Abstract
Background & Aims
Biochemical response to ursodeoxycholic acid (UDCA) therapy is associated with good prognosis in people living with primary biliary cholangitis (PBC). Biochemical response is typically assessed early in disease and it is not known what proportion of patients lose previously attained biochemical response, nor whether this impacts long-term liver transplant (LT)-free survival.
Methods
We identified all UDCA-treated patients with PBC from the Canadian Network for Autoimmune Liver disease with biochemical measurements at 1 year, and evaluated their liver biochemistry over time. Inadequate biochemical response was defined as serum alkaline phosphatase ≥1.67x the upper limit of normal or abnormal serum total bilirubin at 1 year of UDCA therapy and all time points thereafter. Multistate Markov models were used to estimate transition rates between biochemical response states and from each state to LT or death. Results were validated in an external cohort (GLOBAL PBC registry).
Results
A total of 823 patients from eight centers were included. Mean age at diagnosis was 53 years, 91% were female, 33% had inadequate biochemical response to UDCA at 1 year (n = 269). Patients who retained initial adequate response had lower rates of LT or death compared to patients who subsequently lost response (relative rate 0.102, 95% CI 0.047-0.223). Patients who regained adequate response had lower rates than patients who did not (0.016, 95% CI 0.001-0.568), and patients who lost response once more (0.010, 95% CI 0.001-0.340). Patients who regained adequate response for a third time also had lower rates than patients who did not (0.151, 95% CI 0.040-0.566). Analyses in the GLOBAL PBC registry (n = 2,237) validated these results.
Conclusion
Loss of biochemical response at any time is associated with heightened risks of LT or death in people living with PBC. Achievement of biochemical response is an important goal throughout follow-up, regardless of biochemical response profile early in therapy.
Impact and implications:
Early biochemical response to ursodeoxycholic acid is associated with good prognosis in patients with primary biliary cholangitis (PBC). Our work demonstrates that patients with PBC transition between biochemical response states over time, and that these transitions correspond with changes in risk of liver transplantation or death. Clinicians should re-evaluate risk and optimize treatment decisions for patients with PBC throughout follow-up, regardless of early biochemical response to therapy.
Keywords: UDCA, liver transplantation, prognostication, alkaline phosphatase, total bilirubin
Graphical abstract
Highlights:
-
•
Biochemical response to therapy is associated with good prognosis in primary biliary cholangitis.
-
•
Patients with primary biliary cholangitis transition between biochemical response states over time.
-
•
There is a survival benefit when a patient transitions from inadequate to adequate response at any time.
-
•
Clinicians should re-evaluate risk and optimize treatment decisions throughout follow-up.
Introduction
Primary biliary cholangitis (PBC) is a slowly progressive immune-mediated biliary disease that leads to chronic cholestasis, ductopenia, liver fibrosis, and eventual liver transplantation (LT) or death. However, ursodeoxycholic acid (UDCA) when given as first-line treatment for patients with PBC has been demonstrated to slow histological progression[1], [2], [3] and improve LT-free survival.[4], [5], [6], [7] Many criteria have been proposed to assess biochemical response to UDCA,[8], [9], [10], [11], [12], [13] the early attainment of which is associated with improved long-term clinical outcomes. Most biochemical response criteria include alkaline phosphatase (ALP) and/or total bilirubin because values of both biomarkers early in treatment have been associated with long-term clinical outcomes in patients with PBC.4,[14], [15], [16], [17] UDCA response is typically assessed between 6-24 months after UDCA initiation,[8], [9], [10], [11],13 with approximately 40% of patients classified as inadequate responders at 1 year.8 Importantly, inadequate responders to UDCA experience suboptimal survival compared to patients who meet response criteria and to matched control populations.8,18
To date, studies have investigated the association between inadequate biochemical response to UDCA and adverse clinical outcomes based on biochemical measurements at discrete time points after UDCA initiation. The decision to evaluate UDCA response at pre-specified time points early in therapy is somewhat arbitrary and does not consider that a patient with PBC may transition through different disease states over time, altering their risk of a clinical event.19 Longitudinal patterns of UDCA response, and how these patterns may relate to risks of adverse clinical events over time, remain unstudied.
We sought to move beyond frameworks that only consider UDCA response at a single time point early in the course of therapy, and describe how patients transition between response states during the course of their disease. We anchor our definition of biochemical response in real-world criteria for the initiation of second-line therapy, and we evaluate how transitions between these biochemical states associate with eventual risks of LT or death.
Materials and methods
Study design, data source and population
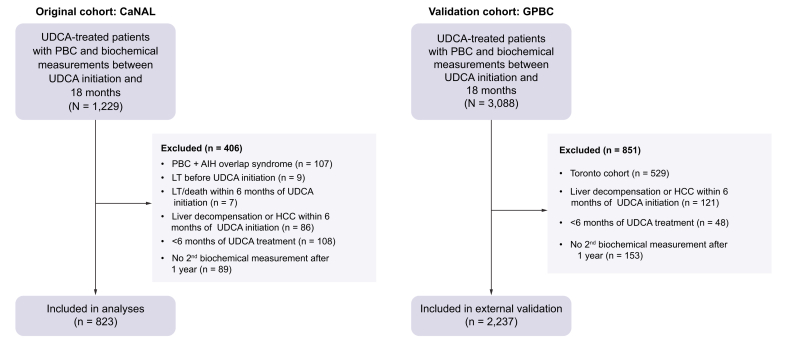
Our study involved two large and distinct cohorts of patients with PBC; one as a primary cohort and the other to validate our findings. We included patients from the Canadian Network for Autoimmune Liver disease (CaNAL), a pan-Canadian registry of patients with autoimmune liver disease including long-term retrospective data and detailed prospective follow-up at tertiary care liver clinics. CaNAL data are collected through detailed manual chart review linked to automated extraction from electronic patient records. Further details about CaNAL are available in the public domain.20,21 We included patients diagnosed with PBC according to established clinical practice guidelines of the American Association for Liver Disease and the European Association for Study of the Liver.22,23 Patients had to be treated with UDCA and have had biochemical measurements at 1 year after UDCA initiation allowing for assessment of UDCA response. We excluded patients who had overlapping clinical diagnoses of autoimmune hepatitis or had end-stage liver disease within the first 6 months of UDCA treatment based on presence of ascites, hepatic encephalopathy, variceal bleeding, hepatorenal syndrome, hepatopulmonary syndrome, gastric antral vascular ectasia, hepatocellular carcinoma, LT, or death. To ensure that patients had adequate opportunity to establish UDCA response, we excluded patients who were treated with UDCA for less than 6 months and defined index date as 1 year after UDCA initiation. Follow-up time was censored at UDCA discontinuation, initiation of second-line therapy (obeticholic acid, bezafibrate, fenofibrate), or the last follow-up. Data from the Global PBC Study Group, an international cohort of patients with PBC,14 were used for external validation. Patients from Toronto exist in both datasets and were excluded from external validation. Both studies hold relevant ethics approval at participating institutions.
Outcome measures
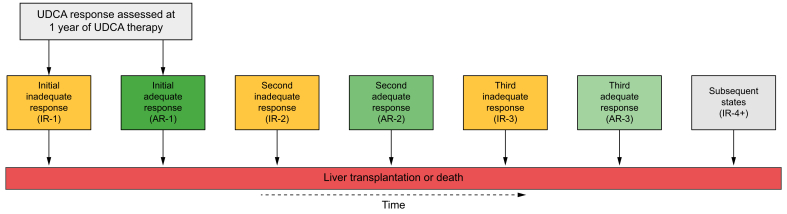
Biochemical response to UDCA was defined at every biochemical measurement throughout follow-up using the following criterion: ALP ≥1.67x the upper limit of normal (ULN) or abnormal total bilirubin.24 Limits of normal were based on site-specific values. We selected this criterion because right now it determines the clinical labelling of biochemical response and is the indication for reimbursement of second-line obeticholic acid therapy, which is immediately relevant for real-world clinical decision making. Each patient was assigned an initial state reflecting adequate or inadequate response to UDCA at 1 year after initiation. UDCA response at 1 year was defined using biochemical measurements closest to 1 year. Patients with inadequate response at 1 year were labelled IR-1 (inadequate response #1) and patients with adequate response were labelled AR-1 (adequate response #1). UDCA response was also assessed at all available biochemical measurements from 1 year until end of follow-up. As a patient’s biochemical response status changed over time, the patient was assigned a new UDCA response state at the time that transition was observed (Fig. 1). Patients could transition to a composite outcome of LT or death from any state at any point in time, and patients without one of these events were censored at last known date alive.
Fig. 1.
Multistate model of the transitions between UDCA response states over time.
This figure outlines biochemical response states through which patients progressively transition in the multistate model. Patients enter the model as IR-1 or AR-1 and progress through states sequentially or remain in a state if no change. Patients may transition to liver transplantation or death from any state at any point in time. For example, a patient with inadequate response at 1 year will begin as IR-1. This patient will transition to AR-1 when they achieve adequate response. This patient’s state will change to IR-2 if they transition back to inadequate response and will change to AR-2 if they achieve adequate response once again. The patient’s state will change to IR-3 if they transition back to inadequate response for a third time and will transition to AR-3 if they achieve adequate response for a third time. State changes beyond the fourth instance of inadequate response are absorbed into the IR-4+ state. We applied a composite endpoint of liver transplantation or death and patients without one of these two events were censored at last known date alive. Patients can transition to liver transplantation or death from any state at any point in time. AR-, adequate response; IR-, inadequate response; LT, liver transplantation; UDCA, ursodeoxycholic acid.
Statistical analysis
We applied Kaplan Meier curves to investigate the association between each UDCA response state and LT-free survival. Kaplan Meier curves employ a clock-reset approach with respect to follow-up time: patients transition to the beginning of the next survival curve when they change UDCA response state. Patients are censored at the time of state transition or the last known date alive. Survival curves are compared using the log-rank test with p values adjusted for multiple comparisons using the Benjamini-Hochberg method.25 We applied time-homogeneous multistate Markov models with progressive state changes to estimate the rate of transition between UDCA response states, and from each state to LT or death. Follow-up time began at 1 year of UDCA therapy and patients entered the model as either adequate responders or inadequate responders at 1 year. Multistate models were fitted without covariates to examine state changes over time for the overall cohort, and with covariates to investigate how patient characteristics were associated with transition between states. Covariates included age at UDCA initiation, sex, ALP x ULN, total bilirubin x ULN, and fibrosis-4 (FIB-4) score indicating advanced fibrosis.26 We compared rates of transition between different states using relative rates, calculated as the ratio of instantaneous rates from the fitted transition intensity matrix. We used the fitted transition probability matrix to estimate the percentage of patients occupying each state at any given time under complete follow-up from 1 to 15 years after UDCA initiation. The time a patient is estimated to spend in each state was defined by the integral of the transition probability matrix from 1 to 15 years after UDCA initiation. Missing biochemical values were imputed with multivariate imputation by chained equations (Table S1). Linear mixed-effects regressions with patient-level random intercepts were used to maintain within-patient correlation of imputed biochemical values and predictive mean matching was used to ensure imputed values were within plausible ranges. Imputation models included age at diagnosis, sex, ALP x ULN, total bilirubin x ULN, alanine aminotransferase x ULN, aspartate aminotransferase x ULN, platelet count, albumin x LLN, year of diagnosis, year of UDCA initiation, follow-up time, and lag values of all above biochemical parameters to better capture mechanisms of missing data.27 We randomly selected one imputed dataset for our main analyses because we did not have sufficient computational resources to repeatedly fit our multivariable model to 50 multiply imputed datasets as per recommendations.28 Instead, we replicated our analyses in a database of completely external patients (external validation) and within a second randomly selected imputed dataset (sensitivity analysis). Analyses were performed in R version 4.0.2 (R Foundation for Statistical Computing). Multistate models were fit using the msm package and imputation was performed using the mice package.29,30
Results
Cohort characteristics
A total of 823 patients from eight Canadian sites were included in the primary cohort (CaNAL, Fig. 2). Mean age at UDCA initiation was 53.0 years (SD 11.8), 91.0% were female, 86.8% were positive for anti-mitochondrial antibody, 12.6% initiated second-line therapy during follow-up, and 4.6% discontinued UDCA at a median of 3.9 years after initiation. Median follow-up after censoring was 6.5 years (25th-75th 3.3-11.6) and median year of diagnosis was 2006 (25th-75th 2000-2013). The cohort experienced 54 LTs and 81 deaths: 6.1% of patients had LT or death by 5 years, 10.8% by 10 years, and 12.6% by 15 years. Serum biochemistry at UDCA initiation and 1 year are presented in Table 1.
Fig. 2.
Study flowchart for the original and validation cohorts.
The same exclusion criteria were applied to both cohorts; exclusion criteria which resulted in 0 patients being excluded are not included in the figure for the validation cohort. Patients from Toronto were excluded from the validation cohort because they were present in the original cohort. AIH, autoimmune hepatitis; CaNAL, Canadian Network for Autoimmune Liver disease; GAVE, gastric antral vascular ectasia; GPBC, Global PBC Study group; HCC, hepatocellular carcinoma; HE, hepatic encephalopathy; HPS, hepatopulmonary syndrome; HRS, hepatorenal syndrome; LT, liver transplantation; PBC, primary biliary cholangitis; SBP, spontaneous bacterial peritonitis; UDCA, ursodeoxycholic acid.
Table 1.
Patient characteristics.
| Characteristic | Main cohort (n = 823) |
|---|---|
| Age at UDCA initiation, mean (SD) | 53.0 (11.8) |
| Female sex, n (%) | 749 (91.0) |
| AMA positive, n (%) | 714 (86.8) |
| Discontinued UDCA, n (%) | 38 (4.6) |
| Initiated second-line therapy, n (%) | 104 (12.6) |
| Obeticholic acid | 60 (7.3) |
| Bezafibrate | 21 (2.6) |
| Fenofibrate | 41 (5.0) |
| Liver transplantation or death - n (%) | |
| 5 years after UDCA initiation | 50 (6.1) |
| 10 years after UDCA initiation | 89 (10.8) |
| 15 years after UDCA initiation | 104 (12.6) |
| Serum biochemistry at UDCA initiation | |
| Alkaline phosphatase x ULN, mean (SD) | 2.36 (1.87) |
| Alkaline phosphatase ≥1.67x ULN, n (%) | 450 (54.7) |
| Total bilirubin x ULN, mean (SD) | 0.76 (0.99) |
| Total bilirubin >1x ULN, n (%) | 125 (15.2) |
| Platelet count (x109/L), mean (SD) | 265 (102) |
| Platelet count <150x109/L, n (%) | 74 (9.0) |
| FIB-4 score ≥4.03, n (%) | 61 (7.4) |
| Serum biochemistry at 1 year | |
| Alkaline phosphatase x ULN, mean (SD) | 1.53 (1.30) |
| Alkaline phosphatase ≥1.67x ULN, n (%) | 228 (27.7) |
| Total bilirubin x ULN, mean (SD) | 0.74 (1.25) |
| Total bilirubin >1x ULN, n (%) | 102 (12.4) |
| Platelet count (x109/L), mean (SD) | 243 (94) |
| Platelet count <150x109/L, n (%) | 120 (14.6) |
| FIB-4 score ≥4.03, n (%) | 51 (6.2) |
ALP, alkaline phosphatase; AMA, anti-mitochondrial antibody; FIB-4, fibrosis-4; UDCA, ursodeoxycholic acid; ULN, upper limit of normal.
Transitions through biochemical response states over time
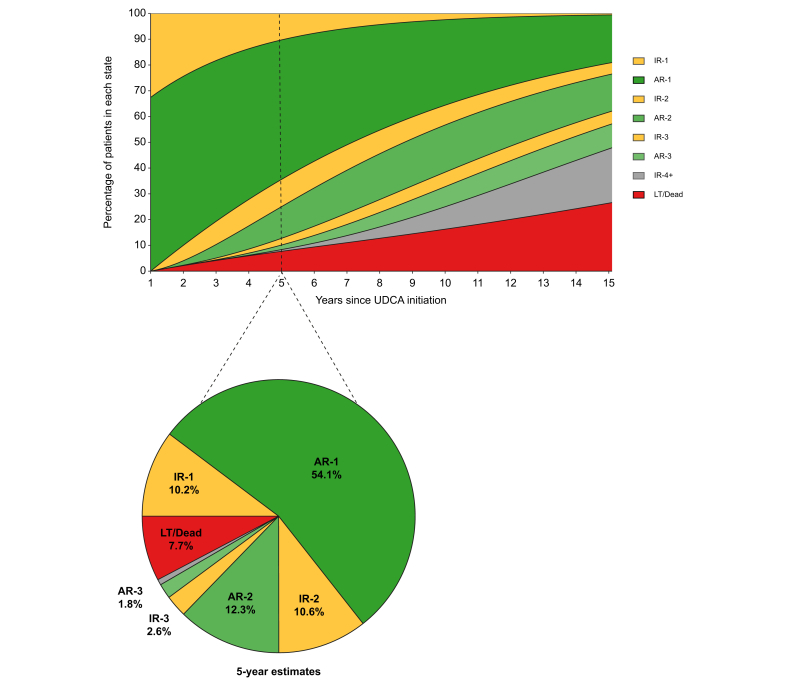
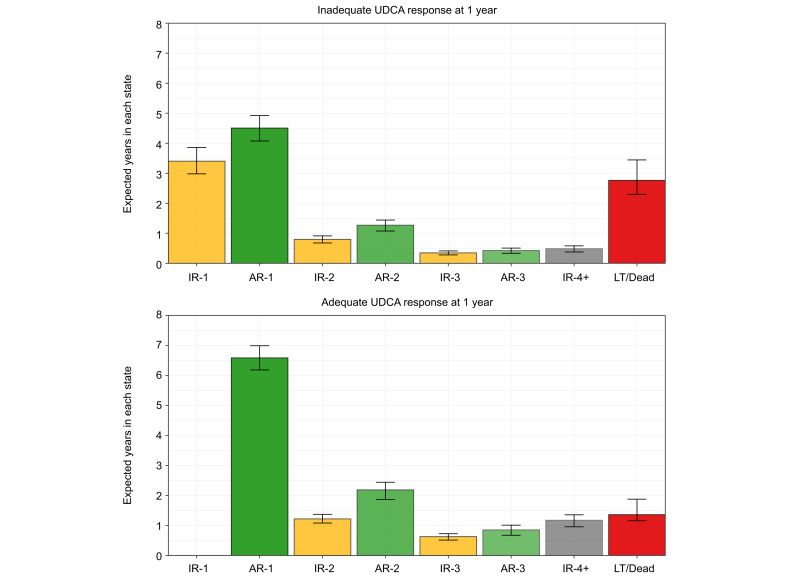
At UDCA initiation, 54.7% of patients had ALP ≥1.67x ULN and 15.2% had total bilirubin >1x ULN. At 1 year of UDCA therapy, 32.6% of patients had inadequate biochemical response: 27.7% had ALP ≥1.67x ULN and 12.4% had total bilirubin >1x ULN. Twenty-three percent of patients with normal total bilirubin and 6.7% of patients with ALP <1.67x ULN were inadequate responders at 1 year. An estimated 10.2% of patients never achieved adequate biochemical response to UDCA by 5 years, 54.1% achieved adequate response and retained their AR-1 state by 5 years, and 7.7% underwent LT or death by 5 years. The remaining 28.0% of patients fluctuated between multiple states: 13.2% lost adequate response and were in IR-2 or IR-3 by 5 years, 14.1% regained adequate response and were in AR-2 or AR-3 by 5 years, and 0.7% transitioned between adequate and inadequate biochemical response at least five times by 5 years (IR-4+ state) (Fig. 3). Patients with inadequate response to UDCA at 1 year were estimated to remain in their IR-1 state for an additional mean of 3.4 years (95% CI 3.0-3.8). If these patients achieved adequate biochemical response, they were expected to retain this response and remain in AR-1 for 4.5 years (95% CI 4.1-4.9). Patients with adequate response at 1 year were estimated to remain in their AR-1 state for an additional 6.6 years (95% CI 6.1-7.0) (Fig. 4).
Fig. 3.
Estimated percentage of patients in each response state over time, whole cohort.
Estimated percentage of patients in each response state over time in the main cohort, derived from the fitted transition probability matrix of the multistate model. Pie chart represents the breakdown of states at 5 years, corresponding to the dashed vertical line in the rectangular figure. The pie chart represents the 5-year values reported in the text. AR-, adequate response; IR-, inadequate response; LT, liver transplantation; UDCA, ursodeoxycholic acid.
Fig. 4.
Estimated mean time spent in each state from 1 to 15 years after UDCA initiation, by UDCA response status at 1 year.
Patients with inadequate response to UDCA at 1 year were estimated to remain in their IR-1 state for an additional mean of 3.4 years (95% CI 3.0-3.8). If these patients achieve adequate biochemical response, they were expected to retain this response and remain in AR-1 for 4.5 years (95% CI 4.1-4.9). Patients with adequate response at 1 year were estimated to remain in their AR-1 state for an additional 6.6 years (95% CI 6.1-7.0). A patient with inadequate response to UDCA at 1 year will enter the IR1 state, and any subsequent achievement of biochemical response moves them to the AR-1 state. A patient with adequate response to UDCA at 1 year will enter the AR-1 state, and any subsequent loss of response moves them to the IR-2 state. The time a patient is estimated to spend in each state was defined by the integral of the transition probability matrix from 1 to 15 years after UDCA initiation. AR-, adequate response; IR-, inadequate response; LT, liver transplant; UDCA, ursodeoxycholic acid.
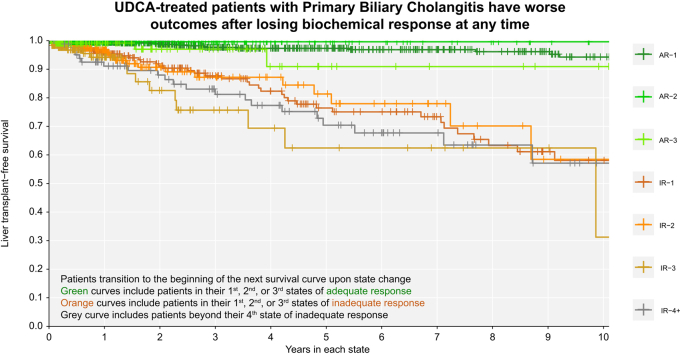
Associations between biochemical response over time and LT-free survival
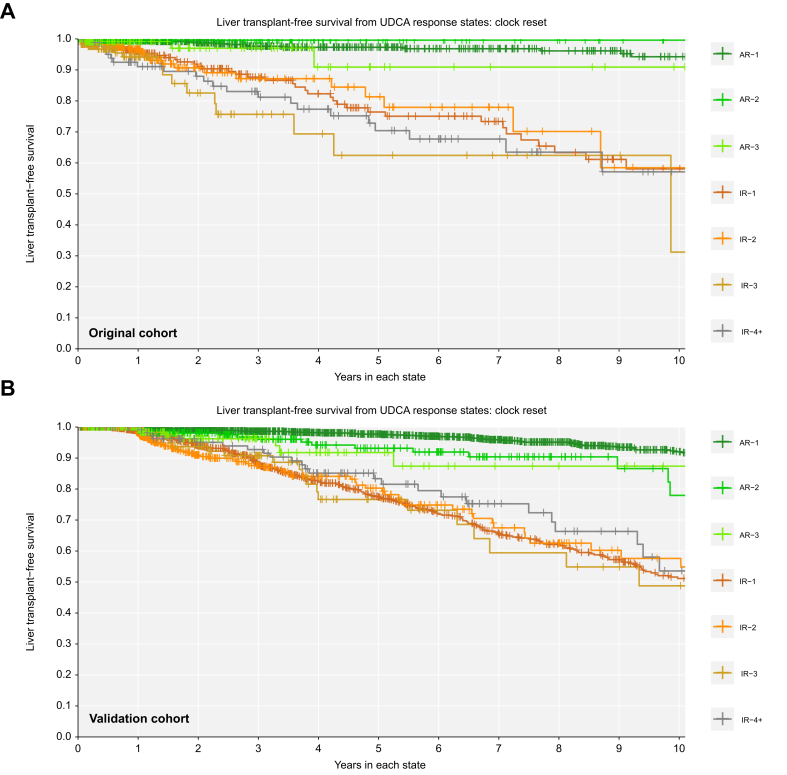
Patients with inadequate biochemical response to UDCA at 1 year had significantly worse LT-free survival over time compared to patients with adequate response at 1 year (Fig. 5). Achieving adequate biochemical response was associated with survival benefit regardless of prior history of inadequate response. Patients in their first, second, and third states of adequate response had better LT-free survival than patients in their first, second, and third states of inadequate response (log-rank p <0.01 all pairwise comparisons to the previous state, Fig. 5). At any time, patients who retained their initial adequate response had significantly lower rates of LT or death than patients who never achieved adequate response (AR-1 vs. IR-1: 0.089-fold, 95% CI 0.046-0.170) or who transitioned back to inadequate response (AR-1 vs. IR-2: 0.102-fold, 95% CI 0.047-0.223). Patients who regained adequate response for the second time had significantly lower rates of LT or death than patients who did not regain response (AR-2 vs. IR-2: 0.016-fold, 95% CI 0.001-0.568) or who transitioned to inadequate response for a third time (AR-2 vs. IR-3: 0.010-fold, 95% CI 0.001-0.340). Patients who regained adequate response for a third time also had lower rates of LT or death compared to patients who did not regain adequate response for a third time (AR-3 vs. IR-3: 0.151-fold, 95% CI 0.040-0.566).
Fig. 5.
Over time, adequate response is always associated with better liver transplant-free survival.
(A) Original cohort; (B) validation cohort. Clock-reset Kaplan Meier curves of survival probability from each UDCA response state. Follow-up time begins 1 year after UDCA initiation. Patients transition from one curve to the next at the time of their state change. Patients are censored at last date known alive or at the time of state change. Log-rank p <0.01 all pairwise comparisons to previous state. p values are corrected for multiple comparisons using the Benjamini-Hochberg method. AR-, adequate response; IR-, inadequate response; UDCA, ursodeoxycholic acid.
Exploratory analysis of associations between patient characteristics and state transitions
Tables S2–S4 present estimates of the association between baseline patient characteristics and state transitions in the main analysis, sensitivity analysis, and external validation. We do not report specific point estimates due to variation between the three analyses and instead comment on associations that were consistent across all analyses (i.e. where 95% CIs exclude 1 in all three analyses). Older age at UDCA initiation was associated with an increased rate of transition from all states of inadequate response to adequate response (Table S2). Higher serum ALP at 1 year was associated with a decreased rate of ever achieving adequate response, an increased rate of losing adequate response, a decreased rate of regaining adequate response again, and an increased rate of losing adequate response again (Table S3). Higher serum total bilirubin at 1 year was also associated with an increased rate of losing adequate response, a decreased rate of regaining adequate response, and an increased rate of losing adequate response again (Table S4). Sex was not associated with rate of initial response, transitions between any response states, or transitions from any state to transplantation or death (all 95% CI exclude 1 in all three analyses). We were unable to comment on FIB-4 score due to too few patients with FIB-4 >4.03 at 1 year.
Study validation and sensitivity analysis
We replicated all analyses in a separate validation cohort of external patients with PBC (n = 2,237, 14 sites, Fig. 2). In the external validation cohort, mean age at UDCA initiation was 54.5 years (SD 11.7), 91.1% were female, mean ALP at 1 year was 1.78x ULN (SD 1.45), mean total bilirubin at 1 year was 0.80 (SD 1.22), and 14.3% had cirrhosis. Median follow-up after censoring was 7.1 years (25th-75th 3.3-11.4) and 7.2% had LT or death by 5 years, 14.4% by 10 years, and 18.2% by 15 years. Median year of diagnosis was 1997 (25th-75th 1991-2004). Patients in the external validation cohort who retained their initial adequate response to UDCA had significantly lower rates of LT or death than patients who never achieved adequate response (AR-1 vs. IR-1: 0.061-fold, 95% CI 0.035-0.105) or transitioned back to inadequate response (AR-1 vs. IR-2: 0.061-fold, 95% CI 0.031-0.119, Fig. 5). Patients who achieved adequate response for the second time also had significantly lower rates of LT or death than patients who did not regain adequate response (AR-2 vs. IR-2: 0.077-fold, 95% CI 0.012-0.481) or who transitioned to inadequate response once more (AR-2 vs. IR-3: 0.073-fold, 95% CI 0.001-0.551). Patients who regained adequate response for a third time also had lower rates of LT or death compared to patients who did not regain adequate response for a third time (AR-3 vs. IR-3: 0.241-fold, 95% CI 0.071-0.823).
Sensitivity analysis in a second imputed dataset selected at random demonstrated the same associations as the main analysis and external validation. Both external validation and sensitivity analysis showed that biochemical states varied during follow-up and often did not reflect biochemical response status at 1 year. All analyses indicated that younger age, higher ALP at 1 year, and higher total bilirubin at 1 year were associated with a tendency towards transitioning to insufficient response throughout follow-up (Tables S2–S4).
Discussion
PBC is a chronic disease where patients are routinely monitored over time, usually for decades. We investigated longitudinal patterns of biochemical response to UDCA therapy and their association with LT or death, first in a multicenter Canadian cohort of patients with PBC and then in an international cohort. We demonstrated, and subsequently validated, that patients transition between adequate and inadequate response to UDCA such that biochemical response status over time may not reflect status at 1 year after UDCA initiation. These transitions between adequate and inadequate biochemical response over time were associated with a significant change in a patient’s risk of LT or death throughout the course of disease. Our findings challenge the current paradigm that long-term risk assessment should be based on biochemical response at 6 months,13 1 year,8,10,11 or 2 years.9 The presence of insufficient biochemical response is a risk factor for worse outcomes at any time and is therefore an indication to optimize treatment when a patient’s biochemical state worsens.
Our findings support that adequate biochemical response to UDCA treatment is a valid surrogate of prognosis throughout a patient’s course of disease. Achievement of adequate biochemical response at any given time was associated with decreased rates of LT or death regardless of a patient’s prior history of inadequate response. In other words, when a patient transitions from inadequate to adequate response, they experience a corresponding survival benefit. When a patient loses adequate response, their risk of LT or death increases. These results emphasize the value in regular monitoring of patients’ biochemical profile over time to re-evaluate their risk of LT or death and intervene proactively. Continuous re-assessment of biochemical response provides important clinical information about a patient’s changing risk profile that may highlight opportunities for intervention to reduce the risk of LT or death.
Importantly, our definition of biochemical response reflects widely used criteria for the indication and reimbursement of second-line therapy. We highlight that our study is not about optimal prognostication of patients with PBC. Research on improving prognostication should build on richer continuous scores (e.g. GLOBE, UK-PBC)31,32 instead of dichotomous biochemical response criteria. Rather, we aim to confirm and spread a critical message about response-guided clinical management anchored in the context of current decision-making criteria. Our analyses focus on a dichotomous biochemical response criterion (ALP ≥1.67x ULN or abnormal total bilirubin) instead of richer continuous prognostic scores because this criterion reflects clinical labelling of biochemical response and is the indication and reimbursement criteria for second-line obeticholic acid.[33], [34], [35], [36] Our results support response-guided management of patients with PBC, and are timely because response-guided management is often poorly implemented in practice (e.g., approximately 1/3 of patients with PBC are under-dosed on UDCA, 1/2 do not receive second-line therapies for insufficient biochemical response).37,38 We acknowledge that applying dichotomous response criteria does not consider that patients have a distribution of risk within their dichotomous response categories, but neither do reimbursement criteria for second-line therapy. Our study does not comment on how changes in the magnitude of continuous biochemistry over time may influence state transitions and clinical outcomes in patients with PBC.
Exploratory results demonstrated that younger age, higher ALP at 1 year, and higher total bilirubin at 1 year were associated with a tendency towards transition to inadequate response throughout follow-up. These results align with the current clinical paradigm that young age, high serum ALP at 1 year, and high serum total bilirubin at 1 year are important factors to consider when assessing future risk in patients with PBC. We did not observe an association between sex and biochemical response or LT and death. Earlier studies have reported that males present with more advanced disease and are less likely to respond to UDCA treatment than females.39,40 Our results are consistent with recent findings from a large international cohort that reported no association between male sex and probability of treatment failure, LT, or death.41 The prognostic role of male sex has been an ongoing subject of debate and while our results suggest that male sex does not meaningfully impact risk after consideration of other important variables, this is yet to be resolved in the literature.
A key strength of our study is our use of all available biochemical measurements over time. We investigated associations using serum biochemistry across continuous time, allowing our estimates to consider the natural times at which patients transition between response states and better reflect the continuous nature of disease progression. This approach allowed our analyses to leverage information from all observations and incorporate more information than an analysis of discrete time points. We were unable to account for adherence to and dose of UDCA therapy.
Our analyses assume that a patient’s future states only depend on their current state and not the time spent in the current state nor previous states (Markov assumption). Our analyses also assume that transition rates between states are consistent across time (time-homogeneity assumption). We were unable to pool results from multiply imputed datasets due to computational limitations. As a result, our 95% CIs are likely underestimated because we were not able to account for between-imputation variability in our data. We remain confident in our results because the external validation was consistent with our main analysis. Our intermittent observation scheme may underestimate the number of true state transitions because patients may transition between response states at times when they are not observed in clinic. This may also contribute to overestimation of the time that patients spend in each state. This limitation is unavoidable and applies to all studies without continuous patient observation. While this may limit our ability to extrapolate our results to the true disease course of PBC, it accurately reflects the information that is available to physicians to evaluate biochemical risk and inform clinical decision making. Second-line therapies are new in PBC; therefore, we were unable to investigate their association with long-term biochemical response and clinical outcomes. As patients accrue more follow-up on second-line therapies, future studies should investigate whether their use has an impact on longitudinal patterns of biochemical response and whether those altered patterns associate with improved clinical outcomes.
In conclusion, achievement of adequate biochemical response to UDCA therapy in people living with PBC is associated with better outcomes at any time during the course of disease. The presence of insufficient biochemical response is a risk factor for worse outcomes and is therefore an indication to optimize treatment throughout follow-up when a patient’s biochemical state worsens. Treatment goals in PBC should target durable biochemical response and move beyond single time point measures early in disease.
Abbreviations
ALP, alkaline phosphatase; AR-1, adequate response #1; CaNAL, Canadian Network for Autoimmune Liver Disease; FIB-4Fibrosis-4; IR-1, inadequate response #1; LT, liver transplant; PBC, primary biliary cholangitis; ULN, upper limit of normal; UDCA, ursodeoxycholic acid.
Financial support
Unrestricted financial support from the Canadian Liver Foundation, Intercept Pharma Canada Inc., and the Toronto General & Western Hospital Foundation support cohort maintenance. Funders had no influence on the study design, data collection, analysis and interpretation of data, or the decision to submit for publication.
Conflict of interest
Authors not listed below report no disclosures. Nora Cazzagon reports consulting fees from Ipsen, Orphalan, and Albireo; honoraria from Advanz, Orphalan, and Albireo; travel support from Orphalan and Ipsen. Hin Hin Ko has received honorarium and worked as consultant and on advisory board for Abbvie, Avir Pharma, Gilead, GSK, Intercept, Ipsen, and Sanofi. Mark Swain reports: Advisory role: Gilead, Ipsen, Pfizer, Roche, Novo Nordisk, GSK. Speaker: Gilead, Abbott. Clinical trial or research support: Gilead, BMS, CymaBay, Intercept, Genfit, Pfizer, Novartis, Astra Zeneca, GSK, Celgene, Novo Nordisk, Axcella Health Inc., Merck, Galectin Therapeutics, Calliditas Therapeutics, Madrigal, AbbVie, Altimmune, Roche, Kowa, Arbutus, Eiger, Janssen, Ipsen, Allergan, Assembly, Arbutus, Enanta, Galmed, Inventiva, Sagimet. Tony Bruns received consulting fees from Intercept/Advanz Pharma, Grifols, and Sobi as well as honoraria for lectures, presentations, or educational events from Falk Foundation, CSL Behring, Merck, Gilead, Intercept/Advanz Pharma, and Gore. Ana Lleo reports receiving consulting fees from Advanz Pharma, AlfaSigma, Takeda, and Albireo Pharma, and speaker fees from Gilead, Abbvie, MSD, Intercept Pharma, AlfaSigma, GSK, and Incyte. Pietro Invernizzi reports consulting fees from Advanz, Ipsen, Zydus, and Calliditas; travel support from Zydus and Ipsen. Christophe Corpechot reports grants from Arrow Génériques and Intercept; consulting fees from Advanz, Ipsen, Cymabay, GSK; honoraria from EchoSens; travel support from Gilead, Ipsen, BioTest. Cyriel Ponsioen reports grants from Perspectum and Gilead; consulting fees from Chemomab and NGM. Adriaan van der Meer reports grants from Cymabay, Gilead, Zambon Nederland B.V; consulting fess from Intercept, Cymabay, Ipsen; honoraria from AOP Health. Maria-Carlota Londoño reports grants from Mirum, consulting fees from Advanz, Ipsen, GSK; honoraria from Advanz, Cymabay, Albireo; travel support from Advanz, Ipsen. Douglas Thorburn reports grants from Advanz; consulting fees from Pilant, Ipsen, ChemomaAb; honoraria from Advanz, Ipsen; travel support from Ipsen. Catherine Vincent reports honoraria from Intercept. Marco Carbone reports consulting fees from Advanz, Cymabay, Ipsen, Mayloy, Kowa; honoraria from Advanz, Ipsen, Mayloy; travel support from Advanz, Ipsen. Nikolaos Gatselis reports honoraria and travel support from Genesis Pharma. Cynthia Levy reports grants from Gilead, Mirum, GSK, Kowa, Intercept, Escient, Calliditas, Zydus, Ipsen, Cymabay; consulting fees from Gilead, Chemomab, Kowa, Intercept, Cymabay, Ipsen, GSK, Calliditas, Mirum; associate editor for Hepatology (journal). Albert Pares reports consulting fees from Calliditas, Falk, and Kowa. Palak Trivedi reports grants from from GSK, LifeArc, PSC Support, Gilead Sciences, Medical Research Foundation, Bristol Myers Squibb, Intercept, Falk Pharma; consulting fees from GSK, Falk Pharma, Pliant, Cymabay, Albireo, Ipsen, Chemomab; honoraria from Advanz, Albireo, Ipsen; leadership role as Chief Investigator of UK-PSC, BASL SIG Chair for Immune-mediated and cholestatic liver disease. Nazia Selzner reports honoraria from Paladin Pharma. Andrew Mason reports grants from Intercept and the Canadian Liver Foundation; consulting fees from the Canadian Agency for Drugs and Technologies in Health, GSK; honoraria from Intercept; travel support from Praespero. Aldo Montano-Loza reports unrestricted grant funding from Intercept. Kris Kowdley reports grants from Gilead, Intercept, 89bio, Cymabay, Genfit, Madrigal, Mirum, Pfizer, NGM, Corcept, GSK, Hanmi, Pliant, Novo Nordisk, Terns, Viking; honoraria from Gilead, Abbvie, Intercept; stock in Inipharm; consulting fees from 89bio, Cymabay, Genfit, Madrigal, Mirum, Pfizer, NGM, Inipharm, Enantha, HighTide, Salix. Pier M Battezzati reports grants from Roche, Tobira Therapeutics, Ultragenyx Pharmaceuticals, Salix Pharmaceuticals, Abbvie, Cymabay, Intercept Pharmaceuticals. Marlyn J Mayo reports grants from Gilead, Intercept, Genfit, Cymabay, Mallinckrodt, Genfit, GSK, Novartis, Target PharmaSolutions; consulting fees from Cymabay, Mallinckrodt, Target PharmaSolutions, Mirum; honoraria from GSK. Aliya F Gulamhusein reports consulting fees from Advanz Pharma and Cymabay; honoraria from Advanz. Harry LA Janssen reports grants from Gilead, GSK, Janssen, Roche, Vir Biotechnilogy Inc; consulting fees from Aligos, Gilead, GSK, Grifols, Roche, Vir Biotechnology Inc, Precision Biosciences. Gideon M Hirschfield reports consulting fees from Kowa, Intercept, Advanz, Mirum, GSK, Escient, Ipsen, Pliant, Gilead, Cymabay. Bettina E Hansen reports grants from Ipsen, Intercept, Cymabay; consulting fees from Intercept, Advanz, Cymabay, Ipsen, Mirum, Calliditas, ChemoMab, Enyo; honoraria from Mirium; travel support from SLO foundation, PBC foundation, PSC Partners; unpaid leadership positions at EASL, AASLD, NAPPED, TreatFIC, Global PBC Study Group, GALA, CaNAL.
Please refer to the accompanying ICMJE disclosure forms for further details.
Authors’ contributions
Conceptualization: SBR, GMH, BEH. Data curation: All authors. Formal analysis: SBR, GMH, WJC, ALM, BEH. Funding acquisition: GMH, BEH. Investigation: SBR, GMH, ALM, BEH. Methodology: SBR, WJC, GMH, BEH. Project administration: All authors. Resources: GMH, HLAJ, ALM, BEH. Software: SBR. Supervision: GMH, BEH. Validation: SBR, GMH, BEH. Visualization: SBR, WJC. Writing – original draft: SBR, WJC. Writing – review & editing: all authors.
Data availability statement
These data are not publicly available due to the sensitive nature of patient data. Researchers interested in using these data will need to comply with all relevant REB requirements of these datasets. Contact the corresponding authors for more information.
Acknowledgements
We would like to thank the Global PBC Study Group for providing their data for our external validation analysis. We would like to thank peer reviewers for their useful feedback that helped us clarify and improve the messaging of our work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2024.101168.
Contributor Information
Gideon M. Hirschfield, Email: gideon.hirschfield@uhn.ca.
Bettina E. Hansen, Email: bettina.hansen@utoronto.ca.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Angulo P., Batts K.P., Therneau T.M., et al. Long-term ursodeoxycholic acid delays histological progression in primary biliary cirrhosis. Hepatology. 1999;29(3):644–647. doi: 10.1002/hep.510290301. [DOI] [PubMed] [Google Scholar]
- 2.Corpechot C., Carrat F., Bonnand A.M., et al. The effect of ursodeoxycholic acid therapy on liver fibrosis progression in primary biliary cirrhosis. Hepatology. 2000;32(6):1196–1199. doi: 10.1053/jhep.2000.20240. [DOI] [PubMed] [Google Scholar]
- 3.Poupon R.E., Lindor K.D., Parés A., et al. Combined analysis of the effect of treatment with ursodeoxycholic acid on histologic progression in primary biliary cirrhosis. J Hepatol. 2003;39(1):12–16. doi: 10.1016/s0168-8278(03)00192-2. [DOI] [PubMed] [Google Scholar]
- 4.Poupon R.E., Poupon R., Balkau B. Ursodiol for the long-term treatment of primary biliary cirrhosis. New Engl J Med. 1994;330(19):1342–1347. doi: 10.1056/NEJM199405123301903. [DOI] [PubMed] [Google Scholar]
- 5.Lindor K.D., Therneau T.M., Jorgensen R.A., et al. Effects of ursodeoxycholic acid on survival in patients with primary biliary cirrhosis. Gastroenterology. 1996;110(5):1515–1518. doi: 10.1053/gast.1996.v110.pm8613058. [DOI] [PubMed] [Google Scholar]
- 6.Poupon R.E., Lindor K.D., Cauch-Dudek K., et al. Combined analysis of randomized controlled trials of ursodeoxycholic acid in primary biliary cirrhosis. Gastroenterology. 1997;113(3):884–890. doi: 10.1016/S0016-5085(97)70183-5. [DOI] [PubMed] [Google Scholar]
- 7.Harms M.H., van Buuren H.R., Corpechot C., et al. Ursodeoxycholic acid therapy and liver transplant-free survival in patients with primary biliary cholangitis. J Hepatol. 2019;71(2):357–365. doi: 10.1016/j.jhep.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Parés A., Caballería L., Rodés J. Excellent long-term survival in patients with primary biliary cirrhosis and biochemical response to ursodeoxycholic acid. Gastroenterology. 2006;130(3):715–720. doi: 10.1053/j.gastro.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 9.Kumagi T., Guindi M., Fischer S.E., et al. Baseline ductopenia and treatment response predict long-term histological progression in primary biliary cirrhosis. Am J Gastroenterol. 2010;105(10):2186–2194. doi: 10.1038/ajg.2010.216. [DOI] [PubMed] [Google Scholar]
- 10.Corpechot C., Abenavoli L., Rabahi N., et al. Biochemical response to ursodeoxycholic acid and long-term prognosis in primary biliary cirrhosis. Hepatology. 2008;48(3):871–877. doi: 10.1002/hep.22428. [DOI] [PubMed] [Google Scholar]
- 11.Corpechot C., Chazouillères O., Poupon R. Early primary biliary cirrhosis: biochemical response to treatment and prediction of long-term outcome. J Hepatol. 2011;55(6):1361–1367. doi: 10.1016/j.jhep.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 12.Kuiper E.M.M., Hansen B.E., de Vries R.A., et al. Improved prognosis of patients with primary biliary cirrhosis that have a biochemical response to ursodeoxycholic acid. Gastroenterology. 2009;136(4):1281–1287. doi: 10.1053/j.gastro.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Angulo P., Lindor K.D., Therneau T.M., et al. Utilization of the Mayo risk score in patients with primary biliary cirrhosis receiving ursodeoxycholic acid. Liver Int. 1999;19(2):115–121. doi: 10.1111/j.1478-3231.1999.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 14.Lammers W.J., van Buuren H.R., Hirschfield G.M., et al. Levels of alkaline phosphatase and bilirubin are surrogate end points of outcomes of patients with primary biliary cirrhosis: an international follow-up study. Gastroenterology. 2014;147(6):1338–1349. doi: 10.1053/j.gastro.2014.08.029. [DOI] [PubMed] [Google Scholar]
- 15.Christensen E., Crowe J., Donaich D., et al. Clinical pattern and course of disease in primary biliary cirrhosis based on an analysis of 236 patients. Gastroenterology. 1980;78(2):236–246. doi: 10.1016/0016-5085(80)90571-5. [DOI] [PubMed] [Google Scholar]
- 16.Trivedi P.J., Bruns T., Cheung A., et al. Optimising risk stratification in primary biliary cirrhosis: AST/platelet ratio index predicts outcome independent of ursodeoxycholic acid response. J Hepatol. 2014;60(6):1249–1258. doi: 10.1016/j.jhep.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 17.Bonnand A.M., Heathcote E.J., Lindor K.D., et al. Clinical significance of serum bilirubin levels under ursodeoxycholic acid therapy in patients with primary biliary cirrhosis. Hepatology. 1999;29(1):39–43. doi: 10.1002/hep.510290140. [DOI] [PubMed] [Google Scholar]
- 18.Lammert C., Juran B.D., Schlicht E., et al. Biochemical response to ursodeoxycholic acid predicts survival in a North American cohort of primary biliary cirrhosis patients. J Gastroenterol. 2014;49(10):1414–1420. doi: 10.1007/s00535-013-0903-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gatselis N.K., Goet J.C., Zachou K., et al. Factors associated with progression and outcomes of early stage primary biliary cholangitis. Clin Gastroenterol Hepatol. 2020;18(3):684–692.e6. doi: 10.1016/j.cgh.2019.08.013. [DOI] [PubMed] [Google Scholar]
- 20.Canadian Network for Autoimmune Liver disease. CaNAL registry. Accessed May 22, 2021. https://www.canalregistry.ca/.
- 21.US National Library of Medicine. Canadian Network for autoimmune liver disease. ClinicalTrials.gov. Accessed May 22, 2021. https://clinicaltrials.gov/ct2/show/NCT03569826.
- 22.Lindor K.D., Bowlus C.L., Boyer J., et al. Primary biliary cholangitis: 2018 practice guidance from the American association for the study of liver diseases. Hepatology. 2019;69(1):394–419. doi: 10.1002/hep.30145. [DOI] [PubMed] [Google Scholar]
- 23.European Association for the Study of the Liver EASL Clinical Practice Guidelines: the diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67(1):145–172. doi: 10.1016/j.jhep.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 24.Nevens F., Andreone P., Mazzella G., et al. A placebo-controlled trial of obeticholic acid in primary biliary cholangitis. New Engl J Med. 2016;375(7):631–643. doi: 10.1056/NEJMoa1509840. [DOI] [PubMed] [Google Scholar]
- 25.Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B (Methodological) 1995;57(1):289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- 26.Murillo Perez C.F., Hirschfield G.M., Corpechot C., et al. Fibrosis stage is an independent predictor of outcome in primary biliary cholangitis despite biochemical treatment response. Aliment Pharmacol Ther. 2019;50(10):1127–1136. doi: 10.1111/apt.15533. [DOI] [PubMed] [Google Scholar]
- 27.Harel O., Mitchell E.M., Perkins N.J., et al. Multiple imputation for incomplete data in epidemiologic studies. Am J Epidemiol. 2018;187(3):576–584. doi: 10.1093/aje/kwx349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jakobsen J.C., Gluud C., Wetterslev J., et al. When and how should multiple imputation be used for handling missing data in randomised clinical trials - a practical guide with flowcharts. BMC Med Res Methodol. 2017;17(1):1–10. doi: 10.1186/s12874-017-0442-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jackson C.H. Multi-state models for panel data: the msm package for R. J Stat Softw. 2011;38(8):1–28. doi: 10.18637/jss.v038.i08. [DOI] [Google Scholar]
- 30.van Buuren S., Groothuis-Oudshoorn K. mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45(3):1–67. doi: 10.18637/jss.v045.i03. [DOI] [Google Scholar]
- 31.Carbone M., Sharp S.J., Flack S., et al. The UK-PBC risk scores: derivation and validation of a scoring system for long-term prediction of end-stage liver disease in primary biliary cholangitis. Hepatology. 2016;63(3):930–950. doi: 10.1002/hep.28017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lammers W.J., Hirschfield G.M., Corpechot C., et al. Development and validation of a scoring system to predict outcomes of patients with primary biliary cirrhosis receiving ursodeoxycholic acid therapy. Gastroenterology. 2015;149(7):1804–1812. doi: 10.1053/j.gastro.2015.07.061. [DOI] [PubMed] [Google Scholar]
- 33.Ministry of Health and Long-Term Care . 2021. Exceptional access program reimbursement criteria for frequently requested Drugs. [Google Scholar]
- 34.Ministry of Health - Province of British Columbia. Limited coverage Drugs – obeticholic acid. Accessed June 8, 2021. https://www2.gov.bc.ca/gov/content/health/practitioner-professional-resources/pharmacare/prescribers/limited-coverage-drug-program/limited-coverage-drugs-obeticholic-acid.
- 35.Alberta Blue Cross. Criteria for coverage OBETICHOLIC ACID.
- 36.Saskatchewan Ministry of Health . 2018. Saskatchewan formulary bulletin. [Google Scholar]
- 37.Abbas N., Smith R., Flack S., et al. Critical shortfalls in the management of PBC: results of a UK-wide, population-based evaluation of care delivery. JHEP Rep. 2024;6(1) doi: 10.1016/j.jhepr.2023.100931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lammers W.J., Leeman M., Ponsioen C.I., et al. How the concept of biochemical response influenced the management of primary biliary cholangitis over time. Neth J Med. 2016;74(6):240–246. [PubMed] [Google Scholar]
- 39.Carbone M., Mells G.F., Pells G., et al. Sex and age are determinants of the clinical phenotype of primary biliary cirrhosis and response to ursodeoxycholic acid. Gastroenterology. 2013;144(3):560–569. doi: 10.1053/j.gastro.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 40.Rubel L.R., Rabin L., Seeff L.B., et al. Does primary biliary cirrhosis in men differ from primary biliary cirrhosis in women? Hepatology. 1984;4(4):671–677. doi: 10.1002/hep.1840040418. [DOI] [PubMed] [Google Scholar]
- 41.Cheung A.C., Lammers W.J., Murillo Perez C.F., et al. Effects of age and sex of response to ursodeoxycholic acid and transplant-free survival in patients with primary biliary cholangitis. Clin Gastroenterol Hepatol. 2019;17(10):2076–2084. doi: 10.1016/j.cgh.2018.12.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
These data are not publicly available due to the sensitive nature of patient data. Researchers interested in using these data will need to comply with all relevant REB requirements of these datasets. Contact the corresponding authors for more information.