Abstract
Neurocysticercosis is one of the main risk factors of seizures and epilepsy in many regions of the world, which are Taenia solium–endemic but resource-constrained to control the parasite. The nosology of seizures and the classification of epilepsy in the context of neurocysticercosis are somewhat uncertain. Many seizures associated with the infection are customarily referred to as “acute symptomatic seizures.” The term, however, seems unsuitable. Neither is the condition acute nor does it allow the avoidance of long-term antiseizure medications, as is the case with acute symptomatic seizures, for instance, associated with traumatic brain injury. We propose that seizures be classified according to the evolutionary stage of parenchymal cysticercosis in addition to the conventional classification of seizures and epilepsy and identification of the epileptogenic zone. An often-ignored aspect is the identification of comorbidities, many of which are specific to neurocysticercosis.
Introduction
Human cysticercosis denotes tissue infection with the larval stage of the tapeworm Taenia solium. Symptoms, disability, and mortality associated with it are almost entirely accounted for by infection of the brain and its coverings, known as neurocysticercosis (NCC). Numerically, the global burden of cysticercosis is estimated at 3.51–6.47 million cases.1 The infection is a significant cause of long-term disability, conservatively estimated at 1.37 million disability-adjusted life years (DALYs), mostly taking the form of seizures and epilepsy. The highest DALY rates have been recorded in sub-Saharan Africa, followed by Latin American, Caribbean, and Southeast Asian regions. In Latin America and the Caribbean, NCC is the most frequent neglected tropical disease causing disability. Clinical cases, however, are encountered worldwide, including in many high-income countries, where it was once deemed eradicated. These cases are among either immigrants from or travelers to endemic regions. Those infested with adult tapeworms in endemic areas can also transmit infection to nonendemic regions.
Seizures are the most common manifestation of NCC. A systematic review of clinical manifestations among symptomatic people from facility-based settings estimated the pooled proportion of seizures/epilepsy to be 79% (95% CI 71%–85%) among children and 63% (95% CI 52%–73%) among adults.2 NCC should be considered in people presenting with new-onset and recurrent seizures everywhere. An overwhelming burden of seizures and epilepsy, however, is encountered in many resource-limited endemic countries. For instance, in Peru, it is the putative risk factor in nearly a third of cases of epilepsy in the community.3 In sub-Saharan Africa, similarly, 29% (95% CI 17%–40%) of epilepsies have been attributed to NCC, diagnosed with CT or serology.4 It is crucial to understand the relationship between NCC, seizures, and epilepsy.
Evolution of Cysticerci in the Human Brain
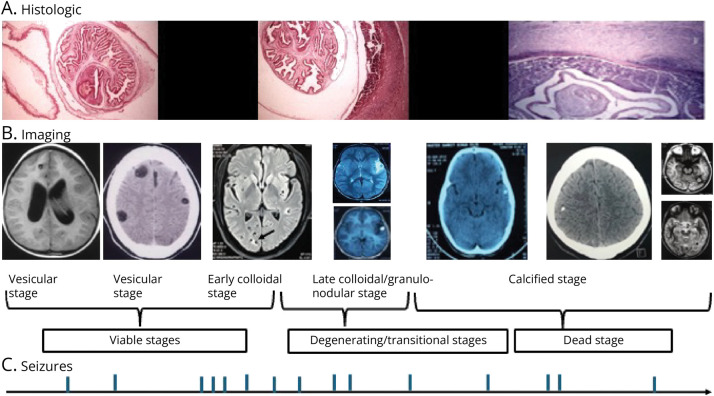
The cysticercus larvae may lodge anywhere in the CNS, including the cerebral parenchyma, subarachnoid spaces, ventricles, and spinal cord. Once the cysticerci lodge in the brain parenchyma, they undergo several evolutionary stages. These stages have been elucidated in histopathologic studies. Imaging studies, mainly MRI but often complemented by CT, can distinguish the evolutionary stages exceedingly well, although correlation with histopathologic materials is rare (Figure 1).5 The initial vesicular stage of cysticercus comprises a scolex with a well-characterized canalicular network and clear cyst fluid surrounded by an intact cyst wall, beyond which there is slight inflammation or edema in the surrounding parenchyma (Figure 1A). An eccentric scolex can be identified on MRI, the cyst fluid contents are isointense with CSF, and there is no edema in the surrounding parenchyma (Figure 1B). Once the host immune system identifies and attacks the vesicular cyst, it transforms into an early degenerating larva, in which the canalicular network becomes less distinct, the cyst fluid becomes turbid, and the cyst wall begins to break. There is inflammation as evidenced by postgadolinium enhancement and perilesional edema on MRI. This is known as the colloidal stage (Figure 1B). The vesicular and at least the early colloidal stages represent viable parasites. From the imaging standpoint, the viable cysts are defined by fluid contents within the cyst wall (Figure 1B).6 The parasite evolves further until no fluid content is discernible, leaving a granuloma with a degenerate and mineralized scolex and surrounding inflammation and edema (Figure 1B). Last, the cysticercus involutes further to a calcified nodule with diminishing inflammation and edema (Figure 1B). The calcified cysticercus, as opposed to the earlier stages, was traditionally considered inactive.7 There is increasing evidence, however, that calcified NCC plays a significant role in triggering seizures and sustaining active epilepsy.8 The staging is somewhat intuitive because the individual stages represent a continuum rather than being rigidly compartmentalized.
Figure 1. Histopathologic and Imaging Stages of Parenchymal Cysticerci and the Occurrence of Seizures.
The top panel (A) depicts the histologic stages of the cysticerci beginning with the vesicular stage with a characteristic canalicular network, an intact cyst wall and minor surrounding inflammation (left), a colloidal stage in which the canalicular network begins to degenerate and there is profound inflammatory infiltrate in the surrounding parenchyma (middle), and the calcified stage with slight surrounding inflammation (hematoxylin and eosin stain; scale bar: 225 μm). The middle panel (B) depicts the evolutionary stages on imaging (from left to right): the vesicular stage on MRI and CT, the colloidal stage on MRI (arrow), the granular-nodular stage on MRI with and without contrast, and the calcified stages on MRI/CT. The bottom panel (C) is a hypothetical depiction of the occurrence of seizures in correspondence with different evolutionary stages (note: the panels merely convey that seizures can occur during all evolutionary stages).5 Reproduced from the study by Singh et al.40 with permission.
The evolution of the cysticercus in the subarachnoid spaces differs and is location-dependent. The evolution is similar to parenchymal cysts when located in the cerebral sulci over the convexity with little available fluid space. In the base of the brain, with abundant fluid space available, the cysts undergo proliferation and hydropic degeneration into large, thin-walled racemose structures.9
After lodging in the brain, the parenchymal cysticercus can remain asymptomatic for long periods. Studies of British soldiers assigned to India during colonial rule in the early 19th century estimated the asymptomatic period from arrival to the onset of cerebral symptoms.10,11 This time could vary from a few months to 30 years, with an average of approximately 3–5 years. Thus, the evolution of these live cysts eventually to a calcified stage is long-drawn and unpredictable.
In one cross-sectional study of people with 1–2 viable cysts and focal seizures, perilesional edema was absent in a third.12 It may be inferred that seizures in this one-third were triggered by either mild inflammation not detectable on MRI, mechanical effects of the cysts on the surrounding cerebral parenchyma, or the culmination of epileptogenesis initiated at the time of lodging (mentioned further). One of the authors' rare observations is that fluid-filled cysts with enhancement and surrounding edema may return to the vesicular stage (Garcia HH, personal communication). This might have implications regarding the generation of seizures during the vesicular stage of the parasite because the inflammation near a reverting cyst could provoke seizures. More often, however, the onset of seizures corresponds to the inflammatory degeneration of the cysticerci, corresponding to the colloidal or granular-nodular stages. Studies documenting findings of a single enhancing lesion or multiple enhancing lesions on CT/MRI in people with new-onset seizures in endemic regions in India and Latin America support this contention.13 When followed up, these people may continue to have seizures despite using antiseizure medications (ASMs). Roughly, up to half of the people with single cysticercus granuloma might have breakthrough seizures over 6–15 months of follow-up.14 Some might continue to have seizures even after the resolution of the granuloma/s. One observational study on solitary cysticercus granuloma with a median follow-up of 66 months reported a 15% incidence of seizures after resolution.15 In those in whom the cyst has resolved, the presence of a calcific residue is a significant risk factor of ongoing seizures. In pooled data of follow-up amounting to 12–24 months after resolution of a solitary cysticercus granuloma from India, the presence of a calcific residue increased the odds of seizure recurrence by 11 (95% CI 5–27) times.14 Another follow-up study of calcified lesions from Peru estimated the 5-year risk of seizures to be 35%.8
While the association between NCC and seizures or epilepsy is indisputable, the evolution might remain asymptomatic without giving rise to seizures or other cerebral symptoms. Reports of NCC first diagnosed at autopsy support this.16 Similarly, CT may detect 1 or more calcifications among asymptomatic individuals. Up to 15% of people in population-based studies from endemic regions may have such calcifications.17,18
Overview of Current International League Against Epilepsy Definitions and Classification of Seizures and Epilepsy
The Epidemiology Commission of the International League Against Epilepsy (ILAE) defines acute symptomatic seizures as those that occur in close temporal relationship to a documented brain insult.19 When the brain insult is acute, such as stroke or traumatic brain injury, the ILAE Commission prescribes a period of 7 days for seizures to be labeled as acute symptomatic. In CNS infections, some of which might have a lengthy course, for example, NCC, seizures occurring during the active phase of infection are labeled acute symptomatic seizures. The Commission also recommends avoiding labels such as reactive seizures, situation-related seizures, and provoked seizures. These acute symptomatic seizures are associated with low recurrence risks in long-term follow-up studies and may not require prolonged treatment with ASMs.
Epilepsy is operationally defined by the occurrence of 2 or more unprovoked seizures or by a single seizure with an estimated probability of recurrence >60% over the next 10 years.20 After characterizing the seizures, epilepsy is further classified as focal, generalized, or unknown and a syndrome is assigned if possible.21,22 Finally, the etiologic factor/s and comorbidities associated are identified.
Epileptogenesis in the Context of NCC
Epileptogenesis is a complex process by which the normal brain is rendered capable of generating spontaneous seizures. It is underpinned by several anatomical and functional alterations commencing with a brain insult but remains ongoing even after spontaneous seizures occur. The time between the initiating insult and the occurrence of the first spontaneous seizure, known as the latent period, is variable and unpredictable.
Little is known about epileptogenesis in the context of NCC. One of the challenges is identifying a suitable NCC animal model.23,24 Many approaches have been attempted using Taenia solium or related helminths and several different animal hosts.24 To understand epileptogenesis in NCC, a parallel could be drawn with seizures associated with brain tumors.25 A variety of pathogenic processes are involved in tumor epileptogenesis. These include but are not limited to the isolation of brain cortical areas, deafferentiation, tissue damage and necrosis, inflammation, swelling and edema, neurotransmitter aberrations, alterations in the microenvironment of the surrounding parenchyma, and gliosis.26 Not least, genetic factors underpin many of the processes. There might, however, be differences between epileptogenesis associated with brain tumors and NCC. Inflammation and ensuing gliosis are the main factors in NCC rather than necrosis, tissue damage, compression, mass effect, swelling, and edema.27,28
In available experimental studies of NCC, the effort has been to discern the initiating and mediating agents responsible for seizures or epileptogenesis. Early rather than late granulomas are, more often, associated with seizures, both having distinct immunologic profiles.29 In another experiment, Substance P was found to be overexpressed in the surrounding brain parenchyma in mice with seizures.30 Seizures did not occur when Substance P was deficient or antagonized. Similarly, axonal spheroids indicating neuronal degeneration in the surrounding brain parenchyma were identified in one study.31 However, more is needed to understand the course of epileptogenesis in NCC. This, in turn, could provide a range of insights into, for instance, seizure recurrence rates and, not least, why drug-resistant epilepsy is rare in the condition.
ILAE Approaches to Seizures and Epilepsy Associated With NCC
Seizure Semiology vs Location of Cysticerci
In people with 1–2 viable or degenerating cysts, there is generally a good correlation between the topographic location of the cysts and the inferred semiological localization of seizure onsets.12 This, however, may not be the case with calcified lesions, in which the correspondence might be less often forthcoming.32 Some calcified lesions might be coincidental, considering an alternative epileptogenic substrate, etiologic factor, or even wholly asymptomatic, as demonstrated in population-based studies from endemic regions.17,18 In any case, a detailed clinical assessment focused on the initial symptoms and signs of the seizure proves invaluable in ascertaining the association between the implied seizure-onset or epileptogenic zone and the location of the cysticercus. However, when multiple parenchymal cysticerci exist, the correspondence between semiological and anatomical localizations can become challenging.
A logical first step is determining seizure types per the current ILAE classification scheme.22 It may be assumed that all seizures are focal in onset. In many instances, a focal onset might not be evident, and in such cases, the label unknown onset is preferred. The initial symptom or manifestation is paramount in delineating seizure types. Accordingly, the terms focal aware and focal with impaired awareness seizures might be used in parallel with or independent of a motor vs nonmotor onset. Generalized seizures, particularly myoclonic, myoclonic-atonic, atonic, absence, atypical absence, eyelid myoclonias, and spasms, do not occur in NCC unless the person has concomitant generalized epilepsy.
What Proportions of the Burden of Seizures Are Accounted For by Different Evolutionary Stages?
Seizures occur during all the cysticercus evolutionary stages but seem more common during the viable and degenerating stages.8,12,14,15 In 3 landmark trials of antiparasitic treatment with follow-up periods between 1 and 2 years, including 2 with albendazole alone and 1 with combination treatment, seizures were fewer among those individuals in whom cysts had resolved by month 6.6,33,34 In a trial of combination antiparasitic treatment, viable cysts were defined by identifying fluid contents within brain T2 MRI scans.6 Seizures were approximately 3–5 times less likely among people with resolved cysts.33 In another controlled trial of albendazole alone, breakthrough seizures were rare among those with resolved cysts.34 This implies that most seizures in the first 1–2 years after antiparasitic treatment occur in association with viable and degenerating cysts. Resolution of cysts with or without antiparasitic treatment considerably reduces the chances of seizures. The interpretation is limited, however, by the short (1–2 years) duration of the 3 trials.6,33,34 When followed over more extended periods, people with calcified cysts continue to experience seizures. Somewhat different from this scenario, when people with calcified cysticerci present in the clinic, they are often likely to have breakthrough seizures on follow-up. In the Peruvian follow-up study of calcified cysts, the 5-year seizure recurrence estimate was 35%.8
The evolutionary stages of NCC identified among people with epilepsy depend on the setting in which these people are sampled. Live and degenerating forms of cysticerci followed by calcified are most frequently identified among people presenting with new-onset seizures in the clinic.13,14 Conversely, calcified lesions are primarily identified in imaging studies performed on people with epilepsy in population-based studies.17,18 There might be some differences between geographic settings explicable by infection pressure because studies from Latin America identified mainly calcified cysts and studies from India identified a more significant proportion of degenerating (or transitional) cysts.35,36
Is the Label, Acute Symptomatic Seizures, Appropriate for NCC-Associated Seizures?
A well-known clinical cliché is that seizures that occur in the setting of viable and degenerating (cf vesicular, colloidal, and granular-nodular stages in histopathologic studies) stages of the cysticerci are labeled as acute symptomatic. Conversely, those occurring in the setting of the calcified stage are termed as unprovoked and amount to epilepsy according to the current ILAE definition. There are, however, several challenges to this concept. First, the pathologic and clinical evolution of NCC cannot be construed as “acute” because it is neither of rapid onset with short duration nor requiring only short-term ASMs commensurate with the implied meaning of the term acute symptomatic seizures. Second, the categorization of seizures as acute symptomatic or provoked critically hinges on the demonstration of the cysticercus' evolutionary stage, ideally on MRI performed in close temporal relationship to the seizure/s. Cost and availability, however, frequently preclude frequent imaging in resource-limited endemic settings. Third, although calcified cysts are considered quiescent and marked by inactivity from a pathologic standpoint, these may periodically and unpredictably demonstrate inflammatory attributes corresponding temporally with seizure/s on follow-up.8 MRI performed in close temporal relationship to the seizure reveals perilesional edema around the calcified lesion in roughly half of them. In the Peruvian study, contemporaneous imaging in controls also with calcified brain lesions but without seizures showed perilesional edema in <10% (p < 0.002).8 This study confirms the association between the development of perilesional edema and the occurrence of seizures on follow-up in people with calcified cysticerci. It did not establish a causal relationship, but it is likely that the events leading to perilesional edema also caused the seizures. Finally, while the association with seizures is indisputable, little is known about the underpinnings and sequence of epileptogenesis in the setting of NCC. Does epileptogenesis begin with structural deformation, deafferentiation, and isolation of surrounding brain parenchyma in the early vesicular stage? Or does it commence with surrounding inflammation that distinguishes the colloidal and granular-nodular stages from the vesicular stage with fluid and electrolyte microenvironment and neurotransmitter aberrations differentially triggered by the inflammatory mediators? Still other, is the process set up during the involution of the cysticercus with the development of gliosis in the surrounding brain parenchyma signaling functional and anatomical changes in astrocytes? Until a clear marker of epileptogenesis is identified, it would be hard, if not impossible, to predict the onset and culmination of epileptogenesis and definitively distinguish between acute symptomatic and unprovoked seizures in the context of brain parenchymal cysticercosis.
How Should Seizures in Association With NCC Be Labeled?
The term “acute symptomatic seizures,” as observed with acute alcohol intoxication and head injury, should be avoided in the context of NCC because this may lead to confusion among treating physicians concerning the duration of ASMs. We contend that instead of being labeled as either acute symptomatic or unprovoked, seizures should be categorized according to the evolutionary stage of the cysticerci as depicted in contemporary imaging studies (Figure 1). Labeling seizures according to the evolutionary stage has many advantages. For one, it would caution the treating physician from considering only short-term ASM use, as is the case with acute symptomatic seizures. It would also be instrumental in determining the need for antiparasitic and anti-inflammatory treatment according to the evolutionary stage of the cysticerci. Antiparasitic treatment hastens the chances of resolution of the cysticerci during the viable, i.e., vesicular and early colloidal stages and perhaps to a somewhat lesser magnitude in the degenerating or granular-nodular stage but has no impact on the course and outcome in the calcific stage of the cysticercus.37
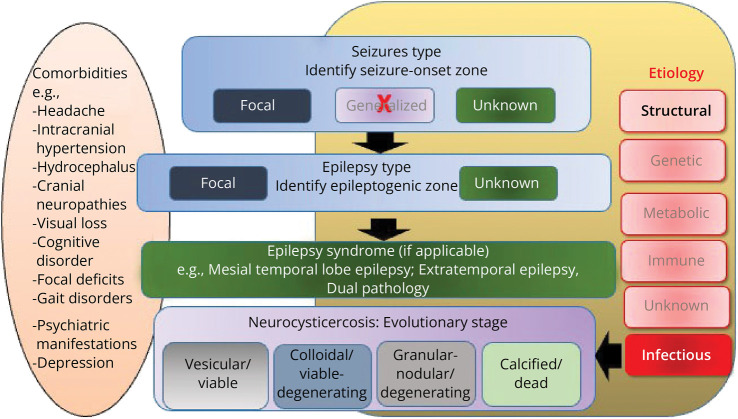
Apart from characterizing semiology and classifying seizures and the corresponding evolutionary stages of the cysticerci, it may be relevant to identify the epilepsy syndrome, if possible, and determine the epileptogenic zone (Figure 2). This is mainly the case when seizures are poorly controlled or are drug-resistant. Overall, while drug-resistant epilepsy is rare in NCC, it is imperative not to miss the association with hippocampal sclerosis because it might crucially affect treatment approaches.38 Of note, only the calcified cysticerci are typically associated with hippocampal sclerosis.39 Still exceedingly rare is the possibility of dual pathology with seizures arising independently from the hippocampus and the calcific lesion. Investigating the association with invasive recordings might be crucial in detecting independent seizure onsets from the hippocampus and the calcified lesion. Finally, the identification of comorbidities, as with any epilepsy diagnosis, is also crucial (Figure 2). The comorbidities comprise but are not limited to a range of neurologic disorders attributed to NCC, for example, headaches, hydrocephalus, raised intracranial tension, vision loss, focal neurologic deficits, cognitive impairments, and depression.
Figure 2. The International League Against Epilepsy Scheme of Classification of Seizures and Epilepsies Applied to Seizures Associated With Neurocysticercosis.
The original scheme has been adapted to suit the categorization of seizures and comorbidities in neurocysticercosis.21,22
A caveat to using the evolutionary stages of cysticercosis in the classification of seizures is the occurrence of multiple cysts in different stages of evolution at any given time point in some individuals.40 We suggest that in such cases, attempts must be made to correlate the seizure-onset zone inferred from electroclinical assessments and the location/s of the cysticerci and, at the same time, label the underlying etiology as multiple parenchymal cysticercosis–mixed stages. Another problematic situation could be the occurrence of several types of seizures. This could occur either with multiple cysticercosis or when cysticercosis is associated with hippocampal sclerosis.38
We recommend a clinical formulation to classify and label seizures associated with NCC, which departs from the usual categorization of seizures into acute symptomatic and unprovoked to an association with the evolutionary stage of the cyst (Figure 2). This concept avoids the categorization of seizures as acute symptomatic and unprovoked, a distinction that is often blurred in cerebral parenchymal cysticercosis, and guides therapeutic decision making apropos antiparasitic treatment. It is also relevant to identify the epileptogenic zone, epilepsy syndrome, and comorbidities, some of which may be related to NCC. Future studies should document long-term seizure recurrence rates after the first seizure in individuals with cerebral parenchymal cysticercosis along with meticulous identification of epileptogenic substrates. In tandem, experimental animal studies should seek to identify functional and anatomical underpinnings of seizures and epilepsy in the setting of the parasitic infection. These are all relevant agendas as we await a policy-driven public health initiative to eliminate cysticercosis.
Glossary
- ASM
antiseizure medication
- DALY
disability-adjusted life year
- ILAE
International League Against Epilepsy
- NCC
neurocysticercosis
Appendix. Authors
| Name | Location | Contribution |
| Gagandeep Singh, MBBS, MD, DM | Department of Neurology, Dayanand Medical College & Hospital, Ludhiana, India | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design |
| Hector H. Garcia, MD, PhD | Center for Global Health and School of Sciences, Universidad Peruana Cayetano Heredia; Cysticercosis Unit, Instituto Nacional de Ciencaia Neurologicas, Lima, Peru | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design |
| Oscar H. Del Brutto, MD | School of Medicine and Research Center, Universidad Espiritu Santo—Ecuador, Samborondón, Ecuador | Drafting/revision of the manuscript for content, including medical writing for content |
| Christina Coyle, MD | Albert Einstein College of Medicine, Bronx, NY | Study concept or design |
| Josemir W. Sander, FMedSci | Department of Clinical & Experimental Epilepsy, UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy, Chalfont St Peter, United Kingdom | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design |
Study Funding
No targeted funding reported.
Disclosure
G. Singh and J.W. Sander are supported by an Academy of Medical Sciences Networking Grant (GCRFNGR6\1073) for work on neurocysticercosis. G. Singh was on the expert panel for the WHO and the Infectious Diseases Society of America and was on the American Society for Tropical Medicine and Hygiene guidelines panel for Neurocysticercosi. J.W. Sander is based at the NIHR University College London Hospitals Biomedical Research Centre (which is sponsored by the UK Department of Health), receives research support from the UK Epilepsy Society, the Marvin Weil Epilepsy Research Fund, and the Christelijke Vereniging voor de verpleging van Lijders aan Epilepsie, Netherlands, and has received personal compensation for serving on the Advisory Boards or Speaker's Bureau for UCB and Angelini Pharma. J.W. Sander or his department has received grants from Esai, Angelini Pharma and UCB. All other authors have no disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Institute of Health Metrics. Global health metrics. Cysticercosis-level 3 cause. 2023. Accessed December 12, 2023. healthdata.org/results/gbd_summaries/2019/cysticercosis-level-3-cause.
- 2.Carabin H, Ndimubanzi PC, Budke CM, et al. Clinical manifestations associated with neurocysticercosis: a systematic review. PLoS Negl Trop Dis. 2011;5(5):e1152. doi: 10.1371/journal.pntd.0001152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montano SM, Villaran MV, Ylquimiche L, et al. ; Cysticercosis Working Group in Peru. Neurocysticercosis: association between seizures, serology, and brain CT in rural Peru. Neurology. 2005;65(2):229-233. doi: 10.1212/01.wnl.0000168828.83461.09 [DOI] [PubMed] [Google Scholar]
- 4.Owolabi LF, Adamu B, Jibo AM, Owolabi SD, Imam AI, Alhaji ID. Neurocysticercosis in people with epilepsy in Sub-Saharan Africa: a systematic review and meta-analysis of the prevalence and strength of association. Seizure. 2020;76:1-11. doi: 10.1016/j.seizure.2020.01.005 [DOI] [PubMed] [Google Scholar]
- 5.Escobar A, Aruffo C, Cruz-Sánchez F, Cervos-Navarro J. Neuropathologic findings in neurocysticercosis [in Spanish]. Arch Neurobiol (Madr). 1985;48(3):151-156. [PubMed] [Google Scholar]
- 6.Garcia HH, Gonzales I, Lescano AG, et al. ; Cysticercosis Working Group in Peru. Efficacy of combined antiparasitic therapy with praziquantel and albendazole for neurocysticercosis: a double-blind, randomised controlled trial. Lancet Infect Dis. 2014;14(8):687-695. doi: 10.1016/S1473-3099(14)70779-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sotelo J, Guerrero V, Rubio F. Neurocysticercosis: a new classification based on active and inactive forms. A study of 753 cases. Arch Intern Med. 1985;145(3):442-445. doi: 10.1001/archinte.1985.00360030074016 [DOI] [PubMed] [Google Scholar]
- 8.Nash TE, Pretell EJ, Lescano AG, et al. ; Cysticercosis Working Group in Peru. Perilesional brain oedema and seizure activity in patients with calcified neurocysticercosis: a prospective cohort and nested case-control study. Lancet Neurol. 2008;7(12):1099-1105. doi: 10.1016/S1474-4422(08)70243-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleury A, Carrillo-Mezo R, Flisser A, Sciutto E, Corona T. Subarachnoid basal neurocysticercosis: a focus on the most severe form of the disease. Expert Rev Anti Infect Ther. 2011;9(1):123-133. doi: 10.1586/eri.10.150 [DOI] [PubMed] [Google Scholar]
- 10.Dixon HBF, Lipscomb FM. Cysticercosis: An Analysis and Follow-Up of 450 Cases. Medical Research Council. 1962. doi: 10.1590/0004-282X-ANP-2021-0001 [DOI] [Google Scholar]
- 11.Singh G, Sander JW. Historical perspective: the British contribution to the understanding of neurocysticercosis. J Hist Neurosci. 2019;28(3):332-344. doi: 10.1080/0964704X.2018.1564523 [DOI] [PubMed] [Google Scholar]
- 12.Duque KR, Escalaya AL, Zapata W, et al. ; Cysticercosis Working Group in Peru. Clinical topography relationship in patients with parenchymal neurocysticercosis and seizures. Epilepsy Res. 2018;145:145-152. doi: 10.1016/j.eplepsyres.2018.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Del Brutto OH. Cysticercus granuloma. Neurology. 1994;44(4):777. doi: 10.1212/wnl.44.4.777 [DOI] [PubMed] [Google Scholar]
- 14.Singh G, Rajshekhar V, Murthy JM, et al. A diagnostic and therapeutic scheme for a solitary cysticercus granuloma. Neurology. 2010;75(24):2236-2245. doi: 10.1212/WNL.0b013e31820202dc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajshekhar V, Jeyaseelan L. Seizure outcome in patients with a solitary cerebral cysticercus granuloma. Neurology. 2004;62(12):2236-2240. doi: 10.1212/01.wnl.0000130471.19171.d8 [DOI] [PubMed] [Google Scholar]
- 16.de Almeida SM, Torres LF. Neurocysticercosis: retrospective study of autopsy reports, a 17-year experience. J Community Health. 2011;36(5):698-702. doi: 10.1007/s10900-011-9389-z [DOI] [PubMed] [Google Scholar]
- 17.Moyano LM, O'Neal SE, Ayvar V, et al. ; Cysticercosis Working Group in Peru. High prevalence of asymptomatic neurocysticercosis in an endemic rural community in Peru. PLoS Negl Trop Dis. 2016;10(12):e0005130. doi: 10.1371/journal.pntd.0005130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fleury A, Gomez T, Alvarez I, et al. High prevalence of calcified silent neurocysticercosis in a rural village of Mexico. Neuroepidemiology. 2003;22(2):139-145. doi: 10.1159/000068748 [DOI] [PubMed] [Google Scholar]
- 19.Beghi E, Carpio A, Forsgren L, et al. Recommendation for a definition of acute symptomatic seizure. Epilepsia. 2010;51(4):671-675. doi: 10.1111/j.1528-1167.2009.02285.x [DOI] [PubMed] [Google Scholar]
- 20.Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55(4):475-482. doi: 10.1111/epi.12550 [DOI] [PubMed] [Google Scholar]
- 21.Scheffer IE, Berkovic S, Capovilla G, et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):512-521. doi: 10.1111/epi.13709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the International League Against Epilepsy: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):522-530. doi: 10.1111/epi.13670 [DOI] [PubMed] [Google Scholar]
- 23.Sitali MC, Schmidt V, Mwenda R, et al. Experimental animal models and their use in understanding cysticercosis: a systematic review. PLoS One. 2022;17(7):e0271232. doi: 10.1371/journal.pone.0271232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia HH, Verastegui MR, Arroyo G, Bustos JA, Gilman RH; Cysticercosis Working Group in Peru. New animal models of neurocysticercosis can help understand epileptogenesis in neuroinfection. Front Mol Neurosci. 2022;15:1039083. doi: 10.3389/fnmol.2022.1039083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Köhling R Epileptogenesis and brain tumors. In: Panayiotopoulos CP, ed. Atlas of Epilepsies. Springer; 2010:47. doi: 10.1007/978-1-84882-128-6 [DOI] [Google Scholar]
- 26.Radin DP, Tsirka SE. Interactions between tumor cells, neurons, and microglia in the glioma microenvironment. Int J Mol Sci. 2020;21(22):8476. doi: 10.3390/ijms21228476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Del Brutto OH, Engel J Jr, Eliashiv DS, García HH. Update on cysticercosis epileptogenesis: the role of the hippocampus. Curr Neurol Neurosci Rep. 2016;16:1. doi: 10.1007/s11910-015-0601-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta RK, Awasthi R, Rathore RK, et al. Understanding epileptogenesis in calcified neurocysticercosis with perfusion MRI. Neurology. 2012;78(9):618-625. doi: 10.1212/WNL.0b013e318248deae [DOI] [PubMed] [Google Scholar]
- 29.Patil S, Robinson P, Actor JK, Baig S, White AC Jr. Proinflammatory cytokines in granulomas associated with murine cysticercosis are not the cause of seizures. J Parasitol. 2006;92(4):738-741. doi: 10.1645/GE-676R1.1 [DOI] [PubMed] [Google Scholar]
- 30.Robinson P, Garza A, Weinstock J, et al. Substance P causes seizures in neurocysticercosis. PLoS Pathog. 2012;8(2):e1002489. doi: 10.1371/journal.ppat.1002489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mejia Maza A, Carmen-Orozco RP, Carter ES, et al. ; Cysticercosis Working Group in Peru. Axonal swellings and spheroids: a new insight into the pathology of neurocysticercosis. Brain Pathol. 2019;29(3):425-436. doi: 10.1111/bpa.12669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh G, Sachdev MS, Tirath A, Gupta AK, Avasthi G. Focal cortical-subcortical calcifications (FCSCs) and epilepsy in the Indian subcontinent. Epilepsia. 2000;41(6):718-726. doi: 10.1111/j.1528-1157.2000.tb00234.x [DOI] [PubMed] [Google Scholar]
- 33.Garcia HH, Pretell EJ, Gilman RH, et al. ; Cysticercosis Working Group in Peru. A trial of antiparasitic treatment to reduce the rate of seizures due to cerebral cysticercosis. N Engl J Med. 2004;350(3):249-258. doi: 10.1056/NEJMoa031294 [DOI] [PubMed] [Google Scholar]
- 34.Carpio A, Kelvin EA, Bagiella E, et al. ; Ecuadorian Neurocysticercosis Group. Effects of albendazole treatment on neurocysticercosis: a randomised controlled trial. J Neurol Neurosurg Psychiatry. 2008;79(9):1050-1055. doi: 10.1136/jnnp.2008.144899 [DOI] [PubMed] [Google Scholar]
- 35.Singh G, Bawa J, Chinna D, et al. Association between epilepsy and cysticercosis and toxocariasis: a population-based case-control study in a slum in India. Epilepsia. 2012;53(12):2203-2208. doi: 10.1111/epi.12005 [DOI] [PubMed] [Google Scholar]
- 36.Hamamoto Filho PT, Singh G, Winkler AS, Carpio A, Fleury A. Could Differences in infection pressure be involved in cysticercosis heterogeneity? Trends Parasitol. 2020;36(10):826-834. doi: 10.1016/j.pt.2020.07.003 [DOI] [PubMed] [Google Scholar]
- 37.White AC Jr, Coyle CM, Rajshekhar V, et al. Diagnosis and treatment of neurocysticercosis: 2017 clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2018;66(8):e49-e75. doi: 10.1093/cid/cix1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh G, Sander JW. Neurocysticercosis as a probable risk factor for hippocampal sclerosis. Arq Neuropsiquiatr. 2018;76(11):783-790. doi: 10.1590/0004-282X20180130 [DOI] [PubMed] [Google Scholar]
- 39.Sánchez SS, Bustos JA, Del Brutto OH, et al. ; Cysticercosis Working Group in Peru. Hippocampal atrophy/sclerosis is associated with old, calcified parenchymal brain neurocysticercosis, but not with more recent, viable infections. Am J Trop Med Hyg. 2021;106(1):215-218. doi: 10.4269/ajtmh.21-0392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh G, Burneo JG, Sander JW. From seizures to epilepsy and its substrates: neurocysticercosis. Epilepsia. 2013;54(5):783-792. doi: 10.1111/epi.12159 [DOI] [PubMed] [Google Scholar]