical Research Centre (CRC) laboratory in Abuja or the 68 Nigerian Army Reference Hospital Yaba (68 NARHY) in Lagos for processing, testing, and storage. Routine urinalysis and malaria tests for all participants and urine pregnancy testing for female participants were performed at the CRC and 68 NARHY laboratories. Rapid HIV tests were performed on site at the PHCs. Results were provided to the participants on the same day and included pre- and post-HIV test counseling. Serum was separated and stored at -80 degrees Celsius until screening for LASV IgM and IgG antibodies, hepatitis B virus (HBV) surface antigen (sAg) and hepatitis C virus (HCV) total antibody was performed at The Defence Reference Laboratory (DRL), Abuja.
Rapid HIV testing was performed in accordance with Nigeria’s national HIV rapid testing algorithm which comprised (1) Determine HIV-1/2 (Abbott, California (CA), USA) for screening followed by (2) Unigold HIV-1/2 (Trinity Biotech Plc., Ireland) for confirmation, and, (3) Statpak HIV-1/2 (Chembio Diagnostic Systems, Inc., New York, USA) if test results for (1) and (2) were discordant [27]. Malaria infection was detected with a USA Food and Drug Administration-cleared rapid diagnostic test (RDT; BinaxNOW™ Malaria, Abbott). The test also differentiated malaria infection with Plasmodium falciparum from less virulent panmalarial infections due to Plasmodium vivax, Plasmodium ovale, or Plasmodium malariae. Urine specimens were tested with a Sure-Vue® STAT Serum/Urine hCG Test Kit (Fisher Scientific, Waltham, Massachusetts, USA) for the detection of pregnancy status. Additionally, urine specimens were tested with Multistix® 10 SG reagent strips (Siemens Healthineers, Malvern, Pennsylvania, USA) for routine urinalysis.
All serum samples were screened for LASV IgG and IgM antibodies using a commercially available ELISA assay (Research Use Only (RUO), ReLASV® Pan-Lassa Combo NP/ Prefusion GP IgG/IgM ELISA Kit, Zalgen Labs, Frederick, Maryland, USA) [28]. To detect a wider range of Lassa virus infections, the assay targets both prefusion glycoprotein (GP) and nucleoprotein (NP) antigens specific to Lassa virus lineages II (Nigeria) and IV (Guinea, Liberia, and Sierra Leone) [29]. Four lineages (I-III and VI/Kako strain) have been identified in Nigeria [30]. Both IgM and IgG are considered markers of prior exposure to Lassa virus since LASV-specific IgM antibodies are not an independent surrogate marker for acute or recent infection and can persist in healthy populations for months to years after infection [22]. Thus LASV seropositivity was defined as positivity on either IgM or IgG testing. Assays were performed according to the manufacturer’s guidance and methods used previously for assay evaluation for laboratory diagnostics for a vaccine development program [28, 29]. Following established methodology from a prior Nigerian study, cutoffs were determined based on the sample data set’s optical density (OD) values [29]. Consistent with the reference, the negative cutoff was set at the 95th percentile (OD < 0.250), and the positive cutoff was set at twice the negative cutoff (OD ≥ 0.500). Samples with OD values between these cutoffs were considered indeterminate. All other serologic assays were conducted with the following: GS HBsAg EIA 3.0 (BioRad Laboratories, Hercules, CA, USA) for screening for HBsAg, GS HBsAg Confirmatory Assay 3.0 (BioRad Laboratories) for confirmation of GS HBsAg EIA 3.0-reactive specimens, Ortho® HCV Version 3.0 ELISA (Chiron Corporation, Emeryville, CA) for screening for antibodies to HCV (anti-HCV), and INNO-LIA™ HCV Score (RUO, Fujirebio, USA) for confirmation of anti-HCV reactive specimens.
Data collection, management, and statistical analysis
At enrollment, a physical examination was conducted, and questionnaires were administered to obtain information such as current sociodemographics, potential LASV exposures, and medical history including past and current symptoms [31, 32]. Sociodemographic data included age, sex, tribe/ethnicity, marital status, occupation, level of education, and residence/housing information. Potential LASV exposures in the past 2 years included animal and other environmental exposures, food hygiene practices, hand hygiene practices, sick contacts, health worker or other occupational exposure, participation in funerals and travel history. For analysis, food and hand hygiene practices were collapsed to a two-level categorical variable (‘Always’ or ‘Other’) from the six-level ordinal variable (coded as ‘never’, ‘rarely’, ‘sometimes’, ‘usually’, ‘almost always’, ‘always’) in the questionnaire. For animal vector exposures in the past 2 years, specific information was elicited about the presence of rodents at home, contact with rodents or rodent excreta, viewing rodent excreta on food and water/drink, rodent consumption, and history of rodent bites. Physical examination included vital signs (height, weight, body temperature, heart rate, blood pressure, and respiratory rate), whereas medical history intake included self-reported prior and current medical history and comorbidities, and self-reported prior and current LF-related symptoms. All the data collected from the hard-copy questionnaires were coded with a unique participant identification number and manually entered into a password-protected REDCap web-based database (Bethesda, Maryland) [33, 34].
Sociodemographic characteristics, LASV exposure and symptom history were described using frequencies and percentages. The seroprevalence of Lassa IgG and IgM, HIV, and HCV antibodies and HBsAg and malaria was calculated by dividing the number of participants with positive test results by the total number of participants tested. Univariate statistical testing was used to identify independent characteristics associated with Lassa seropositivity. For univariate analyses, we assessed associations between characteristics of interest and Lassa seropositivity by using prevalence ratios (PRs) with 95% confidence intervals (CIs) from log-binomial regression. We used prevalence ratios over odds ratios since odds ratios can inflate estimates of the effects of variables when the prevalence is > 10% [35].
Because our study contained a total of 109 initial predictors of interest, variable selection was performed using grouped Least Absolute Shrinkage and Selection Operator (LASSO) regression analyses, performed separately by study site using the glmnet package in R. During the regularization procedure, grouped LASSO shrinks the beta coefficients of variables without predictive power toward zero. Characteristics with nonzero beta coefficients were then selected as predictors. Grouped LASSO expands upon LASSO by selecting or not selecting variables pre-selected to be in a group. Dummy coded variables were grouped together for the selection process including number of pregnancies, number of live births, pregnancy outcome (live birth, spontaneous abortion/miscarriage, still birth, terminated pregnancy), and age at enrollment and place of food preparation (indoor, outdoor, indoor, and outdoor). Because predictor selection is highly variable depending on fold randomization, we iterated the grouped LASSO across 100 randomly generated tenfold partitions. The value for lambda was selected using the minimum cross validation error (MCVE) method across each iteration. Using MCVE is more conservative and selects a smaller number of predictors than the method using one standard error above the MCVE. While neither of the lambda selection techniques demonstrate greater accuracy, the conservative MCVE approach reduces false discovery rates for predictors [36, 37]. Predictors selected by grouped LASSO fifty or more times were included in a log-binomial generalized linear model (GLM) and adjusted for other selected variables by site for estimation of adjusted prevalence ratios (aPRs) [38, 39]. All p-values less than 0.05 were considered to indicate statistical significance. All data were managed and analyzed using SAS® (SAS Institute, Cary, North Carolina, USA, version 9.4) or R Studio software (version 4.0.3, Boston, Massachusetts, USA).
Results
Among 630 participants enrolled in the study, 628 provided blood specimens for the assessment of Lassa IgG and IgM antibodies and were included in the analysis. The participants had a median age of 38 years (interquartile range (IQR) 28–50) and were predominantly female (434, 69%) or married (459, 73%). Almost half (294, 47%) had not completed secondary school. The most common occupations reported were commerce or business (176, 28%) followed by skilled trade (145, 23%). The participants came from low socioeconomic backgrounds. Their median weekly income was ₦8,000 (IQR ₦5,000-₦15,000) (equivalent to roughly USD 6.20 (IQR 3.80–11.50) on April 26th, 2024). Typically, households had a median of 5 other occupants (IQR 4–7) living in a median of 2 rooms (IQR 1–3).
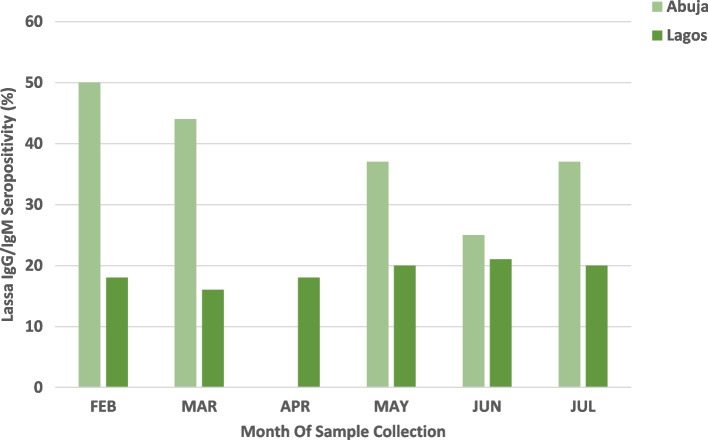
Overall, 27% (171/628) of participants were Lassa seropositive, with significantly more seropositive participants from Abuja (126/376, 33%) than from Lagos (45/252, 18%) (p < 0.05, Table 1). Abuja showed a seasonal difference in seropositivity, with higher rates in the dry season than in Lagos, which did not exhibit seasonal variation (Fig. 2). Compared to those in Lagos, the prevalence of Plasmodium falciparum malaria (12% vs. 0%) and HCV antibodies (11% vs. 2%) in Abuja was significantly greater (p < 0.05, Table 1). Conversely, HBsAg prevalence was greater in Lagos (10%) than in Abuja (8%) (p < 0.05), while HIV prevalence was similar in both cities (Abuja 3%, Lagos 2%, p = 0.3608). Urine pregnancy tests showed a positivity rate of 7% among women in Abuja, and 3% among women in Lagos (Table 1). Overall, LASV seroprevalence was 23% (8/35) among the pregnant women tested (Table 1).
Table 1.
Laboratory findings for Abuja and Lagos, Nigeria, 2022
| Laboratory test | Overall | Abuja | Lagos | p-value | |||
|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | ||
| Lassa IgG antibody | < 0.0001 | ||||||
| Positive | 89 | (14) | 75 | (20) | 14 | (6) | |
| Indeterminate | 132 | (21) | 92 | (24) | 40 | (15) | |
| Negative | 407 | (65) | 209 | (55) | 198 | (79) | |
| Lassa IgM antibody | 0.0058 | ||||||
| Positive | 115 | (18) | 81 | (21) | 34 | (13) | |
| Indeterminate | 160 | (25) | 102 | (27) | 58 | (21) | |
| Negative | 353 | (56) | 193 | (51) | 160 | (63) | |
| Lassa IgG and IgM antibodies | < 0.0001 | ||||||
| Both negative | 270 | (43) | 130 | (35) | 140 | (56) | |
| Both indeterminate | 42 | (7) | 30 | (8) | 12 | (5) | |
| Both positive | 33 | (5) | 30 | (8) | 3 | (1) | |
| IgG negative, IgM indeterminate | 95 | (15) | 56 | (15) | 39 | (15) | |
| IgG negative, IgM positive | 42 | (7) | 23 | (6) | 19 | (7) | |
| IgG indeterminate, IgM negative | 50 | (8) | 34 | (9) | 16 | (6) | |
| IgG indeterminate, IgM positive | 40 | (6) | 28 | (7) | 12 | (5) | |
| IgG positive, IgM negative | 33 | (5) | 29 | (8) | 4 | (2) | |
| IgG positive, IgM indeterminate | 23 | (4) | 16 | (4) | 7 | (3) | |
| Lassa IgG or IgM antibodies | < 0.0001 | ||||||
| Positive | 171 | (27) | 126 | (33) | 45 | (18) | |
| Negative/indeterminate | 457 | (73) | 250 | (66) | 207 | (82) | |
| Rapid HIV test result | 0.3608 | ||||||
| Positive | 17 | (2) | 12 | (3) | 5 | (2) | |
| Negative | 611 | (97) | 364 | (97) | 247 | (98) | |
| Rapid malaria test result | < 0.0001 | ||||||
| Positive, P. falciparum | 47 | (7) | 46 | (12) | 1 | (0) | |
| Negative | 580 | (92) | 329 | (87) | 251 | (100) | |
| Not performed | 1 | (0) | 1 | (0) | 0 | (0) | |
| Hepatitis B surface antigen | 0.0004 | ||||||
| Positive | 56 | (9) | 30 | (8) | 26 | (10) | |
| Negative | 550 | (87) | 324 | (86) | 226 | (90) | |
| Missing | 22 | (4) | 22 | (6) | 0 | (0) | |
| Hepatitis C antibody | < 0.0001 | ||||||
| Positive | 45 | (7) | 40 | (11) | 5 | (2) | |
| Negative | 549 | (87) | 302 | (80) | 247 | (98) | |
| Indeterminate | 2 | (0) | 2 | (1) | 0 | (0) | |
| Missing | 32 | (5) | 32 | (9) | 0 | (0) | |
| Urine pregnancy | < 0.0001 | ||||||
| Positive | 35 | (6) | 27 | (7) | 8 | (3) | |
| Negative | 396 | (63) | 210 | (56) | 186 | (74) | |
| Missing | 4 | (1) | 4 | (1) | 0 | (0) | |
| Not applicable | 193 | (31) | 135 | (36) | 58 | (23) | |
Fig. 2.
Seroprevalence of Lassa IgG/IgM by month of sampling and by site, 2022
In Abuja, ethnicity, electricity in a residence, cleanliness and storage practices in the kitchen, seasonality, prior medical history of an upper respiratory tract infection (URI), and a positive malaria RDT were significantly associated with Lassa seroprevalence in unadjusted analyses (p < 0.05) (Tables 2, 3 and 4). Participants who reported ethnicities other than Hausa/Igbo/Yoruba had 33% lower seroprevalence of Lassa antibodies (PR, 95% CI: 0.67, 0.50–0.89) (Table 2). Participants without electricity in their residence had a 40% greater Lassa seroprevalence (PR, 95% CI: 1.40, 1.00-1.94) than did those with electricity (Table 2). Compared to participants who reported always cleaning their cooking environment or utensils after use, those who reported inconsistent or never cleaning had 40–44% greater prevalence of Lassa (PR, 95% CI: cooking environment 1.44, 1.04–2.01; cooking utensils 1.40, 1.02–1.93) (Table 3). Participants who reported inconsistently washing fruits and vegetables thoroughly before consumptions had a prevalence of Lassa that was 49% (PR, 95% CI: 1.49, 1.12–1.99) greater than participants who reported always washing fruits and vegetables before consumption (Table 3). Compared to participants who reported that they stored food without a cover, those who reported storing food with a cover had a 36% lower prevalence (PR, 95% CI: 0.64, 0.44–0.91) (Table 3). Participants enrolled in the dry season had 68% (PR, 95% CI: 1.68, 1.27–2.22) higher exposure to Lassa virus compared to those who were enrolled in the wet season (Table 3). No past or current symptoms were significantly associated with Lassa seroprevalence (p > 0.05) (Table 4). However, participants who had a self-reported medical history of URIs had a greater Lassa seroprevalence (PR, 95% CI: 1.83, 1.08–3.10) than did those who did not (Table 4). Additionally, participants who had a positive malaria RDT at enrollment had 60% (PR, 95% CI: 1.60, 1.15–2.22) greater Lassa seroprevalence compared to those who tested negative for malaria.
Table 2.
Prevalence of IgM/IgG antibodies to Lassa virus by select socio-demographic characteristics of participants recruited from Abuja and Lagos, Nigeria, 2022a
| Variable | Abuja | Lagos | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Positive | Prevalence | Unadjusted Prevalence Ratio | (95% CI) | p-value | Overall | Positive | Prevalence | Unadjusted Prevalence Ratio | (95% CI) | p-value | |||||
| N | % | N | % | % | N | % | N | % | % | |||||||
| Sex | ||||||||||||||||
| Female | 238 | (63) | 74 | (59) | 31.1 | Referent | — | — | 196 | (78) | 39 | (87) | 19.9 | Referent | — | — |
| Male | 138 | (37) | 52 | (41) | 37.7 | 1.21 | (0.91, 1.61) | 0.1878 | 56 | (22) | 6 | (13) | 10.7 | 0.54 | (0.24, 1.21) | 0.1325 |
| Age | ||||||||||||||||
| 18-24 | 84 | (22) | 27 | (21) | 32.1 | Referent | — | — | 17 | (7) | 1 | (2) | 5.9 | Referent | — | — |
| 25-29 | 58 | (15) | 17 | (13) | 29.3 | 0.91 | (0.55, 1.51) | 0.7210 | 22 | (9) | 7 | (16) | 31.8 | 5.41 | (0.73, 39.86) | 0.0976 |
| 30-39 | 102 | (27) | 32 | (25) | 31.4 | 0.98 | (0.64, 1.49) | 0.9105 | 62 | (25) | 12 | (27) | 19.4 | 3.29 | (0.46, 23.55) | 0.2356 |
| 40-49 | 54 | (14) | 23 | (18) | 42.6 | 1.33 | (0.85, 2.05) | 0.2085 | 65 | (26) | 10 | (22) | 15.4 | 2.62 | (0.36, 19.04) | 0.3425 |
| 50-59 | 52 | (14) | 23 | (18) | 44.2 | 1.38 | (0.89, 2.13) | 0.1508 | 49 | (19) | 10 | (22) | 20.4 | 3.47 | (0.48, 25.13) | 0.2182 |
| ≥ 60 | 26 | (7) | 4 | (3) | 15.4 | 0.48 | (0.18, 1.24) | 0.1299 | 37 | (15) | 5 | (11) | 13.5 | 2.3 | (0.29, 18.18) | 0.4307 |
| Current marital status | ||||||||||||||||
| Married | 262 | (70) | 96 | (76) | 36.6 | Referent | — | — | 197 | (78) | 37 | (82) | 18.8 | Referent | — | — |
| Other | 114 | (30) | 30 | (24) | 26.3 | 0.72 | (0.51, 1.02) | 0.0608 | 55 | (22) | 8 | (18) | 14.5 | 0.77 | (0.38, 1.56) | 0.4763 |
| Highest level of education | ||||||||||||||||
| Completed Secondary School (or above) | 179 | (48) | 61 | (48) | 34.1 | Referent | — | — | 155 | (62) | 26 | (58) | 16.8 | Referent | — | — |
| No Schooling or Less than Secondary School | 197 | (52) | 65 | (52) | 33.0 | 0.97 | (0.73, 1.29) | 0.8241 | 97 | (38) | 19 | (42) | 19.6 | 1.17 | (0.68, 1.99) | 0.5695 |
| Ethnicity | ||||||||||||||||
| Hausa, Igbo, or Yoruba | 89 | (24) | 40 | (32) | 44.9 | Referent | — | — | 226 | (90) | 37 | (82) | 16.4 | Referent | — | — |
| Other Ethnicity | 287 | (76) | 86 | (68) | 30.0 | 0.67 | (0.50, 0.89) | 0.0062 | 26 | (10) | 8 | (18) | 30.8 | 1.88 | (0.98, 3.59) | 0.0561 |
| Total household income per week | ||||||||||||||||
| <=7500 | 214 | (57) | 69 | (55) | 32.2 | Referent | — | — | 88 | (35) | 18 | (40) | 20.5 | Referent | — | — |
| >7500 | 162 | (43) | 57 | (45) | 35.2 | 1.09 | (0.82, 1.45) | 0.5486 | 164 | (65) | 27 | (60) | 16.5 | 0.80 | (0.47, 1.38) | 0.4284 |
| Type of residence currently being occupied | ||||||||||||||||
| Other | 117 | (31) | 38 | (30) | 32.5 | Referent | — | — | 0 | (0) | 0 | (0) | 0.00 | Referent | — | — |
| House or Apartment | 259 | (69) | 88 | (70) | 34.0 | 1.05 | (0.77, 1.43) | 0.7767 | 252 | (100) | 45 | (100) | 17.9 | — | — | |
| Number of rooms in current residence | ||||||||||||||||
| >1 Rooms | 273 | (73) | 86 | (68) | 31.5 | Referent | — | — | 151 | (60) | 28 | (62) | 18.5 | Referent | — | — |
| 1 Room | 103 | (27) | 40 | (32) | 38.8 | 1.23 | (0.91, 1.66) | 0.1700 | 101 | (40) | 17 | (38) | 16.8 | 0.91 | (0.53, 1.57) | 0.7288 |
| Number of years living in current residence | ||||||||||||||||
| Under 15 years | 243 | (65) | 87 | (69) | 35.8 | Referent | — | — | 198 | (79) | 35 | (78) | 17.7 | Referent | — | — |
| 15+ years | 133 | (35) | 39 | (31) | 29.3 | 0.82 | (0.60, 1.12) | 0.2113 | 54 | (21) | 10 | (22) | 18.5 | 1.05 | (0.56, 1.98) | 0.8858 |
| Number of other adults and children living in residence | ||||||||||||||||
| 0-4 | 113 | (30) | 39 | (31) | 34.5 | Referent | — | — | 142 | (56) | 22 | (49) | 15.5 | Referent | — | — |
| 5+ | 263 | (70) | 87 | (69) | 33.1 | 0.96 | (0.71, 1.30) | 0.7863 | 110 | (44) | 23 | (51) | 20.9 | 1.35 | (0.80, 2.29) | 0.2665 |
| Electricity in residence | ||||||||||||||||
| Yes | 317 | (84) | 100 | (79) | 31.5 | Referent | — | — | 242 | (96) | 43 | (96) | 17.8 | Referent | — | — |
| No | 59 | (16) | 26 | (21) | 44.1 | 1.40 | (1.00, 1.94) | 0.0471 | 10 | (4) | 2 | (4) | 20.0 | 1.13 | (0.32, 4.00) | 0.855 |
| Washing facilities in residence | ||||||||||||||||
| Yes | 132 | (35) | 43 | (34) | 32.6 | Referent | — | — | 167 | (66) | 29 | (64) | 17.4 | Referent | — | — |
| No | 244 | (65) | 83 | (66) | 34.0 | 1.04 | (0.77, 1.41) | 0.7783 | 85 | (34) | 16 | (36) | 18.8 | 1.08 | (0.62, 1.88) | 0.7745 |
| Water in residence | ||||||||||||||||
| Yes | 170 | (45) | 63 | (50) | 37.1 | Referent | — | — | 208 | (83) | 37 | (82) | 17.8 | Referent | — | — |
| No | 206 | (55) | 63 | (50) | 30.6 | 0.83 | (0.62, 1.10) | 0.1851 | 44 | (17) | 8 | (18) | 18.2 | 1.02 | (0.51, 2.04) | 0.9506 |
aPrevalence of antibodies to Lassa virus by other socio-demographic characteristics can be found in supplemental table 1
Table 3.
Prevalence of IgM/IgG antibodies to Lassa virus by select self-reported animal, and environmental exposure histories of participants recruited from Abuja and Lagos, Nigeria, 2022a
| Variable | Abuja | Lagos | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Positive | Prevalence | Unadjusted Prevalence Ratio | (95% CI) | p-value | Overall | Positive | Prevalence | Unadjusted Prevalence Ratio | (95% CI) | p-value | |||||
| N | % | N | % | % | N | % | N | % | % | |||||||
| Wet or dry season | ||||||||||||||||
| Wet | 252 | (67) | 69 | (55) | 27.4 | Referent | — | — | 69 | (27) | 14 | (31) | 20.3 | Referent | — | — |
| Dry | 124 | (33) | 57 | (45) | 46.0 | 1.68 | (1.27, 2.22) | 0.0002 | 183 | (73) | 31 | (69) | 16.9 | 0.83 | (0.47, 1.47) | 0.5329 |
| Works as unskilled laborer | ||||||||||||||||
| Yes | 27 | (7) | 13 | (10) | 48.1 | Referent | — | — | 25 | (10) | 5 | (11) | 20.0 | Referent | — | — |
| No | 349 | (93) | 113 | (90) | 32.4 | 0.67 | (0.44, 1.02) | 0.0639 | 227 | (90) | 40 | (89) | 17.6 | 0.88 | (0.38, 2.03) | 0.7657 |
| Contact with sick animal | ||||||||||||||||
| No | 298 | (79) | 99 | (79) | 33.2 | Referent | — | — | 221 | (88) | 35 | (78) | 15.8 | Referent | — | — |
| Yes | 78 | (21) | 27 | (21) | 34.6 | 1.04 | (0.74, 1.47) | 0.8153 | 31 | (12) | 10 | (22) | 32.3 | 2.04 | (1.12, 3.69) | 0.0189 |
| Place of food preparation | ||||||||||||||||
| Indoor | 198 | (53) | 62 | (49) | 31.3 | Referent | — | — | 173 | (69) | 34 | (76) | 19.7 | Referent | — | — |
| Outdoor | 97 | (26) | 38 | (30) | 39.2 | 1.25 | (0.91, 1.73) | 0.1735 | 39 | (15) | 3 | (7) | 7.70 | 0.39 | (0.13, 1.21) | 0.1032 |
| Indoor and Outdoor | 81 | (22) | 26 | (21) | 32.1 | 1.03 | (0.70, 1.50) | 0.8978 | 40 | (16) | 8 | (18) | 20.0 | 1.02 | (0.51, 2.03) | 0.9603 |
| Cleaning of cooking environment | ||||||||||||||||
| Always | 321 | (85) | 101 | (80) | 31.5 | Referent | — | — | 162 | (64) | 30 | (67) | 18.5 | Referent | — | — |
| Other | 55 | (15) | 25 | (20) | 45.5 | 1.44 | (1.04, 2.01) | 0.0296 | 90 | (36) | 15 | (33) | 16.7 | 0.90 | (0.51, 1.58) | 0.7141 |
| Cleaning of cooking utensils after use | ||||||||||||||||
| Always | 310 | (82) | 97 | (77) | 31.3 | Referent | — | — | 191 | (76) | 35 | (78) | 18.3 | Referent | — | — |
| Other | 66 | (18) | 29 | (23) | 43.9 | 1.40 | (1.02, 1.93) | 0.0367 | 61 | (24) | 10 | (22) | 16.4 | 0.89 | (0.47, 1.70) | 0.7335 |
| Handwashing with soap and water before cooking | ||||||||||||||||
| Always | 156 | (41) | 53 | (42) | 34.0 | Referent | — | — | 82 | (33) | 9 | (20) | 11.0 | Referent | — | — |
| Other | 220 | (59) | 73 | (58) | 33.2 | 0.98 | (0.73, 1.30) | 0.8724 | 170 | (67) | 36 | (80) | 21.2 | 1.93 | (0.98, 3.81) | 0.0586 |
| Washing of fruits and vegetables thoroughly before consumption | ||||||||||||||||
| Always | 284 | (76) | 85 | (67) | 29.9 | Referent | — | — | 204 | (81) | 36 | (80) | 17.6 | Referent | — | — |
| Other | 92 | (24) | 41 | (33) | 44.6 | 1.49 | (1.12, 1.99) | 0.0070 | 48 | (19) | 9 | (20) | 18.8 | 1.06 | (0.55, 2.05) | 0.8570 |
| Food preservation by sun drying by the roadside or other surfaces | ||||||||||||||||
| Yes | 121 | (32) | 33 | (26) | 27.3 | Referent | — | — | 45 | (18) | 5 | (11) | 11.1 | Referent | — | — |
| No | 255 | (68) | 93 | (74) | 36.5 | 1.34 | (0.96, 1.87) | 0.0872 | 207 | (82) | 40 | (89) | 19.3 | 1.74 | (0.73, 4.16) | 0.2136 |
| Food storage in container with cover | ||||||||||||||||
| Yes | 237 | (63) | 73 | (58) | 30.8 | Referent | — | — | 238 | (94) | 40 | (89) | 16.8 | Referent | — | — |
| No | 139 | (37) | 53 | (42) | 38.1 | 1.24 | (0.93, 1.65) | 0.1423 | 14 | (6) | 5 | (11) | 35.7 | 2.12 | (1.00, 4.53) | 0.0511 |
| Food storage in container without cover | ||||||||||||||||
| Yes | 36 | (10) | 18 | (14) | 50.0 | Referent | — | — | 8 | (3) | 1 | (2) | 12.5 | Referent | — | — |
| No | 340 | (90) | 108 | (86) | 31.8 | 0.64 | (0.44, 0.91) | 0.014 | 244 | (97) | 44 | (98) | 18.0 | 1.44 | (0.23, 9.2) | 0.6983 |
| Food storage inside locker | ||||||||||||||||
| Yes | 28 | (7) | 9 | (7) | 32.1 | Referent | — | — | 11 | (4) | 4 | (9) | 36.4 | Referent | — | — |
| No | 348 | (93) | 117 | (93) | 33.6 | 1.05 | (0.6, 1.83) | 0.8746 | 241 | (96) | 41 | (91) | 17.0 | 0.47 | (0.20, 1.07) | 0.0728 |
| Food storage in cellophane bags | ||||||||||||||||
| Yes | 63 | (17) | 19 | (15) | 30.2 | Referent | — | — | 24 | (10) | 6 | (13) | 25.0 | Referent | — | — |
| No | 313 | (83) | 107 | (85) | 34.2 | 1.13 | (0.76, 1.7) | 0.5452 | 228 | (90) | 39 | (87) | 17.1 | 0.68 | (0.32, 1.45) | 0.3211 |
| Food storage in sacs | ||||||||||||||||
| Yes | 206 | (55) | 66 | (52) | 32.0 | Referent | — | — | 34 | (13) | 6 | (13) | 17.6 | Referent | — | — |
| No | 170 | (45) | 60 | (48) | 35.3 | 1.10 | (0.83, 1.46) | 0.5051 | 218 | (87) | 39 | (87) | 17.9 | 1.01 | (0.46, 2.21) | 0.9726 |
| Food storage inside cupboard | ||||||||||||||||
| Yes | 47 | (13) | 19 | (15) | 40.4 | Referent | — | — | 60 | (24) | 10 | (22) | 16.7 | Referent | — | — |
| No | 329 | (88) | 107 | (85) | 32.5 | 0.80 | (0.55, 1.18) | 0.2623 | 192 | (76) | 35 | (78) | 18.2 | 1.09 | (0.58, 2.07) | 0.7838 |
| Food storage by unlisted method | ||||||||||||||||
| Yes | 23 | (6) | 7 | (6) | 30.4 | Referent | — | — | 6 | (2) | 3 | (7) | 50.0 | Referent | — | — |
| No | 353 | (94) | 119 | (94) | 33.7 | 1.11 | (0.59, 2.09) | 0.7523 | 246 | (98) | 42 | (93) | 17.1 | 0.34 | (0.15, 0.80) | 0.0128 |
| Contact with a sick person? | ||||||||||||||||
| No | 181 | (48) | 54 | (43) | 29.8 | Referent | — | — | 150 | (60) | 24 | (53) | 16.0 | Referent | — | — |
| Yes | 195 | (52) | 72 | (57) | 36.9 | 0.81 | (0.61, 1.08) | 0.1484 | 102 | (40) | 21 | (47) | 20.6 | 0.78 | (0.46, 1.32) | 0.3501 |
| Contact with sick person, dead or alive? | ||||||||||||||||
| No | 181 | (48) | 54 | (43) | 29.8 | Referent | — | — | 150 | 59.52 | 24 | 53.33 | 16.0 | Referent | — | — |
| Yes | 195 | (52) | 72 | (57) | 36.9 | 1.24 | (0.93, 1.65) | 0.1484 | 102 | 40.48 | 21 | 46.67 | 20.6 | 1.29 | (0.76, 2.18) | 0.3501 |
aPrevalence of antibodies to Lassa virus by other self-reported animal, and environmental exposure histories of participants can be found in supplemental table 2
Table 4.
Prevalence of IgM/IgG antibodies to Lassa virus by select self-reported medical and symptom history, and study laboratory results of participants recruited from Abuja and Lagos, Nigeria, 2022a
| Variable | Abuja | Lagos | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Positive | Prevalence | Unadjusted Prevalence Ratio | (95% CI) | p-value | Overall | Positive | Prevalence | Unadjusted Prevalence Ratio | (95% CI) | p-value | |||||
| N | % | N | % | % | N | % | N | % | % | |||||||
| Medical History | ||||||||||||||||
| High blood pressure | ||||||||||||||||
| No | 327 | (87) | 104 | (83) | 31.8 | Referent | — | — | 207 | (82) | 37 | (82) | 17.9 | Referent | — | — |
| Yes | 49 | (13) | 22 | (17) | 44.9 | 1.41 | (1.00, 2.00) | 0.0524 | 45 | (18) | 8 | (18) | 17.8 | 0.99 | (0.50, 1.99) | 0.9878 |
| Malaria requiring hospitalization or physician diagnosed | ||||||||||||||||
| No | 265 | (70) | 89 | (71) | 33.6 | Referent | — | — | 140 | (56) | 25 | (56) | 17.9 | Referent | — | — |
| Yes | 111 | (30) | 37 | (29) | 33.3 | 0.99 | (0.73, 1.36) | 0.9624 | 112 | (44) | 20 | (44) | 17.9 | 1.00 | (0.59, 1.70) | 1.0000 |
| Typhoid requiring hospitalization or physician diagnosed | ||||||||||||||||
| No | 324 | (86) | 114 | (90) | 35.2 | Referent | — | — | 212 | (84) | 40 | (89) | 18.9 | Referent | — | — |
| Yes | 52 | (14) | 12 | (10) | 23.1 | 0.66 | (0.39, 1.10 | 0.1103 | 40 | (16) | 5 | (11) | 12.5 | 0.66 | (0.28, 1.58) | 0.3515 |
| Symptom history | ||||||||||||||||
| Currently Sick | 1 | (0) | 0 | (0) | 0.0 | |||||||||||
| No | 319 | (85) | 110 | (87) | 34.5 | Referent | — | — | 192 | (76) | 34 | (76) | 17.7 | Referent | — | — |
| Yes | 56 | (15) | 16 | (13) | 28.6 | 0.83 | (0.53, 1.29) | 0.4032 | 60 | (24) | 11 | (24) | 18.3 | 1.04 | (0.56, 1.91) | 0.9120 |
| Upper respiratory tract infection | ||||||||||||||||
| No | 366 | (97) | 120 | (95) | 32.8 | Referent | — | — | 207 | (82) | 34 | (76) | 16.4 | Referent | — | — |
| Yes | 10 | (3) | 6 | (5) | 60.0 | 1.83 | (1.08, 3.10) | 0.0246 | 45 | (18) | 11 | (24) | 24.4 | 1.49 | (0.82, 2.71) | 0.1930 |
| Previous sore throat | ||||||||||||||||
| No | 366 | (97) | 124 | (98) | 33.9 | Referent | — | — | 230 | (91) | 39 | (87) | 17.0 | Referent | — | — |
| Yes | 10 | (3) | 2 | (2) | 20.0 | 0.59 | (0.17, 2.06) | 0.4077 | 22 | (9) | 6 | (13) | 27.3 | 1.61 | (0.77, 3.37) | 0.2081 |
| Previous rhinorrhea (runny nose) | ||||||||||||||||
| No | 363 | (97) | 119 | (94) | 32.8 | Referent | — | — | 171 | (68) | 22 | (49) | 12.9 | Referent | — | — |
| Yes | 13 | (3) | 7 | (6) | 53.8 | 1.64 | (0.97, 2.77) | 0.0636 | 81 | (32) | 23 | (51) | 28.4 | 2.21 | (1.31, 3.72) | 0.0029 |
| Any previous symptoms | ||||||||||||||||
| No | 124 | (33) | 39 | (31) | 31.5 | Referent | — | — | 8 | (3) | 1 | (2) | 12.5 | Referent | — | — |
| Yes | 252 | (67) | 87 | (69) | 34.5 | 1.10 | (0.80, 1.50) | 0.5564 | 244 | (97) | 44 | (98) | 18.0 | 1.44 | (0.23, 9.2) | 0.6983 |
| Laboratory Results | ||||||||||||||||
| Rapid HIV test | ||||||||||||||||
| Negative | 364 | (97) | 122 | (97) | 33.5 | Referent | — | — | 247 | (98) | 45 | (100) | 18.2 | Referent | — | — |
| Positive | 12 | (3) | 4 | (3) | 33.3 | 0.99 | (0.44, 2.24) | 0.9895 | 5 | (2) | 0 | (0) | 0.0 | 0 | (0, .) | 0.9998 |
| Rapid malaria test | ||||||||||||||||
| Negative | 330 | (88) | 103 | (82) | 31.2 | Referent | — | — | 251 | (100) | 44 | (98) | 17.5 | Referent | — | — |
| Positive, P. falciparum | 46 | (12) | 23 | (18) | 50.0 | 1.60 | (1.15, 2.22) | 0.0141 | 1 | (0) | 1 | (2) | 100.0 | 4.58 | (0, .) | 1.000 |
| Hepatitis B surface antigen | ||||||||||||||||
| Negative | 324 | (86) | 111 | (88) | 34.3 | Referent | — | — | 226 | (90) | 40 | (89) | 17.7 | Referent | — | — |
| Missing | 22 | (6) | 6 | (5) | 27.3 | 0.80 | (0.40, 1.60) | 0.5224 | — | — | — | |||||
| Positive | 30 | (8) | 9 | (7) | 30.0 | 0.88 | (0.50, 1.54) | 0.6342 | 26 | (10) | 5 | (11) | 19.2 | 1.09 | (0.47, 2.51) | 0.8458 |
| Hepatitis C antibody | ||||||||||||||||
| Negative | 302 | (80) | 105 | (83) | 34.8 | Referent | — | — | 247 | (98) | 44 | (98) | 17.8 | Referent | — | — |
| Indeterminate | 2 | (1) | 2 | (2) | 100.0 | 2.00 | (2.00, 2.00) | . | — | — | — | |||||
| Missing | 32 | (9) | 7 | (6) | 21.9 | 0.82 | (0.56, 1.19) | 0.2955 | — | — | ||||||
| Positive | 40 | (11) | 12 | (10) | 30.0 | 0.93 | (0.68, 1.27) | 0.6549 | 5 | (2) | 1 | (2) | 20.0 | 1.12 | (0.19, 6.61) | 0.8982 |
| Urine pregnancy | ||||||||||||||||
| Negative | 210 | (56) | 69 | (55) | 32.9 | Referent | — | — | 186 | (74) | 36 | (80) | 19.4 | Referent | — | — |
| Not applicable | 135 | (36) | 49 | (39) | 36.3 | 1.10 | (0.82, 1.48) | 0.5091 | 58 | (23) | 7 | (16) | 12.1 | 0.62 | (0.29, 1.33) | 0.2196 |
| Missing | 4 | (1) | 2 | (2) | 50.0 | 1.52 | (0.56, 4.13) | 0.4100 | — | — | — | |||||
| Positive | 27 | (7) | 6 | (5) | 22.2 | 0.68 | (0.33, 1.41) | 0.2948 | 8 | (3) | 2 | (4) | 25.0 | 1.29 | (0.38, 4.44) | 0.6848 |
aPrevalence of antibodies to Lassa virus by other self-reported medical and symptom histories can be found in supplemental table 3
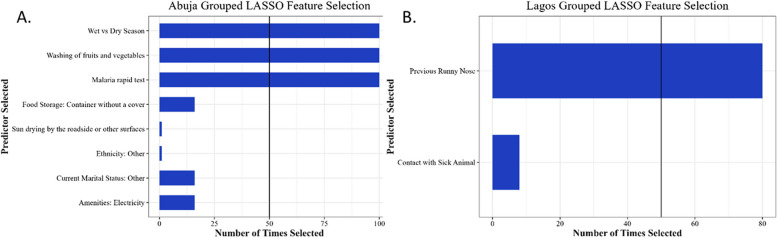
After variable down-selection by grouped LASSO regression and adjustment for other down-selected characteristics in the GLM, only dry season enrollment (aPR, 95% CI: 1.73, 1.33–2.24) compared to wet season, the practice of inconsistently washing fruits and vegetables before consumption (aPR, 95% CI: 1.45, 1.10–1.92), and a positive malaria test at enrollment (aPR, 95% CI: 1.48, 1.09-2.00) were independently associated with Lassa seroprevalence (Table 5). Although marital status, ethnicity, electricity in a residence, food preservation by sun drying on the roadside or other surfaces, and food storage without a cover were selected in the grouped LASSO, these variables were not included in the final multivariate model because they did not meet the > 50 selection criteria (Fig. 3A).
Table 5.
Lassa virus antibody (IgM/IgG) prevalence ratio by site (adjusted for covariates), Abuja and Lagos, Nigeria, 2022
| Variable | Comparison | Adjusted Prevalence Ratio | (95% CI) | p-value |
|---|---|---|---|---|
| Abuja | ||||
| Season | Dry vs. wet | 1.73 | (1.33, 2.24) | < 0.001 |
| Washing fruits and vegetables before consumption | Other vs. always | 1.45 | (1.10, 1.92) | 0.0085 |
| Rapid malaria test | Positive vs. negative | 1.48 | (1.09, 2.00) | 0.0112 |
| Lagosa | ||||
| Previous rhinorrhea (runny nose) | Yes vs. no | 2.21 | (1.31, 3.72) | 0.0029 |
aPrevalence ratio at Lagos was not adjusted for other covariates
Fig. 3.
A Variable selection for Abuja by LASSO regression analysis. B Variable selection for Lagos by LASSO regression analysis
In Lagos, contact with a sick animal, food storage methods, and prior self-reported rhinorrhea symptoms were significantly associated with Lassa seroprevalence in unadjusted analyses (p < 0.05) (Tables 2, 3 and 4). Participants with contact with a sick animal had at least a twofold greater (PR, 95% CI: 2.04, 1.12–3.69) seroprevalence than participants without contact with a sick animal (Table 3). Participants who reported having rhinorrhea (runny nose) in the past had more than two times greater (PR, 95% CI: 2.21, 1.31–3.72) Lassa seroprevalence than participants who did not report having a runny nose in the past (Table 4). In grouped LASSO regression (Table 5), only reported previous rhinorrhea was independently associated with Lassa seroprevalence, although contact with a sick animal was selected by grouped LASSO but did not meet the final selection criteria (> 50 times) (Fig. 3B).
Discussion
This community-based cross-sectional seroprevalence study was conducted to determine the extent of previous exposure to LASV and the risk factors associated with LASV infection. The overall seroprevalence was 27% and almost twofold greater in Abuja than in Lagos, with a prevalence of 33% and 18%, respectively. Seasonality, food washing before consumption, diagnosis of malaria at enrollment, and history of rhinorrhea were linked to LASV exposure.
The burden of LASV exposure estimated in this study is comparable to that in other reports from Nigeria. In 1988 the overall seroprevalence of LASV infection, measured by indirect immunofluorescence antibody testing, was estimated to be 21.3% (range of 13.4–37.5%) in the general population, hospital personnel and their contacts from areas such as Benue, Ondo, Plateau, and Gongola (present day Adamawa and Taraba states) in central, southwestern, and northeastern Nigeria [26]. A review of LF outbreaks occurring in Nigeria from 1952 to 2020 indicated that North-Central states (which include the study site of Abuja) experienced outbreaks for more years (an average of 11 years) compared to 6.8 years in South-Western states (including Lagos) [40]. Lassa virus has historically been found in the drier savannas of northern Nigeria [26]. However, LASV is prevalent in many countries in Africa with variations in population, exposure, and geographic region. A meta-analysis of 82 LASV prevalence studies in 25 sub-Saharan African countries revealed an overall prevalence of 8.7% (95% CI: 6.8–10.8%) with only West African countries having deaths due to LASV [41]. In meta-analysis, the prevalence of LASV was based on studies using various diagnostic tests, such as immunofluorescence, complement fixation, viral culture, RT-PCR, or ELISA, and included acute and convalescent samples.
The seasonality of LF is well known with outbreaks mirroring the ecology of the zoonotic reservoir, the Mastomys rat [42–44]. Mastomys populations flourish during the wet season, providing vegetation cover and facilitating increased reproduction [45]. Human land-use practices such as clearing land for planting and harvesting crops increase human-rodent contact. The resulting food scarcity during the dry season heightens human-rodent contact, by driving rodents to seek nourishment inside human homes thereby increasing exposure to Lassa virus. This may explain the observed increase in prevalence of Lassa among participants enrolled in the dry season (versus the wet season) in Abuja compared to Lagos, where the wet and dry seasons are less distinct.
Food safety may be more of a concern for geographical areas where human land-use practices support rodent populations in homes. In Abuja, food hygiene practices (washing of fruits and vegetables) were associated with LASV seropositivity, which could be due to heightened contact with zoonotic vectors from seasonal variations in animal vector populations. However, in both cities, surprisingly, there was no connection between exposure to rodents (presence, contact, droppings, consumption, bites) and LASV infection. Unintentional and unsought contact with animal excreta has been associated with LASV seropositivity in cross-sectional population-based studies in Nigeria and Guinea. Houses with poor hygiene scores studied in a peri-urban settlement in Edo State in southern Nigeria had 50 times greater odds of reporting cases of LF than did houses with good hygiene scores [46]. In Guinea, uncovered food storage along with other factors was associated with increased LASV seropositivity [47]. Interestingly, in a cross-sectional LASV seroprevalence study in forested regions of Guinea, Kernéis et al. did not find that contact with rats or mice was a major risk factor [48]. Instead, two risk factors were identified: receiving an injection in the past year and living with someone who had bleeding symptoms. The investigators hypothesized that person-to-person transmission, perhaps in healthcare settings or close household contact, might be more important than previously thought. Although food hygiene practices and certain living conditions may be associated with the risk of LF infection, the role of rodents in transmission remains unclear. This finding might be due to limitations of our observational study design, and the established route of transmission through Mastomys rodents should not be discounted. The observed association between food hygiene and LASV seropositivity may be due to an indirect effect of food attracting rodents, rather than direct contact with them [49, 50].
Self-reported rhinorrhea was independently associated with Lassa infection in Lagos, but not in Abuja. Similar associations along with other indistinct symptoms such as fever, pharyngitis, and a clinical presentation with general systemic, respiratory or gastrointestinal symptoms have been reported in other LF studies conducted in West Africa [51, 52]. In a retrospective study analyzing surveillance data from Lassa patients identified in 2018–2019 from all 36 states and FCT in Nigeria, clinical presentations with general systemic, chest/respiratory, ear/nose/throat, or gastrointestinal symptoms were associated with laboratory-confirmed Lassa diagnoses as were occupations in business, trading, farming or agriculture, and male sex [53]. Since LASV infection does not have characteristic symptoms, rhinorrhea and other nonspecific symptoms can be symptoms of LF infection as well as any other respiratory illnesses that occur in the region.
Malaria (Plasmodium falciparum) diagnosis at enrollment was independently associated with Lassa seropositivity in Abuja but not in Lagos. This may represent an incidental association since risk factors for malaria in Abuja likely overlap with those for Lassa infection, despite seasonal variation in malaria burden with higher prevalence in the wet season [54]. Notably, a prior study conducted in Southern Nigeria, reported a high prevalence (37%) of co-infection with malaria in LF patients, but no statistically significant impact of malaria on LF outcome was observed [55]. Risk factors for malaria include poverty, less education, and poor housing conditions [56, 57]. People with lower socioeconomic status likely have limited access to preventive measures and live in housing that is not properly sealed or screened allowing mosquitoes to enter more easily, thus increasing the risk of malaria infection. Further investigation is needed to determine whether the observed association between malaria diagnosis and Lassa seropositivity in Abuja is due to confounding factors, such as socioeconomic status, which can influence both malaria and LF risk.
Our study has a few limitations. The cross-sectional design and reliance on self-reported risk factors limit our ability to definitively establish the temporal relationships between exposures and Lassa infection. Additionally, the lack of Lassa antigen/RNA testing prevents differentiation between acute/recent and past infections. Consequently, the observed associations might include a combination of both types of infections. Furthermore, indeterminate results, potentially due to early infection, low-level antibody presence, non-specific cross-reacting antibodies, or technical variability, were combined with negative results, which may underestimate the true prevalence of Lassa virus exposure. Similarly, while the ReLASV® Pan-Lassa Combo NP/ Prefusion GP IgG/IgM ELISA Kit is designed to detect a wide range of Lassa virus infections, it is important to note that four lineages (I-III and VI/Kako strain) have been identified in Nigeria [30], which could potentially impact the assay’s sensitivity and specificity. Future studies may benefit from incorporating additional assays targeting these specific lineages. While this study observed a trend of higher LF seroprevalence among participants recruited during the dry season, the limited recruitment window (February 2nd, 2022 – July 5th, 2022) likely restricts definitive conclusions regarding seasonality and necessitates further investigation across a full annual cycle to capture potential peak and trough periods. The voluntary nature of the study and purposive selection of LGAs raises concerns about its generalizability to broader LGA communities. Participation may be skewed toward individuals with a history of LF, those motivated by the offered compensation, or those who found participation convenient due to a coinciding healthcare facility visit. Future research could explore a broader range of LGAs and randomized selection of participants to enhance generalizability. Finally, restricting the study to adults only provides an incomplete picture of LASV exposure, transmission dynamics, and risk factors, potentially leading to skewed findings. Children may play a role in transmission within households and communities and may have unique risk factors for LASV infection due to their behavior, immune system development, or reliance on caregivers who might be exposed.
Conclusions
Although LASV has long been endemic to countries in West Africa, it is of global consequence due to the ease of international travel and the potential for the use of LASV as a biological weapon. This study fills a knowledge gap for two major metropolitan areas in Nigeria where LASV exposure was previously unknown. By highlighting priority populations, geographic areas, and preexisting immunity levels, our findings can inform LASV vaccine research and development, and vaccine design and testing strategies. Study findings reinforce prior literature on limiting human-rodent contact to prevent LASV transmission, although our findings focus on behavioral factors such as poor hygiene. Since proper food hygiene protects against various infectious pathogens, not just LASV, educational programs should emphasize this practice for broader public health benefits.
Supplementary Information
Acknowledgements
The authors thank Sandhya Vasan, MD, and Nelson L. Michael, MD, PhD for their mentorship and leadership throughout this project and for their continued support of this work.
EID023 Lassa study team included the following members:
Adefunke Oladipo-Opashina1, Alexus Reynolds5,6, Austin Anikwe1, Bahar Dastgheib2,6, Blessing I. Wilson3,4, Bryce Boron5,6, Bwalya Chama5,6, Daniel Choi5,6, Edward Bloom1, Ekenedirichukwu Okoli1, Gereme Bandong2,6, Helen Nwandu1, Igiri Faith3,4, Jenny Lay2,6, Jumoke T. Nwalozie3,4, Lawrence C. Umeji1, Mekdi Taddese2,6, Mihret Amare2,6, Michelle Imbach5,6, Nkiru Nnadi3,4, Oyerinde Olunsanya4, Sunday Odeyemi1, Susan T. Mason5,6, Zubairu Elayo1.
Disclaimer
The opinions or assertions contained herein are the private views of the author, and are not to be construed as official, or as reflecting true views of the Department of the Army or the Defense Health Agency or the Department of Defense. The investigators have adhered to the policies for protection of human subjects as prescribed in AR 70–25.
Abbreviations
- AMAC
Abuja Municipal Area Council
- anti-HCV
Antibodies to hepatitis C virus
- aPRs
Adjusted prevalence ratios
- CA
California
- CI
Confidence interval
- CRC
Clinical Research Centre
- DRL
Defence Reference Laboratory
- ELISA
Enzyme-linked immunosorbent assay
- FCT
Federal Capital Territory
- GLM
Generalized linear model
- GP
Glycoprotein
- HBV
Hepatitis B virus
- HCV
Hepatitis C virus
- HIV
Human immunodeficiency virus
- IgG
Immunoglobulin G
- IgM
Immunoglobulin M
- Inc
Incorporated
- IQR
Interquartile range
- LASSO
Least Absolute Shrinkage Selection Operator
- LASV
Lassa virus
- LF
Lassa fever
- LGA
Ikorodu Local Government Area
- MCVE
Minimum cross validation error
- ₦
Naira
- NARHY
Nigerian Army Reference Hospital
- NP
Nucleoprotein
- OD
Optical density
- PHC
Primary healthcare center
- Plc
Place
- PR
Prevalence ratio
- RDT
Rapid diagnostic test
- RNA
Ribonucleic acid
- RT-PCR
Reverse transcriptase polymerase chain reaction
- RUO
Research use only
- sAg
Hepatitis B surface antigen
- USA
United States of America
- USD
United States dollar
- VHF
Viral hemorrhagic fever
- WHO
World Health Organization
Authors’ contributions
KM, MOI, ABT, LAE, PP, and ZFP initiated the study. ABT, AZ, EB, DB, KM, LAE, MM, MOI, OA, ORA, PP, SSM, SH, TM, YF, and ZFP designed the research. MJ created new software used in the study. ABT, AO, CA, CE, DE, EI, FA, JF, MA, MM, MOI, NA, NDC, NO, OA, ORA, PD, RA, SSM, TA, VA, YA gathered the data. KL, KM, LAE, NDC, NO, PP, and RA analyzed laboratory or other data. SH, OF, SF, and GS statistically analyzed all data presented in the manuscript. ABT, GS, KL, KM, LAE, NDC, VA, and SH interpreted the data. SH wrote the manuscript and MOI, ABT, AZ, EB, JF, LAE, MA, OA, ON, ORA, PD, SSM, TM, and ZFP contributed to revisions. All authors read and approved the final manuscript.
Funding
Financial support for this study was provided by the Military Infectious Diseases Research Program (MIDRP) and executed through a cooperative agreement (W81XWH-18-2-0040) with the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc.
Availability of data and materials
The anonymized data used in this study are publicly available from the Harvard Dataverse online data repository: https://dataverse.harvard.edu/dataset.xhtml?persistentId=doi:10.7910/DVN/9TN21Y.
Declarations
Ethics approval and consent to participate
Ethical approval was obtained from the Walter Reed Army Institute of Research Institutional Review Board in the USA and the National Health Research Ethics Committee in Nigeria. Administrative approval was obtained from the Federal Capital Territory/Abuja Municipal Area Council and Lagos State/Ikorodu Local Government Area Primary Healthcare Boards and community stakeholders. Written informed consent was obtained from each participant before any study procedures were conducted. The informed consent form was reviewed with volunteers in detail by trained and delegated study staff before written consent was obtained.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shilpa Hakre, Email: shakre@eidresearch.org.
EID023 Lassa study team:
Adefunke Oladipo-Opashina, Alexus Reynolds, Austin Anikwe, Bahar Dastgheib, Blessing I. Wilson, Bryce Boron, Bwalya Chama, Daniel Choi, Edward Bloom, Ekenedirichukwu Okoli, Gereme Bandong, Helen Nwandu, Igiri Faith, Jenny Lay, Jumoke T. Nwalozie, Lawrence C. Umeji, Mekdi Taddese, Mihret Amare, Michelle Imbach, Nkiru Nnadi, Oyerinde Olunsanya, Sunday Odeyemi, Susan T. Mason, and Zubairu Elayo
References
- 1.Pfau CJ. Arenaviruses. In: Baron S, editors. Medical Microbiology. Galveston (TX): University of Texas Medical Branch at Galveston; 1996. Chapter: 57. https://www.ncbi.nlm.nih.gov/books/NBK8193/.
- 2.Auperin DD, Romanowski V, Galinski M, Bishop DH. Sequencing studies of pichinde arenavirus S RNA indicate a novel coding strategy, an ambisense viral S RNA. J Virol. 1984;52(3):897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frame JD, Baldwin JM Jr., Gocke DJ, Troup JM. Lassa fever, a new virus disease of man from West Africa. I. Clinical description and pathological findings. Am J Trop Med Hyg. 1970;19(4):670–6. [DOI] [PubMed] [Google Scholar]
- 4.Ogbu O, Ajuluchukwu E, Uneke CJ. Lassa fever in west African sub-region: an overview. J Vector Borne Dis. 2007;44(1):1–11. [PubMed] [Google Scholar]
- 5.Asogun DA, Adomeh DI, Ehimuan J, Odia I, Hass M, Gabriel M, Olschlager S, Becker-Ziaja B, Folarin O, Phelan E, et al. Molecular diagnostics for lassa fever at Irrua specialist teaching hospital, Nigeria: lessons learnt from two years of laboratory operation. PLoS Negl Trop Dis. 2012;6(9):e1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andersen KG, Shapiro BJ, Matranga CB, Sealfon R, Lin AE, Moses LM, Folarin OA, Goba A, Odia I, Ehiane PE, et al. Clinical sequencing uncovers origins and Evolution of Lassa Virus. Cell. 2015;162(4):738–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, et al. The global distribution and burden of dengue. Nature. 2013;496(7446):504–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richmond JK, Baglole DJ. Lassa fever: epidemiology, clinical features, and social consequences. BMJ. 2003;327(7426):1271–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts L. A spiking fever. Science. 2024;383(6685):810–6. [DOI] [PubMed] [Google Scholar]
- 10.Balogun OO, Akande OW, Hamer DH. Lassa Fever: an evolving emergency in West Africa. Am J Trop Med Hyg. 2020;104(2):466–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lassa Fever. [https://www.who.int/teams/blueprint/lassa-fever]
- 12.Abdulkarim MA, Babale SM, Umeokonkwo CD, Bamgboye EA, Bashorun AT, Usman AA, Balogun MS. Epidemiology of Lassa fever and Factors Associated with deaths, Bauchi State, Nigeria, 2015–2018. Emerg Infect Dis. 2020;26(4):799–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monath TP, Newhouse VF, Kemp GE, Setzer HW, Cacciapuoti A. Lassa virus isolation from Mastomys natalensis rodents during an epidemic in Sierra Leone. Science. 1974;185(4147):263–5. [DOI] [PubMed] [Google Scholar]
- 14.Lassa Fever, Transmission. [https://www.cdc.gov/vhf/lassa/transmission/index.html]
- 15.Lassa fever, Factsheet. [https://www.afro.who.int/health-topics/lassa-fever#:~:text=Lassa%20virus%20infections%20can%20only,antigen%20detection%20tests].
- 16.Khan SH, Goba A, Chu M, Roth C, Healing T, Marx A, Fair J, Guttieri MC, Ferro P, Imes T, et al. New opportunities for field research on the pathogenesis and treatment of Lassa fever. Antiviral Res. 2008;78(1):103–15. [DOI] [PubMed] [Google Scholar]
- 17.McCormick JB, Webb PA, Krebs JW, Johnson KM, Smith ES. A prospective study of the epidemiology and ecology of Lassa fever. J Infect Dis. 1987;155(3):437–44. [DOI] [PubMed] [Google Scholar]
- 18.Kayem ND, Benson C, Aye CYL, Barker S, Tome M, Kennedy S, Ariana P, Horby P. Lassa fever in pregnancy: a systematic review and meta-analysis. Trans R Soc Trop Med Hyg. 2020;114(5):385–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isaac AB, Karolina W, Temitope AA, Anuska R, Joanne E, Deborah A, Bianca OC, Filip T, Zofia P, Oluwasegun OI, et al. Prospects of Lassa Fever candidate vaccines. Afr J Infect Dis. 2022;16(2 Suppl):46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Demby AH, Chamberlain J, Brown DW, Clegg CS. Early diagnosis of Lassa fever by reverse transcription-PCR. J Clin Microbiol. 1994;32(12):2898–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jahrling PB, Niklasson BS, McCormick JB. Early diagnosis of human lassa fever by ELISA detection of antigen and antibody. Lancet. 1985;1(8423):250–2. [DOI] [PubMed] [Google Scholar]
- 22.Branco LM, Grove JN, Boisen ML, Shaffer JG, Goba A, Fullah M, Momoh M, Grant DS, Garry RF. Emerging trends in Lassa fever: redefining the role of immunoglobulin M and inflammation in diagnosing acute infection. Virol J. 2011;8:478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ter Meulen J, Koulemou K, Wittekindt T, Windisch K, Strigl S, Conde S, Schmitz H. Detection of Lassa virus antinucleoprotein immunoglobulin G (IgG) and IgM antibodies by a simple recombinant immunoblot assay for field use. J Clin Microbiol. 1998;36(11):3143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grace JA, Egoh IJ, Udensi N. Epidemiological trends of Lassa fever in Nigeria from 2015–2021: a review. Ther Adv Infect Dis. 2021;8:20499361211058252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dalhat MM, Olayinka A, Meremikwu MM, Dan-Nwafor C, Iniobong A, Ntoimo LF, Onoh I, Mba S, Ohonsi C, Arinze C, et al. Epidemiological trends of Lassa fever in Nigeria, 2018–2021. PLoS ONE. 2022;17(12):e0279467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomori O, Fabiyi A, Sorungbe A, Smith A, McCormick JB. Viral hemorrhagic fever antibodies in Nigerian populations. Am J Trop Med Hyg. 1988;38(2):407–10. [DOI] [PubMed] [Google Scholar]
- 27.Bassey O, Bond K, Adedeji A, Oke O, Abubakar A, Yakubu K, Jelpe T, Akintunde E, Ikani P, Ogundiran A, et al. Evaluation of nine HIV rapid test kits to develop a national HIV testing algorithm in Nigeria. Afr J Lab Med. 2015;4(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.ReLASV® P-L, Combo NP, Prefusion. GP IgG/IgM ELISA Kit (Human anti-LASV NP and GP Antibodies) [https://zalgen.com/wp-content/uploads/PI-35R-04b-ReLASV-Pan-Lassa-Combo-NP_GP-GM-ELISA-Kit-RUO.pdf]
- 29.Heinrich ML, Boisen ML, Nelson DKS, Bush DJ, Cross RW, Koval AP, Hoffmann AR, Beddingfield BJ, Hastie KM, Rowland MM, et al. Antibodies from Sierra Leonean and Nigerian lassa fever survivors cross-react with recombinant proteins representing Lassa viruses of divergent lineages. Sci Rep. 2020;10(1):16030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whitmer SLM, Strecker T, Cadar D, Dienes HP, Faber K, Patel K, Brown SM, Davis WG, Klena JD, Rollin PE, et al. New Lineage of Lassa Virus, Togo, 2016. Emerg Infect Dis. 2018;24(3):599–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.News Release 12-Sep-2017. Joint West Africa Research Group begins infectious disease surveillance study in Nigeria. [https://www.eurekalert.org/news-releases/657644.]
- 32.McCauley M, Parker Z, Iroezindu M, Tiamiyu AB, Akinwale E, Paudel M, Milazzo M, Liu H, Chambers J, Bartolanzo D, Li Q, Harrison NE, Parwon Z, Nyarko E, Happi C, Lombardi K, Eller LA, Nosamiefan I, Asiedu W, Broach E, Mebrathu T, Hakre S, Headley J, Prins P, Adams P, Ake J, Amare M, Koehler J, Ricks K, Schoepp R, Ampofo W, Sahnoon H, Fallah M, Bolay F. Letizia A, Sanders T, Fox A, Diclaro J, Vasan S, Michael N, Modjarrad K, Collins N. Seroprevalence of vector-borne and hemorrhagic fever viruses among a cross-section of adult ambulatory and hospitalized patients with severe illness in Nigeria, Ghana, and Liberia, 2017–2022. Kissimmee: Military Health System Research Symposium (MHSRS); 2023.
- 33.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inf. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Behrens T, Taeger D, Wellmann J, Keil U. Different methods to calculate effect estimates in cross-sectional studies. A comparison between prevalence odds ratio and prevalence ratio. Methods Inf Med. 2004;43(5):505–9. [PubMed] [Google Scholar]
- 36.Chen Y, Yang Y. The one Standard Error Rule for Model Selection: does it work? Stats vol. 2021;4:868–92. [Google Scholar]
- 37.Miller RE, Breheny P. Marginal false discovery rate control for likelihood-based penalized regression models. Biom J. 2019;61(4):889–901. [DOI] [PubMed] [Google Scholar]
- 38.Kuhn M, Kjell J. Applied Predictive modeling. New York: Springer. 2013;26:13.
- 39.Frndak S, Yu G, Oulhote Y, Queirolo EI, Barg G, Vahter M, Manay N, Peregalli F, Olson JR, Ahmed Z, et al. Reducing the complexity of high-dimensional environmental data: an analytical framework using LASSO with considerations of confounding for statistical inference. Int J Hyg Environ Health. 2023;249:114116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Agbonlahor DE, Akpede GO, Happi CT, Tomori O. 52 years of Lassa Fever outbreaks in Nigeria, 1969–2020: an epidemiologic analysis of the temporal and spatial trends. Am J Trop Med Hyg. 2021;105(4):974–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kenmoe S, Tchatchouang S, Ebogo-Belobo JT, Ka’e AC, Mahamat G, Guiamdjo Simo RE, Bowo-Ngandji A, Demeni Emoh CP, Che E, Tchami Ngongang D, et al. Systematic review and meta-analysis of the epidemiology of Lassa virus in humans, rodents and other mammals in sub-saharan Africa. PLoS Negl Trop Dis. 2020;14(8):e0008589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fichet-Calvet E, Lecompte E, Koivogui L, Soropogui B, Dore A, Kourouma F, Sylla O, Daffis S, Koulemou K, Ter Meulen J. Fluctuation of abundance and Lassa virus prevalence in Mastomys natalensis in Guinea, West Africa. Vector Borne Zoonotic Dis. 2007;7(2):119–28. [DOI] [PubMed] [Google Scholar]
- 43.Gibb R, Moses LM, Redding DW, Jones KE. Understanding the cryptic nature of Lassa fever in West Africa. Pathog Glob Health. 2017;111(6):276–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Redding DW, Gibb R, Dan-Nwafor CC, Ilori EA, Yashe RU, Oladele SH, Amedu MO, Iniobong A, Attfield LA, Donnelly CA, et al. Geographical drivers and climate-linked dynamics of Lassa fever in Nigeria. Nat Commun. 2021;12(1):5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leirs H, Verhagen R, Verheyen W. The basis of reproductive seasonally in Mastomys rats (Rodentia: Muridae) in Tanzania. J Trop Ecol. 1994;10(1):55–66. [Google Scholar]
- 46.Ochei O, Abjejegah C, Okoh EC, Abah SO. Housing factors and transmission of Lassa fever in a rural area of south Nigeria. Gen Health Med Sci. 2014;1:15–20. [Google Scholar]
- 47.Ter Meulen J, Lukashevich I, Sidibe K, Inapogui A, Marx M, Dorlemann A, Yansane ML, Koulemou K, Chang-Claude J, Schmitz H. Hunting of peridomestic rodents and consumption of their meat as possible risk factors for rodent-to-human transmission of Lassa virus in the Republic of Guinea. Am J Trop Med Hyg. 1996;55(6):661–6. [DOI] [PubMed] [Google Scholar]
- 48.Kerneis S, Koivogui L, Magassouba N, Koulemou K, Lewis R, Aplogan A, Grais RF, Guerin PJ, Fichet-Calvet E. Prevalence and risk factors of Lassa seropositivity in inhabitants of the forest region of Guinea: a cross-sectional study. PLoS Negl Trop Dis. 2009;3(11):e548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fichet-Calvet E. Chapter 5 - Lassa Fever: A rodent-human interaction. In: Johnson N, editor. The Role of Animals in Emerging Viral Diseases. Boston: Academic Press: 2014, p 89–123. 10.1016/B978-0-12-405191-1.00005-3.
- 50.Clark J, Yakob L, Douno M, Lamine J, Magassouba N, Fichet-Calvet E, Mari-Saez A. Domestic risk factors for increased rodent abundance in a Lassa fever endemic region of rural Upper Guinea. Sci Rep. 2021;11(1):20698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Olayinka AT, Elimian K, Ipadeola O, Dan-Nwafor C, Gibson J, Ochu C, Furuse Y, Iniobong A, Akano A, Enenche L, et al. Analysis of sociodemographic and clinical factors associated with Lassa fever disease and mortality in Nigeria. PLOS Glob Public Health. 2022;2(8):e0000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCormick JB, King IJ, Webb PA, Johnson KM, O’Sullivan R, Smith ES, Trippel S, Tong TC. A case-control study of the clinical diagnosis and course of Lassa fever. J Infect Dis. 1987;155(3):445–55. [DOI] [PubMed] [Google Scholar]
- 53.Olayinka AT, Nwafor CD, Akano A, Jan K, Ebhodaghe B, Elimian K, Ochu C, Okwor T, Ipadeola O, Ukponu W, et al. Research as a pillar of Lassa fever emergency response: lessons from Nigeria. Pan Afr Med J. 2020;37:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Segun OE, Shohaimi S, Nallapan M, Lamidi-Sarumoh AA, Salari N. Statistical modelling of the effects of Weather factors on Malaria occurrence in Abuja, Nigeria. Int J Environ Res Public Health. 2020;17(10). 10.3390/ijerph17103474. [DOI] [PMC free article] [PubMed]
- 55.Okokhere P, Colubri A, Azubike C, Iruolagbe C, Osazuwa O, Tabrizi S, Chin E, Asad S, Ediale E, Rafiu M, et al. Clinical and laboratory predictors of Lassa fever outcome in a dedicated treatment facility in Nigeria: a retrospective, observational cohort study. Lancet Infect Dis. 2018;18(6):684–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Silva PM, Marshall JM. Factors contributing to urban malaria transmission in sub-saharan Africa: a systematic review. J Trop Med. 2012;2012:819563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chiziba C, Mercer LD, Diallo O, Bertozzi-Villa A, Weiss DJ, Gerardin J, Ozodiegwu ID. Socioeconomic, demographic, and Environmental Factors May Inform Malaria Intervention Prioritization in Urban Nigeria. Int J Environ Res Public Health. 2024;21(1). 10.3390/ijerph21010078. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The anonymized data used in this study are publicly available from the Harvard Dataverse online data repository: https://dataverse.harvard.edu/dataset.xhtml?persistentId=doi:10.7910/DVN/9TN21Y.