Abstract
Vaccinia virus is being investigated as a replicating vector for tumor-directed gene therapy. However, the majority of cancer patients have preformed immunologic reactivity against vaccinia virus, as a result of smallpox vaccination, which may limit its use as a vector. The Yaba-like disease (YLD) virus was investigated here as an alternative, replicating poxvirus for cancer gene therapy. We have demonstrated that the YLD virus does not cross-react with vaccinia virus antibodies, and it replicates efficiently in human tumor cells. YLD virus can be expanded and purified to high titer in CV-1 cells under conditions utilized for vaccinia virus. The YLD virus RNA polymerase was able to express genes regulated by a synthetic promoter designed for use in orthopoxviruses. We sequenced the YLD virus TK gene and created a shuttle plasmid, which allowed the recombination of the green fluorescent protein (GFP) gene into the YLD virus. In a murine model of ovarian cancer, up to 38% of cells in the tumor expressed the GFP transgene 12 days after intraperitoneal virus delivery. YLD virus has favorable characteristics as a vector for cancer gene therapy, and this potential should be explored further.
Poxviruses have unique characteristics which make them appealing as vectors for cancer gene therapy (4, 25). They have been investigated as vectors for delivery of tumor-associated antigens, cytokines, and costimulatory molecules in cancer patients, for the development of an antitumor immune response (5, 17, 22, 32). Recently, laboratory experiments have supported the utility of vaccinia virus (VV) as a vector for tumor-directed delivery of genes for enzyme-prodrug therapy and sensitization to systemic treatment with tumor necrosis factor (13, 15, 30). A replicating virus has distinct advantages over nonreplicating vectors for these tumor-directed applications, as it leads to an increase in the percentage of cells within a tumor that express the therapeutic gene over time (23, 35). VV is an efficient, replicating virus that leads to high levels of transgene expression, selectively in tumor tissue when delivered systemically, and this can lead to a significant antitumor response. Selective mutations of the virus may enhance tumor specificity (29) (J. A. McCart, Y. K. Hu, H. R. Alexander, S. K. Libutti, B. Moss, D. L. Bartlett, Abstr. Am. Soc. Gene Ther., abstr. 633, 1999).
Clinical trials with intravascular delivery of mutant VV will likely be hampered by the high percentage of cancer patients with preformed immunity against the virus as a result of vaccination against smallpox. High levels of circulating antibody titers and cytotoxic T cells recognizing VV can be detected many years after vaccination, and it is likely that this preformed immune reactivity will prevent adequate infection and spread of VV throughout a tumor when used as a vector for tumor-directed gene therapy. An alternative replicating poxvirus vector may mediate the selective, high transgene expression within tumors, without immune cross-reactivity. In general, the host range for poxviruses that do not cross-react with orthopoxviruses is quite limited, and although members of the avipoxvirus genus and entomopoxvirus subfamily will infect and express genes in human cells, they will not replicate in human cells (21, 34). Members of the yatapoxvirus genus, on the other hand, have been responsible for zoonotic infections, forming cutaneous nodules in caretakers handling infected monkeys, and replicating virus has been recovered from these lesions (16). These viruses have not been previously explored as expression vectors, nor has their host range been adequately defined.
In this study we explore the Yaba-like disease (YLD) virus as an expression vector. This virus was first recognized in monkey caretakers in 1965 and 1966, in primate centers in the United States, and was traced to a single source (12). YLD infection in caretakers produced a brief fever and the development of a few firm, elevated, round, necrotic maculopapular nodules, followed by complete resolution of the infection. Compared to Tanapox virus and Yaba monkey tumor virus, YLD virus is the least characterized of the yatapoxvirus genus. We demonstrate here that the YLD virus does not cross-react with VV antibodies. It replicates efficiently in human cells and can be grown under normal conditions in CV-1 cells and purified in high titer. We demonstrate that the YLD virus RNA polymerase can express genes regulated by a synthetic promoter designed for use in orthopoxviruses and that a recombinant virus can be made by homologous recombination into the YLD virus thymidine kinase (TK) gene. Finally, we compare the in vitro gene transfer efficiency of YLD virus and VV and explore the in vivo efficiency of gene delivery in a murine model of ovarian cancer.
MATERIALS AND METHODS
Cell lines.
CV-1 (monkey kidney; ATCC CCL 70), RK-13 (rabbit kidney; ATCC CCL 37), CHO (Chinese hamster ovary; ATCC CCL 61), WIDR (human colon cancer; ATCC CCL 218), HT-29 (human colon cancer; ATCC HTB 38), 205 (human colon cancer; ATCC CCL 222), A2780 (human ovarian cancer; used extensively in our branch) and HeLa (human cervical cancer; ATCC CCL 2.2) were all obtained from the American Type Culture Collection. Cell lines 1299 (human non-small cell lung cancer; NCI-H1299) and 460 (human non-small cell lung cancer; NCI-460) were obtained from the NCI tissue bank. MC-38 (murine colon cancer cell line) was originally chemically induced in C57BL/6 mice and has been used extensively in our branch. All cells were maintained in Dulbecco's modified Eagle medium (DMEM) with 10% fetal calf serum (FCS), 200 mM glutamine, 10 kU of penicillin-streptomycin per ml, and 250 μg of Fungizone per ml (all from Biofluids, Rockville, Md.), at 37°C and 5% CO2.
Viruses.
YLD virus was originally obtained as Tanapox virus from the American Type Culture Collection (ATCC VR-937) and further expanded and purified using a common protocol for VV purification (11). However, genomic DNA digestion results (data not shown) confirmed that the virus obtained was YLD virus as previously characterized by Knight et al. (19) instead of Tanapoxvirus. All viruses were stored in 10 mM Tris-HCl (pH 9.0; Quality Biological Inc., Gaithersburg, MD) at −80°C until use.
VV expressing the green fluorescent protein (GFP) was constructed by homologous recombination of the enhanced GFP gene into the TK locus of VJS6, a WR strain VV expressing β-galactosidase (β-Gal) (2). In brief, the enhanced GFP gene (Clontech, Palo Alto, Calif.) was digested with SalI and SpeI and ligated into our shuttle plasmid pCB023-II (30), creating pSEL-EGFP. This shuttle has the GFP gene under the control of the VV synthetic early-late promoter and the guanine phosphoribosyl transferase (gpt) gene under control of the P7.5 promoter (6, 7). The entire cassette is flanked by portions of the VV TK gene, which allows homologous recombination into this locus. Confluent wells of CV-1 cells were infected for 2 h at 37°C with 1.4 × 105 PFU of VJS6 in 1.0 ml of DMEM–2.5% FCS (DMEM-2.5) per well. Supernatants were removed, and a liposomal transfection (SuperFect; Qiagen, Santa Clarita, Calif.) of pSEL-EGFP was performed using 0.7 ml of DMEM–10% FCS (DMEM-10) containing 2 μg of plasmid DNA and 10 μl of liposomes per well at 37°C for 4 h. The transfection medium was removed and replaced with 3.0 ml of DMEM-10 containing mycophenolic acid (25 μg/ml), xanthine (250 μg/ml), and hypoxanthine (15 μg/ml) (gpt selection media, all from Sigma Chemical Co., St. Louis, Mo.). After 3 days of incubation, cells were collected and sonicated in DMEM-2.5. Aliquots (1.0 ml) of serial dilutions (10−2 to 10−4 in gpt selection medium) were used to infect CV-1 cells at 37°C. After 24 to 48 h, cells were observed for green fluorescence and viral plaque formation. Positive plaques were isolated, resuspended in selection medium, and used to reinfect CV-1 cells for plaque purification. After three cycles of plaque purification all plaques were positive for green fluorescence and the virus was expanded in HeLa cells and purified as previously described (11).
ELISA immune cross-reactivity test.
Aliquots (105 PFU) of either VV or YLD virus diluted in Dulbecco's phosphate-buffered saline (Biofluids) were plated and dried overnight in a 96-well enzyme-linked immunosorbent assay (ELISA) plate (Corning Inc. Corning, N.Y.). The wells were blocked with 5% bovine serum albumin (Calbiochem, La Jolla, Calif.) for an hour, followed by incubation with a 1:300 dilution of serum from patients with or without previous smallpox vaccination (kindly provided by S. A. Rosenberg) or serum from mice infected with VV or YLD virus. A 50-μl aliquot of a 1:1,000 to 1:3,000 dilution of anti-human immunoglobulin G (IgG) horseradish peroxidase-conjugated secondary antibody (Amersham Pharmacia Biotech, Piscataway, N.J.) was then applied for 1 h. All incubations were at 37°C and 5% CO2. The plates were washed with washing buffer (Endogen, Woburn, Mass.) in between incubations, and TMB substrate and Stop Solution (Endogen) were added at the end of incubation. Absorbances at 450 nm were determined using a Titertek Multiskan MCC/340 plate reader (Thermo Labsystems, Inc., Beverly, Mass.).
Viral growth curve.
Confluent plates of MC38, CV-1, 1299, RK-13, HT29, HeLa, and CHO cells were infected with YLD virus at a multiplicity of infection (MOI) of 5 and 0.1 for DNA replication analysis and viral expansion assay, respectively. The cells were incubated with the virus in DMEM-2.5 for an hour, prior to removal of the viral suspension and replacement with fresh medium. Cell lysates and supernatants were collected at various time points between 0 and 120 h. The levels of mature YLD virus produced were determined by viral plaque assays on CV-1 cells (11).
DNA replication in various cell lines was examined by DNA dot blot analysis of the cell lysates as previously described (24). cDNA of YLD virus TK gene was randomly labeled with 32P (Loftstrand Laboratories, Rockville, Md.) and used as probe for YLD virus DNA. β-Actin probes from corresponding species were used as a control for cellular DNA.
Assessment of YLD virus polymerase activity.
Cells (80% confluent) were transiently transfected with the expression plasmid pSC65 (4a) carrying the Escherichia coli lacZ gene driven by the VV P7.5 promoter sequence using SuperFect Transfection Reagent (Qiagen). After 3 h, the transfected cells were washed once with 1 ml of phosphate-buffered saline (PBS) and then immediately infected with wild-type YLD virus at an MOI of 1.0 in 500 μl of DMEM-2.5. After 1 h, 2 ml of DMEM-10 was added directly to the cells. β-Gal expression was then qualitatively assessed using a β-Gal staining kit (Roche Diagnostics Corporation, Indianapolis, Ind.) at 48, 72, and 96 h after viral infection.
Molecular analysis of YLD virus TK gene.
YLD virus DNA was obtained from purified YLD virus using a previously described protocol for VV DNA (11). Primers were designed based on homologous regions between the TK genes of VV and Yaba virus. The sequences of the primers were based on the Yaba virus TK cDNA (sense, 5′-GGTCCAATGTTTT CTGGAAAAAGTACAG-3′; antisense, 5′-TCTACATACAG ATTGATA-3′). High-fidelity Supermix (Gibco BRL, Life Technologies, Inc., Rockville, Md.) was used to amplify the DNA. The PCR product was subcloned into pCR-Topo II vector and transformed into One Shot competent bacterial cells (both from Invitrogen, Carlsbad, Calif.). Eight individual colonies were randomly selected and sequenced with M13 forward and reverse primers.
The 5′ and 3′ termini of the TK cDNA were defined by “anchored” PCR using commercial kits for 5′ and 3′ rapid amplification of cDNA ends(RACE) (Gibco BRL), in combination with custom primers designed based on the previously determined TK sequence: 5′-CCATCGTTTGCCATACGTTCGC-3′ for 5′ RACE and 5′-GCGAACGTATGGCAAACGATGG-3′ for 3′ RACE. CV-1 cells were infected with 2 × 105 PFU of purified YLD virus for 5 days. Total RNA was purified using an RNeasy mini kit (Qiagen). The end products of the 5′ and 3′ RACE were subcloned into the pCR-Topo II vector and were sequenced with M13 forward and reverse primers. Six individual colonies were randomly selected and sequenced for each terminus. Sequencing was performed in an automated sequencer (ABI Prism 310 Genetic Analyzer; Perkin-Elmer Co., Norwalk, Conn.).
Generation of recombinant YLD virus.
The YLD virus shuttle plasmid (pYLD-GFP) was made by inserting the initial PCR product, containing bases 37 to 519 of YLD virus TK DNA, into the PUC 19 vector (Worthington Biochemical Co., Lakewood, N.J.). The VV expression cassette, containing enhanced GFP protein under the control of the VV synthetic early-late promoter and GPT under the control of the P7.5 promoter, was excised from pSEL-EGFP using BssHII, Klenow filled, and ligated into the HindIII- and DraIII-digested YLD virus TK gene within the PUC-YLD TK shuttle.
For recombination, confluent CV-1 cells in six-well plates were infected with 4 × 105 PFU of purified YLD virus for 2 h, followed by a 5-h transfection of pYLD-GFP using the SuperFect Reagent and protocol (Qiagen). The cells were washed once with Hanks' balanced salt solution (HBSS) (Biofluids), and incubated in gpt selection medium for 8 days. The cell lysates were harvested, and diluted lysates (1:5) were used for reinfection of overnight serum-starved CV-1 cells in gpt selection medium. Two more rounds of selection were performed, at which time all plaques produced green fluorescence. The YLD-GFP virus was expanded in CV-1 cells and purified on a sucrose gradient according to a standard VV purification protocol (11).
In vitro gene transfer efficiency.
Cells (80% confluent) were infected with YLD-GFP or VV-GFP virus at an MOI of 1.0. After 48 h cells were harvested and counted, and 106 cells were analyzed by fluorescence-activated cell sorter (FACS) analysis for GFP fluorescence.
In vivo gene transfer efficiency.
A total of 106 A2780 cells in 100 μl of PBS/animal was injected intraperitoneally into 6-week-old female athymic nude mice (NIH small animal facility, Frederick, Md.). Tumors were allowed to grow for 2 or 4 weeks prior to virus administration. A total of 108 PFU of YLD-GFP in 1 ml of PBS was then administered by intraperitoneal injection. Mice were sacrificed on days 4 through 16 postinfection. Tumors were harvested from the peritoneal surface and crushed in HBSS using the blunt end of a 1-ml syringe. Half of the fragments were placed in a triple-enzyme solution of 0.1% type IV collagenase, 0.01% type V hyaluronidase, and type IV DNase (30 U/ml) (all from Sigma Chemical Co.) in HBSS and allowed to digest for 2 h at room temperature while continuously stirring with a magnetic stir bar. The solution was then filtered through 112u Nitex nylon mesh (Tetko, Inc., Briarcliff Manor, N.Y.). A total of 106 cells were counted and preserved in 2% methanol-free formaldehyde for FACS analysis. The other half of the tumors were homogenized in lysis buffer and then spun to remove cellular debris. The virus titer in the supernatant was determined by plaque assay in CV-1 cells as described above. All animal studies were approved by the Animal Care and Use Subcommittee of the Animal Sciences Branch, National Cancer Institute.
RESULTS
YLD virus growth and characterization.
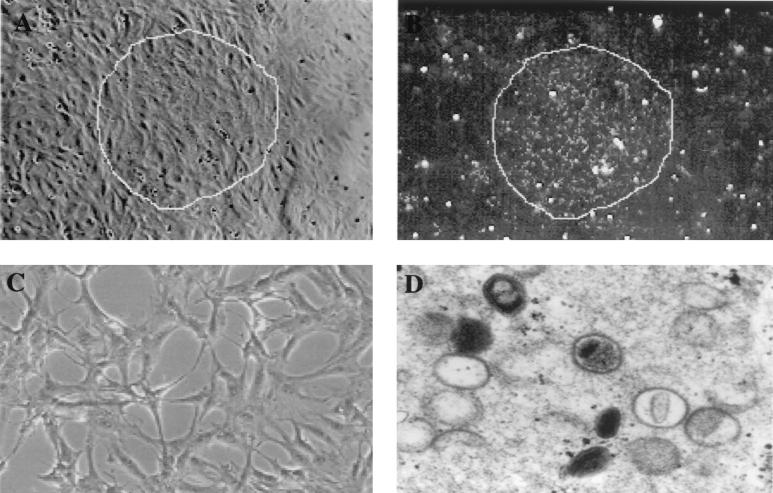
The YLD virus was expanded in CV-1 cells under standard conditions used for VV (37°C, 5%CO2). Fifty confluent T-150 plates yielded a total of 8 × 109 PFU of purified YLD virus as determined on CV-1 cells. The plaques were best visualized by dark-field microscopy, where YLD virus-infected cells had a granular cytoplasm (Fig. 1A and B). Viral cytopathic effect was characterized by elongation and thinning of the infected cells (Fig. 1C). Electron microscopy of YLD virus-infected CV-1 cells demonstrated ovoid, enveloped viral particles in the cytoplasm (Fig. 1D).
FIG. 1.
Characterization of the YLD virus in tissue culture. (A) YLD virus plaque on CV-1 cells 3 days after infection. The majority of cells in the field are infected with the YLD virus; however, no significant cytopathic effect can be demonstrated. (B) Dark-field microscopy of the plaque shown in panel A under the same magnification. The granular nature of the infected cytoplasm is readily apparent, making the identification of plaques much easier. (C) Higher magnification of a single plaque on CV-1 cells 6 days after infection with YLD virus at an MOI of 0.001. The characteristic elongation of the cells represents YLD virus cytopathic effect. (D) Electron microscopic photo of intracellular YLD virus particles in various stages of maturation. The particles appear more ovoid than rectangular as is seen with VV.
VV cross-reactivity.
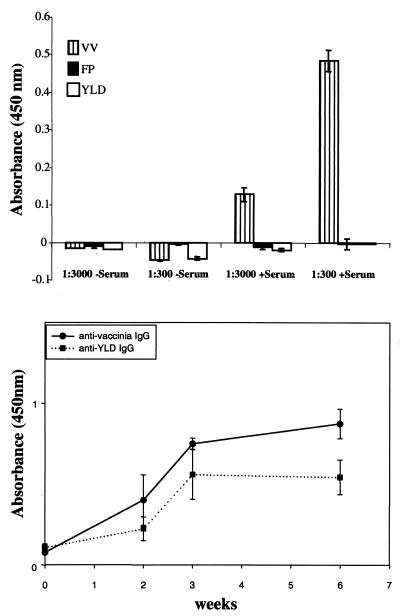
In order to determine YLD virus cross-reactivity with VV, serum from four patients who were recently (<6 months) vaccinated with VV (Wyeth strain) were tested by an ELISA against both YLD virus- and VV-coated plates. Sera from all four patients demonstrated strong reactivity against VV (>1:3,000 dilution) but no reactivity against YLD virus (<1:300 dilution) (Fig. 2A). Serum from mice vaccinated with VV did not react with YLD virus-coated ELISA plates, and mice vaccinated with YLD virus did not react with VV-coated ELISA plates. The time course of antiviral IgG development in mice vaccinated with VV or YLD virus is demonstrated in Fig. 2B. The peak IgG level was measured at about 3 weeks postvaccination, and the level remained high for more than 6 weeks.
FIG. 2.
Antibody cross-reactivity between YLD virus and VV. The top bar graph demonstrates the presence of anti-VV antibodies in serum from a patient recently immunized against VV (+Serum). The serum reacts against VV-coated ELISA plates at a dilution of 1:3,000, whereas no reaction is seen against fowlpox (FP)- or YLD virus-coated ELISA plates. The negative serum (-Serum) represents the serum of a patient not previously immunized with VV. Three other patients who were immunized with VV showed similar results, with reactivity only against VV-coated ELISA plates but not YLD virus-coated plates. The bottom graph demonstrates the development of IgG against VV and YLD virus after immunizing mice with VV or YLD virus (n = 5/group). The antibody titers increased significantly over the first 3 weeks and then leveled off. There was no demonstrable cross-reactivity against VV or YLD virus in these animals.
YLD virus transcription using a VV promoter.
The ability of YLD virus RNA polymerase to transcribe genes utilizing VV promoter was tested by transfection of CV-1 cells with a VV shuttle plasmid (pSC65) which expressed β-Gal regulated by the VV P7.5 promoter followed by infection with YLD virus. After 24 h, X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) staining was performed. Only cells that underwent both YLD virus infection and pSC65 transfection expressed β-Gal (Fig. 3), demonstrating that YLD virus polymerase can utilize a VV promoter.
FIG. 3.
YLD virus RNA polymerase activity using a VV promoter. CV-1 cells were transfected with PSC65 expression plasmid (with the β-Gal gene regulated by the VV synthetic P7.5 promoter), followed by infection with the YLD virus. (A) Cells transfected with PSC65 clone; (B) cells infected with YLD virus alone; (C) cells both transfected and infected. Blue staining is evident after X-Gal staining only in the cells that are both infected and transfected, demonstrating the function of this promoter in YLD virus-infected cells.
Host range studies.
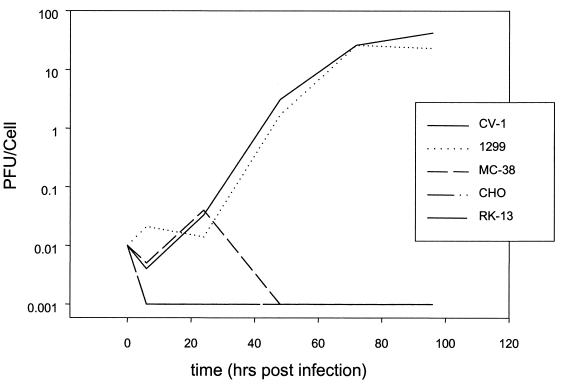
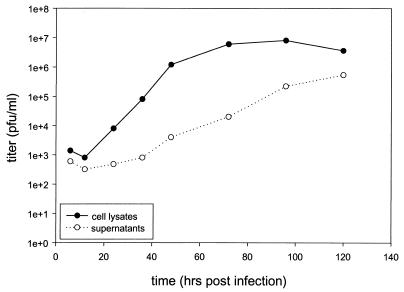
The host range of YLD virus was studied by infecting cell lines from various species with wild-type YLD virus at an MOI of 0.1 and measuring viral expansion over 96 h (Fig. 4). A 3-log-fold expansion was seen at 48 h in CV-1 cells (monkey kidney) and 1299 cells (human non-small cell lung carcinoma). YLD virus did not propagate in RK-13 (rabbit kidney), CHO (Chinese hamster ovary), or MC-38 (murine colon carcinoma) cells. A comparison of cell lysates versus supernatants demonstrated that the majority of infectious virus was cell associated, as is seen with VV (Fig. 5). At 130 h, the extracellular virus titers increase, consistent with cell death and virion release.
FIG. 4.
Host range of YLD. YLD virus was used to infect various cell lines at an MOI of 0.1. Virus was collected from cell lysates at various time points, and titers were determined on CV-1 cells. YLD virus demonstrated replication only in CV-1 and 1299.
FIG. 5.
Viral recovery from cell lysates and supernatants of CV-1 infected cells over time. CV-1 cells were infected at an MOI of 0.01 with YLD virus, and the cells and supernatants were collected for determination of virus titer on CV-1 cells at various time points. The majority of virus appears to be cell associated; however, a significant number of YLD virus particles are recoverable from the supernatant 120 h after infection, suggesting cell death and virion release.
DNA dot blot analyses were performed in the same cell lines after infection at an MOI of 5, and the results demonstrated that no DNA synthesis occurred in RK-13, CHO, or MC-38 cells (Fig. 6). As expected, DNA synthesis occurred in both CV-1 and 1299 cells.
FIG. 6.
Dot blot assay for YLD virus DNA in infected cell lines over time. CHO, RK13: MC38, 1299, and CV-1 cells were infected at an MOI of 5 with YLD virus, and cell lysates were probed with 32P-labeled TK cDNA. Only 1299 and CV-1 cells demonstrate DNA amplification consistent with viral replication. More-efficient DNA synthesis occurs in CV-1 cells than in 1299 cells.
YLD virus TK sequence and recombinant virus production.
Based on conserved, published sequences in the TK gene of VV and Yaba virus, four primers were synthesized that would produce PCR products encompassing approximately 80% of the expected gene sequence. Pairing of the four primers yielded two PCR products of the expected length, and these were cloned into a sequencing vector. Sequencing of multiple clones yielded identical results. 5′ and 3′ RACE was performed to complete the sequencing (using cDNA from YLD virus-infected CV-1 cells). The complete sequence was confirmed from purified YLD virus DNA. There is approximately 69% identity with the VV TK amino acid sequence, 81% identity with Yaba monkey tumor virus (YMTV), and 66% identity with myxomavirus TK amino acid sequence. The entire genome sequence of YLD virus was recently published and is identical to our sequence (available from GenBank) (20). A YLD virus shuttle plasmid was constructed as described in Materials and Methods, and a recombinant YLD virus with the GFP gene inserted into its TK locus was generated without difficulty using positive selection for the expression of gpt. This virus was expanded in CV-1 cells to a titer of 1010 PFU/ml.
In vitro comparison of YLD virus and VV in tumor cells.
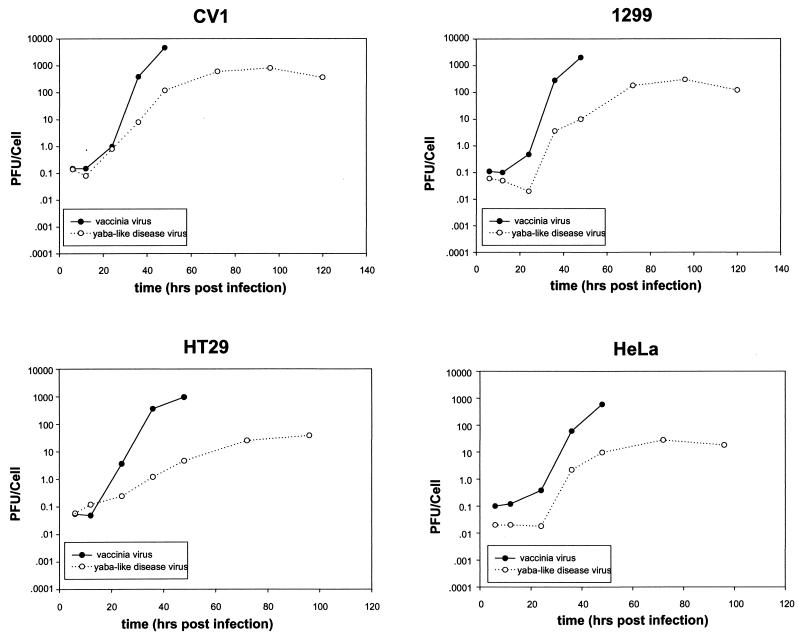
The expansion of YLD virus and VV on various cell lines was tested in parallel as a comparison of replication efficiency. This included CV-1 cells and three human tumor cell lines: 1299 (lung), HT29 (colon), and HeLa (cervical). Cells were infected at an MOI of 0.1, and samples were collected at different times for plaque assay (Fig. 7). VV expansion peaked at 48 h in all cell lines, and the yield was 1 to 2 logs higher than YLD virus, which peaked at 72 to 96 h. The YLD virus expanded 2- to 3-log-fold over 96 h in the human tumor cell lines.
FIG. 7.
Growth curves of YLD virus and VV in various cell lines. Various human tumor cell lines and CV-1 cells were infected with YLD virus or VV at an MOI of 0.1, and samples were taken at various time points for plaque assay on CV-1 cells. The VV replicated more rapidly than the YLD virus in all cell lines tested.
Gene transfer efficiency was compared by infecting cell lines at an MOI of 1.0 with VV-GFP or YLD-GFP, followed by FACS analysis for GFP expression after 48 h (Table 1). As expected, 97 to 100% of VV-infected cells expressed GFP in all cell lines tested. While 97 to 100% of 1299 and HT29 cells infected with YLD virus expressed GFP, only 49% of 460 cells and 11% of WIDR cells expressed GFP. This variability may be related to differences in viral uptake, transcription, or replication efficiency in the different human tumor lines.
TABLE 1.
Percentage of human tumor cells expressing GFP after infection with VV or YLD in vitro
| Cell line | % of tumor cells expressing GFP after infection witha:
|
|
|---|---|---|
| VV | YLD | |
| 1299 | 99.7 | 99.6 |
| HT29 | 99.2 | 99.4 |
| 460 | 97.6 | 48.9 |
| WIDR | 99.4 | 10.8 |
The different cell lines were infected at an MOI of 1, and 48 h later cells were harvested for FACS analysis.
In vivo gene delivery by YLD virus.
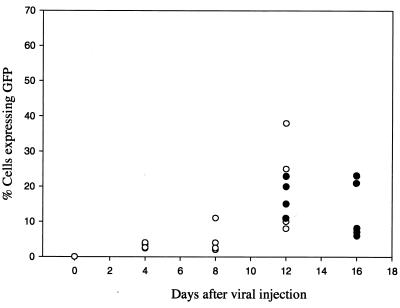
In vivo gene transfer efficiency was tested in a murine xenograft model of ovarian cancer, A2780. (In vitro, 48 h after infection with YLD-GFP at an MOI of 1.0, 58% of A2780 cells expressed GFP.) In vivo gene transfer efficiency was studied after intraperitoneal injection of 108 PFU of YLD-GFP in A2780 peritoneal tumor-bearing nude mice. In the first experiment, the A2780 tumor was allowed to grow intraperitoneally for 4 weeks. At this time the tumor nodules were 5 to 7 mm in size. The virus was injected, and animals were sacrificed on days 4, 8, and 12, after viral injection. The tumor was harvested and tested for GFP expression by FACS. The percentage of cells expressing GFP increased over time, reaching a peak at 12 days after injection (Fig. 8). The mean percentage of tumor cells expressing GFP at 12 days was 22%, ranging from 8 to 38%. The second in vivo experiment was performed after allowing the A2780 tumor to grow only 2 weeks. At this stage the tumor nodules are 1 to 2 mm in diameter. The tumors were collected and tested for GFP expression by FACS on days 12 and 16 after viral injection. On day 12 the mean percentage of GFP expression was 16.5%, ranging from 10 to 23%. At 16 days the mean percentage of cells expressing GFP within the tumor was 13%, ranging from 6 to 23%. In addition to the FACS analysis, tumors were harvested for YLD virus recovery assays on CV1 cells. All tumors on days 12 and 16 had recoverable YLD virus. No virus could be recovered from other organs, including liver, spleen, lung, and brain. The mean recoverable virus from tumor 12 days after YLD virus infection was 4.5 × 103 PFU/mg of protein. The mean virus recovery from tumor 16 days after YLD virus infection was 8.1 × 103 PFU/mg of protein.
FIG. 8.
In vivo tumor GFP expression after infection with YLD virus. Nude mice with established A2780 human ovarian peritoneal carcinomatosis were infected with YLD virus at 108 PFU, injected intraperitoneally. Tumor was sampled every 4 days, and FACS analysis was performed for GFP expression. The graph represents the percentage of cells expressing GFP on the various days after virus delivery. Two experiments were performed. One experiment was performed with a 4-week tumor burden (5- to 7-mm tumor nodules), shown as open circles. The second experiment was performed with 2-week tumors (1- to 2-mm nodules), shown as filled circles. Each circle represents a single animal. There are at least four animals per group. On some days the circles overlap.
DISCUSSION
One primary obstacle to tumor-directed gene therapy is the inefficient transduction of tumor cells in vivo for most vectors. Tumor-specific replicating viruses have gained interest recently as more effective vectors for cancer gene therapy (18, 33). Tumor-selective, replicating adenoviruses and herpesviruses are currently in clinical trials. Spread of the virus throughout the tumor maximizes the percent of cells within a tumor expressing the gene of interest and should improve the antitumor response. This may be significant for delivery of genes expressing cytokines, tumor antigens, or enzymes for enzyme-prodrug therapy. One limitation of the system is the inefficiency of the vector, and any impedance to the spread of the virus will diminish the antitumor response. Initial work with VV as a replicating, tumor-directed virus has been promising. The cytopathic effect of the virus as well as an enzyme-prodrug therapeutic gene can mediate an effective antitumor response (14, 23).
While VV is a promising vector in laboratory studies, its clinical utility may be limited by the fact that the majority of patients more than 35 years old have preformed immune reactivity against the virus as a result of smallpox vaccination. Systemic delivery of VV would be limited by neutralizing preformed antibodies. Such a limitation has been encountered in clinical trials with adenovirus-expressing tumor antigens for the treatment of melanoma (31). While it is impossible to know the exact significance of remote prior vaccination, it is likely that VV will be less efficient in a preimmunized host. We have examined circulating anti-VV IgG levels in people recently vaccinated, recently having received boosters, and remotely vaccinated with VV. We found that high levels of circulating antibodies exist in people vaccinated more than 40 years ago, and that no correlation exists between time from vaccination and antibody levels (unpublished data). Demkowicz et al. demonstrated that specific VV T-cell immunity can persist for up to 50 years after immunization against smallpox in the absence of restimulation (8). Our own observation of laboratory workers and experimentally vaccinated patients receiving VV dermal scarification after prior childhood vaccination has been that limited viral replication occurs, resulting in minimal pox lesions—much less than that seen after primary vaccination. The immune response against VV will also prevent retreatment of patients with this vector (which would improve the chance of response). An alternative poxvirus without immune cross-reactivity with VV may therefore have clinical utility.
The majority of poxviruses that are known not to cross-react with VV (e.g., avipoxvirus genus) do not replicate in human cells (27). While these vectors still express early genes in human cells and are theoretically safer than VV, the lack of replication may limit their effectiveness as a tumor-directed expression vector. Previously, we demonstrated a 4-log-higher in vivo tumor luciferase activity with a replication-competent VV expressing luciferase compared to a psoralen-UV-inactivated VV (29). This increased expression and increased percentage of tumor cells expressing the gene may be important for many forms of tumor-directed gene therapy.
We searched for a poxvirus that was known to replicate in humans that might not cross-react with VV. Of the various genera of poxviruses, perhaps the least well characterized is the Yatapoxvirus genus (26). The Yatapoxvirus genus consists of the YMTV, Tanapoxvirus, and the YLD virus. YMTV was first isolated in 1958 from Rhesus monkeys in Yaba, near Lagos, Nigeria. It was found to induce transmissible benign tumors in Asiatic monkeys and humans and was difficult to grow in cultured cells (1, 28). Tanapox virus was isolated from human skin biopsy specimens during an outbreak of an illness in 1957 and 1962 among natives living along the Tana River Valley in Kenya (10). The clinical manifestations included fever, headache, backache, and prostration. A pock lesion (generally solitary) appeared during the fever, from which Tanapox virus could be isolated. Yaba-like disease virus is closely related to Tanapox virus, but was isolated from epizootic infection of monkey caretakers in California, Oregon, and Texas and was traced to a single primate-importing company (12). YLD was almost identical to Tanapox disease, and the viruses were originally felt to be the same. Knight et al. differentiated Tanapox virus and YLD virus by restrictive mapping (19). All three viruses were cultivated by serial passage at the Center for Disease Control. The serology of these viruses has not been well described. It has previously been shown that anti-VV antisera did not neutralize Tanapox infection in vitro (10). Tanapox virus and YMTV are known to exhibit moderate cross-reactivity. YMTV infection fully protected primates against Tanapox infection (9). YLD virus is felt to be antigenically very similar to Tanapox virus.
We chose to characterize the YLD virus. This virus grows in vitro under conditions similar to VV in monkey and human cells. Visible plaques on CV-1 cells are formed in approximately 7 to 8 days, while for VV this usually takes about 24 to 48 h. We were able to achieve high titers of virus (8 × 109 PFU from 50 flasks) from expansion in CV-1 cells, which is important for consideration of its clinical utility. We demonstrated that the YLD virus RNA polymerase recognizes natural and synthetic promoters designed for use in VV. It is known that these promoters also can be used by other genera of poxviruses, but this had not been demonstrated previously with the Yatapoxvirus genus. We also demonstrated that the YLD virus was not recognized by antiserum from patients recently immunized with VV. In general, different genera of poxviruses do not have antibody cross-reactivity.
The host range of YLD virus was more restrictive than that known for VV. We demonstrated viral replication in monkey and human cell lines, but not in mouse, rabbit, or hamster cells. DNA replication did not occur in cells that were not permissive, but gene expression was demonstrated in those cells (data not shown), indicating that the defect occurs at a postentry step. This suggests that the natural host for this poxvirus is not a rodent as is expected for most orthopoxviruses.
The TK gene has been commonly used as a site for recombination of foreign genes into VV. Insertional deletion of TK attenuates the virus in vivo (3), but replication in transformed cells in vitro and tumor cells in vivo is preserved (30). We demonstrated 69% amino acid identity between VV and YLD virus TK and 81% amino acid identity between YMTV and YLD virus. Recombination into the YLD virus using positive selection with the gpt gene and selection medium was accomplished without difficulty.
YLD virus demonstrated a 3-log expansion over 96 h in human tumor cell lines, and two human tumor lines demonstrated close to 100% gene expression 48 h after infection with YLD virus at an MOI of 1.0. Some variability exists in gene expression in human tumor lines, and the mechanism for this needs to be investigated. While not as efficient as VV, YLD virus compares favorably to other replicating vectors such as adenovirus and herpesvirus currently in clinical trials. As well, the in vivo efficiency of tumor gene delivery by YLD virus in mice with a human ovarian tumor model (A2780) is encouraging, with up to 38% of tumor cells expressing GFP. Unlike with VV, it is not possible to study in vivo tumor selective targeting with YLD virus because of the host range restriction. We believe that the demonstration of increasing percentage of tumor cells expressing GFP over time is the result of YLD virus replication and spread throughout the tumor. As YLD virus does not replicate in murine cells, tumor specificity cannot be studied in this model. This is a limitation for most conditionally replicating viruses for tumor-directed gene delivery (except VV). It should be noted that we have previously compared a psoralen-UV-inactivated VV to a replicating VV in a murine model, and the inactivated virus had no evidence of gene expression by 2 days after delivery (29). We believe efficient viral replication within the tumor is required for measurable gene expression. Other models and other modes of delivery for YLD virus need to be examined.
In summary, we have demonstrated favorable characteristics of the YLD virus as a vector for cancer gene therapy. It replicates in human tumor cell lines, transgenes can be regulated by strong synthetic VV promoters, and it can be produced in high titer. In vivo tumor gene delivery efficacy is encouraging in a model of ovarian carcinomatosis. The potential uses for this vector include expression of cytokines, tumor antigens, or suicide genes. We have previously demonstrated that VV has replication selectivity for tumors with deletion of the VV TK gene and VV growth factor genes (McCart et al, Abstr. Am. Soc. Gene Ther.). A similar secreted epidermal growth factor homolog exists in YLD virus (open reading frame 15L) (20), potentially allowing further manipulations for tumor specificity. YLD virus has the advantage over other viral vectors of not being a virus endemic to the general population, and therefore no preexisting immunity exists.
ACKNOWLEDGMENTS
The first two authors contributed equally to this work.
We thank K. Irvine for advice on the ELISA cross-reactivity assay, A. Chen and D. Schrump for assistance with sequencing, M. Tsokos and M. Abu-Asab for electron microscopy, and R. Sawhney for help with preparation of the manuscript.
REFERENCES
- 1.Bearcroft W G C, Jamieson M F. An outbreak of subcutaneous tumors in rhesus monkeys. Nature. 1958;182:195–196. doi: 10.1038/182195a0. [DOI] [PubMed] [Google Scholar]
- 2.Bronte V, Carroll M W, Goletz T J, Wang M, Overwijk W W, Marincola F, Rosenberg S A, Moss B, Restifo N P. Antigen expression by dendritic cells correlates with the therapeutic effectiveness of a model recombinant poxvirus tumor vaccine. Proc Natl Acad Sci USA. 1997;94:3183–3188. doi: 10.1073/pnas.94.7.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buller R M, Smith G L, Cremer K, Notkins A L, Moss B. Decreased virulence of recombinant vaccinia virus expression vectors is associated with a thymidine kinase-negative phenotype. Nature. 1985;317:813–815. doi: 10.1038/317813a0. [DOI] [PubMed] [Google Scholar]
- 4.Carroll M W, Moss B. Poxviruses as expression vectors. Curr Opin Biotechnol. 1997;8:573–577. doi: 10.1016/s0958-1669(97)80031-6. [DOI] [PubMed] [Google Scholar]
- 4a.Chakrabarti S, Sisler J K, Moss B. Compact, synthetic, vaccinia virus early/late promoter for protein expression. BioTechniques. 1997;23:1094–1097. doi: 10.2144/97236st07. [DOI] [PubMed] [Google Scholar]
- 5.Conry R M, Allen K O, Lee S, Moore S E, Shaw D R, LoBuglio A F. Human autoantibodies to carcinoembryonic antigen (CEA) induced by a vaccinia-CEA vaccine. Clin Cancer Res. 2000;6:34–41. [PubMed] [Google Scholar]
- 6.Davison A J, Moss B. Structure of vaccinia virus early promoters. J Mol Biol. 1989;210:749–769. doi: 10.1016/0022-2836(89)90107-1. [DOI] [PubMed] [Google Scholar]
- 7.Davison A J, Moss B. Structure of vaccinia virus late promoters. J Mol Biol. 1989;210:771–784. doi: 10.1016/0022-2836(89)90108-3. [DOI] [PubMed] [Google Scholar]
- 8.Demkowicz W E, Jr, Littaua R A, Wang J, Ennis F A. Human cytotoxic T-cell memory: long-lived responses to vaccinia virus. J Virol. 1996;70:2627–2631. doi: 10.1128/jvi.70.4.2627-2631.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Downie A W, Espana C. A comparative study of Tanapox and Yaba viruses. J Gen Virol. 1973;19:37–49. doi: 10.1099/0022-1317-19-1-37. [DOI] [PubMed] [Google Scholar]
- 10.Downie A W, Taylor-Robinson C H, Caunt A E, Nelson G S, Manson-Bahr P E C, Matthews T C H. Tanapox: a new disease caused by a pox virus. Br Med J. 1971;1:363–368. doi: 10.1136/bmj.1.5745.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Earl P L, Moss B. Expression of proteins in mammalian cells using vaccinia viral vectors. In: Ausubel F M, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Greene/Wiley Interscience; 1998. pp. 16.15.1–16.18.11. [Google Scholar]
- 12.Espana C. Review of some outbreaks of viral disease in captive nonhuman primates. Lab Anim Sci. 1971;21:1023–1031. [PubMed] [Google Scholar]
- 13.Gnant M, Berger A C, Huang J, Puhlmann M, Wu P C, Merino M J, Bartlett D L, Alexander H R, Jr, Libutti S K. Sensitization of tumor necrosis factor alpha (TNFα) resistant human melanoma by tumor specific in vivo transfer of the gene encoding for endothelial monocyte activating polypeptide (EMAP-II) using recombinant vaccinia virus. Cancer Res. 1999;59:4668–4674. [PubMed] [Google Scholar]
- 14.Gnant M F, Noll L A, Irvine K R, Puhlmann M, Terrill R E, Alexander H R, Jr, Bartlett D L. Tumor-specific gene delivery using recombinant vaccinia virus in a rabbit model of liver metastases. J Natl Cancer Inst. 1999;91:1744–1750. doi: 10.1093/jnci/91.20.1744. [DOI] [PubMed] [Google Scholar]
- 15.Gnant M F X, Puhlmann M, Alexander H R, Jr, Bartlett D L. Systemic administration of a recombinant vaccinia virus expressing the cytosine deaminase gene and subsequent treatment with 5-fluorocytosine leads to tumor specific gene expression and prolongation of survival in mice. Cancer Res. 1999;59:3396–3404. [PubMed] [Google Scholar]
- 16.Grace J T, Jr, Mirand E A. Yaba virus infection in humans. Exp Med Surg. 1965;23:213–216. [PubMed] [Google Scholar]
- 17.Hodge J W, Sabzevari H, Yafal A G, Gritz L, Lorenz M G, Schlom J. A triad of costimulatory molecules synergize to amplify T-cell activation. Cancer Res. 1999;59:5800–5807. [PubMed] [Google Scholar]
- 18.Khuri F R, Nemunaitis J, Ganly I, Arseneau J, Tannock I F, Romel L, Gore M, Ironside J, MacDougall R H, Heise C, Randlev B, Gillenwater A M, Bruso P, Kaye S B, Hong W K, Kirn D H. A controlled trial of intratumoral ONHX-015: a selectively replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat Med. 2000;6:879–886. doi: 10.1038/78638. [DOI] [PubMed] [Google Scholar]
- 19.Knight J C, Novembre F J, Brown D R, Goldsmith C S, Esposito J J. Studies on tanapox virus. Virology. 1989;172:116–124. doi: 10.1016/0042-6822(89)90113-x. [DOI] [PubMed] [Google Scholar]
- 20.Lee H J, Essani K, Smith G L. The genome sequence of Yaba-like disease virus, a yatapoxvirus. Virology. 2001;281:170–192. doi: 10.1006/viro.2000.0761. [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Hall R L, Moyer R W. Transient, nonlethal expression of genes in vertebrate cells by recombinant entomopoxviruses. J Virol. 1997;71:9557–9562. doi: 10.1128/jvi.71.12.9557-9562.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mastrangelo M J, Maguire H C, Jr, Eisenlohr L C, Laughlin C E, Monken C E, McCue P A, Kovatich A J, Lattime E C. Intratumoral recombinant GM-CSF-encoding virus as gene therapy in patients with cutaneous melanoma. Cancer Gene Ther. 1998;6:409–422. doi: 10.1038/sj.cgt.7700066. [DOI] [PubMed] [Google Scholar]
- 23.McCart J A, Puhlmann M, Lee J, Hu Y, Libutti S K, Alexander H R, Bartlett D L. Complex interactions between the replicating oncolytic effect and the enzyme/prodrug effect of vaccinia-mediated tumor regression. Gene Ther. 2000;7:1217–1223. doi: 10.1038/sj.gt.3301237. [DOI] [PubMed] [Google Scholar]
- 24.Merchlinsky M, Moss B. Resolution of vaccinia virus DNA concatemer junctions requires late-gene expression. J Virol. 1989;63:1595–1603. doi: 10.1128/jvi.63.4.1595-1603.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moss B. Genetically engineered poxviruses for recombinant gene expression, vaccination, and safety. Proc Natl Acad Sci USA. 1996;93:11341–11348. doi: 10.1073/pnas.93.21.11341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moss B. Poxviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2637–2671. [Google Scholar]
- 27.Moss B, Carroll M W, Wyatt L S, Bennink J R, Hirsch V M, Goldstein S, Elkins W R, Fuerst T R, Lifson J D, Piatak M, Restifo N P, Overwijk W, Chamberlain R, Rosenberg S A, Sutter G. Host range restricted, non-replicating vaccinia virus vectors as vaccine candidates. Adv Exp Med Biol. 1996;397:7–13. doi: 10.1007/978-1-4899-1382-1_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niven J S F, Armstrong J A, Andrewes C H, Pereira H B, Valentine R C. Subcutaneous growths in monkeys produced by a poxvirus. J Pathol Bacteriol. 1961;81:1–14. [PubMed] [Google Scholar]
- 29.Puhlmann M, Brown C K, Gnant M, Huang J, Libutti S K, Alexander H R, Bartlett D L. Vaccinia as a vector for tumor directed gene therapy. Biodistribution of a thymidine kinase deleted mutant. Cancer Gene Ther. 2000;7:66–73. doi: 10.1038/sj.cgt.7700075. [DOI] [PubMed] [Google Scholar]
- 30.Puhlmann M, Gnant M, Brown C K, Alexander H R, Bartlett D L. Thymidine kinase deleted vaccinia virus expressing purine nucleoside phosphorylase as a vector for tumor directed gene therapy. Hum Gene Ther. 1999;10:649–657. doi: 10.1089/10430349950018724. [DOI] [PubMed] [Google Scholar]
- 31.Rosenberg S A, Zhair Y, Yang J C, Schwartzentruber D J, Hwu P, Marincola F M, Topalian S L, Restifo N P, Seipp C A, Einhorn J H, Roberts B, White D E. Immunizing patients with metastatic melanoma using recombinant adenoviruses encoding MART-1 or gp100 melanoma antigens. J Natl Cancer Inst. 1998;90:1894–1900. doi: 10.1093/jnci/90.24.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanda M G, Smith D C, Charles L G, Hwang C, Pienta K J, Schlom J, Milenic D, Panicali D, Montie J E. Recombinant vaccinia-PSA (Prostvac) can induce a prostate-specific immune response in androgen-modulated human prostate cancer. Urology. 1999;53:260–266. doi: 10.1016/s0090-4295(98)00539-1. [DOI] [PubMed] [Google Scholar]
- 33.Walker J R, McGeach K G, Sundaresan P, Jorgensen T J, Rabkin S D, Martuza R L. Local and systemic therapy of human prostate adenocarcinoma with the conditionally replicating herpes simplex virus vector G207. Hum Gene Ther. 1999;10:2237–2243. doi: 10.1089/10430349950017211. [DOI] [PubMed] [Google Scholar]
- 34.Wang M, Bronte V, Chen P W, Gritz L, Panicali D, Rosenberg S A, Restifo N P. Active immunotherapy of cancer with a nonreplicating recombinant fowlpox virus encoding a model tumor-associated antigen. J Immunol. 1995;154:4685–4692. [PMC free article] [PubMed] [Google Scholar]
- 35.Wildner O, Blaese R M, Morris J C. Therapy of colon cancer with oncolytic adenovirus is enhanced by the addition of herpes simplex virus-thymidine kinase. Cancer Res. 1999;59:410–413. [PubMed] [Google Scholar]