Abstract
Objectives
This study examined the potential association between nucleated red blood cell (NRBC) levels and mortality in critically ill patients with acute pancreatitis (AP) in the intensive care unit, due to limited existing research on this correlation.
Methods
This retrospective cohort study utilized data from the MIMIC-IV v2.0 and MIMIC-III v1.4 databases to investigate the potential relationship between NRBC levels and patient outcomes. The study employed restricted cubic splines (RCS) regression analysis to explore non-linear associations. The impact of NRBC on prognosis was assessed using a generalized linear model (GLM) with a logit link, adjusted for potential confounders. Furthermore, four machine learning models, including Gradient Boosting Classifier (GBC), Random Forest, Gaussian Naive Bayes, and Decision Tree Classifier model, were constructed using NRBC data to generate risk scores and evaluate the potential of NRBC in predicting patient prognosis.
Results
A total of 354 patients were enrolled in the study, with 162 (45.8%) individuals aged 60 years or older and 204 (57.6%) males. RCS regression analysis demonstrated a non-linear relationship between NRBC levels and 90-day mortality. Receiver Operating Characteristic (ROC) analysis identified a 1.7% NRBC cutoff to distinguish survivor from non-survivor patients for 90-day mortality, yielding an Area Under the Curve (AUC) of 0.599, with a sensitivity of 0.475 and specificity of 0.711. Elevated NRBC levels were associated with increased risks of 90-day mortality in both unadjusted and adjusted models (all Odds Ratios > 1, P < 0.05). Assessment of various machine learning models with nine variables, including NRBC, Sex, Age, Simplified Acute Physiology Score II, Acute Physiology Score III, Congestive Heart Failure, Vasopressin, Norepinephrine, and Mean Arterial Pressure, indicated that the GBC model displayed the highest predictive accuracy for 90-day mortality, with an AUC of 0.982 (95% CI 0.970–0.994). Post hoc power analysis showed a statistical power of 0.880 in the study.
Conclusions
Elevated levels of NRBC are linked to an increased mortality risk in critically ill patients with AP, suggesting its potential for predicting mortality.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12876-024-03444-z.
Keywords: Critical care, Cohort study, Nucleated red blood cell, Acute Pancreatitis, Risk factors
Introduction
Acute pancreatitis (AP) is a prevalent gastrointestinal disorder characterized by a spectrum of severity, ranging from a mild form affecting solely the pancreas to a severe condition involving multisystem organ failure and mortality [1, 2]. Over the period from 1961 to 2016, the global incidence of AP demonstrated a 3.07% increase [3]. While most patients with AP exhibit mild interstitial edematous pancreatitis and achieve full recovery with supportive therapy within a few days, a subset exceeding 10% experience a more severe disease course necessitating intensive care unit (ICU) hospitalization [4]. Identifying risk factors associated with disease prognosis is essential for physicians to better anticipate disease evolution and patient outcomes, enhancing the provision of tailored treatment and care protocols.
Nucleated red blood cell (NRBC) represent immature forms of erythrocyte precursors that undergo a developmental process involving nucleus expulsion to become reticulocytes, which are the precursor cells to mature red blood cells [5, 6]. The rapid clearance of NRBC by the spleen underscores the efficient removal process within the peripheral blood, with the identification of circulating NRBC in adults often indicative of heightened erythropoietic activity or possible deficiencies in the filtration mechanisms of blood [7]. Such occurrences often indicate bone marrow damage or stress stimulation, which could suggest underlying serious health conditions [5, 8]. Numerous research studies have corroborated the effectiveness of NRBC as a biomarker for diagnosis and prognostication in critical care adult patients. In a prospective study involving medically intensive care patients, the presence of NRBC was identified in 17.5% of cases, with elevated NRBC levels associated with a significant rise in in-hospital mortality (50.7% vs. 9.8%) [9]. In an observational study conducted by Menk et al. [10], NRBC were found to be a predictive marker for mortality in acute respiratory distress syndrome. The study demonstrated an Area Under the Receiver Operating Characteristic (ROC) Curve of 0.71 (95% CI: 0.66–0.75), indicating the significance of NRBC as a prognostic indicator with statistical significance (P < 0.001) [10]. Furthermore, NRBC were associated with increased mortality in patients with surgical sepsis (27% vs. 12%; P < 0.001) [11].
There is a scarcity of investigations regarding the relationship between NRBC levels and mortality in critically ill AP patients. This study aimed to evaluate the influence of NRBC levels on mortality in critically ill AP patients. The study hypothesized that an escalation in NRBC levels would correlate with an increase in mortality among critically ill patients with AP.
Methods
Study design
The retrospective cohort study drew data from the MIMIC database. The study design procedures are depicted in Supplementary Fig. 1 to offer a comprehensive visual representation.
Data sources
In line with the preceding statements, the data utilized in this study were sourced from the MIMIC databases, specifically the MIMIC-IV v2.0 and MIMIC-III v1.4 databases. These databases encompass comprehensive records of all intensive care patients admitted to the ICU at Beth Israel Deaconess Medical Center, providing a rich and varied patient dataset for our analysis (14). Data extraction and analysis were performed by RUAN (15), an author who has successfully completed the Collaborative Institutional Training Initiative (CITI) online training course and obtained authorization to collect and use data from the MIMIC databases (project approval number: 10520411).
Sample size and power analysis
Given the retrospective design of the study, the sample size was predetermined [12]. Post-hoc power analyses were performed using PASS 15.0 software to assess the study’s statistical power. The calculated power from the analysis resulted in 0.880.
Study population, inclusion, and exclusion criteria
The study focused on adult patients undergoing their initial admission to the ICU at Beth Israel Deaconess Medical Center, specifically diagnosed with AP. Inclusion criteria for the AP group were established according to the International Classification of Diseases (ICD) criteria conforming to both ICD-9 and ICD-10 classifications, as detailed in Supplementary Table 1. The exclusion criteria comprised of repeat admissions, pediatric cases, and patients with missing NRBC records. As depicted in Supplement Fig. 1, the combination of the MIMIC-IV v2.0 and MIMIC-III v1.4 databases initially encompassed 1,916 critically ill patients diagnosed with AP. After excluding minors (n = 2), and patients with missing NRBC recordings (n = 1560), the final study cohort comprised 354 patients.
Grouping and study variables
In this study, the levels of NRBC were assessed within 24 h of the patient’s admission to the ICU. Patient demographic information included gender, age, race, specific treatments, medications, and existing medical conditions. Disease severity assessments were conducted within the first 24 h of admission to the ICU using an array of scoring systems to provide a comprehensive evaluation. These systems encompassed the Sequential Organ Failure Assessment (SOFA) score, which evaluates organ dysfunction, the Glasgow Coma Scale (GCS) for neurological assessment, the Simplified Acute Physiology Score II (SAPS II) for physiological stability, the Oxford Acute Severity of Illness Score (OASIS), the Logistic Organ Dysfunction System (LODS), and the Acute Physiology Score III (APS III) for multifaceted severity assessment [13–17]. The Glasgow Coma Scale (GCS) score was utilized as a standardized tool to evaluate and monitor the patient’s level of consciousness and neurological status [18, 19].
The categorical variables were stratified as follows: sex was categorized as male and female based on genetic sex, race was divided into white or non-white, age was segmented into elderly and non-elderly groups with a threshold age of 60 years, comorbidity was categorized based on the presence or absence of comorbidities, and drug use and non-drug use groups were determined by drug consumption or specialized treatments. The coding of comorbidities follows the definitions provided in the ICD9 and ICD10 codes [20]. Variance inflation factors were utilized to assess the covariance among the variables. The results of the multicollinearity tests are shown in Supplementary Table 2, indicating that all variance inflation factors (VIF) were below 10, suggesting no significant issues with multicollinearity.
Clinical outcome
Approximately half of the fatalities associated with AP occur during the first 14 days, marking a pivotal period in the disease progression [21]. Furthermore, for late deaths, the median time to mortality extends to 56 days, with a range spanning from 19 to 81 days [21]. Consequently, the primary clinical endpoint addressed in this study centered on determining the 90-day all-cause mortality rate among critically ill individuals diagnosed with AP. Secondary clinical outcomes encompassed assessing the rates of 28-day all-cause mortality and in-ICU mortality to provide a comprehensive understanding of the disease course and patient outcomes.
Data cleaning
The presence of missing data is a common challenge in clinical medicine datasets reflecting real-world conditions. In this dataset, only one missing value was detected, specifically in the GCS variable, with a count of n = 1 (Supplementary Fig. 2). This missing value was imputed using the median. Subsequently, an examination was conducted to assess the distribution of outliers for the continuous variables included in the dataset. Outliers were identified as data points exceeding 1.5 times the interquartile range (IQR) beyond the upper quartile (Q3) or falling below 1.5 times the IQR beneath the lower quartile (Q1) [22]. Referring to established methods for managing extreme outliers, the maximum value (Q3 + 1.5*IQR) or minimum value (Q1–1.5*IQR) was utilized to replace outliers (Supplementary Fig. 3) [23]. Moreover, to ensure the integrity and credibility of the study findings, supplementary sensitivity analyses were conducted utilizing the raw data. These additional analyses aimed to validate the robustness of the results and enhance the overall strength of the research outcomes.
Tests for non-linear associations
To investigate the nonlinear relationship between NRBC levels and mortality, restricted cubic spline regression (RCS) was applied, with nodes positioned at the 5th, 35th, 65th, and 95th percentile values [24]. Additionally, a trend analysis was conducted by categorizing NRBC levels into three subsets based on trichotomies, which were included as dummy variables in the regression model.
Constructing an NRBC-based mortality risk score
Creating a mortality risk assessment tool based on NRBC levels involved analyzing various factors using advanced statistical techniques like LASSO regression to support machine learning. Our study utilized four different machine learning models - Gaussian Naive Bayes (GNB), Decision Tree Classifier (DTC), Random Forest (RF), and Gradient Boosting Classifier (GBC) - to develop a predictive model based on NRBC levels for predicting mortality risk in patients with AP [25]. The individuals were randomly allocated into training and validation sets at a ratio of 7:3. The training set was employed to instruct the algorithm, the validation set facilitated model selection, while the test set evaluated the ultimate chosen model. The study assessed the effectiveness of each model using evaluation metrics such as the area under the ROC curve and the precision-recall (PR) curve. To understand better how NRBC levels influence outcomes in critically ill AP patients, the study applied the SHapley additive interpretation (SHAP) theory.
The machine learning algorithm with superior predictive capabilities was employed to calculate the probability of 90-day mortality for each patient. Following this, individuals were classified into high-risk and low-to-moderate risk categories using a tertile division based on their respective mortality probabilities. By comparing 90-day mortality between these risk categories, as depicted in Kaplan-Meier survival curves, we were able to assess the predictive accuracy of our model and identify potential differences in outcomes.
Statistical analysis
Statistical analyses were performed using Stata 17.0 software (StataCorp, based in Texas, USA) and R software (version 4.3.0; developed by R Core Team in Vienna, Austria). Descriptive statistics for continuous variables were presented as either mean with standard deviation (SD) for normally distributed data or median with interquartile range (Q1 - Q3) for data that did not follow a normal distribution [26]. Normality of continuous data distribution in the dataset was assessed visually, as shown in Supplementary Fig. 4. Categorical variables were described as counts (n) and percentages (%) [27]. To compare non-normally distributed data sets, the Wilcoxon rank sum test was applied, while the Kruskal-Wallis test was used for comparisons involving multiple groups [28]. The chi-square test was utilized to evaluate associations between categorical variables.
Subgroup analyses and tests for multiplicative interactions were conducted for gender, race, comorbidity, treatment, and age variables. The influence of NRBC levels on patient prognosis was assessed through a generalized linear model (GLM) with a logit link, adjusting for potential confounders. This analysis aimed to investigate the relationship between NRBC levels and the outcomes of 90-day mortality, 28-day mortality, and in-ICU mortality. Statistical significance was defined as a two-tailed P-value of less than 0.05.
Results
Baseline characteristics of study population
In this study, a total of 354 critically ill patients with AP were included. The study cohort was stratified into survivor and non-survivor subsets based on the patients’ survival at 90 days post-admission. Table 1 shows a significant difference in NRBC levels between the non-survivor and survivor groups, with median values of 1 (1, 2) and 1 (1, 3), respectively (P < 0.001). In addition to these findings, the analysis revealed no significant variances in gender and age distributions between the two cohorts, further emphasizing the distinct NRBC levels as a potential marker for prognostic assessment. However, significant variances were evident in the age distribution, disease severity scores, utilization of vasoactive drugs, and the presence of concurrent heart failure and renal failure between the survivor and non-survivor groups (all P < 0.05).
Table 1.
Baseline information of patients included in this study
| Characteristics | Overall (n = 354) | Survivor (n = 232) | Non-survivor (n = 122) | P value |
|---|---|---|---|---|
| NRBC, % | 1 (1, 2) | 1 (1, 2) | 1 (1, 3) | < 0.001 |
| Sex, n (%) | 0.330 | |||
| Female | 150 (42.4%) | 94 (40.5%) | 56 (45.9%) | |
| Male | 204 (57.6%) | 138 (59.5%) | 66 (54.1%) | |
| Race, n (%) | 0.857 | |||
| White | 217 (61.3%) | 143 (61.6%) | 74 (60.7%) | |
| Non-white | 137 (38.7%) | 89 (38.4%) | 48 (39.3%) | |
| Age, n (%) | 57 (46, 70) | 52 (42, 65) | 67 (52, 78) | < 0.001 |
| <60 | 192 (54.2%) | 146 (62.9%) | 46 (37.7%) | |
| ≥60 | 162 (45.8%) | 86 (37.1%) | 76 (62.3%) | |
| MAP, mmHg | 77 (56, 100) | 79 (58, 104) | 71 (52, 95) | 0.016 |
| Critical Care Score | ||||
| SOFA | 9 (5, 12) | 8 (5, 11) | 10 (7, 13) | < 0.001 |
| SAPSII | 44 (33, 55) | 40 (28.75, 53.25) | 50 (41, 58.75) | < 0.001 |
| OASIS | 39 (31, 46) | 37 (30, 44) | 42 (34, 48) | < 0.001 |
| LODS | 7 (4.25, 10) | 6 (4, 9) | 8 (6, 11) | < 0.001 |
| APSIII | 68.5 (49, 90) | 62 (44, 84) | 78 (63, 101.75) | < 0.001 |
| GCS | 9 (3, 14) | 14 (9, 15) | 13 (9, 15) | 0.417 |
| Co-morbidity | ||||
| Diabetes, n (%) | 100 (28.2%) | 64 (27.6%) | 36 (29.5%) | 0.703 |
| CHF, n (%) | 93 (26.3%) | 49 (21.1%) | 44 (36.1%) | 0.002 |
| CPD, n (%) | 69 (19.5%) | 42 (18.1%) | 27 (22.1%) | 0.363 |
| Renal disease, n (%) | 69 (19.5%) | 36 (15.5%) | 33 (27.0%) | 0.009 |
| Cerebrovascular disease, n (%) | 30 (8.5%) | 22 (9.5%) | 8 (6.6%) | 0.348 |
| Treatment | ||||
| Vasopressin, n (%) | 116 (32.8%) | 54 (23.3%) | 62 (50.8%) | < 0.001 |
| Norepinephrine, n (%) | 183 (51.7%) | 100 (43.1%) | 83 (68%) | < 0.001 |
| RRT, n (%) | 29 (8.2%) | 17 (7.3%) | 12 (9.8%) | 0.413 |
Abbreviations: APS III, Acute Physiology Score III; CHF, Congestive Heart Failure; CPD, Chronic Pulmonary Disease; GCS, Glasgow Coma Scale; LODS, Logistic Organ Dysfunction System; MAP, Mean Arterial Pressure; NRBC, Nucleated Red blood Cells; OASIS, Oxford Acute Severity of Illness Score; RRT, renal replacement therapy; SAPS II, Simplified Acute Physiology Score II; SOFA, Sequential Organ Failure Assessment
NRBC exhibited a correlation with the severity of the disease
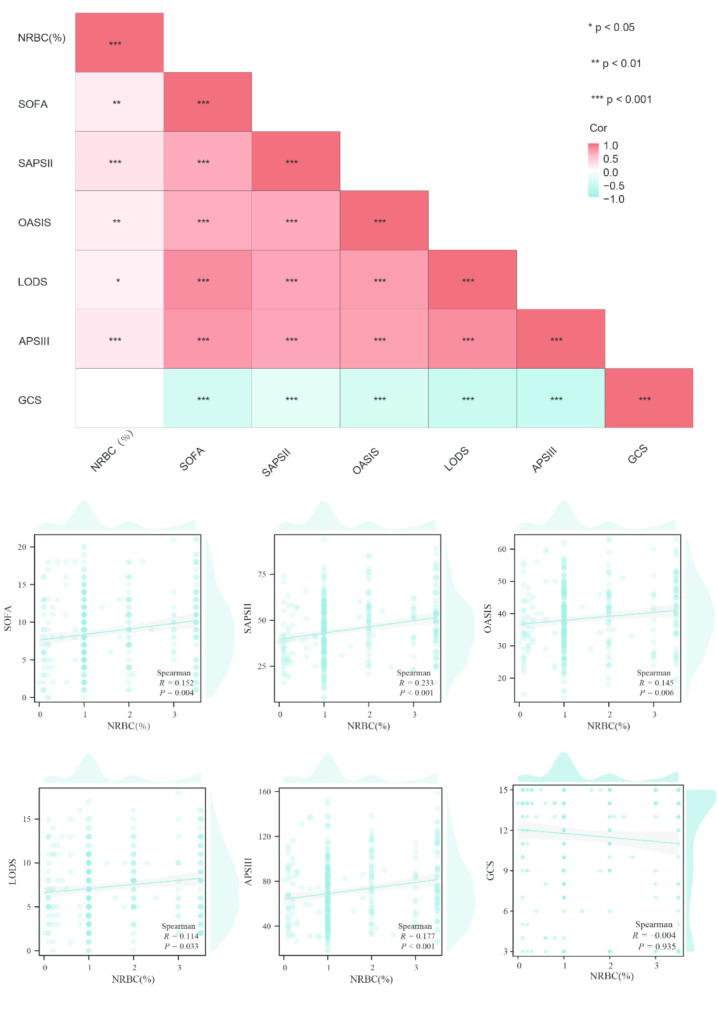
Spearman’s correlation analysis identified significant but weak positive correlations between NRBC levels and various severity scores, except for the GCS score (all Spearman’s |R| < 0.3, Fig. 1). The strongest correlation was observed between NRBC levels and SAPS II (R = 0.233, P < 0.001), followed by APS III score (R = 0.177, P < 0.001), SOFA score (R = 0.152, P < 0.001), OASIS score (R = 0.145, P = 0.006), and LODS score (R = 0.114, P < 0.001). These findings suggest a potential association, indicating that higher NRBC levels may be linked to increased disease severity
Fig. 1.
Correlation analysis between NRBC and disease scores
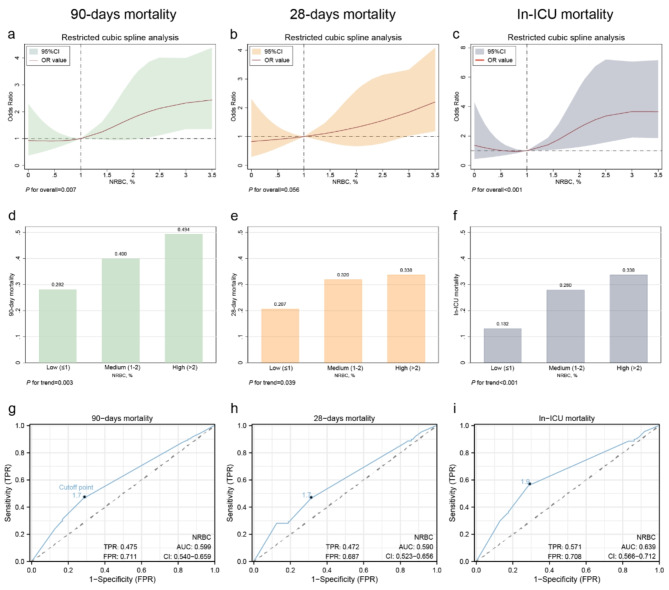
Non-linear association between NRBC and mortality
The RCS regression model identified a nonlinear relationship between NRBC levels and 90-day mortality, as well as in-ICU mortality (P for overall < 0.001, Fig. 2a, b). In contrast, no significant nonlinear association was observed between 28-day mortality and NRBC levels (P for overall > 0.05, Fig. 2c). Subsequently, NRBC levels were stratified into three categories using tertiles: low (NRBC ≤ 1%, n = 227), medium (1–2%, n = 50), and high (NRBC > 2%, n = 77). These groups exhibited increasing trends in all three mortality indicators with higher NRBC levels (90-day mortality: Low vs. Medium vs. High group = 28.2% vs. 40.0% vs. 49.4%; 28-day mortality: Low vs. Medium vs. High group = 20.7% vs. 32.0% vs. 33.8%; In-ICU mortality: Low vs. Medium vs. High group = 13.7% vs. 28.0% vs. 33.8%; all P for trend < 0.05; Fig. 2d-f). ROC curve analysis determined a cutoff NRBC value of 1.7% to differentiate between survivors and non-survivors for 90-day and 28-day mortality, while the cutoff for in-hospital mortality was an NRBC of 1.9% (Fig. 2g-i)
Fig. 2.
Association of NRBC with 90-Day Mortality, 28-Day Mortality, and ICU Mortality (a-c) RCS regression; (d-f) Grouped regression; (g-i) ROC curve. NOTE: The high, medium, and low groupings of NRBC in Fig. 2d-f are established according to the tertile divisions of NRBC levels
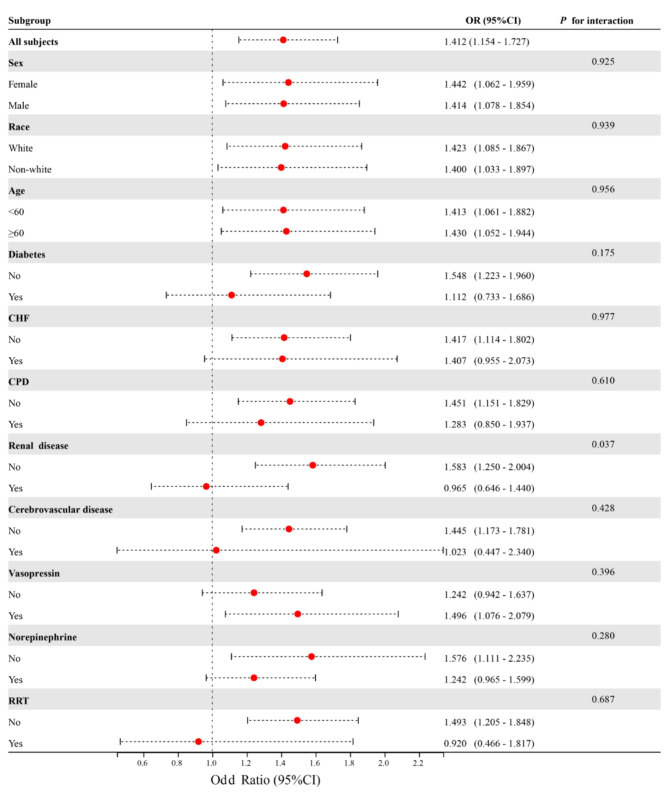
Results of subgroup analyses
To assess potential clinical heterogeneity, this study utilized interaction and stratification analyses (Fig. 3). The findings revealed a significant interaction between NRBC and chronic kidney disease (P for interaction < 0.05). Notably, no significant interactions or stratified analyses were observed for variables such as age (< 60 and ≥ 60 years), sex, race, diabetes mellitus, chronic heart failure, cerebrovascular disease, use of angiotensin, norepinephrine, and Renal Replacement Therapy (RRT)
Fig. 3.
Effect size of NRBC on 90-day mortality in prespecified and exploratory subgroups
NRBC as independent prognostic indicator for 90-day mortality
In this study, GLM-based univariate and multivariate analyses were conducted to assess the prognostic impact of NRBC. The univariate logistic regression analyses revealed a significant association between increased NRBC levels and 90-day mortality (Odds ratio (OR) (95% confidence intervals (CI)) = 1.412 (1.154–1.727), P < 0.001, Table 2), suggesting that heightened NRBC levels act as a risk factor for the occurrence of 90-day mortality in critically ill patients with AP. After a comprehensive multivariate analysis using a GLM regression and adjusting for key variables including age, comorbidities, and critical care scores, the results consistently highlighted the significant association of NRBC levels with 90-day mortality. Specifically, the adjusted OR and its corresponding 95% (CI)were calculated as 1.282 (1.020–1.611), with a statistically significant P-value of 0.033, as detailed in Table 2. These findings underscore the potential prognostic value of NRBC levels in critically ill AP patients.
Table 2.
Univariate and Multivariate GLM Regression Analysis Investigating Association with 90-Day mortality
| Characteristics | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| Odds Ratio (95% CI) | P-value | Odds Ratio (95% CI) | P-value | ||
| NRBC, % | 1.412 (1.154–1.727) | < 0.001 | 1.282 (1.020–1.611) | 0.033 | |
| Sex (Female vs. Male) | 0.803 (0.516–1.249) | 0.330 | |||
| Race (While vs. Non-while) | 1.042 (0.665–1.634) | 0.857 | |||
| Age (< 60 vs. ≥60) | 2.805 (1.783–4.411) | < 0.001 | 2.038 (1.172–3.541) | 0.012 | |
| CHF (No vs. Yes) | 2.107 (1.296–3.424) | 0.003 | 1.743 (0.963–3.155) | 0.066 | |
| CPD (No vs. Yes) | 1.286 (0.747–2.212) | 0.364 | |||
| Diabetes (No vs. Yes) | 1.099 (0.677–1.783) | 0.703 | |||
| Renal disease (No vs. Yes) | 2.019 (1.183–3.446) | 0.010 | 1.057 (0.561–1.993) | 0.864 | |
| Cerebrovascular disease (No vs. Yes) | 0.670 (0.289–1.553) | 0.350 | |||
| Vasopressin (No vs. Yes) | 3.406 (2.134–5.437) | < 0.001 | 1.792 (0.947–3.391) | 0.073 | |
| Norepinephrine (No vs. Yes) | 2.809 (1.772–4.453) | < 0.001 | 1.528 (0.778–3.001) | 0.218 | |
| RRT (No vs. Yes) | 1.380 (0.636–2.991) | 0.415 | |||
| SOFA | 1.096 (1.044–1.150) | < 0.001 | 0.983 (0.887–1.089) | 0.739 | |
| SAPSII | 1.044 (1.028–1.061) | < 0.001 | 1.027 (1.001–1.053) | 0.043 | |
| OASIS | 1.042 (1.019–1.067) | < 0.001 | 0.979 (0.941–1.018) | 0.280 | |
| LODS | 1.129 (1.064–1.199) | < 0.001 | 0.968 (0.844–1.110) | 0.640 | |
| APSIII | 1.019 (1.011–1.027) | < 0.001 | 1.011 (0.993–1.028) | 0.229 | |
| GCS | 0.980 (0.930–1.032) | 0.442 | |||
| MAP, mmHg | 0.991 (0.984–0.998) | 0.015 | 0.994 (0.987–1.002) | 0.160 | |
Abbreviations: APS III, Acute Physiology Score III; CHF, Congestive Heart Failure; CPD, Chronic Pulmonary Disease; GCS, Glasgow Coma Scale; GLM, generalized linear model; LODS, Logistic Organ Dysfunction System; MAP, Mean Arterial Pressure; NRBC, Nucleated Red blood Cells; OASIS, Oxford Acute Severity of Illness Score; RRT, renal replacement therapy; SAPS II, Simplified Acute Physiology Score II; SOFA, Sequential Organ Failure Assessment
NRBC levels as risk factor for mortality
This study further examined the influence of NRBC levels on 90-day mortality, 28-day mortality, and in-ICU mortality using different models (Table 3). In the unadjusted original model, NRBC level emerged as a significant risk factor for all mortality outcomes (90-day mortality: OR (95% CI) = 1.412 (1.154–1.727); 28-day mortality: OR (95% CI) = 1.349 (1.088–1.673); in-ICU mortality: OR (95% CI) = 1.586 (1.258–2.000); all P < 0.01). Model 1, adjusting for age and sex, indicated that NRBC levels remained a risk factor for all mortality endpoints (all OR > 1, P < 0.01). Similarly, in Model 2, which further adjusted for age, sex, and race, NRBC levels were consistently associated with increased mortality risk (all OR > 1, P < 0.01). Model 3, encompassing all confounding factors affecting prognosis, confirmed that NRBC level continued to be a risk factor for both 90-day mortality and In-ICU mortality (90-day mortality: OR (95% CI) = 1.282 (1.020–1.611); In-ICU mortality: OR (95% CI) = 1.444 (1.084–1.923); P < 0.05). However, NRBC levels did not demonstrate a significant impact on 28-day mortality outcomes (OR (95% CI) = 1.162 (0.903–1.494), P = 0.242).
Table 3.
Effect of NRBC on primary and secondary clinical outcomes
| Model (All subjects = 354) |
90-day mortality (n = 122, 34.46%) |
28-day mortality (n = 89, 25.14%) |
In-ICU mortality (n = 70, 19.77%) |
|---|---|---|---|
| Crude model OR (95% CI) | 1.412 (1.154–1.727) | 1.349 (1.088–1.673) | 1.586 (1.258–2.000) |
| P-value | < 0.001 | 0.006 | < 0.001 |
| Adjusted Model1 OR (95% CI) | 1.438 (1.165–1.776) | 1.391 (1.108–1.746) | 1.627 (1.284–2.062) |
| P-value | 0.001 | 0.004 | < 0.001 |
| Adjusted Model2 OR (95% CI) | 1.436 (1.162–1.776) | 1.374 (1.093–1.726) | 1.609 (1.269–2.041) |
| P-value | 0.001 | 0.006 | < 0.001 |
| Adjusted Model3 OR (95% CI) | 1.282 (1.020–1.611) | 1.162 (0.903–1.494) | 1.444 (1.084–1.923) |
| P-value | 0.033 | 0.242 | 0.012 |
Note: Crude model: unadjusted for confounding factors; Model1: adjusted for sex and age; Model2: adjusted for sex, race, and age; Model3: adjusted for age, CHF, Renal disease, Vasopressin, Norepinephrine, SOFA, SAPSII, OASIS, LODS, APSIII, and MAP. Abbreviations: APS III, Acute Physiology Score III; CHF, Congestive Heart Failure; LODS, Logistic Organ Dysfunction System; MAP, Mean Arterial Pressure; NRBC, Nucleated Red blood Cells; OASIS, Oxford Acute Severity of Illness Score; SAPS II, Simplified Acute Physiology Score II; SOFA, Sequential Organ Failure Assessment
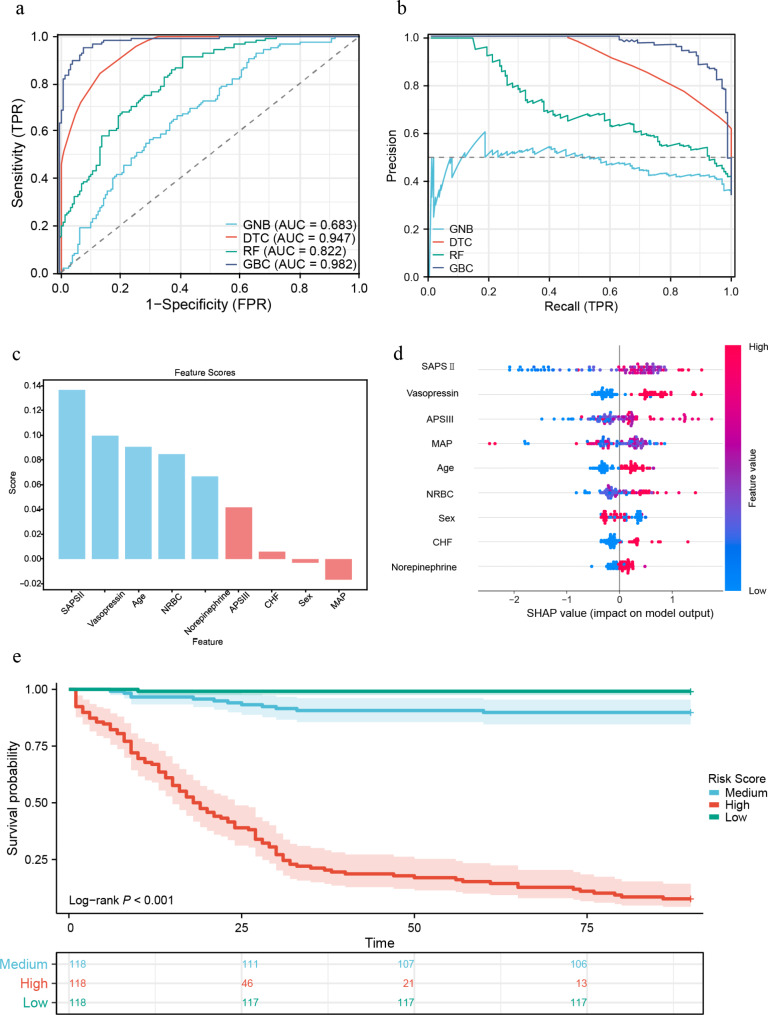
The NRBC-based risk score efficiently predicts 90-day mortality
Through LASSO regression, nine variables, including NRBC, Sex, Age, SAPSII, APSIII, CHF, Vasopressin, Norepinephrine, and MAP, were evaluated for inclusion in the machine learning models (Supplementary Fig. 5a, b). Subsequently, four distinct machine learning models were generated based on the features selected by LASSO regression, with the parameter optimization process demonstrated in Supplementary Fig. 5c-f. Among the models, the GBC model demonstrated the most effective predictive performance, highlighted by both the ROC curve and diagnostic PR curve (AUC (95% CI) = 0.982(0.970–0.994), Fig. 4a, b). Notably, NRBC ranked fourth in terms of feature importance within the model (Feature NRBC score: 0.085, Fig. 4c). Further interpretation from SHAP analysis revealed a direct correlation between elevated levels of NRBC and an increased risk of mortality in patients with AP (Fig. 4d). Based on the risk scores classified as high, medium, and low, survival curves illustrated notable discrepancies among the different risk subgroups (log-rank P < 0.001, Fig. 4e).
Fig. 4.
The NRBC-based risk score efficiently predicts 90-day Mortality (a) ROC curves for the performance of various machine learning models in predicting 90-day mortality are as follows: GBC model AUC (95% CI) = 0.982 (0.970–0.994); DTC model AUC (95% CI) = 0.947 (0.928–0.967); RF model AUC (95% CI) = 0.822 (0.779–0.865); GNB model AUC (95% CI) = 0.683 (0.627–0.739); (b) Precision-Recall curves for various machine learning models illustrate performance metrics, with a greater curve towards the upper right corner indicating superior model performance.; (c) Ranking of feature variable importance in the GBC models; (d) SHapley Additive Explanation visualization illustrating the individual feature impacts on the outcomes of the GBC prediction model. The color spectrum represents the degree of influence, where redder hues indicate a higher association with the risk of 90-day Mortality, while bluer hues suggest a lower association with the risk of 90-day Mortality; (e) Survival curve analysis in subgroups with different risk scores. NOTE: The three subgroups for the survival curve analyses were established based on the tertiles derived from the mortality probability generated by the GBC prognostic model constructed using the 9 variables
Sensitivity analysis
To ensure the robustness of the findings, this study conducted two sensitivity analyses. Firstly, the raw data were reanalyzed, and consistent results were observed across all scenarios (Supplementary Tables 3–4, Supplementary Figs. 6–7). Secondly, NRBC levels were categorized as a discrete variable, dividing participants into three subgroups representing low, intermediate, and high NRBC levels. Kaplan-Meier survival analyses depicted that high NRBC levels were associated with poorer outcomes compared to low NRBC levels (log-rank test, P = 0.001, Supplementary Fig. 8).
Discussion
The primary aim of this study was to explore the correlation between NRBC levels and mortality in patients with AP. Our findings indicated significantly elevated NRBC levels in non-surviving AP patients compared to survivors. Both univariate and multivariate GLM regression analyses recognized NRBC as an independent predictor of 90-day mortality in this patient cohort. NRBC levels exhibited a consistent association with 90-day mortality across various confounder-corrected models, underscoring the robustness of the results. Nonlinear correlation analysis unveiled a non-linear impact of NRBC levels on 90-day mortality. Moreover, the study established the moderate predictive value of NRBC levels for 90-day mortality in AP patients, identifying a cut-off point of 1.7% through ROC curve analysis. Additionally, four machine learning models based on NRBC levels were developed and evaluated, with the optimal prognostic model determined and its predictive performance assessed in patients with AP. Overall, the study highlighted the practical clinical utility of NRBC in prognosticating outcomes in patients with AP.
This finding is supported by prior clinical studies highlighting the importance of NRBC as a significant clinical predictor of mortality in critically ill patients [5, 9, 29–31]. In healthy individuals, NRBC are typically absent in peripheral blood; however, their presence in acute care settings often signifies severely ill patients with a less favorable prognosis [29]. A retrospective study by Shah et al. [29], involving 9,690 patients in a surgical ICU, identified a correlation between elevated NRBC levels and heightened mortality risk among critically ill surgical patients. Similarly, a prospective cohort study in a cardiac ICU, focusing on patients with cardiovascular disease, demonstrated that NRBC could serve as a prognostic indicator for all-cause mortality in individuals with cardiovascular issues [30]. This association was also observed among COVID-19 patients admitted to the ICU [31]. Additionally, in agreement with Stachon et al. [32], our study found that NRBC did not significantly impact individuals with concurrent chronic kidney disease, suggesting that the presence of NRBC in the blood may not be closely related to renal failure.
The study by Xu et al. [33] conducted a retrospective analysis involving 92 severe pancreatitis patients to develop a mortality prediction model for severe acute pancreatitis (SAP) by incorporating NRBC levels. Their research highlighted that NRBC, when integrated with factors such as CCIs, APACHE II, and Ranson score, could serve as indicators for predicting 90-day mortality in SAP patients. Nevertheless, the limited number of positive outcome events (n = 11) in their study might constrain the applicability of their model. Additionally, their study was confined to general ward patients and did not encompass data from ICU patients. Building upon the insights from this prior research, our study delves into a more comprehensive assessment of NRBC levels in critically ill AP patients. In the study, the optimal cut-off point for NRBC levels was determined to be 1.7% based on the ROC curve analysis for predicting 90-day mortality, with a sensitivity of 0.475 and a specificity of 0.711. While these values suggest medium-low sensitivity and specificity in predicting severity, a machine learning strategy was employed in our approach to develop a prognostic model that integrated NRBC levels with other clinical parameters, effectively enhancing the predictive performance of the model (GBC model AUC = 0.982). This illustrates the practical application of utilizing NRBC levels in clinical medicine.
Elevated levels of NRBC in the intensive care setting may signify poor outcomes, but the exact mechanisms by which NRBC contributes to increased mortality in critically ill patients are not yet fully understood. Various studies have provided potential insights from different perspectives. Firstly, hypoxemia is more prevalent in NRBC-positive patients compared to NRBC-negative individuals [34]. Secondly, rapid hemolysis or acute blood loss can result in NRBC presence in the bloodstream, possibly due to enhanced erythropoiesis as a compensatory response to acute anemia [6]. Lastly, infections may also contribute to elevated NRBC levels [35]. NRBC-positive patients tend to have higher levels of cytokines and erythropoietin compared to NRBC-negative patients, suggesting that NRBC can potentially serve as biomarkers for hypoxia and inflammatory damage [36]. Our study demonstrated that elevated NRBC levels are an independent prognostic indicator for 90-day mortality, supporting previous findings while expanding the potential applications of this research.
One of the strengths of this study is its large sample size sourced from a publicly available dataset, enabling a comprehensive exploration of the relationship between NRBC levels and prognosis in critically ill patients with AP. Unlike prior studies that primarily focus on patients in general wards, this study emphasizes patients with AP in ICU, contributing valuable insights to the literature concerning this specific patient population. The study utilized a robust study design and rigorous statistical methods to enhance the reliability of the findings, particularly by investigating the non-linear relationship between NRBC levels and clinical prognosis. However, the study has some limitations. Firstly, being retrospective, it could only establish an association between NRBC levels and 90-day mortality without proving causality and might have overlooked potential confounding variables influencing the results. Secondly, the absence of specific subtypes of AP in the database could have impacted the outcomes.
Overall, this study underscores the significance of NRBC as a prognostic marker for AP and underscores the importance of integrating NRBC assessment into the management of patients with this condition. Future research could expand on this work with larger prospective studies to further validate the prognostic value of NRBC in critically ill AP patients.
Conclusions
The elevation of NRBC levels correlates with an increased risk of 90-day all-cause mortality in critically ill patients with AP, suggesting that NRBC has the potential to be incorporated with other indicators to establish a predictive model for mortality forecasting.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We extend our heartfelt gratitude to all the personnel involved in establishing the Medical Information Marketplace in Intensive Care database, creating a valuable data platform for clinical big data research endeavors.
Abbreviations
- APS III
Acute Physiology Score III
- AP
Acute pancreatitis
- CHF
Congestive Heart Failure
- CI
Confidence intervals
- CITI
Collaborative Institutional Training Initiative
- CPD
Chronic Pulmonary Disease
- DTC
Decision Tree Classifier
- GBC
Gradient Boosting Classifier
- GCS
Glasgow Coma Scale
- GLM
Generalized linear model
- GNB
Gaussian Naive Bayes
- ICD
International Classification of Diseases
- ICU
Intensive care unit
- IQR
Interquartile range
- LODS
Logistic Organ Dysfunction System
- MAP
Mean Arterial Pressure
- NRBC
Nucleated Red Blood Cell
- OASIS
Oxford Acute Severity of Illness Score
- OR
Odds ratio
- PR
Precision-recall
- RBC
Restricted Cubic Spline
- RF
Random Forest Classifier
- ROC
Receiver operating characteristic
- RRT
Renal Replacement Therapy
- SAP
Severe Acute Pancreatitis
- SAPS II
Simplified Acute Physiology Score II
- SD
Standard Deviation
- SHAP
SHapley Additive Interpretation
- SOFA
Sequential Organ Failure Assessment
- STROBE
Strengthening the Reporting of Observational studies in Epidemiology
Author contributions
H-Q. Liu., G-Q. W., X. W., and C-S. Z. was responsible for the study design, and the initial draft of the manuscript. Data collection, and statistical analyses was undertaken by H. R. J-K. S. and F. Q. contributed to revising the manuscript critically for important intellectual content and approved the final version for publication. All authors reviewed the manuscript critically for important intellectual content and have read and approved the final manuscript.
Funding
This work was supported by the Medical and Health Science and Technology Development Project of Shandong Province (202319010449).
Data availability
The datasets and codes employed in this study can be accessed from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Approval to conduct this study was granted by the Ethics Committee of Jining NO.1 People’s Hospital (approval number: KYLL-202311-193), ensuring adherence to ethical standards in the research process. Given the retrospective nature of the study, the need for written informed consent from patients was waived subsequent to a thorough evaluation and approval by the ethics committee, aligning with regulations governing retrospective research protocols.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ji-kui Shi, Email: sjkjnrmyy@126.com.
Feng Qu, Email: rmyyzzeq@163.com.
References
- 1.Sinonquel P, Laleman W, Wilmer A. Advances in acute pancreatitis. 2021, 27(2):193–200. [DOI] [PubMed]
- 2.Pandol SJ, Saluja AK, Imrie CW, Banks PA. Acute pancreatitis: bench to the bedside. Gastroenterology. 2007;132(3):1127–51. [DOI] [PubMed] [Google Scholar]
- 3.Iannuzzi JP, King JA, Leong JH, Quan J, Windsor JW, Tanyingoh D, Coward S, Forbes N, Heitman SJ, Shaheen A-A, et al. Global incidence of Acute Pancreatitis is increasing over time: a systematic review and Meta-analysis. Gastroenterology. 2022;162(1):122–34. [DOI] [PubMed] [Google Scholar]
- 4.Sternby H, Bolado F, Canaval-Zuleta HJ, Marra-López C, Hernando-Alonso AI, del-Val-Antoñana A, García-Rayado G, Rivera-Irigoin R, Grau-García FJ, Oms L et al. Determinants of severity in Acute Pancreatitis: a Nation-wide Multicenter prospective cohort study. 2019, 270(2):348–55. [DOI] [PubMed]
- 5.Pikora K, Kretowska-Grunwald A, Krawczuk-Rybak M, Sawicka-Zukowska M. Diagnostic value and prognostic significance of nucleated red blood cells (NRBC) in selected medical conditions. Cells 2023, 12(14). [DOI] [PMC free article] [PubMed]
- 6.May JE, Marques MB, Reddy VVB, Gangaraju R. Three neglected numbers in the CBC: the RDW, MPV, and NRBC count. Cleve Clin J Med. 2019;86(3):167–72. [DOI] [PubMed] [Google Scholar]
- 7.Amundsen EK, Binde C, Christensen EE, Klingenberg O, Kvale D, Holten AR. Prognostic value of nucleated RBCs for patients with suspected Sepsis in the Emergency Department: a single-center prospective cohort study. Crit care Explorations. 2021;3(7):e0490–0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Constantino BT, Cogionis B. Nucleated RBCs - significance in the peripheral blood film. Lab Med. 2000;31(4):223–9. [Google Scholar]
- 9.Stachon A, Segbers E, Holland-Letz T, Kempf R, Hering S, Krieg M. Nucleated red blood cells in the blood of medical intensive care patients indicate increased mortality risk: a prospective cohort study. Crit Care 2007, 11(3). [DOI] [PMC free article] [PubMed]
- 10.Menk M, Giebelhäuser L, Vorderwülbecke G, Gassner M, Graw JA, Weiss B, Zimmermann M, Wernecke KD, Weber-Carstens S. Nucleated red blood cells as predictors of mortality in patients with acute respiratory distress syndrome (ARDS): an observational study. Ann Intensiv Care 2018, 8. [DOI] [PMC free article] [PubMed]
- 11.Desai S, Jones SL, Turner KL, Hall J, Moore LJ. Nucleated red blood cells are Associated with a higher mortality rate in patients with Surgical Sepsis. Surg Infect. 2012;13(6):360–5. [DOI] [PubMed] [Google Scholar]
- 12.Gauss T, Ageron F-X, Devaud M-L, Debaty G, Travers S, Garrigue D, Raux M, Harrois A, Bouzat P, Abback P, et al. Association of Prehospital Time to In-Hospital trauma mortality in a physician-staffed Emergency Medicine System. Jama Surg. 2019;154(12):1117–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vincent JL, de Mendonca A, Cantraine F, Moreno R, Takala J, Suter PM, Sprung CL, Colardyn F, Blecher S. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Crit Care Med. 1998;26(11):1793–800. [DOI] [PubMed] [Google Scholar]
- 14.Le Gall JR, Lemeshow S, Saulnier F. A new simplified Acute Physiology score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270(24):2957–63. [DOI] [PubMed] [Google Scholar]
- 15.Chen QG, Zhang LS, Ge SH, He WM, Zeng M. Prognosis predictive value of the Oxford Acute severity of illness score for sepsis: a retrospective cohort study. Peerj 2019, 7. [DOI] [PMC free article] [PubMed]
- 16.Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23(10):1638–52. [DOI] [PubMed] [Google Scholar]
- 17.Knaus WA, Wagner DP, Draper EA, Zimmerman JE, Bergner M, Bastos PG, Sirio CA, Murphy DJ, Lotring T, Damiano A. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100(6):1619–36. [DOI] [PubMed] [Google Scholar]
- 18.Teasdale G, Maas A, Lecky F, Manley G, Stocchetti N, Murray G. The Glasgow Coma Scale at 40 years: standing the test of time. Lancet Neurol. 2014;13(8):844–54. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Xia J, Shan Y, Yang Y, Li Y, Sun H. Predictive value of the Oxford Acute severity of illness score in acute stroke patients with stroke-associated pneumonia. Front Neurol 2023, 14. [DOI] [PMC free article] [PubMed]
- 20.Quan HD, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–9. [DOI] [PubMed] [Google Scholar]
- 21.Mutinga M, Rosenbluth A, Tenner SM, Odze RR, Sica GT, Banks PA. Does mortality occur early or late in acute pancreatitis? Int J Pancreatol. 2000;28(2):91–5. [DOI] [PubMed] [Google Scholar]
- 22.Zhao A, Zhang L, Zhang X, Edirisinghe I, Burton-Freeman BM, Sandhu AK. Comprehensive characterization of bile acids in human Biological samples and effect of 4-Week Strawberry Intake on bile acid composition in human plasma. In: Metabolites 11; 2021. [DOI] [PMC free article] [PubMed]
- 23.Reel PS, Reel S, van Kralingen JC, Langton K, Lang K, Erlic Z, Larsen CK, Amar L, Pamporaki C, Mulatero P et al. Machine learning for classification of hypertension subtypes using multi-omics: a multi-centre, retrospective, data-driven study. Ebiomedicine 2022, 84. [DOI] [PMC free article] [PubMed]
- 24.Ruan H, Li S-S, Zhang Q, Ran X. Elevated MMP-8 levels, inversely associated with BMI, predict mortality in mechanically ventilated patients: an observational multicenter study. Crit Care (London England). 2023;27(1):290–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruan H, Ran X, Li SS, Zhang Q. Dyslipidemia versus obesity as predictors of ischemic stroke prognosis: a multi-center study in China. Lipids Health Dis 2024, 23(1). [DOI] [PMC free article] [PubMed]
- 26.Ismaiel A, Spinu M, Leucuta D-C, Popa S-L, Chis BA, Stanculete MF, Olinic DM, Dumitrascu DL. Anxiety and Depression in Metabolic-Dysfunction-Associated fatty liver Disease and Cardiovascular Risk. J Clin Med 2022, 11(9). [DOI] [PMC free article] [PubMed]
- 27.Zhang D, Wang T, Dong X, Sun L, Wu Q, Liu J, Sun X. Systemic Immune-inflammation index for Predicting the prognosis of critically ill patients with Acute Pancreatitis. Int J Gen Med. 2021;14:4491–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruan H, Li Y-z, Zhang Q, Wang B-r, Wu R, Li S-s, Ran X. IDENTIFICATION AND CLINICAL VALIDATION OF HYPOXIA-INDUCIBLE FACTOR 1a PROTEIN AS THE POTENTIAL BIOMARKER IN PATIENTS WITH SEPSIS. Shock. 2023;59(6):855–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah R, Reddy S, Horst HM, Stassinopoulos J, Jordan J, Rubinfeld I. Getting back to zero with nucleated red blood cells: following trends is not necessarily a bad thing. Am J Surg. 2012;203(3):343–5. [DOI] [PubMed] [Google Scholar]
- 30.Monteiro JGD, Torres DDC, da Silva M, Ramos TMD, Alves ML, Nunes WJ, Damasceno EP, Brunet AF, Bittencourt MS, Pedrosa RP et al. Nucleated red blood cells as predictors of all-cause mortality in Cardiac Intensive Care Unit patients: a prospective cohort study. PLoS ONE 2015, 10(12). [DOI] [PMC free article] [PubMed]
- 31.Düz ME, Arslan M, Menek EE, Avci BY. Impact of the seventh day nucleated red blood cell count on mortality in COVID-19 intensive care unit patients: a retrospective case-control study. J Med Biochem. 2023;42(1):138–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stachon A, Holland-Letz T, Kempf R, Becker A, Friese J, Krieg M. Poor prognosis indicated by nucleated red blood cells in peripheral blood is not associated with organ failure of the liver or kidney. Clin Chem Lab Med. 2006;44(8):955–61. [DOI] [PubMed] [Google Scholar]
- 33.Xu CX, Wang J, Jin XX, Yuan Y, Lu GG. Establishment of a predictive model for outcomes in patients with severe acute pancreatitis by nucleated red blood cells combined with Charlson complication index and APACHE II score. Turkish J Gastroenterol. 2020;31(12):936–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuert S, Holland-Letz T, Friese J, Stachon A. Association of nucleated red blood cells in blood and arterial oxygen partial tension. Clin Chem Lab Med. 2011;49(2):257–63. [DOI] [PubMed] [Google Scholar]
- 35.Romero R, Savasan ZA, Chaiworapongsa T, Berry SM, Kusanovic JP, Hassan SS, Yoon BH, Edwin S, Mazor M. Hematologic profile of the fetus with systemic inflammatory response syndrome. J Perinat Med. 2012;40(1):19–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stachon A, Bolulu O, Holland-Letz T, Krieg M. Association between nucleated red blood cells in blood and the levels of erythropoietin, interleukin 3, interleukin 6, and interleukin 12p70. Shock. 2005;24(1):34–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets and codes employed in this study can be accessed from the corresponding author upon reasonable request.