Abstract
Background & Aims
Elevated liver stiffness has been associated with atrial fibrillation (AFib) in the general population. The mechanism underlying this association is unclear.
Methods
Participants were recruited from the general population and prospectively enrolled with follow-up for 5 years. The fibrosis-4 (FIB-4) index was used as a surrogate marker for liver fibrosis. Proteomics analysis was performed using the 92-target Olink inflammation panel. Validation was performed using the NAFLD fibrosis score (NFS), aspartate aminotransferase to platelet index (APRI), and repeat confirmation proteomics.
Results
A sample of 11,509 participants with a mean age of 54.0 ± 11.1 years, 51.3% women, and a median FIB-4 index of 0.85 (0.65/1.12), was used. The FIB-4 index was predictive for prevalent (FIB-4 index adjusted odds ratio (aOR) per SD: 1.100 with 95% CI 1.011-1.196; p = 0.026), but not incident AFib (log[FIB-4 index]) adjusted hazard ratio: 1.125 with 95% CI 0.943-1.342, p = 0.19). Elastic net regularized regression identified CCL20, DNER, and CXCL10 for prevalent AFib, and AXIN1, CXCL10, and Flt3L for the log(FIB-4 index) (per SD) as most important in common regulated proteins. The relationship between the FIB-4 index, the identified proteins, and AFib was relevant and reproduced at the 5-year follow-up for CXCL10 after adjusting for confounders (log[FIB-4 index] per SD - CXCL10 [per SD] adjusted β 0.160 with 95% CI 0.127-0.194, p <0.0001; CXCL10 [per SD] - AFib aOR 1.455 with 95% CI 1.217-1.741, p <0.0001), reproduced using the NFS and APRI, and corresponding to increased serum levels.
Conclusions
CXCL10 is linked to liver fibrosis, as determined by the FIB-4 index, and to prevalent AFib.
Impact and implications:
How elevated liver stiffness relates to atrial fibrillation in the general population remains to be clarified. We hypothesized that systemic inflammation against a background of liver fibrosis produced from metabolic dysfunction-associated steatotic liver disease (MASLD), is involved in the pathophysiology of atrial fibrillation. Using large-scale targeted proteomics, we found that CXCL10 is related to both liver fibrosis, as defined by the fibrosis-4 index, and to atrial fibrillation. These results can aid evidence-based drug development for patients with atrial fibrillation and MASLD-related liver fibrosis.
Keywords: Atrial fibrillation, C-X-C motif chemokine ligand 10 (CXCL10), Fibrosis-4 index (FIB-4 index), Metabolic dysfunction-associated steatotic liver disease (MASLD), Non-invasive test, Proteomics
Graphical abstract
Highlights:
-
•
Liver fibrosis predicts prevalent atrial fibrillation in the general population.
-
•
CXCL10 is at the interface between liver fibrosis and atrial fibrillation.
-
•
Metabolic inflammation could be the link between liver fibrosis and atrial fibrillation.
-
•
CXCL10 could be a novel drug target for atrial fibrillation associated with metabolic inflammation and liver fibrosis.
Introduction
Metabolic dysfunction-associated steatotic liver disease (MASLD) is often associated with cardiovascular disease, including coronary artery disease, congestive heart failure, and atrial fibrillation (AFib), resulting in a high mortality rate.1 Liver fibrosis with resulting stiffening that is associated with the more advanced stages of MASLD has been indicated as a relevant cue to prompt cardiovascular risk assessment and further investigations, including N-terminal pro–B-type natriuretic peptide (NT-proBNP) determination and electrocardiography.2 AFib is the most common cardiac arrhythmia affecting approximately 60 million persons and its prevalence is increasing in parallel with MASLD.[3], [4], [5] The lifetime risk for AFib is 33% and largely depends on modifiable cardiovascular risk factors, including arterial hypertension, type 2 diabetes mellitus, alcohol consumption, and a sedentary lifestyle.4
AFib increases the risk of incident heart failure6 and cardiovascular accidents7 and is consequently an important condition to be treated. The treatment of AFib is, apart from cardiovascular risk management, currently based on anticoagulation therapy with vitamin K antagonists or direct-acting anticoagulants, and rate and rhythm control using pharmacological treatment or ablation. Life-threatening side effects including excessive bleeding and arrhythmias are hence inherent to current treatment modalities.8 Mechanism-based strategies in current drug development for AFib focus on repairing the protein quality control system, DNA damage, and mitochondrial function, as well as dampening inflammatory responses.3,9
Patients with AFib often experience multiple comorbidities based on the presence of shared risk factors, including those involved in MASLD. Considering the lack of both safe and effective therapies for AFib, it is relevant to investigate the interrelationship between these two conditions.3 Recent evidence indicates that liver stiffness when determined by vibration-controlled transient elastography (VCTE) rather than liver steatosis itself, is related to prevalent AFib in the general population.10 The link between liver stiffness and AFib remains undetermined, but could lie in mechanisms involving metabolic inflammation.11 Metabolic inflammation arising from MASLD leads to hepatic fibrosis over time and is the main cause of liver stiffening.2,11,12 In this context, liver-derived inflammatory factors could contribute to, or even trigger AFib.13,14 Several non-invasive tests (NITs) for advanced hepatic fibrosis have been developed in recent years, among which the fibrosis-4 (FIB-4) index has shown utility in the general population.15
In the present study, we investigated circulating inflammatory factors by proteomics analysis and explored targets related to both liver fibrosis, determined by the FIB-4 index, and AFib in a large population-based cohort to better understand the relationship between liver stiffening and AFib and to ultimately provide novel avenues for drug development.
Patients and methods
Study description
The Gutenberg Health Study is a prospective population-based observational cohort study underway in the Rhine-Main Region in Germany. The study has been approved by the local ethics committee and the local and federal data safety commissioners. Written informed consent was obtained from all study participants. The study protocol was in agreement with the ethical guidelines of the Declaration of Helsinki.16
Individuals between 35- and 74-years old from Mainz and the Mainz-Bingen district were invited to enrol to the study. The study sample consisted of 15,010 participants at baseline, enrolled between 2012 and 2017. After 5 years, data was obtained from 12,423 participants.
Exclusion criteria
Participants with cancer, participants consuming alcohol in amounts ≥20 g/day for women and ≥30 g/day for men,17 and participants without available FIB-4 index data were excluded from the study sample.
Definitions of diseases and risk factors
Liver fibrosis was determined using the FIB-4 index according to Sterling et al.18 Two clinically relevant categories were used: FIB-4 index <1.3 (low risk for advanced fibrosis) and ≥1.3 (indeterminant and high risk for advanced fibrosis). The fatty liver index (FLI) was determined according to Bedogni et al.19 and was used a measure of hepatic steatosis with a cut-off of ≥60. Definitions of diseases and risk factors used throughout the text can be found in the supplemental material.
Proteomics analysis for circulating inflammatory proteins
Blood plasma was collected in ethylenediaminetetraacetic acid tubes and analysed with proximity extension assay technology (Olink Proteomics, Uppsala, Sweden) using the Inflammation panel consisting of 92 targets (full list available in Table S1). Briefly, antibody pairs containing unique DNA sequences hybridize upon binding of the specific protein, resulting in proximity extension and amplification by real-time PCR.
Validation of results
The NAFLD fibrosis score (NFS), determined according to Angulo et al.20 and the AST to platelet index (APRI), determined according to Wai et al.21 using aspartate aminotransferase (AST) cut-offs of 35 U/L and 31 U/L for men and women, respectively,22 were used to validate results obtained with the FIB-4 index. Validation of the proteomics results was performed by running a repeated Olink inflammation analysis at the 5-year follow-up.
Statistical analyses
Continuous normally distributed data are presented as mean ± SD and continuous skewed data were presented as median with IQR. Discrete data are described using absolute and relative frequencies. Multivariate logistic regression were used to investigate the relationship between NITs for liver fibrosis and AFib. Cox competing risk analysis was used to adjust for potential confounders in the longitudinal analysis. Multivariate logistic and linear regression for the cross-sectional analyses, and Cox competing risk analysis for longitudinal analyses, were used to investigate the relationships between the NITs for liver fibrosis, AFib, and systemic proteins with adjustments for potential confounders. Elastic net regularized regression models with 10-fold cross-validation were employed to select the most relevant in common modulated inflammatory mediators, with adjustment for age and sex. For that purpose, the λ ratio was used as a scale-invariant measure of predictive robustness to rank proteins according to their relevance. Analyses were performed using R (www.R-project.org, v.4.2.1) and graphs were prepared using GraphPad Prism (v.8.4.3).
Results
Baseline characteristics of the study participants
Of the 15,010 enrolled participants, 3501 participants were excluded resulting in a study sample of 11,509 individuals (Fig. S1). The study participants had a mean age of 54.0 ± 11.1 years and consisted of 51.3% women (Table 1). The median FIB-4 index was 0.85 (0.65/1.12) and 36.1% had hepatic steatosis as determined by a fatty liver index ≥60. Cardiovascular risk factors were more often present in participants with a FIB-4 index ≥1.3 compared with persons with a FIB-4 index <1.3, including dyslipidaemia (45.2% vs. 32.2%), arterial hypertension (66.6% vs. 43.7%), obesity (28.5% vs. 25.0%), and diabetes mellitus (15.9% vs. 7.7%). Consequently, participants with a FIB-index ≥1.3 presented with the metabolic syndrome more frequently than participants with a FIB-4 index <1.3 (28.9% vs. 20.7%). In addition, AFib was present in 6.4% of the persons with a FIB-4 index ≥1.3 compared with 1.7% in participants with a FIB-index <1.3. Other cardiovascular diseases were also more prevalent in participants with a FIB-4 index ≥1.3 compared with those having a FIB-4 index <1.3, among which were congestive heart failure (3.5% vs. 1.0%), coronary artery disease (10.2% vs. 3.0%), and peripheral artery disease (5.4% vs. 2.9%). On the contrary, participants with a FIB-4 index <1.3 were more often smokers compared with participants with a FIB-4 index ≥1.3 (20.7% vs. 10.5%).
Table 1.
Baseline characteristics of the study participants stratified by the fibrosis-4 index.
| Variable | Whole sample (N = 11,509) | Fibrosis-4 index <1.3 (n = 9,795) | Fibrosis-4 index ≥1.3 (n = 1,714) | p value |
|---|---|---|---|---|
| Demographics | ||||
| Sex (female) | 51.3% (5,902) | 53.5% (5,242) | 38.5% (660) | <0.0001 |
| Age, yr | 54.0 ± 11.1 | 52.1 ± 10.5 | 64.8 ± 7.6 | <0.0001 |
| BMI, kg/m2 | 26.6 (23.8/30.1) | 26.5 (23.7/30.0) | 27.3 (24.6/30.7) | <0.0001 |
| Weight, kg | 79.5 ± 16.7 | 79.2 ± 16.8 | 80.9 ± 16.1 | 0.00014 |
| Height, m | 1.70 ± 0.10 | 1.70 ± 0.10 | 1.70 ± 0.09 | 0.28 |
| Waist, cm | 94.1 ± 13.9 | 93.6 ± 13.9 | 97.3 ± 13.9 | <0.0001 |
| Protein data available |
49.9% (5,741) |
48.5% (4,750) |
57.8% (991) |
<0.0001 |
| Cardiovascular risk factors | ||||
| Dyslipidaemia | 34.1% (3,920) | 32.2% (3,146) | 45.2% (774) | <0.0001 |
| Arterial hypertension | 47.1% (5,416) | 43.7% (4,274) | 66.6% (1,142) | <0.0001 |
| Smoking | 19.2% (2,201) | 20.7% (2,021) | 10.5% (180) | <0.0001 |
| Obesity | 25.5% (2,932) | 25.0% (2,443) | 28.5% (489) | 0.0020 |
| Family history of myocardial infarction/stroke | 22.5% (2,587) | 22.7% (2,223) | 21.2% (364) | 0.19 |
| Diabetes mellitus |
8.9% (1,022) |
7.7% (749) |
15.9% (273) |
<0.0001 |
| Comorbidities | ||||
| Metabolic syndrome | 21.9% (2,519) | 20.7% (2,023) | 28.9% (496) | <0.0001 |
| Hyperuricemia | 6.2% (717) | 5.4% (530) | 10.9% (187) | <0.0001 |
| Coronary artery disease | 4.0% (458) | 3.0% (289) | 10.2% (169) | <0.0001 |
| Myocardial infarction | 3.0% (339) | 2.2% (216) | 7.2% (123) | <0.0001 |
| Peripheral artery disease | 3.3% (371) | 2.9% (280) | 5.4% (91) | <0.0001 |
| Atrial fibrillation | 2.4% (275) | 1.7% (168) | 6.4% (107) | <0.0001 |
| Congestive heart failure | 1.3% (154) | 1.0% (94) | 3.5% (60) | <0.0001 |
| Chronic kidney disease |
1.0% (117) |
1.0% (100) |
1.0% (17) |
1.00 |
| Liver parameters | ||||
| Fatty liver index | 45.98 ± 30.47 | 44.90 ± 30.49 | 52.14 ± 29.58 | <0.0001 |
| Fatty liver index ≥60 | 36.1% (4,146) | 34.8% (3407) | 43.1% (739) | <0.0001 |
| Fibrosis-4 index | 0.85 (0.65/1.12) | 0.78 (0.62/0.98) | 1.55 (1.40/1.80) | <0.0001 |
| NAFLD fibrosis score | -2.59 ± 1.30 | -2.87 ± 1.15 | -1.04 ± 0.97 | <0.0001 |
| AST to platelet index | 0.28 (0.23/0.35) | 0.27 (0.22/0.32) | 0.43 (0.36/0.54) | <0.0001 |
| Alanine aminotransferase, U/L | 32.0 (26.0/42.0) | 32.0 (26.0/41.0) | 34.0 (28.0/44.0) | <0.0001 |
| Aspartate aminotransferase, U/L | 25.00 (21.00/29.00) | 24.00 (21.00/28.00) | 29.00 (25.00/36.00) | <0.0001 |
| Gamma-glutamyltransferase, U/L |
23.00 (16.00/35.00) |
23.00 (16.00/34.00) |
26.00 (18.00/42.00) |
<0.0001 |
| Other laboratory measurements | ||||
| Cholesterol, mg/dl | 219.4 ± 40.4 | 220.2 ± 40.2 | 215.1 ± 41.7 | <0.0001 |
| High-density lipoprotein, mg/dl | 56.7 ± 15.4 | 56.8 ± 15.3 | 56.3 ± 16.1 | 0.24 |
| Low-density lipoprotein, mg/dl | 138.6 ± 35.2 | 139.3 ± 34.9 | 134.5 ± 36.7 | <0.0001 |
| Triglycerides, mg/dl | 104.0 (77.0/145.0) | 103.4 (77.0/145.0) | 105.0 (79.0/150.0) | 0.058 |
| C-reactive protein, mg/L | 1.50 (0.52/3.10) | 1.50 (0.50/3.10) | 1.60 (0.68/3.01) | 0.23 |
| Fibrinogen, mg/dl | 321.00 (278.00/375.00) | 320.00 (277.00/373.00) | 332.00 (288.00/386.58) | <0.0001 |
| Glucose, mg/L | 91.0 (85.0/97.2) | 90.0 (85.0/97.0) | 94.0 (88.0/102.0) | <0.0001 |
| HbA1c, % | 5.50 (5.20/5.80) | 5.50 (5.20/5.80) | 5.60 (5.30/6.00) | <0.0001 |
Data presented as mean ± SD (Gaussian-distributed data), median with IQR (non-Gaussian distributed data), or as relative and absolute frequencies (categorical data); a two-sided t-test was used for comparing two Gaussian-distributed continuous variables, a Wilcoxon rank sum test for non-Gaussian distributed variables, and a chi-square for categorical data; p values <0.05 were considered significant.
Relationship between liver fibrosis and atrial fibrillation
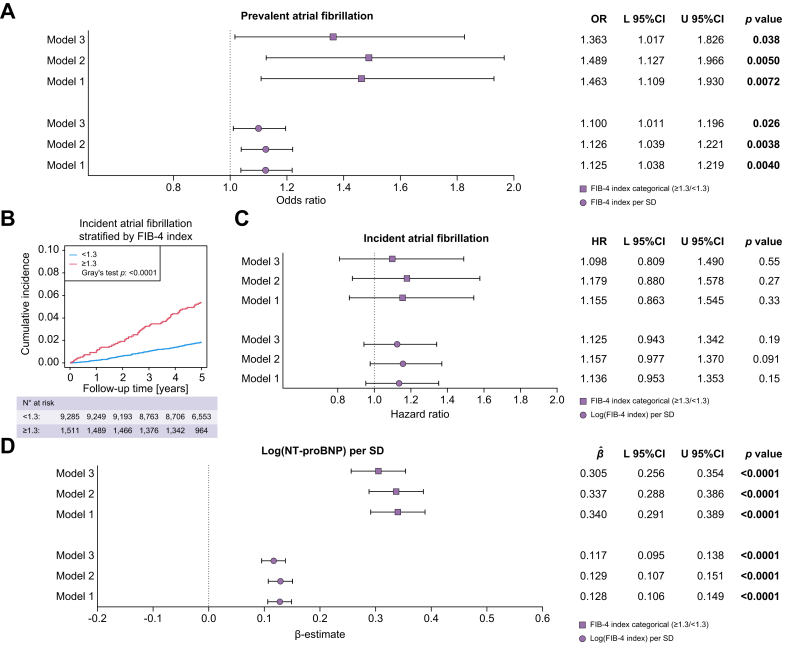
The relationships between liver fibrosis determined by the FIB-4 index and prevalent and incident AFib were investigated employing three additive models (model 1: adjusted for age and sex; model 2: additional adjustment for smoking, arterial hypertension, diabetes mellitus, obesity, and dyslipidaemia; model 3: additional adjustment for coronary artery disease and congestive heart failure) using multivariate logistic regression and Cox competing risk analysis, respectively. The FIB-4 index used both as a continuous and a categorical variable was significantly related to prevalent AFib in all three models (model 3: FIB-4 index per SD: odds ratio (OR) 1.100 with 95% CI 1.011-1.196, p = 0.026; FIB-4 index categorical (≥1.3/<1.3): OR 1.363 with 95% CI 1.017-1.826, p = 0.038) (Fig. 1A). Age, sex, dyslipidaemia, congestive heart failure, and coronary artery disease were factors that significantly influenced the relationship between the FIB-4 index and prevalent AFib (Table S2A and B). The relationship between liver fibrosis and prevalent Afib was replicated using NFS (model 3: NFS per SD: OR 1.253 with 95% CI 1.040-1.509, p = 0.017) and APRI (model 3: log[APRI] per SD: OR 1.158 with 95% CI 1.026-1.308, p = 0.018) (Fig. S2). The data on the FIB-4 index, NFS, and APRI were reproduced through repeated measurements in the same cohort after 5 years (Fig. S3A and B; Table S3 shows the study participant characteristics at the 5-year follow-up). Although a FIB-4 index ≥1.3 predicted incident AFib in an unadjusted model (Gray’s test p <0.0001) (Fig. 1B), it was not the case when confounders were taken into account (model 3: log[FIB-4 index] per SD: hazard ratio [HR] 1.125 with 95% CI 0.943-1.342, p = 0.19; FIB-4 index categorical [≥1.3/<1.3]: HR 1.098 with 95% CI 0.809-1.490, p = 0.55) (Fig. 1C). Age, sex, and congestive heart failure were factors that influenced the relationship between the FIB-4 index and incident AFib (Table S4A and B). Nonetheless, the FIB-4 index related to log(NT-proBNP) levels per SD (model 3: β-estimate log[FIB-4 index] per SD: 0.117 with 95% CI 0.095-0.138, p <0.0001; β-estimate FIB-4 index categorical [≥1.3/<1.3]: 0.305 with 95% CI 0.256-0.354, p <0.0001) (Fig. 1D and Table S5A and B), which is an independent marker for prevalent and incident AFib,23 which was also reproduced in the cohort (Fig. S4). Although the FLI was also related to prevalent AFib (Fig. S5A), it was inversely related to NT-proBNP levels (Fig. S5B).
Fig. 1.
Relationship between the fibrosis-4 index and atrial fibrillation.
(A) Relationship between the FIB-4 index and prevalent atrial fibrillation (symbols represent odds ratios and bars represent 95% CIs). Level of significance: p <0.05 is considered as statistically significant. (multivariate logistic regression, z-test) (model 1: n = 11,395 (275 events); model 2: n = 11,335 (274 events); model 3: n = 11,186 (262 events)). (B) Cumulative incidence of atrial fibrillation based on the FIB-4 index (blue line indicates cumulative incidence of atrial fibrillation with FIB-4 index <1.3 and red line indicates cumulative incidence of atrial fibrillation with FIB-4 index ≥1.3). Level of significance: a p <0.05 is considered as statistically significant (Gray’s test). (C) Relationship between the FIB-4 index and incident atrial fibrillation (symbols represent hazard ratios and bars represent 95% CIs). Level of significance: a p <0.05 is considered as statistically significant (Cox competing risk analysis, event = atrial fibrillation, competing event = death, z-test) (model 1: n = 10,796 (246 events, 166 competing events); model 2: n = 10,737 (246 events, 164 competing events); model 3: n = 10,591 (232 events, 159 competing events)). (D) Relationship between the FIB-4 index and NT-proBNP (symbols represent β-estimates and bars represent 95% CIs). Level of significance: p <0.05 is considered as statistically significant (multivariate linear regression, t-test). Model 1: adjusted for age and sex; model 2: additional adjustment for smoking, arterial hypertension, diabetes mellitus, obesity, and dyslipidaemia; model 3: additional adjustment for coronary artery disease and congestive heart failure. FIB-4, fibrosis-4; L, lower; NT-proBNP, N-terminal pro–B-type natriuretic peptide; U, upper.
Proteomics analysis based on the fibrosis-4 index and atrial fibrillation
As the FIB-4 index was related to prevalent, but not incident AFib, we searched for inflammatory mediators connecting liver fibrosis to prevalent AFib. Fig. 2 shows the relative changes in the %SD of the overall mean of 92 systemic protein levels based on the FIB-4 index (≥1.3/<1.3) and AFib. The top three upregulated proteins in participants with a FIB-4 index of at least 1.3 were CUB domain containing protein 1 (CDCP1) (+63.6%), C-X-C motif chemokine ligand (CXCL) 9 (+49.3%), and CXCL10 (+44.1%), whereas the top three downregulated proteins were AXIN1 (-43.2%), Sirtuin 2 (SIRT2) (-43.0%), and STAM binding protein (STAMBP) (-36.3%). The top three elevated proteins in participants with AFib were CXCL10 (+58.5%), CDCP1 (+56.2%) and fibroblast growth factor 23 (FGF23) (+54.0%) and the top three down-regulated proteins were delta and notch-like epidermal growth factor-related receptor (DNER) (-51.0%), IL-2 (-37.4%), and neurturin (NRTN) (-33.4%) (Fig. S6).
Fig. 2.
Protein expressions in %SD of the overall mean in study participants with a Fibrosis-4 Index ≥1.3 and atrial fibrillation (sorted by Fibrosis-4 Index).
Colour scale indicates protein expression rate; green, lower expression, and red, higher expression, compared with the overall mean). AFib, atrial fibrillation; FIB-4, fibrosis-4.
To find the most relevant and important proteins that could link liver fibrosis to AFib, Elastic net regularized regression analyses were performed with adjustments for age and sex (Fig. 3). As the lower FIB-4 index cut-off of 1.3 performs best in persons ≤65 years,24 and the median age of participants with a FIB-4 index ≥1.3 was 64.8 ± 7.6 years, we used the FIB-4 index on a continuous scale in the protein selection process. The top-three in common modulated proteins were selected for both AFib and log(FIB-4 index) per SD. For AFib, CCL20, DNER, and CXCL10 were selected and for the FIB-4 index, AXIN1, CXCL10, and Fms related receptor tyrosine kinase 3 ligand (Flt3L) were selected, resulting in five unique proteins for further analysis. AXIN1, CXCL10, and Flt3L also had the highest λ ratios based on a FIB-4 index ≥1.3, as common modulated proteins with AFib (Fig. S7).
Fig. 3.
Selection of the most important proteins related to liver fibrosis and atrial fibrillation.
Elastic net regularized regression was used to identify the most relevant circulating proteins in atrial fibrillation and liver fibrosis based on the FIB-4 index on a continuous scale (atrial fibrillation: 10 fold cross-validation AUC = 0.7867, (simple AUC = 0.8202), minimal lambda = 0.004 (10 fold-cross validation), n = 5,672, events = 172, number of proteins = 92, adjusted for age (SD) and sex; log(FIB-4 index): 10-fold cross-validation R2 = 0.6173, (simple R2 = 0.6302), minimal lambda = 0.005 (10-fold-cross validation), m = 5,741, events = 155, number of proteins = 92, (adjusted for age [SD] and sex). Colour scale indicates the lambda ratios within the spectrum of obtained results, green = lower, and red = higher lambda ratio (Lambda ratios prevalent arial fibrillation: CCL20: 6.868, DNER: 5.872, CXCL10: 4.750; log[fibrosis-4 index]: AXIN1: 97.731, CXCL10: 41.078, FlT3L: 32.026). AUC, area under the curve; AXIN1, Axis inhibition protein 1; CCL20, C-C motif chemokine ligand 20; CXCL10, C-X-C motif chemokine ligand 10; DNER, Delta and Notch-like epidermal growth factor-related receptor; Flt3L: Fms related receptor tyrosine kinase 3 ligand.
CXCL10 was an inflammatory nexus between liver fibrosis and prevalent atrial fibrillation
Multivariate linear and logistic regression analyses were used to investigate the exact relationships between the FIB-4 index and the selected proteins, and the selected proteins and AFib, respectively.
When adjusted for age, sex, smoking, arterial hypertension, diabetes mellitus, obesity, dyslipidaemia, coronary artery disease, and congestive heart failure, the standardized log(FIB-4 index) was significantly related with Flt3L (β-estimate 0.189 with 95% CI 0.155-0.222, p <0.0001), AXIN1 (β-estimate -0.451 with 95% CI -0.485 to -0.418, p <0.0001), CXCL10 (β-estimate 0.160 with 95% CI 0.127-0.194, p <0.0001), DNER (β-estimate 0.040 with 95% CI 0.006-0.074, p = 0.021), and CCL20 (β-estimate 0.071 with 95% CI 0.036; 0.105, p <0.0001) (Fig. 4A). When performing the same regression analyses with the FIB-4 index as a categorical variable (≥1.3/<1.3), the relationships with DNER and CCL20 disappeared (Fig. 4B). The relationship between log(FIB-4 index) per SD with Flt3L was influenced by sex, age, and smoking, whereas the relationship with AXIN1 was additionally impacted by arterial hypertension, diabetes mellitus, obesity, dyslipidaemia, and coronary artery disease. The relationship with CXCL10 was influenced by sex, age, smoking, obesity, and dyslipidaemia (Table S6A and B).
Fig. 4.
Relationship between circulating inflammatory proteins with the Fibrosis-4 Index and atrial fibrillation.
(A) Relationship between the log(FIB-4 index) (per SD) and circulating proteins (symbols represent β-estimates and bars represent 95% CIs). Level of significance: p <0.05 is considered as statistically significant (multivariate linear regression, t-test) (model 1: n = 5,741; model 2: n = 5,704; model 3: n = 5,601). (B) Relationship between the FIB-4 index (categorical ≥1.3 vs. <1.3) and circulating proteins (symbols represent β-estimates and bars represent 95% CIs). Level of significance: p <0.05 is considered as statistically significant (multivariate linear regression, t-test) (model 1: n = 5,741; model 2: n = 5,704; model 3: n = 5,601). (C) Relationship between circulating proteins and atrial fibrillation (symbols represent odds ratios and bars represent 95% CIs). Level of significance: p <0.05 is considered as statistically significant (multivariate logistic regression, z-test) (model 1: n = 5,672 [172 events]; model 2: n = 5,635 [171 events]; model 3: n = 5,543 [162 events]). Model 1: adjusted for age and sex; model 2: additional adjustment for smoking, arterial hypertension, diabetes mellitus, obesity, and dyslipidaemia; model 3: additional adjustment for coronary artery disease and congestive heart failure. AXIN1, Axis inhibition protein 1; CCL20, C-C motif chemokine ligand 20; CXCL10, C-X-C motif chemokine ligand 10; DNER, Delta and Notch-like epidermal growth factor-related receptor; FIB-4, fibrosis-4; Flt3L: Fms related receptor tyrosine kinase 3 ligand; L, lower; OR, odds ratio; U, upper.
Logistic regression analyses with the inflammatory proteins as independent variables and AFib as the dependent variable adjusted for the same set of possible confounders as done for the linear regression analyses with the FIB-4 index showed significant relationships for FltL3 (OR 1.186 with 95% CI 1.004-1.401, p = 0.044), AXIN1 (OR 0.816 with 95% CI 0.696-0.958, p = 0.013), CXCL10 (OR 1.455 with 95% CI 1.217-1.741, p <0.0001), DNER (OR 0.766 with 95% CI 0.649-0.904, p = 0.0017), and CCL20 (OR 1.435 with 95% CI 1.234-1.668, p <0.0001) (Fig. 4C). Congestive heart failure was the most important confounder among all investigated proteins in relation to AFib (Table S7).
Multivariate linear regressions with NFS and APRI confirmed an independent relationship of liver fibrosis with Flt3L, AXIN1, and CXCL10 (Fig. S8). Identical multivariate linear regressions using repeated measurements after 5 years validated the relationships between the FIB-4 index, NFS, and APRI, and Flt3L, AXIN1, and CXCL10 (Fig. S9). In contrast, only CXCL10 was validated in multivariate logistic regression analysis as a predictor of prevalent AFib (Fig. S10). Consequently, CXCL10 was identified as the most important inflammatory protein at the interface between liver fibrosis and prevalent AFib. Additional analyses for incident AFib showed that CXCL10 (by tertiles) adds to the risk of incident AFib in a crude competing risk analysis (Gray’s test p <0.0001) (Fig. 5), but not when adjusted for confounders (Table S8).
Fig. 5.
Cumulative incidence of atrial fibrillation based on tertiles of proteins.
Green line indicates the first tertile, blue line indicates the second tertile, and red line indicates the third tertile of protein expression. Level of significance: p <0.05 is considered as statistically significant (Gray’s test). AXIN1, Axis inhibition protein 1; CCL20, C-C motif chemokine ligand 20; CXCL10, C-X-C motif chemokine ligand 10; DNER, Delta and Notch-like epidermal growth factor-related receptor; Flt3L: Fms related receptor tyrosine kinase 3 ligand.
Discussion
Elevated liver stiffness determined by the VCTE was recently found to be associated with AFib in the general population (OR 1.09 per kPa, 95% CI 1.03-1.16).10 We hypothesised that liver-related systemic inflammation against a background of liver fibrosis induced from MASLD could lay at the basis of incident and prevalent AFib.
In this large prospective population-based Western-European cohort, we found an independent relationship between the FIB-4 index and prevalent AFib, which is in line with the findings of a South-Korean study with 74,946 patients with MASLD in which an adjusted OR of 2.255 (with 95% CI 1.744-2.915) for the FIB-4 index (used as categorical variable with <1.30, 1.3–2.67, and >2.67 cut-off values) and prevalent AFib was reported.25 The relationship between liver stiffness and the FIB-4 index and the risk of incident AFib is less clear. A Japanese study including 37,892 unemployed or retired participants aged ≥40 years with a median follow-up period of 5 years reported an adjusted HR of 1.70 (95% CI 1.29-2.23) of developing AFib for subjects with a FIB-4 index in the highest quartile compared with the lowest quartile.26 Although we found a significant predictive role of the FIB-4 index for incident AFib in the crude risk analysis, it was not the case when the analysis was adjusted for age and sex and an additional set of well-established confounders. In contrast, the relationship between the FIB-4 index and NT-proBNP levels in the fully-adjusted model supported the role of liver fibrosis in AFib, as NT-proBNP is even a better marker for prevalent and incident AFib than for heart failure in stable outpatients.23
As mechanism-based treatments for AFib and MASLD are gaining momentum in drug development, and it is well known that AFib can have its basis in systemic inflammation9,[27], [28], [29] we consequently searched for inflammatory proteins that could link liver fibrosis with prevalent AFib.3
In the current analysis, we identified increased CXCL10 levels as an inflammatory nexus between liver fibrosis and prevalent AFib. The association of this circulating protein was preserved after correcting for a broad set of potential confounders in multivariate regression models and was reproduced at the 5-year follow-up.
CXCL10 can bind onto CXCR3 and is a chemoattractant produced by both immune and non-immune cells with pleiotropic functions including the chemoattraction of activated T-cells, macrophages, monocytes, and natural killer cells.30 CXCL10 has reported to be elevated in patients experiencing AFib31 and other cardiovascular diseases including atherosclerosis32 and myocardial infarction.33 In the liver, extracellular vesicles containing CXCL10 are released by hepatocytes mediated by mixed lineage kinase 3 in response to lipotoxicity, which in turn function as an attractant for macrophages.34 Hence, CXCL10 has been identified as a crucial protein in the pathogenesis of MASH, together with other pro-inflammatory cytokines (monocyte chemoattractant protein 1, IL-1β, and tumour necrosis factor-α), and mechanisms including lipogenesis, and oxidative stress, and also correlating with lobular inflammation.35
Mechanistically in relation to AFib, myocardial infarction-associated transcript (MIAT), which is increased in serum extracellular vesicles of patients with AFib, can bind to miR-485-5p to decrease its inhibitory effect on CXCL10, resulting in atrial myocyte fibrosis, inflammation, and oxidative stress in both in vitro and in vivo experimental models.36 Apart from a direct action of CXCL10 on the myocardium, CXCL10 can also promote AFib through its pro-atherogenic properties since subclinical atherosclerosis is an independent risk factor for developing AFib.32,37 Consequently, CXCL10 can act through different mechanisms in the pathophysiology of AFib, which could at least partly be attributed to underlying liver fibrosis. Nonetheless, CXCL10 levels decrease after cryoballoon and radiofrequency balloon ablation, suggesting that CXCL10 secretion is also mediated by AFib itself.38 Furthermore, CXCL10 levels are also increased in patients with non-fibrotic MASLD,39 positioning it as a potential prognostic marker and therapeutic target for progressive MASLD-related cardiovascular disease, including AFib.
Four additional proteins identified as possible connections between liver fibrosis and AFib in this study were AXIN1, Flt3L, DNER, and CCL20. While AXIN1 potently negatively correlated with NITs for liver fibrosis, its relationship with AFib was not reproduced at the 5-year follow-up period. Nonetheless, the link between AXIN1 and AFib has been earlier elegantly identified through exosome sequencing in the serum of patients with AFib. Circulating exosomal miRNA-124-3p was increased in the plasma of patients with AFib, whereas AXIN1 appeared to be its target in a luciferase assay. In addition, miR-124-3p overexpression in rat myocardial fibroblasts resulted in reduced levels of AXIN1, whereas β-catenin, collagen 1, and α-SMA were elevated, suggesting that AXIN1 regulates activation and proliferation of myocardial fibroblasts through Wnt/β-catenin signaling.40 Although Flt3L levels were related to NITs of liver fibrosis and decreases following different ablation techniques used to treat Afib,38 its relationship with AFib was insignificant in the validation study. The relationship of the FIB-4 index as a continuous variable with DNER and CCL20 was of minor importance compared with the other proteins that were identified, whereas the relationship between the FIB-4 index as a clinically-relevant categorical (≥1.3/<1.3) variable and these proteins was insignificant. Therefore, CXCL10 is the most important liver-related factor in MASLD-related liver fibrosis and can maintain a pathogenic environment for AFib, likely a result of atrial myocyte fibrosis and atherosclerosis.36,37 However, the exact mechanism of how CXCL10 contributes to AFib in the setting of liver fibrosis remains to be determined. CXCL10 may be responsible for the recurrence of AFib after ablation, as liver fibrosis determined by the FIB-4 index has been shown to be an independent predictor for AFib recurrence after ablation in a 1-year follow-up study.41
Our study should be interpreted considering several limitations. First, the follow-up period for incident AFib was 5 years which might have been too short to define a predictive role of liver fibrosis in the development of AFib. Secondly, the inflammatory panel was limited to 92 targets using the Olink assay; thus, other markers related to liver fibrosis and AFib may have been missed. Thirdly, participants with chronic viral hepatitis or other causes of liver fibrosis different from MASLD were not specifically assessed through testing and thus occult infections could also have been missed. In contrast, MASLD is by far the most common cause of liver fibrosis in the Western population42 and chronic viral hepatitis has a relatively low prevalence at the general population level in Germany (age-standardized prevalence rate [cirrhosis and other chronic liver diseases associated with to hepatitis] per 100,000: 548.22 for hepatitis C and 284.79 for hepatitis B).43,44 In addition, the sample size of our study, the application of different parameters for assessing hepatic fibrosis, and reproduction of the results after a 5-year interval, allowed for accurate, real-world estimations.
In conclusion, CXCL10 was identified through targeted proteomics and can be considered a biomarker at the interface between the risk of advanced liver fibrosis and prevalent AFib in the general population. Targeting the drivers of hepatic and cardiac inflammation and fibrosis could allow for evidence-based drug development for patients with metabolic inflammation, MASLD, and AFib.
Abbreviations
AFib, atrial fibrillation; APRI, AST to platelet index; AXIN1, axis inhibition protein 1; CCL20, C-C motif chemokine ligand 20; CDCP1, CUB domain containing protein 1; CXCL, C-X-C motif chemokine ligand; DNER, delta and notch-like epidermal growth factor-related receptor; FGF23, fibroblast growth factor 23; FIB-4, fibrosis-4; FLI, fatty liver index; Flt3L, Fms related receptor tyrosine kinase 3 ligand; HCC, hepatocellular carcinoma; HR, hazard ratio; IL, interleukin; MASLD, metabolic dysfunction-associated steatotic liver disease; NFS, NAFLD fibrosis score; NIT, non-invasive test; NRTN, neurturin; NT-proBNP, N-terminal pro–B-type natriuretic peptide; OR, odds ratio; SIRT2, sirtuin 2; STAMBP, STAM Binding Protein; VCTE, vibration-controlled transient elastography.
Financial support
The Gutenberg Health Study is funded by the government of Rhineland-Palatinate (“Stiftung Rheinland-Pfalz für Innovation”, contract AZ 961-386261/733), the research programs “Wissen schafft Zukunft” and “Center for Translational Vascular Biology (CTVB)” of the Johannes Gutenberg-University of Mainz, and its contract with Boehringer Ingelheim and PHILIPS Medical Systems, including an unrestricted grant for the Gutenberg Health Study. J.B. receives funding from Colgate-Palmolive – Society of Toxicology, Onderzoeksraad Vrije Universiteit Brussel, and Chair Mireille Aerens for the Development of Alternative Methods.
Conflicts of interest
A.K.S. received financial and research support by Abbvie, Apellis, Bayer Vital, Heidelberg Engineering, Novartis, Santen and has acted as consultant for Apellis. P.S.W. reports grants from Bayer AG, non-financial grants from Philips Medical Systems, grants and consulting fees from Boehringer Ingelheim, grants and consulting fees from Novartis Pharma, grants and consulting fees from Sanofi-Aventis, grants, consulting and lecturing fees from Bayer Health Care, grants from Daiichi Sankyo Europe, consulting fees from Astra Zeneca, consulting fees and non-financial support from Diasorin and non-financial support from I.E.M. Independent of this research study, J.M.S. has acted as Consultant to Apollo Endosurgery, Albireo Pharma Inc, Bayer, Boehringer Ingelheim, Gilead Sciences, GSK, Intercept Pharmaceuticals, Ipsen, Inventiva Pharma, Madrigal, MSD, Northsea Therapeutics, Novartis, Novo Nordisk, Pfizer, Roche, Sanofi, Siemens Healthineers; has received research Funding from Gilead Sciences, Boehringer Ingelheim, Siemens Healthcare GmbH and Speaker Honorarium from Boehringer Ingelheim, Echosens, MedPublico GmbH, Novo Nordisk, Madrigal Pharmaceuticals. All other authors report no relevant conflict of interest.
Please refer to the accompanying ICMJE disclosure forms for further details.
Authors’ contributions
Conceptualization: JB, MM, AG, TM, PSW, PRG, JMS. Data Curation: TM, PSW, PRG. Formal Analysis: JB, MM, AG, JMS. Funding Acquisition: TM, PSW, PRG. Investigation: JB, MM, AG, PSW, JMS. Methodology: JB, MM, AG, PSW, JMS. Project Administration: TM, PSW, PRG, JMS. Resources: TM, PSW, PRG. Software: J.B., TM, PSW, PRG. Supervision: PSW, JMS. Validation: AG. Visualization: JB, AG. Writing – Original Draft: JB, MM, JMS. Writing – Review & Editing: all authors.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgements
The authors thank all study participants of the Gutenberg Health study for their willingness to participate and all the GHS study team (http://www.gutenberg-gesundheitsstudie.de/ghs/team/studienzentrum.html) for their support. Servier Medical Art was used to make the graphical abstract.
Footnotes
Given their role as Editor, Jörn M. Schattenberg had no involvement in the peer-review of this article and had no access to information regarding its peer-review. Full responsibility for the editorial process for this article was delegated to the Guest Editor Ramon Bataller.
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2024.101171.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Anstee Q.M., Mantovani A., Tilg H., et al. Risk of cardiomyopathy and cardiac arrhythmias in patients with nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2018;15:425–439. doi: 10.1038/s41575-018-0010-0. [DOI] [PubMed] [Google Scholar]
- 2.Boeckmans J., Sandrin L., Knackstedt C., et al. Liver stiffness as a cornerstone in heart disease risk assessment. Liver Int. 2024;44:344–356. doi: 10.1111/liv.15801. [DOI] [PubMed] [Google Scholar]
- 3.Brundel B.J.J.M., Ai X., Hills M.T., et al. Atrial fibrillation. Nat Rev Dis Primers. 2022;8:21. doi: 10.1038/s41572-022-00347-9. [DOI] [PubMed] [Google Scholar]
- 4.Elliott A.D., Middeldorp M.E., Van Gelder I.C., et al. Epidemiology and modifiable risk factors for atrial fibrillation. Nat Rev Cardiol. 2023;6:404–417. doi: 10.1038/s41569-022-00820-8. [DOI] [PubMed] [Google Scholar]
- 5.Wai-Sun Wong V., Ekstedt M., Lai-Hung Wong G., et al. Changing epidemiology, global trends and implications for outcomes of NAFLD. J Hepatol. 2023;79:842–852. doi: 10.1016/j.jhep.2023.04.036. [DOI] [PubMed] [Google Scholar]
- 6.Kotecha D., Piccini J.P. Atrial fibrillation in heart failure: what should we do? Eur Heart J. 2015;36:3250–3257. doi: 10.1093/eurheartj/ehv513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Odutayo A., Wong C.X., Hsiao A.J., et al. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: systematic review and meta-analysis. BMJ. 2016;354 doi: 10.1136/bmj.i4482. [DOI] [PubMed] [Google Scholar]
- 8.Lacoste J.L., Szymanski T.W., Avalon J.C., et al. Atrial fibrillation management: a comprehensive review with a focus on pharmacotherapy, rate, and rhythm control strategies. Am J Cardiovasc Drugs. 2022;22:475–496. doi: 10.1007/s40256-022-00529-6. [DOI] [PubMed] [Google Scholar]
- 9.Van Wagoner D.R., Chung M.K. Inflammation, inflammasome activation, and atrial fibrillation: evidence for causation and new therapeutic targets. Circulation. 2018;138:2243–2246. doi: 10.1161/CIRCULATIONAHA.118.036143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Kleef L.A., Lu Z., Arfan Ikram M., et al. Liver stiffness not fatty liver disease is associated with atrial fibrillation: the Rotterdam study. J Hepatol. 2022;77:931–938. doi: 10.1016/j.jhep.2022.05.030. [DOI] [PubMed] [Google Scholar]
- 11.Gehrke N., Schattenberg J.M. Metabolic inflammation—a role for hepatic inflammatory pathways as drivers of comorbidities in nonalcoholic fatty liver disease? Gastroenterology. 2020;158:1929–1947.e6. doi: 10.1053/j.gastro.2020.02.020. [DOI] [PubMed] [Google Scholar]
- 12.Yang L., Hao Y., Boeckmans J., et al. Immune cells and their derived microRNA-enriched extracellular vesicles in nonalcoholic fatty liver diseases: novel therapeutic targets. Pharmacol Ther. 2023;243 doi: 10.1016/j.pharmthera.2023.108353. [DOI] [PubMed] [Google Scholar]
- 13.Haghbin H., Gangwani M.K., Ravi S.J.K., et al. Nonalcoholic fatty liver disease and atrial fibrillation: possible pathophysiological links and therapeutic interventions. Ann Gastroenterol. 2020;33(6):603–614. doi: 10.20524/aog.2020.0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding Y.H., Ma Y., Qian L.Y., et al. Linking atrial fibrillation with non-alcoholic fatty liver disease: potential common therapeutic targets. Oncotarget. 2017;8:60673–60683. doi: 10.18632/oncotarget.19522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serra-Burriel M., Juanola A., Serra-Burriel F., et al. Development, validation, and prognostic evaluation of a risk score for long-term liver-related outcomes in the general population: a multicohort study. Lancet. 2023;402:988–996. doi: 10.1016/S0140-6736(23)01174-1. [DOI] [PubMed] [Google Scholar]
- 16.Wild P.S., Zeller T., Beutel M., et al. Die Gutenberg gesundheitsstudie. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2012;55:824–830. doi: 10.1007/s00103-012-1502-7. [DOI] [PubMed] [Google Scholar]
- 17.Ratziu V., Bellantani S., Cortez-Pinto H., et al. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol. 2010;53:372–384. doi: 10.1016/j.jhep.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 18.Sterling R.K., Lissen E., Clumeck N., et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 19.Bedogni G., Bellentani S., Miglioli L., et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:1–7. doi: 10.1186/1471-230X-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Angulo P., Hui J.M., Marchesini G., et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–854. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 21.Wai C.T., Greenson J.K., Fontana R.J., et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 22.Huber Y., Schulz A., Schmidtmann I., et al. Prevalence and risk factors of advanced liver fibrosis in a population-based study in Germany. Hepatol Commun. 2022;6:1457–1466. doi: 10.1002/hep4.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Werhahn S.M., Becker C., Mende M., et al. NT-proBNP as a marker for atrial fibrillation and heart failure in four observational outpatient trials. ESC Heart Fail. 2022;9:100–109. doi: 10.1002/ehf2.13703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McPherson S., Hardy T., Dufour J.F., et al. Age as a confounding factor for the accurate non-invasive diagnosis of advanced NAFLD fibrosis. Am J Gastroenterol. 2017;112:740–751. doi: 10.1038/ajg.2016.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park H.E., Lee H., Choi S.Y., et al. The risk of atrial fibrillation in patients with non-alcoholic fatty liver disease and a high hepatic fibrosis index. Sci Rep. 2020;10:5023. doi: 10.1038/s41598-020-61750-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanaka Y., Chuzi S., Morikawa N., et al. Association between the Liver Fibrosis-4 Score and incident atrial fibrillation in a general Japanese population of middle-aged to older adults. Circulation. 2021;143 [Google Scholar]
- 27.Boos C.J., Anderson R.A., Lip G.Y.H. Is atrial fibrillation an inflammatory disorder? Eur Heart J. 2006;27:136–149. doi: 10.1093/eurheartj/ehi645. [DOI] [PubMed] [Google Scholar]
- 28.Nso N., Bookani K.R., Metzl M., et al. Role of inflammation in atrial fibrillation: a comprehensive review of current knowledge. J Arrhythm. 2021;37:1–10. doi: 10.1002/joa3.12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harrison S.A., Allen A.M., Dubourg J., et al. Challenges and opportunities in NASH drug development. Nat Med. 2023;29:562–573. doi: 10.1038/s41591-023-02242-6. [DOI] [PubMed] [Google Scholar]
- 30.Xu Z., Zhang X., Lau J., Yu J. C-X-C motif chemokine 10 in non-alcoholic steatohepatitis: role as a pro-inflammatory factor and clinical implication. Expert Rev Mol Med. 2016;18 doi: 10.1017/erm.2016.16. [DOI] [PubMed] [Google Scholar]
- 31.Martins G.L., Duarte R.C.F., Vieira É.L.M., et al. Evaluation of new potential inflammatory markers in patients with nonvalvular atrial fibrillation. Int J Mol Sci. 2023;24:3326. doi: 10.3390/ijms24043326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heller E.A., Liu E., Tager A.M., et al. Chemokine CXCL10 promotes atherogenesis by modulating the local balance of effector and regulatory T cells. Circulation. 2006;113:2301–2312. doi: 10.1161/CIRCULATIONAHA.105.605121. [DOI] [PubMed] [Google Scholar]
- 33.Altara R., Manca M., Hessel M.H., et al. CXCL10 Is a circulating inflammatory marker in patients with advanced heart failure: a Pilot Study. J Cardiovasc Transl Res. 2016;9:302–314. doi: 10.1007/s12265-016-9703-3. [DOI] [PubMed] [Google Scholar]
- 34.Ibrahim S.H., Hirsova P., Tomita K., et al. Mixed lineage kinase 3 mediates release of C-X-C motif ligand 10-bearing chemotactic extracellular vesicles from lipotoxic hepatocytes. Hepatology. 2015;63:731–744. doi: 10.1002/hep.28252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang X., Shen J., Man K., et al. CXCL10 plays a key role as an inflammatory mediator and a non-invasive biomarker of non-alcoholic steatohepatitis. J Hepatol. 2014;61:1365–1375. doi: 10.1016/j.jhep.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 36.Chen Y., Chen X., Li H., et al. Serum extracellular vesicles containing MIAT induces atrial fibrosis, inflammation and oxidative stress to promote atrial remodeling and atrial fibrillation via blockade of miR-485-5p-mediated CXCL10 inhibition. Clin Transl Med. 2021;11:e482. doi: 10.1002/ctm2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heeringa J., Van Der Kuip D.A.M., Hofman A., et al. Subclinical atherosclerosis and risk of atrial fibrillation. Arch Intern Med. 2007;167:382–387. doi: 10.1001/archinte.167.4.382. [DOI] [PubMed] [Google Scholar]
- 38.Lin M., Bao Y., Du Z., et al. Plasma protein profiling analysis in patients with atrial fibrillation before and after three different ablation techniques. Front Cardiovasc Med. 2023;9 doi: 10.3389/fcvm.2022.1077992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kriss M., Golden-Mason L., Kaplan J., et al. Increased hepatic and circulating chemokine and osteopontin expression occurs early in human NAFLD development. PLoS One. 2020;15 doi: 10.1371/journal.pone.0236353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu P., Li H., Zhang A., et al. MicroRNAs sequencing of plasma exosomes derived from patients with atrial fibrillation: miR-124-3p promotes cardiac fibroblast activation and proliferation by regulating AXIN1. J Physiol Biochem. 2022;78:85–98. doi: 10.1007/s13105-021-00842-9. [DOI] [PubMed] [Google Scholar]
- 41.Wang Z., Wang Y., Luo F., et al. Impact of advanced liver fibrosis on atrial fibrillation recurrence after ablation in non-alcoholic fatty liver disease patients. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.960259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caballería L., Pera G., Arteaga I., et al. High prevalence of liver fibrosis among European adults with unknown liver disease: a population-based study. Clin Gastroenterol Hepatol. 2018;16:1138–1145.e5. doi: 10.1016/j.cgh.2017.12.048. [DOI] [PubMed] [Google Scholar]
- 43.Steffen G., Sperle I., Leendertz S.A., et al. The epidemiology of Hepatitis B, C and D in Germany: a scoping review. PLoS One. 2020;15(3) doi: 10.1371/journal.pone.0229166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cortesi P.A., Fornari C., Conti S., et al. Hepatitis B and C in Europe: an update from the global burden of disease study 2019. Lancet Public Health. 2023;8:e701–e716. doi: 10.1016/S2468-2667(23)00149-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.