Abstract
Purpose
This study aimed to comprehensively assess the safety of rimegepant administration in real-world clinical settings.
Methods
Data from the Food and Drug Administration Adverse Event Reporting System (FAERS) spanning the second quarter of 2020 through the first quarter of 2023 were retrospectively analyzed in this pharmacovigilance investigation. This study focuses on employing subgroup analysis to monitor rimegepant drug safety. Descriptive analysis was employed to examine clinical characteristics and concomitant medication of adverse event reports associated with rimegepant, including report season, reporter country, sex, age, weight, dose, and frequency, onset time, et al. Correlation analysis, including techniques such as violin plots, was utilized to explore relationships between clinical characteristics in greater detail. Additionally, four disproportionality analysis methods were applied to assess adverse event signals associated with rimegepant.
Results
A total of 5,416,969 adverse event reports extracted from the FAERS database, 10, 194 adverse events were identified as the “primary suspect” (PS) drug attributed to rimegepant. Rimegepant-associated adverse events involved 27 System Organ Classes (SOCs), and the significant SOC meeting all four detection criteria was “general disorders and administration site conditions” (SOC: 10018065). Additionally, new significant adverse events were discovered, including “vomiting projectile” (PT: 10047708), “eructation” (PT: 10015137), “motion sickness” (PT: 10027990), “feeling drunk” (PT: 10016330), “reaction to food additive” (PT: 10037977), etc. Descriptive analysis indicated that the majority of reporters were consumers (88.1%), with most reports involving female patients. Significant differences were observed between female and male patients across age categories, and the concomitant use of rimegepant with other medications was complex.
Conclusion
This study has preliminarily identified potential new adverse events associated with rimegepant, such as those involving the gastrointestinal system, nervous system, and immune system, which warrant further research to determine their exact mechanisms and risk factors. Additionally, significant differences in rimegepant-related adverse events were observed across different age groups and sexes, and the complexity of concomitant medication use should be given special attention in clinical practice.
Supplementary Information
The online version contains supplementary material available at 10.1186/s10194-024-01858-4.
Keywords: Adverse events, Sex disparities, Data mining, FAERS, Pharmacovigilance, Rimegepant
Introduction
Migraine, a prevalent and disabling primary headache disorder, impacts around 15% of the global population, resulting in severe disability and a significant socioeconomic burden [1–3]. Conventional medical treatments for migraine encompass preventive therapy (primarily beta-blockers, anti-epileptic medications, onabotulinumtoxin, etc.) as well as acute attack therapy (primarily triptans and nonsteroidal anti-inflammatory drugs, etc.) [4–6]. Triptans have been the most widely used drug for acute migraine, but their failure rate and adverse drug reactions are high [7]. Furthermore, patients with cardiovascular disease and abortive migraines are not eligible to utilize triptans [8, 9, 6]. As for migraine preventive medications, their effectiveness and patient compliance have not been particularly good thus far [10, 11].
One of the strongest peptides with a vasodilating effect, calcitonin gene-related peptide (CGRP) is the primary neuropeptide released by the trigeminal nerve [11, 12]. Its signal transduction may be the primary pathophysiology of a migraine attack [11, 12]. Because of their similar or less toxicity than triptans, CGRP antagonists have shown promise in recent studies, especially when it comes to adverse events [8]. Moreover, in cases of acute migraine with significant cardiovascular risk factors, CGRP antagonists may be the “drug of choice” [3]. Two types of CGRP function-inhibiting modalities have been developed, targeting both CGRP ligands and CGRP receptors: monoclonal antibodies and small molecules (gepants) [13].
Rimegepant is a second-generation gepant with potent, competitive, and selective antagonistic effects on the human CGRP receptor [13, 14]. It is effective in multiple clinical trials for the treatment of acute migraine [13, 14]. The US Food and Drug Administration (FDA) approved rimegepant for the acute treatment of adult migraine on February 27, 2020, and for the prophylactic treatment of episodic migraine on May 27, 2021, due to its innovative medication formulation and general tolerability [3, 14]. Rimegepant’s clinical safety has gained a lot of attention as the first medication with a dual action for treating migraines [6]. Battini et al., for example, used the reporting odds ratio (ROR) algorithm to conduct a disproportionate analysis based on the Food and Drug Administration Adverse Event Reporting System (FAERS) and discovered new safety issues associated with ubrogepant and rimegepant drug treatment, primarily related to psychiatric, nervous system, gastrointestinal, and other adverse events [15]. Porreca et al. conducted a subgroup analysis of previously published FDA data to assess potential sexual differences in response rates of ubrogepant, rimegepant, and zavegepant to acute migraine treatment and discovered that all three drug preventive treatments were effective [16]. However, it is unclear whether the results show a sexual difference [16].
To better understand the safety profile and inherent risks of rimegepant, and to provide more comprehensive insights to support prudent medical decision-making, this study investigated adverse event data related to rimegepant from the FAERS database. The study aimed to conduct a detailed analysis of rimegepant-related adverse events, exploring its patterns, trends, and associated factors to identify safety signals and potential risk factors.
Methods
Data source and collection
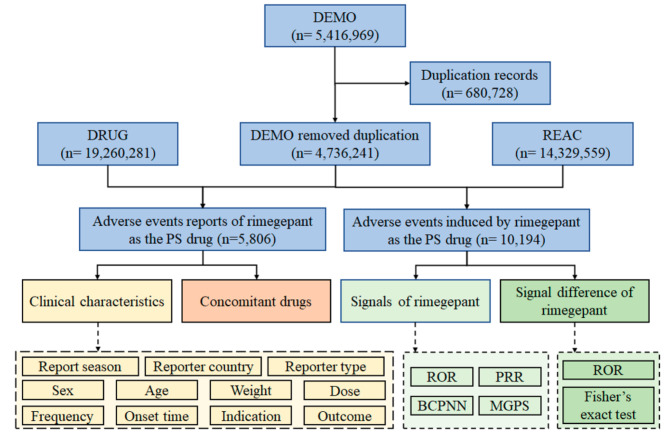
In this retrospective pharmacovigilance study of rimegepant, data were retrieved from the FAERS database spanning the second quarter of 2020 to the first quarter of 2023. Six distinct datasets in ASCII format were employed, encompassing patient demographic and administrative information (DEMO), drug information (DRUG), therapy start dates and end dates for reported drugs (THER), coded for the adverse events (REAC), patient outcomes for the event (OUTC), and indications for use/diagnosis (INDI) [17–19]. The cases involving rimegepant were identified by examining the generic name “RIMEGEPANT SULFATE” in the “prod_ai” column of the DRUG dataset. The role that rimegepant played in adverse events was meticulously classified into four groups: primary suspect, secondary suspect, concomitant, and interacting [20]. Adverse event reports where the “role_cod” column indicated rimegepant as the “primary suspected” (PS) drug were selected, indicating a potential contribution to the adverse event [20]. Following the integration of the DRUG and REAC datasets with the DEMO dataset, the cases and adverse events attributed to rimegepant as the PS drug were identified. The adverse events of rimegepant were coded using Preferred Terms (PT) sourced from the standardized Medical Dictionary for Regulatory Activities 25.1 (MedDRA), encompassing 27 system organ classes (SOCs) [17–19]. All the PTs were categorized into their respective primary SOC levels [17–19]. Figure 1 depicts the flow of data collection and analysis for rimegepant-associated adverse event reports.
Fig. 1.
Flow diagram of data collection and analysis of rimegepant-associated adverse event reports. (DEMO demographic and administrative information, DRUG drug information, REAC coded for the adverse events, PS “primary suspected” drug.)
Statistical analysis
To dissect the clinical characteristics of rimegepant-associated adverse event reports, descriptive analysis methods were employed. This included an examination of report season, reporter country, reporter type, sex, age, weight, dose, frequency, onset time, indication, and outcome, with missing data excluded. Subsequently, correlations between clinical factors were explored using violin plots, parallel category plots, and T-tests. The concomitant medications in the rimegepant-associated adverse event reports underwent further analysis [17, 19]. Medications listed as “UNSPECIFIED INGREDIENT” were excluded from consideration.
The adverse event signals associated with rimegepant were investigated by assessing the frequency and intensity at the SOC and PT levels. The study employed four disproportionality analysis methods, containing reporting odds ratio (ROR) [21, 22], proportional reporting ratio (PRR) [23, 24], Bayesian confidence propagation neural network (BCPNN) [23–25], and the multi-item gamma Poisson shrinker (MGPS) [23–25]. Supplementary Table S1 presents the fourfold table of disproportionality analysis for rimegepant signal detection. Supplementary Table S2 shows equations and criteria for the four algorithms used in rimegepant signal detection. In this study, the detection of an adverse event signal was contingent upon its alignment with all four algorithm criteria simultaneously [17–19].
Furthermore, distinctions in rimegepant-associated adverse event signals were explored regarding sex, age, weight, frequency, onset time, and season. The analysis utilized the ROR algorithm and Fisher’s exact test [17–19], based on a fourfold table outlined in Supplementary Table S3. The criteria for the ROR and Fisher’s exact test in detecting differences in rimegepant signals can be found in Supplementary Table S4. The study conducted all data processing and statistical analyses using Jupyter Notebook version 6.4.12.
Results
Descriptive analysis of clinical characteristics
As shown in Fig. 1, a total of 5,416,969 adverse event reports were extracted from the DEMO dataset. Subsequently, 680,728 duplicate cases were identified and removed by FDA recommendations. This resulted in a reduction in the number of adverse event reports to 4,736,241 for subsequent statistical analysis. A total of 5,806 cases and 10,194 adverse events attributed to rimegepant as the PS drug were identified.
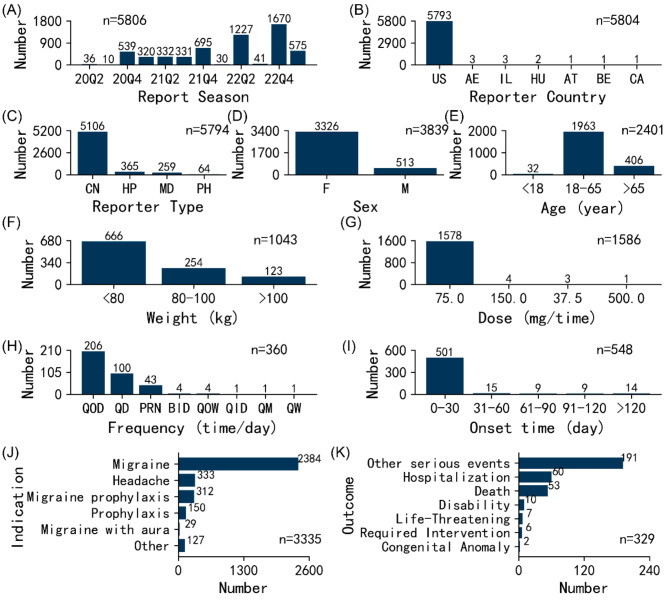
The clinical characteristics of 5,806 adverse event reports associated with rimegepant were analyzed based on available data. Figure 2 illustrates the clinical features of rimegepant-associated adverse event reports. Notably, in terms of report season (the period for which FDA receives reports), during the fourth quarter, a comparatively elevated number of rimegepant-associated adverse event reports was observed in comparison to other quarters. In terms of reporter countries, nearly all reports come from the United States (99.8%, n = 5,793). After excluding reports with unknown sources (n = 12), interestingly, consumers reported the majority of adverse event reports at 88.1% (n = 5,106).
Fig. 2.
Clinical characteristics of rimegepant-associated adverse event reports. (A) Report season. (B) Reporter country. (C) Reporter type. (D) Sex. (E) Age. (F) Weight. (G) Dose. (H) Frequency. (I) Onset time. (J) Indication. (K) Outcome. (CN consumer, HP health-professional, MD medical doctor, PH pharmacist, M male, F female.)
Sex data were accessible for 3,839 cases, revealing that females comprised 86.6% (n = 3,326), while males constituted 13.4% (n = 513). Age data were available for 2,401 cases with an average age of 50.8 years. Notably, patients aged 18–65 years represented the majority of reports, accounting for 81.8% (n = 1,963), followed by those aged > 65 years at 16.9% (n = 406) and < 18 years at 1.3% (n = 32). The characteristics of the age distribution generally match the demographics of migraineurs in general [26]. Weight data were attainable for 1,043 patients, with an average weight of 75.8 kg. The majority of patients weighed < 80 kg, constituting 63.9% (n = 666), followed by those weighing 80–100 kg at 24.4% (n = 254) and > 100 kg at 11.8% (n = 123). Our data aligned well with the baseline characteristics of the migraine treatment group in an open, multicenter, long-term safety study of rimegepant [27].
The recommended dosage for rimegepant in acute migraine treatment is 75.0 mg taken orally as needed, and for episodic migraine prophylaxis, it is 75.0 mg taken orally every other day. After excluding missing data and incomparable doses (“dose_unit” column as “DF” and “MCI”), a total of 1,586 valid dose data points were considered. It was found that 75.0 mg accounted for the vast majority at 99.5% (n = 1,578), followed by 150.0 mg at 0.3% (n = 4), 37.5 mg at 0.2% (n = 3), etc. Regarding dosing frequency, every other day (QOD), daily (QD), and pro re nata (PRN) represented 57.2% (n = 206), 27.8% (n = 100), and 11.9% (n = 43), respectively. Excluding false reports and missing data, 548 cases provided information on the onset time of rimegepant-associated adverse events. Impressively, 91.4% (n = 501) of these events occurred within the first month of administration, followed by 2.7% (n = 15) between 31 and 60 days, 2.6% (n = 14) after 120 days, etc.
After excluding missing data and indications that were ambiguous (“unknown indication”), there were 3,335 valid data points. The predominant indications were “migraine”, “headache”, and “migraine prophylaxis”, constituting 71.5% (n = 2,384), 10.0% (n = 333), and 9.4% (n = 312), respectively. Regarding patient outcomes for rimegepant-associated adverse event reports, 329 outcomes were documented. “Other serious” events and “hospitalizations” were the most frequently reported at 58.1% (n = 191) and 18.2% (n = 60), respectively. Remarkably, 16.1% (n = 53) of reported “deaths” were deemed probably related to rimegepant. Further systematic research is required to determine the causal association between these reported deaths and adverse medication reactions, even though rimegepant is typically well-tolerated and no safety problems were found in clinical trials [6, 27].
Correlation analysis of clinical characteristics
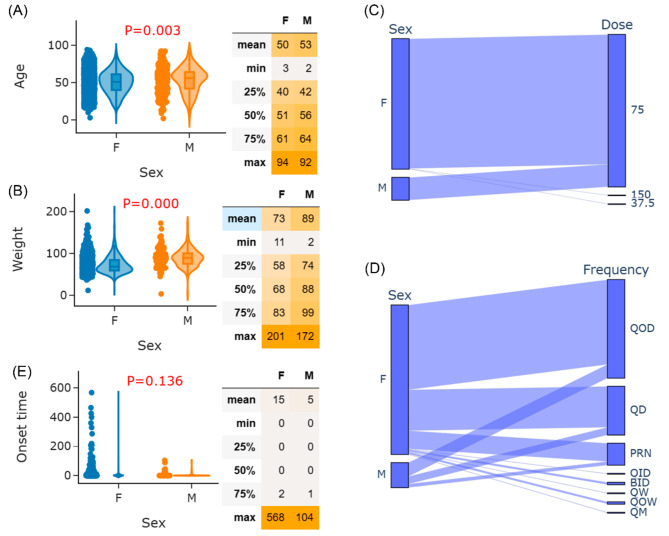
As shown in Fig. 2D, the proportion of rimegepant-associated adverse event reports occurring in females is significantly higher than in males. Correlations between sex and age, weight, dose, frequency, and onset time were further examined for the 5,806 rimegepant-associated adverse event reports. T-tests were first applied to assess differences in age between females and males. The violin plot and T-test result for age by sex are shown in Fig. 3A. A significant age difference was found between females and males (females vs. males: 50 vs. 53 years; P = 0.003), indicating younger females may be more prone to rimegepant-associated adverse events compared to older males. This is closely related to the demographic background of the migraine population, where females consistently outnumber males across all age groups. Specifically, within the 15–39 age range, the incidence rate among females remains higher than that among males [26]. Figure 3B further reveals weight differences. A significant difference in weight was found between females and males (females vs. males: 73 vs. 89 kg; P < 0.001), aligning with population characteristics. These results highlight that close monitoring should be conducted for females using rimegepant, especially those with young age and lightweight.
Fig. 3.
Correlation between typical clinical characteristics of rimegepant-associated adverse event reports. (A) Violin plot and T-test result of age between males and females. (B) Violin plot and T-test result of weight between males and females. (C) Parallel category plot of sex and dose. (D) Parallel category plot of sex and frequency. (E) Violin plot and T-test result of onset time between males and females
For dose, 1,454 cases reported effective dose and sex data simultaneously. To further assess the relevance, a parallel category plot of sex and dose is presented in Fig. 3C. Notably, all patients taking non-recommended doses (150 mg or 37.5 mg) were females. Figure 3D shows a parallel category plot of sex and frequency. Rimegepant frequency among females was more diverse than among males, including QID, BID, QW, QOW, and QM. This underscores the importance of carefully determining appropriate rimegepant dose and frequency for female patients.
Concerning onset time, 512 cases reported effective onset time and sex data simultaneously. T-test examined onset time differences between females and males. The violin plot and T-test result are shown in Fig. 3E. Although the onset time difference between females and males did not reach statistical significance (females vs. males: 15 vs. 5 days; P = 0.136), a discernible trend indicated longer onset times for females. Based on the results of the Global Burden of Disease study, while the age-standardized incidence rates remain relatively stable across different age groups, sex differences persist [26]. Females consistently report higher rates than males, bearing a significantly heavier burden. Moreover, this gap intensifies with age, indicating the need for long-term monitoring of females [26].
Descriptive analysis of concomitant drugs
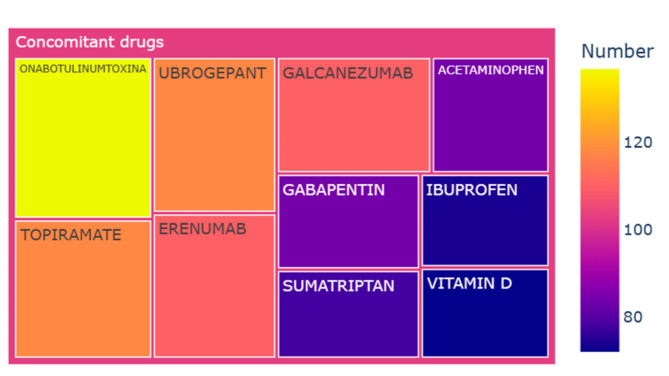
The concomitant drug profiles within the 5,806 rimegepant-associated adverse event reports were notably intricate, encompassing a total of 600 distinct drugs. Figure 4 illustrates the top 10 co-reported drugs in these reports. Of the 5,806 reports, onabotulinumtoxinA was most frequently utilized, accounting for 2.4% (n = 137) of cases. Other commonly used agents included ubrogepant at 2.0% (n = 118), topiramate at 2.0% (n = 118), erenumab at 1.9% (n = 110), galcanezumab at 1.9% (n = 110), gabapentin at 1.4% (n = 84), and acetaminophen at 1.4% (n = 84).
Fig. 4.
Top 10 concomitant drugs co-reported in the rimegepant-associated adverse event reports
Signals of rimegepant
The case number and signal strength of rimegepant at the SOC level are described in Supplementary Table S5. Statistically, rimegepant-associated adverse events involved 27 SOCs. The significant SOC meeting all four detection criteria was “general disorders and administration site conditions” (SOC: 10018065). Additionally, “gastrointestinal disorders” (SOC: 10017947), “nervous system disorders” (SOC: 10029205), “immune system disorders” (SOC: 10021428), and “ear and labyrinth disorders” (SOC: 10013993) were SOCs meeting at least one of the four algorithms.
A total of 57 signals were concurrently identified by all four algorithms. As all PTs originated from the FAERS, 33 signals unrelated to rimegepant were identified. Notably, 9 signals related to therapeutic product effect, including “drug ineffective” (PT: 10013709), “therapeutic product effect incomplete” (PT: 10082200), “therapeutic product effect decreased” (PT: 10082201), “therapeutic product effect variable” (PT: 10082204), etc. may be caused by the progression of the primary disease or reduction in therapeutic effectiveness. The case number and signal strength of unrelated signals at the PT level are listed in Supplementary Table S6.
After excluding the 33 rimegepant-unrelated signals, the case number and signal strength of 24 significant disproportionality PTs are shown in Supplementary Table S7. In this study, “nausea” (PT: 10028813), “abdominal discomfort” (PT: 10000059), “abdominal pain upper” (PT: 10000087), “dyspepsia” (PT: 10013946), “feeling abnormal” (PT: 10016322), “hypersensitivity” (PT: 10020751), “pharyngeal swelling” (PT: 10082270), were consistent with findings from clinical trials. Interestingly, new significant adverse events were uncovered in the label, including “vomiting projectile” (PT: 10047708), “eructation” (PT: 10015137), “motion sickness” (PT: 10027990), “feeling drunk” (PT: 10016330), “reaction to food additive” (PT: 10037977), etc.
Signal differences of rimegepant
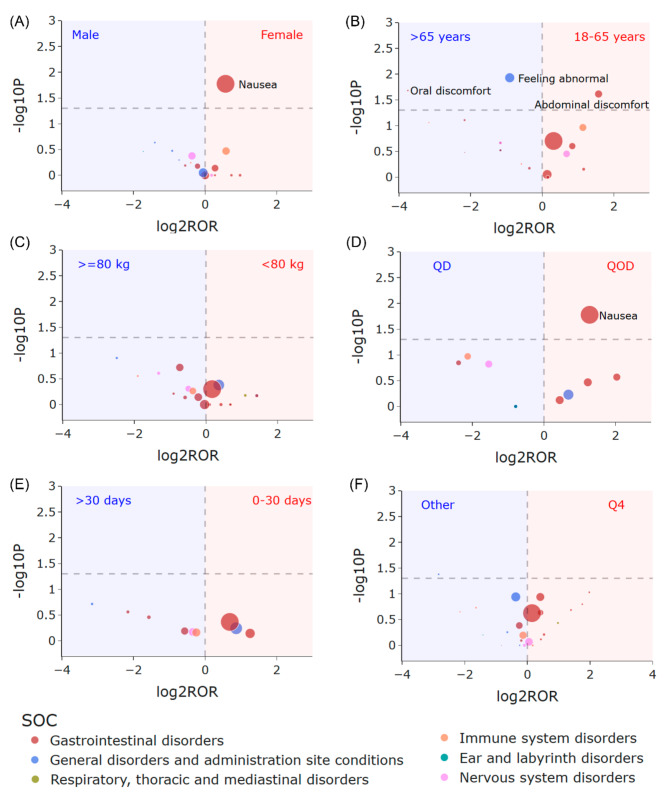
Figure 5 displays volcano plots illustrating the differences in rimegepant signals concerning sex, age, weight, frequency, onset time, and season. No statistically significant differences were observed in terms of weight, onset time, and season. However, notable distinctions were evident concerning sex, age, and frequency. As depicted in Fig. 5A, females exhibited a higher likelihood of experiencing “nausea” compared to males (females vs. males: P 0.017, ROR 1.49, 95% CI 1.07–2.06). Patients over 65 years of age were more prone to reporting “feeling abnormal” (patients with age 18–65 years vs. >65 years: P 0.012, ROR 0.53, 95% CI 0.34–0.85) and “oral discomfort” (patients with age 18–65 years vs. >65 years: P 0.021, ROR 0.074, 95% CI 0.0077-0.71). Conversely, patients aged 18–65 years were more likely to experience “abdominal discomfort” (patients aged 18–65 years vs. >65 years: P 0.024, ROR 2.98, 95% CI 1.08–8.25). Furthermore, individuals taking the medication QOD had a higher propensity for “nausea” compared to those taking it QD (QOD vs. QD: P 0.017, ROR 2.42, 95% CI 1.15–5.10)
Fig. 5.
Volcano plot for difference detection of rimegepant signals. (A) Sex differences between females and males. (B) Age differences between patients with age 18–65 years and > 65 years. (C) Signal difference between patients with weight < 80 kg and > = 80 kg. (D) Signal difference between patients taking frequency of QOD and QD. (E) Signal difference between onset time of 0–30 days and > 30 days. (F) The signal difference between the report season of the fourth quarter and other quarters. The X-axis is the logarithm of the ROR value (log2ROR) based on the ROR algorithm, and the Y-axis is the negative logarithm of the P-value calculated using Fisher’s exact test (− log10P). The colors of the individual points represent different SOCs. The sizes of the individual points represent the case numbers of each PT induced by rimegepant. The larger values in the y-direction represented a strongly significant difference and the bigger size represented a high frequency of each signal at the PT level. In these volcano plots, signals within 24 significant disproportionality PTs of rimegepant are shown
Discussion
Report trends and characteristics analysis
We conducted an in-depth analysis of post-marketing pharmacovigilance data for rimegepant, with a particular focus on reports where rimegepant was identified as the PS drug. The study results revealed several noteworthy phenomena and trends.
First, in terms of reporting season, there was a significant increase in adverse event reports related to rimegepant in 2022. This trend may suggest a growing concern about the safety of rimegepant among the public and healthcare institutions following the FDA’s approval of rimegepant for the preventive treatment of episodic migraine [28]. Additionally, we observed that the number of reports in the fourth quarter of each year was consistently higher than in other quarters. We speculate that this result may be related to environmental changes, though there is a lack of literature to support this hypothesis [29, 30]. To assist direct clinical use, we expect that researchers will continue to examine how environmental changes affect rimegepant’s pharmacokinetics.
Second, reports of adverse events connected to rimegepant are primarily from females, and there is a noteworthy relationship between age and sex. Recent research on the demographics of migraine has revealed that after puberty, females are more likely than males to experience the condition [31]. The Global Burden of Disease study also found that the global burden of migraine increased significantly among those aged 15 to 39, with a higher proportion of females experiencing migraine overall [26]. Although the bulk of reports came from patients between the ages of 18 and 65, our findings suggested that younger females might be more vulnerable to rimegepant-related adverse effects. Females may be more susceptible to recurring negative events in their professional and social life, or more likely to report them, as this age group is going through a vital period in terms of educational attainment, job advancement, and social interactions [26, 28].
In terms of reporter type, adverse events reported by consumers account for the majority. This is an interesting phenomenon. Studies have shown that compared to consumers, adverse events reported by healthcare professionals tend to have higher consistency and more precise categorization [20, 32, 33]. Therefore, the lower proportion of adverse events reported by healthcare professionals suggests that the results should be interpreted with caution [20, 32]. In the future, conducting more detailed subgroup analyses based on whether the reporter is a healthcare professional will be more helpful in assessing the safety of the drug.
Regarding concomitant medication, we found that the number of adverse event reports involving the combined use of rimegepant and erenumab accounted for 1.9% (n = 110), while the combination of rimegepant and topiramate accounted for 2.0% (n = 118). This statistical result may be because rimegepant is primarily metabolized by CYP3A4 and CYP2C9, and concomitant medications could alter the drug’s efficacy and plasma concentration [3, 12]. However, it also highlights that the safety and interaction mechanisms of dual CGRP blockade with combined drugs require further clinical research support.
Existing adverse reactions
We endeavored to conduct a thorough examination of the FAERS database, emphasizing safety indicators that demonstrated a markedly disproportionate correlation with rimegepant. The common and significant SOCs associated with “general disorders and administration site conditions”, “gastrointestinal disorders”, “nervous system disorders”, “ear and labyrinth disorders”, “immune system disorders”, etc., align with the safety data observed in other research [15]. Among the gastrointestinal system category, the most frequently reported adverse events were “nausea”, consistent with recent controlled studies and clinical trials that reported no treatment discontinuations or serious adverse events [14, 34, 35].
New adverse reactions and potential mechanisms
We made an effort to identify novel and unexpected signals of adverse events, but more research is still required to establish causation and verify our findings.
Two gastrointestinal signals were identified: “vomiting projectile” and “eructation”. The effects of CGRP on the gastrointestinal tract include increased intestinal blood flow, gastrointestinal tract smooth muscle relaxation, gastric mucosa protection, decreased gastric emptying, decreased food intake, preservation of oral mucosa integrity, and peristalsis maintenance [15, 36]. Rimegepant’s antagonistic activity on CGRP receptors may result in gastrointestinal symptoms. Further research is necessary to establish the notion that CGRP-mediated modulation of gastrointestinal transit and function may occur through routes that these signals flank. In the interim, we recommend that following the administration of rimegepant, migraine patients undergo routine reviews of their gastroscopy and abdominal ultrasonography to monitor the occurrence of gastrointestinal side effects.
One “motion sickness” signal was found for “ear and labyrinthine disorders”. Uncertainty exists on whether this is a warning sign for safety or a clue that a treatment may not be working [15]. Possible mechanisms involved the participation and prolonged hyperexcitability of the trigeminal vascular system [15].
“Feeling drunk” was the novel neuropsychiatric signal we found, despite the literature’s assertion that rimegepant rarely causes sleepiness. We, therefore, advise patients taking other Central Nervous System (CNS) depressant medications to use rimegepant with caution, abstain from alcohol and marijuana while taking the medication, and refrain from engaging in any activity requiring high alertness, such as operating machinery or driving a car [37].
Three signals about food additives were found in the setting of “Immune system disorders” (“reaction to food additive”, “reaction to excipient”, and “allergic reaction to excipient”). This is the first time that such signals have been documented in the literature. Relevant studies have proposed that individuals with phenylketonuria or any other medical condition should not take rimegepant as it may contain aspartame [37, 38]. Choudhary et al. suggest that aspartame could contribute to adverse neurobehavioral health outcomes, potentially leading to various neurophysiological symptoms like behavioral issues, cognitive problems, anxiety, and migraines [38]. In light of the unclear aspartame dosage in rimegepant and the paucity of information in the pertinent literature, we think more research is necessary to pinpoint the precise mechanism and associated risk factors.
Strengths and limitations
This study has several strengths. First, the FAERS database used represents the most comprehensive repository of post-marketing safety data for pharmaceuticals. Second, our study has identified the largest number of rimegepant cases to date, totaling 5,806 cases and 10,194 adverse events reported. In addition to adverse events consistent with the drug insert and clinical trials, we identified several new significant adverse events, such as “motion sickness”, “reaction to food additive”, and “feeling drunk”. We also found differences and correlations in adverse event reports concerning sex, age, report season, reporter type, and concomitant medications. We hope that this provides a comprehensive and valuable reference for the safety study of rimegepant.
Despite the advantages of large sample size studies and innovative data mining techniques, several limitations must be acknowledged. Firstly, as a spontaneous reporting system, FAERS introduces variability in data quality due to its diverse sources [8]. This diversity complicates controlling for confounding factors such as dosage, treatment duration, comorbidities, and concurrent medications. For instance, medication interactions with onabotulinumtoxinA, ubrogepant, and topiramate might influence rimegepant’s adverse events, while over-the-counter medications like ibuprofen, which protect the gastrointestinal and nervous systems, could mitigate these events [39, 40]. However, this study did not account for these influencing factors.
Secondly, FAERS reports the absolute number of adverse events but lacks data on the total number of patients treated with rimegepant, making it impossible to quantify the incidence of each adverse event or compare event rates across products, which allows only for incidence estimation through signal strength. Thirdly, the voluntary nature of reporting system may lead to underreporting, bias, and a lack of a control group [17]. Consequently, our data cannot be directly compared to the general population. Without baseline data from the general migraine population, determining if specific groups are at higher risk is challenging. Moreover, disproportionality analysis, while assessing signal strength, does not measure risk or establish causality. For instance, while this study found that females were more prone to adverse events, attributing sex as a risk factor is challenging since females have a higher prevalence of migraine and are more likely to use rimegepant.
Another practical issue is the insufficient reporting rate. For example, among the total 5,806 reports, only 360 specified the dosing frequency. It is expected that increased drug usage is expected to yield more reports. Finally, there is no flawless method to identify safety signals from all data sources or adverse event types. Factors like media attention, popu-lation biases, and recent literature may affect reporting behavior. Despite these limitations, our study offers valuable insights for healthcare professionals to monitor patients and adverse events associated with rimegepant.
Conclusion
This study provides important safety information for the clinical use of rimegepant. “Gastrointestinal disorders” remain an area of concern. Issues related to “nervous system disorders”, “immune system disorders”, and “ear and labyrinth disorders” require further investigation to determine their exact mechanisms and risk factors. Furthermore, significant differences in rimegepant-related adverse events were observed across different age groups and sexes, and the complexity of concomitant medication use deserves special attention in clinical practice.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are grateful to all those who reported the rimegepant-associated adverse events in the FAERS database.
Abbreviations
- CGRP
Calcitonin Gene-Related Peptide
- CNS
Central Nervous System
- FDA
Food and Drug Administration
- FAERS
Food and Drug Administration Adverse Event Reporting System
- PS
Primary Suspected
- PT
Preferred Term
- MedDRA
Medical Dictionary for Regulatory Activities
- SOC
System Organ Class
- ROR
Reporting Odds Ratio
- PRR
Proportional Reporting Ratio
- BCPNN
Bayesian Confidence Propagation Neural Network
- MGPS
Multi-item gamma Poisson shrinker
- ESCRS
European Society of Cataract and Refractive Surgeons
Author contributions
J.L. Hu: Formal analysis; Writing original draft; Writing-review & editing. J.Y. Wu: Formal analysis; Writing-review. S.X: Formal analysis; Writing-review. S.Y. Qian: Writing-review & editing. C.J: Formal analysis; Writing-original draft; Writing-review & editing. G.Q. Zheng: Conceptualization; Supervision.All authors have read and agreed to the published version of the manuscript.
Funding
The author(s) declare financial support was received for the research, authorship,and/or publication of this article. This work was supported by the Seed Fund (2021) and the preponderant discipline (2023) of the First Affiliated Hospital of Zhejiang Chinese Medical University (Zhejiang Provincial Hospital of Chinese Medicine).
Data availability
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Declarations
Ethics approval and consent to participate
There was no trackable personal patient or reporter information from the FAERS database.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Cheng Jiang, Email: jiangcheng0818@126.com.
Guo-Qing Zheng, Email: gq_zheng@sohu.com.
References
- 1.Headache Classification Committee of the International Headache Society (IHS) (2018) The international classification of headache disorders 3rd edn. Cephalalgia 38(1): 1–211. 10.1177/0333102417738202 [DOI] [PubMed]
- 2.GBD (2016) Disease and Injury Incidence and Prevalence Collaborators (2017) Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Global Health Metrics 390(10100): 1211–1259. 10.1016/S0140-6736(17)32154-2 [DOI] [PMC free article] [PubMed]
- 3.Rissardo JP, Caprara ALF (2022) Gepants for acute and preventive migraine treatment: a narrative review. Brain Sciences 12(12): 1612. 10.3390/brainsci12121612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiang CC, Schwedt TJ (2020) Calcitonin gene-related peptide (CGRP)-targeted therapies as preventive and acute treatments for migraine—The monoclonal antibodies and gepants. Progress in Brain Research 143–170. 10.1016/bs.pbr.2020.06.019 [DOI] [PubMed]
- 5.Reuter U, Goadsby PJ, et al (2018) Efficacy and tolerability of erenumab in patients with episodic migraine in whom two-to-four previous preventive treatments were unsuccessful: a randomised, double-blind, placebo-controlled, phase 3b study. Lancet 392(10161). 10.1016/S0140-6736(18)32534-0 [DOI] [PubMed]
- 6.Blair HA (2023) Rimegepant: a review in the acute treatment and preventive treatment of migraine. CNS Drugs 37(3):255–265. 10.1007/s40263-023-00988-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lipton R B, Croop R, Stock EG et al (2019) Rimegepant, an oral calcitonin gene–related peptide receptor antagonist, for migraine. New England Journal of Medicine 381(2):142–149. 10.1056/NEJMoa1811090 [DOI] [PubMed] [Google Scholar]
- 8.Xu F, Sun W (2019) Network meta-analysis of calcitonin gene-related peptide receptor antagonists for the acute treatment of migraine. Front Pharmacol 10:795. 10.3389/fphar.2019.00795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao B, Gu S, Shen Z et al (2023) Evaluating ubrogepant-related adverse events using the FDA adverse event reporting system. Expert Opinion on Drug Safety 1–7. 10.1080/14740338.2023.2251390 [DOI] [PubMed]
- 10.Bigal M, Rapoport A et al (2007) Satisfaction with current migraine therapy: experience from 3 centers in US and Sweden. Headache 47(4). 10.1111/j.1526-4610.2007.00752 [DOI] [PubMed]
- 11.Gérard AO, Merino D, Van Obberghen EK et al (2022) Calcitonin gene-related peptide-targeting drugs and Raynaud’s phenomenon: a real-world potential safety signal from the WHO pharmacovigilance database. The Journal of Headache and Pain 23(1):53. 10.1186/s10194-022-01424-w [DOI] [PMC free article] [PubMed]
- 12.Szkutnik-Fiedler D (2020) Pharmacokinetics, pharmacodynamics and drug-drug interactions of new anti-migraine drugs-lasmiditan, gepants, and Calcitonin-Gene-Related Peptide (CGRP) receptor monoclonal antibodies. Pharmaceutics 12(12): 1180. 10.3390/pharmaceutics12121180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berger AA, Winnick A et al (2022) Rimegepant for the treatment of migraine. Health Psychology Research 10(5). 10.52965/001c.38534 [DOI] [PMC free article] [PubMed]
- 14.Tajti J, Szok D et al (2023) The pharmacotherapeutic management of episodic and chronic migraine with gepants. Expert Opin Pharmacother 24(8):947–958. 10.1080/14656566.2023.2201375 [DOI] [PubMed] [Google Scholar]
- 15.Battini V, Carnovale C et al (2023) Ubrogepant and rimegepant: signal detection using spontaneous reports of adverse events from the food and drug administration adverse event reporting System. Expert Opinion on Drug Safety 1–8. 10.1080/14740338.2023.2223958 [DOI] [PubMed]
- 16.Porreca F, Navratilova E et al (2024) Evaluation of outcomes of calcitonin gene-related peptide (CGRP)-targeting therapies for acute and preventive migraine treatment based on patient sex. Cephalalgia: Int J Headache 44(3). 10.1177/03331024241238153 [DOI] [PubMed]
- 17.Jiang C, Qian J et al (2024) Is pitolisant safe for clinical use? A retrospective pharmacovigilance study focus on the post-marketing safety. Pharmacology research & perspectives 12(1). 10.1002/prp2.1161 [DOI] [PMC free article] [PubMed]
- 18.Qi Y, Li J et al (2024) A real-world pharmacovigilance study of FDA adverse event reporting system events for Capmatinib. Scientific reports 14(1). 10.1038/s41598-024-62356-w [DOI] [PMC free article] [PubMed]
- 19.Jiang C, Zheng X et al (2024) A retrospective pharmacovigilance study of post-marketing safety concerns with cefuroxime. Therapeutic Adv Drug Saf 15:20420986241258049. 10.1177/20420986241258049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wen MT, Li JC et al (2024) Indications and adverse events of teriparatide: based on FDA adverse event reporting system (FAERS). Front Pharmacol 15:1391356. 10.3389/fphar.2024.1391356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altebainawi AF, Alfaraj LA et al (2023) Association between proton pump inhibitors and rhabdomyolysis risk: a post-marketing surveillance using FDA adverse event reporting system (FAERS) database. Therapeutic Adv drug Saf 14. 10.1177/20420986231154075 [DOI] [PMC free article] [PubMed]
- 22.Song Y, Xu YL et al (2020) Fractures due to aromatase inhibitor therapy for breast cancer: a real-world analysis of FAERS data in the past 15 Years. Oncology Research and Treatment 43(3). 10.1159/000505376 [DOI] [PubMed]
- 23.Guo M, Shu Y et al (2022) A real-world pharmacovigilance study of FDA adverse event reporting system (FAERS) events for niraparib. Sci Rep 12(1). 10.1038/s41598-022-23726-4 [DOI] [PMC free article] [PubMed]
- 24.Yin Y, Shu Y et al (2022) A real-world pharmacovigilance study of FDA adverse event reporting System (FAERS) events for osimertinib. Sci Rep 12(1):19555. 10.1038/s41598-022-23834-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y, Dong C et al (2022) Post-marketing safety of vemurafenib: a real-world pharmacovigilance study of the FDA adverse event reporting System. J Pharm Pharm Sci 25:377–390. 10.18433/jpps33020 [DOI] [PubMed] [Google Scholar]
- 26.Chen Zfeng, Kong X et al (2024) Global, regional, and national burden and trends of migraine among youths and young adults aged 15–39 years from 1990 to 2021: findings from the global burden of disease study 2021. J Headache Pain 25(1):131. 10.1186/s10194-024-01832-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berman G, Croop R et al (2020) Safety of Rimegepant, an oral CGRP receptor antagonist, plus CGRP monoclonal antibodies for Migraine. Headache 60(8):1734–1742. 10.1111/head.13930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang F, San X et al (2024) Signal mining and risk analysis of Alprazolam adverse events based on the FAERS database. Sci Rep 14(1):7489. 10.1038/s41598-024-57909-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Louis S, Carlson AK et al (2023) Impacts of climate change and air pollution on neurologic health, Disease, and practice: a scoping review. Neurology 100(10):474–483. 10.1212/WNL.0000000000201630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khan S, Siddique R et al (2024) Towards improving the prognosis of stroke through targeting the circadian clock system. Int J Biol Sci 20(2):403–413. 10.7150/ijbs.88370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bugge NS, Vetvik KG et al (2024) Cumulative exposure to estrogen may increase the risk of migraine in women. Cephalalgia: Int J Headache 44(1). 10.1177/03331024231225972 [DOI] [PubMed]
- 32.Liu Y, Li H et al (2024) A real-world pharmacovigilance analysis for transthyretin inhibitors: findings from the FDA adverse event reporting database. Front Pharmacol 15. 10.3389/fphar.2024.1368244 [DOI] [PMC free article] [PubMed]
- 33.Chen C, Ding L et al (2023) Updated insights on dementia-related risk of sacubitril/valsartan: a real-world pharmacovigilance analysis. CNS Neurosci Ther 29(9). 10.1111/cns.14195 [DOI] [PMC free article] [PubMed]
- 34.Scott LJ (2020) Rimegepant First Approval. Drugs 80(7):741–746. 10.1007/s40265-020-01301-3 [DOI] [PubMed] [Google Scholar]
- 35.Dong G, Kjærgaard NA et al (2023) Ubrogepant and Rimegepant: systematic review, meta-analysis, and meta-regression of clinical studies. Exp Opin Drug Saf 22(1):59–70. 10.1080/14740338.2023.2177270 [DOI] [PubMed] [Google Scholar]
- 36.Liang D, Sessa M (2022) Post-marketing safety surveillance of erenumab: new insight from Eudravigilance. Exp Opin Drug Saf 21(9):1205–1210. 10.1080/14740338.2022.2049231 [DOI] [PubMed] [Google Scholar]
- 37.Jinesh S (2023) Pharmaceutical aspects of novel CGRP inhibitors used in the prophylaxis and treatment of migraine. Inflammopharmacology. 10.1007/s10787-023-01276-z [DOI] [PubMed] [Google Scholar]
- 38.Ak C, Yy L (2018) Neurophysiological symptoms and aspartame: What is the connection? Nutritional neuroscience 21(5). 10.1080/1028415X.2017.1288340 [DOI] [PubMed]
- 39.Rainsford KD (2013) Ibuprofen: from invention to an OTC therapeutic mainstay. Int J Clin Pract Suppl (178):9–20. 10.1111/ijcp.12055 [DOI] [PubMed]
- 40.Bjarnason I (2013) Gastrointestinal safety of NSAIDs and over-the-counter analgesics. Int J Clin Pract Suppl (178):37–42. 10.1111/ijcp.12048 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.