Abstract
Solar lentigo is a common condition that can affect people of all ages and ethnicities. The effectiveness of combining laser and light-based modalities for reducing solar lentigines was evaluated. Patients received treatment for facial solar lentigines with an advanced pulsed light device (BBL® HEROTM) followed by nonablative fractionated 1,927nm laser (MOXITM) in a single session. Nonmalignant solar lentigines were evaluated through assessment of photographs and VISIA scans taken before and after treatment. Nine patients aged 29 to 64 years were included in this report. Across all nine patients, there was a significant increase in percentile for brown spots on VISIA scans post-treatment. Follow-up time was variable; in one patient, a marked reduction in hyperpigmentation was observed almost 20 months post-treatment. The results presented in this report demonstrate that combining BBL HERO and MOXI is effective for reducing solar lentigines.
Keywords: Solar lentigines, BroadBand Light, laser
One of the most frequently encountered conditions in dermatology practice is or solar lentigines.1 It can affect all ethnicities and age ranges, and can reach a prevalence of more than 90 percent in some populations.2,3
Solar lentigines are characterized by the appearance of well-defined hyperpigmented lesions on sun-exposed skin. A number of morphological and physiological changes take place in the affected area. In the epidermis there is an accumulation of melanin in the basal layer and an increase in the number of melanocytes.4–7 As solar lentigines progress, the dermal-epidermal junction (DEJ) becomes ridged, and the ridges get longer and extend deeper into the dermis over time.8 Evidence suggests that melanin also migrates into the papillary dermis since it has been detected in dermal melanophages in patients with solar lentigines.9,10
One of the most advanced and widely used devices available for treating solar lentigines is broadband light therapy (BBL). BBL operates by delivering pulses of noncoherent, full-spectrum visible light into the skin. A novel, next-generation BBL device, BBL High Energy Rapid Output (HERO; Sciton, Inc., Palo Alto, California), delivers greater cooling and shorter pulses at four times the rate compared to other intense pulsed light devices, making this a safe and effective treatment for hyperpigmentation in a wide range of skin types.11 The emission spectrum for BBL HERO ranges from 420 to 1,400nm. When this light enters the skin, depending on the filter selected, it will get absorbed by melanin and hemoglobin and generate enough heat to damage melanin-containing cells in the epidermis and blood vessels in the dermis, while keeping surrounding cells and tissues intact.12
As BBL HERO targets melanin in the epidermis, a different modality may be more suitable for targeting melanin in the upper dermis. The nonablative fractionated MOXI (mJouleTM platform; Sciton, Inc., Palo Alto, California) 1,927nm laser is a good candidate since it is primarily absorbed by water and can penetrate into the papillary dermis.13
Both BBL HERO and MOXI have been shown to be effective at treating UV-induced hyperpigmentation as single modalities;11,14 therefore, combining them could further enhance effectiveness of the treatment. The objective of this brief report was to demonstrate effectiveness of combining BBL HERO and MOXI to reduce solar lentigines in a single treatment session.
METHODS
Patient selection. Patient charts from a single medical aesthetics practice were reviewed for patients of Fitzpatrick types I to VI who received a combination of BBL HERO and MOXI therapies to target facial hyperpigmentation. Selected patients consented to share deidentified treatment data, including age, photographs, and VISIA scans, for inclusion in this report. All procedures adhered strictly to the principles outlined in the Declaration of Helsinki (2013).
Treatment. All patients received a single treatment session combining BBL HERO and MOXI. Pre-treatment preparation was not required. Each treatment began with 1 to 4 passes of BBL HERO and, depending on individual needs, some received additional spot treatments over pigmented areas (see Table 1 for settings). Upon completing the treatment, all patients received a high energy (level 3) MOXI treatment (see Table 2 for settings). Administering the pulsed light before laser is an important step since post-laser erythema is very common.
TABLE 1.
Range of settings for BBL HERO treatments
| Treatment type | Technique | Fitzpatrick skin type | Filter (nm) | No. passes | Fluence (J/cm2) | Pulse width (ms) | Pulse rate (Hz) | Cooling (°C) |
|---|---|---|---|---|---|---|---|---|
| Base | Full-face | II | 515 | 1–4 | 4–5 | 3–5 | 4 | 25 |
| III | 515 | 3–4 | 5–6 | 3–5 | 3–4 | 25 | ||
| 560 | 3 | 5 | 3 | 3.5–4 | 20 | |||
| Pigment | Spot | II | 515 | 1–3 | 10–15 | 10–20 | N/A | 15–20 |
| III | 515 | 1–3 | 10–15 | 10–15 | N/A | 15–20 |
BBL: BroadBand Light; N/A: not applicable
TABLE 2.
Range of settings for MOXI treatments
| Fitzpatrick skin type | Level | No. passes | Fluence (mJ) | Density (%) |
|---|---|---|---|---|
| II | 3 | 4 | 20 | 20 |
| III | 3 | 4–6 | 15–20 | 1–20 |
Post-treatment care. Immediately following treatment, all patients were provided with a healing kit that included a barrier cream (Avène Cicalfate+; Pierre Fabre USA, Inc., Parsippany, New Jersey), moisturizer (Avène Thermal Spring Water; Pierre Fabre USA, Inc., Parsippany, New Jersey), and cleanser (Alastin® Gentle Cleanser; Galderma, Dallas, Texas). Patients were asked to apply all contents of the kit daily for the first two weeks following treatment. A sun protection factor (SPF) 30 sunscreen was also provided. Patients were advised to apply sunscreen whenever outdoors in order to minimize sun exposure and prevent any further hyperpigmentation.
RESULTS
A total of nine patients with facial hyperpigmentation, with a mean (standard deviation) age of 44.1 (11.1) years, received one treatment session combining BBL HERO and MOXI. Patient characteristics, including baseline VISIA percentiles for brown spots, are shown in Table 3. Photos and VISIA scans were taken between two weeks and 20 months after the treatment session.
TABLE 3.
Study participant information
| Measure | Value |
|---|---|
| Age, years, mean (SD) | 44.1 (11.1) |
| Fitzpatrick Type, n (%) | |
| II | 3 (33.3) |
| III | 6 (66.7) |
| Brown spots, baseline percentile, mean (SD) | |
| Front view | 20.7 (11.4) |
| Left view | 22.1 (18.7) |
| Average | 21.4 (13.9) |
| BBL treatment type, n (%) | |
| Full-face | 9 (100.0) |
| Spot | 7 (66.7) |
| MOXI treatment type, n (%) | |
| Level 3 | 9 (100.0) |
BBL: BroadBand Light; SD: standard deviation
Photos of the right side of the face taken before and after a single treatment (Figures 1–4) show a marked reduction in pigmentation in patients between the ages of 29 and 64 years at different follow-up times. In one patient (Figure 4), improvement in pigmentation was maintained for almost 20 months. Figure 5 shows photos (Columns A and B) and VISIA scans (Columns C and D) of the front of the face from two patients, aged 29 and 39 years, taken before and after a single treatment. In both cases, there was a noticeable reduction in pigmentation in the post-treatment images, compared to before treatment. Baseline and post-treatment percentiles, as well as the change in percentile from baseline, are shown in Table 4. Across all patients, there was a nonsignificant increase in percentile from baseline (p=0.11, Wilcoxon rank test, α= 0.05). No adverse events were reported in this sample of patients.
FIGURE 1.
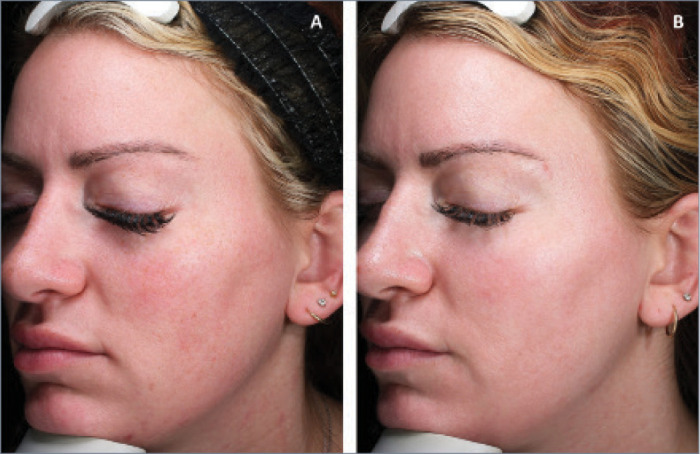
A 29-year-old patient A) before and B) three weeks after combination treatment with broadband light HERO and MOXI.
FIGURE 4.
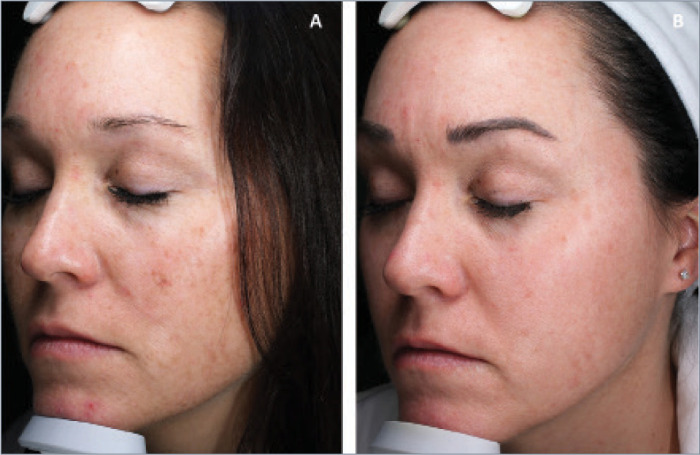
Patient A) before and B) 19.7 months after combination treatment with broadband light HERO and MOXI.
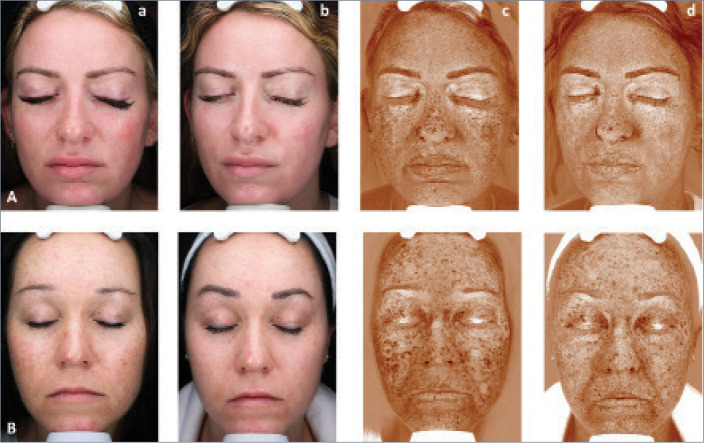
FIGURE 5.
Patients demonstrating a reduction in brown spots on VISIA scans following broadband light (BBL) HERO and MOXI combination treatment. Columns A and B: Photographic assessment of hyperpigmentation before and after treatment. Columns C and D: Brown spots on VISIA scans before and after treatment. Row A: 29-year-old female, Fitzpatrick Type II, follow-up 3 weeks post-treatment; Row B: 39 year-old female, Fitzpatrick type III, follow-up 19.7 months post-treatment.
TABLE 4.
Baseline and post-treatment percentiles for all patients
| Measure | Baseline | Post-treatment | Baseline vs. post-treatment p-value* | Change from baseline |
|---|---|---|---|---|
| Brown spots percentile, mean (SD) | 21.4 (13.9) | 34.1 (19.3) | 0.11 | 12.7 (26.3) |
*Wilcoxon rank test, α=0.05.
SD: standard deviation
FIGURE 2.
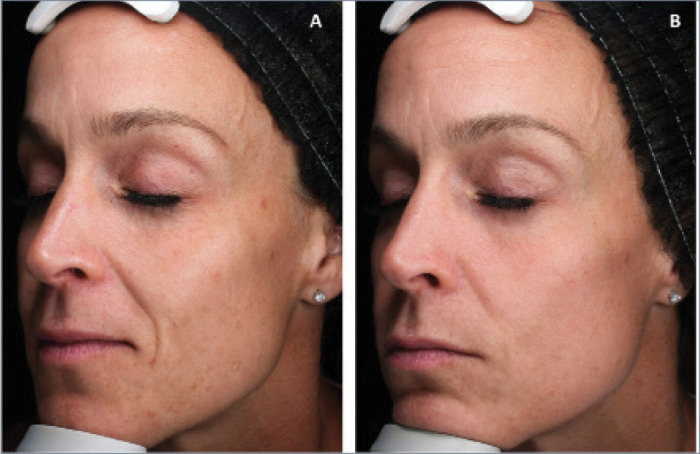
A 41-year-old patient A) before and B) 1.2 months after combination treatment with broadband light HERO and MOXI.
FIGURE 3.
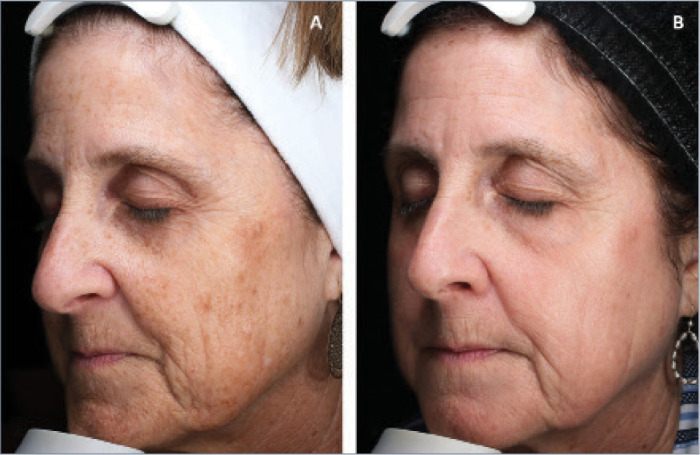
A 64-year-old patient A) before and B) 2.7 months after combination treatment with broadband light HERO and MOXI.
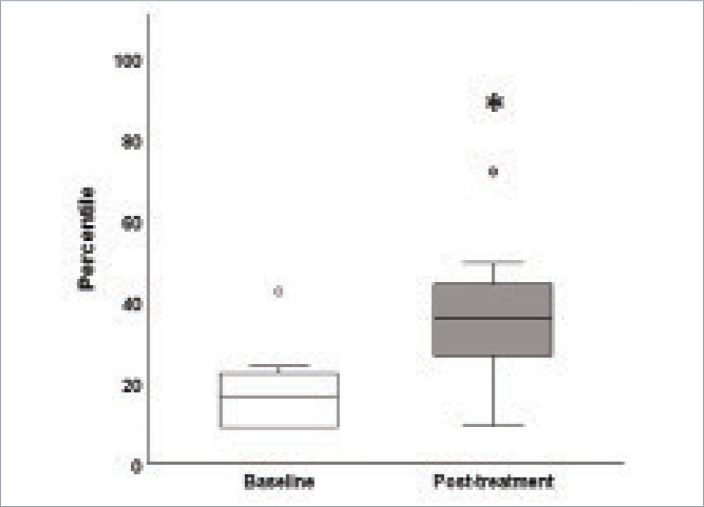
One patient, whose follow-up was approximately 19 months after treatment, did not show any improvement in brown spots. Occasionally, patients who do not adhere to the practice of wearing sunscreen will redevelop hyperpigmentation over time; however, we did not determine whether or not the unimproved patient adhered to the study’s recommended sunscreen protocol. Therefore, a subanalysis of the patients (n=8) who demonstrated an increase in percentile, or a reduction in pigment, was performed. In this subgroup, post-treatment percentile was significantly greater than baseline (p=0.03, Wilcoxon rank test, α=0.05; Figure 6).
FIGURE 6.
Box plots showing percentiles patients (n=8) at baseline and post-treatment.
*p<0.05, Wilcoxon rank test
CONCLUSION
These findings show that a combination of BBL HERO and MOXI can effectively reduce UV-induced hyperpigmentation after just one treatment. While MOXI can significantly improve brown spots as a single treatment modality,14 the magnitude of the improvement is far greater when MOXI is combined with BBL HERO, as shown in this study; this is likely because the two modalities target different components of hyperpigmented lesions. Most of the light emitted by BBL HERO through the 515nm filter is absorbed by melanin in the epidermis,12 while MOXI’s nonablative fractionated microchannels penetrate into the superficial dermis where it can generate enough heat to damage dermal melanophages.13
Both MOXI and BBL HERO are safe and effective treatment for all skin types.11,14 However, patient selection plays an important role with any laser or light-based therapy.15 It is therefore important to identify which patients could benefit most from a MOXI and BBL combination treatment. Other factors, such as the type of hyperpigmentation and overlapping skin conditions, would also need to be considered.16
Limitations. This study was not without limitations. The sample size was very small, and the follow-up time was variable. While we did not find any significant correlation between follow-up time and the amount of improvement in pigment, there may have been some recurrence of hyperpigmentation due to sun exposure, as patients might not have been adherent to regular application of sunscreen following the procedure.
Despite the limitations in this study, the results suggest that combination of BBL HERO and MOXI is an effective option for treating UV-induced hyperpigmentation.
REFERENCES
- Plensdorf S, Livieratos M, Dada N. Pigmentation disorders: diagnosis and management. Am Fam Physician. 2017;96(12):797–804. [PubMed] [Google Scholar]
- Ortonne JP. Pigmentary changes of the ageing skin. Br J Dermatol. 1990;122(Suppl 35):21–28. doi: 10.1111/j.1365-2133.1990.tb16121.x. [DOI] [PubMed] [Google Scholar]
- Tsai J, Chien AL. Photoprotection for skin of color. Am J Clin Dermatol. 2022;23(2):195–205. doi: 10.1007/s40257-021-00670-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakozaki T, Jarrold B, Zhao W et al. Morphological and transcriptional evaluation of multiple facial cutaneous hyperpigmented spots. Skin Health Dis. 2022;2(2):e96.. doi: 10.1002/ski2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi W, Yin L, Smuda C et al. Molecular and histological characterization of age spots. Exp Dermatol. 2017;26(3):242–248. doi: 10.1111/exd.13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WJ, Jo SY, Lee MH et al. The effect of MCP-1/CCR2 on the proliferation and senescence of epidermal constituent cells in solar lentigo. Int J Mol Sci. 2016;17(6):948. doi: 10.3390/ijms17060948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noblesse E, Nizard C, Cario-André M et al. Skin ultrastructure in senile lentigo. Skin Pharmacol Physiol. 2006;19(2):95–100. doi: 10.1159/000091976. [DOI] [PubMed] [Google Scholar]
- Cario-Andre M, Lepreux S, Pain C et al. Perilesional vs. lesional skin changes in senile lentigo. J Cutan Pathol. 2004;31(6):441–447. doi: 10.1111/j.0303-6987.2004.00210.x. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Tahira M, Morino S et al. Novel morphological study of solar lentigines by immunohistochemical and electron microscopic evaluation. J Dermatol. 2013;40(7):528–532. doi: 10.1111/1346-8138.12165. [DOI] [PubMed] [Google Scholar]
- Barysch MJ, Braun RP, Kolm I et al. Keratinocytic malfunction as a trigger for the development of solar lentigines. Dermatopathology (Basel). 2019;6(1):1–11. doi: 10.1159/000495404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitter P, Jr, Gold M, Pozner J. New BBL HERO offers unprecedented results for the face and body. Prime. 2021;11(2):13–15. [Google Scholar]
- Alhallak K, Abdulhafid A, Tomi S, Omran D. Springer, Cham: 2023. Skin, light, and their interactions. In: The Ultimate Guide for Laser and IPL in the Aesthetic Field. pp. 1–38. [Google Scholar]
- Kwon IH, Bae Y, Yeo UC et al. Histologic analyses on the response of the skin to 1,927-nm fractional thulium fiber laser treatment. J Cosmet Laser Ther. 2018;20(1):12–16. doi: 10.1080/14764172.2017.1358455. [DOI] [PubMed] [Google Scholar]
- Vingan NR, Panton JA, Barillas J et al. Investigating the efficacy of a fractionated 1927nm laser for diffuse dyspigmentation and actinic changes. Lasers Surg Med. 2023;55(4):344–358. doi: 10.1002/lsm.23653. [DOI] [PubMed] [Google Scholar]
- Alexis AF. Lasers and light-based therapies in ethnic skin: treatment options and recommendations for Fitzpatrick skin types V and VI. Br J Dermatol. 2013;169(Suppl 3):91–97. doi: 10.1111/bjd.12526. [DOI] [PubMed] [Google Scholar]
- Passeron T, Genedy R, Salah L et al. Laser treatment of hyperpigmented lesions: position statement of the European Society of Laser in Dermatology. J Eur Acad Dermatol Venereol. 2019;33(6):987–1005. doi: 10.1111/jdv.15497. [DOI] [PubMed] [Google Scholar]