Abstract
This study aimed to examine the association between constipation and mild cognitive impairment (MCI); and further elucidate the possible mechanisms involved. A cross-sectional study was conducted among community-dwelling elders (N = 789) in Nanning, China. Trained research staffs collected detailed information through questionnaires and physical examinations. A Bayesian network model was used to explore the hypothesized causal path. Synergistic effects of constipation with infrequent fruit consumption, inactive physical exercise, or history of stroke were observed in the risks of MCI occurrence. The Bayesian network model analyses showed 3 hypothesized causal-association paths leading to MCI occurrence. Among these, constipation, history of stroke, and years of schooling were directly related to the occurrence of MCI. Years of schooling indirectly affected MCI through infrequent fruit consumption and constipation; or through inactive physical exercises and history of stroke. This study demonstrates a direct association between constipation and increased risks of MCI.
Keywords: cognitive function, cognitive impairment, fruit consumption, older adults, stroke, years of schooling
1. Introduction
With the acceleration of world’s population aging, the number of people living with dementia is rapidly growing from 55 million in 2019 to 139 million in 2050 worldwide, according to the latest World Health Organization global status report.[1] Alzheimer disease (AD), the most common form of dementia, is a progressive neurodegenerative disease that causes memory and cognitive decline, accompanied by psychiatric symptoms and behavioral disorders.[2] So far, there is few clinically-approved effective drug to treat AD (e.g. donepezil, rivastigmine, galantamine, memantine, and memantine combined with donepezil). Mild cognitive impairment (MCI), as an intermediate stage of cognitive impairment between physiological cognitive aging and pathological dementia, does not significantly affect daily life.[3,4] A large number of population-based epidemiological investigations have reported that the prevalence of MCI ranged from 3% to 19% among people aged 65 and above.[4,5] Around 30% to 50% of older adults with MCI progress to dementia within 5 years, compared with 3% of those without MCI at the same age.[4–6] But some persons with MCI can remain stable or return to normal over time. That is why MCI can be regarded as a transition stage before the onset of dementia. However, it is yet to be validated whether MCI is a critical stage to prevent or delay the development of dementia by adopting the beneficial factors and/or controlling risk factors of MCI.
Constipation is a common health problem, especially among middle-aged and older people. Previous studies have shown that constipation is closely related to Parkinson disease (PD).[7,8] Constipation can precede clinical motor symptoms of PD by many years.[9] However, only a few studies associated constipation with cognitive function and cognitive diseases. Our previous study in 2020 first reported that infrequent bowel movement was associated with increased risk of MCI in community-living Singaporean older adults in a cross-sectional study.[10] Later in the year of 2022, 3 studies consecutively reported the correlations of constipation with MCI, dementia or AD.[11–13] Although these studies established the positive associations between constipation and cognitive declines, it is yet unclear how constipation promotes cognitive declines during aging. Accumulating evidence highlights the link between constipation and alteration of gut microbiome as a causative factor of mental diseases. Alterations in the structure and functions of gut microbiota may lead to the changes in brain function and behavior, a phenomenon so called microbe–gut–brain axis. This axis may provide a novel research angle to understand the development of mental diseases.
Therefore in the present study, we first determined the correlation between constipation and MCI in a cross-sectional study of adults aged 60 or older in Nanning, China. The second aim was to dissect how constipation affects MCI by analyzing the structure of the hypothesized causal associations and the inter-plays between constipation and other influencing factors of MCI.
2. Materials and methods
2.1. Participants and criteria
This population-based cross-sectional study recruited community-living residents (aged ≥ 60) in Nanning, the capital city of Guangxi province in China using a convenience sampling design in 2021. Well-trained research staffs conducted semi-structured interviews with participants and their caregivers in community health service centers or at their residence. For some contents that cannot accurately remember or report, we asked the older adults’ family members to help them remember or provide accurate answers. For disease history, medication history, and health history, we obtained accurate information by reviewing the older adults’ medical records and health files. The inclusive criteria for this study were community residents who were: (1) aged 60 years or older, (2) local resident for at least 1 year, (3) voluntary and cooperative for the survey. The exclusion criteria were those who: (1) were unable to conduct meaningful face-to-face interviews or complete all questionnaires and evaluations, (2) had been diagnosed with dementia or other major mental diseases (including PD, any acute phase of brain infectious diseases). Finally, a total of 789 participants were included in the study (Table 1). The response rate was 88.1%.
Table 1.
Constipation and some socio-demographic factors were associated with MCI.
| Variables | Control N = 658 n (%) |
MCI N = 131 n (%) |
F/χ2 | P |
|---|---|---|---|---|
| Gender | 5.849 | .016 | ||
| Male | 286 (43.5) | 42 (32.1) | ||
| Female | 372 (56.5) | 89 (67.9) | ||
| Age (mean ± SD) | 69.9 ± 6.8 | 69.6 ± 7.4 | 0.199 | .655 |
| Years of schooling (mean ± SD) | 7.9 ± 4.1 | 6.0 ± 4.6 | 20.993 | <.001 |
| Constipation | 15.677 | <.001 | ||
| Yes | 97 (14.7) | 38 (29.0) | ||
| No | 561 (85.3) | 93 (71.0) | ||
| Ethnic* | 5.052 | .080 | ||
| Han | 419 (63.8) | 70 (53.5) | ||
| Zhuang | 221 (33.6) | 56 (42.7) | ||
| Others | 17 (2.6) | 5 (3.8) | ||
| Marital status | 9.594 | .002 | ||
| Married/cohabitation | 559 (85.0) | 97 (74.0) | ||
| Divorced/widowed/single | 99 (15.0) | 34 (26.0) | ||
| Work status | 0.044 | .834 | ||
| Still working | 33 (5.0) | 6 (4.6) | ||
| Not working | 625 (95.0) | 125 (95.4) | ||
| Annual family income* | 4.572 | .102 | ||
| <30,000 RMB | 308 (46.9) | 74 (56.5) | ||
| 30,000–60,000 RMB | 215 (32.7) | 32 (24.4) | ||
| >60,000 RMB | 134 (20.4) | 25 (19.1) | ||
| Co-living situation | 0.039 | .843 | ||
| Live alone | 47 (7.1) | 10 (7.6) | ||
| Not live alone | 611 (92.9) | 121 (92.4) |
MCI = mild cognitive impairment, SD = standard deviation.
Due to the missing values in some variables, the total numbers may not equal to 658 or 131.
2.2. Variables
Well-trained research staffs collected detailed information through questionnaire survey, including socio-demographic characteristics (age, gender, race, profession, degree of education, annual household income, marital status, and co-living situation), lifestyle and behavior (drinking, smoking, physical exercise, social activities, daytime outdoor activities, mental activities, indoor activities, sleep quality, and post-lunch nap status), medical comorbidities, and dietary habits, as described before.[10]
2.3. Neurocognitive assessment and constipation diagnosis
Cognition function was assessed using the Chinese version of Mini-Mental State Examination (MMSE). The total scores of the MMSE ranged from 0 to 30, with higher scores demonstrating better cognitive function.[10] When the MMSE total scores were less than or equal to the cutoff (17 for illiterate participants, 20 for those with primary school education level, and 24 for individuals with secondary school and above),[14] a further assessment were carried out, including Activity of Daily Living Scale[15] for social functioning assessment, Clinical Dementia Rating[16] for severity of dementia and cognitive decline assessment. The diagnosis of MCI was made via the expert panel consisting of 2 neurologists and neuropsychologists, and other clinical evaluation specialists with expertise in cognitive impairment, according to the diagnostic criteria proposed by Peterson.[17]
Constipation was assessed using a questionnaire according to the Rome Criteria IV. Criteria fulfilled for the last 3 months with symptom onset at least 6 months prior to diagnosis: (1) must include 2 or more of the following: I. straining during more than one-fourth (25%) of defecation; II. lumpy or hard stools (BSFS 1–2) more than one-fourth (25%) of defecation; III. sensation of incomplete evacuation more than one-fourth (25%) of defecation; IV. sensation of anorectal obstruction/blockage more than one-fourth (25%) of defecation; V. manual maneuvers to facilitate more than one-fourth (25%) of defecation (e.g., digital evacuation, support of the pelvic floor); VI. fewer than 3 spontaneous bowel movements per week. (2) Loose stools are rarely present without the use of laxatives. (3) Insufficient criteria for irritable bowel syndrome with constipation.[18]
2.4. Statistical analyses
Categorical variables were described by numbers and frequency, while continuous variables were described by mean and standard deviation. The data were analyzed using the Statistical Package for the Social Sciences 22.0 (IBM Corp., Armonk, NY). The chi-square tests were used to determine significant differences for categorical variables, while one-way analysis of variance were used to evaluate between-group differences for the continuous variables. A two-sided P-value <.05 was considered statistically significant.
To further determine the associations between constipation and MCI occurrence, multivariable logistic regression models was performed. The models were adjusted for gender, age, marital status, work status, smoking, and drinking. Those factors with statistical differences P < .1 under the chi-square test and one-way analysis of variance were included in the multivariable logistic regression models. After identifying the important influence factors of MCI, we explored interactions between constipation with these important influencing factors using the Statistical Package for the Social Sciences 22.0. Lastly, a Bayesian network model was used to reveal the hypothesized causal path between MCI occurrence and the significant factors, as described previously.[10]
After identifying the significant factors underlying the status of MCI, a Bayesian network model, structural equation model, and directed acyclic graph approach were integrated to reveal the structure of hypothesized causal associations between significant factors and MCI. Meanwhile, the probability inferences of the Bayesian network for the influencing factors of MCI were calculated, and the importance of each significant factor was quantitatively measured based on the Tree Augmented Naïve Bayesian network learning. The Bayesian network model was fitted with the bnlearn package via R software 3.5, and the Bayesian network-based causal relationships between exposure variables and MCI were visualized using the R package “dagitty.”[10]
2.5. Ethical approval
The study was approved by the Medical Ethics Committee of Guangxi Medical University (protocol number 20210132). All methods were carried out in accordance with relevant guidelines and regulations. Informed consent was obtained from all participants prior to their participation.
3. Results
3.1. Constipation and year of schooling were associated with MCI
Among the participated 789 older adults, 131 (16.6%) were diagnosed with MCI (MCI group) and 658 (83.4%) with normal cognitive function (control group) by the expert panels (Table 1). The MCI older adults had higher proportions of females (67.9% vs 56.5%, P = .016) and constipation (29.0% vs 14.7%, P < .001) but shorter years of schooling (6.0 ± 4.6 vs 7.9 ± 4.1 years, P < .001) than the control peers. Although few older adults (7.6% and 7.1%) lived alone, a higher proportion of older adults in the MCI group got divorced/widowed or kept single, compared with those in the control group (25.0% vs 15.0%, P = .002). Besides, there were no statistical differences between the 2 groups in age, ethnicity, current work status, and annual family income (all P > .05).
3.2. Other statistically important influencing factors of MCI
As shown in Table 2 of medical comorbidities, the proportions of history of stroke (13.0% vs 4.9%, P < .001), history of head trauma (3.1% vs 0.3%, P = .006) are statistically higher while hypertension (39.7% vs 31.2%, P = .057) tend to be higher in the MCI group than those in the control group, respectively. In contrast, other medical comorbidities did not differ in the 2 groups (Table S1, Supplemental Digital Content, http://links.lww.com/MD/N670). Among the common serum variables (Table 2 and Table S2, Supplemental Digital Content, http://links.lww.com/MD/N670), the concentrations of aspartate aminotransferase (23.58 ± 10.37 vs 21.89 ± 8.43 U/L, P = .045), and low density lipoprotein cholesterol (LDL-C) (3.23 ± 0.89 vs 2.74 ± 0.82 mmol/L, P < .001) were higher, but creatinine (86.29 ± 23.83 vs 80.52 ± 22.85 μmol/L, P = .011) lower, in the MCI group than in the control group. Furthermore among all the surveyed lifestyle factors (Table 2 and Table S3, Supplemental Digital Content, http://links.lww.com/MD/N670), more participants in the MCI group had infrequent (≤3 per week) physical exercise (48.9% vs 21.6%, P < .001), social activities (55.0% vs 39.7%, P = .001) or daytime outdoor activities (36.6% vs 19.9%, P < .001). In line of these inactive lifestyles, more participants in the MCI group had infrequent fruit consumption (≤3 days per week, 36.6% vs 22.2%, P < .001) (Table 2 and Table S4, Supplemental Digital Content, http://links.lww.com/MD/N670).
Table 2.
Other statistically important influential factors of MCI.
| Variables | Control N = 658 n (%) |
MCI N = 131 n (%) |
χ 2 | P |
|---|---|---|---|---|
| Hypertension | 3.628 | .057 | ||
| Yes | 205 (31.2) | 52 (39.7) | ||
| No | 453 (68.8) | 79 (60.3) | ||
| History of stroke | 12.348 | <.001 | ||
| Yes | 32 (4.9) | 17 (13.0) | ||
| No | 626 (95.1) | 114 (87.0) | ||
| History of head trauma | 7.604 | .006 | ||
| Yes | 2 (0.3) | 4 (3.1) | ||
| No | 656 (99.7) | 127 (96.9) | ||
| How often participate in physical exercise | 42.126 | <.001 | ||
| ≤3 per week | 142 (21.6) | 64 (48.9) | ||
| ≥4 per week | 516 (78.4) | 67 (51.1) | ||
| How often participate in social activities | 10.479 | .001 | ||
| ≤3 per week | 261 (39.7) | 72 (55.0) | ||
| ≥4 per week | 397 (60.3) | 59 (45.0) | ||
| How often participate in outdoor activities in daytime | 17.438 | <.001 | ||
| ≤3 per week | 131 (19.9) | 48 (36.6) | ||
| ≥4 per week | 527 (80.1) | 83 (63.4) | ||
| How often consume fruits | 12.307 | <.001 | ||
| ≤3 per week | 146 (22.2) | 48 (36.6) | ||
| ≥4 per week | 512 (77.8) | 83 (63.4) | ||
| Waistline (cm) (mean ± SD) | 85.56 ± 9.11 | 87.18 ± 9.22 | 3.473 | .063 |
| AST (U/L) (mean ± SD) | 21.89 ± 8.43 | 23.58 ± 10.37 | 4.038 | .045 |
| Creatinine (μmol/L) (mean ± SD) | 86.29 ± 23.83 | 80.52 ± 22.85 | 6.494 | .011 |
| LDL-C (mmol/L) (mean ± SD) | 2.74 ± 0.82 | 3.23 ± 0.89 | 38.205 | <.001 |
AST = aspartate aminotransferase, LDL-C = low density lipoprotein cholesterol, MCI = mild cognitive impairment, SD = standard deviation.
3.3. Constipation was independently associated with MCI by multivariate logistic regression analysis
It was found that more years of schooling was associated with decreased risk of MCI occurrence (adjusted odds ratios [AOR] = 0.929, 95% confidence interval [CI] 0.882–0.979, P = .006), after adjusting gender, age, marital status, work status, smoking, and drinking. In contrast, constipation (AOR = 1.937, 95% CI 1.178–3.186, P = .009), history of stroke (AOR = 2.719, 95% CI 1.307–5.654, P = .007), history of head trauma (AOR = 7.996, 95% CI 1.139–56.151, P = .037), inactive physical exercise (AOR = 2.641, 95% CI 1.698–4.108, P < .001), inactive social activities (AOR = 1.632, 95% CI 1.063–2.508, P = .025), infrequent fruits consumption (AOR = 1.692, 95% CI 1.067–2.685, P = .025), and LDL-C (AOR = 2.042, 95% CI 1.578–2.641, P < .001) were statistically associated with increased risks of MCI in older adults (Table 3).
Table 3.
Multivariate analysis for MCI influential factors.
| Variables | β | Wald | P | AOR | 95% CI |
|---|---|---|---|---|---|
| Constipation | 0.661 | 6.791 | .009 | 1.937 | 1.178–3.186 |
| Years of schooling | −0.073 | 7.516 | .006 | 0.929 | 0.882–0.979 |
| Inactive physical exercise | 0.971 | 18.573 | <.001 | 2.641 | 1.698–4.108 |
| Inactive social activity | 0.490 | 5.003 | .025 | 1.632 | 1.063–2.508 |
| History of stroke | 1.000 | 7.165 | .007 | 2.719 | 1.307–5.654 |
| History of head trauma | 2.079 | 4.370 | .037 | 7.996 | 1.139–56.151 |
| Infrequent fruit consumption | 0.526 | 4.990 | .025 | 1.692 | 1.067–2.685 |
| LDL-C (mmol/L) | 0.714 | 29.554 | <.001 | 2.042 | 1.578–2.641 |
Model was adjusted for gender, age, marital status, work status, smoking, and drinking.
AOR = adjusted odds ratios, CI = confidence interval, LDL-C = low density lipoprotein cholesterol.
3.4 . Interactions between constipation with other important influencing factors
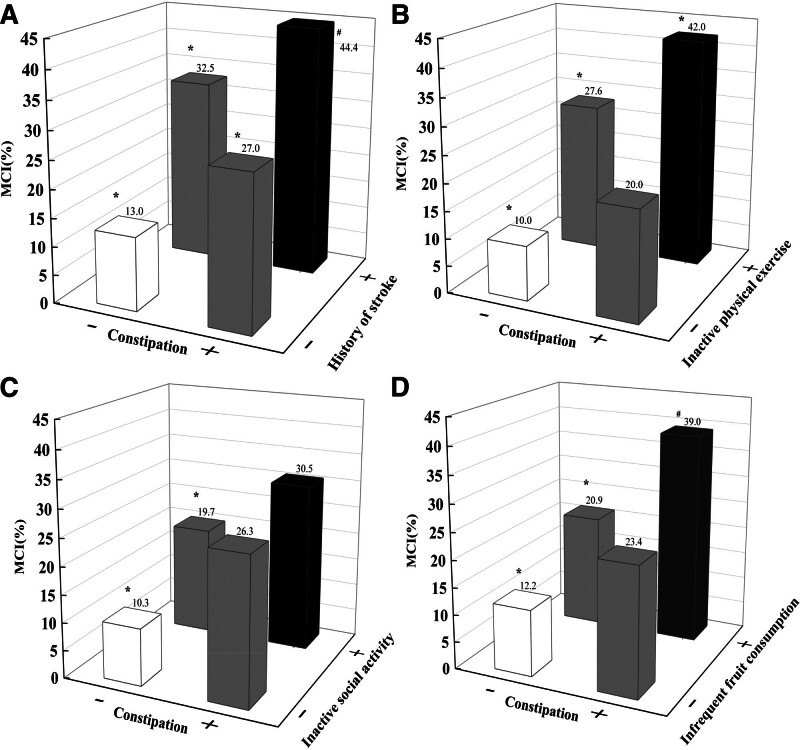
The next question to explore was whether constipation can synergize with other independent influencing factors to affect MCI. We then explored their interactions. As shown in Table 4, constipation can combine with years of schooling, inactive physical exercise, inactive social activity, history of stroke, history of head trauma, and infrequent fruit consumption to affect more MCI (P < .001 for all). Particularly, the combination of constipation with inactive physical exercise affected a higher proportion of MCI than simple addition of the effects of the 2 factors alone (32.0% vs [17.6% + 10.0%], Table 5 and Fig. 1). Similar results was observed in the combination of constipation with infrequent fruit consumption (26.8% vs [8.7% + 11.2%], Table 5 and Fig. 1).
Table 4.
Interactions between constipation with other important influential factors.
| Variables | P | OR | 95% CI |
|---|---|---|---|
| Constipation × years of schooling | <.001 | 1.604 | 1.038–1.090 |
| Constipation × inactive physical exercise | <.001 | 1.790 | 1.509–2.123 |
| Constipation × inactive social activity | <.001 | 1.547 | 1.294–1.850 |
| Constipation × history of stroke | <.001 | 1.641 | 1.353–1.991 |
| Constipation × history of head trauma | <.001 | 1.610 | 1.305–1.987 |
| Constipation × infrequent fruit consumption | <.001 | 1.515 | 1.277–1.797 |
CI = confidence interval, OR = odds ratios.
Table 5.
Stratification analysis between constipation with other important influential factors.
| Constipation | History of stroke | Control | MCI | P | OR | 95% CI |
|---|---|---|---|---|---|---|
| No | No | 534 (87.0) | 80 (13.0) | <.001 | ||
| No | Yes | 27 (67.5) | 13 (32.5) | .001 | 0.311 | 0.154–0.628 |
| Yes | No | 92 (73.0) | 34 (27.0) | <.001 | 0.405 | 0.256–0.641 |
| Yes | Yes | 5 (55.6) | 4 (44.4) | .014 | 0.187 | 0.049–0.712 |
| Constipation | Inactive physical exercise | |||||
| No | No | 448 (90.0) | 50 (10.0) | <.001 | ||
| No | Yes | 113 (72.4) | 43 (27.6) | <.001 | 6.488 | 3.445–12.220 |
| Yes | No | 68 (80.0) | 17 (20.0) | .057 | 1.903 | 0.981–3.691 |
| Yes | Yes | 29 (58.0) | 21 (42.0) | .007 | 2.897 | 1.337–6.276 |
| Constipation | Inactive social activity | |||||
| No | No | 341 (89.7) | 39 (10.3) | <.001 | ||
| No | Yes | 220 (80.3) | 54 (19.7) | <.001 | 3.839 | 2.013–7.321 |
| Yes | No | 56 (73.7) | 20 (26.3) | .070 | 1.789 | 0.954–3.355 |
| Yes | Yes | 41 (69.5) | 18 (30.5) | .591 | 1.229 | 0.579–2.612 |
| Constipation | Infrequent fruit consumption | |||||
| No | No | 440 (87.8) | 61 (12.2) | <.001 | ||
| No | Yes | 121 (79.1) | 32 (20.9) | .007 | 1.908 | 1.189–3.061 |
| Yes | No | 72 (76.6) | 22 (23.4) | .078 | 1.703 | 0.942–3.080 |
| Yes | Yes | 25 (61.0) | 16 (39.0) | <.001 | 6.059 | 3.189–11.479 |
CI = confidence interval, MCI = mild cognitive impairment, OR = odds ratios.
Figure 1.
The respective interactions of constipation with other important influencing factors. The respective interactions of constipation with other important influencing factors included history of stroke (A), inactive physical exercise (B), inactive social activities (C), and infrequent fruit consumption (D). *P < .01, #P < .05.
3.5. Bayesian network model analysis of the causal-association diagram of MCI
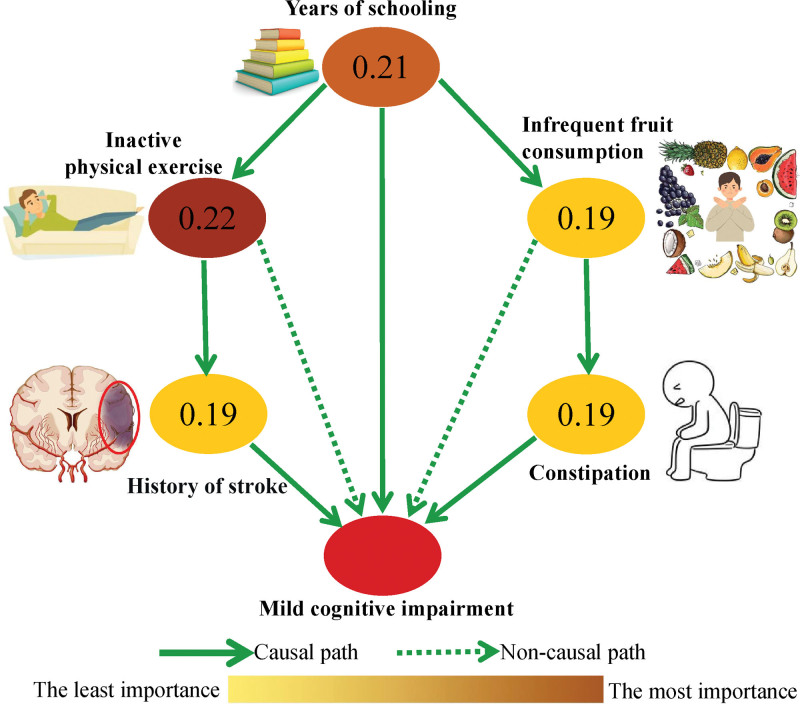
Finally, a Bayesian network model was used to explore the hypothesized causal-association diagram between MCI occurrence and independent influencing factors found in the multivariate logistic regression analysis (Table 3). As shown in Figure 2, the results indicated 3 hypothesized causal-association paths leading to MCI occurrence. Among these, constipation, history of stroke, and years of schooling were directly related to the occurrence of MCI. Years of schooling might cause MCI indirectly through infrequent fruit consumption then constipation. In addition, years of schooling could also result in MCI indirectly through inactive physical exercise then history of stroke. The Bayesian network model causal path analysis further estimated the score of importance of the significant influence factors. Inactive physical exercise was regarded as the most important factor with an inferred score of 0.22, followed by years of schooling (0.21), constipation (0.19), infrequent fruit consumption (0.19), and history of stroke (0.19).
Figure 2.
Bayesian network causal inference of MCI and influencing factors. Bayesian network approach explored the causal-association diagram between MCI and exposure variables, and the importance of exposure variables. The higher magnitude the value is, more important the variable. Meanwhile, the importance of variables was visualized via the gradual changes of color of nodes.
4. Discussion
The present study clearly revealed that constipation was independently associated with the increased risks of MCI occurrence among older adults in Nanning, China. Constipation could combine with inactive physical exercise or infrequent fruit consumption to affect more MCI patients. More importantly, we demonstrated that constipation, together with years of schooling and history of stroke, may directly cause MCI through the analysis of Bayesian network model. Years of schooling may further indirectly affect MCI through infrequent fruit consumption and then constipation.
We previously reported that inactive bowel movement was associated with MCI in a community-living Singaporean cohort.[10] The study was conducted by a survey including bowel movement frequencies, which might be limited by lack of clinical diagnosis and subjective self-report. Thus in the present study, we applied Rome Criteria IV to evaluate constipation. Beside our initial MCI report of inactive bowel movement, 3 other studies recently confirmed the association of constipation with cognitive diseases including MCI, dementia or AD.[11–13] Furthermore, Leta and coauthors reported that constipation was an independent predictor of dementia in 2 independent cohorts of patients with de novo PD (hazard ratio = 2.311, P = .02).[19] The PD patients with constipation of the 2 cohorts also had a faster progression speed to develop dementia.
However, how constipation affects MCI and other cognitive diseases is yet unclear. Our present study suggested that beside the direct effect of constipation, years of schooling, and infrequent fruit consumption, 2 common influencing factors found in our cross-sectional Singaporean study,[10] may sequentially act upstream of constipation (Fig. 2). First, it is well known that higher educational level, which is almost equivalent to longer education years, results in more cognitive reserve.[3,6] Childhood education, as an important modifiable beneficial factor for prevention of AD or MCI, was thus highly recommended as a measure with Class I (strong recommendation) in the Guideline for prevention of AD.[20] Second, higher education level was independently associated with higher consumption of fresh fruits,[15,21] which was in line with the current study. It has long been recognized that fruits positively affected intestinal function. Rich in dietary fiber, fruits play important roles in promoting intestinal function via increasing stool volume, improving stool consistency, and facilitating a healthy microbiota ecosystem.[22] But it is yet not clear why consumption of vegetables which are also rich in dietary fiber and vitamins was not associated with MCI in both the present Nanning cohort and our previous Singaporean cohort.
Constipation is closely related to alteration of gut microbiome. Furthermore, alteration of gut microbiome was able to trigger inflammatory response, and then affected brain function and behavior, ultimately lead to a range of central nervous system diseases such as PD, AD, cognitive disorders, depression, and autism spectrum disorders.[13,23,24] Neurotransmitters and their precursors produced and released by the gut microbiota may also participate in mental diseases.[24] For instance, Clostridium sporogenes and Ruminococcus gnavus from phylum Firmicutes in the human intestine were identified to influence host behavior by regulating the metabolism of tryptophan and serotonin in the brain and peripheral system.[25] Thus, it is possible that constipation might affect MCI through alteration of gut microbiota.
The current study also found that history of stroke was directly associated with increased risks of MCI occurrence among older adults. Furthermore, years of schooling and inactive physical exercises may sequentially reside upstream of stroke (Fig. 2). First, stroke not only contributes to vascular dementia, but also occurs more commonly in older adults with AD than those without.[6] The population-based Oxford Vascular Study demonstrated that the patients with stroke had 50 times higher risk in developing dementia compared with those without stroke.[26] Second, many studies pointed out that physical activity and exercise could prevent stroke through modifying stroke risk factors.[27] Physical exercise could enhance the expression of brain derived neurotrophic factor in peripheral blood, which played an important role in the genesis and development of nerve cells, synaptic plasticity, survival and repair of nerve cells.[28] A systematic review with meta-analysis showed similar results that mind-body exercise (tai chi, yoga, qigong) had the potential to improve various cognitive functions in people with MCI.[29] Third, people with higher education level may pay more attention to physical health, learn more health promotion methods, and then participate in physical exercise more actively.[30] That is why physical exercise was also highly recommended as a measure with Class I (strong recommendation), level B in the Guideline for prevention of AD.[20]
Active engagement in social activities may be potentially protective against progression to MCI or delaying further cognitive decline among older adults.[6,31] Consistent to our results of inactive social activities, a great number of studies indicated that people with little social activity engagement and infrequent social contact might have an increased risk of all-cause dementia.[6] Again, social activities, was a beneficial factor being recommended as a measure with level C in the Guideline for prevention of AD.[20]
This study also revealed an association between history of head trauma and increased risks of MCI. In recent years, some studies showed higher prevalence of self-reported head trauma history in patients with MCI and AD.[6,32,33] Mild traumatic brain injury might contribute to acceleration of cognitive decline, as an independent pathogenic factor to intensify neurodegenerative mechanisms of AD.[33,34] Therefore, active prevention or management of head injuries should be considered to maintain cognitive function in older adults.
Lastly, the present study found that LDL-C was markedly associated with increased risks of MCI among older adults, suggesting that the elevated LDL-C levels might be a potential risk factor for MCI.[35] A longitudinal study of Chinese older adults found that higher blood concentrations of LDL-C in late-life was associated with faster cognitive decline.[36] The concentration of LDL-C was correlated with the incidence and severity of coronary heart disease. Another study reported that LDL-C, together with coronary heart disease, hypertension, and total cholesterol, were independent risk factors for MCI.[37]
Although this study revealed the lack of significant associations between certain factors (BMI, hypertension, diabetes, and heart disease) and MCI, some studies found that these factors were important influencing factors for MCI. The reason for the inconsistent research results may be that the present study was conducted in a single city, which may not be representative of the broader population. Moreover, there were significant differences in lifestyle, economic culture, and dietary habits among populations in different regions. Therefore, it is necessary to conduct a multi-center cohort study to obtain more comprehensive, accurate, and reliable results. A large sample study in China showed that dementia and MCI shared similar risk factors including hypertension, hyperlipidemia, diabetes, heart disease, and cerebrovascular disease.[3] Additionally, our previous study in Singaporean also indicated hypertension was positively associated with MCI occurrence.[10] Therefore, the Guideline for prevention of AD highly recommends that individuals aged <65 years should avoid hypertension via a healthier lifestyle.[20] In recent years, a cohort study suggested that among cognitively intact people, significantly lower BMI occurs beginning approximately 7 years before MCI diagnosis. After MCI diagnosis, BMI declined at the same pace in people who developed dementia and those who did not.[38] The Guideline for prevention of AD also highly recommends that adults aged <65 years should maintain/achieve a BMI between 18.5 and 24.9 kg/m2, and adults aged >65 years should not to be too skinny.[20] These findings highlight the importance of monitoring weight change regularly among older adults.
This study explored a novel influence factor (constipation) on MCI, and analyzed the interactions between constipation with other important influencing factors, and then a Bayesian network model was used to reveal the hypothesized causal path between MCI and the significant influencing factors. The causal path of the influencing factors and MCI were well explained by the Bayesian network model.
Our study has several limitations that have to be acknowledged. First, it is a cross-sectional study that recruited older adults from Nanning, a Southern city of China. This one-city study may not be fully representative of the total population. Second, because many data were collected based on self-report survey from older adults, recalling bias is unavoidable. We thus excluded all dementia patients to minimize the recalling bias. Third, although the diagnosis of constipation was based on the Rome Criteria IV, there is still lack of objective indicators for evaluation.
5. Conclusions
The present study demonstrated that constipation, together with history of stroke, history of head trauma, inactive physical exercise, inactive social activities, infrequency fruit consumption, and LDL-C were closely associated with increased risks of MCI in older adults. In contrast, more years of schooling was associated with decreased risk of MCI. The Bayesian network model analysis further revealed 3 hypothesized causal-association paths leading to MCI occurrence. Among these, constipation, history of stroke and years of schooling were directly related to the occurrence of MCI. Years of schooling might also affect MCI indirectly sequentially through fruit consumption and constipation, or sequentially through inactive physical exercise and stroke. These findings might provide a new direction for the study of the pathogenesis of MCI and a new theoretical basis for the prevention of MCI. We recommend that the health authority conduct a comprehensive assessment of the factors affecting MCI, so as to guide the adoption of measures to prevent MCI. Therefore, active control and reduction risk factors of MCI should be considered to maintain cognitive function and prevent MCI in older adults.
Acknowledgments
The authors are grateful to the participants of this study. The authors also extend their appreciation to the Medical Ethics Committee of Guangxi Medical University, for giving the approval for this study.
Author contributions
Conceptualization: Lei Feng, Guo-Dong Lu.
Data curation: Jin-Meng Huang, Jing Zhou, Andrea B Maier, Kaisy Xinhong Ye, Lei Feng.
Formal analysis: Kai-Yong Huang, Zhen-Zhen Yu, Jia-Jun Tu, Xian-Yan Tang.
Funding acquisition: Kai-Yong Huang, Jing Zhou, Zi Yang, Guo-Dong Lu.
Investigation: Tian-Ming Lu, Yu-Qian Lu, Mei-Chun Huang, Zi Yang.
Methodology: Kai-Yong Huang, Zhen-Zhen Yu.
Software: Jia-Jun Tu, Xian-Yan Tang, Mei-Chun Huang, Zi Yang.
Validation: Jin-Meng Huang, Jing Zhou, Andrea B Maier.
Visualization: Jin-Meng Huang, Tian-Ming Lu, Yu-Qian Lu, Kaisy Xinhong Ye.
Writing – original draft: Kai-Yong Huang, Zhen-Zhen Yu, Jia-Jun Tu, Xian-Yan Tang.
Writing – review & editing: Lei Feng, Guo-Dong Lu.
Supplementary Material
Abbreviations:
- AD
- Alzheimer disease
- AOR
- adjusted odds ratios
- CI
- confidence interval
- LDL-C
- low density lipoprotein cholesterol
- MCI
- mild cognitive impairment
- MMSE
- Mini-Mental State Examination
- PD
- Parkinson disease
This study was supported by the Fudan Professorship Initiating Grant awarded to Guo-Dong Lu; the Natural Science Foundation of China (Grant No. 32160160) awarded to Jing Zhou; the Natural Science Foundation of Guangxi (Grant No. 2023GXNSFAA026155) awarded to Kai-Yong Huang; and the College Students’ Innovative Entrepreneurial Training Plan Program (Grant No. 202310598013) awarded to Zi Yang.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplemental Digital Content is available for this article.
How to cite this article: Huang K-Y, Yu Z-Z, Tu J-J, Tang X-Y, Huang J-M, Lu T-M, Lu Y-Q, Huang M-C, Zhou J, Maier AB, Ye KX, Yang Z, Feng L, Lu G-D. Positive association between constipation and mild cognitive impairment in elders: A cross-sectional study. Medicine 2024;103:40(e39943).
K-YH, Z-ZY, J-JT, and X-YT contributed equally to this work.
Contributor Information
Zhen-Zhen Yu, Email: 302990065@qq.com.
Jia-Jun Tu, Email: 491091707@qq.com.
Xian-Yan Tang, Email: tangxianyan0746@163.com.
Jin-Meng Huang, Email: 2642017088@qq.com.
Tian-Ming Lu, Email: lugd@fudan.edu.cn.
Yu-Qian Lu, Email: lugd@fudan.edu.cn.
Mei-Chun Huang, Email: 2642017088@qq.com.
Jing Zhou, Email: gardenia_zhou@hotmail.com.
Andrea B. Maier, Email: a.maier@nus.edu.sg.
Kaisy Xinhong Ye, Email: kaisy.ye@u.nus.edu.
Zi Yang, Email: 2261890614@qq.com.
Lei Feng, Email: pcmfl@nus.edu.sg.
Guo-Dong Lu, Email: lugd@fudan.edu.cn.
References
- [1].Gauthier S, Webster C, Servaes S, Morais JA, Rosa-Neto P. World Alzheimer report 2022-Life after diagnosis: Navigating treatment, care and support. London: Alzheimer’s Disease International; 2022. [Google Scholar]
- [2].Knopman DS, Amieva H, Petersen RC, et al. Alzheimer disease. Nat Rev Dis Primers. 2021;7:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jia L, Du Y, Chu L, et al. Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: a cross-sectional study. Lancet Public Health. 2020;5:e661–71. [DOI] [PubMed] [Google Scholar]
- [4].Gauthier S, Reisberg B, Zaudig M, et al.; International Psychogeriatric Association Expert Conference on mild cognitive impairment. Mild cognitive impairment. Lancet. 2006;367:1262–70. [DOI] [PubMed] [Google Scholar]
- [5].Langa KM, Levine DA. The diagnosis and management of mild cognitive impairment: a clinical review. JAMA. 2014;312:2551–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390:2673–734. [DOI] [PubMed] [Google Scholar]
- [7].Fu PF, Gao M, Yung KKL. Association of intestinal disorders with Parkinson’s disease and Alzheimer’s disease: a systematic review and meta-analysis. ACS Chem Neurosci. 2020;11:395–405. [DOI] [PubMed] [Google Scholar]
- [8].Simonet C, Bestwick J, Jitlal M, et al. Assessment of risk factors and early presentations of parkinson disease in primary care in a diverse UK population. JAMA Neurol. 2022;79:359–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Savica R, Carlin JM, Grossardt BR, et al. Medical records documentation of constipation preceding Parkinson disease: a case-control study. Neurology. 2009;73:1752–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Huang KY, Tang XY, Yang L, et al. Inactive bowel movement and stroke are associated with increased risks of mild cognitive impairment among community-living Singapore elderly. Aging (Albany NY). 2020;12:17257–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nedelec T, Couvy-Duchesne B, Monnet F, et al. Identifying health conditions associated with Alzheimer’s disease up to 15 years before diagnosis: an agnostic study of French and British health records. Lancet Digit Health. 2022;4:e169–78. [DOI] [PubMed] [Google Scholar]
- [12].Wang F, Fei M, Hu WZ, et al. Prevalence of constipation in elderly and its association with dementia and mild cognitive impairment: a cross-sectional study. Front Neurosci. 2022;15:821654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Nakase T, Tatewaki Y, Thyreau B, et al. Impact of constipation on progression of Alzheimer’s disease: a retrospective study. CNS Neurosci Ther. 2022;28:1964–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Katzman R, Zhang MY, Ouang-Ya-Qu, et al. A Chinese version of the Mini-Mental State Examination; impact of illiteracy in a Shanghai dementia survey. J Clin Epidemiol. 1998;41:971–8. [DOI] [PubMed] [Google Scholar]
- [15].Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–86. [PubMed] [Google Scholar]
- [16].Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–4. [DOI] [PubMed] [Google Scholar]
- [17].Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–92. [DOI] [PubMed] [Google Scholar]
- [18].Sharma A, Rao SSC, Kearns K, Orleck KD, Waldman SA. Review article: diagnosis, management and patient perspectives of the spectrum of constipation disorders. Aliment Pharmacol Ther. 2021;53:1250–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Leta V, Urso D, Batzu L, et al. Constipation is associated with development of cognitive impairment in de novo Parkinson’s disease: a longitudinal analysis of two international cohorts. J Parkinsons Dis. 2021;11:1209–19. [DOI] [PubMed] [Google Scholar]
- [20].Yu JT, Xu W, Tan CC, et al. Evidence-based prevention of Alzheimer’s disease: systematic review and meta-analysis of 243 observational prospective studies and 153 randomised controlled trials. J Neurol Neurosurg Psychiatry. 2020;91:1201–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Althoff T, Nilforoshan H, Hua J, Leskovec J. Large-scale diet tracking data reveal disparate associations between food environment and diet. Nat Commun. 2022;13:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Venancio VP, Kim H, Sirven MA, et al. Polyphenol-rich mango (Mangifera indica L.) ameliorate functional constipation symptoms in humans beyond equivalent amount of fiber. Mol Nutr Food Res. 2018;62:e1701034. [DOI] [PubMed] [Google Scholar]
- [23].Sun Y, Sommerville NR, Liu JYH, et al. Intra-gastrointestinal amyloid-β1-42 oligomers perturb enteric function and induce Alzheimer’s disease pathology. J Physiol. 2020;598:4209–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chen YJ, Xu JY, Chen Y. Regulation of neurotransmitters by the gut microbiota and effects on cognition in neurological disorders. Nutrients. 2021;13:2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Williams BB, Van Benschoten AH, Cimermancic P, et al. Discovery and characterization of gut microbiota decarboxylases that can produce the neurotransmitter tryptamine. Cell Host Microbe. 2014;16:495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Pendlebury ST, Rothwell PM. Incidence and prevalence of dementia associated with transient ischaemic attack and stroke: analysis of the population-based Oxford Vascular Study. Lancet Neurol. 2019;18:248–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Prior PL, Suskin N. Exercise for stroke prevention. Stroke Vasc Neurol. 2018;3:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Yang JL, Lin YT, Chuang PC, Bohr VA, Mattson MP. BDNF and exercise enhance neuronal DNA repair by stimulating CREB-mediated production of apurinic/apyrimidinic endonuclease 1. Neuromolecular Med. 2014;16:161–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zou L, Loprinzi PD, Yeung AS, Zeng N, Huang T. The beneficial effects of mind-body exercises for people with mild cognitive impairment: a systematic review with meta-analysis. Arch Phys Med Rehabil. 2019;100:1556–73. [DOI] [PubMed] [Google Scholar]
- [30].Clemente Remón AL, Jiménez Díaz-Benito V, Jiménez Beatty JE, Santacruz Lozano JA. Levels of physical activity among older adults in the European Union. J Aging Phys Act. 2021;29:242–9. [DOI] [PubMed] [Google Scholar]
- [31].Hughes TF, Sun Z, Chang CH, Ganguli M. Change in engagement in cognitive activity and risk for mild cognitive impairment in a cohort of older adults: the Monongahela-Youghiogheny Healthy Aging Team (MYHAT) study. Alzheimer Dis Assoc Disord. 2018;32:137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Walton SR, Brett BL, Chandran A, et al. Mild cognitive impairment and dementia reported by former professional football players over 50 yr of age: an NFL-LONG study. Med Sci Sports Exerc. 2022;54:424–31. [DOI] [PubMed] [Google Scholar]
- [33].Jacob L, Bohlken J, Kostev K. Risk factors for mild cognitive impairment in german primary care practices. J Alzheimers Dis. 2017;56:379–84. [DOI] [PubMed] [Google Scholar]
- [34].Stovicek PO, Friedmann C, Marinescu D, et al. Mild TBI in the elderly-risk factor for rapid cognitive impairment in Alzheimer’s disease. Rom J Morphol Embryol. 2020;61:61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Liu Y, Zhong X, Shen JJ, et al. Elevated serum TC and LDL-C levels in Alzheimer’s disease and mild cognitive impairment: a meta-analysis study. Brain Res. 2020;1727:146554. [DOI] [PubMed] [Google Scholar]
- [36].Ma CR, Yin ZX, Zhu PF, Luo JS, Shi XM, Gao X. Blood cholesterol in late-life and cognitive decline: a longitudinal study of the Chinese elderly. Mol Neurodegener. 2017;12:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zou Y, Zhu QL, Deng YT, et al. Vascular risk factors and mild cognitive impairment in the elderly population in Southwest China. Am J Alzheimers Dis Other Demen. 2014;29:242–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Guo J, Wang J, Dove A, et al. Body mass index trajectories preceding incident mild cognitive impairment and dementia. JAMA Psychiatry. 2022;79:1180–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.