Abstract
Background
Early secondary hyperparathyroidism (SHPT) diagnosis and treatment are crucial to delay the progression of SHPT and related complications, in particular, cardiovascular events and bone fractures. Extended-release calcifediol (ERC) has been developed for the treatment of SHPT in patients with stage 3/4 chronic kidney disease (CKD) and vitamin D insufficiency (VDI).
Summary
This review compares baseline characteristics and treatment responses of SHPT patients receiving ERC in phase 3 studies with those treated with ERC in a real-world study. Mean ± standard deviation baseline parathyroid hormone (PTH) levels were 147 ± 56 pg/mL and 148 ± 64 pg/mL in the phase 3 ERC cohorts, and 181 ± 98 pg/mL in the real-world study. Other baseline laboratory parameters were consistent between the clinical and real-world studies. ERC treatment increased 25-hydroxyvitamin D (25(OH)D) and significantly reduced PTH levels, regardless of baseline CKD stage, in all studies. In the pooled phase 3 per-protocol populations, 74% of the ERC cohort were uptitrated to 60 μg/day after 12 weeks at 30 μg/day, 97% attained 25(OH)D levels ≥30 ng/mL, and 40% achieved ≥30% PTH reduction. Despite a much lower rate of uptitration in the real-world study, 70% of patients achieved 25(OH)D levels ≥30 ng/mL, and 40% had a ≥30% reduction in PTH.
Key Messages
These data establish a “continuum” of clinical and real-world evidence of ERC effectiveness for treating SHPT, irrespective of CKD stage, baseline PTH levels, and ERC dose. This evidence supports early treatment initiation with ERC, following diagnosis of SHPT, VDI, and stage 3 CKD, to delay SHPT progression.
Keywords: Chronic kidney disease-MBD, Secondary hyperparathyroidism, Extended-release calcifediol, Parathyroid hormone, 25-Hydroxyvitamin D
Introduction
Chronic kidney disease (CKD) is associated with significant morbidity and has risen up the list of leading causes of death over the past 3 decades [1]. CKD patients contribute significantly to the global health burden because progression of CKD may be associated with cardiovascular events, leading to hospitalization and increased risk of death [2].
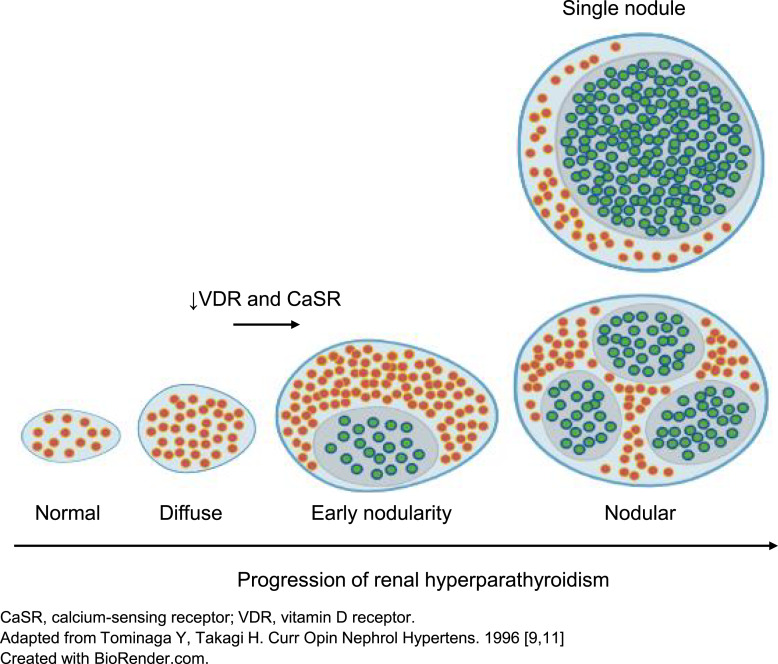
Secondary hyperparathyroidism (SHPT) is characterized by excessive secretion of parathyroid hormone (PTH) and commonly accompanies CKD progression [3]. SHPT is present from as early as stage 2 CKD [3, 4], and 40% of individuals with stage 3 CKD and 82% of individuals with stage 4 CKD are affected [5]. Prolonged and progressive elevations in PTH levels increase the risk of bone disease, fractures, vascular and soft tissue calcification, morbidity, and mortality [3, 6–8]. In the absence of effective treatment, SHPT is progressive both in terms of adverse changes within the parathyroid glands and detrimental effects on multiple organ systems [2, 9, 10]. As the disease advances, the parathyroid glands undergo nodular transformation which is accompanied by reduced vitamin D receptor and calcium-sensing receptor expression and decreased sensitivity to the effects of calcium and 1,25-dihydroxyvitamin D (1,25[OH]2D), the active vitamin D hormone (shown in Fig. 1) [9, 11]. The resulting autonomous and prolific PTH secretion, coupled with therapeutic resistance, may eventually result in the need for surgical intervention, i.e., parathyroidectomy [9, 12, 13].
Fig. 1.
Progressive parathyroid gland hyperplasia and its effects on calcium-sensing receptor and vitamin D receptor expression.
Role of Vitamin D in SHPT in Patients with CKD
SHPT develops as a consequence of mineral metabolism disturbances involving several biochemical parameters, including fibroblast growth factor 23 (FGF-23) and vitamin D metabolites, namely, 25-hydroxyvitamin D (25[OH]D) and 1,25(OH)2D [3, 6]. Vitamin D insufficiency (VDI), along with hypocalcaemia and hyperphosphataemia, are identified as key drivers of SHPT in the 2017 Kidney Disease: Improving Global Outcomes (KDIGO) guideline for the management of CKD-mineral bone disorder [13]. Low levels of 25(OH)D, which occur more frequently in CKD patients than in the general population [14], are independently associated with an increased risk of CKD progression, morbidity, and mortality in non-dialysis CKD patients [15], as well as being linked to elevations in PTH [3]. In the setting of CKD, these PTH elevations are part of an adaptive process that gradually becomes maladaptive in response to declining kidney function [6, 9, 13]. FGF-23 levels increase to facilitate phosphate excretion, leading to reduced production of vitamin D hormone which contributes to hypocalcaemia and increased PTH secretion from the parathyroid gland [13, 16]. The combined effect of these multiple pathways is to promote the progression of SHPT [6, 9, 13].
Management of SHPT in Patients with Non-Dialysis CKD
Published clinical data suggest that higher levels of serum 25(OH)D must be achieved in patients with CKD in order to maximally reduce PTH than in the general population [17–19]. According to the KDIGO guideline, dietary vitamin D supplements (cholecalciferol or ergocalciferol) can be used to suppress PTH, although their efficacy is unproven [13]. Due to the increased risk of hypercalcaemia, calcitriol and vitamin D analogues should not be routinely used in CKD stage 3–4 [13]. Parathyroidectomy can be an effective treatment approach; however, it is associated with a risk of severe hypocalcaemia, and potentially persistence or recurrence of SHPT due to residual or auto-transplanted parathyroid tissue, as well as increased risk of hospitalisation and mortality [7, 9, 12]. The KDIGO guideline, therefore, suggests that parathyroidectomy should be reserved for CKD patients with severe SHPT which is resistant to pharmacological therapy [13].
Early elevations of PTH and accompanying changes in the parathyroid glands are frequently observed from CKD stage 2 onwards (shown in Fig. 1) [3, 4]. Defining exactly when this process shifts from adaptive to maladaptive is challenging, and relying on evidence from commonly used biomarkers, such as serum phosphate, could mean waiting until estimated glomerular filtration rate (eGFR) falls below 30 mL/min/1.73 m2 [9, 20], which could be too late. KDIGO recommends that patients with CKD stages 3 and 4 with progressively rising or persistently elevated PTH levels above the upper limit of normal should be evaluated for VDI, which should be corrected as it is a modifiable risk factor [21]. Initiating an effective management option for SHPT at an earlier stage of CKD may delay progression and reduce the risk of related complications such as cardiovascular-related events and bone fractures [9]. However, as there is currently no globally accepted standard of care for the management of SHPT in non-dialysis CKD patients, practice patterns can vary by region and country. Optimal treatment for SHPT in the early stages of CKD therefore remains undefined [4].
Management of VDI in Non-Dialysis CKD Patients with Secondary Hyperparathyroidism
Dietary vitamin D supplements are widely used to treat SHPT in patients with non-dialysis CKD [13]. Clinical studies show that serum 25(OH)D levels can be raised more rapidly with immediate-release formulations of calcifediol than with supplements [22], but clinically meaningful reductions in PTH are not observed [8]. Analysis of the results of nine randomized controlled trials demonstrates that physiological doses of immediate-release calcifediol are at least 3 times more effective than cholecalciferol in increasing 25(OH)D levels [22]. However, the increases in serum 25(OH)D levels, as well as elevations in 1,25(OH)2D levels, occur rapidly after use of immediate-release calcifediol, and these pharmacological ‘surges’ trigger downregulation of cytochrome P450 (CYP) 27B1 [23] and stimulate upregulation of vitamin D catabolism via CYP24A1 [23–25]. This feedback loop limits further conversion of 25(OH)D to 1,25(OH)2D and leads to inactivation of vitamin D metabolites and accounts for the minimal reductions in plasma PTH levels seen with immediate-release calcifediol [24, 25].
The risks of using vitamin D receptor activators/1α-hydroxylated drugs, namely, calcitriol, paricalcitol, doxercalciferol, and alfacalcidol, include hypercalcaemia and accelerated vascular calcification [26–29]. These were clearly demonstrated in the PRIMO and OPERA studies, which were designed to determine the efficacy of paricalcitol on cardiac endpoints such as left ventricular mass and function in patients with stage 3–5 non-dialysis CKD and SHPT [30, 31]. Hypercalcaemia developed in 23% of patients in the PRIMO study and 43% of patients in the OPERA study. The high rate of hypercalcaemia observed in the OPERA study is of particular note as a lower dose of paricalcitol was used than in the PRIMO analysis. Hypercalcaemia reported in the PRIMO and OPERA studies accounted for a large proportion of the adverse events included in a recent meta-analysis of 6 randomized controlled trials involving almost 800 non-dialysis CKD patients treated with paricalcitol or alfacalcidol. Even when the PRIMO and OPERA studies were excluded, the meta-analysis demonstrated that there was still a significantly increased risk of hypercalcaemia in patients treated with active vitamin D or its analogues [26]. This risk of hypercalcaemia prompted a reevaluation of the benefit/risk profile of these agents, and the 2017 KDIGO guideline no longer recommends routine use of 1,25(OH)2D or active vitamin D analogues in patients with stage 3–5 non-dialysis CKD [13, 21].
Assessing the Role of Extended-Release Calcifediol in Patients with Non-Dialysis CKD: Insights from Clinical Trials and Real-World Analyses
Extended-release calcifediol (ERC) is approved for the treatment of SHPT in patients with stage 3 and 4 CKD and VDI [32, 33]. Data are now available from both randomized clinical trials and real-world studies. This provides an opportunity to evaluate whether baseline characteristics of patients receiving ERC in real-world clinical settings reflect those reported in clinical trials and to assess the clinical changes achieved with treatment in real-world practice.
Baseline Characteristics of Patients Initiating ERC during Phase 3 Clinical Trials and in a Real-World Clinical Setting
Studies 3001 (NCT01651000) and 3002 (NCT01704079) were two identical multicentre, randomized, double-blind, 26-week placebo-controlled studies of ERC in patients with stage 3 or 4 CKD, SHPT, and VDI [34]. The common protocol has been described previously, but to summarize, patients received oral ERC (30 μg for 12 weeks and uptitrated, as needed, to 60 μg until the end of the study), or placebo, once daily at bedtime [32]. A total of 285 patients received ERC during these studies, and an open-label extension phase allowed patients to continue ERC for up to an additional 6 months after completing the double-blind period (NCT02282813) [34, 35]. Patients with stage 3 or 4 CKD were also included in the MBD-AWARE real-world cohort. A retrospective analysis of this cohort reviewed medical records from 15 nephrology clinics in the USA and reported the characteristics of patients who met the study criteria and were treated with ERC (n = 174) [35].
The baseline characteristics of patients included in the clinical trial programme were generally similar to those in the MBD-AWARE cohort (Table 1) [34, 35]. Where the clinical trials aimed to enrol a similar proportion of patients with stage 3 or 4 CKD, in the real-world cohort patients were slightly more likely to be in stage 4 (53%) when being considered for treatment with ERC. Background comorbidities (primarily diabetes and hypertension) were similar in both clinical settings. The primary cause of CKD was unknown in 60% of patients enrolled in the MBD-AWARE cohort, but among patients with a known cause of CKD, a high proportion had CKD attributed to hypertension (52.2%) or diabetes (43.5%). This was consistent with the background data of CKD cause in the randomized phase 3 clinical trial settings.
Table 1.
Baseline demographics and disease characteristics of patients from the ITT population enrolled in the extended-release calcifediol (ERC) treatment arms of the phase 3 clinical trials or included in the MBD-AWARE ERC cohort [29–31]
| Phase 3 clinical trials | Real-world dataset | ||
|---|---|---|---|
| CTAP101-CL-3001 | CTAP101-CL-3002 | MBD-AWAREa (n = 174) | |
| ERC (n = 141) | ERC (n = 144) | ||
| Patient demographics | |||
| Age, years, mean (SD) | 65.1 (10.3) | 66.8 (10.9) | 69.0 (13.2) |
| Male, n (%) | 70.0 (49.6) | 73.0 (50.7) | 84.0 (48.3) |
| Race, n (%) | |||
| White | 85 (60.3) | 98 (68.1) | 113 (64.9) |
| Black or African-Americanb | 50 (35.5) | 43 (29.9) | 34 (19.5) |
| Other | 6 (4.2) | 2 (1.4) | 19 (10.9) |
| Not available | 0.0 | 1 (0.7) | 8 (4.6) |
| Body mass index, kg/m2, mean (SD) | 34.1 (8.3) | 34.7 (7.9) | 34.2 (20.7) |
| CKD characteristics | |||
| CKD stage, n (%) | |||
| 3 | 71 (50.4) | 80 (55.6) | 81 (46.6) |
| 4 | 70 (49.6) | 64 (44.4) | 93 (53.4) |
| Primary cause of CKD, n (%) | |||
| Hypertension | 54 (38.3) | 49 (34.0) | 36 (20.7) |
| Diabetes | 55 (39.0) | 74 (51.4) | 30 (17.2) |
| Other | 5 (3.5) | 5 (3.5) | 3 (1.7) |
| Unknown cause | 27 (19.1) | 16 (11.1) | 105 (60.3) |
| Laboratory parameters | |||
| Serum calcium, mg/dL, mean (SD) | 9.2 (0.29) | 9.3 (0.35) | 9.2 (1.3) |
| Serum phosphorus, mg/dL, mean (SD) | 3.7 (0.55) | 3.8 (0.56) | 3.8 (1.3) |
| Plasma iPTH, pg/mL, mean (SD) | 146.8 (56.01) | 147.6 (64.21) | 181.4 (97.6) |
| eGFR, mL/min/1.73 m2, mean (SD) | 30.3 (11.07) | 30.9 (9.90) | 31.1 (14.5) |
| Serum 25(OH)D, ng/mL, mean (SD) | 20.2 (5.08) | 19.7 (5.56) | 20.3 (9.2) |
25(OH)D, 25-hydroxyvitamin D; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; ERC, extended-release calcifediol; iPTH, intact parathyroid hormone; NVD, nutritional vitamin D; SD, standard deviation; VDA, vitamin D analogues.
aThe MBD-AWARE study included 374 patients in total. In addition to the ERC cohort shown, 55 patients were included in the VDA cohort and 147 patients were included in the NVD cohort.
bOnly includes African-American patients in the MBD-AWARE cohort.
Overall, kidney function was similar between the three populations studied, as reflected by similar baseline eGFR measurements (Table 1). Similarities between the clinical trial and real-world settings were also apparent when comparing baseline levels of serum calcium, phosphorus, and 25(OH)D. Notably, however, patients in the MBD-AWARE cohort had higher levels of baseline intact PTH (iPTH) than was seen in the phase 3 studies: mean iPTH was 181.4 pg/mL in the real-world cohort versus under 150 pg/mL in the clinical trials (Table 1). When iPTH levels in the MBD-AWARE cohort were stratified by CKD stage, higher mean iPTH levels were seen in patients with stage 4 CKD (mean ± standard deviation [SD]: 203.6 ± 109 pg/mL) than in patients with stage 3 CKD (156.0 ± 75 pg/mL) [35].
Dose and Duration of ERC Used in Clinical Trials and in the Real World
In the phase 3 studies, 74% of patients were uptitrated to the maximum dose of 60 μg after the first 12 weeks of the studies. Patients could have received ERC for up to 52 weeks during the double-blind and open-label extension phases with efficacy determined at set intervals during the treatment period [34]. For the MBD-AWARE cohort, only three (1.7%) patients were uptitrated to the 60 μg dose and 1 patient was downtitrated to 30 μg every other day during the review period (reason for dose titration was not specified) [35]. Reasons for the lower rates of dose titration in the real-world setting compared with the phase 3 studies may have included fewer patient visits to the clinic and reduced monitoring of safety laboratory parameters, resulting in less opportunity for dose titration. Alternatively, clinicians may have been unsure of the target PTH level at different stages of CKD due to ambiguity in the guideline and, therefore, were comfortable leaving the dose unchanged as long as PTH was not trending upwards. This low rate of uptitration may also reflect a current approach where clinicians wait to see the impact on clinical laboratory parameters before changing ERC dose. For patients in the MBD-AWARE cohort, the timing of laboratory assessments after initiating therapy could have varied between patients, and in certain instances, these assessments might not have been conducted at all. Both of these factors may have had an impact on the data available for making informed decisions about dose titration.
Effectiveness and Safety of ERC in Patients with CKD Treated during Clinical Trials and in the Real World
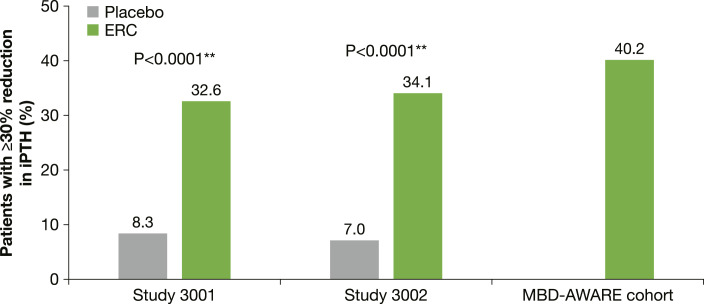
Pre- and post-treatment measurements of serum 25(OH)D, iPTH, serum calcium, serum phosphorus, and eGFR are shown in Table 2. Significantly greater declines in iPTH levels were achieved with ERC than with placebo when assessed at the efficacy assessment phase (EAP), defined as the average of the last 6 weeks of the 26-week double-blind treatment period in the phase 3 studies (primary efficacy assessment), with 33–34% of patients in the intent-to-treat cohorts of the two phase 3 studies achieving a ≥30% reduction in iPTH versus 7–8% in the placebo group (shown in Fig. 2) [34]. In the real-world study, after a mean treatment duration of 23.4 weeks (a similar length of treatment to that assessed in the clinical trial programme), 40.2% of patients had achieved a ≥30% reduction from baseline (shown in Fig. 2) with a mean reduction of 34.1 pg/mL demonstrated [35]. The high levels of iPTH recorded at baseline in the MBD-AWARE cohort may have influenced the results reported.
Table 2.
Pre- and post-treatment measurements of 25-hydroxyvitamin D, iPTH, calcium, phosphorus, and estimated glomerular filtration rate in patients from the ITT population treated with extended-release calcifediol (ERC) in the phase 3 clinical trials or included in the MBD-AWARE ERC cohort [34–36]
| Phase 3 clinical trials | Real-world dataset | |||||
|---|---|---|---|---|---|---|
| CTAP101-CL-3001 | CTAP101-CL-3002 | MBD-AWAREa (n = 174) | ||||
| ERC (n = 141) | ERC (n = 144) | |||||
| pre | post | pre | post | pre | post | |
| Serum 25(OH)D, ng/mL, mean | 20.2 (SD: 5.08) | 67.0 (SD: 22.25)b | 19.7 (SD: 5.56) | 66.8 (SD: 21.41)c | 20.3 (SE: 0.7) | 44.0 (SE: 1.7) |
| Plasma iPTH, pg/mL, mean | 146.8 (SD: 56.01) | 109.6 (SD: 50.95)d | 147.6 (SD: 64.21) | 113.1 (SD: 76.29)c | 181.4 (SE: 7.4) | 147.4 (SE: 7.1) |
| Serum calcium, mg/dL, mean | 9.2 (SD: 0.29) | 9.4 (SD: 0.49)e | 9.3 (SD: 0.35) | 9.4 (SD: 0.35)f | 9.2 (SE: 0.1) | 9.3 (SE: 0.1) |
| Serum phosphorus, mg/dL, mean | 3.7 (SD: 0.55) | 3.9 (SD: 0.62)e | 3.8 (SD: 0.56) | 4.0 (SD: 0.68)f | 3.8 (SE: 0.1) | 3.9 (SE: 0.1) |
| eGFR, mL/min/1.73 m2, mean | 30.3 (SD: 11.07) | 29.8 (SD: 11.84)e | 31.0 (SD: 9.93)g | 29.4 (SD: 11.51)h | 31.1 (SE: 1.1) | 28.0 (SE: 0.9) |
25(OH)D, 25-hydroxyvitamin D; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; ERC, extended-release calcifediol; iPTH, intact parathyroid hormone; ITT, intention-to-treat; NVD, nutritional vitamin D; PP, per protocol; SD, standard deviation; VDA, vitamin D analogues.
aThe MBD-AWARE study included 374 patients in total. In addition to the ERC cohort shown, 55 patients were included in the VDA cohort and 147 patients were included in the NVD cohort.
b n = 118.
c n = 124.
d n = 117.
e n = 131.
f n = 136.
g n = 143.
h n = 132.
Fig. 2.
Proportion of patients treated with ERC with a reduction in iPTH levels of at least 30%* in the phase 3 and real-world patient populations [34, 35].
In the real-world cohort, levels of serum 25(OH)D were assessed after a mean treatment duration of 24.6 weeks, and at this timepoint, 70.1% of patients had 25(OH)D levels ≥30 ng/mL [35]. Mean 25(OH)D levels increased from 20.3 ng/mL at baseline to 44.0 ng/mL during treatment. The proportion of patients achieving the ≥30 ng/mL threshold in the clinical programme was higher with between 80.2% and 83.3% of the intent-to-treat populations achieving this level at the primary efficacy assessment [34]. This difference was more apparent in the per-protocol population, with more than 95% of patients achieving 25(OH)D ≥30 ng/mL, and may be attributed to the use of a lower daily dose of ERC in the real-world cohort given the lack of uptitration [35]. The high levels of iPTH recorded at baseline in the MBD-AWARE cohort may have also influenced the 25(OH)D results. Importantly, the analysis demonstrated that a high proportion of patients obtain benefit from ERC despite the high baseline iPTH levels and use of a low daily ERC dose [35]. It is likely that an increased treatment response may be observed in real-world settings when ERC is uptitrated to its full dose based on clinical decisions made following observed PTH reductions over time.
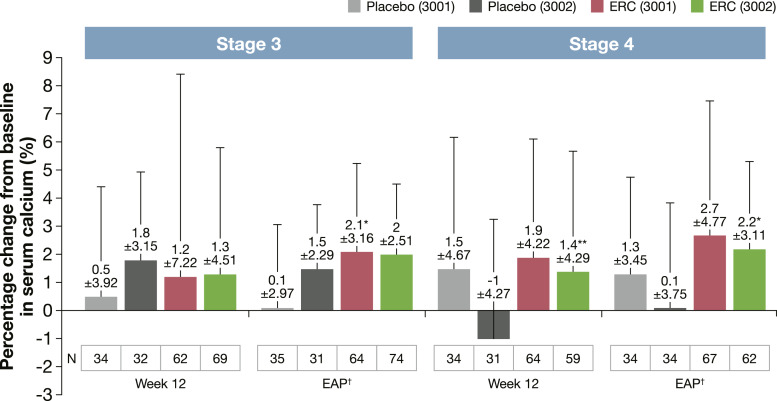
As described in previous publications, the beneficial impact of ERC on iPTH and 25(OH)D levels was not accompanied by safety concerns in the clinical trial programme [34]. Hypercalcaemia is the first sign of vitamin D toxicity, but mean percentage changes in calcium levels at EAP were <3% overall and when stratified by CKD stage (shown in Fig. 3). However, statistically significant increases occurred in the CKD stage 3 group treated with ERC in study 3001 at EAP (p < 0.005, mean ± SD: 2.1 ± 3.16%) and in the CKD stage 4 group treated with ERC in study 3002 at week 12 (p < 0.05, mean ± SD: 1.4 ± 4.29%) and at EAP (p < 0.005, mean ± SD: 2.2 ± 3.11%). Significant increases in serum calcium levels were not observed when ERC was used in the MBD-AWARE cohort with only 1.8% of patients reporting hypercalcaemia, a percentage consistent with the clinical trials [35, 36].
Fig. 3.
Percentage change from baseline in serum calcium levels in patients treated with placebo or ERC at week 12 of treatment or at the end of assessment period in the phase 3 clinical trials.
Discussion: A Data Journey from Phase 3 to Real-World Evidence with ERC
The goal of vitamin D therapy in patients with normal kidney function is to replete an insufficiency of the vitamin. In patients with advanced CKD, higher levels of 25(OH)D need to be reached [17, 18] in order to achieve significant PTH reductions through both renal and extrarenal production of 1,25(OH)2D [19, 37, 38]. ERC supports both mechanisms, as was most recently shown in data from haemodialysis and overweight non-dialysis CKD patients with SHPT [38, 39]. An increasing volume of data now supports the concept that patients with CKD could benefit from early initiation of ERC to control PTH in a physiological manner [34–36, 40]. This approach would likely prevent an increased risk of adynamic bone disease without the risk of adverse events usually associated with immediate-release formulations of calcifediol and activated vitamin analogues like calcitriol and paricalcitol [34–36, 40]. Clinical studies in non-dialysis CKD patients have shown that ERC gradually and reliably increases serum 25(OH)D, resulting in physiologically regulated increases in serum 1,25(OH)2D and sustained clinically relevant reductions in PTH [34], and suggest that initiating ERC early could alleviate the long-term challenges in controlling PTH within the desired range. Following on from the results of the phase 3 clinical studies, the real-world evidence summarized here supports the tolerability and effectiveness of ERC in routine clinical practice [19, 34–36].
Up to 82% of patients with stage 3 or 4 CKD have SHPT [3, 5] and are at increased risk of cardiovascular events and fractures [3, 41, 42]. In both the phase 3 and real-world settings, ERC effectiveness was unaffected by CKD stage [34–36]. Despite the difference of baseline PTH values characterized by higher PTH levels in patients with stage 4 CKD in the MBD-AWARE cohort, the achievement of a clinically relevant response through increasing 25(OH)D levels, alongside the reduction of PTH, shows consistency in both clinical study settings. Similarly, despite lower doses of ERC, a higher percentage reduction of PTH was achieved in the MBD-AWARE cohort, which may be in part due to the high levels of PTH recorded at baseline. Based on the clinical evidence of benefit gained from these datasets, initiating ERC as an early and maintained treatment approach should be considered to prevent SHPT progression and thus limit long-term complications.
The 2017 KDIGO clinical practice guideline recommends correcting VDI in patients with CKD G3a–G5D using treatment strategies recommended for the general population; however, the optimal level of serum 25(OH)D remains undefined [13, 19]. A post hoc analysis of the phase 3 ERC data in non-dialysis patients demonstrated that sufficiently reducing plasma iPTH and bone turnover markers required mean serum 25(OH)D levels of at least 50.8 ng/mL, a target higher than those often adopted in clinical practice (20–30 ng/mL) [19]. During ERC therapy, higher serum 25(OH)D levels progressively reduced plasma iPTH levels, with a steady increase in the percentage of patients showing at least a 30% decrease in PTH up to the highest mean serum 25(OH)D level assessed (92.5 ng/mL) [19]. The gradual elevation of 25(OH)D was not associated with adverse elevation of serum calcium, phosphorus, or FGF-23 levels and did not increase mean serum 1,25(OH)2D above the upper limit of normal. Reduced kidney function and its resultant effect on declining expression of renal CYP27B1 did not impact conversion of 25(OH)D to 1,25(OH)2D, which demonstrates that 25(OH)D can be activated extra-renally by CYP27B1 in the parathyroid gland and other tissues [19].
In conclusion, the combined analysis and outcomes from phase 3 clinical studies and real-world evidence provide a “continuum of clinical evidence” supporting early and sustained treatment with ERC (starting at 30 μg/day for 12 weeks and then uptitrating, as needed, to 60 μg/day) in non-dialysis CKD patients. This therapeutic approach, based on adequate monitoring of key laboratory parameters, should be established as soon as SHPT is diagnosed in patients with stage 3 CKD as its effectiveness and tolerability in this population have been demonstrated. The expectation is that this treatment approach would delay progression of SHPT over time in patients with CKD who are at high risk of bone fractures and cardiovascular events. However, further research is warranted to confirm whether PTH could be lowered to just above the upper limit of normal for the assay and kept at a relatively constant range, which may result in improvement in bone integrity and lower risk of worsening vascular calcifications.
Acknowledgments
Medical writing support was provided by Scarlett Dell-Cronin (Elements Communications Ltd, UK). A portion of the results included in this manuscript were previously presented as an abstract and poster at The American Society of Nephrology’s Kidney Week 2023 in Philadelphia, PA, USA, 2–5 November 2023.
Statement of Ethics
All subjects gave their informed consent for inclusion before they participated in the study.
Conflict of Interest Statement
Akhtar Ashfaq, Charles Bishop, and Stephen Strugnell are employed by and own stock in OPKO Pharmaceuticals. Akhtar Ashfaq is also a member of medical advisory boards AAKP and DPC and an ex-board member of AKF, ex officio. Isabelle Morin is employed by CSL Vifor and owns CSL Vifor shares. Domenico Merante and Henrik Schou were employed by CSL Vifor and owned CSL Vifor shares during the development of the first draft of the manuscript. Marius Manu is employed by CSL Vifor.
Funding Sources
Medical writing support for this manuscript was funded by Vifor Fresenius Medical Care Renal Pharma Ltd., Switzerland. Studies were sponsored by OPKO Pharmaceuticals.
Author Contributions
Domenico Merante was responsible for the research idea and supervision of review writing and contributed to drafting the manuscript, and Marius Manu, Henrik Schou, Isabelle Morin, Akhtar Ashfaq, Charles Bishop, and Stephen Strugnell critically reviewed and amended the manuscript during numerous rounds of reviewal. Domenico Merante, Marius Manu, Henrik Schou, Isabelle Morin, Akhtar Ashfaq, Charles Bishop, and Stephen Strugnell approved the manuscript for publication.
Funding Statement
Medical writing support for this manuscript was funded by Vifor Fresenius Medical Care Renal Pharma Ltd., Switzerland. Studies were sponsored by OPKO Pharmaceuticals.
Data Availability Statement
The data underlying this article are available in the article.
References
- 1. GBD Chronic Kidney Disease Collaboration . Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395(10225):709–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schumock GT, Andress D, E Marx S, Sterz R, Joyce AT, Kalantar-Zadeh K. Impact of secondary hyperparathyroidism on disease progression, healthcare resource utilization and costs in pre-dialysis CKD patients. Curr Med Res Opin. 2008;24(11):3037–48. [DOI] [PubMed] [Google Scholar]
- 3. Levin A, Bakris G, Molitch M, Smulders M, Tian J, Williams LA, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007;71(1):31–8. [DOI] [PubMed] [Google Scholar]
- 4. Ketteler M, Ambühl P. Where are we now? Emerging opportunities and challenges in the management of secondary hyperparathyroidism in patients with non-dialysis chronic kidney disease. J Nephrol. 2021;34(5):1405–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hyder R, Sprague SM. Secondary hyperparathyroidism in a patient with CKD. Clin J Am Soc Nephrol. 2020;15(7):1041–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hu L, Napoletano A, Provenzano M, Garofalo C, Bini C, Comai G, et al. Mineral bone disorders in kidney disease patients: the ever-current topic. Int J Mol Sci. 2022;23(20):12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ureña-Torres PA, Vervloet M, Mazzaferro S, Oury F, Brandenburg V, Bover J, et al. Novel insights into parathyroid hormone: report of the parathyroid day in chronic kidney disease. Clin Kidney J. 2019;12(2):269–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cozzolino M, Ketteler M. Evaluating extended-release calcifediol as a treatment option for chronic kidney disease-mineral and bone disorder (CKD-MBD). Expert Opin Pharmacother. 2019;20(17):2081–93. [DOI] [PubMed] [Google Scholar]
- 9. Cunningham J, Locatelli F, Rodriguez M. Secondary hyperparathyroidism: pathogenesis, disease progression, and therapeutic options. Clin J Am Soc Nephrol. 2011;6(4):913–21. [DOI] [PubMed] [Google Scholar]
- 10. Geng S, Kuang Z, Peissig PL, Page D, Maursetter L, Hansen KE. Parathyroid hormone independently predicts fracture, vascular events, and death in patients with stage 3 and 4 chronic kidney disease. Osteoporos Int. 2019;30(10):2019–25. [DOI] [PubMed] [Google Scholar]
- 11. Tominaga Y, Takagi I. Molecular genetics of hyperparathyoid disease. Curr Opin Nephrol Hypertens. 5:336–42. [DOI] [PubMed] [Google Scholar]
- 12. Steinl GK, Kuo JH. Surgical management of secondary hyperparathyroidism. Kidney Int Rep. 2021;6(2):254–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kidney disease: improving global outcomes (KDIGO) CKD-MBD update work group KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int. 2017;7:1–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Doorenbos CRC, van den Born J, Navis G, de Borst M. Possible renoprotection by Vitamin D in chronic renal disease: beyond mineral metabolism. Nat Rev Nephrol. 2009;5(12):691–700. [DOI] [PubMed] [Google Scholar]
- 15. Ravani P, Malberti F, Tripepi G, Pecchini P, Cutrupi S, Pizzini P, et al. Vitamin D levels and patient outcome in chronic kidney disease. Kidney Int. 2009;75(1):88–95. [DOI] [PubMed] [Google Scholar]
- 16. Kidney Disease Improving Global Outcomes KDIGO CKD-MBD Work Group . KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. 2009(113):S1–130. [DOI] [PubMed] [Google Scholar]
- 17. Ennis JL, Worcester EM, Nephrol J, Coe FL, Sprague SM. Current recommended 25-hydroxyvitamin D targets for chronic kidney disease management may be too low. J Nephrol. 2016;29(1):63–70. [DOI] [PubMed] [Google Scholar]
- 18. Dhillon-Jhattu S, McGill RL, Ennis JL, Worcester EM, Zisman AL, Coe FL. Vitamin D and parathyroid hormone levels in CKD. Am J Kidney Dis. 2023;81(1):122–4. [DOI] [PubMed] [Google Scholar]
- 19. Strugnell SA, Sprague SM, Ashfaq A, Petkovich M, Bishop CW. Rationale for raising current clinical practice guideline target for serum 25-hydroxyvitamin D in chronic kidney disease. Am J Nephrol. 2019;49(4):284–93. [DOI] [PubMed] [Google Scholar]
- 20. Evenepoel P, Rodriguez M, Ketteler M. Laboratory abnormalities in CKD-MBD: markers, predictors, or mediators of disease? Semin Nephrol. 2014;34(2):151–63. [DOI] [PubMed] [Google Scholar]
- 21. Ketteler M, Block GA, Evenepoel P, Fukagawa M, Herzog CA, McCann L, et al. Executive summary of the 2017 KDIGO chronic kidney disease-mineral and bone disorder (CKD-MBD) guideline update: what’s changed and why it matters. Kidney Int. 2017;92(1):26–36. [DOI] [PubMed] [Google Scholar]
- 22. Quesada-Gomez JM, Bouillon R. Is calcifediol better than cholecalciferol for vitamin D supplementation? Osteoporos Int. 2018;29(8):1697–711. [DOI] [PubMed] [Google Scholar]
- 23. Petkovich M, Jones G. CYP24A1 and kidney disease. Curr Opin Nephrol Hypertens. 2011;20(4):337–44. [DOI] [PubMed] [Google Scholar]
- 24. Batacchi Z, Robinson-Cohen C, Hoofnagle A, Isakova T, Kestenbaum B, Martin KJ, et al. Effects of vitamin D2 supplementation on vitamin D3 metabolism in health and CKD. Clin J Am Soc Nephrol. 2017;12(9):1498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Petkovich M, Melnick J, White J, Tabash S, Strugnell S, Bishop CW. Modified-release oral calcifediol corrects vitamin D insufficiency with minimal CYP24A1 upregulation. J Steroid Biochem Mol Biol. 2015;148:283–9. [DOI] [PubMed] [Google Scholar]
- 26. Cozzolino M, Bernard L, Csomor PA. Active vitamin D increases the risk of hypercalcaemia in non-dialysis chronic kidney disease patients with secondary hyperparathyroidism: a systematic review and meta-analysis. Clin Kidney J. 2021;14(11):2437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Svajger BA, Pruss CM, Laverty KJ, Zelt JGE, Jones G, Kaufmann M, et al. PTH suppression by calcitriol does not predict off-target actions in experimental CKD. Pharmacol Res Perspect. 2020;8(3):e00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cardús A, Panizo S, Parisi E, Fernandez E, Valdivielso JM. Differential effects of vitamin D analogs on vascular calcification. J Bone Miner Res. 2007;22(6):860–6. [DOI] [PubMed] [Google Scholar]
- 29. Franchi M, Gunnarsson J, Gonzales-Parra E, Ferreira A, Ström O, Corrao G. Paricalcitol and extended-release calcifediol for treatment of secondary hyperparathyroidism in non-dialysis chronic kidney disease: results from a network meta-analysis. J Clin Endocrinol Metab. 2023;108(11):e1424–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang AYM, Fang F, Chan J, Wen YY, Qing S, Chan IHS, et al. Effect of paricalcitol on left ventricular mass and function in CKD – the OPERA Trial. J Am Soc Nephrol. 2014;25(1):175–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thadhani R, Appelbaum E, Pritchett Y, Chang Y, Wenger J, Tamez H, et al. Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: the PRIMO randomized controlled trial. JAMA. 2012;307(7):674–84. [DOI] [PubMed] [Google Scholar]
- 32. Rayaldee [prescribing information] . OPKO Pharmaceuticals; 2021. [Google Scholar]
- 33. Rayaldee summary of product characteristics. Vifor Fresenius medical care renal Pharma. 2022. [Google Scholar]
- 34. Sprague SM, Crawford PW, Melnick JZ, Strugnell SA, Ali S, Mangoo-Karim R, et al. Use of extended-release calcifediol to treat secondary hyperparathyroidism in stages 3 and 4 chronic kidney disease. Am J Nephrol. 2016;44:316–25. [DOI] [PubMed] [Google Scholar]
- 35. Fadda G, Germain MJ, Broumand V, Nguyen A, McGarvey N, Gitlin M, et al. Real-world assessment: clinical effectiveness and safety of extended-release calcifediol. Am J Nephrol. 2021;52(10–11):798–807. [DOI] [PubMed] [Google Scholar]
- 36. Germain MJ, Paul SK, Fadda G, Broumand V, Nguyen A, McGarvey NH, et al. Real-world assessment: effectiveness and safety of extended-release calcifediol and other vitamin D therapies for secondary hyperparathyroidism in CKD patients. BMC Nephrol. 2022;23(1):362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Almquist M, Isaksson E, Clyne N. The treatment of renal hyperparathyroidism. Endocr Relat Cancer. 2020;27(1):R21–34. [DOI] [PubMed] [Google Scholar]
- 38. Bishop CW, Strugnell SA, Csomor P, Kaiser E, Ashfaq A. Extended-release calcifediol effectively raises serum total 25-hydroxyvitamin D even in overweight nondialysis chronic kidney disease patients with secondary hyperparathyroidism. Am J Nephrol. 2022;53(6):446–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Strugnell S. Initial evaluation of high-dose extended-release calcifediol (ERC) in patients with stage 5 CKD on hemodialysis (HD). American Society of Nephrology | Kidney Week; 2021. Available from: https://www.asn-online.org/education/kidneyweek/2021/program-abstract.aspx?controlId=36053452021 (accessed September 7, 2023). [Google Scholar]
- 40. Sprague SM, Strugnell SA, Bishop CW. Extended-release calcifediol for secondary hyperparathyroidism in stage 3-4 chronic kidney disease. Expert Rev Endocrinol Metab. 2017;12(5):289–301. [DOI] [PubMed] [Google Scholar]
- 41. Nigwekar SU, Tamez H, Thadhani RI. Vitamin D and chronic kidney disease–mineral bone disease (CKD–MBD). Bonekey Rep. 2014;3:498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nickolas TL, McMahon DJ, Shane E. Relationship between moderate to severe kidney disease and hip fracture in the United States. J Am Soc Nephrol. 2006;17(11):3223–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article are available in the article.