Abstract
OBJECTIVE
To determine whether neoadjuvant gemcitabine and cisplatin (GC) vs dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin (ddMVAC) before radical cystectomy improves overall survival (OS), progression-free survival (PFS), and pathologic complete response (pCR) for patients with muscle-invasive bladder cancer with secondary analyses of pathological downstaging and toxicity.
MATERIALS AND METHODS
This systematic review and meta-analysis identified studies of patients with muscle-invasive bladder cancer treated with neoadjuvant GC compared to ddMVAC from PubMed, Web of Science, and EMBASE. Random-effect models for pooled log-transformed hazard ratios (HR) for OS and PFS and pooled odds ratios for pCR and downstaging were developed using the generic inverse variance method and Mantel-Haenszel method, respectively.
RESULTS
Ten studies were identified (4 OS, 2 PFS, and 6 pCR clinical endpoints). Neoadjuvant ddMVAC improved OS (HR 0.71 [95% confidence intervals 0.56; 0.90]), PFS (HR 0.76 [95% confidence intervals 0.60; 0.97]), and pathological downstaging (odds ratio 1.34 [95% confidence interval 1.01; 1.78]) as compared to GC. There was no significant difference between regimens for pCR rates (odds ratio 1.38 [95% confidence interval 0.90; 2.12]). Treatment toxicity was greater with ddMVAC. Limitations result from differences in number of ddMVAC cycles and patient selection between studies.
CONCLUSION
Neoadjuvant ddMVAC is associated with improved OS and PFS vs gemcitabine/cisplatin for patients with muscle-invasive bladder cancer before radical cystectomy. Although rates of pathological complete response were not significantly different, pathological downstaging correlated with OS. ddMVAC should be preferred over gemcitabine/cisplatin for patients with muscle-invasive bladder cancer who can tolerate its greater toxicity.
In nonmetastatic muscle-invasive bladder cancer (MIBC), radical cystectomy is the standard of care for eligible patients.1 The incorporation of neoadjuvant methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) before radical cystectomy has been shown to improve survival in patients with muscle-invasive disease.1 Gemcitabine and cisplatin (GC) have been compared to MVAC in locally advanced and metastatic bladder cancer and found to be noninferior with a favorable safety profile.2 However, in a subsequent phase III study, dose-dense MVAC (ddMVAC) plus granulocyte colony-stimulating factor compared to conventional MVAC yielded a higher complete response (21% vs 9%, P = .009) and overall response rate (64% vs 50%, P = .06) in advanced urothelial carcinoma.3 As a result of these and other studies, conventional MVAC has been largely replaced by ddMVAC. ddMVAC along with GC are the standards of care before radical cystectomy for cisplatin-eligible patients as defined by the Galsky criteria.4–6 The use of chemotherapy for MIBC in the neoadjuvant setting reflects the absence of data from adequately powered randomized trials showing survival benefits with adjuvant chemotherapy.7 A salient clinical question for medical oncologists treating MIBC is whether to proceed with neoadjuvant ddMVAC vs GC for a given patient.
International guidelines are consistent in their recommendation for neoadjuvant cisplatin-based chemotherapy before radical cystectomy. While the European Society of Medical Oncology’s Clinical Practice Guidelines make no distinction between ddMVAC and GC, the National Comprehensive Cancer Network’s Guidelines for bladder cancer indicate that ddMVAC is the preferred regimen.4,5 Until the recent publications of the VESPER and SWOG 1314 trials, no prospective, phase III randomized controlled trial has compared the superiority of neoadjuvant ddMVAC vs GC.8,9 Notably, neither of these trials met their endpoints with respect to overall survival (OS) during initial publication. The recent update on the 5-year OS data from the VESPER trial found ddMVAC to be superior to GC (hazard ratios (HR) = 0.71 [95% confidence intervals (CI) 0.52; 0.97], P = .032).10 Guidelines and clinical decision-making have primarily relied on retrospective data or surrogate endpoints, such as pathologic complete response (pCR) after NAC, which has been shown to correlate with improved OS.11 In this study, we performed a systematic review and meta-analysis to compare the effectiveness and toxicity of neoadjuvant ddMVAC to GC before radical cystectomy for MIBC.
EVIDENCE ACQUISITION
Inclusion and Exclusion Criteria
Studies of patients aged 18 years or older with MIBC treated with neoadjuvant ddMVAC compared to neoadjuvant GC before radical cystectomy were included. The studies must include results of at least one of the following endpoints: OS, progression-free survival (PFS), or pCR. Studies with a combined cohort of patients with upper tract urothelial carcinoma and MIBC without separate outcomes data for patients with MIBC were excluded. Comparison of conventional MVAC vs GC without a ddMVAC arm and data published in a language other than English were also criteria for exclusion.
Search Strategy and Data Extraction
We queried the databases PubMed, Web of Science, and EMBASE to identify eligible studies (last accessed November 10, 2022). The terms “dose dense,” “accelerated,” or “high dose” were used interchangeably for ddMVAC. The name of each chemotherapy in MVAC and GC, “bladder cancer,” and “neoadjuvant” were used in any combination for the search. Two authors (C.J.E. and E.B.C.) independently screened each result for eligibility. Selected studies were reviewed in full, and the clinical endpoints of interest were extracted. Any discrepancies in identified studies or extracted data were resolved by third-party reviewers (S.P.K. and T.W.F.). Studies were categorized as retrospective or prospective to illustrate differences in levels of evidence.12 This study adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement and has been registered with the International Prospective Register of Systematic Reviews (PROSPERO) ID CRD42024521124.13
Statistical Analysis
The primary endpoint of this study was OS, and secondary endpoints were PFS and pCR. Sub-analyses were also conducted for pathological downstaging and treatment toxicity data when available. Meta-analyses using random-effect models were performed for each endpoint of interest with GC serving as the treatment comparisons control arm. Analyses of OS and PFS were calculated from the reported log-transformed HR and 95% CI. The estimated effect size of both is reported in the pooled HR and 95% CI using the generic inverse variance method. Endpoints for pCR and pathological downstaging utilized the data from their respective studies and are presented as pooled odds ratios (OR) and 95% CI using the Mantel-Haenszel method. Subgroup analyses were completed for studies with prospective and retrospective data only. Comparison of treatment toxicities between the 2 regimens of interest is explored with the exact binomial test. Sensitivity analyses included the leave-one-out method to assess changes in overall results and funnel plots to test the probability of publication bias. Analyses were performed using the meta package in R (v4.2.1, R Core Team 2021) and evaluated at a statistical significance level P < .05.14
EVIDENCE SYNTHESIS
Study Selection and Characteristics
A total of 444 articles were identified from the electronic search strategy. After duplicates were removed, 314 abstracts were reviewed for study inclusion and exclusion criteria. The most common reasons for exclusion were combined data for conventional and ddMVAC (n = 11) and combined data for all neoadjuvant chemotherapy (NAC) regimens (Supplemental Fig. 1). Ten studies were identified in the systematic review and included in the meta-analysis (Table 1).8,9,15–22 The VESPER and SWOG 1314 trials were the only prospective studies identified.8,9,15,17 In the VESPER trial, the data for patients who received NAC only, which constituted 88.6% of the overall cohort, were used for the analyses.8 The recently reported 5-year OS data for the VESPER trial in lieu of the 3-year OS data published in the initial manuscript.23 Out of the 10 total studies, 4 included OS, 2 had PFS, and 6 had pCR as clinical endpoints.
Table 1.
Characteristics of the studies included in the meta-analysis.
| Study | Country | Journal | Study Population | Study Period | Median Follow-up (months) | Study Type | Relevant Clinical Endpoints |
|---|---|---|---|---|---|---|---|
| Flaig TW 2021 | USA | Clinical Cancer Review | 167 patients with nonmetastatic (T2-4aN0M0) MIBC randomized to either 4 cycles of ddMVAC (112) or 4 cycles of GC (115) followed by RC | 2014–2017 | NR | Prospective multi-institution phase III clinical trial | pCR |
| Flaig TW 2023 | USA | European Urology | 167 patients with nonmetastatic (T2-4aN0M0) MIBC randomized to either 4 cycles of ddMVAC (112) or 4 cycles of GC (115) followed by RC | 2014–2017 | 53.0 (IQR 43.0–62.0) | Prospective multi-institution phase III clinical trial | OS, PFS |
| Leminski A 2021 | Poland | PLoS One | 79 patients with MIBC treated with ddMVAC (49), GC (24), or gemcitabine-carboplatin (6) as neoadjuvant chemotherapy who underwent RC | 2014–2020 | 19.3 (IQR 9.1–54.2) | Retrospective single-institution cohort | OS |
| Peyton CC 2018 | USA | Journal of Urology | 332 patients with MIBC treated with ddMVAC (46), GC (204), gemcitabine-carboplatin (32), or other (50) as neoadjuvant chemotherapy who underwent RC | 2007–2017 | 13.8 (95% CI 12.3–16.1) | Retrospective single-institution cohort | OS, pCR |
| Pfister C 2021 | France | European Urology | 437 patients with nonmetastatic (T2-4aN0M0) MIBC randomized to either 6 cycles of ddMVAC (199) or 4 cycles of GC (198) followed by RC | 2013–2018 | NR | Prospective multi-institution phase III clinical trial | pCR |
| Pfister C 2022, 2023* | France | Journal of Clinical Oncology | 437 patients with nonmetastatic (T2-4aN0M0) MIBC randomized to either 6 cycles of ddMVAC (199) or 4 cycles of GC (198) followed by RC | 2013–2020 | 63.0 | Prospective multi-institution phase III clinical trial | OS, PFS |
| Ravi P 2021 | International | British Journal of Urology International | 625 patients with cT2-4aN0-1M0 MIBC who received neoadjuvant ddMVAC (151), conventional MVAC (47), or GC (347) and achieved < ypT2N0 at RC | 1996–2019 | 31.2 (IQR 13.2–55.2) | Retrospective multi-institution cohort | pCR |
| Ruplin AT 2022 | USA | Clinical Genitourinary Cancer | 109 patients with MIBC treated with ddMVAC (33) or GC (76) as neoadjuvant chemotherapy followed by RC | 2008–2019 | NR | Retrospective single-institution cohort | pCR |
| Van de Putte EE 2016 | Netherlands | World Journal of Urology | 166 patients with MIBC who received neoadjuvant ddMVAC (80), conventional MVAC (35), or GC (51) | 1990–2014 | NR | Retrospective single-institution cohort | pCR |
| Zargar H 2018 | International | Journal of Urology | 319 patients with locally advanced (cT3-T4aN0M0) MIBC treated with ddMVAC (100) or GC (219) followed by RC | 2000–2015 | 22.8 (IQR 7.2–45.6) | Retrospective multi-institution cohort | pCR |
ddMVAC, dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin; GC, gemcitabine and cisplatin; IQR, interquartile range; MIBC, muscle-invasive bladder cancer; MVAC, methotrexate, vinblastine, doxorubicin, and cisplatin; NR, not reported; OS, overall survival; pCR, progression-free survival; PFS, pathologic complete response; RC, radical cystectomy.
Five-year OS data presented at the American Society of Clinical Oncology Annual Conference 2023.
Overall Survival
Using the 4 studies that included OS as a clinical endpoint, the analysis favors neoadjuvant ddMVAC for improved OS as compared to neoadjuvant GC with an HR of 0.71 (95% CI 0.56; 0.90) (Fig. 1).8,9,16,18 This was statistically significant across and within the prospective and retrospective study subgroups. The intercept of the Eggers test for OS does not statistically differ from zero, indicating no evidence of a possible publication bias (−1.14 [95% CI −5.46; 3.17], P = .37).
Figure 1.

Forest plot comparing OS for patients with MIBC who received neoadjuvant ddMVAC vs neoadjuvant GC. CI, confidence intervals; ddMVAC, dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin; GC, gemcitabine and cisplatin; HR, hazard ratio; MIBC, muscle-invasive bladder cancer; OS, overall survival.
Progression-Free Survival
PFS also favors ddMVAC vs GC for NAC in the 2 included studies with an HR of 0.76 (95% CI 0.60; 0.97) (Fig. 2).8,9 The Eggers test for PFS showed no evidence of a possible publication bias (−2.17 [95% CI −8.21; 12.56], P = .23).
Figure 2.
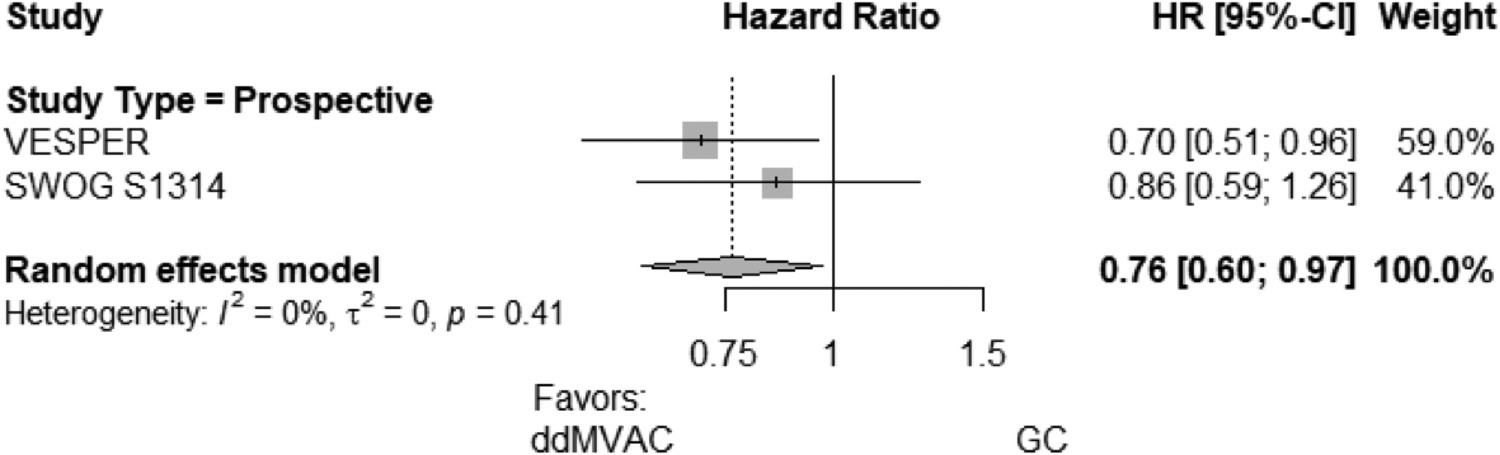
Forest plot comparing PFS for patients with MIBC who received neoadjuvant ddMVAC vs neoadjuvant GC. CI, confidence intervals; ddMVAC, dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin; GC, gemcitabine and cisplatin; HR, hazard ratio; MIBC, muscle-invasive bladder cancer; PFS, pathologic complete response.
Pathologic Complete Response
With respect to rates of pCR, the analysis did not show any statistically significant difference between regimens (OR 1.38 [95% CI 0.90; 2.12]) (Fig. 3).15,17,18,20–22 A signal toward improved pCR with neoadjuvant ddMVAC was observed in both the prospective and retrospective cohorts, although neither was statistically significant.
Figure 3.

Forest plot comparing pCR for patients with MIBC who received neoadjuvant ddMVAC versus neoadjuvant GC. CI, confidence intervals; ddMVAC, dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin; GC, gemcitabine and cisplatin; OR, odds ratio; MIBC, muscle-invasive bladder cancer; pCR, progression-free survival.
Pathological Downstaging—Subgroup Analysis
Rates of pathological downstaging to < ypT2N0 after NAC at the time of radical cystectomy were better with ddMVAC (OR 1.34 [95% CI 1.01; 1.78]) (Supplemental Fig. 2).15–18,20–22 However, this result was driven by the prospective subgroup, because this finding was not statistically significant for the retrospective-only studies.
Treatment Toxicities—Subgroup Analysis
Of the 10 total studies in the meta-analysis, 3 studies—VESPER, SWOG S1314, and Van de Putte EE 2016—included comparative treatment toxicity data.15,17,21 In this subgroup analysis, intention-to-treat rather than NAC-only data was available for the VESPER trial. Rates of Common Terminology Criteria for Adverse Events grade ≥3 for fatigue, gastrointestinal (nausea, vomiting, and diarrhea), anemia, and febrile neutropenia were greater with ddMVAC (Supplemental Table 1).
DISCUSSION
This systematic review and meta-analysis included 1984 total patients with MIBC who received NAC across 10 studies meeting our inclusion criteria. Our study found that ddMVAC was associated with improved OS and PFS compared to GC as NAC for patients with MIBC before radical cystectomy. There were higher pCR rates with the use of ddMVAC, although this was not statistically significant. Conversely, pathologic downstaging showed a statistically significant improvement with the use of ddMVAC, corresponding with OS and PFS. The recently published VESPER and SWOG S1314 trials offered the potential to collectively settle the debate regarding the preferred neoadjuvant cisplatin-based chemotherapy regimen.8,9 To our knowledge, VESPER and SWOG S1314 are the only prospective, randomized controlled trials to compare neoadjuvant ddMVAC to GC before radical cystectomy. In both studies, improvements in PFS and OS were seen following neoadjuvant ddMVAC as compared to GC. However, these findings ultimately were not statistically significant in the intention-to-treat analysis, which in the VESPER trial, included a small cohort (11% of patients) who received adjuvant rather than NAC. The presentation of the 5-year OS data for the neoadjuvant-only chemotherapy cohort of the VESPER trial at the American Society of Clinical Oncology Annual Conference 2023 was the first time a statistically significant OS advantage was shown with ddMVAC vs GC.23 Our meta-analysis, which compiled all neoadjuvant studies published to date, further supports the use of neoadjuvant ddMVAC for MIBC for improved OS and PFS.
Interestingly, pCR rates were not statistically different following neoadjuvant ddMVAC or GC. Our findings are consistent with the meta-analyses performed by Yin et al and Benkhadra et al who found no significant difference between neoadjuvant MVAC and GC on pCR rates.24,25 However, neither of these studies specifically examined ddMVAC. Since this finding was not statistically significant, it raises a question about the validity of this surrogate endpoint. Pathologic complete response at the time of radical cystectomy has previously been associated with improved OS and cancer-specific survival.11,26 As such, pCR has been proposed as a surrogate marker of OS for use in accelerated drug development for NAC.27 In our study, pathologic downstaging (< ypT2N0) showed a statistically significant benefit with neoadjuvant ddMVAC. Additionally, the pathologic downstaging event rate was higher than for pCR (0.456 vs 0.302, respectively; P < .001). Based on these findings, pathologic downstaging rather than pCR may not only be a more reliable surrogate endpoint but also require fewer patients to adequately power future neoadjuvant studies in MIBC.
Rates of Common Terminology Criteria for Adverse Events grade ≥3 for anemia, fatigue, febrile neutropenia, and gastrointestinal toxicities were significantly higher with the use of ddMVAC. The favorable toxicity profile of GC has led to widespread adoption of this neoadjuvant regimen.18 ddMVAC is also contraindicated for patients with significant cardiac disease, such as congestive heart failure.28 Nonetheless, for patients who are candidates for ddMVAC, the OS benefit conferred by ddMVAC is critical in treatment selection also noting the potential side effect differences.
Limitations in our meta-analysis stem from key differences between studies. This pooled analysis included differences in the timing and number of cycles of ddMVAC between trials. For example, 6 cycles of ddMVAC were planned in the VESPER trial compared to 4 cycles of ddMVAC in the SWOG S1314 trial.8,9 In the VESPER trial, 60% of patients went on to receive the full 6 cycles of ddMVAC. As the VESPER trial carried the greatest weight in each analysis, the benefit seen with neoadjuvant ddMVAC may in part be due to the 2 additional cycles given in this trial. Additionally, Zargar H 2018 selected locally advanced non-metastatic MIBC (stage T3 and T4 disease), potentially skewing the analysis in favor of ddMVAC, including the effect of downstaging.19,22 Inclusion of this study led to evidence of moderate statistical heterogeneity in pCR (Zargar H 2018 included: I2 = 61%, P = .02; Zargar H 2018 excluded: I2 = 29%, P = .22), but pCR remained not statistically significant with or without this study in the analysis. Of note, treatment toxicity and pathological downstaging rates were analyzed for descriptive purposes, as the search terms were not designed to capture all studies comparing treatment toxicities between neoadjuvant ddMVAC and GC.
CONCLUSION
In summary, neoadjuvant cisplatin-based chemotherapy remains the recommended approach to treat nonmetastatic MIBC before radical cystectomy in eligible patients. Our meta-analysis demonstrates that ddMVAC confers better oncologic responses with improved PFS and, more importantly, OS as compared to GC. These findings support advancing ddMVAC as first-line neoadjuvant chemotherapy for MIBC considering that another clinical trial comparing neoadjuvant cisplatin-based chemotherapy is unlikely. The correlation between OS, PFS, and pathologic downstaging and lack of statistical significance in pCR rates with ddMVAC suggest pathologic downstaging should be used as a surrogate endpoint in neoadjuvant clinical trials in MIBC. Overall, it is clear ddMVAC is associated with improved OS and PFS, with the cost of more toxicity. Advances in immune checkpoint inhibitors and antibody-drug conjugates have the potential to shift this treatment paradigm in the future. Nonetheless, this analysis shows convincing evidence that ddMVAC should be preferred over GC in appropriately selected cisplatin-eligible patients with MIBC who are radical cystectomy candidates and able to tolerate its increased toxicity profile.
Supplementary Material
Funding Support:
This study received funding from the Robert Rifkin Endowed Chair, William R. Meyn Foundation, and Herbert Crane Prostate Cancer Fund. A.W. and E.M.K. are supported by the Cancer Center Support Grant (P30CA046934). S.P.K. is supported by the Schramm Foundation.
Declaration of Competing Interest
Adam Warren is supported by the Cancer Center Support Grant (P30CA046934). Elizabeth Molina Kuna is supported by the Cancer Center Support Grant (P30CA046934). Simon P. Kim is supported by the Schramm Foundation. Thomas W. Flaig reports leadership, stock, and other ownership interests in Aurora Oncology; consulting for Seagen and Janssen Oncology; research funding from Novartis, Bavarian Nordic, Dendreon, GTx Janssen Oncology, Medication, Sanofi, Pfizer, Bristol-Myers Squibb, Roche/Genentech, Exelixis, Aragon Pharmaceuticals, Sotio, Tokai Pharmaceuticals, Atrazeneca/MedImmune, Lilly, Astellas Pharma, Agensys, Seagen, La Rocher-Posay, Merck, Seagen, Myovant Sciences, and Criterium; and patents, royalties, and other intellectual property related to 2 patents filed by the University of Colorado related to early-stage bladder cancer treatment and detection (not currently commercialized or in active clinical development).
Footnotes
Ethics Statement
As a systematic review and meta-analysis of previously published studies and publicly available data, ethical clearance by an institutional review board was not required.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.urology.2024.04.034.
Data Availability
Research data are stored in an institutional repository and will be shared upon request with the corresponding author.
References
- 1.Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–866. [DOI] [PubMed] [Google Scholar]
- 2.von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18:3068–3077. [DOI] [PubMed] [Google Scholar]
- 3.Sternberg CN, de Mulder P, Schornagel JH, et al. Seven year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumours. Eur J Cancer. 2006;42:50–54. [DOI] [PubMed] [Google Scholar]
- 4.Powles T, Bellmunt J, Comperat E, et al. Bladder cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:244–258. [DOI] [PubMed] [Google Scholar]
- 5.Flaig TW, Spiess PE, Agarwal N, et al. Bladder cancer, version 3.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2020;18:329–354. [DOI] [PubMed] [Google Scholar]
- 6.Galsky MD, Hahn NM, Rosenberg J, et al. A consensus definition of patients with metastatic urothelial carcinoma who are unfit for cisplatin-based chemotherapy. Lancet Oncol. 2011;12:211–214. [DOI] [PubMed] [Google Scholar]
- 7.Pal SK, Agarwal N, Grivas P, Choueiri T. Adjuvant chemotherapy for bladder cancer: using population-based data to fill a void of prospective evidence. J Clin Oncol. 2016;34:777–779. [DOI] [PubMed] [Google Scholar]
- 8.Pfister C, Gravis G, Flechon A, et al. Dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin or gemcitabine and cisplatin as perioperative chemotherapy for patients with nonmetastatic muscle-invasive bladder cancer: results of the GETUG-AFU V05 VESPER trial. J Clin Oncol. 2022;40:2013–2022. [DOI] [PubMed] [Google Scholar]
- 9.Flaig TW, Tangen CM, Daneshmand S, et al. Long-term outcomes from a phase 2 study of neoadjuvant chemotherapy for muscle-invasive bladder cancer (SWOG S1314; NCT02177695). Eur Urol. 2023;84:341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfister C, Gravis G, Flechon A, et al. Perioperative dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin in muscle-invasive bladder cancer (VESPER): survival endpoints at 5 years in an open-label, randomised, phase 3 study. Lancet Oncol. 2024;25:255–264. [DOI] [PubMed] [Google Scholar]
- 11.Sonpavde G, Goldman BH, Speights VO, et al. Quality of pathologic response and surgery correlate with survival for patients with completely resected bladder cancer after neoadjuvant chemotherapy. Cancer. 2009;115:4104–4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burns PB, Rohrich RJ, Chung KC. The levels of evidence and their role in evidence-based medicine. Plast Reconstr Surg. 2011;128:305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balduzzi S, Rucker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22:153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfister C, Gravis G, Flechon A, et al. Randomized phase III trial of dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin, or gemcitabine and cisplatin as perioperative chemotherapy for patients with muscle-invasive bladder cancer. Analysis of the GETUG/AFU V05 VESPER trial secondary endpoints: chemotherapy toxicity and pathological responses. Eur Urol. 2021;79:214–221. [DOI] [PubMed] [Google Scholar]
- 16.Leminski A, Kaczmarek K, Byrski T, Slojewski M. Neoadjuvant chemotherapy with dose dense MVAC is associated with improved survival after radical cystectomy compared to other cytotoxic regimens: a tertiary center experience. PLoS One. 2021;16:e0259526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flaig TW, Tangen CM, Daneshmand S, et al. A randomized phase II study of coexpression extrapolation (COXEN) with neoadjuvant chemotherapy for bladder cancer (SWOG S1314; NCT02177695). Clin Cancer Res. 2021;27:2435–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peyton CC, Tang D, Reich RR, et al. Downstaging and survival outcomes associated with neoadjuvant chemotherapy regimens among patients treated with cystectomy for muscle-invasive bladder cancer. JAMA Oncol. 2018;4:1535–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ravi P, Pond GR, Diamantopoulos LN, et al. Optimal pathological response after neoadjuvant chemotherapy for muscle-invasive bladder cancer: results from a global, multicentre collaboration. BJU Int. 2021;128:607–614. [DOI] [PubMed] [Google Scholar]
- 20.Ruplin AT, Spengler AMZ, Montgomery RB, Wright JL. Downstaging of muscle-invasive bladder cancer using neoadjuvant gemcitabine and cisplatin or dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin as single regimens or as switch therapy modalities. Clin Genitourin Cancer. 2020;18:e557–e562. [DOI] [PubMed] [Google Scholar]
- 21.van de Putte EE, Mertens LS, Meijer RP, et al. Neoadjuvant induction dose-dense MVAC for muscle invasive bladder cancer: efficacy and safety compared with classic MVAC and gemcitabine/cisplatin. World J Urol. 2016;34:157–162. [DOI] [PubMed] [Google Scholar]
- 22.Zargar H, Shah JB, van Rhijn BW, et al. Neoadjuvant dose dense MVAC versus gemcitabine and cisplatin in patients with cT3–4aN0M0 bladder cancer treated with radical cystectomy. J Urol. 2018;199:1452–1458. [DOI] [PubMed] [Google Scholar]
- 23.Pfister C, Gravis G, Flechon A, et al. Multicenter randomized phase III trial of dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin (dd-MVAC) or gemcitabine and cisplatin (GC) as perioperative chemotherapy for muscle-invasive bladder cancer (MIBC): overall survival (OS) data at 5 years in the GETUG/AFU V05 VESPER trial. J Clin Oncol. 2023;41. [DOI] [PubMed] [Google Scholar]
- 24.Yin M, Joshi M, Meijer RP, et al. Neoadjuvant chemotherapy for muscle-invasive bladder cancer: a systematic review and two-step meta-analysis. Oncologist. 2016;21:708–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benkhadra R, Nayfeh T, Patibandla SK, et al. Systematic review and meta-analysis of cisplatin based neoadjuvant chemotherapy in muscle invasive bladder cancer. Bladder Cancer. 2022;8:5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhindi B, Frank I, Mason RJ, et al. Oncologic outcomes for patients with residual cancer at cystectomy following neoadjuvant chemotherapy: a pathologic stage-matched analysis. Eur Urol. 2017;72:660–664. [DOI] [PubMed] [Google Scholar]
- 27.Chism DD, Woods ME, Milowsky MI. Neoadjuvant paradigm for accelerated drug development: an ideal model in bladder cancer. Oncologist. 2013;18:933–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chatterjee K, Zhang J, Honbo N, Karliner JS. Doxorubicin cardiomyopathy. Cardiology. 2010;115:155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Research data are stored in an institutional repository and will be shared upon request with the corresponding author.


