Abstract
Breast cancer is the leading cancer diagnosed among women and environmental studies have produced few leads on modifiable risk factors. Following an Institute of Medicine recommendation for occupational studies of highly exposed women, we took advantage of an existing cohort of 4,503 female hourly autoworkers in Michigan exposed to metalworking fluid (MWF), complex mixtures of oils and chemicals widely used in metal manufacturing worldwide. Cox proportional hazards models were fit to estimate hazard ratios (HR) for incident breast cancer (follow-up 1985–2013) and cumulative exposure (20-year lag) to straight mineral oils (a known human carcinogen), and water-based soluble and synthetic MWF. Because the state cancer registry began decades after the cohort was defined, we restricted analyses to sub-cohorts hired closer to the start of follow-up. Among those hired after 1969, the HR associated with a one interquartile range increase in straight MWF exposure was 1.13 (95% confidence interval: 1.03, 1.23). In separate analyses of premenopausal breast cancer, defined by age at diagnosis, the HR was elevated for exposure to synthetic MWF, chemical lubricants with no oil content, possibly suggesting a different mechanism for the younger cases. This study adds to the limited literature regarding quantitative chemical exposures and breast cancer risk.
Keywords: Breast cancer, Metalworking fluid, Occupational exposure
Breast cancer is the leading cancer diagnosed among women in the United States (1). It is estimated that in 2016 there will be 246,660 new cases and 40,450 deaths attributable to breast cancer among women in the United States (2). Despite the large impact of this disease, well-established risk factors are estimated to account for only 41% of breast cancer cases (3) and studies of environmental exposures and breast cancer risk have produced few additional leads on modifiable risk factors over the past 20 years (4). A 2012 Institute of Medicine review of environmental risk factors for breast cancer noted that the initial identifications of many known human carcinogens were based on studies of high exposures in occupational settings and recommended additional breast cancer studies of worker populations (4).
Despite provocative evidence that metalworking fluid (MWF) contaminants cause mammary gland tumors in laboratory animals (5) and that occupational exposure levels are appreciable, few studies have been conducted on breast cancer risk. MWF are coolants and lubricants widely used in industrial machining and grinding operations, and are categorized into three classes based on composition: straight, soluble, and synthetic. Straight MWF are complex mixtures of paraffinic, naphthenic, and aromatic compounds refined from mineral oil (6). The carcinogenic properties of mineral oil, classified as carcinogenic to humans (7), are thought to be primarily due to their polycyclic aromatic hydrocarbons (PAH) content (8). Soluble MWF are oils emulsified in water. Synthetic fluids are water-soluble chemical lubricants without oil. Ethanolamines and nitrites, added to synthetic MWF to inhibit corrosion and adjust pH, interact to form nitrosamines. Although no toxicologic studies have specifically examined the effects of MWF and breast carcinogenic effects, MWF components including PAH and nitrosamines have been implicated as mammary gland carcinogens (5). Additionally, studies on ambient air PAH exposure are suggestive of increased breast cancer risk (9, 10) and nitrosamines are suspected endocrine disruptors (11). Women comprise a growing proportion of the 4.4 million potentially exposed U.S. workers (12), as well as of the growing workforce employed in metal manufacturing worldwide. The global manufacturing workforce is estimated to be about 30% female (13). The global market volume of MWF in 2013 was 2.3 million tons; Asia had the largest share, 41.5%, compared to North America, 28.0% (14).
Few epidemiologic studies on breast cancer risk and MWF exposure exist (15–22). An early study observed reduced breast cancer mortality among women in automotive manufacturing based on standardized mortality ratios (15, 16). More recently, case-control studies reported elevated breast cancer risk associated with occupation in automotive manufacturing and metal products/metal work (19–21). These studies, however, did not examine a specific compound, but instead used occupation as a proxy for exposure. Three breast cancer studies have been conducted in the United Autoworker-General Motors (UAW-GM) cohort; two were limited in power and relied on either a combination of incident cases and deaths (17) or only deaths (22). The one study to examine only incident breast cancer cases reported slightly elevated risk for straight MWF (23). We took advantage of this large existing cohort of female hourly autoworkers exposed to MWF to examine the relationship between quantitative MWF exposure and breast cancer incidence. We have nine additional years of follow-up and restricted our analysis to registry-identified incident cases. The aim of this study was to examine the exposure-response relationship between cumulative MWF exposure and breast cancer risk in a cohort of occupationally-exposed female autoworkers.
METHODS
Study population
The UAW-GM cohort was a joint labor-management funded study designed in 1984 to examine cancer mortality and its relation to MWF exposure. This cohort has been described elsewhere (24, 25). Briefly, the original study included 46,316 hourly workers from one of three automobile manufacturing plants in Michigan exposed to MWF primarily via inhalation (24). All hourly employees who had worked at least 3 years prior to January 1, 1985 were included in the cohort. Subjects alive on January 1, 1985 (N=33,915) when the Michigan Cancer Registry began were included in the incidence cohort. Analyses for the present study were restricted to female workers in the incidence cohort (N = 4,572). We excluded women who were missing more than 50% of their employment history (N = 59) or were hired prior to 1938 (N = 5). This latter restriction was imposed to address potential left truncation bias in the main cohort (26). The final study population comprised 4,503 female workers.
Outcome assessment
The UAW-GM incidence cohort was linked with the Michigan Cancer Registry to identify incident cancer cases diagnosed between January 1, 1985 and December 31, 2013. Data are collected by the Michigan Department of Community Health as part of the Michigan Cancer Surveillance Program (27), which participates in the National Program of Cancer Registries of the Centers for Disease Control and Prevention (28). We obtained data on first diagnosis of primary breast cancer (International Classification of Disease for Oncology Third Edition codes C50.0-C50.9), including both in-situ and invasive tumors, since 1985. We identified 221 incident cases of breast cancer in the study population, including 72 cases diagnosed at age 55 years or younger. Most cases, 82.8%, had invasive tumors. Data on vital status were obtained from the National Death Index (National Center for Health Statistics, Hyattsville, Maryland) (29).
Exposure assessment
Cumulative exposure estimates for each MWF type were calculated for each subject in the UAW-GM cohort based on detailed employment records available from hire through 1994 and a time-varying job-exposure-matrix. An extensive retrospective exposure assessment was conducted to develop this job-exposure-matrix (30, 31). Size fractionated MWF concentrations were estimated as an 8-hour time-weighted average (mg/m3) based on several hundred personal and area airborne exposure measurements collected by study industrial hygienists during the mid-1980s. A set of multipliers to adjust MWF concentration for temporal trends were developed based on nearly 400 historical air sampling measurements (collected 1958 through 1987), review of historical records, and interviews with plant personnel, and were last updated in 1995. This study relies on the respirable size fraction (<3.5 μm) of the MWF exposure estimates, which mostly deposit in the alveolar region. The job-exposure-matrix was combined with employment records to estimate time-varying annual average daily to straight, soluble, and synthetic MWF (mg/m3) throughout subjects’ entire employment. Missing employment information was interpolated by averaging exposures from previous and subsequent jobs. We then calculated cumulative time-weighted exposure to the three fluid types (mg/m3-years) for each subject for the duration of follow-up. To account for breast cancer latency, cumulative exposures for each fluid type were lagged; because employment data ended 12/31/1994, 19 years prior to end of follow-up, we used a 20-year lag. This assumes exposure accumulated in the twenty years preceding diagnoses does not affect cancer risk.
Data analyses
Cox proportional hazards models were fit to estimate hazard ratios and 95% confidence intervals associated with exposure to straight, soluble, and synthetic MWF (20-year lag) on breast cancer incidence. Exposure was defined as cumulative exposure to each fluid type for the respirable size fraction. MWF exposures were modeled both as continuous and categorical. For continuous exposure, hazard ratios (HR) were computed for an increase of one interquartile range among exposed subjects in the full study population; for the three fluid types, straight, soluble, and synthetic, these are 0.318, 0.979, and 0.270 mg/m3-years, respectively. For categorical exposure, the referent groups were subjects with no fluid type exposure. For each fluid type, the median level among exposed cases was used as the cut points for the exposure categories. Tests for trend were conducted by setting the value to the median for exposure category, modeling exposure as a continuous variable, and testing for non-zero slope using a likelihood ratio test. Age was the time metric for all models. All models included baseline covariates for race (white/black), an established breast cancer risk factor, manufacturing plant to adjust for plant-specific characteristic not captured elsewhere, as well as social-economic status and regional difference, and year of hire (B-spline with 3 degrees of freedom and equally spaced knots (32)) to account for secular trends in exposure including PAH content, personal protective equipment, and plant ventilation. A time-varying covariate for calendar year (B-spline with 3 degrees of freedom and equally spaced knots) was included to account for secular trends in breast cancer diagnosis. To assess the linearity of exposure-response relationships we evaluated additional Cox models with penalized splines (2 degrees of freedom) for MWF exposure.
By necessity only UAW-GM cohort members alive on January 1, 1985 were included in the incidence cohort, thereby creating a left-truncated cohort. Left truncation occurs when not all otherwise eligible subjects are enrolled in the cohort. Downward bias arises from left truncation because the proportion of subjects susceptible to the effect of exposure decreases over time (26). To reduce this potential bias, we restricted analyses to a series of sub-cohorts defined by year of hire. Narrowing the time interval between hire and start of the cancer registry reduces the opportunity for susceptible subjects to die prior to start of follow-up in 1985. We defined two sub-cohorts; hired after 1959 and hired after 1969 (20 and 10 years, respectively, prior to the last year of hire among cases). The greater the restriction, the less left truncation bias we expect.
We also examined premenopausal breast cancer as an outcome using age at diagnosis as an indicator for menopausal status. The statistical methods employed were the same as those described above for the main analysis. For these analyses, however, a series of four age cut points, 55, 54, 53, and 52 years of age, were used to define premenopausal breast cancer cases and follow-up ended upon reaching that age. A separate model was made for each age cut point. The younger cut points improved specificity but reduced sensitivity in identifying premenopausal breast cancer cases. For these analyses, we did not examine sub-cohorts defined by year of hire since these cases were hired later in time. Among cases diagnosed at age 55 or younger, the earliest year of hire was 1966. Thus, left truncation bias is less of a concern here. No apparent violation of the underlying assumption of proportional hazards was detected based on correlations between the Schoenfeld residuals for each MWF of interest and the ranked failure times. SAS software version 9.4 (SAS Institute, Cary, NC) was used for all analyses, except for Cox models with penalized splines which were conducted in R (version 3.2.3, R Core Team, Vienna, Austria). Use of human subjects data in this study was reviewed and approved by the Office for the Protection of Human Subjects at the University of California, Berkeley.
RESULTS
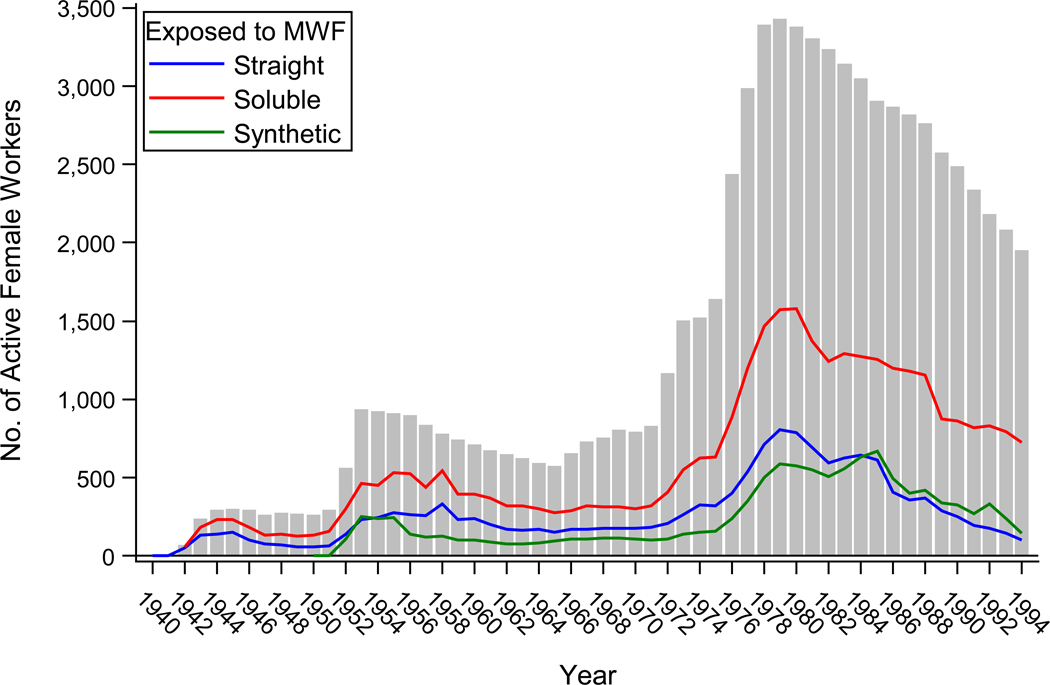
Table 1 provides demographic and exposure characteristics of the 221 incident breast cancer cases and 4,503 female autoworkers comprising the study population. The cohort is predominantly white, but does have more than a quarter African American women. Total number of active female workers in the three plants and their MWF exposure by year is presented in Figure 1. Just over half of the women, 50.4%, were hired in 1974 or later. As of December 31, 1994, the last data of known employment, 39.0% of the women remained actively employed in one of the three plants. The percentages of subjects ever exposed to straight, soluble, and synthetic fluids were 53.7, 84.1, and 38.0, respectively. We observed elevated rates of incident breast cancer among those with greater straight MWF exposure after reducing bias due to left truncation. When we examined the full study population using continuous exposure, results were null for all fluids types (Table 2). But in the analyses using sub-cohorts restricted by year of hire, the HR for continuous straight MWF exposure were increasingly farther from the null with more restrictive sub-cohorts (i.e., with decreased time between hire and the start of follow-up). Examining the exposure-response relation using categorized exposure revealed elevated HR, but with wide confidence intervals, for straight fluids in the full study population (Table 2). Results were stronger and presented a more positive exposure-response in the sub-cohort analyses. No elevated HR were observed for either soluble or synthetic fluids. Models with penalized spline coding for straight MWF exposure showed an approximately linear exposure-response relationship with the hazard ratio on the log scale, up to the 99th percentile of exposure among cases for both the 1959 and 1969 year of hire restricted sub-cohorts (Web Figure 1).
Table 1.
Demographic and Exposure Characteristics of Female Breast Cancer Cases and Cohort Members in the UAW-GM Incidence Cohort Who Were Alive in 1985, Michigan, 1985–2013.
| Cases | Cohort | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| No. | % | Mean (range) | Mean (SD) | No. | % | Mean (range) | Mean (SD) | |
|
| ||||||||
| No. subjects | 221 | 4,503 | ||||||
| No. person-years | 3,519 | 108,595 | ||||||
| Year of birth | 1938 (1907–1959) | 1940 (1893–1961) | ||||||
| Year of hire | 1969 (1942–1979) | 1969 (1940–1981) | ||||||
| Age at hire (years) | 30.2 (18.0–55.1) | 29.3 (16.7–58.0) | ||||||
| Duration of employment (years) | 18.1 (3.2–47.8) | 16.2 (2.9–47.8) | ||||||
| Race | ||||||||
| White | 155 | 70.1 | 3,256 | 72.3 | ||||
| African American | 66 | 29.9 | 1,247 | 27.7 | ||||
| Plant | ||||||||
| Plant 1 | 25 | 11.3 | 509 | 11.3 | ||||
| Plant 2 | 122 | 55.2 | 2,612 | 58.0 | ||||
| Plant 3 | 74 | 33.5 | 1,382 | 30.7 | ||||
| Vital status as of 2013 | ||||||||
| Alive | 132 | 59.7 | 2,947 | 65.5 | ||||
| Deceased | 89 | 40.3 | 1,556 | 34.6 | ||||
| Year of diagnosis | 1999 (1985–2013) | |||||||
| Age at diagnosis (years) | 62.1 (34.8–94.1) | |||||||
| Cumulative exposure (mg/m3-years)a | ||||||||
| Straight | 0.29 (1.08) | 0.20 (1.75) | ||||||
| Soluble | 0.68 (1.28) | 0.51 (1.42) | ||||||
| Synthetic | 0.09 (0.43) | 0.08 (0.44) | ||||||
|
| ||||||||
Abbreviations: UAW-GM, United Autoworkers-General Motors.
Cumulative exposure lagged 20 years
Figure 1.
Number of active workers (gray vertical bars) and annual exposure prevalence to straight (blue line), soluble (red line), and synthetic (green line) metalworking fluids (MWF) by year among female members of the United Autoworker-General Motor incidence cohort who were alive in 1985 (n = 4,503), Michigan, 1940–1994.
Table 2.
Adjusted Hazard Ratiosa of Breast Cancer Incidence (1985–2013) in Relation to Cumulative Exposure to Metalworking Fluid Size <3.5μm (20-Year Lag) in Female Autoworkers Based on the Full UAW-GM Incidence Cohort and in Sub-cohorts Defined by Year of Hire.
| Restricted by Year of Hire | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| All N = 4,503 (221 Cases) | Hired ≥ 1959 N = 3,431 (172 Cases) | Hired ≥ 1969 N = 3,182 (157 Cases) | ||||||||||
|
|
||||||||||||
| Cumulative Exposure (mg/m3-years) | No. of Cases | Person-years | HR | 95% CI | No. of Cases | Person-years | HR | 95% CI | No. of Cases | Person-years | HR | 95% CI |
|
| ||||||||||||
| Categorical | ||||||||||||
| Straight | ||||||||||||
| 0 | 124 | 74,802 | 1.00 | Referent | 106 | 69,019 | 1.00 | Referent | 95 | 65,022 | 1.00 | Referent |
| 0.0001–0.1120 | 48 | 17,440 | 1.39 | 0.90, 2.14 | 36 | 12,760 | 1.64 | 0.99, 2.73 | 33 | 11,888 | 1.67 | 0.99, 2.83 |
| ≥0.1121 | 49 | 16,353 | 1.32 | 0.86, 2.01 | 30 | 9,251 | 1.64 | 1.01, 2.67 | 29 | 8,406 | 1.74 | 1.05, 2.86 |
| P for trend | 0.43 | 0.13 | 0.08 | |||||||||
| Soluble | ||||||||||||
| 0 | 80 | 52,727 | 1.00 | Referent | 74 | 50,819 | 1.00 | Referent | 65 | 48,070 | 1.00 | Referent |
| 0.0001–0.4999 | 70 | 29,660 | 0.90 | 0.61, 1.34 | 61 | 25,716 | 0.90 | 0.58, 1.39 | 59 | 24,371 | 1.00 | 0.63, 1.59 |
| ≥0.5000 | 71 | 26,208 | 0.88 | 0.56, 1.40 | 37 | 14,496 | 0.73 | 0.42, 1.26 | 33 | 12,875 | 0.77 | 0.43, 1.39 |
| P for trend | 0.87 | 0.34 | 0.33 | |||||||||
| Synthetic | ||||||||||||
| 0 | 168 | 86,772 | 1.00 | Referent | 133 | 75,619 | 1.00 | Referent | 121 | 71,094 | 1.00 | Referent |
| 0.0001–0.0699 | 26 | 10,213 | 0.89 | 0.53, 1.49 | 22 | 9,099 | 0.72 | 0.40, 1.31 | 19 | 8,555 | 0.62 | 0.33, 1.17 |
| ≥0.0700 | 27 | 11,610 | 0.77 | 0.47, 1.26 | 17 | 6,312 | 0.80 | 0.43, 1.48 | 17 | 5,667 | 0.87 | 0.46, 1.64 |
| P for trend | 0.39 | 0.71 | 0.96 | |||||||||
| Continuous b | ||||||||||||
| Straight | 1.00 | 0.98, 1.02 | 1.11 | 1.01, 1.22 | 1.13 | 1.03, 1.23 | ||||||
| Soluble | 1.00 | 0.91, 1.09 | 0.97 | 0.74, 1.28 | 0.98 | 0.73, 1.30 | ||||||
| Synthetic | 0.98 | 0.90, 1.07 | 0.82 | 0.53, 1.29 | 0.91 | 0.60, 1.38 | ||||||
|
| ||||||||||||
Abbreviations: UAW-GM, United Autoworkers-General Motors; CI, confidence interval; HR, hazard ratio.
Each Cox regression models included cumulative exposure to the three fluid types, used age as time scale, and adjusted for year of hire, calendar year, race, and manufacturing plant.
HR per increase of one interquartile range among exposed subjects in the full study population; for the three fluid types these are 0.318, 0.979, and 0.270 mg/m3-years, respectively.
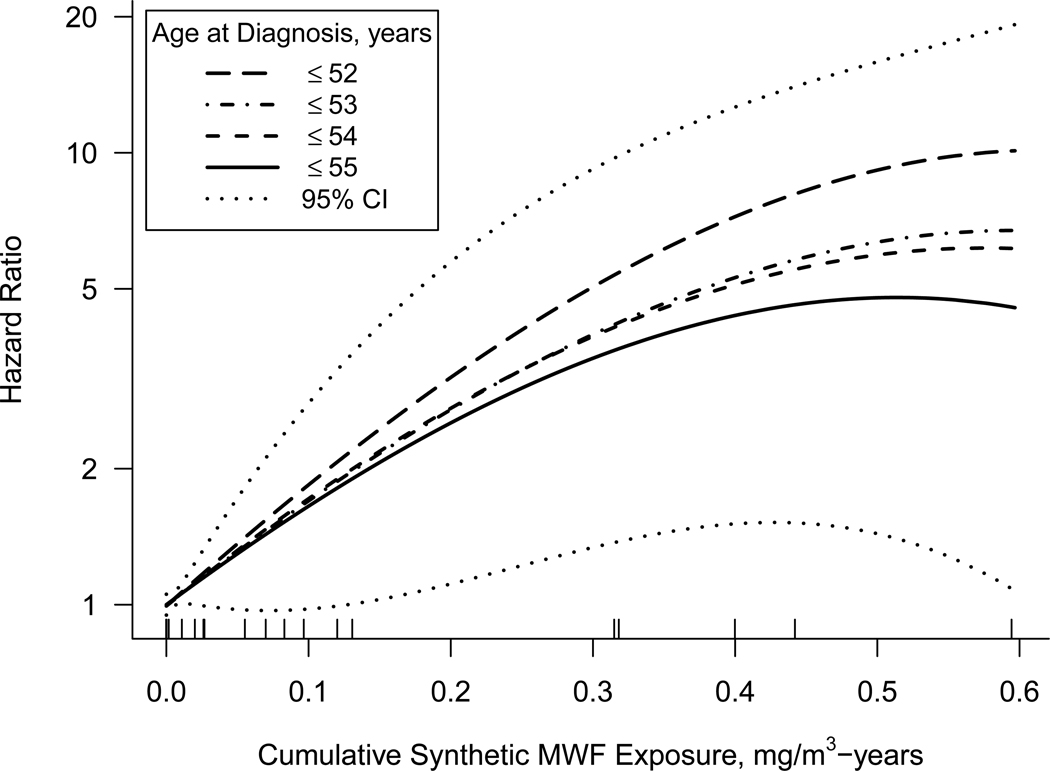
Results from the premenopausal breast cancer incidence analyses suggest an increased hazard associated with synthetic fluid exposure, but null associations for straight and soluble MWF. The HR for continuous synthetic fluid exposure was elevated when using age 54 as the cut point to define premenopausal cases and increased in magnitude with lower age cut points (Table 3). When using a categorical exposure metric, a strong exposure-response was observed for synthetic MWF, particularly with younger age cut points (Table 3). Models with penalized spline coding for synthetic MWF exposure showed a positive exposure-response relationship regardless of age cut point used through the 99th percentile of exposure among cases (Figure 2). As with the other models, HR were higher when the premenopausal case definition was based on younger age cut points.
Table 3.
Adjusted Hazard Ratiosa of Premenopausal Breast Cancer Incidence (1985–2013) in Relation to Cumulative Exposure to Metalworking Fluid Size <3.5μm (20-Year Lag) in Female Autoworkers in the UAW-GM Cohort Using Multiple Age Cut Points for Premenopausal Definition.
| Age at Diagnosis | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||||||
| Age 55 or Younger N = 3,263 (72 Cases) | Age 54 or Younger N = 3,211 (65 Cases) | Age 53 or Younger N = 3,148 (60 Cases) | Age 52 or Younger N = 3,092 (49 Cases) | |||||||||||||
|
|
||||||||||||||||
| Cumulative Exposure (mg/m3-years) | No. of Cases | Person-years | HR | 95% CI | No. of Cases | Person-years | HR | 95% CI | No. of Cases | Person-years | HR | 95% CI | No. of Cases | Person-years | HR | 95% CI |
|
| ||||||||||||||||
| Categorical | ||||||||||||||||
| Straight | ||||||||||||||||
| 0 | 54 | 48,105 | 1.00 | Referent | 48 | 46,151 | 1.00 | Referent | 45 | 44,131 | 1.00 | Referent | 39 | 42,082 | 1.00 | Referent |
| 0.0001–0.0799 | 9 | 5,470 | 0.95 | 0.36, 2.52 | 8 | 4,968 | 1.08 | 0.37, 3.10 | 7 | 4,488 | 0.82 | 0.26, 2.52 | 5 | 4,022 | 0.88 | 0.23, 3.31 |
| ≥0.0800 | 9 | 4,208 | 1.08 | 0.44, 2.66 | 9 | 3,692 | 1.47 | 0.56, 3.82 | 8 | 3,196 | 1.23 | 0.45, 3.37 | 5 | 2,728 | 1.15 | 0.34, 3.91 |
| P for trend | 0.89 | 0.41 | 0.54 | 0.80 | ||||||||||||
| Soluble | ||||||||||||||||
| 0 | 42 | 39,347 | 1.00 | Referent | 39 | 38,184 | 1.00 | Referent | 36 | 36,963 | 1.00 | Referent | 33 | 35,675 | 1.00 | Referent |
| 0.0001–0.3319 | 15 | 11,070 | 0.79 | 0.37, 1.71 | 14 | 10,189 | 0.81 | 0.36, 1.85 | 13 | 9,324 | 0.81 | 0.35, 1.89 | 9 | 8,488 | 0.60 | 0.22, 1.63 |
| ≥0.3320 | 15 | 7,366 | 0.83 | 0.33, 2.09 | 12 | 6,437 | 0.72 | 0.26, 2.00 | 11 | 5,529 | 0.76 | 0.27, 2.19 | 7 | 4,669 | 0.55 | 0.16, 1.88 |
| P for trend | 0.81 | 0.61 | 0.72 | 0.47 | ||||||||||||
| Synthetic | ||||||||||||||||
| 0 | 56 | 50,721 | 1.00 | Referent | 50 | 48,481 | 1.00 | Referent | 46 | 46,207 | 1.00 | Referent | 39 | 43,914 | 1.00 | Referent |
| 0.0001–0.0899 | 8 | 5,059 | 1.04 | 0.37, 2.96 | 8 | 4,601 | 1.16 | 0.39, 3.48 | 8 | 4,155 | 1.42 | 0.46, 4.39 | 5 | 3,725 | 1.33 | 0.34, 5.28 |
| ≥0.0900 | 8 | 2,003 | 2.40 | 0.88, 6.54 | 7 | 1,729 | 2.71 | 0.92, 7.98 | 6 | 1,453 | 2.78 | 0.88, 8.73 | 5 | 1,193 | 3.76 | 1.04, 13.51 |
| P for trend | 0.07 | 0.05 | 0.09 | 0.04 | ||||||||||||
| Continuous b | ||||||||||||||||
| Straight | 0.81 | 0.51, 1.27 | 0.87 | 0.57, 1.35 | 0.87 | 0.54, 1.39 | 0.82 | 0.42, 1.58 | ||||||||
| Soluble | 0.90 | 0.64, 1.27 | 0.91 | 0.59, 1.39 | 0.84 | 0.51, 1.38 | 0.75 | 0.39, 1.44 | ||||||||
| Synthetic | 1.06 | 0.95, 1.17 | 1.13 | 0.99, 1.30 | 1.15 | 1.00, 1.32 | 1.17 | 1.01, 1.35 | ||||||||
|
| ||||||||||||||||
Abbreviations: UAW-GM, United Autoworkers-General Motors; CI, confidence interval; HR, hazard ratio.
Each Cox regression models included cumulative exposure to the three fluid types, used age as time scale, and adjusted for year of hire, calendar year, race, and manufacturing plant.
HR per increase of one interquartile range among exposed subjects age 55 or younger; for the three fluid types these are 0.157, 0.474, and 0.092 mg/m3-years, respectively.
Figure 2.
Adjusted hazard ratios for premenopausal breast cancer incidence as a smoothed function of 20-year lagged cumulative synthetic metalworking fluid (MWF) as estimated in a Cox regression model using penalized splines (2 degrees of freedom) based on a cohort of female workers in the United Autoworker-General Motor incidence cohort who were alive in 1985, Michigan, 1985–2013. Premenopausal breast cancer was defined as diagnosis by four select age cut points. The 95% confidence interval (95% CI) is shown for the model using age 55 years as the cut point. Models used age as the time scale and adjusted for cumulative exposure to straight and soluble MWF, year of hire, calendar year, race, and manufacturing plant. Graph truncated at the 99th percentile of synthetic fluid exposure among cases (0.60 mg/m3-years). The rug plot indicates exposure of the cases 55 years old or younger.
DISCUSSION
In a prospective cohort of 4,503 female autoworkers from the UAW-GM study we examined MWF exposure and its association with incident breast cancer. Exposure to straight MWF, but neither soluble nor synthetic, was found to be positively associated with breast cancer. This was evident in the analyses using sub-cohorts restricted by year of hire. When restricted to subjects hired 1959 or later, the HR for straight MWF exposure was elevated in both the continuous and categorical analyses. Results became stronger when using the sub-cohort restricted to subjects hired 1969 or later. This more restrictive sub-cohort was designed to reduce left truncation bias, and may explain the stronger results observed in these analyses.
Though power was adequate for the main analysis, when we restricted to presumed premenopausal cases, the number of subjects in each exposure category was sparse. Results however, were modestly suggestive of an increased risk associated with higher synthetic MWF exposure. The associations were more pronounced when younger age cut points defined the cases. These younger case definitions were more specific for premenopausal breast cancer, which is more important than sensitivity for reducing bias when the outcome is rare (33). Our interpretation of these results, however, was constrained by the small number of premenopausal cases. Moreover, these results were apparently inconsistent with the inverse association with synthetic fluids observed in the main analysis. One possible explanation for this inconsistency is that synthetic MWF were introduced later in time. Exposed older subjects therefore had to have remained employed longer, introducing survivor bias among the older cases.
Three previous breast cancer studies relied on data from the UAW-GM study (17, 22, 23). The first study combined incident cases and deaths, and imputed date of diagnosis if missing (17). The modest risk increase for cumulative soluble MWF exposure observed was inconsistent with the literature on latency for breast cancer, suggesting higher risk associated with exposure in the decade preceding diagnosis. The present analysis was restricted to incident cases and we imposed a 20-year lag on cumulative exposures based on the latency period for breast cancer (34–36). The second study examined the incidence of several cancers and reported an increased and borderline significant HR associated with exposure to straight MWF among younger women in the cohort (23). The third study, based on 43 breast cancer deaths, found a HR of 1.4 (95% confidence interval: 0.7, 2.5) associated with higher straight MWF exposure (22). Mortality, however, is a poor surrogate for breast cancer incidence given a five-year survival rate of 88% (37). After accounting for left truncation, we observed similar but marginally larger HR associated with straight fluid exposure both when using a continuous and categorical metric. The additional of nine years of follow-up compared to the second study and the use of the more appropriate breast cancer outcome measure of incidence rather than mortality compared to the third study may have led to the slightly more positive results.
The contrasting results in the pooled analysis versus the sub-analysis restricted to premenopausal breast cancer, suggest a possibly distinct biologic mechanism for this type of breast cancer. However, the distribution of cumulative straight MWF exposure was markedly lower among premenopausal cases, with a mean of 0.02 mg/m3-years (standard deviation (SD): 0.05) based on an age cut point of 55 years compared to all cases at 0.29 (SD: 1.08), and may also have contributed to a lack of association for straight fluids in the premenopausal analyses. The PAH content of straight MWF may be the causal factor in the elevated risk. Air pollution exposure, as a proxy for PAH, has been associated with postmenopausal breast cancer. Total suspended particulate exposure was associated with postmenopausal, but not premenopausal, breast cancer in a population-based, case-control study in Western New York State (9). A later study in the same population modeled total PAH exposure and similarly found exposure at first birth associated with postmenopausal, but not premenopausal, breast cancer (10). The only study to examine occupational PAH exposure found an increased odds of premenopausal breast cancer among those exposed (35). Because benzene was a co-exposure in the study, the authors additionally evaluated exclusive PAH exposed and found null results. Studies of the Long Island Breast Cancer Study Project have found PAH-DNA adducts, which are short-term PAH biomarkers of exposure, to be positively associated with incident breast cancer (38, 39).
There is less evidence for synthetic fluids and premenopausal breast cancer risk. Nitrosamines are a class of potentially hazardous contaminants found in synthetic fluids (40). N-nitrosodiethanolamine, a type of nitrosamine, has been found in synthetic fluids (41, 42) and in post-shift urine samples of workers exposed to water-based MWF (43). It has been demonstrated to induce DNA damage in animal and human cells (44) and metal workers exposed to higher levels of N-nitrosodiethanolamine were found to have increased levels of DNA single strand breaks in blood cells (45). Although it is classified as possibly carcinogenic to humans and is an animal carcinogen (46), there is no specific research on this compound and breast cancer risk.
Bias due to the healthy worker survivor effect is a concern in occupational epidemiology (47–49). We recently assessed the presence of the healthy worker survivor effect in the UAW-GM incidence cohort based on three necessary underlying conditions: 1) leaving work predicts future exposure, 2) leaving work is associated with disease outcome, and 3) prior exposure increases probability of leaving work (50). The first condition is a given, since subjects who leave work are no longer exposed. We found prior soluble and synthetic, but not straight, MWF exposure associated with leaving work among female subjects. This supports the third condition for soluble and synthetic, but not straight, fluids. Although breast cancer was not examined in that assessment, we used the same statistical method and found that having left work was not associated with breast cancer incidence, indicating a lack of evidence for the second condition. Overall, these results imply that healthy worker survivor bias did not influence our estimates of the association between MWF exposure and breast cancer risk, particularly for straight fluids.
Our main limitation is unmeasured potential confounders. Although we control for age and race, we do not account for several other breast cancer risk factors including social economic status, family history of breast cancer, age at menarche or menopause, age at first-full term pregnancy, parity, breastfeeding history, use of oral contraceptives or postmenopausal hormones, and alcohol consumption. Because these are all blue-collar workers employed at the same three plants, we do not expect large differences in social economic status. Additionally, we do not expect family history of breast cancer, age at menarche or menopause, or use of oral contraceptives or postmenopausal hormones to be related to MWF exposure. Lastly, prior studies of this cohort have found no association between MWF exposure and cirrhosis death, a proxy for alcohol consumption (51). Therefore, the potential confounders of most concern are those related to childbearing: age at first full-term pregnancy, parity, and breastfeeding history. Women with a younger age at first full-term pregnancy, higher parity, and who breastfed longer have reduced breast cancer risk and are likely to have lower MWF exposure due to taking maternity leave, entering the workforce later, and/or leaving the workforce earlier. This would produce positive confounding and bias results upward. In the above evaluation, however, we saw no evidence of leaving work being associated with reduced breast cancer risk. Furthermore, we would expect such a bias to affect the results for all three fluid types, not just the straight fluids in the pooled analyses or the synthetic fluids in the premenopausal analyses. Although unmeasured confounding may account for some portion of the results observed, this study makes a contribution to the literature.
This study adds to the limited literature regarding quantitative chemical exposures and breast cancer risk in humans. This study utilized a well-characterized occupational cohort of blue collar women with long cancer incidence follow-up and provided evidence of an increased risk of breast cancer associated with cumulative straight MWF exposure.
Supplementary Material
ACKNOWLEDGEMENTS
This work was financially supported by the National Institute of Occupational Safety and Health at the Center for Disease Control and Prevention (Grants T42OH008429 and R01OH010028).
ABBREVIATIONS
- HR
hazard ratio
- MWF
metalworking fluid
- PAH
polycyclic aromatic hydrocarbons
- UAW-GM
United Autoworker-General Motors
Footnotes
Contact for editorial queries (approval of galleys and answering questions during editing): Dr. Erika Garcia, Environmental Health Sciences Division, School of Public Health, University of California, 50 University Hall #7360, Berkeley, CA 94720-7360, egarcia@berkeley.edu
Conflicts of interest: none declared.
Contributor Information
Erika Garcia, Environmental Health Sciences Division, School of Public Health, University of California at Berkeley, Berkeley, California.
Patrick T. Bradshaw, Epidemiology Division, School of Public Health, University of California at Berkeley, Berkeley, California
Ellen A. Eisen, Environmental Health Sciences Division, School of Public Health, University of California at Berkeley, Berkeley, California
REFERENCES
- 1.U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999–2011 Incidence and Mortality Web-based Report. Atlanta, GA: Department of Health and Human Services, Center for Disease Control and Prevention, and National Cancer Institute; 2014. [Google Scholar]
- 2.SEER. Cancer Statistics Factsheets: Female Breast Cancer. Bethesda, MD: National Cancer Institute. (http://seer.cancer.gov/statfacts/html/breast.html). (Accessed November 7 2016). [Google Scholar]
- 3.Madigan MP, Ziegler RG, Benichou J, et al. Proportion of breast cancer cases in the United States explained by well-established risk factors. J Natl Cancer Inst 1995;87(22):1681–1685. [DOI] [PubMed] [Google Scholar]
- 4.Institute of Medicine (IOM). Chapter 7: Recommendations for Future Research. Breast Cancer and the Environment: A Life Course Approach. Washington, DC: The National Academies Press, 2012:325–345. [Google Scholar]
- 5.Rudel RA, Attfield KR, Schifano JN, et al. Chemicals causing mammary gland tumors in animals signal new directions for epidemiology, chemicals testing, and risk assessment for breast cancer prevention. Cancer 2007;109(12 Suppl):2635–2666. [DOI] [PubMed] [Google Scholar]
- 6.Neale M Tribiology Handbook. New York, NY: John Wiley and Sons; 1973. [Google Scholar]
- 7.International Agency for Research on Cancer (IARC). Mineral Oils, Untreated or Mildly Treated. Lyon, France: IARC; 2012. [Google Scholar]
- 8.Catchpole WM, Macmillan E, Powell H. Specifications for cutting oils with special reference to carcinogenicity. Ann Occup Hyg 1971;14(2):171–179. [DOI] [PubMed] [Google Scholar]
- 9.Bonner MR, Han D, Nie J, et al. Breast cancer risk and exposure in early life to polycyclic aromatic hydrocarbons using total suspended particulates as a proxy measure. Cancer Epidemiol Biomarkers Prev 2005;14(1):53–60. [PubMed] [Google Scholar]
- 10.Nie J, Beyea J, Bonner MR, et al. Exposure to traffic emissions throughout life and risk of breast cancer: the Western New York Exposures and Breast Cancer (WEB) study. Cancer Causes Control 2007;18(9):947–955. [DOI] [PubMed] [Google Scholar]
- 11.U.S. Environmental Protection Agency. Endocrine Disruptor Screening Program: Universe of Chemicals for Potential Endocrine Disruptor Screening and Testing. Office of Chemical Safetly and Pollution Prevention, Office of Water, and Office of Research and Development; 2012. [Google Scholar]
- 12.Steenland K, Burnett C, Lalich N, et al. Dying for work: The magnitude of US mortality from selected causes of death associated with occupation. Am J Ind Med 2003;43(5):461–482. [DOI] [PubMed] [Google Scholar]
- 13.International Labour Office. Global Employment Trends for Women: March 2009. Geneva, Switzerland: ILO, 2009. [Google Scholar]
- 14.Frost and Sullivan. Analysis of the Global Metalworking Fluids Market. Mountain View, CA, 2014. [Google Scholar]
- 15.Delzell E, Beall C, Macaluso M. Cancer mortality among women employed in motor vehicle manufacturing. J Occup Med 1994;36(11):1251–1259. [DOI] [PubMed] [Google Scholar]
- 16.Beall C, Delzell E, Macaluso M. Mortality patterns among women in the motor vehicle manufacturing industry. Am J Ind Med 1995;28(3):325–337. [DOI] [PubMed] [Google Scholar]
- 17.Thompson D, Kriebel D, Quinn MM, et al. Occupational exposure to metalworking fluids and risk of breast cancer among female autoworkers. Am J Ind Med 2005;47(2):153–160. [DOI] [PubMed] [Google Scholar]
- 18.Brophy JT, Keith MM, Gorey KM, et al. Occupation and breast cancer: a Canadian case-control study. Ann N Y Acad Sci 2006;1076:765–777. [DOI] [PubMed] [Google Scholar]
- 19.Hansen J Breast cancer risk among relatively young women employed in solvent-using industries. Am J Ind Med 1999;36(1):43–47. [DOI] [PubMed] [Google Scholar]
- 20.Villeneuve S, Fevotte J, Anger A, et al. Breast cancer risk by occupation and industry: analysis of the CECILE study, a population-based case-control study in France. Am J Ind Med 2011;54(7):499–509. [DOI] [PubMed] [Google Scholar]
- 21.Brophy JT, Keith MM, Watterson A, et al. Breast cancer risk in relation to occupations with exposure to carcinogens and endocrine disruptors: a Canadian case-control study. Environ Health 2012;11:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friesen MC, Betenia N, Costello S, et al. Metalworking fluid exposure and cancer risk in a retrospective cohort of female autoworkers. Cancer Causes Control 2012;23(7):1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friesen MC, Costello S, Thurston SW, et al. Distinguishing the common components of oil- and water-based metalworking fluids for assessment of cancer incidence risk in autoworkers. Am J Ind Med 2011;54(6):450–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eisen EA, Tolbert PE, Monson RR, et al. Mortality studies of machining fluid exposure in the automobile industry I: A standardized mortality ratio analysis. Am J Ind Med 1992;22(6):809–824. [DOI] [PubMed] [Google Scholar]
- 25.Eisen EA, Bardin J, Gore R, et al. Exposure-response models based on extended follow-up of a cohort mortality study in the automobile industry. Scand J Work Environ Health 2001;27(4):240–249. [DOI] [PubMed] [Google Scholar]
- 26.Applebaum KM, Malloy EJ, Eisen EA. Left truncation, susceptibility, and bias in occupational cohort studies. Epidemiology 2011;22(4):599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michigan Cancer Surveillance Program. Cancer Statistics. Michigan Department of Health and Human Services. (http://www.michigan.gov/mdch/0,4612,7-132-2945_5221-16586--,00.html). (Accessed May 5 2015).
- 28.Dividion of Cancer Prevention and Control National Center for Chronic Disease Prevention and Health Promotion. National Program of Cancer Registries. Centers for Disease Control and Prevention; 2014. (http://www.cdc.gov/cancer/npcr/). (Accessed November 5 2014).
- 29.Cowper DC, Kubal JD, Maynard C, et al. A primer and comparative review of major US mortality databases. Ann Epidemiol 2002;12(7):462–468. [DOI] [PubMed] [Google Scholar]
- 30.Hallock MF, Smith TJ, Woskie SR, et al. Estimation of historical exposures to machining fluids in the automotive industry. Am J Ind Med 1994;26(5):621–634. [DOI] [PubMed] [Google Scholar]
- 31.Woskie SR, Smith TJ, Hallock MF, et al. Size-selective pulmonary dose indices for metal-working fluid aerosols in machining and grinding operations in the automobile manufacturing industry. Am Ind Hyg Assoc J 1994;55(1):20–29. [DOI] [PubMed] [Google Scholar]
- 32.De Boor C A practical guide to splines. New York, NY: Springer-Verlag; 1978. [Google Scholar]
- 33.Rothman KJ, Greenland S, Lash TL. Modern epidemiology. 3rd ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 34.Aschengrau A, Paulu C, Ozonoff D. Tetrachloroethylene-contaminated drinking water and the risk of breast cancer. Environ Health Perspect 1998;106 Suppl 4:947–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petralia SA, Vena JE, Freudenheim JL, et al. Risk of premenopausal breast cancer in association with occupational exposure to polycyclic aromatic hydrocarbons and benzene. Scand J Work Environ Health 1999;25(3):215–221. [DOI] [PubMed] [Google Scholar]
- 36.Kelsey JL, Berkowitz GS. Breast cancer epidemiology. Cancer Res 1988;48(20):5615–5623. [PubMed] [Google Scholar]
- 37.ACS. Cancer Facts & Figures 2014. Atlanta, GA: American Cancer Society, 2014. [Google Scholar]
- 38.Gammon MD, Sagiv SK, Eng SM, et al. Polycyclic aromatic hydrocarbon-DNA adducts and breast cancer: a pooled analysis. Arch Environ Health 2004;59(12):640–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gammon MD, Santella RM, Neugut AI, et al. Environmental toxins and breast cancer on Long Island. I. Polycyclic aromatic hydrocarbon DNA adducts. Cancer Epidemiol Biomarkers Prev 2002;11(8):677–685. [PubMed] [Google Scholar]
- 40.NIOSH. Criteria for a Recommended Standard: Occupational Exposure to Metalworking Fluids. Cincinnati, OH: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health,, 1998. [Google Scholar]
- 41.Fan TY, Morrison J, Rounbehler DP, et al. N-Nitrosodiethanolamine in synthetic cutting fluids: a part-per-hundred impurity. Science 1977;196(4285):70–71. [DOI] [PubMed] [Google Scholar]
- 42.Keefer LK, Goff U, Stevens J, et al. Persistence of N-nitrosodiethanolamine contamination in American metal-working lubricants. Food Chem Toxicol 1990;28(7):531–534. [DOI] [PubMed] [Google Scholar]
- 43.Ducos P, Gaudin R. N-nitrosodiethanolamine urinary excretion in workers exposed to aqueous metalworking fluids. Int Arch Occup Environ Health 2003;76(8):591–597. [DOI] [PubMed] [Google Scholar]
- 44.Holzer J, Voss B, Karroum S, et al. A comparative study of chemically induced DNA damage in isolated nasal mucosa cells of humans and rats assessed by the alkaline comet assay. J Toxicol Environ Health A 2008;71(13–14):936–946. [DOI] [PubMed] [Google Scholar]
- 45.Fuchs J, Burg J, Hengstler JG, et al. DNA damage in mononuclear blood cells of metal workers exposed to N-nitrosodiethanolamine in synthetic cutting fluids. Mutat Res 1995;342(1–2):95–102. [DOI] [PubMed] [Google Scholar]
- 46.International Agency for Research on Cancer (IARC). Some Industrial Chemicals. Lyon, France: IARC; 2000. [Google Scholar]
- 47.Arrighi HM, Hertz-Picciotto I. The evolving concept of the healthy worker survivor effect. Epidemiology 1994;5(2):189–196. [DOI] [PubMed] [Google Scholar]
- 48.Buckley JP, Keil AP, McGrath LJ, et al. Evolving methods for inference in the presence of healthy worker survivor bias. Epidemiology 2015;26(2):204–212. [DOI] [PubMed] [Google Scholar]
- 49.Picciotto S, Hertz-Picciotto I. Commentary: healthy worker survivor bias: a still-evolving concept. Epidemiology 2015;26(2):213–215. [DOI] [PubMed] [Google Scholar]
- 50.Garcia E, Picciotto S, Costello S, et al. Assessment of the healthy worker survivor effect in cancer studies of the United Autoworkers-General Motors cohort. Occup Environ Med 2017;74(4):294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eisen EA, Tolbert PE, Hallock MF, et al. Mortality studies of machining fluid exposure in the automobile industry. III: A case-control study of larynx cancer. Am J Ind Med 1994;26(2):185–202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.