Abstract
Background
How to reduce the high incidence rate and mortality of colorectal cancer (CRC) effectively is the focus of current research. Endoscopic treatment of early-stage CRC and colorectal adenomas (CAC) has a high success rate, but although several treatments are available for advanced CRC, such as surgery, radiotherapy, chemotherapy, and immunotherapy, the 5-year survival rate remains low. In view of the high incidence rate and mortality of CRC, early rational drug prevention for high-risk groups and exploration of alternative treatment modalities are particularly warranted.
Summary
Gut microbiota is the target of and interacts with probiotics, prebiotics, aspirin, metformin, and various Chinese herbal medicines (CHMs) for the prevention of CRC. In addition, the anti-cancer mechanisms of probiotics differ widely among bacterial strains, and both bacterial strains and their derivatives and metabolites have been found to have anti-cancer effects. Gut microbiota plays a significant role in early drug prevention of CRC and treatment of CRC in its middle and late stages, targeting gut microbiota may be a new strategy for colorectal cancer treatment.
Keywords: Gut microbiota, Colorectal cancer, Probiotics, Prebiotics, Medicinal
Key message
This review covers current progress in the role of gut microbiota and drugs in CRC.
Introduction
Nowadays, colorectal cancer has a high incidence rate and fatality rate, ranking third and second among frequent malignant tumors, respectively [1, 2]. Most colorectal cancers follow an “adenoma-carcinoma” (CAC) pattern and are influenced by various environmental and genetic influences, early prevention and screening of high-risk individuals with risk factors such as advanced age and obesity are the main measures to reduce the incidence of CRC [3]. One of the risk factors for colorectal cancer is dysbiosis of the gut microbiota, the stability of the intestinal microenvironment prevents colorectal carcinogenesis by preserving the functionality of the intestinal barrier and by mediating intestinal inflammation and immune responses [4]. The gut microbiota is closely related to the development of CRC. 16S rRNA gene sequencing revealed a decrease in bacterial diversity in fecal microbiota of CRC patients, accompanied by an increase in the abundance of pathogenic bacteria such as Gammaproteobacteria, Enterobacteriales, and Fusobacteria [5]. Moreover, gut microbiota has already undergone changes in the early stage of colorectal cancer, i.e. the stage of colorectal adenoma [6].
Probiotics and prebiotics have been found to prevent colorectal cancer, the mechanism by which probiotics and prebiotics prevent colorectal cancer by regulating gut microbiota homeostasis is widely recognized, which will be further summarized in this review. The effective treatment measures for intermediate and advanced colorectal cancer mainly include surgical treatment, radiotherapy, and immunotherapy. Using probiotics to modulation of gut microbiota homeostasis can reduce postoperative adverse effects, improve radiotherapy and immunotherapy efficacy, reduce adverse drug reactions, and ultimately reduce mortality from advanced colorectal cancer. It is noteworthy that the mechanisms of prevention of CRC by aspirin, metformin, and Chinese herbal medicines are also associated with the gut microbiota, which will be discussed in detail in this review. The gut microbiota is central to the early prevention and treatment of the middle and late stages of CRC (Fig. 1). Morbidity and mortality of Reducing CRC by targeting gut microbiota may be a novel strategy for the treatment of colorectal cancer.
Fig. 1.
The gut microbiota is central to the early prevention and treatment of the middle and late stages of CRC
Probiotics
Dysbiosis of the gut microbiota is closely associated with CRC development and progression. Probiotics regulate gut homeostasis by acting directly with the gut [7]. Attention to the role of probiotics in the primary prevention of colorectal cancer is the current research hotspot. The majority of studies have focused on exploring the mechanism of single probiotics and their derivatives, and metabolites in colorectal cancer. The main probiotics that are currently receiving attention are Lactobacillus, Clostridium butyricum, Akkermansia muciniphila, and Bifidobacterium. Several animal and external cell experiments had proved that there was a difference in the anti-cancer mechanism of different genera and species (Table 1). β- Galactosidase, a key protein secreted by Streptococcus thermophilus, induces apoptosis in CRC cancer cells by activating the AMPK pathway and Inhibition of the " Warburg effect " phenomenon [8]. Clostridium casei exert anticancer effects by producing short-chain fatty acids and Butyricicoccus pullicaecorum by upregulating short-chain fatty acid receptors (SLC5A8 and gpr43) [9–11]. Specific protein in Akkermansia muciniphila, Amuc _ 1434 * and Amuc_ 1100, has special anti-cancer properties. Amuc_ 1434 * can degrade CRC cell mucin 2 to promote CRC cell apoptosis [12, 13], and Amuc_ 1100 activates cytotoxic T lymphocytes in mesenteric lymph nodes and enhances their cytotoxicity to inhibit colitis-induced CRC [14].
Table 1.
Main mechanisms of CRC prevention by probiotics in animal experiments and in vitro cell experiments
| References | Probiotics | Cell/animal experiments | Treatments | Effects | Potential mechanisms |
|---|---|---|---|---|---|
| Zhang Y, et al. (2017)[16] | Lactobacillus casei Zhang |
Animals: Male C57BL/6J mice AOM/DSS-induced CRC model; Standard sterile mouse chow. |
The probiotic was mixed in the diet (4 × 109 CFU/d), Once a day for 9 weeks. |
①↓Number and size of tumors, ②↓Histological scores. |
①Activated the anti-inflammatory CLCN3 signaling and anti-oncogenic TGF-β/CLIC4 signaling ②↑SCFAs in caecum contents; ③ Altered gut microbiota. |
| Li Q , et al(2021)[8] | Streptococcus thermophilus |
Cells: CRC cell lines (HCT116, HT29, and Caco-2); Human normal colon epithelial cell line NCM460 |
①Supplemented with 10% (vol/vol) fetal bovine serum, and 1% penicillin/streptomycin in a humidified atmosphere containing 5% CO2. ② pcDNA3.1(+)/YAP1 and empty vector were transfected into HCT116 cells to get the YAP1-overexpressed cell line. |
①↓cells vitality ②↓Cells proliferation |
The Antitumor Effect of Streptococcus thermophilus Is Mediated by the Secretion of β-Galactosidase. Which inhibited both the Hippo signaling and the Warburg metabolic phenotype in CRC cells. |
|
Animals: Male C57BL/6 wild-type mice (5 wks old) underwent 6 consecutive intraperitoneal injections of azoxymethane (AOM; 10 mg/kg) at 1-week intervals. |
The probiotic was administered by intraperitoneal injection (1 × 108 CFU) | ↓Number and size of tumors. | |||
| Liu M, et al. (2020)[11] | Clostridium butyricum |
Animals: Male C57BL/6 mice (6–8 wks old) AOM/DSS-induced CRC model; Standard sterile mouse chow. |
The probiotic was administered by oral gavage (2 × 108 CFU/0.2mL physiological saline), 3 times a week for 11 weeks (78 days). |
①↓Number and size of tumors. ②↓Histological scores. |
① Inhibited activation of NF-κB signaling; ② Anti-inflammation through↓TNF-a、IL-6 and COX-2 expression; ③ Did not alter gut microbiota. |
|
Chen D, et al. (2020)[10] |
Clostridium butyricum (ATCC 19398) |
Cells: Human CRC cell lines (HCT116, Caco-2, and HCT8). |
Treated with Clostridium butyricum supernatant at 37℃for 1 h. |
①↓Cells proliferation, ②↑Cells apoptosis. |
① Suppressed the Wnt/β-catenin signaling; ②↑GPR43 and GPR109A expression; ③↓DCA and LCA,↑SCFAs in fecal contents; ④ Altered gut microbiota. |
|
Animals: Female BALB/c SPF Apcmin/+ mice (4 wks old); A diet rich in fat (60% fat, 20% protein, and 20% carbohydrates). |
The probiotic was administered by oral gavage (2 × 109 CFU/0.2mL) after being given a 3-day antibiotic cocktail, 3 times a week for 12 weeks. |
①↓Number and size of tumors. ②↓Intestinal tumor burden. ③↑Representative and histological appearance |
|||
| Chang SC, et al. (2020)[9] | Butyricicoccus pullicaecorum (A gut butyrate-producing bacterium) |
Cells: SW480 colon cancer cell line and SW620 lymph node metastatic derivative cell line |
① Treated with Butyricicoccus pullicaecorum supernatant at 37 °C for 5 days; ② Treated or not treated with sodium butyrate (NaB, 5mM) |
↓Cells proliferation | Butyricicoccus pullicaecorum increased the expression of SLC5A8 and GPR43 by producing butyrate. |
|
Animals: Male BALB/cByJNarl mice (4–6 wks old) DMH/DSS-induced CRC model; Standard sterile mouse chow |
The probiotic was administered by oral gavage (3.125 × 107 CFU/0.1mL), Once a week for 7 weeks |
①↓ACF and tumor incidence ②↓Tumor invasion depth ③↑Survival rates ④↓Body weight loss and anal bleeding ⑤↓Serum CEA levels |
|||
| Wang L, et al. (2020)[14] | Akkermansia muciniphila (Amuc_1100: The membrane protein) |
Cells: CT26 cells cocultured with CTLs (isolated from the spleen of normal mice) |
Treat with Amuc_1100 (10 µg/mL) at 37℃for 24 h. |
①↑CT26 cells apoptosis ②↑proportion of CTLs |
①A. muciniphila and Amuc_1100 activated CTLs in the MLN and enhanced their cytotoxic effect, as indicated by TNF-α induction and PD-1 downregulation. ② Anti-inflammatory (↓TNF-α、IFN-γ、IL-1β/6/18/33 expression) ③↓DNA damage (γH2AX staining) and↓cells proliferation (Ki67 staining) |
|
Animals: Male C57BL/6J mice (6–8 wks old) AOM/DSS-induced CRC model; Standard sterile mouse chow. |
A. muciniphila (1.5 × 108 CFU) or the Amuc_1100 (3 µg) was administered by oral from 2 weeks before AOM injection until sacrifice (Total 23 weeks). |
①↓Numbers and area of tumors ②↓Splenomegaly ③↓DAI |
|||
| Meng X, et al(2019)[12] | Akkermansia muciniphila (Amuc_1434*) |
Cells: LS174T colon cancer cell lines |
Treat with five different volumes (0, 50, 100, 200, and 400 µL) of 50 µg/mLAmuc_1434* (50 µg/mL) at 37℃for 3 h. | Amuc_1434* Degradation of Muc2 |
① Amuc_1434* could associate with Muc2 and participate in its degradation ② Amuc_1434 was mainly distributed in the colon of BALB/c mice |
| Meng X, et al(2020)[12] | Akkermansia muciniphila ( Amuc_1434*) |
Cells: LS174T cells |
Teat with various concentrations (0, 8, and 64 µg/mL) of Amuc_1434* for 24 h |
①↓Cells proliferation ② ↓Cell cycle ③↑Cell apoptosis |
① Amuc_1434* Promoted the Change of Cellular Redox Status and Mitochondrial Dysfunction in LS174T Cells ② Amuc_1434* Activated the LS174T Cells’ Apoptotic Pathway via TRAIL |
| Peng M, et al. (2020) [118] | Lactobacillus casei |
Cells: HCT116 |
0.1, 0.2, 0.3, 0.4, and 0.5 mM of the probiotic cell-free supernatant were added to cells at 37 °C for 24 h. | ↓Cells proliferation |
① Anti-inflammatory (↓COX-2、PGE-2、IL-17/21/22/23、INF-γ and↑IL-10、 TGF-βexpression). ② Anti-oxidative effects (↑RCA). ③↓CDK1/2/6, PLK1, and SKP2 expression. ④ Altered gut microbiota. |
|
Animals: Gender-mixed BALB/c mice (3 wks old); Standard sterile mouse chow |
The probiotic was mixed in the diet (1 × 109 CFU/mL), Once a day for 7 days. |
Prevention of CRC | |||
| Sugimura N, et al. (2021)[15] | Lactobacillus gallinarum |
Cells: Two CRC cell lines (HCT116 and LoVo) |
Different concentrations of culture supernatant were used for culturing CRC cell lines (5%/10%20%) for 5 days. |
①↓Cells viability ②↑Cells apoptosis |
① Lactobacillus gallinarum produces and catabolizes L-tryptophan to release indole-3-lactic acid to protect against CRC. (High-throughput targeted L-tryptophan metabolic profiling using culture supernatant) ② Altered gut microbiota. |
|
Animals: ① Gender-mixed apcmin/+ mice (5–6 wks old) ② Gender-mixed C57BL/6 mice (6wks old) AOM/DSS-induced CRC model |
The probiotic was mixed in the PBS (1 × 108 CFU/0.1mL) and administered by oral gavage. Once a day for 12 weeks. |
↓Number and size of tumors. | |||
| Li S.C, et al(2019)[17] | Lactobacillus acidophilus |
Animals: Male F344 rats (3 or 5 wks old) |
Feeding containing 10% GBR, 2.5%,5%,10% FGBR 10 weeks |
①↓Aberrant Crypt Foci (ACF) ②↓Mucin and Mucin-Depleted Foci (MDF) ③↓Serum Pro-Inflammatory Cytokines in Rats ④↑the Expression of Apoptosis-Related Proteins in the Colon of Rats |
①GBR and FGBR reduced the primary ACF number and decreased TNF-α, IL-6, and IL-1β levels ②GBR and FGBR at the 2.5% level increased pro-apoptotic cleaved caspase-3 and decreased anti-apoptotic B-cell lymphoma 2 (Bcl-2) expressions. ③FGBR at the 2.5% level further reduced the number of sialomucin-producing ACF (SIM-ACF) and increased Bax expression. |
| Xu H, et al. (2021)[119] | Lactobacillus rhamnosus M9 |
Animals: Male C57BL/6NCrSlc mice (8 wks old) AOM/DSS-induced CRC model; Standard sterile mouse chow. |
The probiotic was administered by oral (2 × 109 CFU/d) on weeks 8 and 10, a total of 14 days. |
①↓Number and size of tumors. ②↑Colon length |
①↓Cells proliferation (PCNA and Ki67 staining) ②↓CD68+/CD163+ expression ③↓P-Akt and p-STAT3 expression ④ Altered gut microbiota. |
| Wang T, et al. (2022)[120] | Lactobacillus coryniformis MXJ32 |
Animals: Male C57BL/6 mice (6 wks old) AOM/DSS-induced CRC model; Standard sterile mouse chow. |
The probiotic was administered by oral (1 × 109 CFU/0.2mL) from 4 weeks after AOM injection until sacrifice (Total 14 wks). |
①↓DAI ②↓Thymus and spleen weight ②↓Number and size of tumors. |
① Protected the intestinal barrier integrity (↑occludin, claudin-1, ZO 1, MUC-2/3 expression) ② Anti-inflammatory: (↓TNF-α, TNF-1β, IL-6, IL-γ, and IL-17a expression), (↓serum LPS and↓TLR4, MyD88 and NF-κB mRNA expression) ③↑The level of SCFAs ④ Altered gut microbiota. |
| Shujie C, et al.(2023)[120] | Bifidobacterium adolescentis(B.a) |
Cells: Cancer-associated fibroblasts(CAFs) |
CAFs were incubated with B.a or E.coli for 48 h, and co-cultured with HCT-116 cells | ↓CRC cell proliferation |
①CD143 CAFs was activated by B.a and then reshaped TME+ ②Wnt/β-catenin signaling was involved in the induction of CD143 CAFs by B.a+ |
| Yifeng L, et al.(2023)[44] | Bifidobacterium adolescentis(B.a) |
Animals: AOM/DSS-induced CRC model in C57BL/6 mice |
Pretreated with streptomycin (2 mg/mL) for 7 days and administrated with B.a, Escherichia coli (E. coli) or vehicle (PBS) control |
①↓size of tumors ②↓marker Ki67 and marker CD31 |
①B.adolescentis facilitated the infiltration of Decorin macrophages to suppress CRC+ ②The activation of TLR2 is essential for inducing DCN macrophages by B.a+ ③B.a regulated DCN macrophages through TLR2/YAP axis+ |
In addition, the anti-cancer mechanisms of the same genera but different species of probiotics are different (Table 1). L-tryptophan and its metabolite indole-3-lactic acid, produced by Lactobacillus galinarum, were found to be a protective metabolite able to induce apoptosis of human CRC cells in vitro cell experiments [15]. Lactobacillus casei Zhang induces inflammation by activating the anti-inflammatory pathway CLCN3 signaling pathway and TGF-βAnti cancer signaling pathways [16]. L.acidophilus exerts anticancer effects by inhibiting precancerous lesions, reducing proinflammatory cytokines, and increasing the expression of apoptosis-related proteins [17]. Lactobacillus casei Zhang, Lactobacillus galinarum, and Lactobacillus acidophilus are all grouped in the genus Lactobacillus, but they have different anticancer mechanisms. The anticancer mechanisms of probiotics are complex and diverse, and even if the same bacterial strain has been found to have multiple anticancer pathways," individualized " studies of probiotics can identify superior anticancer bacterial strains.
Bacteriocins are peptides or precursor peptides synthesized by bacteria through ribosomes during metabolic processes, which can inhibit bacterial activity [18]. Currently, some studies suggest that bacteriocins such as nisin, plantaricin A, and pyocins have anti-cancer capabilities [19], and many bacteriocins also play a role in the treatment of CRC [20, 21]. For example, Nisin is produced by Lactococcus lactis subsp. and can significantly inhibit the cell viability of colorectal cancer cells (SW480), which may be related to its ability to reduce the expression of cyclin D1 [22]. Nisin also has an inhibitory effect on the proliferation of colorectal cancer cells Cac2 and HT29 [23]. The study by Hesam S et al. found that Nisin can interfere with the expression of MMP2 and MMP9 genes related to cell metastasis, thereby reducing the metastasis and migration ability of CRC cells, demonstrating its potential to resist CRC progression and metastasis [24]. Caspase-3 is a key factor in executing cell apoptosis, and plantaginomycin BM-1 produced by Lactobacillus plantarum BM-1 can induce SW480 cell death through a caspase-dependent apoptotic pathway [25]. Azurin is secreted by Pseudomonas aeruginosa and its 28 amino acid fragments (P28) derived from Azurin have shown anti-tumor activity in a phase I clinical trial of short-term injection therapy in 15 patients with advanced solid tumors (including 4 cases of colorectal cancer). In addition, engineering probiotics expressing azuron reduced the tumor burden induced by AMO/DSS in mice and also improved intestinal microbiota dysbiosis in mice [26].
In clinical trials, probiotics have been found to have the potential to prevent CRC. Yogurt is produced by the fermentation of lactobacilli and Streptococcus thermophilus, and it was proposed as early as 2004 that can inhibit CRC progression [27–30]. An epidemiological study of 120,000 people over 32 years found that taking yogurt regularly was associated with a lower risk of colorectal cancer proximally [30].The population taking Lactobacillus casei has a lower risk of developing moderate to high-grade colorectal tumors compared to the normal group [31]. Hatakka K et al., found that daily dosing of Lactobacillus rhamnosus lc705 and Propionibacterium Freudenreichii SSP shermanii JS for 4 weeks was capable of reducing the activity of fecal β- Glucosidase activity in healthy male volunteers [32], which were found to promote CRC development by producing carcinogens [33]. Probiotics hold great promise in the prevention of colorectal cancer, and the anticancer mechanisms are diverse in animal and in vitro cell experiments, but the effective activity of probiotics decreases after they pass through the GI tract, and how to increase their colonization in the gut needs to be further addressed in clinical trials.
Gut microbes modulate the response of CRC patients to chemo - and immunotherapeutic drugs [34]. High-quality evidence indicates that the gut microbiota is closely associated with chemotherapeutic agents such as 5-uf [35], oxaliplatin [35], cyclophosphamide [36] and immunotherapeutic agents such as PD-1 inhibitors [37, 38], and clta-4 inhibitors [37, 39]. Multiple studies have found significant differences in gut microbiota between treatment-responsive and non-treatment-responsive patients, and between patients with no adverse effects and mild or severe adverse effects [40–43], mainly including probiotics such as Bifidobacterium spp., Lactobacillus spp., and Bacteroides spp., which increase efficacy. 16S rRNA sequencing of stool samples from patients with advanced rectal cancer undergoing neoadjuvant chemoradiotherapy revealed that the relative abundance of butyrate-producing bacterial strains, such as Roseburia., Dorea., and Anaerostipes., were significantly elevated in the treatment-responsive group. The regulation of immune therapy drug response by probiotics may be related to their own impact on immune cells. Bifidobacterium is a common anti-cancer probiotic. Studies have shown that after oral administration of Bifidobacterium to AOM/DSS mice, the tumor size is significantly reduced compared to the control group. This is because Bifidobacterium can promote the infiltration of Decorin macrophages into colorectal tumors by activating the TLR2/YAP axis, thereby exerting its anti-cancer effect [44]. Macrophages with different polarization states exhibit different immune responses, and M2 phenotype macrophages exhibit anti-inflammatory properties. Lactic acid can promote macrophage polarization towards the M2 phenotype [45], while brewing yeast strain BY4741 is a probiotic that can produce lactic acid. It not only promotes macrophage polarization, but also inhibits macrophage necrosis, thereby alleviating colitis and edema symptoms in DSS induced mouse colitis models, significantly reducing histopathological scores [46]. A study published in Nature, in which 11 low-abundance rare bacterial strains were isolated from the feces of healthy volunteers, was able to significantly improve the efficacy of PD-1 inhibitors and reduce adverse effects [37], possibly through the enrichment of gut bacteria with product γ- Interferon - γ functions of CD8 + T lymphocytes. Recently Zhang et al., similarly found that Lactobacillus casei increased the efficacy of PD-1 inhibitors through a CD8 + T lymphocyte-dependent manner [38], suggesting that CD8 + T-lymphocytes may be a key target for probiotics to improve the efficacy of immunotherapy.
Gut microbes are important in modulating the adverse effects of drugs. The main adverse effects caused by chemo - and immunotherapeutic drugs are intestinal mucositis, diarrhea, and weight loss, while gut microbes are closely related to intestinal immunity and inflammatory response. An animal study in which feces from healthy mice were transplanted into the intestines of CRC mice receiving chemotherapeutic agents found that the symptoms of diarrhea and intestinal mucositis were alleviated, and the expression of Toll-like receptor (TLR), MyD88 mRNA was inhibited and serum IL-6 concentrations were significantly decreased in the intestinal tissues of mice measured [47], whereas diarrheal symptoms were also significantly improved in chemotherapy-treated mice with a knockout of Toll-like receptor (TLR-4 / MyD88 NF) [48], which led to the speculation that the gut microbes regulated TLR-4 / MyD88 NF-κB-il6 signaling alleviates intestinal inflammation in mice. Clinical experiments have further confirmed that adverse effects, such as intestinal mucositis and diarrhea, are associated with intestinal microbial disorders. 16 S rRNA sequencing of feces from CRC patients who developed diarrhea compared with those who did not, after completing 8 cycles (a total of 2 years) of capeox chemotherapy in stage III CRC patients, revealed that a total of 75 bacterial strains differed in abundance [40], while simultaneous oral administration of probiotics during chemotherapy significantly reduced the incidence and severity of diarrhea in CRC patients [49], Simultaneous oral administration of probiotics during chemotherapy in CRC patients is a novel therapeutic strategy to alleviate adverse effects.
Surgical treatment is a common treatment for CRC. According to the British Cancer Treatment Center, 65.7% of colon cancer patients and 63.2% of rectal cancer patients receive surgical treatment [50], but the incidence rate of CRC surgery-related complications is as high as 23 − 34.6% [51, 52]. Being well-prepared preoperatively is one of the strategies to reduce the risk of postoperative complications in CRC patients, and there are currently multiple studies suggesting that preoperative gut microbial disorders in CRC patients are associated with the risk of postoperative complications [53–55]. The postoperative complications of CRC patients mainly include early diarrhea, anastomotic leakage, intestinal obstruction, and postoperative infection, preoperative oral administration of probiotics can modulate the gut microbiota, thereby significantly reducing the risk of multiple postoperative complications [56, 57]. The administration of probiotics significantly decreased the incidence of a number of complications, such as postoperative incision infection, pneumonia, urinary tract infection, and anastomotic leak, according to a meta-analysis of 19 high-quality clinical controlled studies by Zeng et al., which included a total of 1975 CRC patients [56]. Furthermore, administration of probiotics significantly reduces postoperative serum IL-6 / 10 / 12 / 22 and other inflammatory factors in patients with CRC [58], and in a rat model of anastomotic leak, butyrate administered transrectally was found to promote intestinal anastomotic healing in rats [59, 60], suggesting a potential efficacy of probiotics in preventing postoperative CRC infections and related complications. Maintaining the homeostasis of the gut microbiota in preoperative patients is one of the keys to reducing postoperative complications, preoperative oral administration of probiotics is an effective way to maintain gut microbial homeostasis, but gut microbial homeostasis is also influenced by numerous factors, such as preoperative enteroscopy, antibiotics, preoperative neoadjuvant chemoradiotherapy, etc. Finding a balance point between probiotics and preoperative tests / therapeutic measures is a matter of concern for clinicians.
The gut microbiome is a biomarker for predicting chemotherapy and surgical complications. In recent years, with the development of metagenomics technology, researchers have found that gut derived microbiome also exists in the blood [61]. Yang et al. proposed that the diversity of blood microbiome is a promising indicator for predicting the clinical efficacy of DC-CIK combined chemotherapy. Compared with late stage CRC patients without treatment response, Bifidobacterium, Lactobacillus, Enterococcus, and Pseudomonas are more abundant in the blood of patients with DC-CIK combined chemotherapy efficacy [42]. However, in the monotherapy group (oxaliplatin and capecitabine), there was no significant difference in the pre-treatment blood microbiome between responders and non responders. The reason may be due to the different pharmacological effects of chemotherapy drugs and DC-CIK. The characteristics of preoperative gut microbiota are significantly correlated with postoperative complications. Acinetobacter Iwoffii and Acinetobacter jhonsonii bacteria are significantly enriched in patients with anastomotic fistula [54], and Faecalibacterium is significantly reduced in patients with postoperative intestinal obstruction in CRC. Jin et al. used Faecalibacterium as a biomarker to predict postoperative intestinal obstruction. The AUC values in the early onset intestinal obstruction group (occurring during the perioperative period after surgery) and the late onset intestinal obstruction group (occurring within 6 months after discharge) were 0.74 and 0.67. In the cohort of another 38 CRC patients, the AUC value was confirmed to be 0.79 [53]. Finding effective microbial biomarkers to construct screening models, accurately predicting chemotherapy efficacy and the risk of postoperative complications, and providing personalized prevention and treatment for patients in advance is another strategy to improve the prognosis of advanced CRC patients.
Prebiotics
Compared with probiotics, prebiotics are not affected by digestive enzymes and a strong acid environment in the upper digestive tract, providing conditions for the sustainable regulation of gut microbial homeostasis. In the body, prebiotics can promote the proliferation of beneficial bacteria by stimulating their metabolism and metabolism without being digested and absorbed. Functional oligosaccharides, polysaccharides, cereals, vegetables, and Chinese herbs in plants are among the common prebiotics. The preventive effect of prebiotics on CRC is mainly reflected in two aspects. On one hand, both beneficial and harmful bacteria are regulated by prebiotics to maintain gut microbial homeostasis [62–65]. In animal experiments, alisol B 23 acetate (ab23a) is a naturally occurring prebiotic, and ab23a is capable of reducing bacterial pathogens such as Klebsiella, and Citrobacter and increasing the abundance of beneficial bacteria such as Bacteroides, Lactobaci, llus and Alloprevotella [66]. Triterpene saponins (ginsenoside-rb3 and ginsenoside rd) can promote the growth of probiotics such as Bifidobacterium spp., Lactobacillus spp., Bacteroides acidifies, and Bacteroides xylanisolvens and reduce the abundance of CRC associated bacteria such as Dysgonomonas spp., and Helicobacter spp [67]. Some prebiotics have species specificity for the growth-promoting effects of probiotics. For example, fructooligosaccharides (FOS), xylooligosaccharides (XOS), and galactooligosaccharides (GOS) all enhance the growthbutyrate-producingcing probiotics, but display different growth profiles [68, 69].
Prebiotics are not only able to modulate gut microbiota homeostasis as a whole but can also activate against key pathogenic bacteria [70, 71]. Fusobacterium nucleatum is a key pathogenic bacterium in the development of CRC and plays an important role in promoting CRC liver metastasis [72–74]. Coculture of L-fucose and Fusobacterium nucleatum with human CRC cell lines revealed a significant decrease in the proliferation, migration, and invasion of CRC cells. The underlying mechanism is through inhibition of STAT3 signaling andepithelial-mesenchymall transition [70]. Another in vitro cell experiment also similarly found that derivatives of vanillin from vanilla have specific activity against Fusobacterium nucleatum [71]. L-fucose and vanillin derivatives have unique effects against Fusobacterium nucleatum. Deeply studying the mechanism of action of prebiotics on a specific strain may be a new treatment strategy for prebiotics to prevent CRC.
On the other hand, prebiotics are converted by certain special gut strains into metabolites with anticancer activity [75–77]. For example, nondigestible dietary fibers in food, functional oligosaccharides that are metabolized to short-chain fatty acids by intestinal probiotics, play an important role in improving human intestinal health and suppressing intestinal tumors [77, 78]. Ferulic acid, a well-known natural prebiotic, is abundant in Chinese herbs such as Angelica Sinensis and cohoshi and has medicinal value. Luo y et al. simulated the intestinal environment in vitro, metabolized ferulic acid to its metabolite (2-methoxy-4-vinyl phenol) with human intestinal microbes and commercial probiotics such as Lactic acid bacteria, Bifidobacteria, and Streptococcus, and found that 2-methoxy-4-vinyl phenol displayed more potent anticancer effects than ferulic acid [76]. Short chain fatty acids are currently widely known anticancer metabolites, which have anti-inflammatory, inhibitory effects on tumor proliferation, and anti-tumor cell immunity [79]. Dietary fiber and functional oligosaccharides that are not easily digestible in food can increase the content of short chain fatty acids in the intestine through fermentation by intestinal microorganisms [80], playing an important role in improving human intestinal health and inhibiting intestinal tumors.
Aspirin
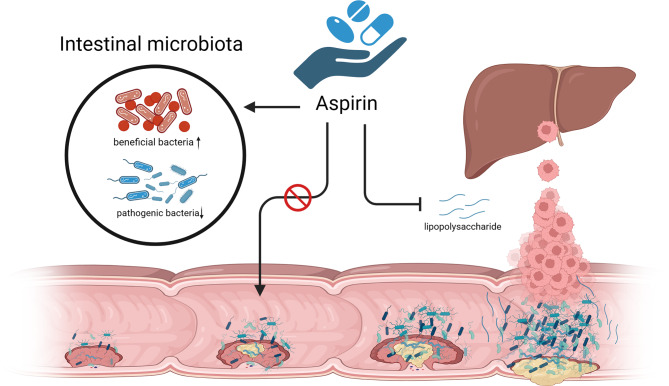
Aspirin has antipyretic analgesic and antiplatelet aggregation functions and is a nonsteroidal anti-inflammatory and antiplatelet aggregation drug acting on cyclooxygenase. The evidence of high-qualitylity research supports the benefits of taking aspirin regularly for reducing the risk of CAC and CRC [81–83], which led the US to recommend aspirin in 2016 for the primary prevention of CRC in most populations [84]. Although anti-inflammatory effects have received much attention as the most likely mechanism of aspirin’s prevention of CRC, there is evidence that gut microbes are involved in aspirin’s anti-cancer effects (Fig. 2) and that there is significant interaction between gut microbes and aspirin. Under the condition of regular administration of aspirin, antibiotic-depleted germ-free CRC mice have higher plasma aspirin concentrations than intestinal microbiota intact CRC mice, owing to the fact that certain intestinal aerobes degrade aspirin and reduce its circulating bioavailability [85].
Fig. 2.
The mechanism of aspirin in anti-CRC
Beyond the effects of gut microbes on the metabolism of aspirin, studies have found that aspirin affects the gut microbiota’s composition, 16sRNA sequencing of intestinal tissue and fecal samples from rats given low-dose aspirin and from normal rats revealed significant differences in the gut microbiota [86]. Aspirin would increase intestinal beneficial bacteria and decrease CRC-related pathogenic bacteria [85, 87, 88]. A small clinical study randomized 50 healthy volunteers to aspirin (325 mg / D, n = 30) or placebo (n = 20) for 6 weeks confirmed that regular aspirin administration increased beneficial bacteria such as Akkermansia, Prevotella, and Ruminococcaceae and decreased the abundance of pathogenic bacteria such as Parabacteroides, Bacteroides, and Dorea by 16 S rRNA sequencing of fecal samples [87]. In vitro assays, aspirin was shown to effectively inhibit the proliferation of Fusobacterium nucleatum to counteract the carcinogenic effects [88], demonstrating the huge potential of aspirin in maintaining gut microbial homeostasis.
The liver is a common metastatic organ of CRC, and the inhibitory effect of aspirin on CRC liver metastasis is associated with gut microbes, with one study finding that lipopolysaccharide (LPS) derived from gut microbes can induce colon cancer cell metastasis in vitro and in vivo, whereas aspirin inhibits LPS induced CRC metastasis by inhibiting TLR4 [89]. To sum up, aspirin may prevent CRC development and improve prognosis by maintaining intestinal microbial homeostasis, but drug absorption and metabolism are influenced by gut microbes, and there are serious adverse effects of the drugs, such as bleeding due to the antiplatelet effects of excess aspirin. Further studies on the balance between drug efficacy, circulating bioavailability, and adverse drug effects by gut microbiota are needed.
Metformin
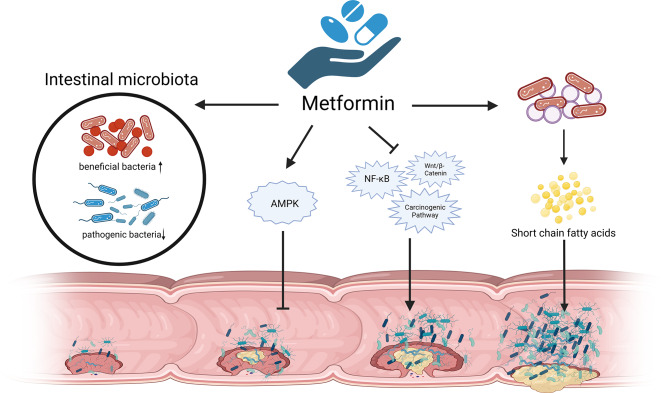
Metformin is the first-line drug for the management of diabetes, which is believed to prevent several malignancies including CRC [90, 91]. Low-dose (250 mg / D) metformin reduces the incidence and number of metachronous adenomas or polyps in patients after polypectomy [90]. How metformin prevents CRC is incompletely explored, and currently the major research direction is the AMPK pathway-dependent anti-inflammatory effect [92]. It is noteworthy that some mechanisms of metformin and probiotics in preventing colorectal cancer are consistent, such as inhibiting NF-κB、Wnt/β-Catenin, and other carcinogenic signaling pathways [93, 94] (Fig. 3). High-quality evidence suggests that metformin exerts indirect non-AMPK pathway anti-CRC effects by directly regulating intestinal homeostasis [95, 96]. A clinical study of patients with new-onset diabetes found that the abundance of a total of 86 bacterial strains changed significantly after 4 months of metformin treatment compared with pretreatment, whereas only 1 bacterium changed in the placebo group [97]. Similarly performed 16 S rRNA sequencing of fecal samples, metformin led to changes in the gut microbiota of young non-diabetic men, mainly characterized by decreased abundance of Fusobacterium and Enterobacter, and increased abundance of Escherichia and Shigella, with the gut microbiota returning to pretreatment levels after discontinuing metformin [98]. Metformin has also been found to reverse Fusobacterium nucleatum-induced CRC development in animal experiments [99]. Perhaps metformin protects against CRC by affecting the gut microbial structure.
Fig. 3.
The mechanism of metformin in anti-CRC
In addition, the gut microbiota mediates the hypoglycemic effects and adverse effects of metformin, including improvement of insulin resistance, regulation of glucose and energy metabolism, reduction of body mass index, and alleviation of gastrointestinal adverse effects such as diarrhea, bloating, and nausea [100]. Obesity is one of the risk factors for CRC, in an HFD-fed mouse model of colon adenoma, metformin inhibited tumor growth and increased the abundance of short-chain fatty acid-producing bacteria such as Alistipes, Lachnospiraceae and Ruminococcaceae [101], which may be achieved by restoring SGLT1 dependent glucose sensitive pathways in the small intestine [102]. Gut microbes are involved in the hypoglycemic effect of metformin and control risk factors for CRC while being involved in the anti-CRC effect of metformin.
Chinese herbal medicines
Many Chinese herbs, such as berberine [103], Sidi Decoction [104], and Gegen Decoction [105], play a role in the prevention and treatment of CRC and are involved in regulating the proliferation, apoptosis, migration, and angiogenesis of CRC cells. Available evidence suggests that the gut microbiota is perhaps the key to many Chinese herbal medicines exerting anti-CRC effects. Administration of berberine 0.6 g daily was effective in reducing the recurrence risk of CAC in the high-risk group (or = 0.77, 95% CI 0.66–0.91, P < 0.001) [103]. In animal experiments, Berberine itself is also able to delay the generation of colitis-associated cancer tumors by remodeling the composition of the gut microbiota in mice with CAC-producing short-chain fatty acids [106]. Mechanistically, CRC mice treated with berberine exhibited decreased inflammatory cell expression (IL-1 β, TNF- α, CCL1, CCL6, and CXCL9), and NF- κ B expression was greatly suppressed [107]. In addition, a clinical controlled study found that compared with pretreatment, oral administration of Gegen Decoction upregulated the abundance of Bacteroides spp., Akkermansia spp., and Prevotella spp., and downregulated the abundance of Macromonas spp., Veillonella spp., which was confirmed by measuring immune factors such as CD4 + T, CD8 + T and inflammatory factors such as IL-2 and IL-6, and proved that Gegen Decoction improved the immunity and alleviated the inflammatory status of CRC patients by regulating gut microbes [105]. Similar effects of modulating gut microbiota composition were also demonstrated in Chinese herbs such as Sidi Decoction [104], Evodia [108], and San-Wu-Huang-Qin [109].
Paris polyphylla is a well-known herb with anticancer activity, the active components of Paris polyphylla were confirmed in vitro experiments to not only inhibits Fusobacterium nucleatum growth directly, but also reverse the promoting proliferation and migration of CRC cells by Fusobacterium nucleatum [110], Paris polyphylla has antibiotic like effects on Fusobacterium nucleatum. In animal experiments, berberine can reverse the luminal microbiota imbalance induced by Fusobacterium nucleatum and block the activation of tumorigenesis-related pathways [111]. Further exploration of the mechanism of Chinese herbal medicine on Fusobacterium nucleatum in vivo is a direction of concern. Additionally, whether some special intestinal microflora participate in the metabolism of Chinese herbal medicine, thus weakening or improving the anti-tumor activity of Chinese herbal medicine is also a problem that researchers need to pay attention to.
Conclusions
Gut microbes play an important role in early chemoprevention of colorectal cancer. Probiotics have been widely studied in vitro and animal experiments through direct anti-CRC effects, but the anticancer mechanisms of probiotics with different bacterial strains have significant characteristics(Table 1). It is worth mentioning that the gut microbiota is influenced by many factors in the clinic, such as gastrointestinal endoscopy, enema, application of antibiotics, and other examinations and treatment measures. Therefore, under the influence of these examinations and therapeutic measures, the effective activity of probiotics after passing through the gastrointestinal tract needs to be further confirmed in clinical studies. Mechanisms regarding the prevention of CRC by drugs are divided into two aspects, including gut microbe-dependent anticancer mechanisms and non-gut microbe-dependent anticancer mechanisms. On the one hand, healthy gut microbial homeostasis is able to prevent CRC, and these drugs prevent CRC by up-regulation of beneficial bacteria and down-regulation of harmful bacteria, and on the other hand, these drugs have activity against CRC. But certain intestinal microbes are involved in drug metabolism and absorption, thereby attenuating or enhancing the anti-CRC activity of drugs, thus, the triangular relationship between gut microbes, preventative drugs, and CRC requires further exploration.
Expectation
FMT has been widely demonstrated in animal experiments as a novel therapeutic modality to alleviate intestinal inflammation [112, 113]. Identifying strains with superior anticancer properties is perhaps the main direction of future research. Among patients with advanced CRC, surgical treatment, radiotherapy, and chemotherapy and immunotherapy are common treatment methods, but surgical complications, adverse drug reactions, and drug resistance can easily increase the mortality of CRC patients. Modulation of the gut microbiota by FMT or oral probiotics is effective in alleviating surgical complications and adverse drug reactions, furthermore, FMT was also effective in treating radiation enteritis, a common post-radiation complication [114, 115]. Therefore, identifying strains with superior anticancer properties to aid FMT applications may be a major direction for future research.
In colorectal cancer, mismatch repair-deficient (MMRd) tumors exhibit more anti-tumor immunity than mismatch repair-proficient (MMRp) tumors. MMRd colorectal cancer tumor tissues are infiltrated with a large number of cytotoxic T cells while responding to About 50% respond to immune checkpoint inhibitors, whereas MMRp hardly responds to immunotherapy [116]. However, whether gut microbiota composition differs between MMRd and MMRp colorectal cancer tissues, and whether the gut microbiota influences the phenomenon of differential antitumor immunity in MMRd and MMRp colorectal cancers is unknown, this could perhaps be a new area for future research.
Developing vaccines to target CRC-associated focused pathogenic bacteria is a new strategy to prevent colorectal cancer, Fusobacterium nucleatum acts as a " Star " bacterium to promote CRC development and progression, and studies have found that there are multiple antigenic virulence factors in extracellular vesicles secreted by gut tissue derived Fusobacterium nucleatum, such as Fada and fap2, etc., which may play important roles in the development and design of vaccines against Fusobacterium nucleatum [49]. It is worth mentioning that advanced CRC patients are more likely to translocate the gut microbiome into the blood due to the obstruction of the intestinal lumen by the tumor mass causing an elevated pressure in the intestinal lumen, suggesting that the blood microbiome might be a new biomarker to suggest the CRC progression situation. Similarly, 16 S RNA sequencing also revealed differences in gut microbiota composition at different stages of CRC [6], and tissue gut microbes exhibit differences between normal and cancer [117]. Gut microbes play an important role in CRC early prevention as well as in the treatment of middle and late-stage CRC, and targeting gut microbes could be a new strategy for treating colorectal cancer.
Acknowledgements
Not applicable.
Author contributions
All authors participated in the research and writing of the manuscript, and Hu Kefeng and Zhou Yuping made key revisions to the knowledge of the paper.
Funding
Supported by Zhejiang Provincial Natural Science Foundation of China, No.LTGD23C040008, No.LBY23H200006; Ningbo Natural Science Foundation, No.2022J229; Project of NINGBO Leading Medical & Health Discipline, No.2022-S04; Ningbo Top Medical and Health Research Program, No.2023020612.
Data availability
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yuping Zhou, Email: nbuzhouyuping@126.com.
Kefeng Hu, Email: fyhukefeng@nbu.edu.cn.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. [DOI] [PubMed] [Google Scholar]
- 2.Morgan E, Arnold M, Gini A, Lorenzoni V, Cabasag CJ, Laversanne M, Vignat J, Ferlay J, Murphy N, Bray F. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut 2022:gutjnl–2022. [DOI] [PubMed]
- 3.Ionescu VA, Gheorghe G, Bacalbasa N, Chiotoroiu AL, Diaconu C. Colorectal Cancer: from risk factors to Oncogenesis. Med (Kaunas) 2023, 59(9). [DOI] [PMC free article] [PubMed]
- 4.Saus E, Iraola-Guzmán S, Willis JR, Brunet-Vega A, Gabaldón T. Microbiome and colorectal cancer: roles in carcinogenesis and clinical potential. Mol Aspects Med. 2019;69:93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang Y, Misra BB, Liang L, Bi D, Weng W, Wu W, Cai S, Qin H, Goel A, Li X, et al. Integrated microbiome and metabolome analysis reveals a novel interplay between commensal bacteria and metabolites in colorectal cancer. Theranostics. 2019;9(14):4101–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yachida S, Mizutani S, Shiroma H, Shiba S, Nakajima T, Sakamoto T, Watanabe H, Masuda K, Nishimoto Y, Kubo M, et al. Metagenomic and metabolomic analyses reveal distinct stage-specific phenotypes of the gut microbiota in colorectal cancer. Nat Med. 2019;25(6):968–76. [DOI] [PubMed] [Google Scholar]
- 7.Eslami M, Yousefi B, Kokhaei P, Hemati M, Nejad ZR, Arabkari V, Namdar A. Importance of probiotics in the prevention and treatment of colorectal cancer. J Cell Physiol. 2019;234(10):17127–43. [DOI] [PubMed] [Google Scholar]
- 8.Li Q, Hu W, Liu WX, Zhao LY, Huang D, Liu XD, Chan H, Zhang Y, Zeng JD, Coker OO, et al. Streptococcus thermophilus inhibits colorectal tumorigenesis through secreting β-Galactosidase. Gastroenterology. 2021;160(4):1179–e11931114. [DOI] [PubMed] [Google Scholar]
- 9.Chang SC, Shen MH, Liu CY, Pu CM, Hu JM, Huang CJ. A gut butyrate-producing bacterium Butyricicoccus pullicaecorum regulates short-chain fatty acid transporter and receptor to reduce the progression of 1,2-dimethylhydrazine-associated colorectal cancer. Oncol Lett. 2020;20(6):327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen D, Jin D, Huang S, Wu J, Xu M, Liu T, Dong W, Liu X, Wang S, Zhong W, et al. Clostridium butyricum, a butyrate-producing probiotic, inhibits intestinal tumor development through modulating wnt signaling and gut microbiota. Cancer Lett. 2020;469:456–67. [DOI] [PubMed] [Google Scholar]
- 11.Liu M, Xie W, Wan X, Deng T. Clostridium butyricum modulates gut microbiota and reduces colitis associated colon cancer in mice. Int Immunopharmacol. 2020;88:106862. [DOI] [PubMed] [Google Scholar]
- 12.Meng X, Wang W, Lan T, Yang W, Yu D, Fang X, Wu H. A purified aspartic protease from Akkermansia Muciniphila plays an important role in degrading Muc2. Int J Mol Sci 2019, 21(1). [DOI] [PMC free article] [PubMed]
- 13.Meng X, Zhang J, Wu H, Yu D, Fang X. Akkermansia muciniphila Aspartic protease Amuc_1434* inhibits human colorectal Cancer LS174T cell viability via TRAIL-Mediated apoptosis pathway. Int J Mol Sci 2020, 21(9). [DOI] [PMC free article] [PubMed]
- 14.Wang L, Tang L, Feng Y, Zhao S, Han M, Zhang C, Yuan G, Zhu J, Cao S, Wu Q, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurised bacterium blunts colitis associated tumourigenesis by modulation of CD8(+) T cells in mice. Gut. 2020;69(11):1988–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sugimura N, Li Q, Chu ESH, Lau HCH, Fong W, Liu W, Liang C, Nakatsu G, Su ACY, Coker OO, et al. Lactobacillus gallinarum modulates the gut microbiota and produces anti-cancer metabolites to protect against colorectal tumourigenesis. Gut. 2021;71(10):2011–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Ma C, Zhao J, Xu H, Hou Q, Zhang H. Lactobacillus casei Zhang and vitamin K2 prevent intestinal tumorigenesis in mice via adiponectin-elevated different signaling pathways. Oncotarget. 2017;8(15):24719–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li SC, Lin HP, Chang JS, Shih CK. Lactobacillus acidophilus-fermented Germinated Brown Rice suppresses preneoplastic lesions of the Colon in rats. Nutrients 2019, 11(11). [DOI] [PMC free article] [PubMed]
- 18.Heilbronner S, Krismer B, Brötz-Oesterhelt H, Peschel A. The microbiome-shaping roles of bacteriocins. Nat Rev Microbiol. 2021;19(11):726–39. [DOI] [PubMed] [Google Scholar]
- 19.Baindara P, Korpole S, Grover V. Bacteriocins: perspective for the development of novel anticancer drugs. Appl Microbiol Biotechnol. 2018;102(24):10393–408. [DOI] [PubMed] [Google Scholar]
- 20.Thoda C, Touraki M. Probiotic-derived bioactive compounds in Colorectal Cancer Treatment. Microorganisms 2023, 11(8). [DOI] [PMC free article] [PubMed]
- 21.Ebrahimzadeh S, Ahangari H, Soleimanian A, Hosseini K, Ebrahimi V, Ghasemnejad T, Soofiyani SR, Tarhriz V, Eyvazi S. Colorectal cancer treatment using bacteria: focus on molecular mechanisms. BMC Microbiol. 2021;21(1):218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hosseini SS, Goudarzi H, Ghalavand Z, Hajikhani B, Rafeieiatani Z, Hakemi-Vala M. Anti-proliferative effects of cell wall, cytoplasmic extract of Lactococcus lactis and nisin through down-regulation of cyclin D1 on SW480 colorectal cancer cell line. Iran J Microbiol. 2020;12(5):424–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maher S, McClean S. Investigation of the cytotoxicity of eukaryotic and prokaryotic antimicrobial peptides in intestinal epithelial cells in vitro. Biochem Pharmacol. 2006;71(9):1289–98. [DOI] [PubMed] [Google Scholar]
- 24.Soleimanifar H, Mahmoodzadeh Hosseini H, Samavarchi Tehrani S, Mirhosseini SA. The Anti-adhesion Effect of Nisin as a Robust Lantibiotic on the Colorectal Cancer cells. Adv Biomed Res. 2023;12:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang H, Jin J, Pang X, Bian Z, Zhu J, Hao Y, Zhang H, Xie Y. Plantaricin BM-1 decreases viability of SW480 human colorectal cancer cells by inducing caspase-dependent apoptosis. Front Microbiol. 2022;13:1103600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang H, Zhou T, Jin W, Zong S, Mamtimin T, Salama ES, Jeon BH, Liu P, Han H, Li X. Tumor-targeting engineered probiotic Escherichia coli Nissle 1917 inhibits colorectal tumorigenesis and modulates gut microbiota homeostasis in mice. Life Sci. 2023;324:121709. [DOI] [PubMed] [Google Scholar]
- 27.de Moreno de Leblanc A, Perdigón G. Yogurt feeding inhibits promotion and progression of experimental colorectal cancer. Med Sci Monit. 2004;10(4):Br96–104. [PubMed] [Google Scholar]
- 28.Pala V, Sieri S, Berrino F, Vineis P, Sacerdote C, Palli D, Masala G, Panico S, Mattiello A, Tumino R, et al. Yogurt consumption and risk of colorectal cancer in the Italian European prospective investigation into cancer and nutrition cohort. Int J Cancer. 2011;129(11):2712–9. [DOI] [PubMed] [Google Scholar]
- 29.Martinez-Gonzalez MA, Sayon-Orea C, Ruiz-Canela M, de la Fuente C, Gea A, Bes-Rastrollo M. Yogurt consumption, weight change and risk of overweight/obesity: the SUN cohort study. Nutr Metab Cardiovasc Dis. 2014;24(11):1189–96. [DOI] [PubMed] [Google Scholar]
- 30.Michels KB, Willett WC, Vaidya R, Zhang X, Giovannucci E. Yogurt consumption and colorectal cancer incidence and mortality in the nurses’ Health Study and the Health professionals Follow-Up study. Am J Clin Nutr. 2020;112(6):1566–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishikawa H, Akedo I, Otani T, Suzuki T, Nakamura T, Takeyama I, Ishiguro S, Miyaoka E, Sobue T, Kakizoe T. Randomized trial of dietary fiber and Lactobacillus casei administration for prevention of colorectal tumors. Int J Cancer. 2005;116(5):762–7. [DOI] [PubMed] [Google Scholar]
- 32.Hatakka K, Holma R, El-Nezami H, Suomalainen T, Kuisma M, Saxelin M, Poussa T, Mykkänen H, Korpela R. The influence of Lactobacillus rhamnosus LC705 together with Propionibacterium freudenreichii ssp. Shermanii JS on potentially carcinogenic bacterial activity in human colon. Int J Food Microbiol. 2008;128(2):406–10. [DOI] [PubMed] [Google Scholar]
- 33.Arafa HM. Possible contribution of beta-glycosidases and caspases in the cytotoxicity of novel glycoconjugates in colon cancer cells. Invest New Drugs. 2010;28(3):306–17. [DOI] [PubMed] [Google Scholar]
- 34.Cheng WY, Wu CY, Yu J. The role of gut microbiota in cancer treatment: friend or foe? Gut. 2020;69(10):1867–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu T, Guo F, Yu Y, Sun T, Ma D, Han J, Qian Y, Kryczek I, Sun D, Nagarsheth N, et al. Fusobacterium nucleatum promotes Chemoresistance to Colorectal Cancer by modulating Autophagy. Cell. 2017;170(3):548–e563516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillère R, Hannani D, Enot DP, Pfirschke C, Engblom C, Pittet MJ, et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342(6161):971–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanoue T, Morita S, Plichta DR, Skelly AN, Suda W, Sugiura Y, Narushima S, Vlamakis H, Motoo I, Sugita K, et al. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature. 2019;565(7741):600–5. [DOI] [PubMed] [Google Scholar]
- 38.Zhang SL, Han B, Mao YQ, Zhang ZY, Li ZM, Kong CY, Wu Y, Chen GQ, Wang LS. Lacticaseibacillus Paracasei sh2020 induced antitumor immunity and synergized with anti-programmed cell death 1 to reduce tumor burden in mice. Gut Microbes. 2022;14(1):2046246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vétizou M, Pitt JM, Daillère R, Lepage P, Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong CP, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350(6264):1079–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fei Z, Lijuan Y, Xi Y, Wei W, Jing Z, Miao D, Shuwen H. Gut microbiome associated with chemotherapy-induced diarrhea from the CapeOX regimen as adjuvant chemotherapy in resected stage III colorectal cancer. Gut Pathog. 2019;11:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi W, Shen L, Zou W, Wang J, Yang J, Wang Y, Liu B, Xie L, Zhu J, Zhang Z. The gut microbiome is Associated with therapeutic responses and toxicities of Neoadjuvant Chemoradiotherapy in rectal Cancer Patients-A Pilot Study. Front Cell Infect Microbiol. 2020;10:562463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang D, Wang X, Zhou X, Zhao J, Yang H, Wang S, Morse MA, Wu J, Yuan Y, Li S, et al. Blood microbiota diversity determines response of advanced colorectal cancer to chemotherapy combined with adoptive T cell immunotherapy. Oncoimmunology. 2021;10(1):1976953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yi Y, Shen L, Shi W, Xia F, Zhang H, Wang Y, Zhang J, Wang Y, Sun X, Zhang Z, et al. Gut Microbiome Components Predict response to Neoadjuvant Chemoradiotherapy in patients with locally advanced rectal Cancer: a prospective, longitudinal study. Clin Cancer Res. 2021;27(5):1329–40. [DOI] [PubMed] [Google Scholar]
- 44.Lin Y, Fan L, Qi Y, Xu C, Jia D, Jiang Y, Chen S, Wang L. Bifidobacterium adolescentis induces decorin(+) macrophages via TLR2 to suppress colorectal carcinogenesis. J Exp Clin Cancer Res. 2023;42(1):172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC, Phillips GM, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513(7519):559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun S, Xu X, Liang L, Wang X, Bai X, Zhu L, He Q, Liang H, Xin X, Wang L, et al. Lactic acid-producing Probiotic Saccharomyces cerevisiae attenuates Ulcerative Colitis via suppressing macrophage pyroptosis and modulating gut microbiota. Front Immunol. 2021;12:777665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chang CW, Lee HC, Li LH, Chiang Chiau JS, Wang TE, Chuang WH, Chen MJ, Wang HY, Shih SC, Liu CY et al. Fecal microbiota transplantation prevents intestinal Injury, Upregulation of Toll-Like receptors, and 5-Fluorouracil/Oxaliplatin-Induced toxicity in Colorectal Cancer. Int J Mol Sci 2020, 21(2). [DOI] [PMC free article] [PubMed]
- 48.Secombe KR, Crame EE, Tam JSY, Wardill HR, Gibson RJ, Coller JK, Bowen JM. Intestinal toll-like receptor 4 knockout alters the functional capacity of the gut microbiome following irinotecan treatment. Cancer Chemother Pharmacol. 2022;89(2):275–81. [DOI] [PubMed] [Google Scholar]
- 49.Mego M, Chovanec J, Vochyanova-Andrezalova I, Konkolovsky P, Mikulova M, Reckova M, Miskovska V, Bystricky B, Beniak J, Medvecova L, et al. Prevention of irinotecan induced diarrhea by probiotics: a randomized double blind, placebo controlled pilot study. Complement Ther Med. 2015;23(3):356–62. [DOI] [PubMed] [Google Scholar]
- 50.Pallan A, Dedelaite M, Mirajkar N, Newman PA, Plowright J, Ashraf S. Postoperative complications of colorectal cancer. Clin Radiol. 2021;76(12):896–907. [DOI] [PubMed] [Google Scholar]
- 51.Paszat LF, Sutradhar R, Corn E, Luo J, Baxter NN, Tinmouth J, Rabeneck L. Morbidity and mortality following major large bowel resection for colorectal cancer detected by a population-based screening program. J Med Screen. 2021;28(3):252–60. [DOI] [PubMed] [Google Scholar]
- 52.Calmels M, Collard MK, O’Connell L, Voron T, Debove C, Chafai N, Parc Y, Lefevre JH. Redo-surgery after failed colorectal or coloanal anastomosis: morbidity, mortality and factors predictive of success. A retrospective study of 200 patients. Colorectal Dis. 2022;24(4):511–9. [DOI] [PubMed] [Google Scholar]
- 53.Jin Y, Geng R, Liu Y, Liu L, Jin X, Zhao F, Feng J, Wei Y. Prediction of postoperative ileus in patients with Colorectal Cancer by Preoperative Gut Microbiota. Front Oncol. 2020;10:526009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Palmisano S, Campisciano G, Iacuzzo C, Bonadio L, Zucca A, Cosola D, Comar M, de Manzini N. Role of preoperative gut microbiota on colorectal anastomotic leakage: preliminary results. Updates Surg. 2020;72(4):1013–22. [DOI] [PubMed] [Google Scholar]
- 55.Liu Y, He W, Yang J, He Y, Wang Z, Li K. The effects of preoperative intestinal dysbacteriosis on postoperative recovery in colorectal cancer surgery: a prospective cohort study. BMC Gastroenterol. 2021;21(1):446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zeng J, Ji Y, Liang B, Zhang G, Chen D, Zhu M, Wu S, Kuang W. The effect of pro/synbiotics on postoperative infections in colorectal cancer patients: a systematic review and meta-analysis. Complement Ther Clin Pract. 2021;43:101370. [DOI] [PubMed] [Google Scholar]
- 57.Chen Y, Qi A, Teng D, Li S, Yan Y, Hu S, Du X. Probiotics and synbiotics for preventing postoperative infectious complications in colorectal cancer patients: a systematic review and meta-analysis. Tech Coloproctol. 2022;26(6):425–36. [DOI] [PubMed] [Google Scholar]
- 58.Zaharuddin L, Mokhtar NM, Muhammad Nawawi KN, Raja Ali RA. A randomized double-blind placebo-controlled trial of probiotics in post-surgical colorectal cancer. BMC Gastroenterol. 2019;19(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bloemen JG, Schreinemacher MH, de Bruine AP, Buurman WA, Bouvy ND, Dejong CH. Butyrate enemas improve intestinal anastomotic strength in a rat model. Dis Colon Rectum. 2010;53(7):1069–75. [DOI] [PubMed] [Google Scholar]
- 60.Bosmans JW, Jongen AC, Boonen BT, van Rijn S, Scognamiglio F, Stucchi L, Gijbels MJ, Marsich E, Bouvy ND. Comparison of three different application routes of butyrate to improve colonic anastomotic strength in rats. Int J Colorectal Dis. 2017;32(3):305–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bittel M, Reichert P, Sarfati I, Dressel A, Leikam S, Uderhardt S, Stolzer I, Phu TA, Ng M, Vu NK, et al. Visualizing transfer of microbial biomolecules by outer membrane vesicles in microbe-host-communication in vivo. J Extracell Vesicles. 2021;10(12):e12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Geier MS, Butler RN, Howarth GS. Probiotics, prebiotics and synbiotics: a role in chemoprevention for colorectal cancer? Cancer Biol Ther. 2006;5(10):1265–9. [DOI] [PubMed] [Google Scholar]
- 63.Bai D, Sun T, Zhao J, Du J, Bu X, Cao W, Zhao Y, Lu N. Oroxylin A maintains the colonic mucus barrier to reduce disease susceptibility by reconstituting a dietary fiber-deprived gut microbiota. Cancer Lett. 2021;515:73–85. [DOI] [PubMed] [Google Scholar]
- 64.Chang ZY, Liu HM, Leu YL, Hsu CH, Lee TY. Modulation of gut microbiota combined with upregulation of intestinal tight Junction explains anti-inflammatory effect of Corylin on Colitis-Associated Cancer in mice. Int J Mol Sci 2022, 23(5). [DOI] [PMC free article] [PubMed]
- 65.Weng W, Goel A. Curcumin and colorectal cancer: an update and current perspective on this natural medicine. Semin Cancer Biol. 2022;80:73–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhu HC, Jia XK, Fan Y, Xu SH, Li XY, Huang MQ, Lan ML, Xu W, Wu SS. Alisol B 23-Acetate ameliorates Azoxymethane/Dextran Sodium Sulfate-Induced Male Murine Colitis-Associated Colorectal Cancer via modulating the composition of gut microbiota and improving intestinal barrier. Front Cell Infect Microbiol. 2021;11:640225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang G, Khan I, Li X, Chen L, Leong W, Ho LT, Hsiao WLW. Ginsenosides Rb3 and rd reduce polyps formation while reinstate the dysbiotic gut microbiota and the intestinal microenvironment in apc(Min/+) mice. Sci Rep. 2017;7(1):12552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rawi MH, Zaman SA, Pa’ee KF, Leong SS, Sarbini SR. Prebiotics metabolism by gut-isolated probiotics. J Food Sci Technol. 2020;57(8):2786–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scott KP, Grimaldi R, Cunningham M, Sarbini SR, Wijeyesekera A, Tang MLK, Lee JC, Yau YF, Ansell J, Theis S et al. Developments in understanding and applying prebiotics in research and practice-an ISAPP conference paper. J Appl Microbiol 2020, 128(4):934–949. [DOI] [PubMed]
- 70.Duan C, Tang X, Wang W, Qian W, Fu X, Deng X, Han C, Hou X. L-fucose ameliorates the carcinogenic properties of Fusobacterium nucleatum in colorectal cancer. Oncol Lett. 2021;21(2):143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhou Z, Wang Y, Ji R, Zhang D, Ma C, Ma W, Ma Y, Jiang X, Du K, Zhang R, et al. Vanillin derivatives reverse Fusobacterium nucleatum-Induced Proliferation and Migration of Colorectal Cancer through E-Cadherin/β-Catenin pathway. Front Pharmacol. 2022;13:841918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sakamoto Y, Mima K, Ishimoto T, Ogata Y, Imai K, Miyamoto Y, Akiyama T, Daitoku N, Hiyoshi Y, Iwatsuki M, et al. Relationship between Fusobacterium nucleatum and antitumor immunity in colorectal cancer liver metastasis. Cancer Sci. 2021;112(11):4470–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yin H, Miao Z, Wang L, Su B, Liu C, Jin Y, Wu B, Han H, Yuan X. Fusobacterium nucleatum promotes liver metastasis in colorectal cancer by regulating the hepatic immune niche and altering gut microbiota. Aging. 2022;14(4):1941–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang Y, Zhang L, Zheng S, Li M, Xu C, Jia D, Qi Y, Hou T, Wang L, Wang B, et al. Fusobacterium nucleatum promotes colorectal cancer cells adhesion to endothelial cells and facilitates extravasation and metastasis by inducing ALPK1/NF-κB/ICAM1 axis. Gut Microbes. 2022;14(1):2038852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang P, Chen H, Zhu Y, McBride J, Fu J, Sang S. Oat avenanthramide-C (2c) is biotransformed by mice and the human microbiota into bioactive metabolites. J Nutr. 2015;145(2):239–45. [DOI] [PubMed] [Google Scholar]
- 76.Luo Y, Wang CZ, Sawadogo R, Yuan J, Zeng J, Xu M, Tan T, Yuan CS. 4-Vinylguaiacol, an active metabolite of Ferulic Acid by Enteric Microbiota and Probiotics, possesses significant activities against drug-resistant human colorectal Cancer cells. ACS Omega. 2021;6(7):4551–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hou H, Chen D, Zhang K, Zhang W, Liu T, Wang S, Dai X, Wang B, Zhong W, Cao H. Gut microbiota-derived short-chain fatty acids and colorectal cancer: ready for clinical translation? Cancer Lett. 2022;526:225–35. [DOI] [PubMed] [Google Scholar]
- 78.Van Loo J. The specificity of the interaction with intestinal bacterial fermentation by prebiotics determines their physiological efficacy. Nutr Res Rev. 2004;17(1):89–98. [DOI] [PubMed] [Google Scholar]
- 79.Zeng H, Umar S, Rust B, Lazarova D, Bordonaro M. Secondary bile acids and short chain fatty acids in the Colon: a focus on colonic microbiome, cell proliferation, inflammation, and Cancer. Int J Mol Sci 2019, 20(5). [DOI] [PMC free article] [PubMed]
- 80.Jang EY, Hong KB, Chang YB, Shin J, Jung EY, Jo K, Suh HJ. In Vitro Prebiotic effects of Malto-Oligosaccharides Containing Water-Soluble Dietary Fiber. Molecules 2020, 25(21). [DOI] [PMC free article] [PubMed]
- 81.Brusselaers N, Lagergren J. Maintenance use of non-steroidal anti-inflammatory drugs and risk of gastrointestinal cancer in a nationwide population-based cohort study in Sweden. BMJ Open. 2018;8(7):e021869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Burn J, Sheth H, Elliott F, Reed L, Macrae F, Mecklin JP, Möslein G, McRonald FE, Bertario L, Evans DG, et al. Cancer prevention with aspirin in hereditary colorectal cancer (Lynch syndrome), 10-year follow-up and registry-based 20-year data in the CAPP2 study: a double-blind, randomised, placebo-controlled trial. Lancet. 2020;395(10240):1855–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mädge JC, Stallmach A, Kleebusch L, Schlattmann P. Meta-analysis of aspirin-guided therapy of colorectal cancer. J Cancer Res Clin Oncol. 2022;148(6):1407–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bibbins-Domingo K. Aspirin use for the primary Prevention of Cardiovascular Disease and Colorectal Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2016;164(12):836–45. [DOI] [PubMed] [Google Scholar]
- 85.Zhao R, Coker OO, Wu J, Zhou Y, Zhao L, Nakatsu G, Bian X, Wei H, Chan AWH, Sung JJY, et al. Aspirin reduces colorectal Tumor Development in mice and gut microbes reduce its Bioavailability and Chemopreventive effects. Gastroenterology. 2020;159(3):969–e983964. [DOI] [PubMed] [Google Scholar]
- 86.Chi T, Zhao Q, Wang P. Fecal 16S rRNA Gene Sequencing Analysis of Changes in the Gut Microbiota of Rats with Low-Dose Aspirin-Related Intestinal Injury. Biomed Res Int 2021, 2021:8848686. [DOI] [PMC free article] [PubMed]
- 87.Prizment AE, Staley C, Onyeaghala GC, Vivek S, Thyagarajan B, Straka RJ, Demmer RT, Knights D, Meyer KA, Shaukat A, et al. Randomised clinical study: oral aspirin 325 mg daily vs placebo alters gut microbial composition and bacterial taxa associated with colorectal cancer risk. Aliment Pharmacol Ther. 2020;52(6):976–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brennan CA, Nakatsu G, Gallini Comeau CA, Drew DA, Glickman JN, Schoen RE, Chan AT, Garrett WS. Aspirin modulation of the Colorectal Cancer-Associated Microbe Fusobacterium nucleatum. mBio 2021, 12(2). [DOI] [PMC free article] [PubMed]
- 89.Ying J, Zhou HY, Liu P, You Q, Kuang F, Shen YN, Hu ZQ. Aspirin inhibited the metastasis of colon cancer cells by inhibiting the expression of toll-like receptor 4. Cell Biosci. 2018;8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Higurashi T, Hosono K, Takahashi H, Komiya Y, Umezawa S, Sakai E, Uchiyama T, Taniguchi L, Hata Y, Uchiyama S, et al. Metformin for chemoprevention of metachronous colorectal adenoma or polyps in post-polypectomy patients without diabetes: a multicentre double-blind, placebo-controlled, randomised phase 3 trial. Lancet Oncol. 2016;17(4):475–83. [DOI] [PubMed] [Google Scholar]
- 91.Chan AT. Metformin for cancer prevention: a reason for optimism. Lancet Oncol. 2016;17(4):407–9. [DOI] [PubMed] [Google Scholar]
- 92.Zheng Z, Bian Y, Zhang Y, Ren G, Li G. Metformin activates AMPK/SIRT1/NF-κB pathway and induces mitochondrial dysfunction to drive caspase3/GSDME-mediated cancer cell pyroptosis. Cell Cycle. 2020;19(10):1089–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jaromy M, Miller JD. Pharmacologic mechanisms underlying antidiabetic drug metformin’s chemopreventive effect against colorectal cancer. Eur J Pharmacol. 2021;897:173956. [DOI] [PubMed] [Google Scholar]
- 94.Zhang T, Hu L, Tang JF, Xu H, Tian K, Wu MN, Huang SY, Du YM, Zhou P, Lu RJ et al. Metformin inhibits the Urea cycle and reduces Putrescine Generation in Colorectal Cancer Cell lines. Molecules 2021, 26(7). [DOI] [PMC free article] [PubMed]
- 95.Sun L, Xie C, Wang G, Wu Y, Wu Q, Wang X, Liu J, Deng Y, Xia J, Chen B, et al. Gut microbiota and intestinal FXR mediate the clinical benefits of metformin. Nat Med. 2018;24(12):1919–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pryor R, Norvaisas P, Marinos G, Best L, Thingholm LB, Quintaneiro LM, De Haes W, Esser D, Waschina S, Lujan C, et al. Host-microbe-drug-nutrient screen identifies bacterial effectors of Metformin Therapy. Cell. 2019;178(6):1299–e13121229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wu H, Esteve E, Tremaroli V, Khan MT, Caesar R, Mannerås-Holm L, Ståhlman M, Olsson LM, Serino M, Planas-Fèlix M, et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med. 2017;23(7):850–8. [DOI] [PubMed] [Google Scholar]
- 98.Bryrup T, Thomsen CW, Kern T, Allin KH, Brandslund I, Jørgensen NR, Vestergaard H, Hansen T, Hansen TH, Pedersen O, et al. Metformin-induced changes of the gut microbiota in healthy young men: results of a non-blinded, one-armed intervention study. Diabetologia. 2019;62(6):1024–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huang X, Hong X, Wang J, Sun T, Yu T, Yu Y, Fang J, Xiong H. Metformin elicits antitumour effect by modulation of the gut microbiota and rescues Fusobacterium nucleatum-induced colorectal tumourigenesis. EBioMedicine. 2020;61:103037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Forslund K, Hildebrand F, Nielsen T, Falony G, Le Chatelier E, Sunagawa S, Prifti E, Vieira-Silva S, Gudmundsdottir V, Pedersen HK, et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528(7581):262–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Broadfield LA, Saigal A, Szamosi JC, Hammill JA, Bezverbnaya K, Wang D, Gautam J, Tsakiridis EE, Di Pastena F, McNicol J, et al. Metformin-induced reductions in tumor growth involves modulation of the gut microbiome. Mol Metab. 2022;61:101498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bauer PV, Duca FA, Waise TMZ, Rasmussen BA, Abraham MA, Dranse HJ, Puri A, O’Brien CA, Lam TKT. Metformin alters Upper Small Intestinal Microbiota that Impact a Glucose-SGLT1-Sensing glucoregulatory pathway. Cell Metab. 2018;27(1):101–e117105. [DOI] [PubMed] [Google Scholar]
- 103.Chen YX, Gao QY, Zou TH, Wang BM, Liu SD, Sheng JQ, Ren JL, Zou XP, Liu ZJ, Song YY, et al. Berberine versus placebo for the prevention of recurrence of colorectal adenoma: a multicentre, double-blinded, randomised controlled study. Lancet Gastroenterol Hepatol. 2020;5(3):267–75. [DOI] [PubMed] [Google Scholar]
- 104.Wang Y, Zhang X, Li J, Zhang Y, Guo Y, Chang Q, Chen L, Wang Y, Wang S, Song Y, et al. Sini Decoction ameliorates Colorectal Cancer and modulates the composition of gut microbiota in mice. Front Pharmacol. 2021;12:609992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li Y, Li ZX, Xie CY, Fan J, Lv J, Xu XJ, Lv J, Kuai WT, Jia YT. Gegen Qinlian decoction enhances immunity and protects intestinal barrier function in colorectal cancer patients via gut microbiota. World J Gastroenterol. 2020;26(48):7633–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yan S, Chang J, Hao X, Liu J, Tan X, Geng Z, Wang Z. Berberine regulates short-chain fatty acid metabolism and alleviates the colitis-associated colorectal tumorigenesis through remodeling intestinal flora. Phytomedicine. 2022;102:154217. [DOI] [PubMed] [Google Scholar]
- 107.Chen H, Ye C, Cai B, Zhang F, Wang X, Zhang J, Zhang Z, Guo Y, Yao Q. Berberine inhibits intestinal carcinogenesis by suppressing intestinal pro-inflammatory genes and oncogenic factors through modulating gut microbiota. BMC Cancer. 2022;22(1):566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhu LQ, Zhang L, Zhang J, Chang GL, Liu G, Yu DD, Yu XM, Zhao MS, Ye B. Evodiamine inhibits high-fat diet-induced colitis-associated cancer in mice through regulating the gut microbiota. J Integr Med. 2021;19(1):56–65. [DOI] [PubMed] [Google Scholar]
- 109.Zhou Y, Feng Y, Cen R, Hou X, Yu H, Sun J, Zhou L, Ji Q, Zhao L, Wang Y, et al. San-Wu-Huang-Qin decoction attenuates tumorigenesis and mucosal barrier impairment in the AOM/DSS model by targeting gut microbiome. Phytomedicine. 2022;98:153966. [DOI] [PubMed] [Google Scholar]
- 110.Lin LT, Shi YC, Choong CY, Tai CJ. The fruits of Paris polyphylla inhibit Colorectal Cancer Cell Migration Induced by Fusobacterium nucleatum-Derived Extracellular vesicles. Molecules 2021, 26(13). [DOI] [PMC free article] [PubMed]
- 111.Yu YN, Yu TC, Zhao HJ, Sun TT, Chen HM, Chen HY, An HF, Weng YR, Yu J, Li M, et al. Berberine may rescue Fusobacterium nucleatum-induced colorectal tumorigenesis by modulating the tumor microenvironment. Oncotarget. 2015;6(31):32013–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wen X, Wang HG, Zhang MN, Zhang MH, Wang H, Yang XZ. Fecal microbiota transplantation ameliorates experimental colitis via gut microbiota and T-cell modulation. World J Gastroenterol. 2021;27(21):2834–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang W, Zou G, Li B, Du X, Sun Z, Sun Y, Jiang X. Fecal microbiota transplantation (FMT) alleviates experimental colitis in mice by Gut Microbiota Regulation. J Microbiol Biotechnol. 2020;30(8):1132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ding X, Li Q, Li P, Chen X, Xiang L, Bi L, Zhu J, Huang X, Cui B, Zhang F. Fecal microbiota transplantation: a promising treatment for radiation enteritis? Radiother Oncol. 2020;143:12–8. [DOI] [PubMed] [Google Scholar]
- 115.Liu T, Su D, Lei C, Liu Z. Treatment of Radiation Enteritis with Fecal Transplantation. Am Surg 2022:31348221091954. [DOI] [PubMed]
- 116.Pelka K, Hofree M, Chen JH, Sarkizova S, Pirl JD, Jorgji V, Bejnood A, Dionne D, Ge WH, Xu KH, et al. Spatially organized multicellular immune hubs in human colorectal cancer. Cell. 2021;184(18):4734–e47524720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu W, Zhang X, Xu H, Li S, Lau HC, Chen Q, Zhang B, Zhao L, Chen H, Sung JJ, et al. Microbial Community Heterogeneity within Colorectal Neoplasia and its correlation with colorectal carcinogenesis. Gastroenterology. 2021;160(7):2395–408. [DOI] [PubMed] [Google Scholar]
- 118.Peng M, Lee SH, Rahaman SO, Biswas D. Dietary probiotic and metabolites improve intestinal homeostasis and prevent colorectal cancer. Food Funct. 2020;11(12):10724–35. [DOI] [PubMed] [Google Scholar]
- 119.Xu H, Hiraishi K, Kurahara LH, Nakano-Narusawa Y, Li X, Hu Y, Matsuda Y, Zhang H, Hirano K. Inhibitory effects of breast milk-derived Lactobacillus rhamnosus Probio-M9 on Colitis-Associated Carcinogenesis by Restoration of the gut microbiota in a mouse model. Nutrients 2021, 13(4). [DOI] [PMC free article] [PubMed]
- 120.Wang T, Zhang L, Wang P, Liu Y, Wang G, Shan Y, Yi Y, Zhou Y, Liu B, Wang X, Chen S, Fan L, Lin Y, Qi Y, Xu C, Ge Q, Zhang Y, Wang Q, Jia D, Wang L et al. Lactobacillus coryniformis MXJ32 administration ameliorates azoxymethane/dextran sulfate sodium-induced colitis-associated colorectal cancer via reshaping intestinal microenvironment and alleviating inflammatory response. Eur J Nutr: Bifidobacterium adolescentis orchestrates CD143(+) cancer-associated fibroblasts to suppress colorectal tumorigenesis by Wnt signaling-regulated GAS1. Cancer Commun (Lond) 2023, 43(9):1027–1047. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.