Abstract
Objective
To assess medication adherence and factors associated with poor adherence in youth with bipolar disorder followed from adolescence through young adulthood.
Method
Participants with bipolar disorder recruited through the Course and Outcome of Bipolar Youth (COBY) study were included in this study if they were prescribed psychotropic medications and had at least 3 follow-up assessments of medication adherence (N = 179, ages 12-36). Medication adherence had been evaluated for a median of 8 years using a questionnaire derived from the Coronary Artery Risk Development in Young Adults (CARDIA) study. For the longitudinal evaluation, adherence was measured as the percentage of follow-up assessments in which the participants did not endorse any of the nonadherence items included in the questionnaire. Concurrent and future predictors of poor adherence were assessed using both univariate and multivariate longitudinal analyses.
Results
Among the participants, 51% reported poor adherence in more than 50% of their follow-up assessments. Younger age, family conflicts, polypharmacy, lower functioning, greater severity of mood symptoms, and comorbid disorders were associated with poor adherence in the univariate analyses. In the multivariate analyses, comorbid attention-deficit/hyperactivity disorder was the single most influential factor associated with concurrent and future poor adherence in all age groups. Participants’ most reported reasons for poor adherence were forgetfulness (56%), negative attitudes toward medication treatment (10.5%), and disturbed daily routine (7%).
Conclusion
Poor medication adherence is a significant problem in youth with bipolar disorder, with the most influential factor being the presence of comorbid attention-deficit/hyperactivity disorder. Thus, it is important to identify and appropriately treat comorbid attention-deficit/hyperactivity disorder to improve medication adherence and prognosis of patients. Providers should also recommend tools to enhance consistent medication intake and address patients’ concerns and negative beliefs about their illness and treatment.
Key words: ADHD, bipolar disorder, child and adolescent, medication adherence, transition to adulthood
Bipolar disorder (BD) affects 1% to 3% of the world’s population and represents a major cause of disability accounting for the loss of about 10 million disability-adjusted life-years.1,2 BD is associated with significant psychosocial impairment, recurrent hospitalizations, and a high risk for substance abuse and suicidal behaviors.3 Up to 60% of individuals with BD report the onset of symptoms during adolescence,4 and early-onset BD is found to be associated with a worse prognosis, higher rates of comorbidities, and marked functional and social impairment, indicating the need for early identification and treatment.5,6
Several psychotropic medications are found to be effective in the management of BD,7,8 but “drugs do not work in patients who do not take them” (C. Everett Koop, US Surgeon General, 1985). The World Health Organization, recognizing the important role of medication adherence in improving health care outcomes, has emphasized that “increasing the effectiveness of adherence interventions may have a far greater impact on the health of the population than any improvement in specific medical treatment.”9 Poor medication adherence can be attributed to a variety of causes including, among others, doubt about the diagnosis and necessity of the treatment, the actual or perceived role of side effects, fear of addiction to the medication, economic barriers, lack of family support, and peer pressure.9,10 In psychiatric disorders, lack of insight about the illness and depressive symptoms such as hopelessness, social isolation, and lack of motivation also play an important role in poor adherence.10,11
In fact, poor medication adherence is common in patients with psychiatric disorders, including BD. A recent meta-analysis found that nearly half of adults with BD fail to adhere to the prescribed medication regimen.12 Youth with BD also have low medication adherence rates, an issue that has been addressed in several studies.13 For example, in a 3-month naturalistic study of adherence in adolescents with BD, patients were found to take less than 60% of doses as prescribed, based on objective data obtained from an electronic pillbox.14 Such poor adherence rates are found to be a leading cause of treatment failure, frequent relapses, recurrent hospitalizations, higher health care costs, and worse outcomes.15, 16, 17
However, no prior study has followed youth with BD longitudinally for a long time to assess how their trajectories of adherence change over time, through the transition from adolescence to adulthood. Such a study is warranted given that medication adherence is a dynamic process, and it is critical to understand how the patient’s behavior and attitude change over time and the course of the illness.18 Additionally, adolescence is associated with significant developmental and social changes, and the transition into adulthood is associated with increased independence and autonomy, which can impact medication adherence in this specific age group.9,19 To our knowledge, this is the first study to assess medication adherence prospectively, over a median of 8 years, in a large sample of youth with BD through the transition to adulthood and examine the concurrent and future predictors of poor adherence, using both univariate and multivariate longitudinal analyses.
Method
Participants
Participants for the current study were recruited through the parent Course and Outcome of Bipolar Youth (COBY) study, a multisite (University of Pittsburgh Medical Center, University of California Los Angeles, and Brown University) longitudinal study of 413 youth (7-17 years old at intake) with a DSM-IV20 diagnosis of BD-I, BD-II, or research operationalized criteria for BD not otherwise specified (BD-NOS).21,22 Participants with schizophrenia, intellectual disability, autism, or mood disorders secondary to medical conditions or substance abuse were excluded from the parent study.
For this longitudinal study, we included only participants who were prescribed psychotropic medications and had at least 3 assessments of adherence after the introduction of the adherence assessment scale (Supplement 1, available online) into the COBY study. Of excluded participants, 159 were excluded because they did not receive treatment with psychotropic medications during the period of this study, and 75 were excluded because they had fewer than 3 assessments of adherence. Excluded participants were more likely to be male, to be non-White, and to have less history of suicidal ideation, psychosis, and anxiety disorders. There were no significant differences in the severity of mood symptoms or rates of comorbid substance use disorder (SUD), disruptive behavior disorders (DBDs) (incorporating oppositional defiant disorder and/or conduct disorder), or attention-deficit/hyperactivity disorder (ADHD). The institutional review board at each site approved the study, and informed consent or assent was obtained from participating youth and their parent or primary caretakers.
Measures
The methods of the COBY study have been previously described in the literature.21,22 Briefly, at intake, participants were evaluated for psychiatric disorders using the Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (K-SADS-PL).23 The severity of mood symptoms was ascertained using the K-SADS Mania Rating Scale (MRS) and Depression Rating Scale (DRS),24 and the severity of anxiety symptoms was assessed using the Screen for Child Anxiety Related Emotional Disorders (SCARED).25 The Longitudinal Interval Follow-up Evaluation (LIFE) and the psychiatric status ratings of LIFE were used to assess the week-by-week longitudinal changes in psychiatric symptoms as well as pharmacological treatment.8,26 General functioning was assessed using the Children’s Global Assessment Scale (CGAS)27 and the Global Assessment of Functioning (GAF) scale.20 Family psychiatric history was assessed using a modified version of the Family History Screen.28 Family functioning was evaluated using the child and parent versions of the Conflict Behavior Questionnaire (CBQ) and the Family Adaptability and Cohesion Evaluation Scale II (FACES II).29,30 Socioeconomic status (SES) was determined using the Hollingshead 4-factor index.31
Longitudinal adherence to prescribed medications was measured via the Coronary Artery Risk Development in Young Adults (CARDIA) Psychotropic Medication Adherence Questionnaire (Supplement 1, available online).32,33 After the introduction of the questionnaire to the COBY protocol in May 2011, adherence was measured at every follow-up assessment over a median of 8 years. The CARDIA adherence questionnaire included 4 questions to screen for medication adherence that were derived from the widely used Morisky, Green, and Levine Medication Adherence Questionnaire (MAQ).34 These questions, with “yes” or “no” responses, included the following:
-
1.
Do you ever forget to take your medications?
-
2.
Are you ever careless in taking your medications?
-
3.
Do you ever miss taking your medications when you are feeling better?
-
4.
Do you ever miss taking any of your medications because you are feeling sick?
In keeping with the literature, poor adherence was defined as the endorsement of at least one nonadherence item in the MAQ.35 Thereafter, for the purpose of a longitudinal evaluation of the participants’ adherence, adherence was measured as the percentage of follow-up assessments in which the participants did not endorse any of the 4 nonadherence items in the MAQ. Participants were considered to have a consistent good adherence if they were adherent in >75% of their assessments during the follow-up period.15 The questions of the MAQ are designed to facilitate disclosure of poor adherence. The MAQ has shown adequate psychometric properties and has been validated when compared with objective measures of medication adherence in a variety of psychiatric disorders and other medical conditions, particularly at a threshold score of >1.35,36 The MAQ has shown sensitivity values between 72% and 84% in detecting poor adherence in psychiatric patients.35
In addition to the above-mentioned screening questions derived from the MAQ, the CARDIA adherence questionnaire added contextual information about the participant’s adherence. The questionnaire assessed the date of the last missed/skipped dose, the main reported reason for medication nonadherence, the usual method(s) of remembering to take medications, and who pays for the medications.
Statistical Analysis
First, we evaluated the overall rate of poor adherence across all the assessments performed during the length of the study. Then, the associations between potential predictor variables, chosen based on the literature and data available in COBY, and the concurrent probability of poor adherence (dichotomously defined as the endorsement of at least one nonadherence item) were tested using mixed logistic regression models fitting a random intercept to account for within-subject correlation across repeated measures (estimating standardized odds ratios [OR] for continuous predictor effects). All models covaried for the number of prescribed medications to account for possible confounding effects of this factor. We further tested interactions between each hypothesized predictor and age to account for possible moderation effects. To test lagged effects, we fit mixed logistic regression models of poor adherence odds on predictor values from the previous assessment (approximately 7 months prior). To ensure the temporal directionality of lag-regression effects, we also reran models to test the effects of lagged poor adherence on future symptoms. Post hoc sensitivity analyses reran the models described above adjusting the poor adherence threshold to require more endorsed nonadherence items.
To identify the strongest predictors of poor adherence, we used the least absolute shrinkage and selection operator (LASSO),37 a modified form of generalized linear regression that penalizes overfit models via a regularization parameter that proportionally shrinks predictor coefficients toward zero. Predictor selection is implicitly performed as less important predictor coefficients are shrunken to zero without the potential biases of other variable selection techniques such as multiple comparisons and collinearity between predictor variables. We fit separate LASSOs to test concurrent and lagged predictor effects (including all possible age interactions), and we also reran LASSO analyses differentially on adolescent and adult subsamples (ages <18 vs ≥18, allowing crossover cases to contribute observations to both subgroups) to identify differences in results between the 2 age groups as well as account for potential differences in some variable definitions in adulthood vs adolescence (eg, socioeconomic status).
Results
Participants
In this study, 179 participants (52.5% females, 86.6% White, mean [SD] SES = 3.4 [1.2]) who met the inclusion criteria described earlier were included (Table 1). These participants had more severe mood symptoms and higher rates of comorbidities than the other COBY participants who did not meet the inclusion criteria. Among the participants, 135 (75%) were diagnosed with BD-I, 23 (13%) were diagnosed with BD-II, and 21 (12%) were diagnosed with BD-NOS, as of the last follow-up assessment. Mean (SD) age at BD onset was 9.2 [4.1] years, and the most common comorbidities were anxiety disorders (73%), ADHD (65%), DBDs (52%), and SUD (38%).
Table 1.
Demographic and Clinical Characteristics (N = 179 Participants)
| Demographic variables | ||
|---|---|---|
| Age, y | ||
| Mean | (SD) | |
| 23.2 | (5.1) | |
| Median | ||
| 23.2 | ||
| Range | ||
| 12.2-36.6 | ||
| % | ||
| Female sex | 52.5 | |
| White race | 86.6 | |
| Hispanic ethnicity | 7.8 | |
| Living with both biological parents | 47.5 | |
| Mean | (SD) | |
| SESa | 3.4 | (1.2) |
| Clinical variables | ||
|---|---|---|
| % | ||
| BD-I | 75.4 | |
| BD-II | 12.8 | |
| BD-NOS | 11.7 | |
| Mean | (SD) | |
| Age of mood disorder onset | 9.2 | (4.1) |
| % | ||
| Anxiety disorders | 73.2 | |
| ADHD | 65.4 | |
| DBDs | 52.0 | |
| SUD | 38.0 | |
| Mean | (SD) | |
| Intake CGAS | 54.1 | (12.3) |
| Intake Depression Rating Scale | 15.4 | (10.8) |
| Intake Mania Rating Scale | 23.3 | (12.3) |
| Psychotropic medications | ||
|---|---|---|
| Number of medications | ||
| Mean | (SD) | |
| 2.2 | (1.2) | |
| Median | ||
| 2.0 | ||
| % | ||
| Antipsychotics | 87.7 | |
| Antidepressants | 77.7 | |
| Anticonvulsants | 77.7 | |
| Stimulants | 65.4 | |
| Lithium | 54.2 | |
| Other medicationsb | 60.9 | |
Note: Disorder diagnosis rates reflect lifetime status, and medication rates reflect rates of any use over follow-up. ADHD = attention-deficit/hyperactivity disorder; BD = bipolar disorder; DBDs = disruptive behavior disorders; NOS = not otherwise specified; SES = socioeconomic status; SUD = substance use disorder.
SES score 1-2 indicates low socioeconomic status, 3-4 indicates moderate socioeconomic status, and 5 indicates high socioeconomic status.
Other medications include all other psychotropic medications such as atomoxetine, α-2 agonists, and benzodiazepines.
During the study, 1,246 assessments were performed with a median of 7 assessments per participant (range, 3-14) and a median duration of 7 months between the assessments. Through the follow-up period, the age range was 12 to 36 years old with a median age of 23.2 years old.
Over the follow-up period, the participants were prescribed on average 2 psychotropic medications. The most frequently prescribed medications were antipsychotics (88%) followed by antidepressants (78%), anticonvulsants (78%), stimulants (65%), and lithium (54%). Through the follow-up period, 74% of participants were covered by private health insurance, 58% were covered by Medicaid, and 28% were covered by Medicare. Additionally, 49% of participants reported paying for the medication themselves, and a family member paid for medications for 35% of participants.
Prevalence of Poor Adherence
During the length of the study, poor medication adherence was reported in 56% of all assessments (Table 2). Also, participants reported missed or skipped doses during the last 2 weeks before the assessment in 37% of assessments. Only 17% (30/179) of the participants were found to have consistent good adherence (adherence >75% of the assessments) during the follow-up period. Moreover, among the 149 participants with inconsistent adherence, 92 (51% of all participants) reported poor adherence in >50% of their assessments.
Table 2.
Medication Adherence Questions (N = 179 Participants)
| Nonadherence over follow-up | % |
|---|---|
| Do you ever forget to take your medications? | 44.0 |
| Are you ever careless in taking your medications? | 12.5 |
| Do you ever miss taking your medications when you are feeling better? | 12.1 |
| Do you ever miss taking your medications because you are feeling sick? | 8.3 |
| Assessments with any poor adherence | 55.9 |
| Reasons reported for skipping doses | |
|---|---|
| Forgot | 56.1 |
| Changes in daily routine | 7.1 |
| Do not want to be on medication | 6.5 |
| Side effects | 4.0 |
| Were busy with other things | 3.9 |
| Do not need them | 3.6 |
| Unable to refill | 3.5 |
| Were away from home | 2.8 |
| Feel better | 1.6 |
| Feel sick with medication | 1.3 |
| How do you usually remember to take your medications? | |
|---|---|
| Memory only | 65.3 |
| Friend/family member | 27.4 |
| Pillbox | 13.1 |
| Alarm | 7.4 |
| Pain or other symptoms | 4.7 |
| Calendar/diary | 0.8 |
| Who pays for your medication? | |
|---|---|
| Private health insurance | 74.3 |
| Medicaid | 58.1 |
| Participant | 48.6 |
| Family member | 35.2 |
| Medicare | 28.5 |
| Friend | 0.0 |
| When was the last time you missed or skipped any of your medications? | |
|---|---|
| Within the past week | 20.2 |
| 1 to 2 weeks ago | 16.9 |
| 3 to 4 weeks ago | 9.3 |
| More than a month ago | 16.8 |
| Never missed a dose | 36.8 |
Note: All other reasons (listed in Supplement 1, available online) that were reported in less than 1% of the assessments are not included in this table.
Reported Reasons for Poor Adherence
Among all the reported reasons for poor adherence, the most frequent reason was forgetting (56%), followed by changes in daily routine (7%), not wanting to be on medication (6.5%), being busy with other things (4%), side effects of the medication (4%), the belief that they do not need medication (4%), problems with refill (3.5%), and being away from home (3%). Each of the other reasons included in the questionnaire was reported in less than 2% of the assessments (Table 2).
How Participants Remembered to Take Their Medications
Most participants relied only on their memory (65%) to take their medications, followed by relying on a friend or family member (27%), using a pillbox (13%), using an alarm (7%), having symptoms (5%), and using a calendar or diary (1%) (Table 2). Participants endorsed multiple responses, so the percentages did not add up to 100%.
Factors Associated With Poor Adherence
Mixed Logistic Regressions
In general, poor adherence was associated with a higher number of prescribed medications (OR = 1.26, 95% CI 1.05-1.50, p = .01), but not the class of medications (Table 3) (the result is nearly identical when controlling for age). After controlling for the number of prescribed medications, younger age was significantly associated with poor adherence (OR = 0.72, 95% CI 0.60-0.86, p = .0002) with most improvement in adherence occurring during the transition from adolescence into adulthood. More precisely, the estimated adherence probability at the mean assessment age (23.2) was 44% and ranged from 36% at 1 SD below the mean age (18.1) to 52% at 1 SD above the mean age (28.3). Further, participants in young adulthood (≥18 years) had about half the odds of reporting poor adherence compared with adolescents (OR = 0.59, 95% CI 0.41-0.85, p = .004), and the average adherence rates improved by 21% when the same participants aged into adulthood (t = 2.36, df = 64, p = .02). Increased family conflicts as reported by parents (but not by the child) were also associated with less adherence (OR = 1.35, 95% CI 1.03-1.78, p = .03). Further, living independently after age 18 was associated with less adherence (OR = 1.44, 95% CI 1.27-1.63, p = .003). Meanwhile, there were no effects of sex, race, SES, body mass index, family functioning, or family psychiatric history, including parental psychopathology, on the rates of adherence (all ps > .05). Controlling for the number of prescribed medications, poor adherence was found to be significantly associated with more severe symptoms of depression (DRS) (OR = 1.21, 95% CI 1.03-1.42, p = .02) and mania (MRS) (OR = 1.17, 95% CI 1.01-1.35, p = .04), the presence of subthreshold hypo/mania (LIFE) (OR = 1.64, 95% CI 1.15-2.34, p = .005), DBD (OR = 1.54, 95% CI 1.02-2.33, p = .03) and ADHD (OR = 1.90, 95% CI 1.30-2.77, p = .0008) symptoms, and lower overall functioning (CGAS) (OR = 0.83, 95% CI 0.70-0.99, p = .03). There were no significant associations between the age of mood disorder onset or BD subtype and medication adherence.
Table 3.
Tests of Association With Probability of Poor Adherence (N=179)
| Variable | Odds ratio | (95% CI) | p |
|---|---|---|---|
| Number of prescribed medicationsa | 1.26 | (1.05, 1.50) | .0118 |
| Age, continuous | 0.72 | (0.60, 0.86) | .0002 |
| Adulthood vs adolescence | 0.59 | (0.41, 0.85) | .0041 |
| SES | 0.98 | (0.84, 1.14) | .7098 |
| Body mass index | 0.82 | (0.60, 1.14) | .2400 |
| Living with both parents | 0.83 | (0.55, 1.26) | .3770 |
| Female sex | 0.78 | (0.50, 1.21) | .2740 |
| White race | 1.17 | (0.61, 2.24) | .6690 |
| Living independently after age 18 | 1.44 | (1.27, 1.63) | .0031 |
| Family psychiatric history | |||
| Depression | 0.98 | (0.51, 1.90) | .9652 |
| BD | 0.76 | (0.49, 1.19) | .2217 |
| ADHD | 1.17 | (0.76, 1.81) | .4773 |
| CD | 1.33 | (0.85, 2.08) | .2123 |
| Schizophrenia | 0.74 | (0.31, 1.76) | .4929 |
| Psychosis | 0.98 | (0.55, 1.72) | .9306 |
| Anxiety | 0.69 | (0.42, 1.14) | .1484 |
| SUD | 0.74 | (0.46, 1.18) | .2060 |
| Suicidality | 0.95 | (0.62, 1.46) | .8186 |
| Mood disorder onset age | 0.82 | (0.66, 1.02) | .0738 |
| BD-I/II vs BD-NOS | 0.80 | (0.39, 1.62) | .5068 |
| DRS | 1.21 | (1.03, 1.42) | .0222 |
| MRS | 1.17 | (1.01, 1.35) | .0397 |
| CGAS | 0.83 | (0.70, 0.99) | .0331 |
| SCARED Child version | 1.06 | (0.82, 1.36) | .6675 |
| SCARED Parent version | 1.15 | (0.88, 1.50) | .3183 |
| BCS | 1.07 | (0.84, 1.37) | .5888 |
| PSRs | |||
| Euthymia | 0.80 | (0.53, 1.20) | .2757 |
| Threshold MDE | 1.32 | (0.93, 1.87) | .1298 |
| Subthreshold or worse MDE | 1.18 | (0.85, 1.64) | .3109 |
| Threshold hypo/mania | 1.22 | (0.70, 2.14) | .4373 |
| Subthreshold or worse hypo/mania | 1.64 | (1.15, 2.34) | .0051 |
| Anxiety | 1.18 | (0.81, 1.72) | .3831 |
| ADHD | 1.90 | (1.30, 2.77) | .0008 |
| DBDs | 1.54 | (1.02, 2.33) | .0341 |
| SUD | 1.04 | (0.64, 1.71) | .8736 |
| FACES II | |||
| Cohesion, parent | 0.92 | (0.62, 1.37) | .6790 |
| Adaptability, parent | 0.92 | (0.62, 1.36) | .6801 |
| Cohesion, child | 0.95 | (0.66, 1.36) | .7767 |
| Adaptability, child | 0.72 | (0.50, 1.04) | .0793 |
| CBQ | |||
| Parent | 1.35 | (1.03, 1.78) | .0305 |
| Child about mother | 1.12 | (0.91, 1.38) | .2823 |
| Child about father | 1.03 | (0.82, 1.30) | .7926 |
Note: Boldface type indicates significant associations (p < .05). ADHD = attention-deficit/hyperactivity disorder; BD = bipolar disorder; CBQ = Conflict Behavior Questionnaire; CD = conduct disorder; CGAS = Children’s Global Assessment Scale; DBDs = disruptive behavior disorders; DRS = K-SADS Depression Rating Scale; FACES II = Family Adaptability and Cohesion Evaluation Scale II; MDE = manic/depressive episode; MRS = K-SADS Mania Rating Scale; NOS = not otherwise specified; PSRs = psychiatric status ratings; SCARED = Screen for Child Anxiety Related Emotional Disorders; SES = socioeconomic status; SUD = substance use disorder.
All models except number of prescribed medications covary for the number of prescribed medications.
Analyses were also performed to evaluate the demographic and clinical factors that predicted poor adherence at the next assessment. The only significant, unmoderated, predictive factor of future poor adherence was the presence of ADHD symptoms (OR = 1.73, 95% CI 1.19-2.52, p = .004). Meanwhile, child-reported anxiety symptoms (SCARED) predicted future poor adherence in adolescents (OR = 1.51, 95% CI 1.10-2.06, p = .01), but not in young adults. Rerunning lag-regression models to test the effects of lagged adherence on future symptoms found no significant reverse effects.
LASSO Analyses
After fitting LASSOs to model both concurrent associations with and lagged predictors of poor adherence, the only factor selected was ADHD symptoms. After noting the age effect described above and to assess the effects of the transition from adolescence to adulthood, we repeated LASSO analyses separately on the adolescent and adult subsamples. Results indicated that the most influential concurrent correlates of poor adherence during adolescence were younger age and symptoms of anxiety and ADHD, and the most influential lagged predictor of poor adherence was ADHD symptoms. Meanwhile, the most influential concurrent correlates of poor adherence during adulthood were lower SES, living independently, and ADHD symptoms; no predictors were selected in the lag regression model.
Supplemental Analyses
As ADHD was the strongest factor associated with both concurrent and future poor adherence, we conducted a sensitivity analysis using a more conservative cutoff to define poor adherence, increasing the poor adherence threshold to require at least 2 endorsed items on the MAQ, rather than only one. Implementing this adjustment effectively yielded no changes to the ADHD findings described above. Further, we found that participants with a lifetime ADHD diagnosis had more than twice the odds of reporting 3 or more nonadherence items (OR = 2.28, 95% CI 1.15-4.50, p = .018), and if they had current threshold ADHD symptoms, their odds were even worse (OR = 2.67, 95% CI 1.54-4.65, p = .0005).
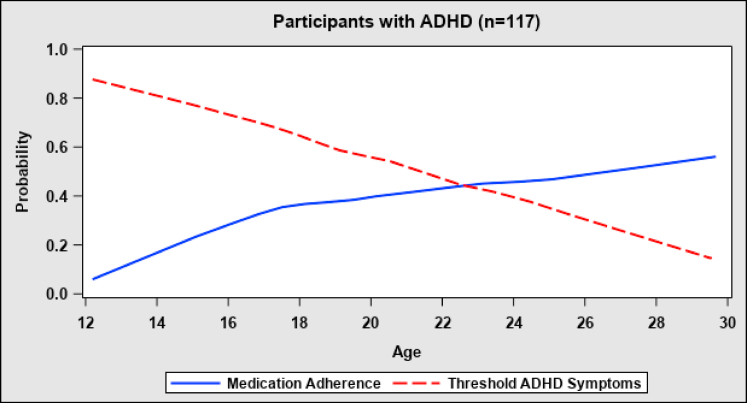
As adolescents were found to have significantly lower rates of adherence compared with young adults, we also performed further post hoc analyses to identify factors associated with the improvement of adherence with increasing age. The improvement could not be explained by the presence of a secondary informer (OR = 0.86, 95% CI 0.58-1.30, p = .1), dropout rates over time, or the course of BD. Further, living independently was associated with greater odds of poor adherence when participants aged into adulthood. However, comparing adherence trends in the subsamples with comorbid ADHD (n = 117) and without ADHD (n = 62) showed that the association between younger age and poor adherence was highly significant in the subsample with ADHD (OR = 0.57, 95% CI 0.45-0.73, p < .0001) and nonsignificant in the subsample without ADHD (OR = 1.08, 95% CI 0.80-1.47, p = .6). Additionally, in the subsample with ADHD, the ADHD symptoms precipitously declined with age and this decline was accompanied by a marked decrease in the concurrent (OR = 0.60, 95% CI 0.39-0.93, p = .02) and lagged (OR = 0.65, 95% CI 0.42-0.99, p = .045) odds of poor adherence (Figure 1). Moreover, the results of clinical associations obtained after adjustment to age were replicated after adjustment to both age and ADHD. Hence, the effect of ADHD explains the effect of age on the improvement of adherence, and this finding was independent of the other significant clinical associations.
Figure 1.
Medication Adherence and Attention-Deficit/Hyperactivity Disorder (ADHD) Symptoms by Age in Participants With ADHD
Lastly, as there was possible collinearity between the number of prescribed medications and the various predictors tested in this study (eg, symptom severity, comorbidities), we reran all models without covarying for the number of prescribed medications to assess whether results changed. There were no significant changes to the results or any effect sizes.
Discussion
This longitudinal study offers additional evidence of the alarming rates of poor psychotropic medication adherence among adolescents and young adults with BD. Also, it highlights the role of comorbid ADHD and changes in adherence determinants through the transition from adolescence into adulthood.
Poor adherence was reported in 56% of all assessments over the course of the study. Only 1 out of 6 participants reported consistent good adherence in more than 75% of their follow-up evaluations, while half of the sample reported poor adherence in more than 50% of their follow-up evaluations. Most of the participants relied only on memory to take their medications. Thus, it was not surprising that their main reported reason for poor adherence was forgetfulness. In addition, they reported that changes in their daily routines (eg, being away from home) and negative attitudes toward medication treatment also influenced their adherence.
In the univariate analyses, several factors were associated with poor medication adherence, including younger age; polypharmacy; lower functioning; greater severity of mood symptoms; comorbid ADHD, DBD, and anxiety disorders; and family conflicts. However, in the multivariate analyses, using LASSOs, only comorbid ADHD was found to be associated with concurrent and future poor adherence in all age groups. Moreover, using a stricter definition of poor adherence showed an even stronger association between ADHD and poor adherence.
Considering the findings relating age effects and to assess the influence of the transition from adolescence to adulthood, a multivariate analysis separating adolescents and young adults was performed. This analysis showed that younger age, anxiety disorders, and ADHD were the most influential factors associated with poor adherence during adolescence. Meanwhile, during young adulthood, poor adherence was associated with lower SES, living independently, and ADHD.
The rates of poor adherence in our sample are comparable with other studies on medication adherence in adults and youth with BD. However, there is wide variability in the reported rates of poor adherence in the literature (20%-66%).11,13 These inconsistencies could be explained by the methodological differences among the studies, including the study design (ie, cross-sectional or longitudinal), the use of different measures and definitions of adherence, the age and size of the sample, and the duration of follow-up.12 For example, a 12-month prospective cohort study in adolescents with BD after hospitalization reported that nearly 65% of the patients were partially adherent or nonadherent.15 Also, in 2 large 1-year longitudinal studies in adult patients with BD, partial adherence or nonadherence was identified in nearly 50% of the patients.38,39 In contrast, in a large 6-year longitudinal study that included both adolescents and adults with BD, only 24% of the participants were found to be nonadherent in 20% of their visits.40 However, the authors reported lower rates of adherence among younger participants and participants with early-onset BD.40
The rates of poor adherence found in our study are also consistent with the rates reported in longitudinal studies for other psychiatric disorders. For example, a 2-year longitudinal study on adherence to medications in adults with unipolar depression that also used the MAQ scale found that 69% of patients were consistently or intermittently nonadherent throughout the follow-up period.41 Additionally, a 16-year longitudinal study of individuals with ADHD from childhood to adulthood found that about 66% of the patients had inconsistent adherence patterns, 25% had negligible adherence, and less than 10% had consistent medication adherence.42
The factors associated with poor adherence in our univariate analyses were similar to most of the longitudinal studies of youth with BD, including the severity of illness, the number of doses, and comorbid ADHD.14,15,18 Comparable findings were reported in adults with BD, with a general agreement between the studies on the significant effect of severity of illness and the presence of comorbid disorders, but there were disparate findings regarding the demographic factors (eg, age, sex, and SES).11 Moreover, a systematic review and meta-analysis of 28 studies on medication adherence in children and adolescents with a variety of psychiatric disorders, of which 8 included BD, concluded that demographic factors and medication classes were less predictive of adherence, while the severity of illness and comorbidities such as ADHD and SUD were significantly associated with poor medication adherence.43
Surprisingly, we did not find an effect of SUD. However, most of the studies on adherence in youth with BD did not report such a correlation, and only one longitudinal study reported that poor adherence was associated specifically with alcohol use.15 In contrast, comorbid SUD was found to be significantly associated with poor medication adherence in the literature on BD in adults.11
Across all ages, univariate and multivariate analyses showed that ADHD was the most influential factor associated with both concurrent and future poor adherence in our study, and improvement in ADHD resulted in better adherence to medications. Another longitudinal study in adolescents with BD also found that ADHD was the main factor associated with poor adherence (F = 9.7, df = 1.70, p = .003),15 and the meta-analysis on adherence across youth with several psychiatric disorders, noted above, also showed the substantial negative effects of ADHD on adherence (pooled OR = 0.61, 95% CI 0.41-0.91).43 However, it is important to note that not all adherence studies in youth with BD have found this association.14,44 Meanwhile, large and longitudinal studies, in which ADHD was the primary disorder of the investigation, have shown remarkably low rates of adherence to medications, especially among adolescents.42,45
Consistent with the above ADHD findings, we found that most participants reported forgetfulness as the most important reason for poor adherence, and the vast majority relied only on memory to take their medications. Impairment of cognitive and executive functions, such as planning and organization, has been reported to be a leading cause of poor adherence in patients with BD, as it prevents the patients from following a routine of regular medication intake.11 This is even more relevant in patients with comorbid ADHD, who often experience marked forgetfulness, distractibility, and impaired attention and organization.45 This could be worsened by the lack of supervision and social support when adolescents transition into adulthood and live independently as reported in this study.
Consistent with the literature, we found that participants’ negative beliefs about medications and their illnesses also affected their adherence to treatment.11,12 The studies on adherence in BD and other psychiatric disorders show a significant correlation between stronger beliefs about the necessity of the prescribed medication and good adherence and between greater concerns about the risks of dependence or side effects of the medication and poor adherence.46,47
To improve outcomes of BD treatment, interventions should address barriers to medication adherence, especially forgetfulness and patients’ negative attitudes toward medication treatment. Routine intake of medications can be encouraged by using tools such as pill boxes, alarms, and calendars. Mobile applications have particularly shown promising results in improving medication adherence in patients with medical and psychiatric illnesses.48 Also, it is important to build a therapeutic alliance with patients; address their and their families’ beliefs and concerns; and educate them about their illness, treatment, and the need for good adherence.
The results presented above need to be taken considering the following limitations. An interview that included a patient-report scale was used to assess adherence to psychotropic medications in our sample. Although the assessment was done longitudinally, it is still subject to recall bias. Moreover, despite that we interviewed participants for adherence to medications, the nature of the patient-report scale used may yield underestimates or, more commonly, overestimates of participants’ adherence.9,14,49 However, patient-report adherence scales are cheap, noninvasive, time-efficient, and easy to administer making them more suitable for routine clinical use than blood tests and other objective measures such as pill count and electronic monitoring devices that have their own problems; none of these are regarded as a gold standard tool.9,50 Additionally, the generalizability of the observations obtained from a subsample of the COBY study to other populations is arguable because most participants were White, and they were recruited primarily from outpatient settings.
In conclusion, this study prospectively assessed medication adherence in youth with BD followed from adolescence through adulthood, with a much longer follow-up time than most of the other studies. Half of the adolescents and young adults with BD showed significantly poor medication adherence, and only a small minority showed consistent good adherence. The presence of comorbid ADHD was the most influential factor associated with poor adherence, indicating the importance of prompt recognition and treatment of this comorbidity. Moreover, interventions should also include tools to reduce forgetfulness and enhance the routine intake of medications, such as phone applications, alarms, and pill boxes. Additionally, building a therapeutic alliance with the patients and addressing their and their families’ beliefs and concerns are crucial to improving treatment adherence and outcome.
Footnotes
This research was supported by the National Institute of Mental Health (NIMH), Course and Outcome of Bipolar Youth (COBY) study grants RO1 MH059929 (PI: Birmaher), RO1 MH59691 (PIs: Keller/Yen), and RO1 MH59977 (PI: Strober), and Predicting Adult Outcomes in Bipolar Youth (PROBY) study grants RO1 MH112544 (PI: Birmaher) and RO1 MH5270580 (PI: Yen).
The research was performed with permission from the University of Pittsburgh Institutional Review Board.
This study was presented as an abstract at the American Academy of Child & Adolescent Psychiatry (and the Canadian Academy of Child & Adolescent Psychiatry) 69th Annual Meeting; October 17-22, 2022; Toronto, Ontario, Canada.
Mr. Merranko served as the statistical expert for this research.
Author Contributions
Conceptualization: Elhosary, Merranko, T. Goldstein, Hafeman, B. Goldstein, Axelson, Hunt, Yen, Diler, Ryan, Keller, Strober, Birmaher
Formal analysis: Merranko
Fundingacquisition: Axelson, Yen, Keller, Strober, Birmaher
Investigation: Gill, Hower
Methodology: Elhosary, Merranko, T. Goldstein, Hafeman, Birmaher
Project administration: Gill, Hower, Weinstock
Supervision: Birmaher
Writing – original draft: Elhosary, Merranko, Birmaher
Writing – review and editing: T. Goldstein, Hafeman, B. Goldstein, Gill, Hower, Axelson, Hunt, Yen, Diler, Ryan, Keller, Weinstock, Strober, Birmaher
The authors would like to thank the COBY study participants and their families and the COBY research team. The authors also thank Rita Scholle, BA, of UPMC Western Psychiatric Hospital, for her immense help with the preparation of the manuscript.
Disclosure: Dr. T. Goldstein has received grants from the NIMH, the American Foundation for Suicide Prevention (AFSP), the University of Pittsburgh Clinical and Translational Science Institute, and the Pittsburgh Foundation and royalties from Guilford Press. Dr. Hafeman has received grants from the NIMH and the Brain and Behavior Research Foundation. Dr. B. Goldstein has received research grant support from Brain Canada, the Canadian Institutes of Health Research, the Heart and Stroke Foundation, the NIMH, and the University of Toronto Department of Psychiatry. He also acknowledges his position as RBC Investments Chair in Children’s Mental Health and Developmental Psychopathology at Centre for Addiction and Mental Health (CAMH), a joint Hospital-University Chair between the University of Toronto, CAMH, and the CAMH Foundation. Dr. Axelson has received grants from the NIMH and royalties from UpToDate. Dr. Hunt has received an honorarium from Wiley Publishers and has received support from the NIMH. Dr. Yen has received research support from the NIMH, the National Center for Complementary and Integrative Health (NCCIH), and the AFSP and has served as a consultant at Janssen Global Services. Dr. Diler has received research support from the NIMH. Dr. Ryan has received grants from the National Institutes of Health. Dr. Keller has received research support from the NIMH. Dr. Weinstock has received grant funding from the NIMH, the NCCIH, the Patient-Centered Outcomes Research Institute, the US Department of Veterans Affairs (VA), and the Warren Alpert Foundation and book royalties from Oxford University Press. Dr. Strober has received research support from the NIMH and as the Resnick Endowed Chair in Eating Disorders at the University of California, Los Angeles. Dr. Birmaher has received grants from the NIMH and royalties from Random House, UpToDate, and Lippincott Williams & Wilkins. Ms. Hower has received funding from the NIMH and honoraria from the US Department of Defense. Dr. Elhosary, Mr. Merranko, and Ms. Gill have reported no biomedical financial interests or potential conflicts of interest.
Supplemental Material
References
- 1.Merikangas K.R., Jin R., He J.P., et al. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry. 2011;68(3):241–251. doi: 10.1001/ARCHGENPSYCHIATRY.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He H., Hu C., Ren Z., Bai L., Gao F., Lyu J. Trends in the incidence and DALYs of bipolar disorder at global, regional, and national levels: results from the global burden of Disease Study 2017. J Psychiatr Res. 2020;125:96–105. doi: 10.1016/J.JPSYCHIRES.2020.03.015. [DOI] [PubMed] [Google Scholar]
- 3.Grande I., Berk M., Birmaher B., Vieta E. Bipolar disorder. Lancet. 2016;387(10027):1561–1572. doi: 10.1016/S0140-6736(15)00241-X. [DOI] [PubMed] [Google Scholar]
- 4.Perlis R.H., Dennehy E.B., Miklowitz D.J., et al. Retrospective age at onset of bipolar disorder and outcome during two-year follow-up: results from the STEP-BD study. Bipolar Disord. 2009;11(4):391–400. doi: 10.1111/J.1399-5618.2009.00686.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baldessarini R.J., Tondo L., Vázquez G.H., et al. Age at onset versus family history and clinical outcomes in 1,665 international bipolar-I disorder patients. World Psychiatry. 2012;11(1):40–46. doi: 10.1016/J.WPSYC.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birmaher B., Goldstein T., Axelson D.A., Pavuluri M. In: Lewis’s Child and Adolescent Psychiatry: A Comprehensive Textbook. 5th ed. Martin A., Volkmar F.R., Bloch M., editors. Wolters Kluwer; Philadelphia: 2018. Bipolar spectrum disorders. [Google Scholar]
- 7.Geddes J.R., Miklowitz D.J. Treatment of bipolar disorder. Lancet. 2013;381(9878):1672–1682. doi: 10.1016/S0140-6736(13)60857-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hafeman D.M., Rooks B., Merranko J., et al. Lithium versus other mood-stabilizing medications in a longitudinal study of youth diagnosed with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2020;59(10):1146–1155. doi: 10.1016/J.JAAC.2019.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization . World Health Organization; 2003. Adherence to long-term therapies: evidence for action.https://apps.who.int/iris/handle/10665/42682 [Google Scholar]
- 10.Robin Dimatteo M., Giordani P.J., Lepper H.S., Croghan T.W. Patient adherence and medical treatment outcomes: a meta-analysis. Med Care. 2002;40(9):794–811. doi: 10.1097/00005650-200209000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Levin J.B., Krivenko A., Howland M., Schlachet R., Sajatovic M. Medication adherence in patients with bipolar disorder: a comprehensive review. CNS Drugs. 2016;30(9):819–835. doi: 10.1007/S40263-016-0368-X. [DOI] [PubMed] [Google Scholar]
- 12.Semahegn A., Torpey K., Manu A., Assefa N., Tesfaye G., Ankomah A. Psychotropic medication non-adherence and its associated factors among patients with major psychiatric disorders: a systematic review and meta-analysis. Syst Rev. 2020;9(1):17. doi: 10.1186/S13643-020-1274-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanchez M., Lytle S., Neudecker M., McVoy M. Medication adherence in pediatric patients with bipolar disorder: a systematic review. J Child Adolesc Psychopharmacol. 2021;31(2):86–94. doi: 10.1089/CAP.2020.0098. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein T.R., Krantz M., Merranko J., et al. Medication adherence among adolescents with bipolar disorder. J Child Adolesc Psychopharmacol. 2016;26(10):864–872. doi: 10.1089/cap.2016.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DelBello M.P., Hanseman D., Adler C.M., Fleck D.E., Strakowski S.M. Twelve-month outcome of adolescents with bipolar disorder following first hospitalization for a manic or mixed episode. Am J Psychiatry. 2007;164(4):582–590. doi: 10.1176/AJP.2007.164.4.582. [DOI] [PubMed] [Google Scholar]
- 16.Colom F., Vieta E., Tacchi M.J., Sánchez-Moreno J., Scott J. Identifying and improving non-adherence in bipolar disorders. Bipolar Disord. 2005;7(Suppl 5):24–31. doi: 10.1111/J.1399-5618.2005.00248.X. [DOI] [PubMed] [Google Scholar]
- 17.Walker S., Mackay E., Barnett P., et al. Clinical and social factors associated with increased risk for involuntary psychiatric hospitalisation: a systematic review, meta-analysis, and narrative synthesis. Lancet Psychiatry. 2019;6(12):1039–1053. doi: 10.1016/S2215-0366(19)30406-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coletti D.J., Leigh E., Gallelli K.A., Kafantaris V. Patterns of adherence to treatment in adolescents with bipolar disorder. J Child Adolesc Psychopharmacol. 2005;15(6):913–917. doi: 10.1089/CAP.2005.15.913. [DOI] [PubMed] [Google Scholar]
- 19.Hack S., Chow B. Pediatric psychotropic medication compliance: a literature review and research-based suggestions for improving treatment compliance. J Child Adolesc Psychopharmacol. 2001;11(1):59–67. doi: 10.1089/104454601750143465. [DOI] [PubMed] [Google Scholar]
- 20.American Psychiatric Association . 4th ed. American Psychiatric Association; Washington, DC: 2000. Diagnostic and Statistical Manual of Mental Disorders. text rev. [Google Scholar]
- 21.Birmaher B., Axelson D., Strober M., et al. Clinical course of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry. 2006;63(2):175–183. doi: 10.1001/ARCHPSYC.63.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Birmaher B., Axelson D., Goldstein B., et al. Four-year longitudinal course of children and adolescents with bipolar spectrum disorders: the Course and Outcome of Bipolar Youth (COBY) study. Am J Psychiatry. 2009;166(7):795–804. doi: 10.1176/APPI.AJP.2009.08101569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaufman J., Birmaher B., Brent D., et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 24.Axelson D., Birmaher B.J., Brent D., et al. A preliminary study of the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children mania rating scale for children and adolescents. J Child Adolesc Psychopharmacol. 2003;13(4):463–470. doi: 10.1089/104454603322724850. [DOI] [PubMed] [Google Scholar]
- 25.Birmaher B., Brent D.A., Chiappetta L., Bridge J., Monga S., Baugher M. Psychometric properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED): a replication study. J Am Acad Child Adolesc Psychiatry. 1999;38(10):1230–1236. doi: 10.1097/00004583-199910000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Keller M.B., Lavori P.W., Friedman B., et al. The Longitudinal Interval Follow-up Evaluation: a comprehensive method for assessing outcome in prospective longitudinal studies. Arch Gen Psychiatry. 1987;44(6):540–548. doi: 10.1001/ARCHPSYC.1987.01800180050009. [DOI] [PubMed] [Google Scholar]
- 27.Shaffer D., Gould M.S., Brasic J., Fisher P., Aluwahlia S., Bird H. A Children’s Global Assessment Scale (CGAS) Arch Gen Psychiatry. 1983;40(11):1228–1231. doi: 10.1001/ARCHPSYC.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- 28.Weissman M.M., Wickramaratne P., Adams P., Wolk S., Verdeli H., Olfson M. Brief screening for family psychiatric history: the family history screen. Arch Gen Psychiatry. 2000;57(7):675–682. doi: 10.1001/ARCHPSYC.57.7.675. [DOI] [PubMed] [Google Scholar]
- 29.Robin A.L., Foster S.L. In: Dictionary of Behavioral Assessment Techniques. Hersen M., Bellack A.S., editors. Pergamon Press; Oxford: 1995. The Conflict Behavior Questionnaire; pp. 148–150. [Google Scholar]
- 30.Olson D.H., Portner J., Bell R.Q. Family Inventories: Inventories Used in a National Survey of Families Across the Family Life Cycle. University of Minnesota; Minneapolis: 1982. FACES II: Family Adaptability and Cohesion Evaluation Scales; pp. 4–24. [Google Scholar]
- 31.Hollingshead A. Four factor index of social status. Yale Journal of Sociology, Volume 8. 2011. https://sociology.yale.edu/sites/default/files/files/yjs_fall_2011.pdf#page=21
- 32.Oates G.R., Juarez L.D., Hansen B., Kiefe C.I., Shikany J.M. Social risk factors for medication nonadherence: findings from the CARDIA Study. Am J Health Behav. 2020;44(2):232. doi: 10.5993/AJHB.44.2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salas M., Kiefe C.I., Schreiner P.J., et al. Obesity modifies the association of race/ethnicity with medication adherence in the CARDIA Study. Patient. 2008;1(1):41–54. doi: 10.2165/01312067-200801010-00007. [DOI] [PubMed] [Google Scholar]
- 34.Morisky D.E., Green L.W., Levine D.M. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 35.George C.F., Peveler R.C., Heiliger S., Thompson C. Compliance with tricyclic antidepressants: the value of four different methods of assessment. Br J Clin Pharmacol. 2000;50(2):166–171. doi: 10.1046/J.1365-2125.2000.00244.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lavsa S.M., Holzworth A., Ansani N.T. Selection of a validated scale for measuring medication adherence. J Am Pharm Assoc. 2011;51(1):90–94. doi: 10.1331/JAPHA.2011.09154. [DOI] [PubMed] [Google Scholar]
- 37.Hastie T., Tibshirani R., Friedman J. Springer; New York: 2009. The Elements of Statistical Learning. [DOI] [Google Scholar]
- 38.Sajatovic M., Valenstein M., Blow F.C., Ganoczy D., Ignacio R.V. Treatment adherence with antipsychotic medications in bipolar disorder. Bipolar Disord. 2006;8(3):232–241. doi: 10.1111/J.1399-5618.2006.00314.X. [DOI] [PubMed] [Google Scholar]
- 39.Sajatovic M., Valenstein M., Blow F., Ganoczy D., Ignacio R. Treatment adherence with lithium and anticonvulsant medications among patients with bipolar disorder. Psychiatr Serv. 2007;58(6):855–863. doi: 10.1176/ps.2007.58.6.855. [DOI] [PubMed] [Google Scholar]
- 40.Perlis R.H., Ostacher M.J., Miklowitz D.J., et al. Clinical features associated with poor pharmacologic adherence in bipolar disorder: results from the STEP-BD study. J Clin Psychiatry. 2010;71(3):2763. doi: 10.4088/JCP.09M05514YEL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.ten Doesschate M.C., Bockting C.L.H., Schene A.H. Adherence to continuation and maintenance antidepressant use in recurrent depression. J Affect Disord. 2009;115(1-2):167–170. doi: 10.1016/J.JAD.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 42.Greenhill L.L., Swanson J.M., Hechtman L., et al. Trajectories of growth associated with long-term stimulant medication in the multimodal treatment study of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2020;59(8):978–989. doi: 10.1016/J.JAAC.2019.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Edgcomb J.B., Zima B. Medication adherence among children and adolescents with severe mental illness: A systematic review and meta-analysis. J Child Adolesc Psychopharmacol. 2018;28(8):508–520. doi: 10.1089/CAP.2018.0040. [DOI] [PubMed] [Google Scholar]
- 44.Drotar D., Greenley R.N., Demeter C.A., et al. Adherence to pharmacological treatment for juvenile bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(7):831–839. doi: 10.1097/CHI.0B013E31805C7421. [DOI] [PubMed] [Google Scholar]
- 45.Lawson K.A., Johnsrud M., Hodgkins P., Sasané R., Crismon M.L. Utilization patterns of stimulants in ADHD in the Medicaid population: a retrospective analysis of data from the Texas Medicaid Program. Clin Ther. 2012;34(4):944–956.e4. doi: 10.1016/J.CLINTHERA.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 46.Clatworthy J., Bowskill R., Parham R., Rank T., Scott J., Horne R. Understanding medication non-adherence in bipolar disorders using a Necessity-Concerns Framework. J Affect Disord. 2009;116(1-2):51–55. doi: 10.1016/J.JAD.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 47.Brown C., Battista D.R., Bruehlman R., Sereika S.S., Thase M.E., Dunbar-Jacob J. Beliefs about antidepressant medications in primary care patients: relationship to self-reported adherence. Med Care. 2005;43(12):1203–1207. doi: 10.1097/01.MLR.0000185733.30697.F6. [DOI] [PubMed] [Google Scholar]
- 48.Corden M.E., Koucky E.M., Brenner C., et al. MedLink: a mobile intervention to improve medication adherence and processes of care for treatment of depression in general medicine. Digit Health. 2016;2 doi: 10.1177/2055207616663069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sajatovic M., Velligan D.I., Weiden P.J., Valenstein M.A., Ogedegbe G. Measurement of psychiatric treatment adherence. J Psychosom Res. 2010;69(6):591–599. doi: 10.1016/J.JPSYCHORES.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Osterberg L., Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.