Abstract
Invasive group A streptococci infections are increasing worldwide, mainly due to the emm1 lineage M1UK emergence. Although this variant has recently been described in adult Italian patients, its circulation in children has not yet been established. Characterizing by whole genome sequencing 6 invasive group A streptococci strains isolated between 2022 and 2023, we highlighted M1UK lineage circulation in pediatric patients in Italy.
Keywords: Streptococcus pyogenes, pediatrics, meningitis, acute liver failure, whole genome sequencing, Italy
INTRODUCTION
Streptococcus pyogenes (group A streptococci, GAS), an exclusive human bacterial pathogen, is equipped with a large set of virulence factors involved in pathogenesis and disease manifestations, ranging from asymptomatic carriage to life-threatening invasive disease (such as sepsis, meningitis, necrotizing fasciitis and puerperal sepsis).1 GAS isolates can be typed using a serotype-specific antiserum against the surface M protein or, more recently, by sequencing the 5′ end of the emm gene that encodes the M protein.2 To date, more than 250 emm types have been recorded with over 1900 distinct emm subtypes.2 In 2016, after being the main culprit of invasive infections in Western countries for nearly four decades, S. pyogenes serotype M1 (also known as “genotype emm1,” the “M1T1 clone,” and “M1global”) showed the rapid emergence of a new clonal lineage. This variant, designated as M1UK, contributed to the seasonal rise of scarlet fever and the increase in invasive infections in the United Kingdom.3 This new emm1 lineage shows an increased expression of SpeA toxin in both noninvasive and invasive strains.3 After the first notifications, the M1UK lineage expanded rapidly accounting for 91% of the invasive emm1 isolates in England in 2020.4 Subsequently, it spread worldwide, with cases notified in Australia, the United States, Canada, Denmark, the Netherlands and the United Kingdom.4 During 2022, other European countries observed an increase in invasive GAS (iGAS) infections, though not related to a specific variant, with emm types 1 and 12 reported as the most common.5 Several cases of fatal iGAS infection in children younger than 10 years of age have been reported from European countries with pediatric iGAS cases higher than pre-Covid-19 levels.5 This epidemiology has led the World Health Organization and the European Centre for Disease Prevention and Control to invite European Union/European Economic Area countries and the United Kingdom to share information on GAS and iGAS infections.5 In Italy, the Ministry of Health reported an increase in scarlet fever cases starting from January 2023, especially in children for which epidemiological information is scarce worldwide, as most studies include predominantly adults. Indeed, investigating the GAS strains responsible for the increase in invasive infections recorded in Milan (Italy), a recent study found the M1UK variant exclusively in adult patients.6
Embracing the call for improved surveillance activities, we carried out molecular surveillance through whole genome sequencing (WGS) of eight GAS isolates. Six strains were isolated from pediatric patients with iGAS infection, 1 causing meningitis and 1 liver failure, and 2 strains were isolated from patients without documented invasive disease, to serve as virulence control.
METHODS
In the period December 2022 to February 2024, the Bambino Gesù Children’s Hospital registered 19 cases of iGAS infections, with S. pyogenes identified from normally sterile body sites through the FilmArray blood culture identification panel (BCID, BioFire Diagnostics). Only in 6 cases, it was possible to cultivate and isolate S. pyogenes from the respective biological material, namely, blood (patients 1, 6 and 7), cerebrospinal fluid (patient 2), pleural fluid (patient 5) and synovial liquid (patient 8). All the isolated iGAS and 2 additional noninvasive GAS isolated in the same period from nonsterile sites, namely, ear (patient 3) and skin (patient 4), were tested for antimicrobial susceptibility and subjected to Next Generation Sequencing (see Table, Supplemental Digital Content 1, http://links.lww.com/INF/F618). Detailed methods are reported in Supplemental Digital Content 2, http://links.lww.com/INF/F619.
RESULTS
Clinical Presentation
In the whole cohort, 5 patients were male; the mean age at the time of the infection was 4.9 years. Among the patients with iGAS infection, 2 had otomastoiditis, complicated by meningitis and sepsis, 1 had pneumonia with pleural effusion, 2 had arthritis/osteoarthritis and 1 had a streptococcal toxic shock syndrome (STSS) with acute liver failure (see Table, Supplemental Digital Content 1, http://links.lww.com/INF/F618). None of the patients had comorbidities or a known diagnosis or history suggestive of primary immunodeficiency, and all showed normal results on first-level immunological screening. A viral coinfection was reported for 3 patients, 1 with adenovirus and 1 with influenza virus, while the patient with STSS had parainfluenza 3 virus and enterovirus on the rhino-pharyngeal swab. Three patients required surgical intervention or drainage of the infected site. Two patients, one with meningitis and the other with STSS, required intensive care unit admission and the first died. To understand bacterial differences responsible for the invasiveness, we included in our analysis 2 patients with noninvasive GAS infections (see Table, Supplemental Digital Content 1, http://links.lww.com/INF/F618).
Strain Characterization
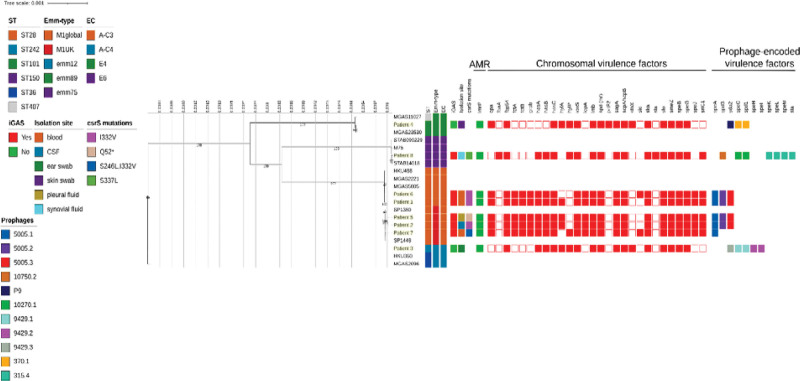
Two GAS and 6 iGAS strains were isolated from pediatric children from December 2022 to February 2024. Emm typing revealed the presence of emm type/ST emm12.101/ST242 and emm89.0/ST101 among GAS, whereas the 6 iGAS strains belonged to emm1/ST28 (5/6, 83%) and emm75.0/ST150 (1/6, 17%; Fig. 1). Among the 5 emm1 strains, 3 subtypes could be identified, namely, emm1.0 (2/5, 40%), emm1.52 (2/5, 40%) and emm1.25 (1/6, 20%). Phylogenetic analysis revealed strains belonged to the M1global clade whereas 3 to the M1UK clade. Among them, 2 carried 27/27 of the clone-defining SNPs and 1 presented a deletion in the region corresponding to phage 5005.3, carrying sda2 and containing 1 of the 27 positions that define M1UK (Fig. 1).
FIGURE 1.
Estimated maximum likelihood phylogenetic analysis of Streptococcus pyogenes isolates (n = 8) and reference genomes (n = 12). The phylogeny was estimated on a coreSNP of 18,839 bp with IqTree using the best-fit model of nucleotide substitution GTR+F+G4 with 1000 replicates fast bootstrapping. Leaves’ number represents the sample IDs (with isolates collected in this study highlighted in yellow); bootstraps values higher than 90 are shown on branches. Information regarding the samples is reported: sequence type (ST), emm type, emm-type cluster (EC), presence of invasive group A streptococci (iGAS) infection, isolation site, csrS mutations identified, presence (filled squared) or absence of antimicrobial resistance genes (AMR) presence (filled square) or absence of virulence factors and divided in chromosomal (red) or phage-associated (color based on phage on which they are present).
Regardless of the presence of the lmrP resistance gene, all bacterial isolates showed susceptibility to the standard dosing regimen or increased exposure to all antibiotics tested (data not shown). A total of 40 virulence factors were identified, of which 11 were common to all strains. Of the remaining 29, 18 were located on the chromosome and 11 were phage-associated. Four of 5 emm1 strains carried 3 prophages (Φ5005.1, Φ5005.2 and Φ5005.3) encoding virulence factors, speA, spd3 and sda2, respectively, while one carried only 2 of them (Φ5005.1 and Φ5005.2). The emm75.0 iGAS strain carried 5 prophages, of which 3 (Φ315.4, Φ10270.1 and Φ10750.1) encoding the virulence factors, speK/speL/speM/sla, speC/spd1 and spd3, respectively. Even noninvasive strains showed the presence of prophage-encoded virulence factors: emm89.0 strain carried 3 phages (Φ9429.1, Φ9429.2 and Φ9429.3) encoding the speC/spd1, speH/speI and sda2 virulence factors, respectively, while emm12.101 strain carried 3 phages, of which only 2 (Φ370.1 and P9) encoding speC/spd1 and sda2 virulence factors, respectively. However, only the 6 invasive strains carried mutations in the csrS gene, which is part of the CsrRS 2-component regulatory systems (Fig. 1).
DISCUSSION
To the best of our knowledge, this study demonstrates, for the first time, the circulation of the new M1UK clone among children in Italy. Additionally, being the M1UK lineage characterized by greater invasive potential, our results explain the increase in S. pyogenes invasiveness recently recorded in our pediatric hospital.
As previously observed in adult settings, multiple emm types contributed to the increase in iGAS infection observed in the last 2 years in our pediatric hospital. Despite belonging to distinct, and in some cases unrelated, emm types, all the iGAS that we analyzed possessed mutations in the csrS gene, which, presumably, allows expression of the phage-encoded virulence factors.7 Indeed, bacteriophages encoding virulence factors were also recovered in the 2 noninvasive GASs in our cohort, which, in contrast, had no mutations in csrS. Naturally occurring mutations in the 2-component gene regulatory systems CsrRS (also called CovRS) appear critical for GAS pathogenesis and result in hypervirulent strains with upregulated expression of many virulence factor genes (eg, capsule, cell-surface proteases and speA)7; in fact, csrS alteration is notably linked to the invasive spread of the mutant strains.7 Moreover, the 5 emm1 strains analyzed possess the bacteriophage-encoded toxin SpeA, whose transcription and production have been seen to increase approximately tenfold in M1UK strains.8
Among the 3 M1UK strains analyzed in our study, 1 was responsible for meningitis and 1 for fulminant liver failure, 2 uncommon clinical manifestations in pediatric GAS infections. Our data add to recent evidence identifying M1UK as the main culprit of meningitis in the Netherlands.9 GAS meningitis, associated with significant mortality and morbidity, can be a consequence of bacteremia or intracranial extension of head and neck infections.10 Consistently, the onset of infection in the patient of our cohort who presented with meningitis and who had a fatal outcome was attributable to otomastoiditis. On the other hand, liver involvement is referred to in streptococcal toxic shock syndrome, but fulminant liver failure has been described only once so far in a patient with iGAS infection sustained by the M1global clade ancestor (ie, M1T1).11 Thus, we first observed this complication in association with M1UK infection.
Increased iGAS reports worldwide indicate a need for ongoing surveillance of novel lineages, given the potential public health effects.4 The emm-typing scheme, widely used in diagnostics and public health surveillance, does not enable the identification of strains harboring pathogenic/virulence genes, which can differ greatly among isolates of the same serotype/emm type.2 Molecular characterization by WGS allows a deeper understanding of GAS pathogenicity and identification of high-risk clones and potential new sublineages. Consistently, we resorted to WGS and de novo genome assembly to properly identify iGAS in our pediatric patients. Continued bacteriological surveillance of isolates from both invasive disease and asymptomatic carriers remains necessary to inform public health officials in a timely manner regarding circulating clones with possibly altered transmission or virulence potential. Genomic analysis is required to effectively achieve this goal, as classical typing techniques (e.g. emm typing), although valuable, lack the necessary resolution to provide comprehensive information for strain comparison.
Probably the most important challenge to address the increase in iGAS infections is to implement molecular surveillance, particularly in severe clinical diseases, ideally through WGS to identify virulence gene repertoire and regulator system mutations responsible for altered pathogenicity. Early identification of a variant with hypervirulence features could direct clinicians in adopting ad hoc therapeutic strategies, tailoring empiric antibiotic and anti-inflammatory therapy in accordance with the microbiological/genomic data. In addition, early identification of such variants would allow to implement prophylactic measures to prevent their spread in both hospital and community settings.
In conclusion, the identification, for the first time, of the circulation of M1UK clone in Italian children, alongside the presence of other iGAS strains sharing similar dysregulation of phage-encoded virulence factors, underlines the need for continuous molecular surveillance of S. pyogenes strains to provide information on possible changes in clone prevalence and virulence content.
INSTITUTIONAL REVIEW BOARD STATEMENT
Not applicable. No research on humans was conducted, and only bacterial isolates recovered from routine screening and diagnostic laboratory tests were assessed, without direct use of clinical specimens. At the time of hospitalization, the parents of the patient consent to the use of personal data for diagnosis and treatment activities and for future scientific research purposes connected to them. Basic demographic and clinical information collected in this study did not infringe upon the rights or welfare of the patient. Considering the retrospective nature of the analysis, the current study did not require the approval of the local ethics committee according to current legislation, but a notification was sent. Data were retrospectively analyzed in line with personal data protection policies, and patient consent was not required. For these reasons, the approval of the local ethics committee was not deemed necessary.
Supplementary Material
Footnotes
This research was supported by the ANIA Foundation, EU funding within the NextGenerationEU-MUR PNRR Extended Partnership initiative on Emerging Infectious Diseases (Project no. PE00000007, INF-ACT). This work was supported also by the Italian Ministry of Health with “Current Research Funds.”
The authors have no conflicts of interest to disclose. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
G.V., M.R., and M.A. contributed equally to this work and share the first authorship.
C.F.P. and P.B. contributed equally to this work and share the last authorship.
G.V., M.R., M.A., and V.C. conceived the idea up to the drafting of the manuscript. A.G., V. Fini, and K.Y.L.R. gave valuable technical support. V. Fox assembled the data for the bioinformatic analysis and critically analyzed it. M.A., L.P., and A.S. contributed to the recording and evaluating the various isolates that were analyzed in this manuscript. M.D.L., L.L., and L.G. took care of the drafting of clinical data and manifestations of the patients. M.R. and A.V. have drawn attention to this emerging pathogen. C.F.P. and P.B. provided supervision and founding. All authors have read and agreed to the published version of the manuscript.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com).
Contributor Information
Gianluca Vrenna, Email: gianluca.vrenna@opbg.net.
Martina Rossitto, Email: martina.rossitto@opbg.net.
Marilena Agosta, Email: marilena.agosta@opbg.net.
Maia De Luca, Email: maia.deluca@opbg.net.
Laura Lancella, Email: laura.pansani@opbg.net.
Livia Gargiullo, Email: livia.gargiullo@opbg.net.
Annarita Granaglia, Email: annarita.granaglia@opbg.net.
Vanessa Fini, Email: vanessa.fini@opbg.net.
Katia Yu La Rosa, Email: katia.yularosa@opbg.net.
Marta Argentieri, Email: marta.argentieri@opbg.net.
Laura Pansani, Email: laura.pansani@opbg.net.
Annamaria Sisto, Email: annamaria.sisto@opbg.net.
Massimiliano Raponi, Email: massimiliano.raponi@opbg.net.
Alberto Villani, Email: alberto.villani@opbg.net.
Carlo Federico Perno, Email: carlofederico.perno@opbg.net.
REFERENCES
- 1.Brouwer S, Rivera-Hernandez T, Curren BF, et al. Pathogenesis, epidemiology and control of group A Streptococcus infection. Nat Rev Microbiol. 2023;21:431–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Efstratiou A, Lamagni T. Epidemiology of Streptococcus pyogenes. In: Ferretti JJ, Stevens DL, Fischetti VA, eds. Streptococcus pyogenes: Basic Biology to Clinical Manifestations. 2nd ed. University of Oklahoma Health Sciences Center; 2022 Oct 8 Chapter 19. Published online 2022. https://www.ncbi.nlm.nih.gov/books/ [Google Scholar]
- 3.Lynskey NN, Jauneikaite E, Li HK, et al. Emergence of dominant toxigenic M1T1 Streptococcus pyogenes clone during increased scarlet fever activity in England: a population-based molecular epidemiological study. Lancet Infect Dis. 2019;19:1209–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhi X, Li HK, Li H, et al. Emerging invasive group a streptococcus M1UK lineage detected by allele-specific PCR, England, 20201. Emerg Infect Dis. 2023;29:1007–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.European Centre for Disease Prevention and Control. Communicable disease threats report, 12-18 March 2023, week 11. Accessed May 2, 2024. https://www.ecdc.europa.eu/en/publications-data/communicable-disease-threats-report-12-18-march-2023-week-11. [Google Scholar]
- 6.Mangioni D, Fox V, Saltini P, et al. Increase in invasive group A streptococcal infections in Milan, Italy: a genomic and clinical characterization. Front Microbiol. 2023;14:1287522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horstmann N, Myers KS, Tran CN, et al. CovS inactivation reduces CovR promoter binding at diverse virulence factor encoding genes in group A Streptococcus. PLoS Pathog. 2022;18:e1010341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li HK, Zhi X, Vieira A, et al. Characterization of emergent toxigenic M1UK Streptococcus pyogenes and associated sublineages. Microb Genom. 2023;9:mgen0000994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Putten BCL, Vlaminckx BJM, de Gier B, et al. Group A streptococcal meningitis with the M1UK variant in the Netherlands. JAMA. 2023;329:1791–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee J, Blackburn J, Pham-Huy A. Uncommon clinical presentation of a common bug: Group A streptococcus meningitis. Paediatr Child Health. 2021;26:e129–e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biesel-Desthieux MN, Tissières P, Belli DC, et al. Fulminant liver failure in a child with invasive group A streptococcal infection. Eur J Pediatr. 2003;162:245–247. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.