Abstract
The rapid spread of herpes simplex virus type 1 (HSV-1) in mucosal epithelia and neuronal tissue depends primarily on the ability of the virus to navigate within polarized cells and the tissues they constitute. To understand HSV entry and the spread of virus across cell junctions, we have previously characterized a human keratinocyte cell line, HaCaT. These cells appear to reflect cells infected in vivo more accurately than many of the cultured cells used to propagate HSV. HSV mutants lacking gE/gI are highly compromised in spread within epithelial and neuronal tissues and also show defects in cell-to-cell spread in HaCaT cells, but not in other, nonpolarized cells. HSV gD is normally considered absolutely essential for entry and cell-to-cell spread, both in cultured cells and in vivo. Here, an HSV-1 gD mutant virus, F-US6kan, was found to efficiently enter HaCaT cells and normal human keratinocytes and could spread from cell to cell without gD provided by complementing cells. By contrast, entry and spread into other cells, especially highly transformed cells commonly used to propagate HSV, were extremely inefficient. Further analyses of F-US6kan indicated that this mutant expressed extraordinarily low (1/500 wild-type) levels of gD. Neutralizing anti-gD monoclonal antibodies inhibited entry of F-US6kan, suggesting F-US6kan utilized this small amount of gD to enter cells. HaCaT cells expressed high levels of an HSV gD receptor, HveC, and entry of F-US6kan into HaCaT cells could also be inhibited with antibodies specific for HveC. Interestingly, anti-HveC antibodies were not fully able to inhibit entry of wild-type HSV-1 into HaCaT cells. These results help to uncover important properties of HSV and human keratinocytes. HSV, with exceedingly low levels of a crucial receptor-binding glycoprotein, can enter cells expressing high levels of receptor. In this case, surplus gD may be useful to avoid neutralization by anti-gD antibodies.
Herpes simplex virus (HSV) enters host cells following sequential interactions with several host cell surface molecules. The sequential nature of virus attachment and entry into cells was first proposed during the characterization of HSV type 1 (HSV-1) mutants lacking gD that could adsorb onto but not enter cells (14, 19). These studies suggested that there were sequential interactions with cells: adsorption onto very numerous cell surface sites followed by secondary interactions with so-called gD receptors present on cells in much more limited numbers (13, 14). Further support for the hypothesis came from the observation that cells expressing gD were resistant to infection (5) because gD interfered with endogenous receptors. Moreover, gD receptors could be blocked by using soluble forms of gD that bound to saturable cell surface sites (13). Subsequently, it was shown that HSV adsorbs onto cell surface heparan sulfate molecules, which involves two other HSV glycoproteins, gC and gB (12, 33). Adsorption onto heparan sulfate apparently precedes interactions with gD receptors, leading to fusion of the virion envelope with the plasma membrane.
Several gD receptors have been identified by expression cloning, including HveA, HveB, and HveC (11, 17, 23, 30, 31). Of these three recently described receptors, HveC appears to be the most important in adherent cells, epithelial cells, and other cells that HSV normally infects in vivo. HveB is not used by wild-type HSV-1 but can act as a receptor for HSV-2. HveA is highly expressed in and, to some extent, restricted to lymphocytes, monocytes, and other nonadherent cells and is not found in the brain (18). Moreover, anti-HveA antibodies do not block HSV-1 entry into several adherent cell lines (J. C. Whitbeck, G. Cohen, and R. Eisenberg, unpublished results). By contrast, HveC antibodies block entry into several cell types normally used to propagate HSV (16).
HveC and HveB, immunoglobulin superfamily members related to the poliovirus receptor, are also known as nectin-1 and nectin-2, respectively, and can act as homotypic, Ca2+-independent cell adhesion molecules (1, 20, 27, 28). Nectins localize to cadherin-based adherens junctions through interactions between their cytoplasmic domains and PDZ domains of afadin, an actin filament-binding protein (21, 22, 27, 28). As nectins are found largely at cell junctions, it is not surprising that these cell adhesion molecules play important roles in the cell-to-cell spread of HSV (6), a process involving movement of virions across adherens junctions (15). However, nectins also mediate entry of HSV into cells (11, 30), and thus, some fraction of nectins must also be distributed on the apical surfaces of polarized cells as well as more uniformly on nonpolarized cells.
There are several reasons to believe that HveA, -B, and -C are not the only HSV and gD receptors. For example, mannose-6-phosphate (M6P) receptors can act as HSV receptors: anti-M6P receptor antibodies block entry of HSV-1, and there is reduced cell-to-cell spread in cells unable to add M6P to gD (4). However, there is also evidence that HSV does not depend solely on M6P receptors (4). Furthermore, HveC-neutralizing antibodies can effectively block entry of HSV-1 into some cells, but these antibodies are much less effective in other cells (16; C. Krummenacher, G. Cohen, and R. Eisenberg, unpublished results). HSV has a wide host range, and this may hinge on using a variety of cell surface receptors, including molecules that have not yet been described.
HSV replication and spread in mucosal epithelium and in the nervous system depends largely on polarized cells, e.g., dermal keratinocytes and neurons. As part of our efforts to understand HSV entry and cell-to-cell spread across epithelial cell junctions, we have studied a human keratinocyte cell line, HaCaT (32; T. McMillan and D. C. Johnson, unpublished data). Studies involving HSV gE− and gI− mutants illustrate the rationale for examining these cells. A complex of HSV glycoproteins, gE/gI, plays an important role in cell-to-cell spread in both epithelial and neuronal tissues (reviewed in McMillan and Johnson, unpublished). gE− and gI− mutant viruses displayed a particularly pronounced phenotype in HaCaT cells, producing plaques eightfold smaller than those produced by wild-type HSV-1 (32). By contrast, gE− and gI− mutants frequently do not display this phenotype in most of the highly transformed cells commonly used to propagate HSV. HSV gD is usually considered to be absolutely essential for entry and cell-to-cell spread, both in cultured cells and in vivo (9, 19). However, the relative importance of HSV-1 gD in entry and cell-to-cell spread in epithelial cells and keratinocytes has not been carefully examined. In this study, we found that an HSV-1 gD mutant, F-US6kan, could spread in and efficiently enter keratinocytes without gD provided by complementing cells. Analyses of F-US6kan indicated that this mutant expressed 1/500 the gD expressed by wild-type HSV-1. HaCaT cells expressed high levels of HveC, suggesting that high receptor concentrations may alleviate the requirement for normal amounts of gD.
MATERIALS AND METHODS
Cells and viruses.
A human keratinocyte cell line, HaCaT (3) (a gift of N. E. Fusenig, Heidelberg, Germany), was grown in Dulbecco's modified essential medium (DMEM; BioWhittaker Inc., Walkersville, Md.) supplemented with 10% heat-inactivated fetal bovine serum (FBS; HyClone). Vero, R970, HEp-2, 293, Colo (human skin squamous carcinoma cells; a gift from R. McKenzie, Sunnybrook Hospital, Toronto, Canada), and U373-MG glioblastoma (American Type Culture Collection [ATCC]) cells were all grown in DMEM supplemented with 5 to 10% FBS. A431 (ATCC), HeLa (ATCC), and MDBK (ATCC) cells were grown in Eagle's modified essential medium (BioWhittaker) supplemented with 10% FBS. HEC-1A (human endometrial epithelial) (2) cells were grown in RPMI medium (BioWhittaker) supplemented with 10% FBS. ARPE-19 cells (ATCC) were grown in DMEM and F12 media (50:50) supplemented with 10% FBS. CHO and COS-1 cells were grown in α-MEM supplemented with 10% FBS. Human keratinocytes derived from human foreskins were obtained from Cascade Biologics Inc. (Portland, Oreg.) or from Paul Cooke (Department of Dermatology, Oregon Health Sciences University, Portland), and both were grown in Epilife medium supplemented with human keratinocyte growth supplement (Cascade Biologics) and 60 μM Ca2+. HSV-1 wild-type strain F and F-gEβ, a virus unable to express gE (9), were propagated on Vero cells, and titers were determined. The HSV-1 gD mutants F-gDβ (19), F-US6kan (14), RR1097 (25*), and KOS gD− (8) were all propagated on VD60 cells that express gD. To produce noncomplemented stocks of these viruses (lacking gD), Vero or HaCaT cells were infected with 5 PFU/cell, and after 24 h, the cells were scraped and sonicated, and the cell-derived stocks were frozen at −70°C in supernatant from the infected cell culture. Infected cell culture supernatants were also harvested, clarified by centrifugation at 500 × g, and frozen at −70°C.
Infection of cells and staining of HSV plaques.
Cells were infected for 2 h in appropriate medium supplemented with 1 to 2% FBS, virus was removed, and fresh medium containing 1 to 2% FBS and 0.2% human gamma globulin (a source of anti-HSV neutralizing antibodies) was added for 2 days. The cells were washed, fixed, and stained with polyclonal anti-HSV-1 antibodies, peroxidase-conjugated secondary antibodies, and peroxidase substrate as described previously (32).
Radiolabeling of infected cells and immunoprecipitation.
HaCaT or Vero cells were infected with 1 or 5 PFU of wild-type HSV-1 F or F-US6kan/cell or with a similar number of particles (normalized for nucleocapsid protein by using Western dot blot analyses). After 2 h, the cells were labeled with [35S]methionine-cysteine (Amersham) (50 μCi/ml) for 5 h, lysed with NP-40–deoxycholate (DOC) lysis buffer (100 mM NaCl, 50 mM Tris-HCl [pH 7.5], 1.0% NP-40, 0.5% DOC) supplemented with 2 mg of bovine serum albumin/ml and 1 mM phenylmethylsulfonyl fluoride, and cell extracts were frozen at −70°C. HSV gD was immunoprecipitated by using a pool of the anti-gD monoclonal antibodies (MAbs) DL6 and LP2. HSV-1 ICP47 was immunoprecipitated from cell extracts using anti-ICP47 rabbit antiserum (ICP47-5) and subjected to electrophoresis as described previously (29). The dried gels were placed in contact with a phosphorimager, and viral proteins were quantified.
Western blot analysis for HSV gD or nucleocapsid proteins.
HaCaT cells infected with F-US6kan or wild-type HSV-1 were lysed in NP-40–DOC lysis buffer containing 0.1% sodium dodecyl sulfate (SDS), insoluble material was removed by centrifugation, 2% SDS and 2% β-mercaptoethanol were added, and samples were boiled for 5 min and then subjected to electrophoresis through SDS–10% polyacrylamide gels. Proteins were transferred to polyvinylidene difluoride (Immobilon-P; Millipore, Bedford, Mass.) membranes, and the membranes were blocked by incubation with 5% nonfat milk–0.1% Tween 20 and then incubated with anti-gD MAb ID3. The blots were washed and stained with horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G as described previously (32). The proteins were visualized with an enhanced-chemiluminescence kit (New England Nuclear) and exposed to X ray film. To ensure that there were equal quantities of virus particles in stocks of noncomplemented gD mutants, the amount of viral nucleocapsid in each stock was quantified. Preparations of virus were serially diluted in phosphate-buffered saline (PBS) and passed through Immobilon P membranes mounted in a 96-well dot blot apparatus (Gibco/BRL, Gaithersburg, Md.) under suction. The membranes were blocked by incubation with PBS containing 2% normal goat serum, 1% fish gelatin (Sigma, St. Louis, Mo.), 0.5% polyvinylpyrrolidone, and 0.1% Tween 20 (blocking buffer). The membranes were then incubated with rabbit anti-VP5 serum (anti-NC-1 [7]) diluted 1:750 in PBS containing 1% nonfat milk, 0.5% normal goat serum, 0.25% polyvinylpyrrolidone, and 0.1% Tween 20 for 12 to 16 h; washed five times with PBS containing 1% bovine serum albumin and 0.1% Tween 20 (wash buffer); and incubated with horseradish peroxidase-conjugated donkey anti-rabbit antibodies (Amersham) for 1.5 h. The membranes were washed and incubated with chemiluminescence reagent (New England Nuclear) for 1 min, wrapped with plastic sheets, and analyzed with a Lumi Imager (Boehringer Mannheim).
Flow cytometric analysis for HveC expression.
Cells were removed from plastic dishes after treatment with 53 mM EDTA for 10 to 20 min, washed in fluorescence-activated cell sorter (FACS) buffer (PBS containing 1% FBS–0.05% sodium azide), and suspended in duplicate 96-well U-bottom microtiter plates. The cells were stained with 50 μg of anti-HveC MAb CK41 or CK8 or anti-major histocompatibility complex class I MAb W6/32/ml or no antibody for 1 h at 4°C. The cells were pelleted in the plates, washed three times with FACS buffer, and stained with fluorescein isothiocyanate-conjugated goat anti-mouse antibodies for 40 min at 4°C. The cells were washed three times with FACS buffer and analyzed by using a Becton Dickinson FACSCalibur flow cytometer.
Inhibition of HSV by anti-gD and anti-HveC antibodies.
Preparations of virus were preincubated with anti-gD MAb LP2 or DL11, anti-gE MAb 3114, anti-gI MAb 3104, or anti-HCMV gH MAb 14-4b for 30 min at 37°C prior to addition to HaCaT cells for 2 h. In other experiments, HaCaT cells were preincubated with anti-HveC or -HveA MAb or anti-HLA DRα DA6.147 for 30 min at 37°C, and then F-US6kan or HSV-1 F was added for an additional 2 h at 37°C. In both sets of experiments, the virus and antibodies were removed, residual virus was inactivated with citrate buffer (pH 3.0) (4), and the cells were washed and then incubated with medium supplemented with 1% FBS and 0.2% human gamma globulin for 2 days. The plaques were stained with anti-HSV antibodies as described above.
RESULTS
F-US6kan can spread cell to cell in human keratinocytes.
A human keratinocyte cell line, HaCaT, was derived from a culture of primary keratinocytes obtained from adult skin (3). These cells are aneuploid, spontaneously transformed in vitro, and express several keratinocyte markers, forming orderly structured and differentiated epidermal tissue when transplanted into nude mice (3). In characterizing HaCaT cells, we tested various virus mutants for cell-to-cell spread. F-US6kan is a recombinant HSV-1 in which a kanamycin gene cassette was inserted between the gD promoter and coding sequences, and it was reported that F-US6kan does not express gD (14). F-US6kan was derived from F-gDβ, a gD− virus in which gD and gI coding sequences were replaced by β-galactosidase coding sequences (by replacing the gD-gI deletion with sequences into which the kanamycin gene cassette was inserted) (19). Both F-gDβ and F-US6kan produced syncytial plaques on complementing VD60 cells that provide gD (14). For all of the present studies, we identified and used a nonsyncytial variant of F-US6kan that produced nonsyncytial plaques in a stable fashion on VD60 cell monolayers.
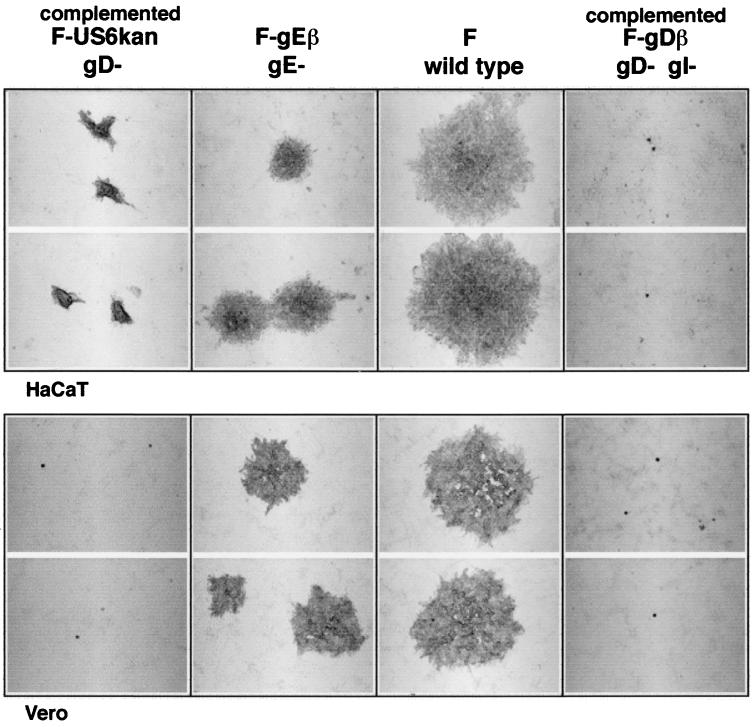
F-US6kan derived from complementing VD60 cells produced plaques composed of approximately 100 cells on HaCaT cells, while wild-type HSV-1 F plaques contained approximately 1,000 cells (Fig. 1, top). It was quite striking that these F-US6kan plaques were observed in numbers (1 × 109 to 2 × 109 PFU/ml) equal to those of wild-type HSV-1 F. The plaques produced by complemented F-US6kan on HaCaT cells were similar in number and size to those on complementing VD60 cells. It should be noted that the cells in F-US6kan plaques piled on top of one another, so that the diameters of the plaques did not accurately reflect the numbers of cells infected when making comparisons to plaques formed by wild-type HSV-1. This piling up was not observed to the same extent with wild-type HSV-1. By contrast, F-US6kan infected only single cells on monolayers of Vero cells (Fig. 1, bottom). F-gDβ, a second gD− HSV-1, infected only single cells on monolayers of HaCaT and Vero cells. F-gEβ, a gE− virus, produced plaques that were composed of approximately 150 to 200 cells. Therefore, F-US6kan derived from complementing VD60 cells can spread in HaCaT cell monolayers, although not as well as wild-type HSV-1.
FIG. 1.
Plaques formed by complemented F-US6kan on HaCaT and Vero cells. Confluent monolayers of HaCaT and Vero cells were infected with various dilutions of F-US6kan (derived from complementing VD60 cells), F-gEβ, wild-type HSV-1 F, or F-gDβ (derived from VD60 cells). After 2 days, the cells were fixed and stained with anti-HSV-1 polyclonal antibodies, peroxidase-conjugated secondary antibodies, and peroxidase substrate.
F-US6kan produced on noncomplementing cells can enter HaCaT cells.
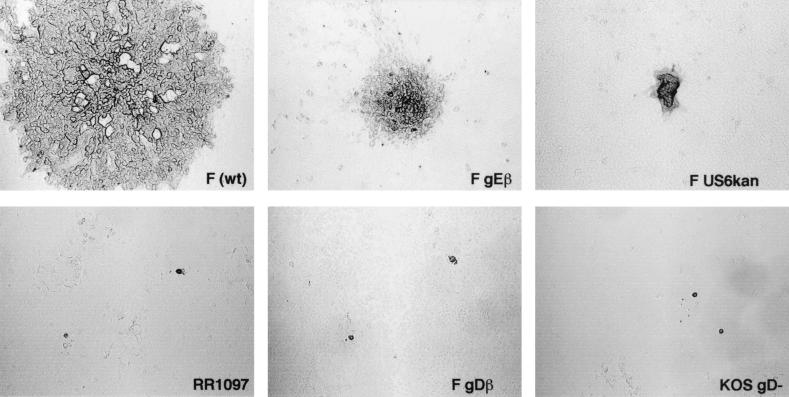
To investigate whether F-US6kan could enter HaCaT cells without the gD provided by VD60 cells, stocks of virus were produced by infecting Vero cells with VD60-derived virus. Previously, similar virus preparations produced by using 10 PFU/cell were shown to contain normal numbers of virus particles lacking gD (19). Noncomplemented F-US6kan produced plaques of comparable size to those produced by complemented F-US6kan on HaCaT cells, encompassing 75 to 125 cells per plaque, compared with approximately 1,000 cells per plaque for wild-type HSV-1 (Fig. 2). Again, it was quite striking that the numbers of plaques produced by noncomplemented F-US6kan were similar to those of wild-type HSV-1 (i.e., the titers were 1 × 109 to 2 × 109 PFU/ml for both wild-type HSV-1 and F-US6kan) (Table 1). Moreover, F-US6kan and wild-type HSV-1 stocks derived from Vero cell culture supernatants both produced 1 × 107 to 2 × 107 plaques/ml on HaCaT cells (data not shown). These preparations of noncomplemented F-US6kan contained low levels of wild-type virus derived by recombination with cellular copies of the gD gene (14, 19). However, these wild-type contaminants produced plaques of normal size on HaCaT (1,000 cells/plaque) or Vero cells and were extremely rare, diminished in number by 4 to 5 log units relative to those produced by the gD mutant (not shown). It is unlikely that this nonsyncytial variant of F-US6kan had acquired a second-site mutation that accounted for virus entry, because the original syncytial F-US6kan (14) also produced numerous plaques (titers were 1 × 109 to 2 × 109 PFU/ml) on HaCaT cells and there was no evidence of cell fusion (not shown).
FIG. 2.
Plaques for noncomplemented HSV-1 gD mutants on HaCaT cells. HaCaT cells were infected for 2 days with wild-type (wt) HSV-1 F, F-gEβ, F-US6kan, RR1097, F-gDβ, or KOS gD−. Each of these viruses was derived from Vero cells, and thus, in the case of the gD− viruses, there was no complementation.
TABLE 1.
Numbers and sizes of plaques formed by F-US6kan and F-gDβ
| Cell type | Virus titera (PFU/ml)
|
Approximate no. of cells/plaque
|
||||
|---|---|---|---|---|---|---|
| F | F-US6kan | F-gDβ | F | F-US6kan | F-gDβ | |
| HaCat | 1.3 × 109 | 1.4 × 109 | 7.0 × 106 | 1,000 | 100–150 | 1 |
| Vero | 3.6 × 109 | 2.5 × 107 | 2.5 × 106 | 200 | 1 | 1 |
| VD60 | 1.1 × 109 | 1.7 × 107 | 8.4 × 106 | 75 | 75 | 75 |
| R970 | 7.3 × 108 | 4.1 × 106 | 6.0 × 105 | 500 | 1 | 1 |
| Hep-2 | 5.6 × 108 | 9.7 × 106 | 1.0 × 106 | 50–100 | 1 | 1 |
Stocks of wild-type HSV-1 strain F, F-US6kan, and F-gDβ were all grown on Vero cells and then diluted to infect monolayers of various cells. The numbers and sizes of these plaques are indicated.
Noncomplemented F-US6kan also produced plaques composed of approximately 100 infected cells on two different preparations of primary human foreskin keratinocytes, compared with approximately 1,000 cells/plaque for wild-type HSV-1 (data not shown). The titers of both F-US6kan and wild-type HSV-1 on these primary cells were similar to those on HaCaT cells (data not shown).
We also characterized the entry of three other HSV-1 gD mutants on HaCaT cells. F-gDβ was described above. RR1097 was derived from HSV-1 F by replacing most of the gD coding sequences with a green fluorescent protein gene (25). KOS gD− was derived from HSV-1 strain KOS by replacing all gD coding sequences with β-galactosidase sequences (8). In other experiments, we verified that RR1097 and KOS gD− expressed both gI and gE (not shown). Stocks of these three mutants were produced together with F-US6kan on Vero cells so that virions did not contain gD. RR1097, KOS gD−, and F-gDβ infected very few HaCaT cells, and the virus did not spread beyond a single infected cell (Fig. 2). The numbers of these single infected cells were 300- to 10,000-fold lower than with F-US6kan. For example, in one experiment, F-US6kan produced 1.5 × 109 plaques, F-gDβ produced 5 × 106 single infected cells, and RR1097 and KOS gD− produced 1 × 104 to 2 × 104 single infected cells.
It was conceivable that the entry of F-US6kan into HaCaT cells was mediated by residual gD present in the virion envelope, originally produced in VD60 cells and carried over into Vero cells during production of virus stocks. However, this possibility was ruled out by the observations that F-gDβ, RR1097, and KOS gD−, all grown in parallel with F-US6kan, did not efficiently enter HaCaT cells and could not spread between cells. Moreover, stocks of F-US6kan produced by first passaging the virus on Vero cells and then secondarily on HaCaT cells could efficiently produce plaques on HaCaT cells, yielding titers of 1 × 109 to 2 × 109 PFU/ml (not shown).
To investigate whether F-US6kan could enter other cell types, noncomplemented F-US6kan derived from Vero cells and stocks of wild-type HSV-1 F and F-gDβ produced in parallel were used to infect a number of normal and transformed cells. On Vero, R970 (human osteosarcoma), and Hep-2 (human epidermoid larynx carcinoma) cell monolayers, F-US6kan produced only single infected cells, and their numbers were approximately 60- to 200-fold lower than the plaques formed on HaCaT cells (Table 1). F-gDβ also produced single infected cells on Vero, R970, and Hep-2 monolayers, and these were 500- to 1,500-fold fewer than the plaques produced by the wild type on these cells. All three viruses produced small (75-cell) plaques on complementing VD60 cells. In Table 2, HaCaT cells are compared with other epithelial cells, including Colo (human skin squamous carcinoma), MDBK (bovine kidney epithelial), A431 (human epidermoid carcinoma), ARPE-19 (human retinal epithelial), and HEC-1A (human endometrial epithelial) cells. The numbers of plaques produced by noncomplemented F-US6kan were reduced by 8- to 50-fold on these cells, and the plaques were 4- to 100-fold smaller than on HaCaT cells. In Table 3, other transformed cells infected with noncomplemented F-US6kan are compared with HaCaT cells. As with Vero and R970 cells, only a single HeLa cell was infected, although 5 to 20 cells were infected in monolayers of COS-1, 293, and U373 cells. Therefore, in general, F-US6kan entered highly transformed cell lines very poorly and did not spread beyond a single infected cell or, in some cases, only a few cells. Cell-to-cell spread was better in epithelial cell lines, but the numbers of plaques were also reduced compared with those in HaCaT keratinocytes.
TABLE 2.
Plaques formed by F-US6kan on various epithelial cells
| Cell type | Virus titer (PFU/ml) | No. of cells/plaque |
|---|---|---|
| HaCat | 7.8 × 108 | 100–150 |
| Colo | 1.0 × 108 | 15–50 |
| MDBK | 6.6 × 107 | 5–25 |
| A431 | 6.5 × 107 | 4–20 |
| ARPE-19 | 2.8 × 107 | 10–50 |
| HEC-1A | 1.5 × 107 | 1–6 |
TABLE 3.
Plaques formed by F-US6kan on various transformed cell lines
| Cell type | Virus titer (PFU/ml) | No. of cells/plaque |
|---|---|---|
| HaCaT | 5.7 × 108 | 100–150 |
| COS-1 | 1.5 × 107 | 1–5 |
| 293 | 3.0 × 107 | 5–20 |
| U373-His16 | 2.0 × 107 | 5–15 |
| HeLa | 1.0 × 107 | 1 |
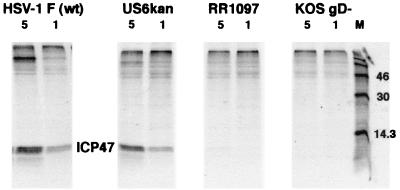
The entry of noncomplemented F-US6kan into HaCaT cells was characterized further by analyzing the expression of an HSV immediate-early protein, ICP47, shortly after infection. HaCaT cells were infected with similar amounts of noncomplemented F-US6kan, RR1097, KOS gD−, or wild-type HSV-1 F particles. For F and F-US6kan, the cells were infected with 1 or 5 PFU/cell, based on virus titers on HaCaT cells. However, for RR1097 and KOS gD−, equal numbers of virus particles were used by quantifying viral nucleocapsid protein in virus preparations, using Western blot analysis. The cells were labeled with [35S]methionine-cysteine, and ICP47 immunoprecipitated from cell extracts. ICP47 was expressed at similar levels in cells infected with noncomplemented F-US6kan and wild-type HSV-1, but no ICP47 was detected in cells infected with RR1097 or KOS gD− (Fig. 3). In other experiments, no detectable ICP47 was expressed in Vero cells infected with noncomplemented F-US6kan (not shown). Therefore, noncomplemented F-US6kan can efficiently enter HaCaT cells but not Vero cells.
FIG. 3.
Entry of noncomplemented HSV-1 gD mutants into HaCaT cells. HaCaT cells were infected with 5 or 1 PFU of wild-type HSV-1 F or F-US6kan or with an equal number of particles (normalized for nucleocapsid protein) of RR1097 or KOS gD−. After 2 h, the virus was removed, and the cells were labeled for 5 h with [35S]methionine-cysteine. The HSV immediate-early protein ICP47 was immunoprecipitated from cell extracts.
F-US6kan expresses very low levels of gD.
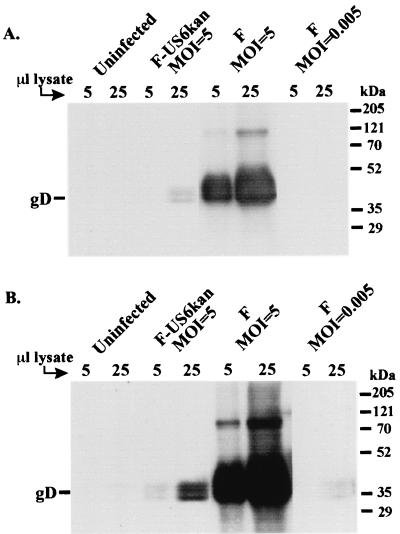
The differences between F-US6kan and the other three gD mutants were striking. F-US6kan could infect HaCaT cells well, as efficiently as wild-type HSV-1, although spread was partially compromised. By contrast, F-gDβ, RR1097, and KOS gD− all produced 1,000 to 100,000 fewer plaques, and only single cells were infected. In F-US6kan, the coding sequences for gD are intact, although the promoter and RNA start site are separated by a kanamycin gene cassette from the coding sequences. By contrast, in RR1097, F-gDβ, and KOS gD−, the coding sequences are largely or completely removed. Previous analyses of F-US6kan had indicated that gD was not produced in infected cells (14). However, it was possible that a low level of gD was produced and remained undetected in the earlier studies. To quantify gD expression, HaCaT cells were infected with noncomplemented F-US6kan or wild-type HSV-1 F, and cell extracts were subjected to Western blot analyses using MAb ID3. Measurable gD was expressed in cells infected with 5 PFU of F-US6kan/cell, although this was much lower than extracts from cells infected with wild-type HSV-1 (Fig. 4). Since the noncomplemented F-US6kan stocks contained contaminating levels of wild-type HSV-1, in some cases amounting to 1/1,000th that of F-US6kan, we also infected cells with wild-type HSV-1 using 0.005 PFU/cell to determine whether wild-type contamination could account for the gD observed. Cells infected in this way (Fig. 4, right lanes) displayed much lower levels of gD than F-US6kan-infected cells (Fig. 4, middle lanes), and thus, we concluded that F-US6kan did express very low levels of gD. When lighter exposures were scanned and quantified, we determined that there was 503-fold less gD in F-US6kan-infected HaCaT cells than in cells infected by wild-type HSV-1. Other blots probed with anti-gD polyclonal antisera produced similar results.
FIG. 4.
Western blot analysis of the gD expressed in F-US6kan-infected cells. HaCaT cells were infected with wild-type HSV-1 F or noncomplemented F-US6kan using 5 PFU/cell (multiplicity of infection [MOI]) or with wild-type HSV-1 using 0.005 PFU/cell or were left uninfected. After 9 h, the cells were lysed with detergent, and extracts (either 5 or 25 μl) were subjected to electrophoresis and then transferred to Immobilon P membranes that were probed with anti-gD MAb ID3. (A) Blot exposed to film for 5 s; (B) the same blot exposed for 30 s.
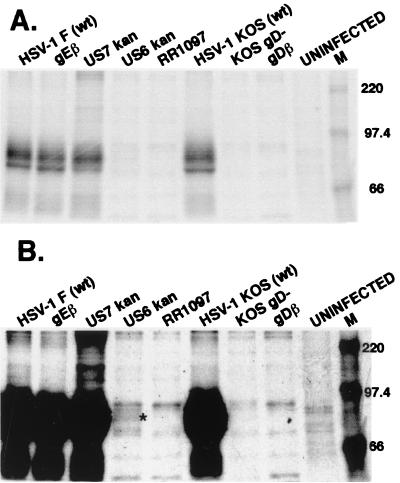
As a second measure of gD expression in F-US6kan-infected cells, HaCaT cells were labeled with [35S]methionine-cysteine, and gD was immunoprecipitated using a pool of two anti-gD MAbs. A small amount of gD was detected in F-US6kan-infected cells, just slightly below background bands seen in uninfected cells (Fig. 5B). gD was readily radiolabeled and immunoprecipitated from cells infected with wild-type HSV-1, F-gEβ, or F-US7kan, a gI− mutant. Quantification of this gel indicated that there was 436-fold less gD in cells infected with F-US6kan than in wild-type HSV-1. Therefore, F-US6kan expresses gD at a level approximately 500-fold lower than does wild-type HSV-1.
FIG. 5.
Immunoprecipitation of radiolabeled gD from cells infected with F-US6kan and other HSV gD mutants. HaCaT cells were infected with wild-type (wt) HSV-1 F, F-gEβ (a gE− HSV), or F-US7kan (a gI− HSV), all derived from Vero cells, or with F-US6kan, RR1097, KOS gD−, or F-gDβ, all derived from VD60 cells, or were left uninfected. In every case, 5 PFU/cell was used. The cells were labeled from 7 to 11 h after infection with [35S]methionine-cysteine, and then gD was immunoprecipitated from cell extracts using a pool of monoclonal antibodies, LP2 and DL6. (A) A 24-h exposure of the gel to a phosphorimager plate; (B) the same exposure with contrast adjusted upwards. The asterisk in panel B designates gD bands.
F-US6kan utilizes gD to enter HaCaT cells.
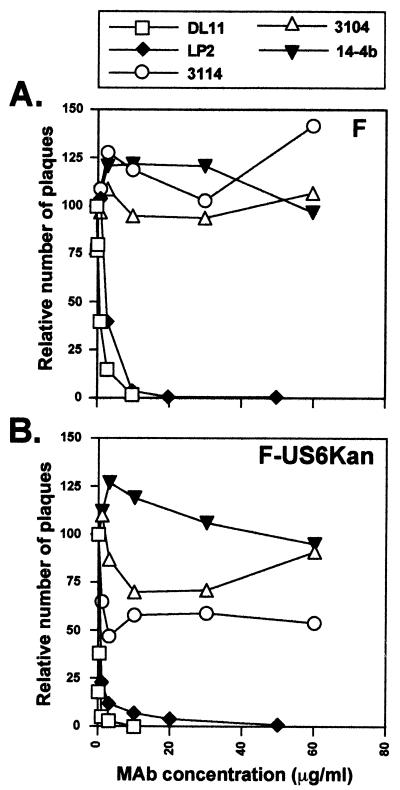
Virus mutants lacking gD coding sequences could not enter HaCaT cells well, yet F-US6kan could do so efficiently, and it expresses small amounts of gD. Therefore, it was of interest to determine whether entry could be blocked by anti-gD antibodies. F-US6kan and wild-type HSV-1, grown in parallel on Vero cells, were incubated with various concentrations of LP2 or DL11, two potent neutralizing anti-gD MAbs, and then added to HaCaT cells. Both gD MAbs effectively neutralized wild-type HSV-1 and F-US6kan: at 5 to 10 μg/ml, there were few plaques, although F-US6kan was neutralized with lower antibody concentrations (Fig. 6). Anti-gE MAb 3114 and anti-gI MAb 3104 had little effect on wild-type HSV-1 F but did inhibit F-US6kan, reducing the number of plaques by 30 to 50% (Fig. 6). Whether this reflects the use of gE/gI for entry is not yet clear and is under further investigation.
FIG. 6.
Inhibition of wild-type HSV-1 and F-US6kan plaques by anti-gD and anti-gE MAbs. Wild type HSV-1 F and noncomplemented F-US6kan were incubated with increasing concentrations of purified antibodies (anti-gD MAbs LP2 and DL11, anti-gE MAb 3114, anti-gI MAb 3104, or anti-HCMV gH MAb 14-4b) for 30 min at 37°C prior to addition to HaCAT cells. Two hours after infection, the virus inoculum was removed, the cells were washed with Na citrate buffer (pH 3.0) for 1 min, and then medium containing 0.2% human gamma globulin was added. At 2 days postinfection, the cells were fixed and stained, and the plaques were counted. The numbers of plaques observed in cell monolayers infected with viruses treated with antibodies are expressed as a percentage of the number of plaques observed when the viruses were not treated with MAbs. (A) HSV-1 F; (B) F-US6kan.
HaCaT cells express high levels of HveC, and anti-HveC antibodies neutralize F-US6kan entry.
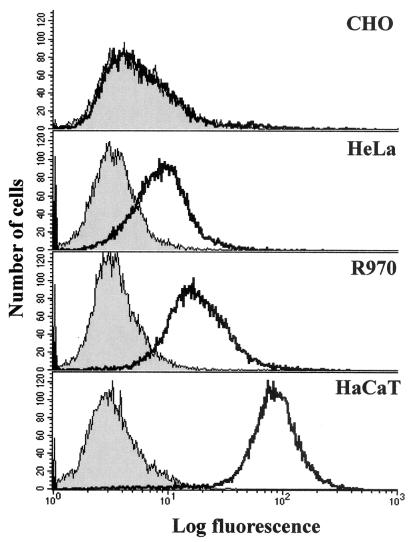
F-US6kan could efficiently enter HaCaT cells and other keratinocytes but not most other cell lines. We reasoned that keratinocytes might express high levels of gD receptors, especially HveC. Two anti-HveC MAbs, CK8 and CK41 (16), were used in FACS experiments. CHO cells, known to lack HveC, were negative for staining by both CK8 (not shown) and CK41 (Fig. 7). There was significant staining of HeLa and R970 cells, above background, with both antibodies (CK41 [Fig. 7] and CK8 [not shown]). However, there was more intense staining of HaCaT cells with both antibodies (CK41 [Fig. 7] and CK8 [not shown]). We estimated that there was approximately 10-fold more HveC present on the surfaces of HaCaT than on those of R970 and HeLa cells, and a fraction of both R970 and HeLa cells expressed levels of HveC similar to those of CHO cells that are negative for HveC. Therefore, HaCaT cells express relatively high levels of HveC, and this might explain the entry of F-US6kan into keratinocytes and not other cells.
FIG. 7.
Flow cytometric analyses of HveC on various cells. CHO, HeLa, R970, and HaCaT cells were removed from plastic dishes and stained with anti-HveC MAb CK41 and secondary fluorescent antibodies. Shaded curves represent cells stained with a control antibody, and open curves represent cells stained with MAb CK41.
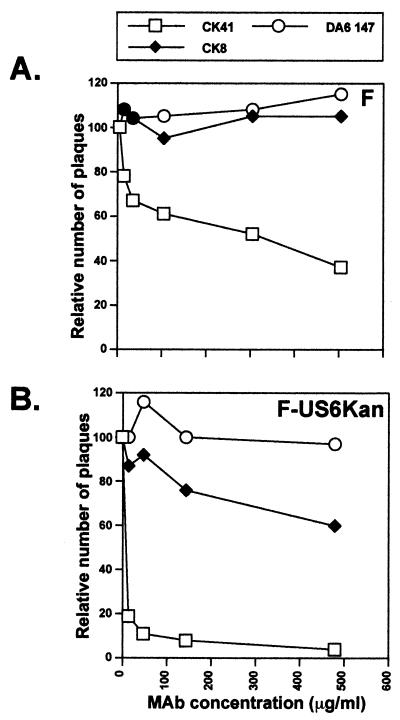
To determine whether HveC might affect F-US6kan entry into HaCaT cells, cells were pretreated with anti-HveC or irrelevant antibodies and then infected with wild-type HSV-1 F or F-US6kan. Anti-HveC MAb CK41 effectively inhibited entry of F-US6kan into HaCaT cells at concentrations of 10 to 20 μg/ml (Fig. 8). However, with wild-type HSV-1, inhibition by anti-HveC MAb CK41 was only 60% at concentrations of 500 μg/ml. There was no inhibition by anti-HveA antibody, CW3 (not shown), or a control MAb, DA6.147 to HLA DRα. These results suggest that F-US6kan uses small amounts of gD to interact with the relatively large amounts of HveC to gain entry into HaCaT cells. Inhibition of wild-type HSV-1 by anti-HveC antibody on HaCaT cells is much less effective, either because there is too much HveC to neutralize or because there are other receptors.
FIG. 8.
Inhibition of wild-type HSV-1 and F-US6kan plaques by anti-HveC MAb. HaCaT cells were incubated with various concentrations of purified anti-HveC MAb CK41 or CK8 or control anti-HLA DRα MAb DA6.147 for 30 min at 37°C, followed by addition of wild-type HSV-1 F or noncomplemented F-US6kan. After 2 h, the inoculum was removed, the cells were washed for 1 min with Na citrate buffer (pH 3.0), and then medium containing 0.2% human gamma globulin was added. After 2 days, the cells were fixed and stained for HSV antigens, and the plaques were counted. The numbers of plaques observed in monolayers treated with MAbs were plotted as a percent of the number of plaques in untreated monolayers. (A) Wild-type HSV-1 F; (B) F-US6kan.
DISCUSSION
Previous studies with HSV-1 gD mutants had led to the conclusion that gD was essential for entry and cell-to-cell spread (14, 19). By contrast, the related alphaherpesvirus pseudorabies virus requires gD for entry but not for cell-to-cell spread (24, 26). Here, we observed that F-US6kan could spread in human keratinocytes, apparently without gD. Subsequent studies indicated that F-US6kan could also efficiently enter keratinocytes, since the same number of plaques were formed by viruses with or without gD. The low level of gD produced by F-US6kan was not sufficient for entry or cell-to-cell spread of F-US6kan on other cells, such as Vero, R970, HeLa, HEp-2, and COS-1 cells. With other epithelial cells, such as ARPE-19, MDBK, Colo, and A431 cells, there was some limited spread of F-US6kan, as well as inefficient entry. Therefore, the ability of F-US6kan to enter and spread between cells appears to be most efficient in keratinocyte cell lines and primary keratinocytes.
On careful inspection, we found that F-US6kan expressed extremely low levels of gD, approximately 1/500 that expressed by wild-type HSV-1. F-US6kan contains a kanamycin gene cassette inserted into the gD promoter and separating the TATAA box from the mRNA start site (14). We sequenced the promoter region of F-US6kan and found no obvious changes in the gD promoter or N-terminal coding sequences, other than the insertion (not shown). Thus, the most likely explanation for the low, but detectable, levels of gD is that there is some very inefficient initiation of transcription of the gD gene at the mRNA start site without the TATAA box.
Given the low levels of gD in F-US6kan, it is likely that there are also low levels of gD in virions. Our analysis of gD did not extend to extracellular virions because the levels of gD were so low as to make analyses of relatively rare extracellular particles difficult. More important than quantification of gD in the virion was the question of whether this small amount of gD could mediate entry into HaCaT cells. This appears to be the case, based on two observations. First, gD− viruses in which the coding sequences were completely or partially deleted did not form plaques on HaCaT cells, as only single infected cells were observed, and these were exceedingly rare. Second, two different anti-gD MAbs could effectively block entry of F-US6kan into HaCaT cells. The results of these antibody inhibition experiments are certainly consistent with a role for the small amounts of gD in F-US6kan entry. However, based on past experience with neutralizing antibodies, these observations do not exclude the possibility that there is entry into keratinocytes that does not involve gD. For example, these anti-gD antibodies may bind to the surfaces of F-US6kan virions in such a way as to preclude entry by indirect means, e.g., steric hindrance. We note that gE−- and gI−-specific MAbs also reduced the entry of F-US6kan substantially (30 to 50%), yet there is no evidence to date that gE-gI is required for entry into these cells.
F-US6kan could also spread from cell to cell in HaCaT and keratinocyte monolayers, although plaques were smaller than those produced by wild-type HSV-1. It appeared possible that this spread was mediated by gE/gI, a glycoprotein that is especially important for cell-to-cell spread in keratinocytes (32). Indeed, a mutant derived from F-US6kan with a deletion of the gE gene could not spread beyond a single infected keratinocyte (K. Goldsmith and D. C. Johnson, unpublished results). Thus, it appears that gE/gI is necessary for cell-to-cell spread of F-US6kan in keratinocyte monolayers, although this result does not directly address whether gE/gI partially substitutes for gD in this process.
HaCaT cells express relatively high levels of HveC, a receptor for HSV-1 and gD ligand (11, 17). This 10-fold difference in HveC levels compared with those in R970 or HeLa cells might entirely explain the observation that F-US6kan can enter HaCaT cells quite efficiently, forming normal numbers of plaques and productively expressing viral early proteins. In this scenario, the higher surface concentrations of gD receptors compensate for the low levels of gD in F-US6kan virions. Consistent with this, anti-HveC antibodies, especially MAb CK41, efficiently blocked entry of F-US6kan into HaCaT cells. One might wonder why HSV-1 expresses relatively high levels of gD when virions containing 500-fold less gD can enter keratinocytes efficiently. There may be cells in vivo that have lower levels of HveC (or other receptors) that might require higher levels of gD in the virion. Also, efficient cell-to-cell spread appears to require higher concentrations of gD than does efficient virus entry into keratinocytes. This is consistent with the notion that entry and cell-to-cell spread show some differences (9, 10, 25). Moreover, since gD is an important target of neutralizing antibodies, surplus gD might benefit HSV by allowing entry into cells such as keratinocytes, even when a fraction of gD is bound by antibodies.
Anti-HveC antibodies inefficiently blocked entry of wild-type HSV-1 into keratinocytes, and only at relatively high antibody concentrations. Similar observations were previously made by Krummenacher et al., who found that relatively high concentrations of anti-HveC MAb were required to block entry into neuroblastoma cell lines and that this inhibition was not always complete (16). This may relate to the fact that, in the presence of high quantities of gD, as in wild-type HSV-1 virions, moderate reductions in HveC (in cells that express relatively high levels of the receptor) are not sufficient to effectively block HSV entry. Consistent with this, F-US6kan was blocked by anti-HveC MAb more efficiently than was wild-type HSV-1. Alternatively, there may be other forms of nectins or other as-yet-uncharacterized HSV receptors on keratinocytes and neuronal cells, which are particularly relevant in vivo.
ACKNOWLEDGMENTS
We are grateful to Pat Spear for providing KOS gD−, Norbert Fusenig for providing HaCaT cells, Bill Britt for providing MAb 14-4b, and Paul Cook for providing primary keratinocytes. D.C.J. is grateful to Roman Tomazin for his faithful devotion to M.T.H throughout this work.
This work was supported by grants CA73996 from the National Cancer Institute to D.C.J.; EY07029 from the National Eye Institute to M.T.H.; RPG-97-070-01-VM from the American Cancer Society and AI07533 from the National Cancer Institute and National Institute of Allergy and Infectious Diseases to R.J.R. and D.A.R.; and Public Health Service grants NS36631 and NS30606 to C.K., R.J.E., and G.H.C.
REFERENCES
- 1.Aoki J, Koike S, Asou H, Ise I, Suwa H, Tanaka T, Miyasaka M, Nomoto A. Mouse homolog of poliovirus receptor-related gene 2 product, mPRR2, mediates homophilic cell aggregation. Exp Cell Res. 1997;235:374–384. doi: 10.1006/excr.1997.3685. [DOI] [PubMed] [Google Scholar]
- 2.Ball J M, Moldoveaunu Z, Melsen L R, Kozlowski P A, Jackson S, Mulligan M J, Mestecky J F, Compans R W. A polarized human endometrial cell line that binds and transports polymeric IgA in vitro. Cell Dev Biol. 1995;31:196–206. doi: 10.1007/BF02639434. [DOI] [PubMed] [Google Scholar]
- 3.Boukamp P, Petrussevska R T, Breitkreutz D, Hornung J, Markham A, Fusenig N E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunetti C R, Burke R L, Hoflack B, Ludwig T, Dingwell K S, Johnson D C. Role of mannose-6-phosphate receptors in herpes simplex virus entry into cells and cell-to-cell transmission. J Virol. 1995;69:3517–3528. doi: 10.1128/jvi.69.6.3517-3528.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campadelli-Fiume G, Arsenakis M, Farabegoli F, Roizman B. Entry of herpes simplex virus 1 in BJ cells that constitutively express viral glycoprotein D is by endocytosis and results in degradation of the virus. J Virol. 1988;62:159–167. doi: 10.1128/jvi.62.1.159-167.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cocchi F, Menotti L, Dubreuil P, Lopez M, Campadelli-Fiume G. Cell-to-cell spread of wild-type herpes simplex virus type 1, but not of syncytial strains, is mediated by the immunoglobulin-like receptors that mediate virion entry, nectin1 (PRR1/HveC/HIgR) and nectin2 (PRR2/HveB) J Virol. 2000;74:3909–3917. doi: 10.1128/jvi.74.8.3909-3917.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen G H, Ponce de Leon M, Diggelmann H, Lawrence W C, Vernon S K, Eisenberg R J. Structural analysis of the capsid polypeptides of herpes simplex virus types 1 and 2. J Virol. 1980;34:521–531. doi: 10.1128/jvi.34.2.521-531.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dean H J, Terhune S S, Shieh M T, Susmarski N, Spear P G. Single amino acid substitutions in gD of herpes simplex virus 1 confer resistance to gD-mediated interference and cause cell-type-dependent alterations in infectivity. Virology. 1994;199:67–80. doi: 10.1006/viro.1994.1098. [DOI] [PubMed] [Google Scholar]
- 9.Dingwell K S, Brunetti C R, Hendricks R L, Tang Q, Tang M, Rainbow A J, Johnson D C. Herpes simplex virus glycoproteins E and I facilitate cell-to-cell spread in vivo and across junctions of cultured cells. J Virol. 1994;68:834–845. doi: 10.1128/jvi.68.2.834-845.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dingwell K S, Johnson D C. Herpes simplex virus gE/gI facilitates cell-to-cell spread and binds to components of cell junctions. J Virol. 1998;72:8933–8942. doi: 10.1128/jvi.72.11.8933-8942.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geraghty R J, Krummenacher C, Cohen G H, Eisenberg R J, Spear P. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science. 1998;280:1618–1620. doi: 10.1126/science.280.5369.1618. [DOI] [PubMed] [Google Scholar]
- 12.Herold B C, WuDunn D, Soltys N, Spear P G. Glycoprotein C of herpes simplex virus type 1 plays a principal role in the adsorption of virus to cells and in infectivity. J Virol. 1991;65:1090–1098. doi: 10.1128/jvi.65.3.1090-1098.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson D C, Burke R L, Gregory T. Soluble forms of herpes simplex virus glycoprotein D bind to a limited number of cell surface receptors and inhibit virus entry into cells. J Virol. 1990;64:2569–2576. doi: 10.1128/jvi.64.6.2569-2576.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson D C, Ligas M W. Herpes simplex viruses lacking glycoprotein D are unable to inhibit virus penetration: quantitative evidence for virus-specific cell surface receptors. J Virol. 1988;62:4605–4612. doi: 10.1128/jvi.62.12.4605-4612.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson D C, Webb M, Wisner T W, Brunetti C. Herpes simplex virus gE/gI sorts nascent virions to epithelial cell junctions, promoting virus spread. J Virol. 2001;75:821–833. doi: 10.1128/JVI.75.2.821-833.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krummenacher C, Baribaud I, Ponce de Leon M, Whitbeck J C, Lou H, Cohen G H, Eisenberg R J. Localization of a binding site for herpes simplex virus glycoprotein D on herpesvirus entry mediator C by using antireceptor monoclonal antibodies. J Virol. 2000;74:10863–10872. doi: 10.1128/jvi.74.23.10863-10872.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krummenacher C, Nicola A V, Whitbeck J C, Lou H, Hou W, Lambris J D, Geraghty R J, Spear P G, Cohen G H, Eisenberg R J. Herpes simplex virus glycoprotein D can bind to poliovirus receptor-related protein 1 or herpesvirus entry mediator, two structurally unrelated mediators of virus entry. J Virol. 1998;72:7064–7074. doi: 10.1128/jvi.72.9.7064-7074.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwon B S, Tan K B, Ni J, Oh K O, Lee Z H, Kim K K, Kim Y J, Wang S, Gentz R, Yu G L, Harrop J, Lyn S D, Silverman C, Porter T G, Truneh A, Young P R. A newly identified member of the tumor necrosis factor receptor superfamily with a wide tissue distribution and involvement in lymphocyte activation. J Biol Chem. 1997;272:14272–14276. doi: 10.1074/jbc.272.22.14272. [DOI] [PubMed] [Google Scholar]
- 19.Ligas M W, Johnson D C. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by beta-galactosidase sequences binds to but is unable to penetrate into cells. J Virol. 1988;62:1486–1494. doi: 10.1128/jvi.62.5.1486-1494.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez M, Aoubala M, Jordier F, Isnardon D, Gomez S, Dubreuil P. The human poliovirus receptor related 2 protein is a new hematopoietic/endothelial homophilic adhesion molecule. Blood. 1998;92:4602–4611. [PubMed] [Google Scholar]
- 21.Mandai K, Nakanishi H, Satoh A, Obaishi H, Wada M, Nishioka H, Itoh M, Mizoguchi A, Aoki T, Fujimoto T, Matsuda Y, Tsukita S, Takai Y. Afadin: a novel actin filament-binding protein with one PDZ domain localized at cadherin-based cell-to-cell adherens junction. J Cell Biol. 1997;139:517–528. doi: 10.1083/jcb.139.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyahara M, Nakanishi H, Takahashi K, Satoh-Horikawa K, Tachibana K, Takai Y. Interaction of nectin with afadin is necessary for its clustering at cell-cell contact sites but not for its cis dimerization or trans interaction. J Biol Chem. 2000;275:613–618. doi: 10.1074/jbc.275.1.613. [DOI] [PubMed] [Google Scholar]
- 23.Montgomery R I, Warner M S, Lum B J, Spear P G. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 1996;87:427–436. doi: 10.1016/s0092-8674(00)81363-x. [DOI] [PubMed] [Google Scholar]
- 24.Peeters B, Pol J, Gielkens A, Moormann R. Envelope glycoprotein gp50 of pseudorabies virus is essential for virus entry but is not required for viral spread in mice. J Virol. 1993;67:170–177. doi: 10.1128/jvi.67.1.170-177.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rauch D A, Rodriguez N, Roller R J. Mutations in herpes simplex virus glycoprotein D distinguish entry of free virus from cell-cell spread. J Virol. 2000;74:11437–11446. doi: 10.1128/jvi.74.24.11437-11446.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rauh I, Mettenleiter T C. Pseudorabies virus glycoproteins gII and gp50 are essential for virus penetration. J Virol. 1991;64:5348–5346. doi: 10.1128/jvi.65.10.5348-5356.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Satoh-Horikawa K, Nakanishi H, Takahashi K, Miyahara M, Nishimura M, Tachibana K, Mizoguchi A, Takai Y. Nectin-3, a new member of immunoglobulin-like cell adhesion molecules that shows homophilic and heterophilic cell-cell adhesion activities. J Biol Chem. 2000;275:10291–10299. doi: 10.1074/jbc.275.14.10291. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi K, Nakanishi H, Miyahara M, Mandai K, Satoh K, Satoh A, Nishioka H, Aoki J, Nomoto A, Mizoguchi A, Takai Y. Nectin/PRR: an immunoglobulin-like cell adhesion molecule recruited to cadherin-based junctions through interaction with Afadin, a PDZ domain-containing protein. J Cell Biol. 1999;145:539–549. doi: 10.1083/jcb.145.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomazin R, Hill A B, Jugovic P, York I, van Endert P, Ploegh H L, Andrews D W, Johnson D C. Stable binding of the herpes simplex virus ICP47 protein to the peptide binding site of TAP. EMBO J. 1996;15:3256–3266. [PMC free article] [PubMed] [Google Scholar]
- 30.Warner M S, Geraghty R J, Martinez W M, Montgomery R I, Whitbeck J C, Xu R, Eisenberg R J, Cohen G H, Spear P G. A cell surface protein with herpesvirus entry activity (HveB) confers susceptibility to infection by mutants of herpes simplex virus type 1, herpes simplex virus type 2, and pseudorabies virus. Virology. 1998;246:179–189. doi: 10.1006/viro.1998.9218. [DOI] [PubMed] [Google Scholar]
- 31.Whitbeck J C, Peng C, Lou H, Xu R, Willis S H, Ponce de Leon M, Peng T, Nicola A V, Montgomery R I, Warner M S, Soulika A M, Spruce L A, Moore W T, Lambris J D, Spear P G, Cohen G H, Eisenberg R J. Glycoprotein D of herpes simplex virus (HSV) binds directly to HVEM, a member of the tumor necrosis factor receptor superfamily and a mediator of HSV entry. J Virol. 1997;71:6083–6093. doi: 10.1128/jvi.71.8.6083-6093.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wisner T, Brunetti C, Dingwell K, Johnson D C. The extracellular domain of herpes simplex virus gE is sufficient for accumulation at cell junctions but not for cell-to-cell spread. J Virol. 2000;74:2278–2287. doi: 10.1128/jvi.74.5.2278-2287.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.WuDunn D, Spear P G. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J Virol. 1989;63:52–58. doi: 10.1128/jvi.63.1.52-58.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]