Abstract
Background:
Anterior cruciate ligament (ACL) injury is frequently associated with injuries to other parts of the knee, including the menisci and articular cartilage. After ACL injury and reconstruction, there may be progressive chondral degradation. Biomarkers in blood, urine, and synovial fluid can be measured after ACL injury and reconstruction and have been proposed as a means of measuring the associated cellular changes occurring in the knee.
Purpose:
To systematically review the literature regarding biomarkers in urine, serum, or synovial fluid that have been associated with an outcome measure after ACL reconstruction.
Study Design:
Systematic review; Level of evidence, 3.
Methods:
This review was performed according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. The MEDLINE, Embase, CINAHL, and Web of Science databases were searched to identify studies published before September 2023 that reported on patients undergoing ACL reconstruction where a biomarker was measured and related to an outcome variable. Of 9360 results, 16 studies comprising 492 patients were included. Findings were reported as descriptive summaries synthesizing the available literature.
Results:
A total of 45 unique biomarkers or biomarker ratios were investigated (12 serum, 3 urine, and 38 synovial fluid; 8 biomarkers were measured from >1 source). Nineteen different outcome measures were identified, including the International Knee Documentation Committee Subjective Knee Form, Knee injury and Osteoarthritis Outcome Score, numeric pain scores, radiological outcomes (magnetic resonance imaging and radiography), rates of arthrofibrosis and cyclops lesions, and gait biomechanics. Across the included studies, 17 biomarkers were found to have a statistically significant association (P < .05) with an outcome variable. Serum interleukin 6 (s-IL-6), serum and synovial fluid matrix metalloproteinase-3 (s-MMP-3 and sf-MMP-3), urinary and synovial fluid C-terminal telopeptide of type 2 collagen (u-CTX-II and sf-CTX-II), and serum collagen type 2 cleavage product (s-C2C) showed promise in predicting outcomes after ACL reconstruction, specifically regarding patient-reported outcome measures (s-IL-6 and u-CTX-II), gait biomechanical parameters (s-IL-6, sf-MMP-3, s-MMP-3, and s-C2C), pain (s-IL-6 and u-CTX-II), and radiological osteoarthritis (ratio of u-CTX-II to serum procollagen 2 C-propeptide).
Conclusion:
The results highlight several biomarkers that have been associated with clinically important postoperative outcome measures and may warrant further research to understand if they can provide meaningful information in the clinical environment.
Keywords: anterior cruciate ligament, biomarker, osteoarthritis, patient-reported outcome measures, blood, serum, urine, synovial fluid
Injury to the anterior cruciate ligament (ACL) results in many changes to the knee joint occurring at many different levels. In addition to changes to the biomechanical environment and joint kinematics, synovial fluid sampling studies suggest changes also occur at the cellular level very soon after ACL injury22,27,30,43 and ACL reconstruction.22,30,35
The measurement of biomarkers has been proposed as a means of evaluating these cellular changes, and there has been significant interest in the context of osteoarthritis outside of the ACL arena.5,22,33,34,52 In the osteoarthritis setting, it has been proposed that biomarkers may have a role in evaluating Burden of disease, be Investigative or Prognostic, have use in evaluating the Efficacy of an Intervention or a Diagnostic role - the BIPED approach as described by Bauer et al. 5 Biomarkers with potential clinical utility have been broadly classified into biomarkers of collagen metabolism, biomarkers of aggrecan metabolism, biomarkers of noncollagenous proteins, and biomarkers of other processes such as inflammation. 42
Posttraumatic arthritis after ACL injury is multifactorial in origin, but cellular-level changes are a part of this process.9,20,39,44,67 The concept of biomarkers predicting osteoarthritis is particularly attractive in this unique patient population who are typically young but at increased risk of developing osteoarthritis, and where disease onset and progression could potentially be identified and monitored before clinical or radiological signs become apparent. However, biomarkers are not only limited to evaluating osteoarthritis but may also have a role in predicting other outcomes after ACL reconstruction. Patient-reported outcome measures (PROMs),7,29,36 pain scores, 7 and gait biomechanics15,53 are some examples of outcomes that have been associated with various biomarkers in the population who underwent ACL reconstruction.
Internationally, biomarker databases are being developed and tissue and fluid sample banks exist for patient cohorts with ACL injuries in Sweden, 58 the Netherlands, 20 the United States, 27 and Australia,2,16 for example. Correlation of biomarkers to outcome variables over the long term is an emerging area and likely a future direction for research in patients with ACL injuries. The aim of this study was to systematically review the literature and synthesize the currently available evidence where biomarkers in blood, urine, or synovial fluid have been measured and associated with an outcome measure after ACL reconstruction.
Methods
This review was performed according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines and registered with the International Prospective Register of Systematic Reviews (PROSPERO; reference No. CRD42022343980).
Search Strategy
A systematic search was conducted on March 18, 2022, and updated on September 4, 2023, in conjunction with a senior librarian at the institution at which the search was conducted. The following databases were used: MEDLINE, Embase, CINAHL, and Web of Science. Search terms were entered under 5 concepts: (1) anterior cruciate ligament, ACL, ACL reconstruction; (2) blood, plasma, serum; (3) synovial fluid; (4) urine; and (5) biomarker, biomarkers. In addition, 13 specific biomarkers of interest were searched for individually (eg, “CTX-2” and “interleukin”). The details of the search strategy utilized for MEDLINE are included in Appendix Table A1. To supplement electronic searches, the reference lists of relevant studies were also cross-checked for any additional references. The final list of candidate studies was then scanned and duplicates were removed. The results of the search were imported into Covidence.
Selection Criteria
Studies were included in the review if they reported on associations between biomarkers from blood, urine, or synovial fluid and an outcome measure after ACL reconstruction. All reported outcome measures were included, grouped as “PROMs,”“radiological outcomes,” or “other.” We excluded reports on biomarkers after ACL injury without subsequent surgical management; animal studies; non–English-language studies; and reviews, commentaries, or conference proceedings/abstracts where no full text could be identified. Selection criteria were applied by 2 independent reviewers (C.M. and C.L.). A consensus was used to resolve any discrepancies, with a third reviewer (L.M.B.) adjudicating. All levels of evidence were considered.
Methodological Assessment
The methodological quality of the included articles was assessed using the US National Heart, Lung, and Blood Institute (NHLBI) study quality assessment tools.50,51 The appropriate NHLBI tool was used for observational cohort studies and case-control studies.
Data Extraction and Synthesis
Data were extracted concurrently by 2 reviewers (C.M. and C.L.) for the following variables: number of included patients and patient characteristics, details of the biomarkers measured including the time points of measurement, fluid sampled (urine, serum/blood, or synovial fluid), and biomarker levels. Details of the outcome measures were recorded including measurement/assessment protocols as well as the time points of the measurements.
Lotz et al 42 provided a structure to conceptualize the numerous biomarkers that have been investigated in relation to osteoarthritis. This was published after a working meeting of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis. The authors consider 4 main biomarker groups: biomarkers of collagen metabolism, biomarkers related to aggrecan metabolism, biomarkers related to noncollagenous proteins, and biomarkers related to other processes (eg, inflammation and fibrosis). Where appropriate, the results in the present review are presented according to this structure to provide a framework for organization.
Given the heterogeneity of the included studies, no statistical analysis was performed. Data are presented in a descriptive manner and in tables where appropriate.
Results
Literature Identification
The electronic search yielded 9360 results, with a further single reference 59 identified via citation tracking. After removing duplicates, 7930 titles and abstracts were screened, and 47 full-text articles were assessed for eligibility. A total of 31 results were excluded, some for multiple reasons, but the main reasons have been counted in this review (Figure 1). One paper combined operatively and nonoperatively managed ACL injuries and was excluded after we confirmed with the authors that no ACL reconstruction subgroup data were available. 59
Figure 1.
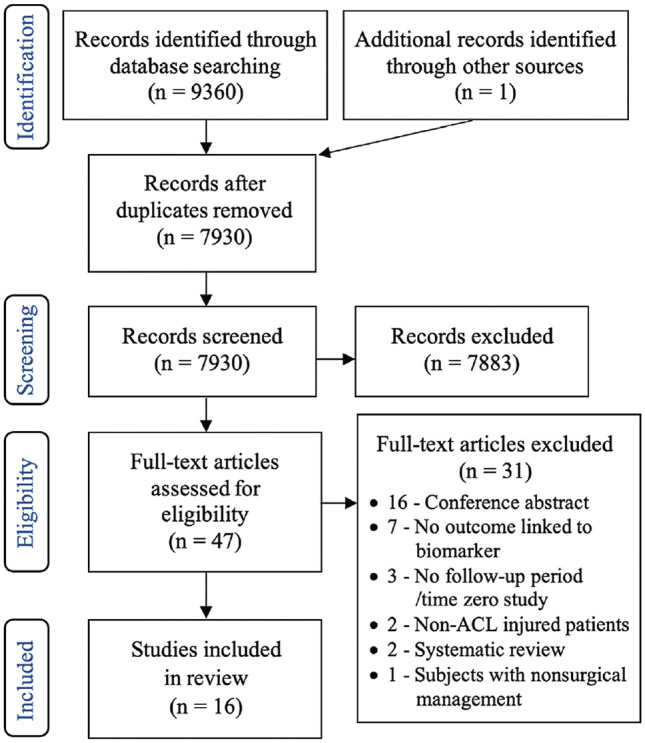
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flowchart of the study-inclusion process.
Methodological Assessment
We identified 16 studies (492 patients who underwent ACL reconstruction) in which a biomarker was measured and linked to an outcome measure. There were 11 cohort studies, ¶ 4 case-control studies,4,7,15,32 and 1 cross-sectional study. 54 Ten studies had a prospective design, # while 6 studies were retrospective.4,25,32,44,54,56 NHLBI quality-assessment scores ranged from 5 60 to 9 56 for cohort studies (maximum possible score, 14) and 77,32,63 to 84,15 for case series (maximum possible score, 12). Of the included studies, 0 were good, 12 were fair, ** and 4 were poor quality.3,25,54,60 Considerable heterogeneity of study design and outcome measures were identified, and it was decided that a meta-analysis was not appropriate.
Overview of Included Studies and Description of Available Literature
Of the 492 included patients, the weighted mean patient age was 24.58 years (mean ages in the studies ranged from 18.4 to 34.04 years); 207 (42%) were female. Of the 16 studies, 9 studies (56%) took biomarker samples over multiple time points (33% took ≥3 samples), and 7 studies (44%) took samples at a single time point (70% of these were on the day of ACL reconstruction). A total of 45 unique biomarkers or biomarker ratios were investigated (42 biomarkers and 3 biomarker ratios). Of these markers, 12 were identified in serum, 3 in urine, and 38 in synovial fluid. Seven markers were measured in 2 different mediums, and 1 marker was measured in all 3 mediums. A summary of the biomarkers identified as well as a summary of their basic function is presented in Appendix Table A2; refer to Appendix Table A2 for expansions of abbreviations throughout the text. Any prefixes before the biomarkers indicate the source (s-, serum; sf-, synovial fluid; u-, urinary).
A summary of the identified biomarkers stratified by source (urine, synovial fluid, or serum) and functional grouping as per the Lotz et al 42 classification is presented in Table 1. The majority of the biomarkers (n = 39) were measured in synovial fluid, with a wide variety of physiological processes including inflammatory pathways and metabolism of cartilage, bone, and synovium. In contrast, only 3 urinary biomarkers were identified, all being markers of chondral metabolism.
Table 1.
Summary of Biomarkers and Biomarker Ratios Studied Across the 16 Included Studies a
| Biomarker Classification b | ||||||
|---|---|---|---|---|---|---|
| Biomarker Source | Biomarker of Chondral Metabolism | Biomarker of Aggrecan Metabolism | Biomarker of Noncollagenous Proteins | Biomarkers of Other Processes (ie, Inflammation) | ||
| Urine |
CTX-II
C1,2C C2C |
|||||
| Serum |
C2C
C2C/CPII CPII |
Aggrecan |
COMP
MMP-3 |
ALT AST |
IL-6
MCP-1 TNF-α TTT |
|
| Synovial fluid | C2C C2C/KS CPII CTX-II NTX-1 |
C4S C6S C6S/C4S C2C/KS KS sGAG |
BMP-2 COMP MMP-1 MMP-3 MMP-9 TIMP-1 TIMP-2 |
bFGF
Bilirubin + biliverdin IFN-γ IL-1 IL-1a IL-1b IL-1Ra |
IL-2 IL-4 IL-6 IL-8 IL-10 IL-12p70 IL-13 |
MCP-1
MIP-1B NO RANTES TNF-α TSG-6 VEGF |
Markers in bold have a statistically significant association with an outcome measure in at least 1 included study. See Appendix Table A2 for list of abbreviations.
Per Lotz et al. 42
Outcome Measures
The outcome measures evaluated across the included studies are summarized in Table 2. There were 6 PROMs, 5 radiological outcome measures, and 8 outcome measures marked as “other,” including rates of arthrofibrosis and cyclops lesions, gait biomechanics and speed, ACL laxity measures, and arthroscopic assessment of chondral surfaces. Table 2 details the outcome measures used in the included studies and lists the biomarkers with and without an association demonstrated. Overall, the majority of biomarkers investigated were not found to be associated with the selected outcome variables (Table 2).
Table 2.
Outcome Measures Used in the Included Studies a
| Outcome | Biomarkers Investigated and Associated With Outcome During at Least 1 Time Point | Biomarkers Investigated and Not Associated With Outcome |
|---|---|---|
| Patient-Reported Outcome Measures | ||
| VAS pain or NPRS | sf-IL-6, sf-CTX-II (Sullivan et al
60
) u-CTX-II (Chmielewski et al 7 ) sf-IL-6, sf-IL-1 (Gupta et al 19 ) |
sf-IL-6, sf-IL-1Ra, sf-MIP-B, sf-MCP-1, sf-RATNES, sf-VEGF, sf-bFGF, sf-MMP-3, sf-TIMP-1, sf-TIMP2 (Markus et al
44
) sf IL-1β (Sullivan et al 60 ) sf-TNF-α (Gupta et al 19 ) |
| Lysholm score | sf-IL-6 (Gupta et al 19 ) | sf-IL-6, sf-IL-1Ra, sf-MIP-B, sf-MCP-1, sf-RATNES, sf-VEGF, sf-bFGF, sf-MMP-3, sf-TIMP-1, sf-TIMP2 (Markus et al
44
) sf-IL-1, sf-TNF-α (Gupta et al 19 ) |
| KOOS/KOOS-QOL | sf-IL-1α, sf-IL-1Ra, sf-MMP-9 (Lattermann et al 36 ) | sf-IL-6, sf-IL-1Ra, sf-MIP-B, sf-MCP-1, sf-RATNES, sf-VEGF, sf-bFGF, sf-MMP-3, sf-TIMP-1, sf-TIMP2 (Markus et al
44
) sf-COMP, sf-CTX-II, u-CTX-II, sGAG, sf-IL-1β, sf-MMP-1, sf-MMP-3, sf-NTX-I, sf-TSG-6 (Lattermann et al 36 ) s-MCP-1/s-COMP biochemical profile (Lisee et al 40 ) |
| Tegner activity scale | sf-IL-6 (Gupta et al 19 ) | sf-IL-6, sf-IL-1Ra, sf-MIP-B, sf-MCP-1, sf-RATNES, sf-VEGF, sf-bFGF, sf-MMP-3, sf-TIMP-1, sf-TIMP2 (Markus et al
44
) sf-IL-1, sf-TNF-α (Gupta et al 19 ) |
| IKDC/IKDC-SKF | u-CTX-II (Chmielewski et al
7
) sf-IL-1α (Lattermann et al 36 ) |
sf-COMP, sf-CTX-II, u-CTX-II, sGAG, sf-IL-1β, sf-IL-1Ra, sf-MMP-1, sf-MMP-3, sf-MMP-9, sf-NTX-I, sf-TSG-6 (Lattermann et al 36 ) |
| Marx activity scale | — | s-MCP-1/s-COMP biochemical profile (Lisee et al 40 ) |
| Imaging-Based Outcome Measures | ||
| Osteoarthritis (modified Outerbridge assessment per Colak et al 10 on 3-T MRI) | sf-MCP-1, sf-VEGF, sf-IL-1Ra (Markus et al 44 ) | sf-IL-6, sf-MIP-B, sf-RATNES, sf-bFGF, sf-MMP-3, sf-TIMP-1, sf-TIMP2 (Markus et al 44 ) |
| Osteoarthritis (joint-space width on weightbearing radiograph per Dupuis et al 13 ) | — | u-C2C/s-CP-II ratio (Tourville et al 63 ) |
| Osteoarthritis (T1ρ and T2 quantitative assessment per Li et al37,38 on 3-T MRI) | High sf-GAG/low sf-IL-6; IL-8; IL-10; TNF-α; MMP-1; MMP-3 biochemical profile (Amano et al 3 ) | High sf-IL-6; IL-8; IL-10; TNF-α; MMP-1; MMP-3/low sf-GAG biochemical profile (Amano et al 3 ) |
| Osteoarthritis (MRI T1ρ relaxation times) | High s-MCP-1/s-COMP biochemical profile (Lisee et al 40 ) | — |
| Tunnel enlargement (measured on plain radiograph) | sf-IL-6, sf-TNF-α, sf-NO (Zysk et al 69 ) | sf-IL-1β, sf-BMP-2 (Zysk et al 69 ) |
| Other Outcome Measures | ||
| Arthrofibrosis (requiring manipulation or arthrolysis) | sf-RANTES, sf-bFGF (Avila et al 4 ) | sf-IL-6, sf-VEGF-A, sf-TIMP-1, sf-IL-1Ra, sf-MMP-3, sf-MCP-1, sf-MIP-1B (Avila et al 4 ) |
| Gait biomechanics (vertical ground-reaction force, knee flexion angle, internal knee extension moment) | sf-MMP-3, sf-IL-6 (Evans-Pickett et al 15 ) | — |
| ACL laxity (KT-1000 arthrometer) | sf-IL-6 (Gupta et al 19 ) | sf-IL-1, sf-TNF-α (Gupta et al 19 ) |
| Physical Activity Score | sf-IL-1β (Inoue et al 25 ) | sf-TNF-α, sf-IL-2, sf-IL-6, sf-IL-8, sf-IL-10, sf-IFN-γ (Inoue et al 25 ) |
| Cyclops lesion | TTT (IgM) (Kodama et al 32 ) | |
| Walking speed | s-C2C (Pietrosimone et al 54 ) | s-Aggrecan (Pietrosimone et al 54 ) |
| Gait biomechanics (peak vertical ground-reaction force, vertical ground-reaction loading rate, knee adduction moment) | s-IL-6, s-MMP-3, s-C2C/CPII (Pietrosimone et al 53 ) | — |
| Osteoarthritis (arthroscopic chondral assessment) | sf-Δdi-C6S, sf-KS, sf-C6s/C4S ratio (Sobue et al 56 ) | sf-C2C, sf-Δdi-C4S, sf-C2C/KS ratio (Sobue et al 56 ) |
See Appendix Table A2 for a list of biomarker abbreviations. Biomarker prefixes: s-, serum; sf-, synovial fluid; u-, urinary. ACL, anterior cruciate ligament; IKDC, International Knee Documentation Committee; KOOS, Knee injury and Osteoarthritis Outcome Score; MRI, magnetic resonance imaging; NPRS, numeric pain rating scale; QOL, Quality of Life; SKF, Subjective Knee Evaluation Form; VAS, visual analog scale.
Patient-Reported Outcome Measures
PROMs assessed included the visual analog scale (VAS) for pain, Lysholm score, Knee injury and Osteoarthritis Outcome Score (KOOS), Tegner activity scale, International Knee Documentation Committee (IKDC) score, and Marx activity scale. Two studies found that higher baseline (time of surgery) synovial fluid interleukin levels correlated with worse Lysholm and Tegner scores at 12 months 19 and lower levels of reaching the Patient Acceptable Symptom State for the IKDC and KOOS 36 at 2 years. Increased sf-IL-6 at baseline was also associated with increased VAS pain scores in 2 studies with follow-up periods of 4 weeks 60 and 1 year. 19 Pain as measured by VAS had the highest number of biomarkers with a statistically significant association (Table 2). Lower u-CTX-II levels were associated with reduced pain levels in 1 study 7 ; however, higher sf-CTX-II levels were associated with reduced pain in another study. 60
Radiological Outcomes
Radiological outcomes of osteoarthritis were included in 4 studies (Table 2). Three were magnetic resonance imaging (MRI)–based assessments,3,40,44 and 1 was based on weightbearing radiographs. 63 All 3 MRI studies used different outcome assessment protocols (Table 2). Of the 4 studies correlating biomarkers to radiological outcomes, the follow-up period ranged from 12 months 40 to 7.8 years, 44 and sample sizes were small, ranging from 18 patients 44 to 35 patients. 63 In the study with the longest follow-up (mean, 7.8 years), increasing levels of sf-MCP-1, sf-VEGF, and sf-IL-1Ra taken at the time of surgery were associated with increasing degenerative change on MRI at the final follow-up. 44 In 1 study of 35 patients with ACL injuries, increased ratios of u-CTX-II/s-CPII (a ratio of type 2 collagen cleavage to synthesis) were associated with joint-space narrowing on weightbearing radiographs at the 4-year follow-up. 63 One study of 24 patients demonstrated that increasing serum levels of MCP-1 and COMP (markers of inflammation and matrix degradation, respectively) between the preoperative and 6-month mark were associated with inferior cartilage composition on 12-month postoperative MRI. 40
Other Outcome Measures
Three studies15,53,54 evaluating gait speed or biomechanics originated from the same center (Table 2). The authors found that patients with slower walking speeds had higher s-C2C concentrations. 54 A stiffened knee gait strategy was associated with higher sf-IL-6 and sf-MMP-3 levels, 15 and increased s-MMP-3 was associated with reduced limb symmetry indices in terms of knee adduction moment and peak vertical ground-reaction force loading rate. 53
Two studies applying case-control methodology demonstrated an association between a biomarker and local or generalized arthrofibrosis, specifically a cyclops lesion formation 32 or a postoperative procedure for stiffness. 4 Increased synovial fluid biomarker RANTES as measured at the time of surgery was associated with increasing rates of manipulation under anesthesia/lysis of adhesions in 11 patients matched to 21 controls at a median of 92 days. 4 High presurgery s-TTT values were associated with increased rates of cyclops lesion formation at 3 months postoperatively. 32
One study investigated the association between biomarkers and knee stability as assessed by KT-1000 arthrometer testing. 19 In a study of 59 patients who underwent ACL reconstruction, higher sf-IL-6 levels preoperatively were associated with poorer KT-1000 arthrometer laxity measures at 2, 6, and 12 months. 19
Biomarkers Summarized by Source (Urine, Serum, Synovial Fluid)
In terms of urinary biomarkers, there were 3 studies7,36,63 including 85 patients in which a urinary biomarker was measured. Three different urinary biomarkers were identified: u-C2C, u-C1,2C, and u-CTX-II; all are biomarkers of type 2 collagen degradation (Appendix Table A2). The mean follow-up across these 3 studies was 77.1 months (median, 46 months). Increased u-CTX-II correlated with increased pain scores over the short term (up to 16 weeks postsurgery) in a study of 28 patients who underwent ACL reconstruction. 7 Increased u-CTX-II levels correlated with worse IKDC-SKF scores at up to a 16-week follow-up in the same study. 7 Although not reaching statistical significance, increased u-CTX-II levels trended toward reduced rates of achieving the Patient Acceptable Symptom State for KOOS–Quality of Life scores (P = .08) at mean follow-up of 2.4 years in a series of 22 patients after ACL reconstruction. 36
There were 5 studies32,40,53,54,63 with 113 patients overall (minimum sample size, n = 16 32 ; maximum, n = 35 63 ) in which a serum or blood biomarker was measured. Twelve different blood or serum biomarkers were reported on: s-aggrecan, s-ALT, s-AST, s-C2C, s-C2C:CPII, s-COMP, s-CRP, s-IL-6, s-MCP-1, s-MMP-3, s-CPII, and s-TTT. Where reported, the mean follow-up ranged from 6 months 53 to 46 months. 63 Increasing levels of s-MMP-3 and s-IL-6 were associated with reduced loading of the injured limb compared with the uninjured contralateral limb at a 6-month follow-up in a series of 18 patients using 3-dimensional gait analysis. 53 Slower walking speeds were associated with higher s-C2C levels taken at 3-dimensional gait analysis assessment at a minimum of 6 months postoperatively in 20 patients. 54 Increasing s-COMP and s-MCP-1 from the time of surgery to the 6-month follow-up was associated with inferior MRI-based cartilage proteoglycan density at the 12-month follow-up in a series of 24 patients. 40
A total of 39 different synovial fluid biomarkers were identified in 10 studies †† reporting on a cumulative 351 patients (minimum sample size, n = 11 4 ; maximum, n = 79 25 ). The most frequently included biomarkers were sf-IL-6, sf-MMP-3, sf-IL-1b, sf-TNF-α, sf-IL-1Ra, and sf-CTX-II, which were reported in at least 3 separate studies. Where reported, the mean follow-up ranged from a minimum of 1 month 60 to 7.8 years. 44 Synovial fluid biomarkers of aggrecan metabolism, noncollagenous protein activity, and inflammation were associated with chondral degeneration and inferior PROMs in a number of studies. Baseline levels of sf-MCP-1, sf-VEGF, and sf-IL-1Ra correlated with increased MRI-assessed chondral degeneration at a mean follow-up of 7.8 years in 18 patients. 44 Higher sf-sGAG concentrations (a marker of cartilage degeneration) at the time of surgery were associated with inferior cartilage composition on sequential MRI assessments during the first 3 years after ACL reconstruction in a study of 26 patients. 3 Lower levels of sf-KS and sf-C6S/C4S at the time of surgery were associated with an increase in the number of high-grade cartilage lesions at 2-year arthroscopic assessment in a study of 62 patients. 56
Overall, 17 biomarkers across 16 studies had a statistically significant association to an outcome measure on at least 1 time point. In contrast to Table 2, where the literature is summarized according to outcome measure, Table 3 highlights the 17 biomarkers found to have an association to an outcome measure and summarizes these findings. While Table 2 highlights the outcomes that can potentially be assessed with biomarkers in patients with ACL injuries, Table 3 highlights the specific candidate biomarkers that have shown some early promise.
Table 3.
Biomarkers With a Statistically Significant Correlation or Between-Group Difference in Relation to an Outcome Measure in the Included Studies a
| Biomarker | First Author (Year) | N | Follow-up | Key Findings |
|---|---|---|---|---|
| Biomarkers of Chondral Metabolism | ||||
| CTX-II | Chimielewski (2012) 7 | 28 | 16 wk | u-CTX-II concentrations decreased over time and correlated with numeric pain rating scores at 4, 8, 12, and 16 wk postsurgery (r = 0.406; P = .039) Negative correlation between u-CTX-II concentrations and IKDC-SKF scores at 4, 8, 12, and 16 wk postsurgery (r = −0.402; P = .034) |
| Sullivan (2023) 60 | 23 | 4 wk | sf-CTX-II negatively correlated with VAS pain score over the first 4 wk postoperatively (r = −0.39; P = .002) | |
| Tourville (2013) 63 | 35 | 4 y | Higher u-CTX-II/s-CPII ratios positively correlated with joint-space narrowing at 4 years on weightbearing radiograph; 11 patients with joint-space narrowing had significantly higher u-CTX-II/s-CPII ratios at 4 y compared with 31 ACL-intact controls | |
| C2C | Pietrosimone (2016) 54 | 20 | 43 ± 36 mo | s-C2C levels negatively correlated with walking speed (r = −0.52; P = .02), even after accounting for variance of stance phase duration (partial r = −0.53; P = .02) |
| Pietrosimone (2017) 53 | 18 | 6 mo | s-C2C/s-CPII ratios measured within 2 wk of surgery were negatively related to vertical ground-reaction force LSI at 6 mo postoperatively (r = −0.5; P = .04), but this was not significant after controlling for walking speed (r = −0.24; P = .36) | |
| Biomarkers of Aggrecan Metabolism | ||||
| sGAG | Amano (2018) 3 | 26 | 3 y | Patient group characterized by high sf-sGAG and low sf-IL-6, IL-8, IL-10, TNF-α, MMP-1, and MMP-3 positively correlated with higher T1ρ relaxation times (medial tibia: β = 3.29, P = .001; patella: β = 2.46, P = .007) and T2 relaxation times (medial tibia: β = 1.48, P = .32; patella: β = 1.74, P = .37) in the medial tibia and patella |
| C6S | Sobue (2017) 56 | 62 | 2 y | Median baseline sf-Δdi-C6S levels at the time of surgery were significantly higher in the group who showed an increase in the number of high-grade cartilage lesions at 2-y follow-up compared with the group who did not show an increase in the number of high-grade cartilage lesions (53.4 vs 73.5; P = .004); high-grade cartilage lesions were defined as an increase in Outerbridge grade 3 or 4 lesions arthroscopically |
| KS | Sobue (2017) 56 | 62 | 2 y | Lower sf-KS levels at the time of surgery were associated with an increase in the number of high-grade cartilage lesions at 2 years, as defined by increased Outerbridge grade 3 or 4 lesions arthroscopically (P = .021) |
| C6S/C4S | Sobue (2017) 56 | 62 | 2 y | Lower sf-C6S/C4S levels at the time of surgery were associated with an increase in the number of high-grade cartilage lesions at 2 years, as defined by increased Outerbridge grade 3 or 4 lesions arthroscopically (P = .028) |
| Biomarkers of Noncollagenous Proteins | ||||
| Serum biochemical profile of increasing COMP and MCP-1 | Lisee (2021) 40 | 24 | 12 mo | Patients who had a serum biochemical profile of increasing s-COMP and increasing s-MCP-1 between the preoperative and 6-mo postoperative time points were associated with greater lateral femoral (β = 12.71; P = .04) and lateral tibial (β = 3.88; P = .001) MRI T1p relaxation times at 12 mo postoperatively; a k-means cluster analysis was used to create the different biomarker profile groups based on biomarker changes with time |
| MMP-3 | Pietrosimone (2017) 53 | 18 | 6 mo | Higher s-MMP-3 measured within the first 2 wk postsurgery correlated with reduced knee adduction moment LSI at 6-mo follow-up (r = −0.64; P = .01) Higher s-MMP-3 measured at 6 mo postoperatively correlated with reduced knee adduction moment LSI (r = −0.67; P = .01) and reduced vertical ground-reaction force loading rate LSI (r = −0.6; P = .01) at 6-mo follow-up |
| Evans-Pickett (2021) 15 | 38 | 6 mo | High sf-MMP-3 collected day 7 postinjury correlated with aberrant biomechanics at 6 mo postoperatively, including underloading and a stiffened knee gait strategy | |
| MMP-9 | Latterman (2018) 36 | 22 | 2 y | Patients who failed to meet the Patient Acceptable Symptom state for the KOOS–Quality of Life had significantly higher sf-MMP-9 on the day of surgery compared with those did meet this (mean ± SD, 30.99 ± 35.96 vs 6.94 ± 10.30 ng/mL; P = .01; Cohen d = 1.07) |
| Biomarkers of Other Processes (ie, inflammation) | ||||
| IL-6 | Pietrosimone (2017) 53 | 18 | 6 mo | Higher s-IL-6 measured at the 6-mo postoperative mark correlated with reduced knee adduction moment LSI (r = −0.60; P = .02) after controlling for walking speed |
| Evans-Pickett (2021) 15 | 38 | 6 mo | High sf-IL-6 collected day 7 postinjury correlated with aberrant biomechanics at 6 mo postoperatively, including underloading and a stiffened knee gait strategy | |
| Gupta (2021) 19 | 59 | 12 mo | Preoperative sf-IL-6 was associated with VAS scores, KT-1000 arthrometer testing, Lysholm knee scores, and Tegner scores at 12 mo; higher IL-6 preoperatively was associated with increased pain scores, decreased mechanical stability, and poorer Lysholm and Tegner scores | |
| Sullivan (2023) 60 | 23 | 4 wk | sf-IL-6 was correlated with VAS pain scores preoperatively and over the first 4 wk postoperatively (r = 0.52; P < .001) | |
| IL-1b | Inoue (2016) 25 | 79 | 3 mo | Higher sf-IL-1b taken at 3-4 days postoperatively was seen in patients with a delayed recovery according to the authors’ 5-point ordinal recovery grading system (P = .03) |
| IL-1a | Latterman (2018) 36 | 22 | 2 y | Patients who failed to meet the Patient Acceptable Symptom State for the KOOS–Quality of Life had significantly higher sf-IL-1a on the day of surgery (P = .004) Patients who failed to meet the Patient Acceptable Symptom State for the IKDC had significantly higher sf-IL-1a on the day of surgery (P = .02) |
| IL-1Ra | Latterman (2018) 36 | 22 | 2 y | Patients who failed to meet the Patient Acceptable Symptom State for the KOOS–Quality of Life had significantly higher sf-IL-1Ra on the day of surgery (P = .03) |
| Markus (2023) 44 | 18 | 7.8 y | Lateral tibial plateau chondral lesion size as assessed by modified Outerbridge classification on 3-T MRI correlated with sf-IL-1Ra levels at the time of surgery (R2 = 0.271; P = .032) | |
| MCP-1 | Markus (2023) 44 | 18 | 7.8 y | sf-MCP-1 levels at the time of surgery correlated with lateral femoral condyle chondral lesion depth (R2 = 0.362; P = .01) and size (R2 = 0.292; P = .025) as assessed by the modified Outerbridge classification on 3-T MRI |
| VEGF | Markus (2023) 44 | 18 | 7.8 y | sf-VEGF levels at the time of surgery correlated with patellar chondral lesion depth (R2 = 0.606; P = .001) and size (R2 = 0.403; P = .006) as well as trochlear lesion size (R2 = 0.57; P = .001) as assessed by the modified Outerbridge classification on 3-T MRI |
| RANTES | Avila (2022) 4 | 32 | 92 days b | sf-RANTES taken before surgical incision was significantly higher in the ACLR group with stiffness requiring manipulation under anesthesia or arthroscopic arthrolysis as compared with matched controls (OR, 2.28; P = .019) |
| bFGF | Avila (2022) 4 | 32 | 92 days b | sf-bFGF taken before surgical incision was significantly higher in the ACLR group with stiffness requiring manipulation under anesthesia or arthroscopic arthrolysis as compared with matched controls (OR, 1.91; P = .047) |
| TTT | Kodama (2018) 32 | 120 | 12 mo | Patients with cyclops nodule formation 12 mo after double-bundle hamstring ACLR had significantly higher TTT as measured at the time of surgery (OR, 9.34; 95% CI, 1.94-90.3; P = .002) |
Table stratified according to biomarker classification described by Lotz et al. 42 See Appendix Table A2 for a list of biomarker abbreviations. Biomarker prefixes: s-, serum; sf-, synovial fluid; u-, urinary. ACLR, anterior cruciate ligament reconstruction; KOOS, Knee injury and Osteoarthritis Outcome Score; LSI, Limb Symmetry Index; MRI, magnetic resonance imaging; VAS, visual analog scale.
Time to diagnosis of stiffness; actual follow-up time not stated.
Discussion
This systematic review has demonstrated that multiple biomarkers in the blood, urine, and synovial fluid have been measured after ACL reconstruction and associated with clinically important outcome measures. However, this review suggests that there are not enough data on any of them to suggest that they are ready to be used in the clinical setting. The associations found are in the context of a plethora of biomarkers investigated across multiple series, typically with small patient numbers (median, 43; range, 13 69 to 94 32 ) and short follow-up periods (where reported: median, 9 months; range, 4 weeks 60 to 7.8 years 44 ). The chance of type 1 and 2 errors in the existing body of literature is high, and the methodological quality of the included studies was fair to poor. From a clinical perspective, the questions of what biomarker to measure, when to measure it, and what outcomes can reliably be predicted are far from having definitive answers.
Despite these challenges, this review highlights some encouraging results and a number of biomarkers worthy of further research. Serum IL-6, s-MMP-3, sf-MMP-3, u-CTX-II, sf-CTX-II, and s-C2C show promise in predicting outcomes after ACL reconstruction, Specifically, PROMs (s-IL-6,19,60 sf- and u-CTX-II7,60), gait biomechanical parameters (sf- and s-IL-6,15,53 sf- and s-MMP-3, 53 s-C2C 54 ), pain (s-IL-6,19,60 u-CTX-II7,60), and radiological osteoarthritis (u-CTX-II/s-CPII) 63 were reported to be associated with these biomarkers.
In the existing literature, biomarker use has predominantly been explored as a prognostic tool. For example, biomarkers of chondral metabolism being used to predict future degenerative change ahead of time.3,56 Of note, a number of biomarker associations with specific short-term complications or outcomes such as cyclops lesion formation, 33 arthrofibrosis, 4 or postoperative pain 60 were identified in this review. This may open the door for the concept of biomarkers being used as a management tool. Theoretically, serial preoperative measurements could guide surgical decision-making in terms of timing of surgery by waiting for biomarkers to reach a defined level. Preoperative sf-RANTES (associated with increased interventions for stiffness 4 ) or s-TTT (associated with cyclops development 32 ) concentrations could be monitored to optimize the timing of any surgical intervention. However, this concept assumes that levels normalize with time, something that is yet to be demonstrated. Although attractive, this would require serial biomarker measurements. Of the included studies, 56% (9/16) took samples over multiple time points, with 33% taking ≥3 samples. Only 38% (6/16) of the studies in this review took biomarker samples before ACL reconstruction, and no study assessed serial preoperative samples to define trends.
Patients undergoing ACL reconstruction represent a unique patient population. In this young and active population, there is estimated to be an 8-fold increase in the rate of osteoarthritis after ACL reconstruction, and the prevalence of osteoarthritis was estimated to be 36% at 10 years after ACL reconstruction in 1 umbrella systematic review. 66 Predicting osteoarthritis with accuracy at the individual level is difficult. Rates of osteoarthritis vary widely, from 0% to 100% at 10 years in another systematic review, 39 highlighting the inherent challenges of defining and investigating this phenomenon. The multifactorial origin of posttraumatic osteoarthritis makes determining the significance of individual risk factors difficult. Biomarkers represent an attractive way to try to better understand the pathophysiology of this process and would ideally predict who is at risk of developing premature posttraumatic osteoarthritis. This has been a focus of the research efforts to date, with 3 studies correlating biomarkers to chondral degeneration or osteoarthritis on MRI,3,40,44 1 correlating with radiographic changes, 63 and 1 with arthroscopic findings 56 (Table 2). Furthermore, the IKDC, KOOS, and Lysholm PROMs were assessed across 5 studies7,19,36,40,44 (Table 2), which include an assessment of pain potentially reflecting degenerative change.
Six biomarkers or biomarker ratios (u-CTX-II/s-CPII, sf-MCP-1, sf-VEGF, sf-IL-1Ra, high sf-GAG/cytokine ratio, high s-MCP-1/COMP ratio) were associated with MRI-diagnosed chondral pathology.3,40,44,63 Although more data are needed, by aiming to directly measure chondral metabolism as a mechanism of osteoarthritis pathogenesis, biomarkers may reflect the influence of many, if not all, of the multiple factors contributing to the development of osteoarthritis. For example, in the multifactorial osteoarthritis development process, it is difficult to quantify the individual contributions of factors such meniscal injuries, residual instability, malalignment, or genetic factors, which are inherently heterogeneous and difficult to quantify. However, these factors may all contribute to a common pathway of chondral degradation that can potentially be measured directly with biomarkers of chondral breakdown. U-CTX-II, a breakdown product of type 2 collagen, appears to be a leading candidate for further investigation given the ease of collection from the urine and the association with radiological osteoarthritis, 63 pain7,60 and PROMs 7 demonstrated in the existing literature.
A 2015 systematic review by Harkey et al 22 analyzed the available literature until June 2014 regarding osteoarthritis-related biomarkers after ACL injury and reconstruction. The authors identified 8 studies in which biomarkers of osteoarthritis were studied in the context of ACL reconstruction. The authors identified decreased levels of biomarkers indicating collagen breakdown in the serum, but increased levels of biomarkers indicating collagen breakdown in the urine after ACL reconstruction as compared with controls. When compared with preoperative values, synovial inflammatory cytokine biomarkers increased, while plasma biomarkers did not change after ACL reconstruction. 22 In contrast, the current systematic review had a different focus, specifically looking at the association of biomarkers with outcome variables. Building on the data from the systematic review by Harkey et al, 22 biomarkers of chondral metabolism were associated with PROMs and radiological outcomes (Table 3). Although encouraging, the available literature is nonetheless limited by heterogeneous outcome measures and small sample sizes.
It is worth noting that many of the included studies found associations between specific biomarker profiles3,40 or ratios53,56,63 and an outcome of interest. This may indicate that it is not one specific biomarker that is associated with the outcome but rather a combination of biomarkers. Interpreting multiple biomarkers together may allow assessment of both chondral anabolism and catabolism, or pro- and anti-inflammatory agents, for example, and may be more reflective of the net status or homeostasis at the cellular level within the knee. An “inflammatory phenotype” was proposed by Avila et al 4 as one example of this concept.
If biomarkers are to become adopted clinically in the context of ACL reconstruction, there are a number of requirements. The ideal biomarker would be easily accessible; urine and serum biomarkers would be preferable because they avoid the need for arthrocentesis. If a synovial fluid marker were required, it would ideally need sampling only at the time of surgery to avoid the need for multiple joint aspirations. Other desirable attributes would be for a biomarker to be cheap and easy to measure, with high accuracy and precision of measurement. This has not been demonstrated in the current literature. Ideally, the marker would have a strong association with an important outcome demonstrated in multiple prospective series in differing patient cohorts. The outcome measured would also need to be something that could help guide treatment to optimize outcomes or, at a minimum, help counsel patients if the marker correlated with an unmodifiable factor. This may include using the biomarker to help guide timing of surgery, graft choice, or extra-articular procedures or counsel a patient about the probability of posttraumatic osteoarthritis. As one example of how this might be used clinically, elevated preoperative TTT levels (shown to be associated with cyclops lesion development) 32 may lead surgeons to mitigate risk by taking a smaller-diameter quadricep tendon graft for male patients (another risk factor for cyclops development in other series). 21 Even if this was a not modifiable factor, it may be beneficial to help with decision-making regarding return to sport, lifestyle, and occupation. For example, if a biomarker profile could accurately predict early-onset arthritis, this may be taken into consideration by patients and steer them away from occupations with high demands on the knee. Furthermore, accurate identification of patient subgroups at risk of an outcome like arthritis may provide a target group for therapeutic interventions.
Limitations
This systematic review is limited by the quality of the included studies and marked heterogeneity in the biomarkers evaluated, timing of biomarker collection, reporting of concomitant knee injuries, and outcomes assessed. There may be other biomarkers strongly associated with ACL injuries; however, the lack of correlation to an outcome variable excluded such papers from the scope of this review. These other markers may still warrant investigation, so the markers listed here cannot be considered a comprehensive list of potential ACL-related biomarkers. The conclusions of this review have been tempered in light of these limitations, but it still provides a comprehensive update on this rapidly evolving topic and a platform for future work that appears justified based on the many promising biomarkers identified.
Conclusion
Biomarkers in the blood, urine, and synovial fluid can be measured after ACL injury and reconstruction and have been associated with clinically important outcome measures after surgery. The current results highlight several biomarkers that may warrant further research to understand if these biomarkers can provide meaningful information in the clinical environment.
Acknowledgments
The authors acknowledge Angela Johns-Hayden, senior research librarian at La Trobe University, for her assistance.
Appendix
Appendix Table A1.
MEDLINE Search Strategy
| Search No. | Search Terms | Search Notes | Results |
|---|---|---|---|
| S1 | Anterior Cruciate Ligament/ | MeSH | 11,660 |
| S2 | “anterior cruciate ligament*” | 24,973 | |
| S3 | ACL OR ACLR | 19,613 | |
| S4 | 1 OR 2 OR 3 | 29,234 | |
| S5 | Blood/ | MeSH | 53,764 |
| S6 | Blood OR bloods | 3,997,776 | |
| S7 | Plasma/ | MeSH | 24,156 |
| S8 | Plasma | 984,856 | |
| S9 | Serum/ | MeSH | 11,184 |
| S10 | Serum | 1,163,613 | |
| S11 | Urine/ | MeSH | 37,942 |
| S12 | Urine | 381,615 | |
| S13 | Synovial fluid/ | MeSH | 13,528 |
| S14 | “synovial fluid*” | 18,724 | |
| S15 | Biomarkers/ | MeSH | 324,330 |
| S16 | Biomarker* | 693,576 | |
| S17 | CTX-2 | 183 | |
| S18 | Matrix metalloproteinases/ | MeSH | 10,592 |
| S19 | “matrix metalloproteinases” OR MMp | 68,354 | |
| S20 | TNF-related apoptosis-Inducing Ligand/ | MeSH | 5929 |
| S21 | TNF | 199,434 | |
| S22 | Interleukins/ | MeSH | 18,120 |
| S23 | Interleukin* | 386,929 | |
| S24 | Cartilage Oligomeric Matrix Protein/ | MeSH | 793 |
| S25 | “cartilage oligomeric matrix protein” OR COMP | 4648 | |
| S26 | Transforming Growth Factor beta/ OR Transforming growth factor beta1/ OR Latent TGF-beta Binding Proteins/ | MeSH | 64,016 |
| S27 | TGF | 82,006 | |
| S28 | Tissue Inhibitor OR Metalloproteinases/ | MeSH | 3600 |
| S29 | “tissue inhibitor or metalloproteinases” OR TIMP | 14,688 | |
| S30 | NTX | 2279 | |
| S31 | ARGS | 3065 | |
| S32 | Interferons/ | MeSH | 24,838 |
| S33 | Interferons OR IFN | 152,113 | |
| S34 | OR/5-33 | ||
| S35 | 4 AND 34 | 3670 | |
| S36 | 35 not (Animals/ not (Animals/ and Humans/)) | MEDLINE human studies filter | 2834 |
| S37 | Limit 36 to English language | 2617 |
Table A2.
List of Biomarker Abbreviations With a Summary of Biomarker Function
| Abbreviation | Full Name | Basic Function | Included Study Reporting on Biomarker |
|---|---|---|---|
| Aggrecan | Aggrecan | Major proteoglycan in articular cartilage 28 | Pietrosimone 54 |
| ALT | Alanine transaminase (also known as alanine aminotransferase) | Enzyme found mostly in hepatocytes that catalyzes the reversible interconversion of l-alanine and 2-oxoglutalate to pyruvate and l-glutamate 41 ; has also been found to be elevated in skeletal muscle diseases 49 | Kodama 32 |
| AST | Aspartate transaminase (also known as aspartate aminotransferase) | Enzyme found mostly in hepatocytes; catalyzes the transamination reaction between l-aspartate and 2-oxoglutarate into oxaloacetate and l-glutamate 31 ; also found to be elevated in skeletal muscle diseases 49 | Kodama 32 |
| Bilirubin + biliverdin | Bilirubin and biliverdin | By-product of heme catabolism and is subsequently reduced to bilirubin 47 | Amano 3 |
| BMP-2 | Bone morphogenic protein 2 | Role in signaling osteoblast proliferation and differentiation 6 | Zysk 69 |
| C1,2C | Collagen type 1 and 2 cleavage product | By-product of type 1 and 2 collagen cleavage by collagenases 61 | Tourville 63 |
| C2C | Collagen type 2 cleavage product | By-product of cleavage of type 2 collagen cleavage by collagenases 61 | Lisee, 40 Pietrosimone,53,54 Sobue, 56 Tourville 63 |
| C2C/CPII | Collagen type 2 cleavage product/procollagen 2 carboxypeptide | Ratio of type 2 collagen degradation relative to synthesis 40 | Lisee, 40 Pietrosimone 53 |
| C2C/KS | Collagen type 2 cleavage product/keratan sulfate | Ratio of C2C/KS in synovial fluid that may offer greater ability to identify early, pre-radiographic high-grade cartilage damage better than either biomarker individually56,68 | Sobue 56 |
| C4S | Chondroitin-4-sulfate | In osteoarthritic cartilage, C6S is replaced by C4S 48 | Sobue 56 |
| C6S | Chondroitin-6-sulfate | Major glycosaminoglycan found in articular cartilage 48 | Sobue 56 |
| C6S/C4S | Chondroitin-6-sulfate/chondroitin-4-sulfate | Ratio of C6S to C4S, considered to be a marker of osteoarthritis progression 48 | Sobue 56 |
| COMP | Cartilage oligomeric matrix protein | Extracellular matrix protein that binds to aggrecan; considered to be a biomarker of matrix degeneration 40 | Latterman, 36 Lisee 40 |
| CPII | Procollagen 2 C-propeptide | Marker of type 2 collagen synthesis 61 | Amano, 3 Tourville 63 |
| CRP | C-reactive protein | Acute inflammatory protein synthesized primarily in the liver 57 | Kodama 32 |
| CTX-II | C-terminal cross-linked telopeptide of type 2 collagen | By-product of type 2 collagen and articular cartilage degradation 7 | Chmielewski, 7 Latterman, 36 Tourville 63 |
| FGF-2 (bFGF) | Fibroblast growth factor 2 (also known as basic fibroblast growth factor) | Growth factor promoting chondrogenesis, angiogenesis, wound healing, and granulation tissue formation; excess may contribute to onset or progression of osteoarthritis4,24 | Avila, 4 Markus 44 |
| IFN-γ | Interferon gamma | Pro-inflammatory cytokine that has been used as a marker of cartilage degeneration3,62 | Amano, 3 Inoue 25 |
| IL-1 | Interleukin 1 | Refers to IL-1a and IL-1b, 2 cytokines that play a major role in inflammation 26 | Gupta 19 |
| IL-1a | Interleukin 1 alpha | Pro-inflammatory cytokine produced by macrophages and epithelial cells 3 | Amano, 3 Latterman 36 |
| IL-1b | Interleukin 1 beta | Pro-inflammatory cytokine produced by macrophages and epithelial cells 3 | Amano, 3 Inoue, 25 Latterman, 36 Sullivan, 60 Zysk 69 |
| IL-1Ra | Interleukin 1 receptor antagonist | Anti-inflammatory cytokine that inhibits the effect of IL-1b4,26 | Amano, 3 Avila, 4 Latterman, 36 Markus 44 |
| IL-2 | Interleukin 2 | Cytokine produced by activated T cells with a role in mediating the immune system by promoting growth and differentiation of T cells, T-helper cells, and NK cells 23 | Amano, 3 Inoue 25 |
| IL-4 | Interleukin 4 | Cytokine produced by Th2 cells and mast cells; involved in B-cell activation, IgE switch, and suppression of Th1 cells 26 | Amano 3 |
| IL-6 | Interleukin 6 | Cytokine secreted by T cells, macrophages, and endothelial cells during infection or trauma, stimulating IL-10 and IL-1Ra to act as a negative feedback mechanism to inflammation4,17 | Amano, 3 Avila, 4 Evans-Pickett, 15 Gupta, 19 Inoue, 25 Markus, 44 Pietrosimone, 53 Sobue, 56 Sullivan, 60 Zysk 69 |
| IL-8 | Interleukin 8 | Cytokine produced by monocytes that attracts neutrophils, basophils, and T cells 23 | Amano, 3 Inoue 25 |
| IL-10 | Interleukin 10 | Anti-inflammatory cytokine produced by Th2 cells and macrophages that inhibits Th1 cells, increases MHC expression on B cells, and stimulates mast cell growth 23 | Amano, 3 Inoue 25 |
| IL-12p70 | Interleukin 12p70 | Active form of cytokine IL-12 produced by B cells and macrophages that induces NK-cell activation and Th1-cell differentiation 23 | Amano 3 |
| IL-13 | Interleukin 13 | Anti-inflammatory cytokine produced by T cells; acts to promote B-cell growth/differentiation, inhibit macrophage cytokine production, and inhibit Th1 cells 23 | Amano 3 |
| KS | Keratan sulfate | A glycosaminoglycan that is one of the constituent proteins of aggrecan when attached to a core protein 56 ; levels are decreased in cartilage degradation 68 | Sobue 56 |
| MCP-1 (CCL2) | Monocyte chemotactic protein 1 (also known as chemokine ligand 2) | Chemokine that recruits monocytes, memory T cells, and dendritic cells to inflammatory sites4,12 | Avila, 4 Lisee, 40 Markus 44 |
| MIP-1B | Macrophage inflammatory protein 1B | Chemokine produced by neutrophils that activates granulocytes; recruits neutrophiles/monocytes/macrophages/immature dendritic cells/Th1 cells to inflammatory sites 4 | Avila, 4 Markus 44 |
| MMP-1 | Matrix metalloproteinase 1 | Metalloproteinase that degrades type 1, 2, 3, 7, 8, and 10 collagen and gelatin, aggrecan, and proteoglycan link proteins 12 | Amano, 3 Latterman 36 |
| MMP-3 | Matrix metalloproteinase 3 | Metalloproteinase that degrades type 2, 4, 9, 10, and 11 collagen and proteoglycans, fibronectin, laminin, and elastin; can activate other MMPs4,14 | Amano, 3 Avila, 4 Evans-Pickett, 15 Latterman, 36 Lisee, 40 Markus, 44 Pietrosimone 53 |
| MMP-9 | Matrix metalloproteinase 9 | Metalloproteinase that degrades type 4, 5, 7, 10, and 14 collagen and gelatin, aggrecan, elastin, fibronectin, laminin, and proteoglycan link protein 11 | Amano, 3 Latterman 36 |
| NO | Nitric oxide | A free radical shown to have a role on potentiating cytokine and inflammation-induced bone resorption65,69 | Zysk 69 |
| NTX-1 | N-terminal telopeptide of type 1 collagen | Marker of breakdown of type 1 collagen and indicator of bone turnover3,36 | Latterman, 36 Amano 3 |
| RANTES (CCL5) | Regulated on activation, normal T cell expressed and presumably secreted (also known as chemokine ligand 5) | Chemokine that recruits leukocytes to the site of inflammation and activates natural killer cells4,55 | Avila, 4 Markus 44 |
| sGAG | Sulfated glycosaminoglycan | A class of long, linear polysaccharides that includes the chondroitin sulfate (eg, C4S, C6S) and keratan sulfate (KS) groups 64 | Amano, 3 Latterman 36 |
| TIMP-1 | Tissue inhibitor of metalloproteinases 1 | Protein that inhibits MMPs; promotes cell growth of chondrocytes and has chondroprotective effect4,18 | Avila, 4 Markus 44 |
| TIMP-2 | Tissue inhibitor of metalloproteinases 2 | Protein that inhibits MMPs, favoring MMP-2 46 | Markus 44 |
| TNF-α | Tumor necrosis factor alpha | Pro-inflammatory cytokine produced by macrophages1,3 | Amano, 3 Gupta, 19 Inoue, 25 Zysk 69 |
| TSG-6 | Tumor necrosis factor–stimulated gene 6 protein | Anti-inflammatory protein significantly upregulated in osteoarthritis progression 8 | Latterman 36 |
| TTT | Thymol turbidity test | A marker of inflammatory conditions such as chronic hepatitis, chronic infection, or collagen disease 32 | Kodama 32 |
| VEGF | Vascular endothelial growth factor | Growth factor that induces angiogenesis4,45 | Avila, 4 Markus 44 |
Footnotes
Final revision submitted February 10, 2024; accepted March 5, 2024.
One or more of the authors has declared the following potential conflict of interest or source of funding: L.M.B. has received consulting fees from Arthrex and nonconsulting fees from Arthrex, Smith & Nephew, and Device Technologies. J.A.F. has received consulting fees from Arthrex and Smith & Nephew and is a paid associate editor for the Orthopaedic Journal of Sports Medicine. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
ORCID iD: Lachlan M. Batty  https://orcid.org/0000-0002-3914-1666
https://orcid.org/0000-0002-3914-1666
References
- 1. Adams SB, Setton LA, Bell RD, et al. Inflammatory cytokines and matrix metalloproteinases in the synovial fluid after intra-articular ankle fracture. Foot Ankle Int. 2015;36(11):1264-1271. [DOI] [PubMed] [Google Scholar]
- 2. Alvarez-Romero J, Laguette MN, Seale K, et al. Genetic variants within the COL5A1 gene are associated with ligament injuries in physically active populations from Australia, South Africa, and Japan. Eur J Sport Sci. 2023;23(2):284-293. [DOI] [PubMed] [Google Scholar]
- 3. Amano K, Huebner JL, Stabler TV, et al. Synovial fluid profile at the time of anterior cruciate ligament reconstruction and its association with cartilage matrix composition 3 years after surgery. Am J Sports Med. 2018;46(4):890-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Avila A, Petrera M, Duenes M, et al. RANTES concentration at the time of surgery is associated with postoperative stiffness in patients undergoing ACL reconstruction. Am J Sports Med. 2022;50(14):3838-3843. [DOI] [PubMed] [Google Scholar]
- 5. Bauer D, Hunter D, Abramson S, et al. Classification of osteoarthritis biomarkers: a proposed approach. Osteoarthritis Cartilage. 2006;14(8):723-727. [DOI] [PubMed] [Google Scholar]
- 6. Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22(4):233-241. [DOI] [PubMed] [Google Scholar]
- 7. Chmielewski TL, Trumble TN, Joseph A-M, et al. Urinary CTX-II concentrations are elevated and associated with knee pain and function in subjects with ACL reconstruction. Osteoarthritis Cartilage. 2012;20(11):1294-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chou CH, Attarian DE, Wisniewski HG, Band PA, Kraus VB. TSG-6—a double-edged sword for osteoarthritis (OA). Osteoarthritis Cartilage. 2018;26(2):245-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cinque ME, Dornan GJ, Chahla J, Moatshe G, LaPrade RF. High rates of osteoarthritis develop after anterior cruciate ligament surgery: an analysis of 4108 patients. Am J Sports Med. 2018;46(8):2011-2019. [DOI] [PubMed] [Google Scholar]
- 10. Colak C, Polster JM, Obuchowski NA, et al. Comparison of clinical and semiquantitative cartilage grading systems in predicting outcomes after arthroscopic partial meniscectomy. AJR Am J Roentgenol. 2020;215(2):441-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cui N, Hu M, Khalil RA. Biochemical and biological attributes of matrix metalloproteinases. Prog Mol Biol Transl Sci. 2017;147:1-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29(6):313-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dupuis DE, Beynnon BD, Richard MJ, Novotny JE, Skelly JM, Cooper SM. Precision and accuracy of joint space width measurements of the medial compartment of the knee using standardized MTP semi-flexed radiographs. Osteoarthritis Cartilage. 2003;11(10):716-724. [DOI] [PubMed] [Google Scholar]
- 14. Emonard H, Grimaud JA. Matrix metalloproteinases. A review. Cell Mol Biol. 1990;36(2):131-153. [PubMed] [Google Scholar]
- 15. Evans-Pickett A, Longobardi L, Spang JT, et al. Synovial fluid concentrations of matrix metalloproteinase-3 and interluekin-6 following anterior cruciate ligament injury associate with gait biomechanics 6 months following reconstruction. Osteoarthritis Cartilage. 2021;29(7):1006-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Feldmann DC, Rahim M, Suijkerbuijk MAM, et al. Investigation of multiple populations highlight VEGFA polymorphisms to modulate anterior cruciate ligament injury. J Orthop Res. 2022;40(7):1604-1612. [DOI] [PubMed] [Google Scholar]
- 17. Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res Ther. 2006;8(suppl 2):S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gomis-Ruth FX, Maskos K, Betz M, et al. Mechanism of inhibition of the human matrix metalloproteinase stromelysin-1 by TIMP-1. Nature. 1997;389(6646):77-81. [DOI] [PubMed] [Google Scholar]
- 19. Gupta R, Khatri S, Malhotra A, Bachhal V, Masih GD, Kaur J. Pre-operative joint inflammation has no bearing on outcome of arthroscopic anterior cruciate ligament reconstruction at 1-year follow-up; a prospective study. Indian J Orthop. 2021;55(2):360-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hagemans FJA, Larsson S, Reijman M, Frobell RB, Struglics A, Meuffels DE. An anterior cruciate ligament rupture increases levels of urine N-terminal cross-linked telopeptide of type I collagen, urine C-terminal cross-linked telopeptide of type II collagen, serum aggrecan ARGS neoepitope, and serum tumor necrosis factor-alpha. Am J Sports Med. 2021;49(13):3534-3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Haley RM, Lamplot JD, Myer GD, et al. Localized anterior arthrofibrosis after soft-tissue quadriceps tendon anterior cruciate ligament reconstruction is more common in patients who are female, undergo meniscal repair, and have grafts of larger diameter. Arthroscopy. 2023;39(6):1472-1479. [DOI] [PubMed] [Google Scholar]
- 22. Harkey M, Luc B, Golightly Y, et al. Osteoarthritis-related biomarkers following anterior cruciate ligament injury and reconstruction: a systematic review. Osteoarthritis Cartilage. 2015;23(1):1-12. [DOI] [PubMed] [Google Scholar]
- 23. Hodi FS, Soiffer RJ. Interleukins. In: Bertino JR, ed. Encyclopedia of Cancer. 2nd ed. Academic Press; 2002:523-535. [Google Scholar]
- 24. Im HJ, Muddasani P, Natarajan V, et al. Basic fibroblast growth factor stimulates matrix metalloproteinase-13 via the molecular cross-talk between the mitogen-activated protein kinases and protein kinase Cdelta pathways in human adult articular chondrocytes. J Biol Chem. 2007;282(15):11110-11121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Inoue M, Muneta T, Ojima M, et al. Inflammatory cytokine levels in synovial fluid 3, 4 days postoperatively and its correlation with early-phase functional recovery after anterior cruciate ligament reconstruction: a cohort study. J Exp Orthop. 2016;3(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jacques C, Gosset M, Berenbaum F, Gabay C. The role of IL-1 and IL-1Ra in joint inflammation and cartilage degradation. Vitam Horm. 2006;74:371-403. [DOI] [PubMed] [Google Scholar]
- 27. Kaplan DJ, Cuellar VG, Jazrawi LM, Strauss EJ. Biomarker changes in anterior cruciate ligament-deficient knees compared with healthy controls. Arthroscopy. 2017;33(5):1053-1061. [DOI] [PubMed] [Google Scholar]
- 28. Kiani C, Chen L, Wu YJ, Yee AJ, Yang BB. Structure and function of aggrecan. Cell Res. 2002;12(1):19-32. [DOI] [PubMed] [Google Scholar]
- 29. Kingery MT, Adams AC, Manjunath AK, et al. Synovial fluid cytokine profile at the time of arthroscopy explains intermediate-term functional outcomes. Am J Sports Med. 2022;50(5):1261-1271. [DOI] [PubMed] [Google Scholar]
- 30. Kingery MT, Anil U, Berlinberg EJ, Clair AJ, Kenny L, Strauss EJ. Changes in the synovial fluid cytokine profile of the knee between an acute anterior cruciate ligament injury and surgical reconstruction. Am J Sports Med. 2022;50(2):451-460. [DOI] [PubMed] [Google Scholar]
- 31. Kirsch JF, Eichele G, Ford GC, et al. Mechanism of action of aspartate aminotransferase proposed on the basis of its spatial structure. J Mol Biol. 1984;174(3):497-525. [DOI] [PubMed] [Google Scholar]
- 32. Kodama Y, Furumatsu T, Hino T, et al. Thymol turbidity test is associated with the risk of cyclops syndrome following anterior cruciate ligament reconstruction. BMC Musculoskelet Disord. 2018;19(1):367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kraus VB, Burnett B, Coindreau J, et al. Application of biomarkers in the development of drugs intended for the treatment of osteoarthritis. Osteoarthritis Cartilage. 2011;19(5):515-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kraus VB, Collins JE, Hargrove D, et al. Predictive validity of biochemical biomarkers in knee osteoarthritis: data from the FNIH OA Biomarkers Consortium. Ann Rheum Dis. 2017;76(1):186-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Larsson S, Struglics A, Lohmander LS, Frobell R. Surgical reconstruction of ruptured anterior cruciate ligament prolongs trauma-induced increase of inflammatory cytokines in synovial fluid: an exploratory analysis in the KANON trial. Osteoarthritis Cartilage. 2017;25(9):1443-1451. [DOI] [PubMed] [Google Scholar]
- 36. Lattermann C, Conley CE, Johnson DL, et al. Select biomarkers on the day of anterior cruciate ligament reconstruction predict poor patient-reported outcomes at 2-year follow-up: a pilot study. Biomed Res Int. 2018;2018:9387809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li X, Han ET, Busse RF, Majumdar S. In vivo T(1rho) mapping in cartilage using 3D magnetization-prepared angle-modulated partitioned k-space spoiled gradient echo snapshots (3D MAPSS). Magn Reson Med. 2008;59(2):298-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li X, Pedoia V, Kumar D, et al. Cartilage T1rho and T2 relaxation times: longitudinal reproducibility and variations using different coils, MR systems and sites. Osteoarthritis Cartilage. 2015;23(12):2214-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lie MM, Risberg MA, Storheim K, Engebretsen L, Oiestad BE. What’s the rate of knee osteoarthritis 10 years after anterior cruciate ligament injury? An updated systematic review. Br J Sports Med. 2019;53(18):1162-1167. [DOI] [PubMed] [Google Scholar]
- 40. Lisee C, Spang JT, Loeser R, et al. Tibiofemoral articular cartilage composition differs based on serum biochemical profiles following anterior cruciate ligament reconstruction. Osteoarthritis Cartilage. 2021;29(12):1732-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu Z, Que S, Xu J, Peng T. Alanine aminotransferase-old biomarker and new concept: a review. Int J Med Sci. 2014;11(9):925-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lotz M, Martel-Pelletier J, Christiansen C, et al. Value of biomarkers in osteoarthritis: current status and perspectives. Ann Rheum Dis. 2013;72(11):1756-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Markus DH, Berlinberg EJ, Strauss EJ. Current state of synovial fluid biomarkers in sports medicine. JBJS Rev. 2021;9(8):e21.00024. [DOI] [PubMed] [Google Scholar]
- 44. Markus DH, Hurley ET, Mojica ES, et al. Concentration of synovial fluid biomarkers on the day of anterior cruciate ligament (ACL)-reconstruction predict size and depth of cartilage lesions on 5-year follow-up. Knee Surg Sports Traumatol Arthrosc. 2023;31(5):1753-1760. [DOI] [PubMed] [Google Scholar]
- 45. Moens S, Goveia J, Stapor PC, Cantelmo AR, Carmeliet P. The multifaceted activity of VEGF in angiogenesis—implications for therapy responses. Cytokine Growth Factor Rev. 2014;25(4):473-482. [DOI] [PubMed] [Google Scholar]
- 46. Morgunova E, Tuuttila A, Bergmann U, Tryggvason K. Structural insight into the complex formation of latent matrix metalloproteinase 2 with tissue inhibitor of metalloproteinase 2. Proc Natl Acad Sci U S A. 2002;99(11):7414-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mosqueda L, Burnight K, Liao S. The life cycle of bruises in older adults. J Am Geriatr Soc. 2005;53(8):1339-1343. [DOI] [PubMed] [Google Scholar]
- 48. Nakajima A, Nakagawa K, Aoki Y, et al. Changes in synovial fluid biochemical markers following arthroscopic surgery in patients with knee osteoarthritis. Rheumatol Int. 2013;33(1):209-214. [DOI] [PubMed] [Google Scholar]
- 49. Nathwani RA, Pais S, Reynolds TB, Kaplowitz N. Serum alanine aminotransferase in skeletal muscle diseases. Hepatology. 2005;41(2):380-382. [DOI] [PubMed] [Google Scholar]
- 50. National Heart, Lung, and Blood Institute of the National Institutes of Health. Quality assessment tools for controlled intervention and cohort observation studies. Updated 2021. Accessed August 24, 2024. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
- 51. National Heart, Lung, and Blood Institute of the National Institutes of Health. Study quality assessment tools. Updated 2021. Accessed August 24, 2024. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
- 52. Neuman P, Dahlberg LE, Englund M, Struglics A. Concentrations of synovial fluid biomarkers and the prediction of knee osteoarthritis 16 years after anterior cruciate ligament injury. Osteoarthritis Cartilage. 2017;25(4):492-498. [DOI] [PubMed] [Google Scholar]
- 53. Pietrosimone B, Loeser RF, Blackburn JT, et al. Biochemical markers of cartilage metabolism are associated with walking biomechanics 6-months following anterior cruciate ligament reconstruction. J Orthop Res. 2017;35(10):2288-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pietrosimone B, Troy Blackburn J, Harkey MS, et al. Walking speed as a potential indicator of cartilage breakdown following anterior cruciate ligament reconstruction. Arthritis Care Res (Hoboken). 2016;68(6):793-800. [DOI] [PubMed] [Google Scholar]
- 55. Raghu H, Lepus CM, Wang Q, et al. CCL2/CCR2, but not CCL5/CCR5, mediates monocyte recruitment, inflammation and cartilage destruction in osteoarthritis. Ann Rheum Dis. 2017;76(5):914-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sobue Y, Kojima T, Kurokouchi K, et al. Prediction of progression of damage to articular cartilage 2 years after anterior cruciate ligament reconstruction: use of aggrecan and type II collagen biomarkers in a retrospective observational study. Arthritis Res Ther. 2017;19(1):265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. 2018;9:754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Struglics A, Larsson S, Kumahashi N, Frobell R, Lohmander LS. Changes in cytokines and aggrecan ARGS neoepitope in synovial fluid and serum and in C-terminal crosslinking telopeptide of type II collagen and N-terminal crosslinking telopeptide of type I collagen in urine over five years after anterior cruciate ligament rupture: an exploratory analysis in the knee anterior cruciate ligament, nonsurgical versus surgical treatment trial. Arthritis Rheumatol. 2015;67(7):1816-1825. [DOI] [PubMed] [Google Scholar]
- 59. Struglics A, Turkiewicz A, Larsson S, et al. Molecular and imaging biomarkers of local inflammation at 2 years after anterior cruciate ligament injury do not associate with patient reported outcomes at 5 years. Osteoarthritis Cartilage. 2020;28(3):356-362. [DOI] [PubMed] [Google Scholar]
- 60. Sullivan B, Stone AV, Conley CEW, Hunt ER, Lattermann C, Jacobs CA. Human synovial fluid interleukin-6, but not type II collagen breakdown, positively correlated with pain after anterior cruciate ligament injury and reconstruction. J Orthop Res. 2023;41(2):300-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Svoboda SJ, Harvey TM, Owens BD, Brechue WF, Tarwater PM, Cameron KL. Changes in serum biomarkers of cartilage turnover after anterior cruciate ligament injury. Am J Sports Med. 2013;41(9):2108-2116. [DOI] [PubMed] [Google Scholar]
- 62. Tau G, Rothman P. Biologic functions of the IFN-gamma receptors. Allergy. 1999;54(12):1233-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tourville TW, Johnson RJ, Slauterbeck JR, Naud S, Beynnon BD. Relationship between markers of type II collagen metabolism and tibiofemoral joint space width changes after ACL injury and reconstruction. Am J Sports Med. 2013;41(4):779-787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Uyama T, Kitagawa H, Sugahara K. Biosynthesis of glycosaminoglycans and proteoglycans. In: Kamerling H, ed. Comprehensive Glycoscience. Vol 3. Elsevier; 2007:79-104. [Google Scholar]
- 65. van’t Hof RJ, Ralston SH. Nitric oxide and bone. Immunology. 2001;103(3):255-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Webster KE, Hewett TE. Anterior cruciate ligament injury and knee osteoarthritis: an umbrella systematic review and meta-analysis. Clin J Sport Med. 2022;32(2):145-152. [DOI] [PubMed] [Google Scholar]
- 67. Webster KE, Hewett TE. Meta-analysis of meta-analyses of anterior cruciate ligament injury reduction training programs. J Orthop Res. 2018;36(10):2696-2708. [DOI] [PubMed] [Google Scholar]
- 68. Yoshida H, Kojima T, Kurokouchi K, et al. Relationship between pre-radiographic cartilage damage following anterior cruciate ligament injury and biomarkers of cartilage turnover in clinical practice: a cross-sectional observational study. Osteoarthritis Cartilage. 2013;21(6):831-838. [DOI] [PubMed] [Google Scholar]
- 69. Zysk SP, Fraunberger P, Veihelmann A, et al. Tunnel enlargement and changes in synovial fluid cytokine profile following anterior cruciate ligament reconstruction with patellar tendon and hamstring tendon autografts. Knee Surg Sports Traumatol Arthrosc. 2004;12(2):98-103. [DOI] [PubMed] [Google Scholar]


