Abstract
Background:
There are few studies demonstrating how kidney function affects the risk of developing delirium in older adult surgical patients administered opioids. This study determined whether baseline kidney function influences the relationship between morphine equivalent dose and the development of delirium on postoperative day (POD) 2 in patients with hip fracture.
Methods:
This retrospective study analyzed emergency department (ED) estimated glomerular filtration rate (eGFR), perioperative serum creatinine, intravenous morphine equivalents and POD2 delirium assessment by the Confusion Assessment Method in 652 patients age ≥ 65 years without preoperative delirium. ED eGFR was used to divide subjects into groups by presence or absence of chronic kidney disease (CKD) and associations of opioid dose with POD2 delirium were compared using multivariable logistic regression.
Results:
POD2 delirium incidence was 29.8% (N=194). Intraoperative and post anesthesia care unit (PACU) morphine equivalent dosage as well as ED eGFR was similar comparing patients with and without POD2 delirium. Age, American Society of Anesthesiologist (ASA) status, and dementia were associated with delirium on POD2. The odds of POD2 delirium increased significantly with increase of intraoperative opioid in patients with CKD (OR=1.6, 95% CI: 1.2-2.2), but not in patients without CKD (p-interaction=0.04). PACU or POD1 opioid doses were not associated with POD2 delirium after covariate adjustment.
Conclusion:
This study suggests that incremental increases in intraoperative opioids combined with CKD increase odds of POD2 delirium after hip fracture repair, compared to patients without CKD..
Keywords: delirium, postoperative, hip fracture, analgesics, opioids, kidney
Background
Delirium is the most common complication after hip fracture surgery.1 Postoperative delirium is associated with poorer cognitive outcomes, increased length of stay, increased complications and nursing home placement.2 Therefore, it is imperative to optimize management strategies to prevent delirium.
One controversy in delirium prevention is opioid administration for pain management. There is evidence that opioids can predispose to delirium in a dose dependent manner.3 Alternatively, undertreating pain can increase the chance of developing delirium.4 Individualized opioid dosing strategies must take into account age and organ function such as kidney function. Chronic kidney disease (CKD) is a recognized risk factor for postoperative delirium.5 Since some opioids and their metabolites are excreted by the kidney,6 decreases in baseline kidney function with age and co-morbidity7 as well as changes in kidney function during the perioperative period8 could contribute to delirium onset.
CKD is common in hip fracture patients. Reduced kidney function was present on admission in approximately one-third of patients with hip fracture and is a risk factor for postoperative complications and increased mortality.9-11 Currently, there are few studies demonstrating how CKD affects the risk of developing delirium in older surgical patients administered opioids.
The primary aim of this study was to determine whether CKD influences the relationship between opioid dose and the development of postoperative delirium in patients with hip fracture. In addition, we sought to determine whether perioperative changes in kidney function affect the relationship between opioid dose and the onset of postoperative delirium.
Methods
The study was approved by the Institutional Review Board for Johns Hopkins University. Between 1999 and 2019, patients admitted to the hip fracture service were consented for collection of perioperative data. The hip fracture service is multidisciplinary12 and includes geriatric co-management, in which patients are assessed for delirium pre and postoperatively. Baseline demographics, at-home medications, intraoperative characteristics and postoperative outcomes are collected on all consenting individuals. Preoperative “probable dementia” was diagnosed if the patient had no delirium by Confusion Assessment Method (CAM) and either Mini Mental State Examination (MMSE) cutoff score <24 or clinical diagnosis of dementia by a geriatrician during the pre-hip fracture repair evaluation.13 Exclusion criteria for the analysis included preoperative delirium, age less than 65 years, and incomplete data on opioid dosing and/or kidney function.
Delirium:
The presence of delirium was assessed preoperatively and on postoperative day 2 using the Confusion Assessment Method14 as performed by either the attending geriatrician or a trained research assistant.
Opioid dose:
All opioid doses were converted to intravenous morphine equivalents in milligrams divided by weight in kilograms. Morphine equivalent dose was calculated as per standard ratio15 of:
IV Morphine: IV Hydromorphone of 10:1.5
IV Morphine: IV Fentanyl of 1mg: 10mcg
IV Morphine: PO Morphine of 1:3
PO Morphine: PO Oxycodone of 1.5:1
PO Morphine: PO Tramadol of 1:4
The cumulative opioid dose in intravenous morphine equivalents was calculated by adding intraoperative opioid + PACU opioid + POD1 opioid. Intraoperative opioid included opioids administered from entry to operating room exit. PACU opioid included opioids administered from entry until PACU exit. POD1 opioid included opioids administered from 7am the day after surgery until 7am the following day. Total morphine equivalents for each time period included both pro re nata (prn) and regular scheduled dosing.
Kidney function:
Serum creatinine values were obtained on routine perioperative blood draws including arrival in the Emergency Department (ED), as the baseline, POD1 and POD2. The eGFR in the ED was calculated using the patient’s age, sex, race and creatinine using the Chronic Kidney Disease Epidemiology Collaboration Equation.16
Statistical Analysis
Participants were divided into two groups based on presence or absence of CKD. CKD was defined as eGFR <60mL/min/1.73m2. Frequency data between presence or absence of CKD were compared using Fisher’s exact test, continuous data were analyzed with 2-sample t-test. Unless otherwise indicated, frequency data are reported as number and percentage, and continuous data are reported as mean with standard deviation. Pearson Correlation was performed between creatinine values obtained from the ED and creatinine values that were obtained within six months prior to the patient’s index hospitalization in order to determine if baseline ED creatinine was reflective of the patient’s true baseline kidney function or if the circumstances that led to a hip fracture also may have affected kidney function
Multivariable logistic regression was performed to test the interaction of CKD and morphine equivalents given intraoperatively, in the PACU and on POD1, on the odds of delirium on POD2. To examine impact of dosing across time periods on the outcome, incremental changes based on one standard deviation of dosing amount for each time period were used as the measurement units rather than using the absolute amounts in multivariable modeling. The regression model evaluated effect modification by level of ED eGFR above or below the CKD cutoff, through inclusion of the morphine equivalent variables, presence or absence of CKD, and their cross-product interaction terms, adjusting for relevant confounders including age, ASA status and probable dementia at baseline and change in creatinine from the ED to POD1.
All p values reported are two-tailed and a p < 0.05 is considered statistically significant. Statistical analyses were carried out using SAS version 9.4.
Results
From 1999 to 2019, 1,924 patients underwent hip fracture repair at Johns Hopkins Bayview Medical Center. Of this group, 711 patients provided written consent to be included in our database, among them 652 had information on kidney function, delirium and opioid administration.
Pearson Correlation between ED creatinine and creatinine values obtained within six months prior to the patients index hospitalization was 0.747 (p<0.0001).
Table 1 shows baseline demographics and clinical characteristics grouped according to CKD. Patients with CKD were older with higher ASA status. Patients with CKD were more likely to have coronary artery disease, congestive heart failure, peripheral vascular disease, and higher ED creatinine.
Table 1:
Baseline Demographics and Clinical Characteristics
| Variable | No Chronic Kidney Disease (N = 321) |
Chronic Kidney Disease (N = 331) |
p |
|---|---|---|---|
| Age in years, Mean (SD) | 79 (8) | 83 (7) | 0.000 |
| Female | 224 (70%) | 251 (76%) | 0.094 |
| Caucasian* | 310 (97%) | 313 (95%) | 0.266 |
| ASA Status* | 0.014 | ||
| 2 | 90 (28%) | 62 (19%) | |
| 3 | 203 (63%) | 228 (69%) | |
| 4 | 28 (9%) | 40 (12%) | |
| Diagnoses | |||
| Coronary Artery Disease* | 59 (18%) | 91 (28%) | 0.007 |
| Congestive Heart Failure* | 32 (10%) | 70 (21%) | 0.000 |
| Stroke* | 51 (16%) | 66 (20%) | 0.185 |
| Diabetes* | 67 (21%) | 82 (25)% | 0.263 |
| Peripheral Vascular Disease (N=292/304) | 21 (7%) | 40 (13)% | 0.021 |
| Dementia | 59 (18%) | %74 (22%) | 0.243 |
| Depression (N=291/203) | 47 (16%) | 47 (16%) | 0.911 |
| COPD* | 72 (22%) | 60 (18%) | 0.205 |
| At-home Medications | |||
| Antipsychotics (N=247/273) | 14 (6%) | 16 (6%) | 0.999 |
| Benzodiazepines (N=247/274) | 20 (8%) | 14 (5%) | 0.214 |
| Antidepressants (N=247/274) | 38 (15%) | 39 (14%) | 0.713 |
| Statins (N=247/274) | 36 (15%) | 32 (12%) | 0.363 |
| Opioids (N=276/300) | 122 (44%) | 127 (42%) | 0.674 |
| ED Creatinine | 0.8 (0.2) | 1.5 (1.0) | 0.000 |
| Femoral Neck Fracture* | 154 (48%) | 151 (46%) | 0.313 |
N = 1 or 2 with missing value
ASA=American Society of Anesthesiologists. SD=standard deviation.
Table 2 shows perioperative characteristics grouped by CKD. Patients with CKD received lower cumulative opioid doses and had higher mortality in hospital. Patients with CKD received a significantly lower amount of midazolam but not propofol intraoperatively compared to patients without CKD.
Table 2:
Perioperative variables
| Variable | No Chronic Kidney Disease (N = 321) |
Chronic Kidney Disease (N = 331) |
p |
|---|---|---|---|
| Intraoperative | |||
| Surgical Procedure* | 0.234 | ||
| Bipolar or Monopolar | 119 (37%) | 127 (38%) | |
| Nail | 116 (36%) | 133 (40%) | |
| Length of Surgery in Minutes (N=317/330) | 113 (50) | 108 (52) | 0.225 |
| Regional Anesthesia | 184 (57%) | 203 (61%) | 0.196 |
| Midazolam (mg/kg) (N=314/326) | 0.007(0.015) | 0.004 (0.012) | 0.013 |
| Propofol (mg/kg) (N=286/287) | 5 (5) | 5 (5) | 0.911 |
| Postoperative | |||
| Delirium on POD2 | 91 (28%) | 103 (31%) | 0.442 |
| Change in creatinine from ED to POD1, per mg/dl increase (N=307/325) | −0.04 (0.17) | −0.16 (0.36) | 0.000 |
| Time to Aldrete Score of 9 or Greater (min) (N=234/262) | 25 (44) | 29 (57) | 0.479 |
| Average Pain Score on PACU, No (%)Discharge (N=246/270) | 1 (2) | 1 (2) | 0.955 |
| Average Pain Score on POD1 (N=268/280) | 4 (3) | 3 (3) | 0.137 |
| Average Pain Score on POD2 (N=255/271) | 3 (3) | 3 (3) | 0.902 |
| Cumulative Opioid Dose (INTRAOP + PACU + POD1) mg/kg morphine equivalents (n=282/301) | 0.5 (0.5) | 0.4 (0.4) | 0.021 |
| ICU Days Postop (N=248/267) | 1 (3) | 1 (2) | 0.613 |
| RBC Units Transfused, No (%) perioperatively (N=283/297) | 1 (2) | 1 (2) | 0.952 |
| Hospital Length of Stay Days, Mean (SD) (N=315/326) | 6 (4) | 6 (4) | 0.751 |
| Discharge from Hospital Alive* | 316 (99%) | 316 (95%) | 0.012 |
| Discharge Location (N=306/310) | 0.880 | ||
| Home | 23 (7%) | 14 (5%) | |
| Skilled Rehabilitation | 275 (84%) | 251 (85%) |
N = 1 or 2 had missing value
N = 316 in the upper half and N = 327 in the chronic kidney disease group had data.
RBC= red blood cell. ICU= intensive care unit. PACU= post anesthesia care unit. POD= postoperative day. Intraop= intraoperative or intraoperatively. UTI= Urinary Tract Infection. Postop = postoperative or postoperatively. No =Number.
The incidence of delirium on POD2 was 29.8% (N=194). The baseline (ED) eGFR was similar in patients with and without delirium on POD2 (eGFR: 59.0 ± 24.1 vs 61.6 ± 22.7 mL/min, p=0.18). Patients with delirium on POD2 received similar amounts of morphine equivalents intraoperatively and in the PACU, but lower amounts of morphine equivalents on POD1, as compared to their counterparts who were delirium free on POD2 (morphine equivalents: intraoperative 0.19 ± 0.21 vs 0.16 ± 0.21 mg/kg, p=0.09; PACU 0.017 ± 0.049 vs 0.021 ± 0.057 mg/kg, p=0.47; POD1 0.13 ± 0.17 vs 0.18 ± 0.22 mg/kg, p=0.03). Cumulative opioid dose, intraoperative + PACU + POD1, was similar between these two groups of patients (morphine equivalents: 0.35 ± 0.31 vs 0.35 ± 0.34 mg/kg, p=0.99).
In multivariable analyses, older age, elevated ASA status and having a probable dementia diagnosis at baseline were associated with significantly increased odds of delirium on POD2 independent of kidney function at baseline and opioid dosing perioperatively in all 4 models. Change in creatinine from ED to POD1 was not associated with odds of delirium on POD2 (Supplementary Table S1). One SD increase of intraoperative morphine equivalent dose was associated with a 35% increase in odds of delirium on POD2 in patients with CKD, compared to patients without CKD where odds of delirium was not associated with opioid dose (Supplementary Table S1, Model 1). However, this effect modification of opioid dosage on the odds of delirium by CKD was not found with PACU or POD1 dosages (Supplementary Table S1, Models 2 and 3) where opioid dosage was not associated with odds of delirium regardless of presence or absence of CKD. Similar results were observed using the data from only patients who had all 3 measurements of intravenous morphine equivalents perioperatively (Supplementary Table S1, Model 4 and Figure 1), where the odds ratio of delirium on POD2 associated with 1 SD increase in morphine equivalents received intraoperatively was estimated as 1.60 (95% Confidence Interval (CI): 1.19 to 2.15) for patients with CKD, compared to the odds ratio of 1.06 (95% CI: 0.82 to 1.38) in patients without CKD (p-interaction = 0.04).
Figure 1:
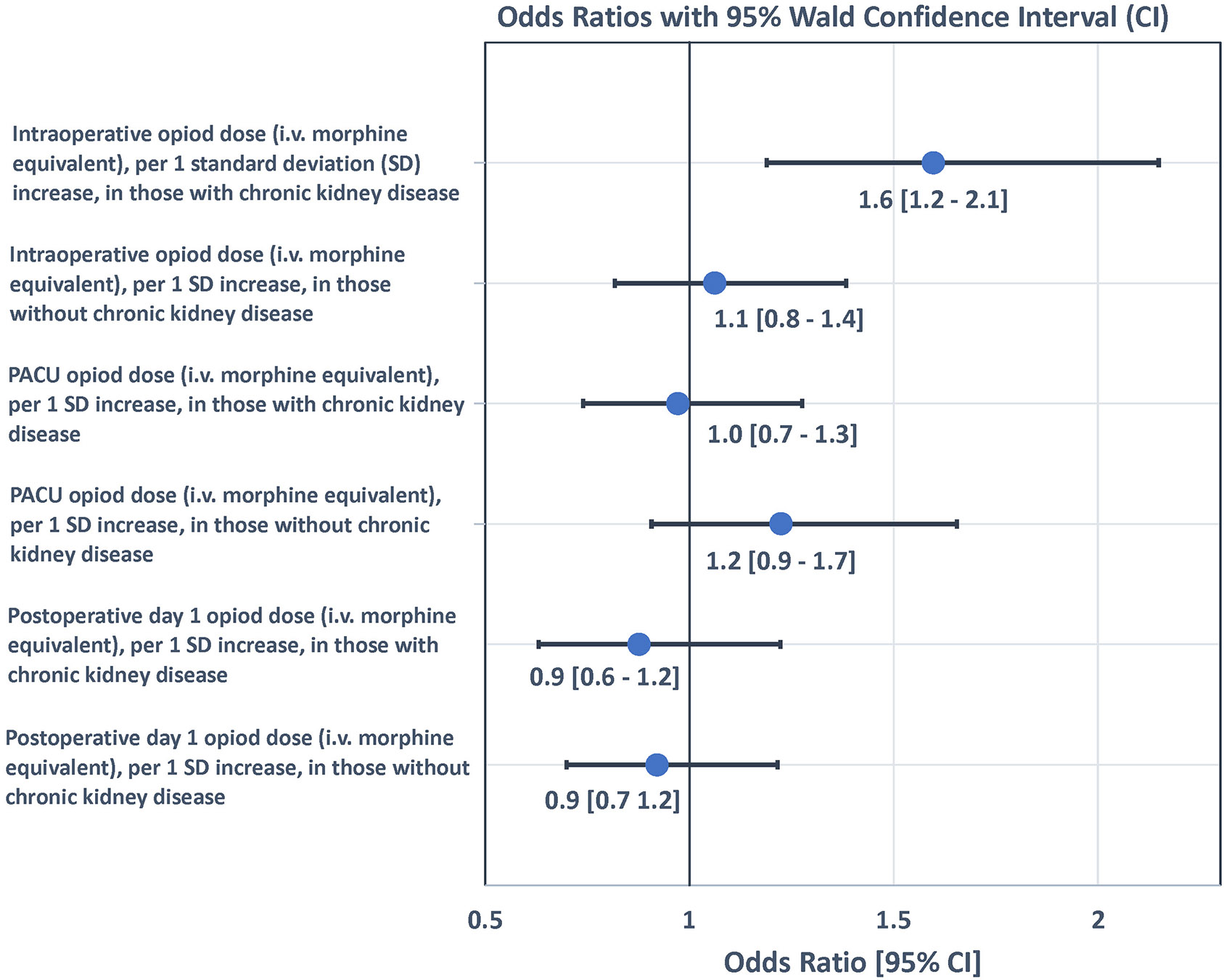
Forrest plot of odds ratios for opioid dose for prediction of delirium on postoperative day 2 by presence or absence of chronic kidney disease. OR = Odds Ratio. Odds Ratio adjusted for age, ASA, dementia status, and change in creatinine from ED to POD1. SD=standard deviation. PACU=post anesthesia care unit. POD=postoperative day.
Discussion
This study shows that in hip fracture patients, CKD has a modifying effect on the relationship between intraoperative opioid dose and risk of delirium on POD2. No modifying effect was found with either PACU or POD1 opioid dose. In addition, perioperative changes in kidney function were not associated with development of delirium on POD2. These results emphasize the importance of considering baseline kidney function for intraoperative opioid dosing in preventing early postoperative delirium.
Previous studies report an association between intraoperative opioid doses and postoperative delirium in orthopedic patients.3 Similarly, intraoperative opioid dose has been related to PACU symptoms of delirium.17 The current study found POD2 delirium associated with intraoperative dose to be specific in older patients with CKD undergoing hip fracture repair, but did not find associations or effect modification by CKD of opioid dose in PACU or on POD1. These findings are most likely related to variations in opioid dose between the intraoperative, PACU, and POD1 time periods. The PACU dose is much lower than intraoperatively and therefore less likely to have effect. Patients who had delirium receive significantly less opioid on POD1 compared to those without delirium. This would tend to negate the opioid effect at this timepoint.
Kidney function decreases after hip fracture repair in approximately 13% of patients.18 We found no association between creatinine changes from ED to POD1 and delirium on POD2. Whether changes in creatinine after POD 1 affect delirium is still unclear. However, data from cardiac surgery suggests that acute kidney injury in the two days postoperatively increases the risk of delirium.19
Although opioids are primarily metabolized in the liver, kidney function affects opioid pharmacokinetics and removal of some metabolites.6 Patients with reduced kidney function are at higher risk for delirium following opioid administration.20,21 Adverse opioid effects when administered for pain management can be avoided by decreasing the opioid dosage based on the severity of kidney disease.22 In the current study, the SD for intraoperative opioid is 0.21 mg/kg so the odds ratio reported for intraoperative opioid is associated with such a dosing increase. In clinical terms this roughly equates to intravenous doses of 150 mcg fentanyl or 2.2mg hydromorphone in a 70 kg individual with CKD during the intraoperative period.
Advantages of this study include the large sample size with complete intraoperative and postoperative data on kidney function and opioid dosage. Limitations include that the effects after POD2 were not analyzed. Different opioids were converted to intravenous morphine equivalents and lumped together in the analysis, yet we did not account for differences in pharmacokinetics. Even though the morphine equivalent calculations were originally obtained as outlined in the methods section, our database contains only total morphine equivalents calculations and not data concerning the specific opioid administered. Our analysis included hip fractures occurring over a 20-year period, prohibiting the ascertainment of specifics concerning type of opioid administered in older cases occurring prior to electronic medical records. Our database does contain information on postoperative complications. However, it does not allow us to establish the temporal relationship between the occurrence of these complications and delirium on POD2. Since the health status of those with lower kidney function is in general worse, there are likely other factors in this group that could contribute to delirium. Complete data concerning known risk factors for delirium in an older adult population are not available in our dataset. Given this, the variables chosen for the final model are limited and residual confounding cannot be ruled out.
In summary, CKD in older patients with hip fracture has a modifying effect on intraoperative opioid dose such that incremental dose increases have significantly greater odds of precipitating POD2 delirium in patients with than without CKD. Further study is required to determine whether adjusting intraoperative opioid dose in hip fracture patients with CKD is a possible intervention to prevent early postoperative delirium.
Supplementary Material
Supplementary Table S1. Analysis of Maximum Likelihood Estimates for delirium on postoperative day 2
Acknowledgement
Conflict of Interest
Arman B. Davani: none
Scott H. Snyder: The views expressed in this article are those of the author and do not reflect the official policy of the Department of Army/Navy/Air Force, Department of Defense, or U.S. Government.
Esther S. Oh: none
Simon C. Mears: none
Deidra C. Crews: Dr. Crews is supported, in part, by grant K24 HL148181 from the National Heart, Lung and Blood Institute (NHLBI), NIH.
Nae-Yuh Wang: Dr. Wang’s work is partially supported by the grant UL1 TR003098 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research.
Frederick E. Sieber: none
Sponsor’s Role
The manuscript’s contents are solely the responsibility of the authors and do not necessarily represent the official view of NCATS, NHLBI, or NIH.
References
- 1.Guenther U, Radtke FM. Delirium in the postanaesthesia period. Curr Opin Anaesthesiol. 2011;24(6):670–675. [DOI] [PubMed] [Google Scholar]
- 2.Marcantonio ER, Flacker JM, Michaels M, Resnick NM. Delirium is independently associated with poor functional recovery after hip fracture. J Am Geriatr Soc. 2000;48(6):618–624. [DOI] [PubMed] [Google Scholar]
- 3.Weinstein S, Poultsides L, Baaklini L, et al. Postoperative delirium in total knee and hip arthroplasty patients: A study of perioperative modifiable risk factors. Br J Anaesth. 2018;120(5):999–1008. [DOI] [PubMed] [Google Scholar]
- 4.Morrison RS, Magaziner J, Gilbert M, et al. Relationship between pain and opioid analgesics on the development of delirium following hip fracture. J Gerontol A Biol Sci Med Sci. 2003;58(1):M76–M81. [DOI] [PubMed] [Google Scholar]
- 5.American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults. Postoperative delirium in older adults: Best practice statement from the American Geriatrics Society. J Am Coll Surg. 2015;220(2):136–48.e1. [DOI] [PubMed] [Google Scholar]
- 6.Mercadante S, Arcuri E. Opioids and renal function. J Pain. 2004;5(1):2–19. [DOI] [PubMed] [Google Scholar]
- 7.Epstein M. Aging and the kidney. J Am Soc Nephrol. 1996;7(8):1106–1122. [DOI] [PubMed] [Google Scholar]
- 8.Zarbock A, Koyner JL, Hoste EA, Kellum JA. Update on perioperative acute kidney injury. Anesth Analg. 2018;127(5):1236–1245. [DOI] [PubMed] [Google Scholar]
- 9.Mangat KS, Mehra A, Yunas I, Nightingale P, Porter K. Is estimated peri-operative glomerular filtration rate associated with post-operative mortality in fractured neck of femur patients?. Injury. 2008;39(10):1141–1146. [DOI] [PubMed] [Google Scholar]
- 10.White S, Rashid N, Chakladar A. An analysis of renal dysfunction in 1511 patients with fractured neck of femur: The implications for peri-operative analgesia. Anaesthesia. 2009;64(10):1061–1065. [DOI] [PubMed] [Google Scholar]
- 11.Porter CJ, Moppett IK, Juurlink I, Nightingale J, Moran CG, Devonald MA. Acute and chronic kidney disease in elderly patients with hip fracture: Prevalence, risk factors and outcome with development and validation of a risk prediction model for acute kidney injury. BMC Nephrol. 2017;18(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Jonge KE, Christmas C, Andersen R, et al. Hip fracture service—an interdisciplinary model of care. J Am Geriatr Soc. 2001;49(12):1737–1738. [DOI] [PubMed] [Google Scholar]
- 13.Oh ES, Sieber FE, Leoutsakos J, Inouye SK, Lee HB. Sex differences in hip fracture surgery: Preoperative risk factors for delirium and postoperative outcomes. J Am Geriatr Soc. 2016;64(8):1616–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inouye SK, Charpentier PA. Precipitating Factors for Delirium in Hospitalized Elderly Persons: Predictive Model and Interrelationship With Baseline Vulnerability. JAMA. 1996;275(11):852–857. [PubMed] [Google Scholar]
- 15.McPherson ML. Demystifying Opioid Conversion Calculations: A Guide For Effective Dosing. 2nd ed. Bethesda, MD: American Society of Health-System Pharmacists; 2014. [Google Scholar]
- 16.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Card E, Pandharipande P, Tomes C, et al. Emergence from general anaesthesia and evolution of delirium signs in the post-anaesthesia care unit. Br J Anaesth. 2015;115(3):411–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pedersen AB, Christiansen CF, Gammelager H, Kahlert J, Sørensen HT. Risk of acute renal failure and mortality after surgery for a fracture of the hip: A population-based cohort study. Bone Joint J. 2016;98-B(8):1112. [DOI] [PubMed] [Google Scholar]
- 19.Kotfis K, Ślozowska J, Listewnik M, Szylińska A, Rotter I. The impact of acute kidney injury in the perioperative period on the incidence of postoperative delirium in patients undergoing coronary artery bypass Grafting—Observational cohort study. Int J Environ Res Public Health. 2020;17(4):1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siew ED, Fissell WH, Tripp CM, et al. Acute kidney injury as a risk factor for delirium and coma during critical illness.Am J Respir Crit Care Med. 2017;195(12):1597–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishida JH, McCulloch CE, Steinman MA, Grimes BA, Johansen KL. Opioid analgesics and adverse outcomes among hemodialysis patients. Clin J Am Soc Nephrol. 2018;13(5):746–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tawfic QA, Bellingham G. Postoperative pain management in patients with chronic kidney disease. J Anaesthesiol Clin Pharmacol. 2015;31(1):6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1. Analysis of Maximum Likelihood Estimates for delirium on postoperative day 2


