Abstract
Backgrounds:
Renal tubular injury caused by oxidative stress and inflammation results in acute kidney injury. Recent research reported that antibiotics may protect renal tubules from progressive deterioration, but the underlying mechanism remains unclear. Therefore, we investigated the efficacy and mechanism of action of antibiotics against renal tubular injury.
Methods:
We screened ciprofloxacin, ceftizoxime, minocycline, and netilmicin and selected ciprofloxacin to examine further because of its low toxicity towards renal tubular cells. We evaluated the effect of ciprofloxacin on cell survival by analyzing apoptosis and autophagy.
Results:
Terminal deoxynucleotidyl transferase-mediated d-UTP nick end labeling (TUNEL) assay results showed that the ciprofloxacin group had less apoptotic cells than the control group. The ratio of cleaved caspase 3 to caspase 3, the final effector in the apoptosis process, was decreased, but the ratio of B-cell lymphoma 2 (Bcl-2)-associated X protein (Bax) to Bcl-2 located upstream of caspase 3 was not decreased in the ciprofloxacin group. Therefore, apoptosis inhibition does not occur via Bax/Bcl-2. Conversely, the levels of phosphorylated Bcl-2, and Beclin-1, an autophagy marker, were increased, and that of caspase-3 was decreased in the ciprofloxacin group.
Conclusion:
This indicates that ciprofloxacin enhances autophagy, increasing the amount of free Beclin-1 via phosphorylated Bcl-2, and inhibits caspase activity. Therefore, ciprofloxacin might protect against renal tubular injury through the activation of autophagy in the setting of acute kidney injury.
Keywords: acute kidney injury, apoptosis, autophagy, ciprofloxacin
1. Introduction
Studies on the homeostasis of mammalian cells have been actively conducted for a long time, and it is well known that autophagy, apoptosis, and necrosis are the most important mechanisms of programmed cell death in cells. The fate of cells is decided by the coordinated action of this programmed cell death.[1] Among them, apoptosis has been considered the important mechanism of programmed cell death in mammalian cells, but recent studies have reported that autophagy plays a dual role in both the survival and death mechanisms, according to the cell type and degree of stimulation.[2] Cell homeostasis can be controlled by the interaction between autophagy and apoptosis at the molecular level.[3] In particular, autophagy and apoptosis play important roles in the homeostasis of kidney cells, such as podocytes and tubular epithelial cells, in hypoxia, nutrient deprivation, oxidative stress, and inflammation conditions.[4,5] In other words, renal tubular injury caused by several injuries results in acute kidney injury, and the cells damaged by acute kidney injury recover or die based on the relationship between autophagy and apoptosis.
Recent research has reported that antibiotics may influence deteriorated kidney cells or tissues, although antibiotics have their own action mechanisms against bacteria, such as inhibition of replication and differentiation.[6] For example, tigecycline, which is a new broad-spectrum antibiotic drug that structurally and functionally resembles tetracycline, has been reported to have anti-cancer effects by activating the adenosine monophosphate (AMP) -activated protein kinase/mammalian target of rapamycin (AMPK/mTOR) pathway, but not apoptosis, in human gastric cancer cells.[7] Minocycline, a protein synthesis inhibitor (30S inhibitor), and a representative tetracycline antibiotic, has been reported to be associated with apoptosis in acute kidney injury and chronic inflammation.[8] In other words, antibiotics may restore renal tubules, but the underlying mechanism remains unclear.
Therefore, we aimed to investigate the effect of antibiotics on the survival of kidney cells, and the mechanism of action of antibiotics on cell viability associated with apoptosis and autophagy.
2. Materials and methods
2.1. Materials
We used the Madin-Darby canine kidney (MDCK) cell line, which is a tubular epithelial kidney cell line, which was purchased from the Korean Cell Line Bank (Seoul, South Korea).[9,10] We used 4 representative antibiotics from each spectrum, ciprofloxacin (CJ Healthcare Corp., Seoul, South Korea), ceftizoxime (DONG-A ST, Seoul, South Korea), minocycline (Sigma-Aldrich Co., St. Louis), and netilmicin (KUHNIL Pharm. Seoul, South Korea), to screen for effective antibiotics that improve MDCK cell viability after 48 hours of antibiotic treatment using a 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay. Ciprofloxacin is one of the quinolones which block DNA replication via inhibition of DNA gyrase. Besides its antibiotic activity, some studies have reported the immunomodulatory effects of ciprofloxacin in rodent models and human clinical trials.[11,12] Ceftizoxime is a cell wall inhibitor and a member of the third-generation cephalosporin available for parenteral administration. It binds to and inactivates penicillin-binding proteins of the inner membrane of the bacterial cell wall. This leads to weakness of the bacterial cell wall. Minocycline is a protein synthesis inhibitor (30S inhibitor) and a representative tetracycline antibiotic that has been reported to have immunomodulatory and anti-inflammatory effects. In particular, minocycline inhibits apoptosis in certain neurodegenerative disorders and has potent anti-apoptotic and anti-inflammatory effects, as observed in a rat model of ischemia-reperfusion injury.[13] Minocycline improved streptozotocin-induced kidney damage by protecting against long-term hyperglycemia-induced kidney cell apoptosis.[14] Netilmicin is a protein synthesis inhibitor (30S inhibitor) and one of the members of the aminoglycoside family. It is used to kill a wide range of bacteria. Its action resembles that of gentamicin, but it is less nephrotoxic.
Primary antibodies for B-cell lymphoma 2 (Bcl-2), caspase-3, cleaved caspase-3 (Cell Signaling Technology, Beverly), Bcl-2-associated X protein (Bax; Santa Cruz Biotechnology, Santa Cruz), phosphorylated Bcl-2 (pBcl-2), and phosphorylated Bax (pBax; Thermo Fisher Scientific, Waltham) were used as apoptosis markers. Beclin-1 primary antibody (OriGene Technology, Rockville) was used as an autophagy marker. A primary antibody against β-actin (Santa Cruz Biotechnology, Santa Cruz) was used as the control. The secondary antibody used was horseradish peroxidase-conjugated secondary antibody (Enzo, Farmingdale).
2.2. Methods
2.2.1. Cell viability measurement
We divided the operating conditions into normoxia (21% O2) and hypoxia (1% O2) conditions. Hypoxic conditions are achieved with specific gas mixtures, generally (1% oxygen; with 5% carbon dioxide and the balance 94% nitrogen).[15] In general, oxygen concentrations of <2% are considered hypoxic, however, normoxic levels must be considered when selecting the experimental hypoxic conditions (Kaur B et al, 2023).[16] The glucose media were not supplemented. We evaluated the effect of antibiotics on cell viability according to the type and concentration of the 4 antibiotics: ciprofloxacin, ceftizoxime, minocycline, and netilmicin. MDCK cells were plated (at a density of 7 × 104 cells/well) in a 48-well plate and grown in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with penicillin (100 units/mL), streptomycin (100 µg/mL), and fetal bovine serum (FBS, 10%; Gibco BRL, Gaithersburg) at 37°C for 48 hours under normoxia conditions (5% CO2 balanced with air) in a humidified chamber (Forma Scientific Inc., Marietta). After the media were replaced by DMEM without glucose or serum, ciprofloxacin was added, and the cells were cultured under normoxia and hypoxia conditions. Four antibiotics were added to each well at different concentrations. Ciprofloxacin (0, 50, 100, 150, and 200 µg/mL), ceftizoxime (0, 10, 100, 200, 500, and 1000 µg/mL), minocycline (0, 10, 50, 100, 200, and 500 µg/mL), and netilmicin (0, 1, 10, 100, 500, and 1000 µg/mL) were used. The effects of the antibiotics on cell viability were evaluated using an MTT assay. An MTT assay, which is a tetrazolium-based colorimetric assay, was used to assess cell viability because it allows an easy and rapid reading of many samples.[17] MTT (5 mg/mL) was added to each well. Because MTT solutions are sensitive to light, the exposure to light was minimized. The plates were placed in a 37°C and 5% CO2 incubator for 2 hours. Dimethyl sulfoxide (DMSO) was dispensed after removing the MTT solution. The plates were shaken for 10 to 15 minutes while being protected from light. The absorbance was measured using a plate reader at a wavelength of 570 nm.
2.2.2. TUNEL assay
MDCK cells were plated (at a density of 1 × 105 cells/well) on a Lab-Tek® 4 well chamber slide (Thermo Fisher Scientific, Waltham) and grown in DMEM culture medium, as described above. Twenty-four hours after plating, a terminal deoxynucleotidyl transferase-mediated d-UTP nick end labeling (TUNEL) assay was performed to detect the nicks generated from DNA strand breaks.[18] The aim of the assay was to identify indicators of damage due to apoptosis.[17] We used ciprofloxacin (100 µg/mL) and ApopTag® Peroxidase In Situ Apoptosis Detection Kit (Merck Millipore, Burlington). We fixed cells in 1% paraformaldehyde in phosphate-buffered saline (PBS), pH 7.4 and washed them twice with PBS for 5 minutes. We performed post-processing using precooled ethanol: acetic acid (2:1) at −20°C in a Coplin jar for 5 minutes, and drained it. We quenched the cells in 3.0% hydrogen peroxide in PBS at room temperature for 5 minutes. We rinsed the specimen twice with PBS for 5 minutes each time in a Coplin jar. An equilibration buffer (75 µL/5 cm2) was immediately added to the sample. We immediately pipetted the working strength terminal deoxynucleotidyl transferase (TdT) enzyme solution (55 µL/5 cm2) onto the section. The cells were incubated for 1 hour at 37°C in a humidified chamber. The specimen was placed in a Coplin jar containing the working strength stop/wash buffer and stirred for 15 seconds and then incubated at room temperature for 10 minutes. We applied anti-digoxigenin peroxidase conjugate to the slide at room temperature (approximately 65 µL/5 cm2 of the surface covered). The cells were incubated in a humidified chamber for 30 minutes at room temperature. We applied sufficient peroxidase substrate to completely cover the specimen (75 µL/5 cm2). We stained the cells for 3 to 6 minutes at room temperature. To determine the optimal staining time, we monitored the color change of cells under the microscope. We counterstained the cells in a Coplin jar for 10 minutes at room temperature using 0.5% methyl green. The specimens were dehydrated by moving the slides through 3 xylene jars while incubating for 2 minutes in each Coplin jar. We removed the slides from the Coplin jar and mounted them under a glass coverslip in a mounting medium.
2.2.3. Western blot analysis
Cell extracts were prepared by incubating the cells in cell lysis buffer [1X radioimmunoprecipitation assay buffer (RIPA) Buffer: 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid disodium salt dehydrate (Na2EDTA), 1 mM ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), 1% nonyl phenoxypolyethoxylethanol (NP)-40, 1% sodium deoxycholate, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, and 1 µg/mL leupeptin]. A total of 70 µg of caspase 3, cleaved caspase 3, and Beclin-1 proteins were separated using electrophoresis by using a 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene fluoride (PVDF) membrane. After the membranes were blocked with buffer [TBS-T buffer: 20 mM Tris (pH 7.5), 150 mM NaCl, and 0.1% Tween 20] containing 5% skim milk, the membranes were incubated with the primary antibodies. After washing, the membranes were incubated with the secondary antibody. Then, the membranes were washed with TBS-T buffer and developed using an enhanced chemiluminescence (ECL) Western blot assay detection reagent (GE Healthcare, Little Chalfont, UK). The intensity of each band was quantified using a ChemiDoc™ XRS + System with Image Lab™ Software (Bio-Rad, Hercules). A Western blotting assay was performed more than 3 times to investigate the apoptosis and autophagy markers in order to assess the reproducibility, and thus, the reliability of the results.
The Institutional Review Board of Keimyung University Dongsan Hospital approved this study (NON2024-002).
2.3. Statistical analysis
Experiments were repeated at least 3 times to obtain consistent results. One-way analysis of variance (ANOVA) was performed. Shapiro–Wilk statistics were used to verify the normal distribution, and Levene statistics were used to analyze the homogeneity of variance. Tukey’s test was performed for post hoc multiple comparisons. Student t test was performed and the data were expressed as the mean ± standard deviation. In the non-normal distribution, the Kruskal–Wallis test was performed. The Statistical Package for the Social Science (SPSS) version 18.0 (SPSS Inc., Chicago) was used. The results were statistically significant at a P value lower than .05.
3. Results
3.1. Screening of the ideal concentration of antibiotics that assures MDCK cell viability in normoxia and hypoxia conditions
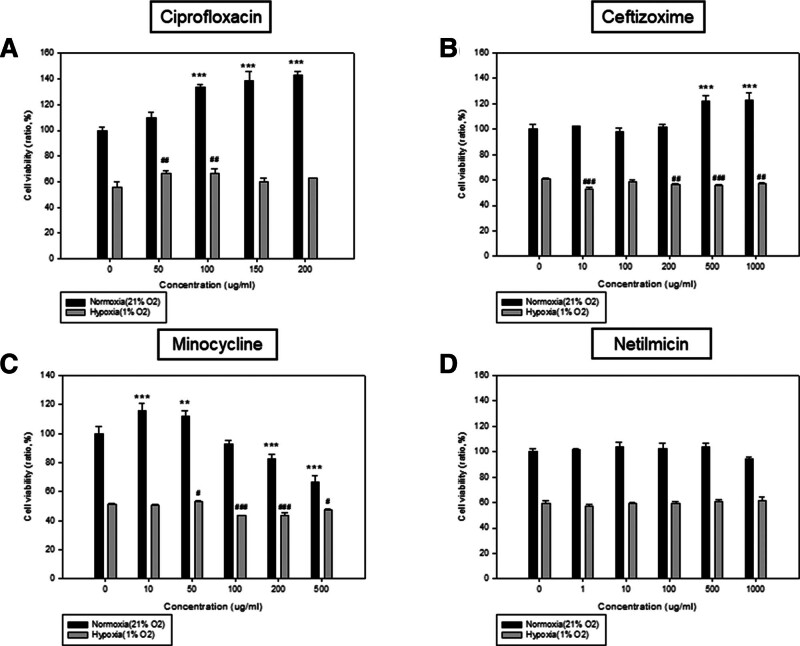
Ciprofloxacin, ceftizoxime, minocycline, and netilmicin were used for the screening of the concentration of antibiotics that assures the cell viability of MDCK cells after 48 hours of treatment using a 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay. Under normoxia (21% O2) conditions, ciprofloxacin showed a consistent increase in cell viability since the beginning of ciprofloxacin induction, compared with the control group, especially at a concentration higher than 100 µg/mL (P < .001). However, cell viability was mildly increased with the increase in the concentration (50–100 µg/mL) of ciprofloxacin, in comparison with the control group under hypoxia conditions (1% O2; Fig. 1A). This result indicates that ciprofloxacin can improve cell viability under both normoxia and hypoxia conditions. The maximal efficacies (cell viability of ciprofloxacin vs control) of ciprofloxacin were 42.6% at 200 µg/mL under normoxia conditions, and 10.1% at 100 µg/mL under hypoxia conditions. Under normoxia conditions, ceftizoxime also showed an increase in cell viability after the introduction of ceftizoxime at a high concentration (500–1000 µg/mL). However, there were no changes in cell viability with increasing concentrations of ceftizoxime under hypoxia conditions (Fig. 1B). Under normoxia and hypoxia conditions, minocycline (10 and 50 µg/mL) resulted in an increase in cell viability. However, cell viability rapidly decreased when the concentration of minocycline surpassed 50 µg/mL in normoxia and hypoxia conditions (Fig. 1C). Under normoxia and hypoxia conditions, the concentration of netilmicin was not correlated with cell viability (Fig. 1D).
Figure 1.
The relationship between the concentration of (A) ciprofloxacin, (B) ceftizoxime, (C) minocycline, and (D) netilmicin and cell viability. MDCK cells were plated in DMEM with glucose (1 g/L) and FBS (10%) at a density of 7 × 104 per well in 48-well plates under normoxia condition (21% O2). After 48 hours of incubation, the medium was exchanged with DMEM without glucose or FBS. After antibiotics were added at appropriate concentrations, respectively, the cells were incubated further under normoxia condition (21% O2) and hypoxia condition (1% O2). Concentrations of antibiotics added were as follows; (A) Ciprofloxacin (0, 50, 100, 150, and 200 µg/mL); (B) Ceftizoxime (0, 10, 100, 200, 500, and 1000 µg/mL); (C) Minocycline (0, 10, 50, 100, 200, and 500 µg/mL); (D) Netilmicin (0, 1, 10, 100, 500, and 1000 µg/mL). Cell viability was assessed through MTT assay at 48 hours of antibiotics treatment. The experiment at ciprofloxacin concentration was performed more than 3 times (Normoxia vs *** P < .001; Hypoxia vs ## P < .01). DMEM = Dulbecco’s Modified Eagle’s Medium, FBS = fetal bovine serum, MDCK = Madin-Darby canine kidney, MTT = 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyl-2H-tetrazolium bromide.
Under normoxia conditions, ciprofloxacin showed the highest efficacy (30–45%) in comparison with other antibiotics. Minocycline had a 20% efficacy at a lower concentration, but the toxic effect increased as the concentration of minocycline was increased (Table 1). Based on these results, we chose ciprofloxacin to examine further, an antibiotic with a similar efficacy but a lower toxicity compared to other antibiotics.
Table 1.
Efficacies of antibiotics on cell viability between normoxia and hypoxia.
| Spectrum | Name | Efficacy | |||
|---|---|---|---|---|---|
| Normoxia (21% O2) | Hypoxia (1% O2) | ||||
| Con. (µg/mL) | Efficacy (%) | Con. (µg/mL) | Efficacy (%) | ||
| Quinolones | Ciprofloxacin | 50 | 10.00 ± 2.36 | 50 | 9.27 ± 2.26** |
| 100 | 33.49 ± 1.22*** | 100 | 10.07 ± 2.91** | ||
| 150 | 38.87 ± 3.99*** | ||||
| 200 | 42.62 ± 1.71*** | ||||
| Cephalosporins | Ceftizoxime | 500 | 21.77 ± 2.19*** | No effect | |
| 1000 | 22.61 ± 2.93*** | ||||
| Tetracyclines | Minocycline | 10 | 15.98 ± 2.48*** | 50 | 4.42 ± 0.3* |
| 50 | 12.13 ± 1.91** | ||||
| Aminoglycosides | Netilmicin | No effect | No effect | ||
Efficacies of 4 different antibiotics on improving cell viability under normoxic (21% O2) and hypoxic (1% O2) conditions at 48 hours of incubation with each antibiotic are presented. “Efficacy (%)” indicates a percentage for cell viability of group by treated antibiotics compared to the control group.
P < .05.
P < .01.
P < .001.
3.2. Effect of ciprofloxacin on apoptosis under normoxia conditions
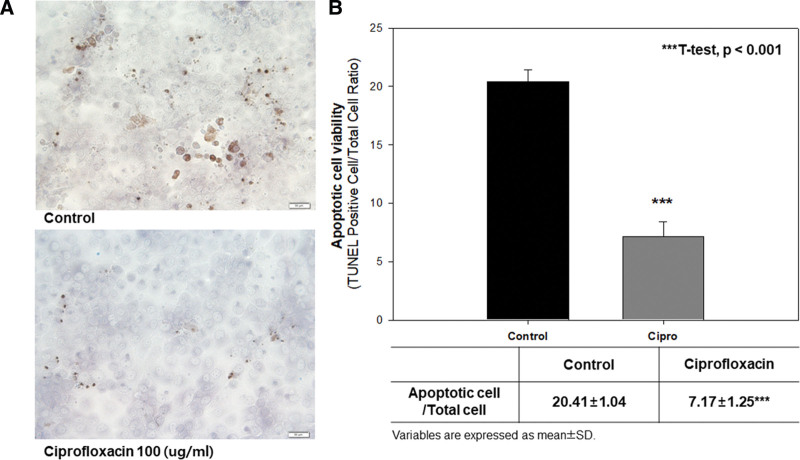
We first investigated the effect of TUNEL assay to determine whether ciprofloxacin improved cell viability by inhibiting apoptosis. We carried out the experiments using a 100 µg/mL concentration under normoxia conditions because ciprofloxacin improved cell viability under both normoxia and hypoxia conditions at this concentration, but its efficacy was more prominent under normoxia conditions. In the TUNEL assay, there were no apoptotic bodies at a concentration of 100 µg/mL in comparison with the control group (Fig. 2A). The ratio of apoptotic cells to total cells was significantly lower in the ciprofloxacin group than in the control group (7.17 ± 1.25 vs 20.41 ± 1.04, P < .001; Fig. 2B). Therefore, the efficacy of ciprofloxacin on the viability of MDCK cells was significantly higher in comparison with the control group under normoxia conditions by inhibiting apoptosis.
Figure 2.
(A) The qualitative and (B) quantitative comparison of the difference of apoptotic bodies between in the control group and ciprofloxacin group. ApopTag® Peroxidase In Situ Apoptosis Detection Kit was used. A concentration of 100 µg/mL of ciprofloxacin was used in comparison with control group. The examination was done under normoxia condition (21% O2). In the TUNEL assay, apoptotic cells from nicks of DNA strand breaks by labeling DNA strand breaks with fluorochromes were identified and quantified by fluorescence microscopy. The ratio of TUNEL positive cell to total cells was calculated for apoptotic cell viability. All data were performed more than 3 times in order to assess reproducibility and reliability of the results. Student t test was used statistically; ***t test, P < .001 vs control. TUNEL = transferase-mediated d-UTP nick end labeling.
3.3. The efficacy of ciprofloxacin on cell viability under stressful conditions via the interaction between autophagy and apoptosis
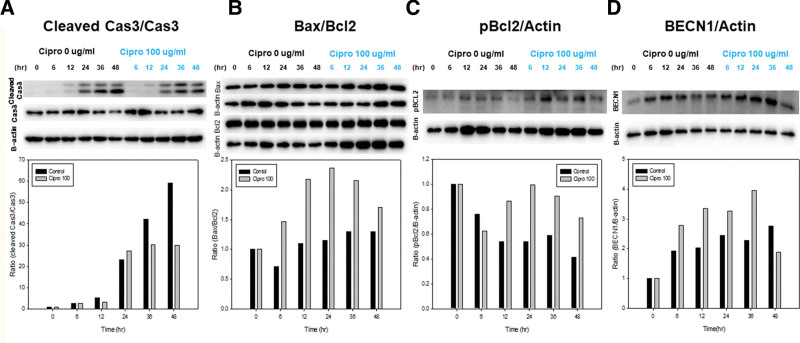
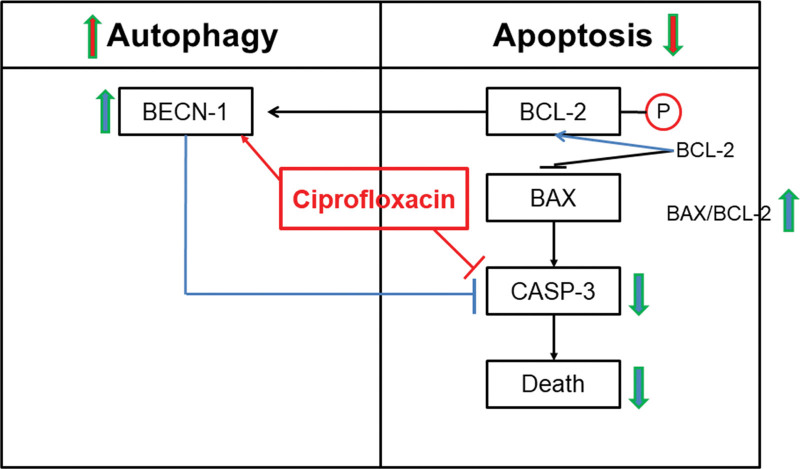
To elucidate underlying mechanisms further, we investigated apoptosis and autophagy markers. First, the upstream production of caspase 3, which causes apoptosis, was investigated. Cleaved caspase-3 to caspase-3, which is the final effector caspase in apoptosis, was lower in the ciprofloxacin group than in the control group after 36 hours at the beginning of treatment (Fig. 3A). Second, the ratio of Bax to Bcl-2 was investigated located upstream of caspase 3. The ratio of Bax to Bcl-2 was higher in the ciprofloxacin group than in the control group 6 hours after the beginning of the treatment (Fig. 3B). Third, the ratio of pBcl2 (phosphorylated Bcl-2, which is an inactive form of Bcl-2) to β-actin was higher in the ciprofloxacin group than in the control group 12 hours after the beginning of treatment (Fig. 3C). The results of caspase-3 experiments favor anti-apoptosis whereas Bcl-2 experiments favor apoptosis. To accommodate apparent contradictory results, we assumed that ciprofloxacin may also affect autophagy. The ratio of Beclin-1, an autophagy marker, to β-actin increased with time and was higher in the ciprofloxacin group at all times, indicating that ciprofloxacin increases autophagy (Fig. 3D). Finally, this indicates that ciprofloxacin enhanced autophagy, increasing the amount of free Beclin-1 via phosphorylated Bcl-2, and inhibited caspase activity (Fig. 4).
Figure 3.
The comparison of (A) cleaved caspase 3/caspase 3, (B) Bax/Bcl2, (C) pBcl2/β-actin, and (D) BECN1/β-actin between in the control group and ciprofloxacin group. A concentration of 100 µg/mL of ciprofloxacin was used in comparison with control group. β-actin was used for the control. The examination was done under normoxia condition (21% O2). Proteins were detected by western blot analysis. Cell viability was checked at 0, 6, 12, 24, and 48 hours after treatments. All data were performed more than 3 times in order to assess reproducibility and reliability of the results. Bax = Bcl-2-associated X protein, B-cell lymphoma 2.
Figure 4.
Overview of the molecular mechanisms of underlying the autophagy-apoptosis crosstalk by ciprofloxacin. This figure shows the relationship between autophagy and apoptosis for the effect of ciprofloxacin. The ratio of cleaved caspase 3 to caspase 3 (cleaved caspase 3/caspase 3) was decreased, but the ratio of Bax to Bcl-2 (Bas/Bxl-2) upstream of caspase 3 was not decreased in the ciprofloxacin group. Conversely, the levels of phosphorylated Bcl-2 and Beclin-1 were increased, and that of caspase-3 was decreased in the ciprofloxacin group. Bax = Bcl-2-associated X protein, B-cell lymphoma 2.
4. Discussion
In our study, we showed that ciprofloxacin protected against the injury of MDCK cells by inhibiting apoptosis through an autophagy mechanism. Although all cells eventually undergo apoptosis, it was noteworthy that ciprofloxacin could protect cell injury in the process of apoptotic cell death.
When energy is lacking, the organism tries to save cells by recycling dead cells through autophagy activation. At this time, it is not yet clear whether autophagy is being promoted or whether apoptosis is being suppressed. Apoptosis refers to programmed cell death, which can be observed through DNA breakage.[1] In our study, the TUNEL assay demonstrated a status of apoptosis. Recently, several antibiotics have been reported to control cell viability. Therefore, for the first time, we screened using an MTT assay whether cell viability increased when antibiotics representing each class were administered. We examined 4 antibiotics: ciprofloxacin, a quinolone, ceftizoxime, a cephalosporin, minocycline, a tetracycline, and netilmicin, an aminoglycoside. Ceftizoxime was effective only at high concentrations under normoxia conditions, but not under hypoxia conditions. Netilmicin was not effective under normoxia or hypoxia conditions. Minocycline was effective at lower concentrations but deteriorated cell viability at higher concentrations under both normoxia and hypoxia conditions. Therefore, we used the most effective and safe antibiotic, ciprofloxacin, for further studies.
The first experiment was performed to investigate whether ciprofloxacin improved cell viability by inhibiting apoptosis using the TUNEL assay. The results showed that apoptosis was reduced qualitatively and quantitatively when ciprofloxacin was added under the normoxia conditions. Therefore, ciprofloxacin saves cells by inhibiting apoptosis.
The second experiment was performed to investigate the mechanism of increased ciprofloxacin-induced cell viability. In previous studies, apoptosis has been reported to be mainly responsible for programmed cell death,[19] but autophagy has also been reported to play important roles.[2] In addition, although apoptosis and autophagy have been considered as different concepts, many studies have shown that the homeostasis of cells is maintained through the interaction between these 2 molecular mechanisms.[18,20] Firstly, we investigated apoptosis mechanism with the relationship among caspase-3, Bax, and Bcl-2. In general, when Bax/Bcl-2 decreases and caspase-3 decreases, apoptosis is suppressed. Our study showed reduction of the caspase-3 level in the ciprofloxacin-treated group, and this result means that the inhibition of apoptosis occurs inhibiting caspase-3 activity by ciprofloxacin. However, conversely, ciprofloxacin-treated cells showed an increase in Bax/Bcl-2 level in this study. This may not be explained by the fact that the whole process is caused by apoptosis because this means that an increased Bax/Bcl-2 level caused more apoptosis, which leads to conflicting results (Figs. 2 and 3B). Therefore, we investigated the influence of ciprofloxacin on autophagy in an energy-insufficient environment. Since Beclin-1 is inhibited by Bcl-2, when Bcl-2 is phosphorylated and inactivated, Beclin-1 and Bcl-2 are separated from each other and an increased level of free Beclin-1 becomes operational, resulting in an increase in pBcl-2. This means that the inhibition of autophagy induced by Bcl-2 is reduced, which can lead to a better progression of autophagy. In other words, an increase in the amount of free Beclin-1 promotes autophagy.[21] First, the level of Beclin-1 increases steeply. Second, when the level of unbounded pBcl-2 is increased, the level of free Beclin-1 is increased due to a decrease in the binding to Beclin-1. Third, as Bax increases, more Bcl-2 and more bonds can be obtained, and the amount of free Beclin-1 can be increased. Finally, caspase-3 cleaves and deactivates Beclin-1, and the decreasing level of caspase-3 increases the level of free Beclin-1. Finally, in our study, ciprofloxacin enhanced autophagy, increasing the level of free Beclin-1, and inhibited caspase activity and reduced cell death due to the energy produced during autophagy as summarized in Figure 4. To survive in the absence of energy, cells die to generate energy sources for the living cells. In other words, when the energy source is insufficient, apoptosis is promoted upstream, but at the same time, autophagy is promoted to decompose the unnecessary proteins in the cell and use them as energy sources. Accordingly, the activity of caspase-3 is inhibited, thereby suppressing apoptosis.
The strength of this study is that it has shown a positive effect on cell viability using antibiotics, and this is the first study to apply the concept of autophagy and apoptosis. In previous studies, minocycline had potent anti-apoptotic and anti-inflammatory effects and protected renal function in a rat model of ischemia-reperfusion injury.[8,13,14] Because minocycline showed toxic effects at high concentrations in the MDCK cell line in our study, we could explain the effect of ciprofloxacin, which is safer than other antibiotics for the action of autophagy, on cell viability. Recently, these concepts of autophagy have been applied in several clinical fields, such as acute kidney injury,[22] chronic kidney disease (obstructive nephropathy, immunoglobulin A nephropathy, autoimmune kidney diseases),[23] diabetic nephropathy,[24] autosomal dominant polycystic kidney disease,[25] cystinosis,[26] and chronic cyclosporine A renal toxicity.[27] Our findings may have a positive impact on acute kidney injury and kidney diseases associated with chronic inflammation.
However, our study has some limitations. First, the order of apoptosis and the autophagy process is still unclear. Autophagy is known to precede apoptosis.[28] However, our study did not show the preceding autophagy process, although the apoptosis and autophagy markers were checked at 0, 6, 12, 24, 36, and 48 hours. Second, in our study, research on other markers of autophagy and apoptosis was insufficient, and further studies are needed. Third, a slightly stronger stimulus leads directly to apoptosis without the action of autophagy. Although nutrient depletion is the most powerful factor in inducing autophagy, the combined metabolic stress of hypoxia and nutrient depletion damages organs, proteins, and DNA, which ultimately leads to apoptosis. Recent studies have shown that autophagy mitigates metabolic stress to protect cells against extreme cell damage.[4] Autophagy is not only necessary as a tool to generate energy in a nutrient-free environment, but it also plays an important role in regulating protein and organ function and maintaining homeostasis. It is also important in situations of metabolic stress, that is, when energy is limited and intracellular damage is accelerated. However, it is important to recognize that autophagy cannot inhibit apoptosis completely in conditions in which apoptosis is induced. In other words, when cells are exposed to stresses, autophagy takes place temporally, but it cannot completely prevent the progression to apoptosis. Finally, extreme environments with no nutrient supply and no oxygen are unlikely to reveal the mechanisms by which antibiotics affect cell viability and are most likely to be biased by apoptosis processes. In the future, it will be necessary to study the difference between normoxia and hypoxia conditions in a nutrient-free environment.
In conclusion, the effect of ciprofloxacin on renal tubular injury under nutrient-free conditions was associated with autophagy. Therefore, ciprofloxacin might be helpful in protection against renal tubular injury through activation of autophagy in the setting of acute kidney injury. Further studies are needed to confirm the mechanism by which ciprofloxacin affects cell viability.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (Ministry of Science and ICT, MSIT; 2021R1F1A1061572).
Author contributions
Conceptualization: Ki Sung Ahn, Jongwon Lee.
Data curation: Sun-Ha Lim, Yaerim Kim, Jin Hyuk Paek, Kyubok Jin, Seungyeup Han.
Formal analysis: Sun-Ha Lim.
Funding acquisition: Woo Yeong Park.
Investigation: Woo Yeong Park.
Supervision: Ki Sung Ahn, Jongwon Lee.
Writing – original draft: Woo Yeong Park.
Writing – review & editing: Woo Yeong Park.
Abbreviations:
- Bax
- Bcl2-associated X protein
- Bcl-2
- B-cell lymphoma 2
- MDCK
- Madin-Darby canine kidney
- TUNEL
- transferase-mediated d-UTP nick end labeling
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (Ministry of Science and ICT, MSIT) (2021R1F1A1061572).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
How to cite this article: Park WY, Lim S-H, Kim Y, Paek JH, Jin K, Han S, Ahn KS, Lee J. Impact of ciprofloxacin with autophagy on renal tubular injury. Medicine 2024;103:40(e39888).
Contributor Information
Sun-Ha Lim, Email: sunha1977@hanmail.net.
Yaerim Kim, Email: yaerim86@gmail.com.
Jin Hyuk Paek, Email: novawang@naver.com.
Kyubok Jin, Email: mdjin922@gmail.com.
Seungyeup Han, Email: hansy@dsmc.or.kr.
Ki Sung Ahn, Email: ksahn211@gmail.com.
Jongwon Lee, Email: leejw@cu.ac.kr.
References
- [1].Fuchs Y, Steller H. Programmed cell death in animal development and disease. Cell. 2011;147:742–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Eisenberg-Lerner A, Bialik S, Simon HU, Kimchi A. Life and death partners: apoptosis, autophagy and the cross-talk between them. Cell Death Differ. 2009;16:966–75. [DOI] [PubMed] [Google Scholar]
- [3].Gump JM, Thorburn A. Autophagy and apoptosis: what is the connection? Trends Cell Biol. 2011;21:387–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Havasi A, Dong Z. Autophagy and tubular cell death in the kidney. Semin Nephrol. 2016;36:174–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Han SJ, Lee HT. Mechanisms and therapeutic targets of ischemic acute kidney injury. Kidney Res Clin Pract. 2019;38:427–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bbosa GS, Mwebaza N, Odda J, Kyegombe DB, Ntale MJH. Antibiotics/antibacterial drug use, their marketing and promotion during the post-antibiotic golden age and their role in emergence of bacterial resistance. Health (N Y). 2014;06:410–25. [Google Scholar]
- [7].Tang C, Yang L, Jiang X, et al. Antibiotic drug tigecycline inhibited cell proliferation and induced autophagy in gastric cancer cells. Biochem Biophys Res Commun. 2014;446:105–12. [DOI] [PubMed] [Google Scholar]
- [8].Sutton TA, Kelly KJ, Mang HE, Plotkin Z, Sandoval RM, Dagher PC. Minocycline reduces renal microvascular leakage in a rat model of ischemic renal injury. Am J Physiol Renal Physiol. 2005;288:F91–7. [DOI] [PubMed] [Google Scholar]
- [9].Arthur JM. The MDCK cell line is made up of populations of cells with diverse resistive and transport properties. Tissue Cell. 2000;32:446–50. [DOI] [PubMed] [Google Scholar]
- [10].Dukes JD, Whitley P, Chalmers AD. The MDCK variety pack: choosing the right strain. BMC Cell Biol. 2011;12:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fukumoto R, Cary LH, Gorbunov NV, Lombardini ED, Elliott TB, Kiang JG. Ciprofloxacin modulates cytokine/chemokine profile in serum, improves bone marrow repopulation, and limits apoptosis and autophagy in ileum after whole body ionizing irradiation combined with skin-wound trauma. PLoS One. 2013;8:e58389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Park CH, Lee J, Jung HY, et al. Identification, biological activity, and mechanism of the anti-ischemic quinolone analog. Bioorg Med Chem. 2007;15:6517–26. [DOI] [PubMed] [Google Scholar]
- [13].Wu Z, Zou X, Zhu W, Mao Y, Chen L, Zhao F. Minocycline is effective in intracerebral hemorrhage by inhibition of apoptosis and autophagy. J Neurol Sci. 2016;371:88–95. [DOI] [PubMed] [Google Scholar]
- [14].Yuan H, Zhang X, Zheng W, Zhou H, Zhang BY, Zhao D. Minocycline attenuates kidney injury in a rat model of streptozotocin-induced diabetic nephropathy. Biol Pharm Bull. 2016;39:1231–7. [DOI] [PubMed] [Google Scholar]
- [15].Wu D, Yotnda P. Induction and testing of hypoxia in cell culture. J Vis Exp. 2011;54:2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kaur B, Miglioranza Scavuzzi B, Abcouwer SF, Zacks DN. A simplified protocol to induce hypoxia in a standard incubator: a focus on retinal cells. Exp Eye Res. 2023;236:109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].van Meerloo J, Kaspers GJ, Cloos J. Cell sensitivity assays: the MTT assay. Methods Mol Biol. 2011;731:237–45. [DOI] [PubMed] [Google Scholar]
- [18].Kim JH, Kim KM, Jeong JU, Shin JH, Shin JM, Bang KT. Nrf2-Heme oxygenase-1 modulates autophagy and inhibits apoptosis triggered by elevated glucose levels in renal tubule cells. Kidney Res Clin Pract. 2019;38:318–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Galluzzi L, Vitale I, Abrams JM, et al. Molecular definitions of cell death subroutines: recommendations of the nomenclature committee on cell death 2012. Cell Death Differ. 2012;19:107–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rubinstein AD, Kimchi A. Life in the balance - a mechanistic view of the crosstalk between autophagy and apoptosis. J Cell Sci. 2012;125:5259–68. [DOI] [PubMed] [Google Scholar]
- [21].Gordy C, He YW. The crosstalk between autophagy and apoptosis: where does this lead? Protein Cell. 2012;3:17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kaushal GP, Shah SV. Autophagy in acute kidney injury. Kidney Int. 2016;89:779–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Liu N, Shi Y, Zhuang S. Autophagy in chronic kidney diseases. Kidney Dis (Basel). 2016;2:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kume S, Koya D. Autophagy: a novel therapeutic target for diabetic nephropathy. Diabetes Metab J. 2015;39:451–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ravichandran K, Edelstein CL. Polycystic kidney disease: a case of suppressed autophagy? Semin Nephrol. 2014;34:27–33. [DOI] [PubMed] [Google Scholar]
- [26].Gahl WA, Thoene JG, Schneider JA. Cystinosis. N Engl J Med. 2002;347:111–21. [DOI] [PubMed] [Google Scholar]
- [27].Kim HS, Choi SI, Jeung EB, Yoo YM. Cyclosporine A induces apoptotic and autophagic cell death in rat pituitary GH3 cells. PLoS One. 2014;9:e108981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Marino G, Niso-Santano M, Baehrecke EH, Kroemer G. Self-consumption:the interplay of autophagy and apoptosis. Nat Rev Mol Cell Biol. 2014;15:81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]