Abstract
We report a case involving an aortic arch anomaly that has not been previously documented. The patients were a 76-year-old female who was urgently transported to the hospital because of a sudden disturbance of consciousness. Neurological symptoms indicated impending brain herniation, and the patient was diagnosed with hypertensive intracerebral hemorrhage (left putamen-thalamus) and acute hydrocephalus, thereafter she died approximately 6 hours after arrival. Head and neck-to-chest computed tomography angiography revealed an atypical aortic arch branching pattern. From the proximal side, the left common carotid artery, right common carotid artery, right subclavian artery, and left subclavian artery originated sequentially, suggesting an aberrant left common carotid artery. In the context of acute stroke management, understanding such aortic arch variations is crucial for planning access routes and treatment strategies for neuroendovascular procedures.
Keywords: Aberrant left common carotid artery, Atypical left aortic arch, Four branches left aortic arch, Intracerebral hemorrhage, Acute stroke
Introduction
Congenital anomalies of the aortic arch (AA) are frequently encountered during stroke diagnosis and treatment. Although these anomalies generally do not pose significant issues in the medical management of stroke, they provide crucial information for neuroendovascular procedures. They can influence the choice of access routes and devices, necessitating thorough preoperative planning.
Several classifications of the AA branching pattern variations have been reported [1,2]. Among these, the brachiocephalic (BC) - left common carotid artery (LCCA) trunk (commonly known as the bovine arch), the aortic origin of the left vertebral artery (LVA), and aberrant right subclavian artery (ARSubA) are relatively common. The AA branching pattern variation observed in this case has not been documented in previous reports, highlighting its clinical significance and warranting detailed presentation.
Case presentation
Patient: A 76-year-old, right-handed female.
Current Medical History: In December 2023, the patient suddenly lost consciousness and collapsed at home, prompting emergency calls. Owing to severe impairment of consciousness (Japan Coma Scale: JCS 200), the doctor at the scene secured her airway (nasotracheal intubation), initiated 100% oxygen administration, and transported her to our hospital. The time from onset to hospital arrival was 78 min. She had a preonset modified Rankin Scale score of 0.
Comorbidities/Medical History: No family members were available to provide a detailed medical history.
Vital Signs: blood pressure, 210/96 mmHg; pulse rate, 55 bpm; SpO2, 100% (FiO2 1.0); respiratory rate, 26/min.
Neurological Findings: Consciousness level was JCS 200, Glasgow Coma Scale E1V2M4, pupils 6.0 mm/6.0 mm with no light reflex, decerebrate posture, and a National Institutes of Health Stroke Scale score of 36.
Laboratory Findings: Complete blood counts revealed a white blood cell count of 9360/µL, hemoglobin level of 11.9 g/dL, red blood cell count of 4.1 × 106/µL, and platelet count of 300 × 103/µL, with no remarkable abnormalities noted. Biochemical tests revealed no remarkable abnormalities in the liver, kidney, or electrolytes. Coagulation tests showed PT-INR of 0.95, aPTT of 24.3 s, D-dimer level of 0.5 µg/mL, with no remarkable abnormalities observed.
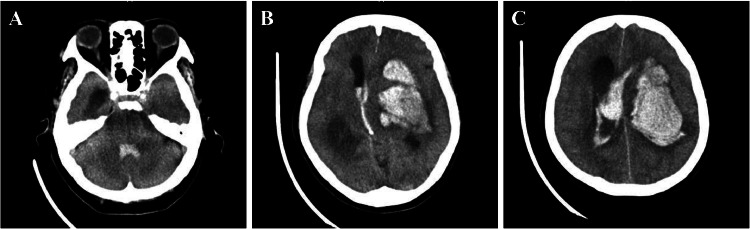
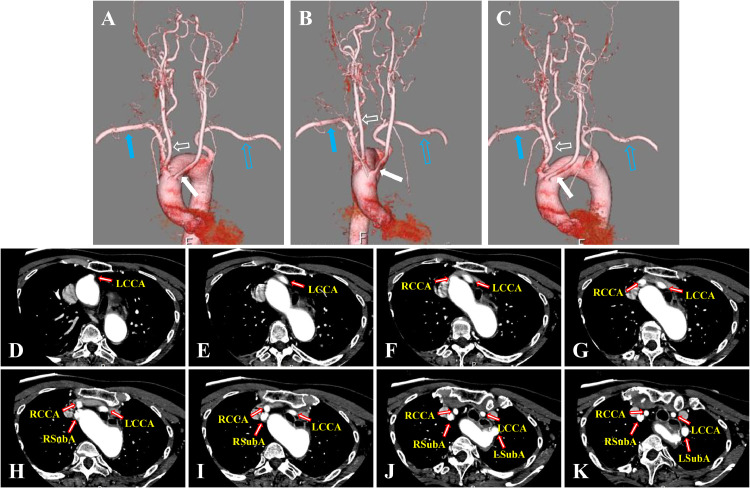
Radiological Examinations: After confirming stable vital signs, head computed tomography (CT) was performed to diagnose a mixed-type hemorrhage in the left putamen-thalamus, ventricular rupture, and acute hydrocephalus (Fig. 1). CT angiography was also performed to investigate potential intracranial vascular diseases such as arteriovenous malformation, as the source of bleeding. No obvious source was identified, leading to a diagnosis of hypertensive intracerebral hemorrhage. However, the AA branching pattern was atypical, with the LCCA, right common carotid artery (RCCA), right subclavian artery (RSubA), and left subclavian artery (LSubA) originating sequentially from the proximal side, suggesting an aberrant left common carotid artery (ALCCA) (Fig. 2).
Fig. 1.
Neuro-radiological findings on admission. Computed tomography head scan showing a large hemorrhage in the left putamen-thalamus and hydrocephalus (A, B, and C).
Fig. 2.
Neuro-angiographical findings. Computed tomography angiography in volume-rendering (A-C, A: anterior-posterior view, B: right anterior oblique view, C: left anterior oblique view) and source axial images (D-K) showing 4 atypical branches of the left aortic arch (LAA), proximal-to-distal progression, left common carotid artery (LCCA, white closed arrows in A-C), right common carotid artery (RCCA, white open arrows in A-C), right subclavian artery (RSubA, blue closed arrows in A-C) and left subclavian artery (LSubA, blue open arrows in A-C) originate from the LAA.
Clinical Course: Neurologically, the patient exhibited decerebrate rigidity and dilated pupils. Imaging findings led to a diagnosis of brain herniation. Considering neurological prognosis and life expectancy, conservative treatment was chosen over aggressive surgical intervention. The patient's blood pressure began to decrease after approximately 5 hours of arrival, and she died approximately 6 hours after arrival.
Discussion
Normal development of AA begins around the third week of gestation [3]. In the early stages, the ventral aorta ascends continuously from the terminal aortic sac, whereas the left and right dorsal aortas also descend. Sequentially 6 pairs of AA between the ventral aorta and dorsal aortas from the head side. In humans, the first and second AAs regress early, while the fifth AA either does not form or regresses quickly. The third AA develops into the carotid system, which includes the proximal part of the BC artery, common carotid artery, and distal part of the internal carotid artery. The fourth AA forms the AA and RSubA, and the sixth AA develops into the pulmonary artery and ductus arteriosus. The dorsal aortas develop symmetrically, branching into intersegmental arteries (ISA), with both subclavian arteries originating from the seventh ISA.
The typical AA is the left-sided aortic arch (LAA), which accounts for 99.7% of cases. The common branching pattern of the LAA and AA includes 3 branches in a proximal-to-distal progression: the BC trunk (BCT), which serves as a common trunk for the RCCA and RSubA; the LCCA; and the LSubA. This typical branching pattern is observed in 61.2%-99.3% of cases. However, branching anomalies occur in 38.8% of cases and are thus relatively common in clinical practice [1,4].
Several reports have detailed variations in AA branching patterns. Acar et al. [1] reported typical 3-branch patterns in 76.12%, atypical 3-branch patterns in 1.66%, 2-branch patterns in 17.79%, 4-branch patterns in 4.08%, and 5-branch patterns in 0%. Natsis et al. [2] reported typical 3-branch patterns in 78% of the cases, atypical 3-branch patterns in 1%, 2-branch patterns in 13%, 4-branch patterns in 5%, and 5-branch patterns in 0%.
Acar et al. [1] classified 4-branch LAA patterns into 5 types, in a proximal-to-distal progression, in the following order: 1) BCT/LCCA/aberrant left vertebral artery (ALVA)/LSubA, 2) BCT/LCCA/LSubA/ALVA, 3) BCT/thyroidea ima artery/LCCA/LSubA, 4) ARSubA/RCCA/LCCA/LSubA, 5) ARSubA/bilateral CCA trunk/LVA/LSubA. Natsis et al. [2] classified 7 patterns, of proximal-to-distal progression, in the following order: 1) BCT/LCCA/LVA/LSubA, 2) RCCA/LCCA/LSubA/ARSubA, 3) BCT/thyroidea ima artery/LCCA/LSubA, 4) BCT/LCCA/LSubA/LVA, 5) BCT/LVA/LSubA/ARSubA, 6) RSubA/RCCA/LCCA/LSubA, 7) LCCA/LVA/LSubA/aberrant BCT.
Our patient had an LAA with 4 branches in proximal-to-distal progression in the following order: LCCA, RCCA, RSubA, and LSubA, indicating an ALCCA. Despite extensive research, no previous studies have matched this branching pattern.
Anomalies in the LAA can present challenges to vascular access during endovascular treatments of head and neck vascular disorders and tumors. In cases of an ALCCA, where the LCCA branches proximally from the BCT, guiding a catheter into the LCCA during a femoral artery approach may be challenging, even with a type I or type II AA. Therefore, careful consideration must be given to the shape and type of guiding catheters and inner catheters used. Additionally, if the presence of an ALCCA is not known beforehand, there is a risk of misidentifying it as a bovine arch, potentially leading to disorientation during the procedure. Similarly, catheter navigation to the ALCCA via the right brachial artery or right radial artery approach is also likely to be difficult, and careful selection of catheter shape and type is necessary. Guiding the catheter may be even more challenging compared to the femoral artery approach, necessitating careful consideration of the approach site itself. If the presence of an ALCCA is recognized in advance, selecting a catheter with a longer Simmons shape, Neuro-EBU (SILUX, Saitama, Japan), Axcelguide MSK-guide (Medikit, Tokyo, Japan), Axcelguide Stiff-J (Medikit, Tokyo, Japan), or a balloon-tipped guiding catheter may be useful. Additionally, these anomalies are associated with peripheral and central nervous system vascular diseases, as well as aortic dissections [2,5]. This necessitates careful consideration of treatment plans and the risk of complications [1,[6], [7], [8], [9]]. For elective treatment, preoperative evaluation can identify these anomalies, allowing for comprehensive preoperative planning. In emergencies, particularly acute ischemic stroke requiring mechanical thrombectomy, evaluation of the AA and thoracic aorta can help identify anomalies and inform device selection and procedural strategies. At our facility, in addition to identifying anomalies in the AA branching pattern, we routinely perform CT angiography from the intracardiac and thoracic aorta to the intracranial regions for the preoperative evaluation of candidates for thrombectomy. This procedure aims to identify potential embolic sources, such as thrombi in the aortic wall or within the heart chambers.
Given that approximately 40% of AA branching anomalies are observed, they are highly likely to be encountered in clinical practice. It is essential to consider this during preoperative evaluations to ensure the precision and safety of emergency neuroendovascular treatments.
Conclusions
This case report presents an extremely rare LAA branching pattern in an ALCCA identified during the initial evaluation of an acute intracerebral hemorrhage. Awareness of these branching patterns is crucial for planning endovascular treatments for head and neck diseases.
Patient consent
Consent was obtained from the patient's family for the publication of this case report.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments: We would like to thank Editage (www.editage.jp) for English language editing.
References
- 1.Açar G, Çiçekcibaşı AE, Uysal E, Koplay M. Anatomical variations of the aortic arch branching pattern using CT angiography: a proposal for a different morphological classification with clinical relevance. Anat Sci Int. 2022;97:65–78. doi: 10.1007/s12565-021-00627-6. [DOI] [PubMed] [Google Scholar]
- 2.Natsis K, Piagkou M, Lazaridis N, Kalamatianos T, Chytas D, Manatakis D, et al. A systematic classification of the left-sided aortic arch variants based on cadaveric studies’ prevalence. Surg Radiol Anat. 2021;43:327–345. doi: 10.1007/s00276-020-02625-1. [DOI] [PubMed] [Google Scholar]
- 3.Kau T, Sinzig M, Gasser J, Lesnik G, Rabitsch E, Celedin S, et al. Aortic development and anomalies. Semin Interv Radiol. 2007;24:141–152. doi: 10.1055/s-2007-980040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pandalai U, Pillay M, Moorthy S, Sukumaran TT, Ramakrishnan S, Gopalakrishnan A, et al. Anatomical variations of the aortic arch: a computerized tomography-based study. Cureus. 2021;13:e13115. doi: 10.7759/cureus.13115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shalhub S, Schäfer M, Hatsukami TS, Sweet MP, Reynolds JJ, Bolster FA, et al. Association of variant arch anatomy with type B aortic dissection and hemodynamic mechanisms. J Vasc Surg. 2018;68:1640–1648. doi: 10.1016/j.jvs.2018.03.409. [DOI] [PubMed] [Google Scholar]
- 6.Lazaridis N, Piagkou M, Loukas M, Piperaki ET, Totlis T, Noussios G, et al. A systematic classification of the vertebral artery variable origin: clinical and surgical implications. Surg Radiol Anat. 2018;40:779–797. doi: 10.1007/s00276-018-1987-3. [DOI] [PubMed] [Google Scholar]
- 7.Popieluszko P, Henry BM, Sanna B, Hsieh WC, Saganiak K, Pękala PA, et al. A systematic review and meta-analysis of variations in branching patterns of the adult aortic arch. J Vasc Surg. 2018;68:298–306.e10. doi: 10.1016/j.jvs.2017.06.097. [DOI] [PubMed] [Google Scholar]
- 8.Syperek A, Angermaier A, Kromrey ML, Hosten N, Kirsch M. The so-called “bovine aortic arch”: a possible biomarker for embolic strokes? Neuroradiology. 2019;61:1165–1172. doi: 10.1007/s00234-019-02264-3. [DOI] [PubMed] [Google Scholar]
- 9.Woraputtaporn W, Ananteerakul T, Iamsaard S, Namking M. Incidence of vertebral artery of aortic arch origin, its level of entry into transverse foramen, length, diameter and clinical significance. Anat Sci Int. 2019;94:275–279. doi: 10.1007/s12565-019-00482-6. [DOI] [PubMed] [Google Scholar]