Abstract
Aim
Nanotechnology presents a promising approach for managing chronic periodontitis, a common oral disease characterized by gum inflammation and loss of supporting bone around teeth. This study aimed to evaluate the antimicrobial efficacy of acerola-mediated silver nanoparticles (AgNPs) gel and copper oxide nanoparticles (CuONPs) gel in periodontitis patients with and without diabetes.
Materials and methods
The antimicrobial efficacy of acerola-mediated AgNPs gel and CuONPs nanogel was assessed using the agar well diffusion technique, Minimum Inhibitory Concentration (MIC) assay, Minimum Bactericidal Concentration (MBC) analysis, time-kill curve assay, and cytoplasmic and protein leakage analysis from periodontitis patients with and without diabetes.
Results
The study found that acerola-mediated AgNPs gel demonstrated more consistent and effective antimicrobial activity against periodontitis, with lower MIC and MBC values compared to the CuONPs gel, across all tested concentrations. These results suggest that acerola-mediated AgNPs gel may be a more effective and targeted therapeutic agent for periodontal disease management.
Conclusion
The findings emphasize the importance of nanoparticle gel concentration in optimizing periodontal treatment outcomes. Acerola-mediated AgNPs gel, with its superior efficacy and consistency in bactericidal activity, shows significant potential for periodontal therapy.
Clinical significance
Innovative nanoparticles like copper and silver oxides exhibit antibacterial, anti-inflammatory, and antioxidant properties, making them promising agents for targeting periodontal pathogens. Acerola (Malpighia emarginata), with its high vitamin C content and antioxidant properties, is beneficial in mitigating oxidative stress associated with chronic periodontitis.
Keywords: Vitamin C, Chronic periodontitis, Acerola cherry, Green synthesis, Silver nanoparticles, Copper oxide nanoparticles, Antimicrobial activity
1. Introduction
Nanotechnology presents a promising approach in the treatment and management of chronic periodontitis, a prevalent oral condition characterized by gum inflammation and loss of supporting bone around teeth. The primary cause of this disease plaque biofilm on teeth and gingival surfaces, is often challenging to treat due to bacterial resistance and the body's natural defenses.1 Antimicrobial nanoparticles, such as silver and copper oxide, show promise in targeting and eradicating bacteria responsible for periodontal damage. Iron oxide nanoparticles inhibit enzymes associated with tissue destruction, while nickel nanoparticles combat key plaque-forming bacteria.2,3.
Silver nanoparticles, widely used in dentistry, offer antibacterial activity beneficial in reducing bacterial growth and inflammation associated with periodontitis. Copper Oxide Nanoparticles (CuONPs) also hold promise, specifically by enhancing the regenerative capabilities of periodontal ligament (PDL) cells. Beyond their antimicrobial applications, nanotechnology contributes to periodontitis treatment through the development of biocompatible materials for periodontal regeneration.4, 5, 6
Green synthesis of nanoparticles provides a sustainable and environmentally friendly alternative to traditional chemical synthesis methods, making it particularly for chronic periodontitis. This approach utilizes fruit and plant extracts, enzymes, and microbes to produce non-toxic, eco-friendly nanoparticles (NPs) beneficial for periodontal health.7. This method avoids the use of hazardous compounds and does not require high pressure or temperature, making it a simple and environmentally friendly process. Plant-based preparation is recognized for its cost-effectiveness and suitability for large-scale NP synthesis, making it an attractive green synthesis approach for metal and metal oxide NPs.8
Plant extracts, derived from various parts of plants, contain phytochemicals that act as capping, reducing, and stabilizing agents in NP synthesis processes. This simplicity and eco-friendliness make green synthesis an appealing choice for the synthesis of metal NPs such as Ag, Cu, and AuNPs with applications in periodontal health. These NPs, incorporated into hygiene products like toothpaste and mouthwash, exhibit antimicrobial properties that can help control the progression of periodontitis by eliminating bacterial biofilms and reducing inflammation. The use of green-synthesized NPs is considered safer and more environmentally friendly than conventional synthesis methods.9
The utilization of Vitamin C-rich Acerola for synthesizing nanoparticles as a treatment for chronic periodontitis is an innovative approach that capitalizes on Vitamin C's natural antioxidant properties. Vitamin C, or L-ascorbic acid, is essential for various physiological functions, including collagen synthesis, hormone production, and its involvement in gene regulation. Acerola, with its high Vitamin C content, ranging from 820 to 4023 mg/100g of fresh weight, becomes an appealing source for nanoparticle synthesis and is particularly relevant for combating oxidative stress in chronic periodontitis.10,11The synthesis of nanoparticles using Acerola extract aligns with green and environmentally friendly practices, avoiding the use of toxic compounds.12,13
In this current study, the comparative analysis of acerola-mediated silver nanoparticles (AgNPs) gel and copper oxide nanoparticles (CuONPs) gel, employing various assays such as agar well diffusion, Minimum Inhibitory Concentration (MIC), Minimum Bactericidal Concentration (MBC), and time kill curve, cytoplasmic leakage, Protein leakage analysis offers a comprehensive insight into their antimicrobial effectiveness. This study specifically investigates distinct patterns of antimicrobial activity against plaque samples from periodontitis patients with and without diabetes at different concentrations. The outcomes from these analyses contribute to a complete understanding of the potential applications of acerola-mediated AgNPs and CuONPs gels in managing periodontal health, particularly for diabetes and non-diabetic periodontitis individuals. This information is pivotal for advancing our knowledge and guiding the development of targeted and effective antimicrobial strategies for periodontal health management.
2. Materials and Methods
Using freeze-dried Acerola powder (Brut Appetit) from RMCA Ventures, Bangalore, this in vitro investigation was carried out in Chennai between August to November 2023, adhering to acceptable laboratory procedures.
Dental plaque biofilm samples were collected from subgingival and supragingival sites of mild periodontitis patients with or without diabetes mellitus, using a sterile wooden toothpick by mechanical scraping in the interproximal, vestibular, lingual, or palatal surface in a single direction. The toothpicks with the biofilm samples were immediately placed in a tube containing 5 mL of Müller–Hinton broth (MH, BD™ Difco™, Rockville, MD, USA), and stored for further investigations.
2.1. Preparation of acerola cherry extract
Two grams of freeze dried acerola cherry powder was measured and added to 100 mL of distilled water. The mixed solution was heated using a heating mantle at 55 °C for 15–20 min. Subsequently, the extract was filtered using Whatman No. 1 filter paper to remove the impurities. The filtered extract was mixed and boiled using a heating mantle for 5–10 min and was used for further processing.
2.2. Green synthesis of acerola-mediated silver and copper nanoparticles
A quantity of 2 mM of silver nitrate was added to 80 mL of distilled water to make a silver nitrate solution. 20 mL of acerola cherry was added to the prepared silver nitrate solution (Fig. 1-a). The reaction solution was kept in an orbital shaker at 160–180 RPM (rotation per minute) for 48 h. After that, centrifugation was carried out at 8000 RPM (rotation per minute) for 10 min to segregate the pellet. Then the remaining supernatant was discarded, and the pellet was collected and stored in the refrigerator at 4° C for further use. A quantity of 30 mM of copper sulphate was added to 50 mL of distilled water to make copper sulphate solution (Fig. 1-b). 50 mL of acerola cherry was added to the prepared copper sulphate solution. The reaction solution was kept in an orbital shaker at 160–180 RPM (rotation per minute) for 48 h. After that, centrifugation was carried out at 8000 RPM (rotation per minute) for 10 min to segregate the pellet. Then the remaining supernatant was discarded, and the pellet was collected and stored in the refrigerator at 4 °C for further use.
Fig. 1.
Green synthesis of acerola-mediated silver nanoparticles and copper nanoparticles using vitamin C rich acerola cherry extract. a) Acerola cherry filtered extract b) silver nanoparticle solution c) copper oxide nanoparticle solution.
2.3. Formulation of acerola mediated silver nanoparticles (AgNPs) and copper oxide nanoparticles based gel
The gel preparation (Fig. 2) involved dissolving 2.5g of Carboxymethylcellulose in 25 mL of distilled water. Separately, 2.5g of Carbopol was dissolved in another 25 mL of distilled water. After achieving a homogeneous mixture for each component, the two solutions were combined and thoroughly mixed using a homogenizer. Once complete homogenization was achieved, the prepared gel served as the base for formulating the AgNPs and CuONPs based gel. To this gel, 100 mg of AgNPs and CuONPs, were added and further mixed thoroughly using a homogenizer to ensure a uniform blend. The resultant green-synthesized AgNPs and CuONPs based gel was stored in a refrigerator for subsequent evaluation studies.
Fig. 2.
Formulation of acerola mediated a) AgNPs gel b) CuONPs gel.
2.4. Evaluation of gel
2.4.1. pH measurement
A precise amount of gel was weighed and dissolved in 100 mL of purified water. The pH of the resulting mixture was determined using a digital pH meter.
2.4.2. Viscosity measurement
The viscosity of the formulation was evaluated without dilution using a Rheometer with a spindle 05 at a weight of 0.1 g.
2.5. Antimicrobial activity
Anaerobic organisms were cultured in brain-heart infusion (BHI) agar incubated in an anaerobic environment. The antimicrobial activity of the nanoparticles was evaluated using the agar well diffusion technique. Brain-heart infusion (BHI) agar plates were prepared and sterilized using an autoclave at 121 °C for 15–20 min. After sterilization, the medium was poured on to the surface of sterile Petri plates and allowed to cool to room temperature. The bacterial suspension (Periodontal pathogens-Plaque-I and II) was spread evenly onto the agar plates using sterile cotton swabs. Wells of 9 mm diameter were created in the agar plates using a sterile polystyrene tip. The wells were then filled with different concentrations of both AgNPs gel, CuONPs gel and standard (amoxyrite) on each well. McIntosh and Filde's anaerobic jar was used for anaerobic microorganism culturing and incubation. The plates were incubated for 24 h. The antimicrobial activity was evaluated by measuring the diameter of the inhibition zone surrounding the wells.
2.5.1. Minimum inhibitory concentration assay (MIC assay) and minimum bactericidal concentration (MBC)
The Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) assays are pivotal in periodontal research for assessing the efficacy of antimicrobial agents against bacteria associated with periodontitis. These assays were conducted under controlled conditions to ensure precise results, providing valuable insights into the effectiveness of antimicrobial interventions for periodontal care.14
2.5.1.1. Minimum inhibitory concentration assay (MIC assay)
Mueller Hinton broth was prepared, sterilized, and 6 mL was dispensed into each of the three test tubes. Plaque Sample I (periodontitis without diabetes) and Plaque Sample II (periodontitis with diabetes) were introduced into each of the five test tubes at a concentration of 5 × 105 CFU/ml. The first three tubes contained the Vitamin C-rich acerola-mediated silver nanoparticles gel at three different concentrations (25, 50, 100 μg/mL), while the fourth (Plaque I) and fifth tubes (Plaque II) served as the standard group with amoxyrite. The sixth tube contained the Vitamin C-rich acerola-mediated copper nanoparticles gel at three different concentrations (25, 50, 100 μg/mL), with the ninth (Plaque I) and tenth tubes (Plaque II) as the standard group with amoxyrite. Two additional tubes acted as growth controls for Plaque I and Plaque II samples. Incubation was carried out under suitable conditions for various time intervals (0h, 1h, 2h, 3h, 4h). Subsequently, the percentage of dead cells was calculated at a wavelength of 540 nm at regular intervals.
2.5.1.2. Minimum bactericidal concentration (MBC) assay
MHA agar was prepared, autoclaved, cooled, and poured onto sterile petri plates, allowing it to solidify. Following solidification, the bacterial suspension from all tubes for each sample (Periodontitis without diabetes and Periodontitis with diabetes) treated with both nanogels was evenly spread across the agar surface using a sterile glass spreader. The plates underwent a 24-h incubation period at 37 °C, after which the number of colonies was observed and enumerated.
2.5.2. Time-kill curve assay
A time-kill curve assay was performed to evaluate the bactericidal properties and concentration-dependent relationship between AgNPs and CuONPs gels, derived from acerola, and their impact on the growth rate of anaerobic microbes isolated from plaque samples (Periodontitis without diabetes and Periodontitis with diabetes) over time. This assay utilized a standardized protocol, including the preparation of Brain Heart Infusion Broth (BHI) supplemented with varying concentrations of acerola-mediated AgNPs and CuONPs gels (25 μg, 50 μg, 100 μg) and the subsequent time-kill curve analysis. Initially, the pathogens were cultured in BHI broth without antimicrobial agents for a pre-incubation period of 4 h to ensure they reached a stable early-to-mid log phase. An inoculum of 0.5 McFarland of each plaque sample was prepared from cultures grown on Brine heart infusion agar plates under anaerobic conditions with controlled CO2. This inoculum was then diluted in pre-heated BHI medium to a final concentration of 90 μL per well in a 96-well ELISA plate. Each well containing 90 μL of the pre-incubated plaque samples was supplemented with 10 μL of both nanogels at three different concentrations, along with a standard (amoxyrite) and untreated control. The loaded ELISA well plate were measured using ELISA plate reader at 600 nm.15 This protocol allowed for the assessment of the bactericidal effects of acerola-mediated AgNPs and CuONPs gels on the anaerobic microbes, providing insights into their potential as antimicrobial agents. The results obtained from such assays are crucial for understanding the effectiveness of these nanogels in controlling bacterial growth, especially in challenging environments such as periodontal plaque, where traditional antibiotic treatments may not be effective.16
2.5.3. Cytoplasmic leakage
In the study, an attempt was made to assess the cytoplasmic leakage of bacterial cells when subjected to treatments with nanoparticles (NPs) based gel derived from acerola, specifically silver nanoparticles (AgNPs) and copper oxide nanoparticles (CuONPs). The cytoplasmic leakage refers to the release of cytoplasmic materials, such as DNA and proteins, from bacterial cells, which is a critical aspect of understanding the mechanisms of bacterial cell death. For the assessment of cytoplasmic leakage, plaque samples I and II, each containing 10 mL of bacterial cultures, were cultured in Brain Heart Infusion (BHI) broth under anaerobic conditions for an overnight period. The following day, the cultures were harvested by centrifugation at 5000 rpm for 10 min. The pellet was washed and resuspended in PBS buffer at a pH of 7.2, with the cell concentration adjusted to 1 × 105 cells per mL. Different aliquots of the cell suspension were then treated with both acerola-mediated AgNPs and CuONPs gels, as well as a control group without nanogels, and incubated at room temperature for durations of 3 and 5 h. After incubation, the cultures were centrifuged again at 5000 rpm for 10 min, and the absorbance of the supernatants was measured at a wavelength of 260 nm. This methodology allows for the quantification of cytoplasmic leakage, providing insights into the bactericidal mechanisms of acerola-mediated AgNPs and CuONPs gels. The results from this study contribute to a better understanding of the antimicrobial properties of nanoparticles derived from natural sources, such as acerola, and their potential applications in combating periodontal infections.17
2.5.4. Protein leakage
The Bradford test was employed to investigate protein leakage by exposing periodontal pathogens containing plaque samples (Periodontitis without diabetes) and Periodontitis with diabetes) to different concentrations of acerola mediated AgNPs gel and CuONPs gel, specifically 25 μg, 50 μg, and 100 μg, for a duration of 24–48 h. After treatment, the plaque suspensions were centrifuged at 6000 rpm for 15 min, which separated the supernatant phase. A total of 200 μL of this supernatant was then added to 96-well ELISA plates. To these, 800 μL of Bradford reagent was added and incubated for 10 min in a dark environment. Amoxyrite served as the standard, and the optical density (OD) of the samples was measured at 595 nm.15,18
3. Results
3.1. Evaluation of Gel
3.1.1. Gel viscosity analysis
The viscosity analysis of acerola-mediated silver nanoparticles (AgNPs) gel and copper oxide nanoparticles (CuONPs) gel was analysed using a viscometer (Fig. 3), revealing consistent flow properties for both formulations. The acerola-mediated AgNPs gel demonstrated a viscosity that allowed it to flow for 1 min and 30 s when dispensing 1 ml, indicating its stable consistency with 11.50 Pa s under the tested conditions. Similarly, the acerola-mediated CuONPs gel exhibited a comparable viscosity of 15.35 Pa s, with a flow time of 1 min and 30 s for the same volume, highlighting its ability to maintain stability.
Fig. 3.

Gel viscosity analysis using viscometer.
3.1.2. pH of Gel
The recorded pH for acerola based AgNPs gel and CuONPs gel were 6.23 and 6.11, falling within the neutral to slightly acidic range. This pH range is considered favorable for dental applications as it ensures the stability of the gel and minimizes the likelihood of irritation to oral tissues. The neutral to slightly acidic pH of the gels adds to their suitability for potential use in dental and periodontal applications.
3.2. Antimicrobial Activity
3.2.1. Agar well diffusion technique
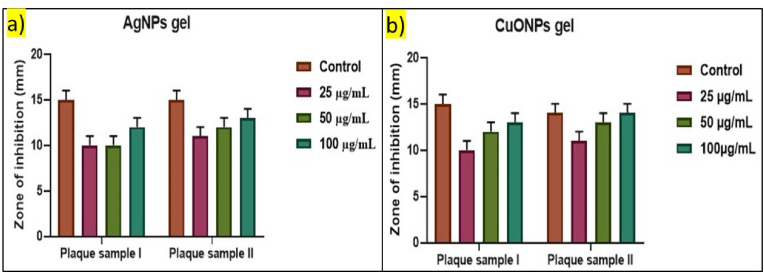
A comparative analysis between the antimicrobial activity of acerola-mediated silver nanoparticles (AgNPs) gel and copper oxide nanoparticles (CuONPs) gel (Fig. 4), using the agar well diffusion technique, reveals distinct patterns of antimicrobial efficacy against two plaque samples, one from periodontitis samples without diabetes (Plaque sample I) and the other from periodontitis samples with diabetes (Plaque sample II), at varying concentrations. For the acerola-mediated AgNPs gel, the study showed generally larger zones of inhibition for Plaque sample II compared to Plaque sample I across all tested concentrations, indicating a consistent inhibitory effect against bacterial growth in both samples. This suggested that the acerola-mediated AgNPs gel demonstrates promising antimicrobial activity in both diabetes-associated and non-diabetes periodontitis samples, regardless of the presence of diabetes.15,19,20
Fig. 4.
Antimicrobial activity of acerola-mediated a) silver nanoparticles (AgNPs) gel and b) copper oxide nanoparticles (CuONPs) gel using the agar well diffusion technique.
In contrast, the acerola-mediated CuONPs gel exhibited variable antimicrobial activity against the plaque samples. For Plaque sample I, the gel initially showed a decrease in the zone of inhibition at 25 μg/mL compared to the control, followed by an increase at higher concentrations (50 μg/mL and 100 μg/mL). Plaque sample II demonstrated a consistent increase in the zone of inhibition with escalating concentrations of the acerola-mediated CuONPs gel. This variation in antimicrobial activity suggested potential differences in the microbial compositions or sensitivities to copper oxide nanoparticles between the samples. Both acerola-mediated AgNPs and CuONPs gels could potentially contribute to the management and treatment of chronic periodontitis by reducing the growth of bacteria responsible for the disease.21 Further research, including microbiological characterization and clinical studies, is needed to fully understand the potential therapeutic applications of these nanoparticles in the treatment of chronic periodontitis and other oral health conditions.22
3.2.2. Minimum inhibitory concentration assay
3.2.2.1. Plaque sample I (without diabetes)
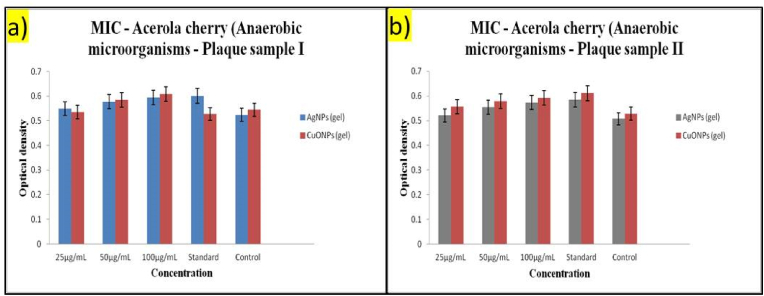
The analysis of Plaque sample I (Fig. 5a), which was tested without diabetes, indicated a difference in the Minimum Inhibitory Concentration (MIC) between AgNPs (Silver Nanoparticles) and CuONPs (Copper Oxide Nanoparticles) gels at various concentrations. The result showed that AgNPs gel has a lower MIC than CuONPs gel, which implies that AgNPs gel might be more effective in inhibiting bacterial growth, which is crucial for preventing plaque buildup.
Fig. 5.
Comparative MIC analysis of a) Plaque Samples I and b) Plaque II treated with acerola based AgNPs and CuONPs Gel.
3.2.2.2. Plaque sample II (with diabetes)
The analysis of Plaque sample II (Fig. 5a), which was tested with diabetes, who are at an increased risk of dental complications, including plaque buildup, leading to gum disease and tooth decay also revealed differences in the mean values between AgNPs gel and CuONPs gel across the various concentrations examined. The Tukey post-hoc test showed that AgNPs gel exhibited a higher mean value compared to CuONPs gel, suggesting that AgNPs gel may have a more potent effect or a different interaction with the periodontitis sample with diabetes.
Both samples showed a difference in the MIC between AgNPs and CuONPs gel, with AgNPs gel being more effective in inhibiting bacterial growth. This effectiveness was observed in both samples, indicating that the type of gel used significantly affects the MIC, which measures the ability of the gel to inhibit bacterial growth. The analysis of periodontitis sample II, with diabetes, further supports the hypothesis that individuals with diabetes may benefit less from a more effective gel in preventing or treating periodontitis. The lower MIC of AgNPs gel compared to CuONPs gel in this context suggests that AgNPs gel might be particularly beneficial for individuals with diabetes, who are at an increased risk of dental complications. While both samples demonstrated the effectiveness of AgNPs gel over CuONPs gel, the specific differences in the mean values and the overall trend indicate that AgNPs gel is more effective in inhibiting bacterial growth, which is crucial for preventing plaque buildup and associated dental issues.
3.2.3. Minimum bactericidal concentration
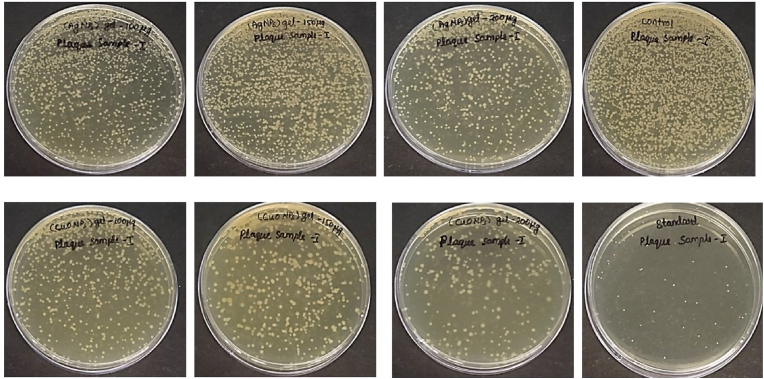
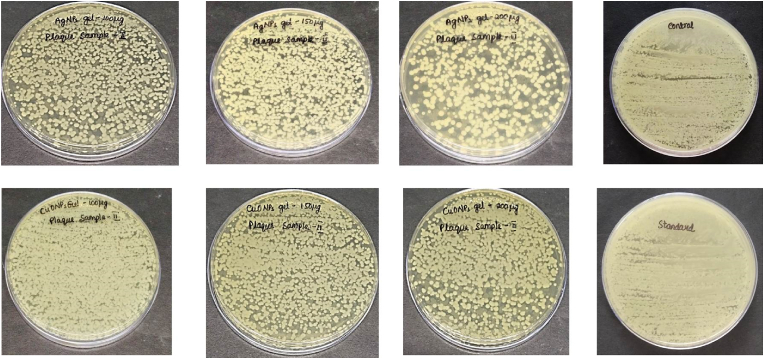
The Minimum Bactericidal Concentration (MBC) analysis of Plaque Sample I (Fig. 6) and Plaque Sample II (Fig. 7) treated with AgNPs gel and CuONPs gel provides insights into the antimicrobial efficacy of these nanoparticles against bacterial growth in two different plaque samples. The MBC represents the lowest concentration of the respective nanoparticles at which no bacterial growth was observed after treatment, offering a critical measure of their effectiveness.
Fig. 6.
Minimum bactericidal concentration of both AgNPs and CuONPs gel against Plaque I sample (without diabetes).
Fig. 7.
Minimum bactericidal concentration of both AgNPs and CuONPs gel against Plaque II sample (with diabetes).
3.2.3.1. AgNPs gel
Plaque Sample I: The MBC was consistently observed at a concentration of 100 μg/mL, indicating a high level of efficacy at this concentration.
Plaque Sample II: Similar to Plaque Sample I, the MBC was consistently observed at a concentration of 100 μg/mL, with an observed "enormous" level of inhibition.
3.2.3.2. CuONPs gel
Plaque Sample I: The MBC varied across concentrations, with 200 μg/mL being the lowest concentration at which no bacterial growth was observed consistently across all trials.
Plaque Sample II: Similar to Plaque Sample I, the MBC varied across concentrations, with 200 μg/mL being the lowest concentration at which no bacterial growth was observed consistently across all trials.
AgNPs gel demonstrated a more consistent and potentially more effective antimicrobial activity at a lower concentration of 100 μg/mL compared to CuONPs gel in both Plaque Sample I and Plaque Sample II. CuONPs gel required a higher concentration to achieve the same level of bactericidal effect as AgNPs gel in both Plaque Sample I and Plaque Sample II.
The comparative analysis of the Minimum Bactericidal Concentration (MBC) between Plaque Sample I and Plaque Sample II, as well as between AgNPs gel and CuONPs gel, reveals that both AgNPs gel and CuONPs gel exhibit bactericidal effects against the bacteria present in both plaque samples. However, AgNPs gel demonstrates a more consistent and potentially more effective antimicrobial activity at a lower concentration of 100 μg/mL compared to CuONPs gel, which requires a higher concentration to achieve similar levels of bactericidal effect. These findings highlight the potential advantages of AgNPs gel in terms of its efficacy and consistency in bactericidal activity, which could be crucial for its application in periodontitis management. The consistent MBC at 100 μg/mL for both plaque samples suggests that AgNPs gel may have a broader applicability across for periodontal problems, potentially offering a more effective and targeted therapeutic strategy.
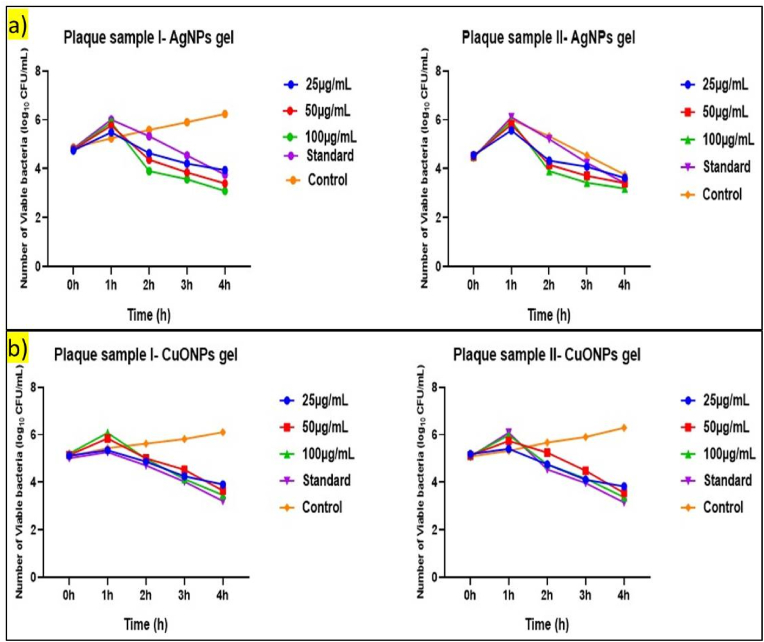
3.2.4. Time kill curve assay
The study aimed to compare the antibacterial efficacy of acerola-mediated AgNPs gel and CuONPs gel on plaque samples I and II (Fig. 8), derived from individuals without and with diabetes. The experiments were conducted using concentrations of 25 μg/mL, 50 μg/mL, and 100 μg/mL for both gels, with results compared against standard and control groups.
Fig. 8.
Acerola mediated a) AgNPs gel b) CuONPs gel on Plaque Samples I and II.
3.2.4.1. Plaque sample I (periodontitis without diabetes)
At the initiation of the experiment, bacterial counts were recorded for all concentrations, showing minimal variations among different concentrations and control groups. After 1 h of exposure, a noticeable increase in antibacterial effectiveness was observed, with a reduction in bacterial counts for all concentrations. The antibacterial activity continued to escalate after 2 h, with a more pronounced reduction in bacterial counts across all concentrations. At the 3-h time point, the antibacterial effect remained evident, with a significant decrease in bacterial counts for the gel-treated groups compared to the control and standard groups. The final time point, 4 h, demonstrated a sustained and concentration-dependent antibacterial activity of the AgNPs gel. The bacterial counts continued to decline, indicating the effectiveness of the treatment. Similar to the AgNPs gel, the CuONPs gel also showed a sustained and concentration-dependent antibacterial activity across all time points. The antibacterial effectiveness escalated over time, particularly after 2 h of exposure. The continued decline in bacterial counts over time suggests that the CuONPs gel could be effective in maintaining oral health over extended periods for non-diabetic periodontitis individuals.
3.2.4.2. Plaque sample II (periodontitis with diabetes)
The AgNPs gel demonstrated a sustained and concentration-dependent antibacterial activity across all time points, with the antibacterial effectiveness escalating over time, particularly after 2 h of exposure. The continued decline in bacterial counts over time suggests that the AgNPs gel could be effective in maintaining oral health over extended periods for non-diabetic individuals. The CuONPs gel also exhibited a sustained and concentration-dependent antibacterial activity across all time points.
Both AgNPs and CuONPs gels showed a sustained and concentration-dependent antibacterial activity across all time points with the effectiveness increasing with higher concentrations. The continued decline in bacterial counts over time suggests that these gels can be tailored to achieve desired levels of antibacterial activity, making them a promising candidate for periodontal health management and maintenance.
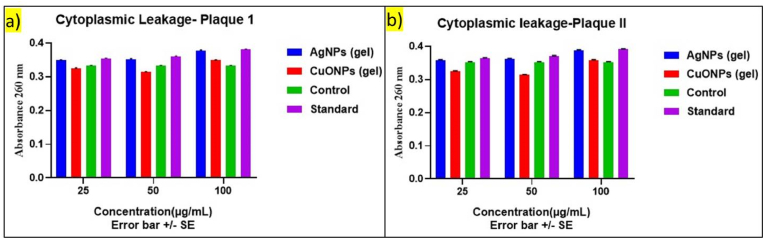
3.2.5. Cytoplasmic leakage analysis
The cytoplasmic leakage analyses were performed on plaque samples from periodontitis individuals without diabetes (Sample I) (Fig. 9a) and those with diabetes (Sample II) (Fig. 9b) to assess the differential impact of AgNPs gel and CuONPs gel at varying concentrations. Control samples and standard samples served as references for both groups.
Fig. 9.
Cytoplasmic Leakage Analysis on Plaque Samples with and without Diabetes treated with AgNPs and CuONPs gel a) Plaque I b) Plaque II sample.
3.2.5.1. Plaque samples of periodontitis without diabetes (sample I)
At a nanoparticle concentration of 25 μg/mL, both AgNPs gel and CuONPs gel exhibited similar cytoplasmic leakage values in plaque samples. The leakage values were 0.348 for AgNPs gel and 0.324 for CuONPs gel, indicating minimal impact on cellular integrity at this lower concentration. Increasing the concentration to 50 μg/mL resulted in a slight rise in cytoplasmic leakage for both gels compared to controls, with values of 0.352 for AgNPs gel and 0.313 for CuONPs gel. These changes suggest a potential concentration-dependent effect on cellular integrity. At 100 μg/mL, both gels caused increased cytoplasmic leakage, with values of 0.377 for AgNPs gel and 0.348 for CuONPs gel, indicating a more pronounced impact on cellular integrity at higher concentrations. Control and standard samples maintained consistent leakage values across all concentrations, ranging from 0.332 for controls to 0.354 for standards.
3.2.5.2. Plaque samples from periodontitis individuals with diabetes (sample II)
At 25 μg/mL, cytoplasmic leakage was similar for both AgNPs gel and CuONPs gel, with values of 0.358 for AgNPs gel and 0.324 for CuONPs gel, suggesting minimal impact at this concentration. Increasing to 50 μg/mL led to a slight elevation in leakage for both gels compared to controls, with values of 0.362 for AgNPs gel and 0.313 for CuONPs gel. This indicates a concentration-dependent effect on cellular integrity. At 100 μg/mL, both gels increased cytoplasmic leakage, with values of 0.387 for AgNPs gel and 0.358 for CuONPs gel, suggesting a more pronounced effect at higher concentrations. Control and standard samples showed consistent leakage values, ranging from 0.352 to 0.354 for controls and 0.364 to 0.366 for standards.
In summary, the comparative analysis indicates that cytoplasmic leakage responses to AgNPs gel and CuONPs gel vary with the presence of diabetes, showing concentration-dependent effects on cellular integrity.
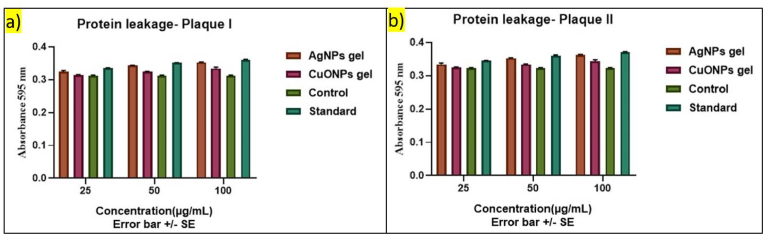
3.2.6. Protein leakage analysis
The protein leakage analysis (Fig. 10) on plaque samples I and II, derived from periodontitis individuals without and with diabetes, using acerola-mediated AgNPs gel and CuONPs gel, provides valuable insights into the effectiveness of these nanoparticle gels in managing oral health. The concentrations tested were 25 μg/mL, 50 μg/mL, and 100 μg/mL, with results compared against a standard and a control group for both diabetic and non-diabetic individuals.
Fig. 10.
Protein leakage Analysis on Plaque Samples with and without Diabetes treated with AgNPs and CuONPs gel a) Plaque I b) Plaque II sample.
3.2.6.1. Plaque samples of periodontitis without diabetes (sample I)
AgNPs Gel: At all concentrations, the AgNPs gel demonstrated a moderate level of protein leakage, with a slight increase in leakage as the concentration increased. The 100 μg/mL concentration showed the highest level of leakage, suggesting a significant impact on the integrity of the plaque. This indicates that the AgNPs gel may have a slightly greater effect on oral health in non diabetic individuals compared to the CuONPs gel at the same concentration.
CuONPs Gel: The CuONPs gel also exhibited moderate levels of protein leakage across all tested concentrations, with a slight decrease in leakage as the concentration increased. The 100 μg/mL concentration showed a moderate level of leakage, suggesting a moderate impact on the integrity of the plaque. This indicates that the CuONPs gel may have a slightly lesser effect on oral health in non diabetic individuals compared to the AgNPs gel at the same concentration.
3.2.6.2. Plaque samples from periodontitis individuals with diabetes (sample II)
AgNPs Gel: Similar to the non diabetic group, the AgNPs gel demonstrated a moderate level of protein leakage across all concentrations, with a slight increase in leakage as the concentration increased. The 100 μg/mL concentration showed the highest level of leakage, suggesting a significant impact on the integrity of the plaque. This indicates that the AgNPs gel may have a slightly greater effect on periodontitis individuals with diabetes compared to the CuONPs gel at the same concentration.
CuONPs Gel: The CuONPs gel also exhibited moderate levels of protein leakage across all tested concentrations, with a slight decrease in leakage as the concentration increased. The 100 μg/mL concentration showed a moderate level of leakage, suggesting a moderate impact on the integrity of the plaque. This indicates that the CuONPs gel may have a slightly lesser effect on periodontitis individuals with diabetic individuals compared to the AgNPs gel at the same concentration.
Both AgNPs and CuONPs gels exhibited moderate levels of protein leakage across all tested concentrations for both diabetic and non-diabetic periodontitis individuals. However, the AgNPs gel showed a slightly higher level of leakage compared to the CuONPs gel at the 100 μg/mL concentration for both groups. This suggests that while both gels may have a moderate impact on the integrity of the plaque, the AgNPs gel may have a slightly greater effect, which could be relevant for assessing the effectiveness of the gel in managing periodontal health in both diabetic and non-diabetic individuals. The control and standard groups showed protein leakage levels indicating a baseline level of leakage, providing a reference point for comparing the impact of the AgNPs and CuONPs gels on protein leakage.
The protein leakage analysis results underscore the potential of both AgNPs and CuONPs gels as agents for managing periodontal health in both diabetic and non-diabetic individuals. The AgNPs gel, particularly at higher concentrations, may have a slightly greater effect on protein leakage compared to the CuONPs gel. These findings warrant further investigation into their effectiveness and potential applications in dental hygiene and periodontal health management. The results also highlight the importance of considering the concentration of the nanoparticle gels in tailoring their application for optimal periodontal health outcomes.
4. Discussion
The comprehensive microbiological evaluation conducted in this study provides compelling evidence for the superior efficacy of acerola-mediated silver nanoparticles (AgNPs) gel over copper oxide nanoparticles (CuONPs) gel in the treatment of periodontitis, with a specific emphasis on cases involving diabetes mellitus. Additionally, acerola which is rich in vitamin C enhances the production of nitric oxide (eNO), prostaglandins PGE1 and PGI2, and cytoprotection; it also has antimutagenic, vasodilatory, and inhibitory effects on platelet aggregation beneficial in diabetes. The diverse analyses, including viscosity, gel pH, antimicrobial activity, minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC), time kill curve assay and cytoplasmic, protein leakage assessments, consistently highlight acerola-mediated AgNPs gel as the more significant candidate for oral health management.
Both acerola-mediated AgNPs and CuONPs gels demonstrated comparable flow properties, ensuring their practicality for oral applications. However, the neutral to slightly acidic pH range of acerola-mediated AgNPs gel enhances its suitability for dental use, minimizing the risk of irritation to oral tissues. This characteristic positions acerola-mediated AgNPs gel as a more favorable option for potential clinical applications.23
The agar well diffusion technique revealed distinct advantages of acerola-mediated AgNPs gel over acerola-mediated CuONPs gel in terms of antimicrobial efficacy. Acerola-mediated acerola-mediated AgNPs gel consistently exhibited larger zones of inhibition against bacterial growth in both diabetes-associated and non-diabetes periodontitis samples. This robust inhibitory effect suggests that acerola-mediated AgNPs gel holds promise for effectively managing periodontitis, regardless of the presence of diabetes.The MIC and MBC analyses unequivocally favored acerola-mediated AgNPs gel, showcasing its lower MIC and more consistent bactericidal effects at lower concentrations compared to acerola-mediated CuONPs gel. This implies that acerola-mediated AgNPs gel is not only more potent in inhibiting bacterial growth but also requires lower concentrations for effective bactericidal action. Such characteristics position acerola-mediated AgNPs gel as a more efficient and targeted solution for combating periodontal bacteria.The time-dependent antibacterial activity of acerola-mediated AgNPs gel demonstrated sustained and concentration-dependent effectiveness, underscoring its potential for long-term oral health maintenance.24 Acerola-mediated CuONPs gel exhibited similar characteristics, but the consistently superior performance of acerola-mediated AgNPs gel further solidifies its position as the preferred choice for extended antibacterial effects. The cytoplasmic and protein leakage analyses accentuated the slightly higher impact of acerola-mediated AgNPs gel on cellular integrity at the 100 μg/mL concentration compared to acerola-mediated CuONPs gel. While both gels exhibited moderate effects, acerola-mediated AgNPs gel displayed a slightly greater influence, especially in individuals with diabetes. This finding reinforces the notion that acerola-mediated AgNPs gel may offer a more profound and targeted impact on plaque integrity.25
The use of gels in the treatment of chronic periodontitis has been evaluated in previous research works. Studies have investigated the effectiveness of different gel formulations in improving clinical parameters such as pocket depth (PD) and clinical attachment level (CAL) in patients with periodontitis.26 These studies have examined the use of gels containing antimicrobial agents such as tetracycline, doxycycline, chlorhexidine, and metronidazole, as well as natural agents like chitosan and Tea polyphenols (TP).27 The results have shown that the use of gels in combination with non-surgical therapy can lead to improved clinical outcomes in patients with periodontitis. Additionally, research has also explored the use of gels loaded with osteogenesis drugs and other therapeutic agents to promote bone regeneration and enhance wound healing in periodontal tissues. These findings suggest that gels can be a promising option for the treatment of chronic periodontitis.28,29
In a prior research study, the integration of silver nanoparticles synthesized with Ocimum sanctum leaf extract (AgNP) was investigated and notable antimicrobial efficacy was reported against various periodontal pathogens, including Fusobacterium nucleatum (Fn), Porphyromonas gingivalis (Pg), Aggregatibacter actinomycetemcomitens (Aa), and Prevotella intermedia (Pi). The dose-dependent nature of AgNP's antimicrobial activity, along with its superiority over pure Ocimum sanctum extract (OSE) and silver nitrate (SN), underscores its potential for controlled local drug delivery in chronic periodontitis management. The Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) tests further support the robust antimicrobial effects of AgNP, particularly against Aggregatibacter actinomycetemcomitens (Aa). These results position AgNP synthesized with Ocimum sanctum leaf extract as a promising candidate for the targeted treatment of chronic periodontitis.16,30
In other study, silver nanoparticles (AgNPs) have exhibited inhibitory effects on oral biofilms associated with dental caries and periodontal disease, showcasing their potential as antimicrobial agents for oral health maintenance.9 The study emphasizes the influence of particle size, time, and gender on the antimicrobial and substantivity effects of AgNPs.31 The identification of specific oral microbiomes affected, including S. mutans, S. sobrinus, S. sanguinis, S. gordonii, S. oralis, P. gingivalis, T. forsythia, and P. intermedia, further highlights the broad-spectrum activity of AgNPs. The study suggests that AgNPs could be a valuable tool for controlling and preventing dental caries and periodontal disease across diverse patient profiles.23
In contrast, copper nanoparticles (CuNPs) have also demonstrated antimicrobial efficacy against periodontal pathogens, particularly Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans. The minimal inhibitory concentration (MIC) for nano-copper against these pathogens indicates its potential as an effective antimicrobial agent.32 The bactericidal and bacteriostatic effects at different concentrations underscore the versatility of CuNPs in combating periodontal pathogens. Incorporating nano-copper into restorative materials or local drug delivery systems emerges as a promising treatment option for periodontal diseases. Additionally, the sustained delivery of bactericidal properties and notable germicidal effects of CuNP gel further support its potential as a futuristic drug delivery agent against periodontal pathogens.33
The cumulative evidence presented strongly suggests that acerola-mediated AgNPs gel outperforms acerola-mediated CuONPs gel in various aspects of microbiological efficacy. These findings have profound implications for the development of tailored therapeutic strategies in periodontal health management. Acerola mediated AgNPs gel's consistent superiority across multiple analyses positions it as the more significant and promising candidate for further exploration and potential clinical applications.34,35 Future research avenues could delve deeper into the specific mechanisms behind the enhanced efficacy of AgNPs gel, exploring its interactions with periodontal bacteria and the unique benefits it offers in diabetic conditions. Additionally, continued investigation into the safety, cytotoxicity, and biocompatibility of acerola-mediated AgNPs gel is crucial for ensuring its suitability for prolonged oral use.
5. Conclusion
While both acerola-mediated AgNPs and CuONPs gels demonstrate antimicrobial activity against periodontal diseases, acerola-mediated AgNPs gel emerges as highly significant, especially in addressing chronic periodontitis with diabetes, where its consistent and potent effects at lower concentrations hold immense promise. The observed antimicrobial mechanisms, such as disrupting bacterial cell membranes and causing leakage of cellular contents, align with the superior efficacy of the AgNPs gel. These findings offer valuable insights into the optimization and application of nanoparticle gels for managing chronic periodontitis, particularly in individuals with diabetes. In conclusion, the robust microbiological evaluation presented in this study unequivocally establishes acerola-mediated AgNPs gel as the more significant and potent candidate for the treatment of periodontitis, providing a foundation for advancing targeted and effective interventions in oral health care, particularly for diabetes mellitus.
Funding
-
1
Source(s) of support in the form of grants, equipment, drugs, or all of these- Nil
Patient consent
-
1
Patient Consent- Not applicable
-
2
Ethical Clearance- Obtained institutional ethics clearance (MADC/IEC-1/02/2022).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Nil.
Contributor Information
C. Burnice Nalina Kumari, Email: drburnice.perio@madch.edu.in.
N. Ambalavanan, Email: drambal@gmail.com.
S. Rajesh Kumar, Email: rajeshkumars.sdc@saveetha.com.
Jaideep Mahendra, Email: jaideep_m_23@yahoo.co.in.
Uma Sudhakar, Email: ums_570@yahoo.co.in.
References
- 1.Nasiri K., et al. Recent advances in metal nanoparticles to treat periodontitis. J Nanobiotechnol. Aug. 2023;21(1):283. doi: 10.1186/s12951-023-02042-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basudan A.M. Nanoparticle based periodontal drug delivery - a review on current trends and future perspectives. Saudi Dent. J. Dec. 2022;34(8):669–680. doi: 10.1016/j.sdentj.2022.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen H., et al. Nano-based drug delivery systems for periodontal tissue regeneration. Pharmaceutics. Oct. 2022;14(10) doi: 10.3390/pharmaceutics14102250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uskoković V., Pejčić A., Koliqi R., Anđelković Z. Polymeric nanotechnologies for the treatment of periodontitis: a chronological review. Int J Pharm. Sep. 2022;625(122065) doi: 10.1016/j.ijpharm.2022.122065. [DOI] [PubMed] [Google Scholar]
- 5.Kennedy S., Srinivasan S., Jayavel K., Sundaram R. Nanotechnology in periodontal management. Int. J. Orofac. Biol. 2019;3(1):8. [Google Scholar]
- 6.Flores-Rábago K.M., Rivera-Mendoza D., Vilchis-Nestor A.R., Juarez-Moreno K., Castro-Longoria E. Antibacterial activity of biosynthesized copper oxide nanoparticles (CuONPs) using Ganoderma sessile. Antibiotics (Basel) Jul. 2023;12(8) doi: 10.3390/antibiotics12081251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kiarashi M., et al. Spotlight on therapeutic efficiency of green synthesis metals and their oxide nanoparticles in periodontitis. J Nanobiotechnol. 2024;22(1) doi: 10.1186/s12951-023-02284-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmed O., et al. Plant extract-synthesized silver nanoparticles for application in dental therapy. Pharmaceutics. Feb. 2022;14(2):380. doi: 10.3390/pharmaceutics14020380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emmanuel R., et al. Antimicrobial efficacy of green synthesized drug blended silver nanoparticles against dental caries and periodontal disease causing microorganisms. Mater Sci Eng C. Nov. 2015;56:374–379. doi: 10.1016/j.msec.2015.06.033. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Z., et al. The emerging role of plant-derived exosomes-like nanoparticles in immune regulation and periodontitis treatment. Front Immunol. Jun. 2022;13 doi: 10.3389/fimmu.2022.896745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vitamin C—Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients. 2021;13(2):615. doi: 10.3390/nu13020615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murererehe J., Uwitonze A.M., Nikuze P., Patel J., Razzaque M.S. Beneficial effects of vitamin C in maintaining optimal oral health. Front Nutr. 2021;8 doi: 10.3389/fnut.2021.805809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Functional Foods. Apple Academic Press; 2013. Vitamin C in human health and disease is still a mystery? An overview; pp. 179–202. [Google Scholar]
- 14.Belanger C.R., Hancock R.E.W. Testing physiologically relevant conditions in minimal inhibitory concentration assays. Nat Protoc. Aug. 2021;16(8):3761–3774. doi: 10.1038/s41596-021-00572-8. [DOI] [PubMed] [Google Scholar]
- 15.Tharani M., Rajeshkumar S., Al-Ghanim K.A., Nicoletti M., Sachivkina N., Govindarajan M. Terminalia chebula-assisted silver nanoparticles: biological potential, synthesis, characterization, and ecotoxicity. Biomedicines. May 2023;11(5) doi: 10.3390/biomedicines11051472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Espinosa-Cristóbal L.F., et al. Antimicrobial and substantivity properties of silver nanoparticles against oral microbiomes clinically isolated from young and young-adult patients. J Nanomater. Nov. 2019;2019:1–14. [Google Scholar]
- 17.Sahoo B., Leena Panigrahi L., Jena S., Jha S., Arakha M. Oxidative stress generated due to photocatalytic activity of biosynthesized selenium nanoparticles triggers cytoplasmic leakage leading to bacterial cell death. RSC Adv. Apr. 2023;13(17):11406–11414. doi: 10.1039/d2ra07827a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kielkopf C.L., Bauer W., Urbatsch I.L. Bradford assay for determining protein concentration. Cold Spring Harb Protoc. Apr. 2020;2020(4) doi: 10.1101/pdb.prot102269. [DOI] [PubMed] [Google Scholar]
- 19.Charannya S., Duraivel D., Padminee K., Poorni S., Nishanthine C., Srinivasan M.R. Comparative evaluation of antimicrobial efficacy of silver nanoparticles and 2% chlorhexidine gluconate when used alone and in combination assessed using agar diffusion method: an in vitro study. Contemp Clin Dent. Sep. 2018;9(Suppl 2):S204–S209. doi: 10.4103/ccd.ccd_869_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marinescu L., et al. Comparative antimicrobial activity of silver nanoparticles obtained by wet chemical reduction and solvothermal methods. Int J Mol Sci. May 2022;23(11):5982. doi: 10.3390/ijms23115982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ssekatawa K., et al. Phyto-mediated copper oxide nanoparticles for antibacterial, antioxidant and photocatalytic performances. Front Bioeng Biotechnol. Feb. 2022;10 doi: 10.3389/fbioe.2022.820218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cavassin E.D., et al. Comparison of methods to detect the in vitro activity of silver nanoparticles (AgNP) against multidrug resistant bacteria. J Nanobiotechnol. Oct. 2015;13(1):64. doi: 10.1186/s12951-015-0120-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steckiewicz K.P., et al. Silver nanoparticles as chlorhexidine and metronidazole drug delivery platforms: their potential use in treating periodontitis. Int J Nanomed. Feb. 2022;17:495–517. doi: 10.2147/IJN.S339046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hernández-Venegas P.A., et al. Bactericidal activity of silver nanoparticles on oral biofilms related to patients with and without periodontal disease. J Funct Biomater. Jun. 2023;14(6) doi: 10.3390/jfb14060311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu T., et al. Evaluation of the anti-biofilm effect of poloxamer-based thermoreversible gel of silver nanoparticles as a potential medication for root canal therapy. Sci Rep. Jun. 2021;11(1) doi: 10.1038/s41598-021-92081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahmood A., Abdul-Wahab G., Al-Karawi S. Effect of hyaluronan and metronidazole gels in management of chronic periodontitis. J Int Oral Health. 2019;11(3):158. [Google Scholar]
- 27.Elnagdy S., Raptopoulos M., Kormas I., Pedercini A., Wolff L.F. Local oral delivery agents with anti-biofilm properties for the treatment of periodontitis and Peri-implantitis. A narrative review. Molecules. Sep. 2021;26(18):5661. doi: 10.3390/molecules26185661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopes P.P., Barroca N.B., Daniel-Da-Silva A.L., Ferreira Leite B. Application of chitosan based materials for drug delivery. Front. Biomater. Chitosan Based Mater. Its Appl. 2017;3:181–248. [Google Scholar]
- 29.Atia G.A.N., et al. Drug-loaded chitosan scaffolds for periodontal tissue regeneration. Polymers. Aug. 2022;14(15):3192. doi: 10.3390/polym14153192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.P S., Gv G., Sm D., Ks A. Antimicrobial effect of silver nanoparticles synthesised with Ocimum sanctum leaf extract on periodontal pathogens. J. Oral Health Dent. Sci. Nov. 2017;1(1) [Google Scholar]
- 31.Castro M.M.L., et al. Antioxidants as adjuvants in periodontitis treatment: a systematic review and meta-analysis. Oxid Med Cell Longev. Jul. 2019;2019 doi: 10.1155/2019/9187978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shimpi S., Mahale S., Sethi K., Chaudhari D., Kadam P., Katkurwar A. Antimicrobial efficacy of copper nanoparticles against Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis: an in-vitro study. Sci. Dent. J. 2021;5(3):128. [Google Scholar]
- 33.Mahale S., Dhadse P., Mahale A. In vitro assessment of copper nanoparticle gel as a futuristic drug delivery agent against periodontal pathogens. Research sq preprint. 2023:1–16. [Google Scholar]
- 34.Fuloria N.K., Fuloria S., Chia K.Y., Karupiah S., Sathasivam K. Response of green synthesized drug blended silver nanoparticles against periodontal disease triggering pathogenic microbiota. J Appl Biol Biotechnol. 2019;7(4):46–56. [Google Scholar]
- 35.Numan A.A., Ahmed M., Galil M.S.A., Al-Qubati M., Raweh A.A., Helmi E.A. Bio-fabrication of silver nanoparticles using Catha edulis Extract: procedure optimization and antimicrobial efficacy encountering antibiotic-resistant pathogens. Adv Nanoparticles. 2022;11(2):31–54. doi: 10.4236/anp.2022.112004. 10.4236/anp.2022.112004. Available: [DOI] [Google Scholar]