Abstract
Esophageal cancer (EC) poses a significant global health burden, necessitating effective treatment strategies. Immune checkpoint inhibitors have emerged as a promising therapeutic option for EC, but the identification of predictive biomarkers remains crucial for optimizing patient outcomes. We conducted a retrospective analysis of medical records from advanced esophageal squamous cell carcinoma patients treated with first-line programmed death 1 inhibitors. Peripheral blood lymphocyte subpopulations were evaluated using flow cytometry, while hematological tests provided data on neutrophil, lymphocyte, and monocyte counts. Cox regression and logistic regression analyses were employed to explore the association between lymphocyte subpopulations, baseline characteristics, and progression-free survival (PFS). Among the 100 initially included patients, 70 met eligibility criteria. Multivariate Cox regression analysis revealed a significant association between high CD16+CD56+ lymphocyte proportions and longer PFS, independent of other clinical variables. Similarly, a high CD4+/CD8+ ratio was correlated with prolonged PFS. Kaplan–Meier survival curves supported these findings. Logistic regression analysis indicated no significant differences in the CD4+/CD8+ ratio and CD16+CD56+ lymphocytes concerning baseline characteristics, suggesting their potential as independent prognostic markers. Our study highlights the predictive value of peripheral blood CD16+CD56+ lymphocytes and the CD4+/CD8+ ratio for the efficacy of programmed death 1 inhibitors in advanced esophageal squamous cell carcinoma patients. These findings underscore the importance of peripheral blood biomarkers in guiding personalized immunotherapy strategies and improving outcomes for EC patients.
Keywords: biomarkers, esophageal squamous cell carcinoma, immune checkpoint inhibitors, peripheral blood lymphocyte subpopulation, progression-free survival
1. Introduction
Esophageal cancer (EC) stands as one of the most prevalent and deadly malignancies globally, ranking seventh in incidence and 6th in mortality among cancers.[1] Within China, it is the 6th most common cancer and 4th in terms of mortality.[2] EC predominantly comprises 2 histological types: esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma.[3] ESCC is the most common histological type of EC in Asian countries, whereas esophageal adenocarcinoma predominates in North American and Western European nations.[4] The conventional treatment modalities for EC encompass surgery, chemotherapy, chemoradiotherapy, and targeted therapy.[3] Nevertheless, EC prognosis remains bleak, with a 5-year survival rate of only 20% across all stages, dropping to <5% for stage IV disease.[3,5]
In recent years, the introduction of immune checkpoint inhibitors (ICIs) has revolutionized the prognosis for numerous cancer types, including EC. ICIs primarily encompass cytotoxic T lymphocyte-associated antigen-4 inhibitors and programmed death 1 (PD-1)/programmed death ligand-1 (PD-L1) inhibitors.[5] Pivotal clinical trials such as KEYNOTE-181 and KEYNOTE-590 have demonstrated that ICIs, specifically pembrolizumab, enhance overall survival and progression-free survival (PFS) in advanced EC patients.[6,7] Consequently, ICIs combined with chemotherapy are now considered a standard of care for first-line treatment in advanced EC.
Despite the significant advancement in patient outcomes attributed to ICIs, it remains evident that not all patients derive equal benefit from this therapeutic approach. Thus, the quest for predictive biomarkers to estimate ICIs efficacy is imperative. Given the reliance of certain biomarkers, such as PD-L1 expression and microsatellite instability-H, on tumor tissue, there is an imperative need to identify peripheral blood-based biomarkers that are more practical and readily accessible.[8,9] Past research has intimated that lymphocyte subpopulations in peripheral blood have the potential to predict ICIs efficacy in patients with lung cancer and head and neck squamous cell carcinoma.[10,11] Additionally, higher CD8+ T cell infiltration is associated with better prognosis in gastric and esophageal cancers.[12] However, the relevance of peripheral blood lymphocyte subpopulations in forecasting ICIs efficacy within advanced ESCC patients remains ambiguous. This study endeavors to explore the correlation between peripheral blood lymphocyte subpopulations and the effectiveness of PD-1 inhibitors in advanced ESCC patients, thereby delving into their potential role as valuable biomarkers.
2. Methods
2.1. Patients
We conducted a retrospective analysis of medical records from advanced ESCC patients diagnosed between January 2021 and October 2023 at Jiangsu Provincial People’s Hospital. The inclusion criteria comprised patients aged >18 years with histologically or cytologically confirmed ESCC, unresectable, locally advanced, recurrent, or metastatic disease, complete demographic and clinical information, and receipt of first-line PD-1 inhibitors treatment, with or without chemoradiotherapy therapy. Exclusion criteria encompassed the presence of other concurrent malignancies, autoimmune diseases, and the use of more than 2 ICIs.
For each eligible patient, data encompassed various parameters include age, sex, body mass index, tumor location (upper/middle/lower), presence or absence of visceral metastasis, presence or absence of surgery, presence or absence of other treatments prior to immunotherapy (radiotherapy/chemotherapy/targeted therapy), smoking and alcohol use history, history of hypertension, diabetes and hyperlipidemia history, neutrophil count, lymphocyte count, monocyte count, lactate dehydrogenase (LDH) level, albumin level, and baseline peripheral blood lymphocyte subpopulations (CD3+, CD3+CD4+, CD3+CD8+, CD19+, CD16+CD56+ lymphocyte proportions, and CD4+/CD8+ ratio), treatment details including the type of PD-1 inhibitors and its combination with chemotherapy, and treatment outcomes. CD3+ lymphocytes, CD3+CD4+ lymphocytes, CD3+CD8+ lymphocytes, CD19+ lymphocytes, and CD16+CD56+ lymphocytes represent T lymphocytes, helper T lymphocytes, cytotoxic T lymphocytes, B cells, and natural killer (NK) cells (CD3‐CD16+CD56+), respectively. All hematological tests were completed within 1 week before initial treatment of PD-1 inhibitors.
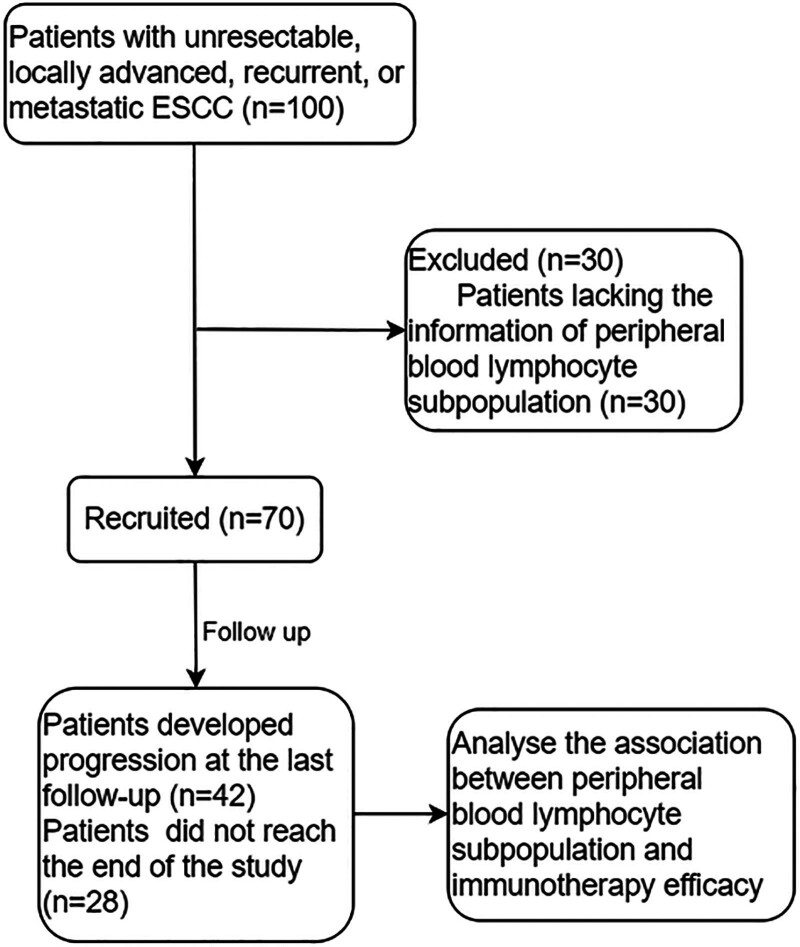
The primary outcome was PFS and defined as the time from treatment initiation to radiological or clinical progression or death from any cause. No progression or death at the last follow-up was defined as censored. The median was used as the cutoff value for the lymphocyte subpopulation and other continuous variables. Detailed steps are shown in Figure 1.
Figure 1.
Flow chart.
The study design adhered to the ethical principles of the Helsinki Declaration and received ethical approval from the Ethics Review Committee of the First Affiliated Hospital of Nanjing Medical University.
2.2. Hematological tests
Peripheral blood lymphocytes were evaluated using flow cytometry. A volume of 100 μL of blood sample was incubated with primary antibodies against anti-CD3, anti-CD4, anti-CD8, anti-CD16, anti-CD56, and anti-CD19 at room temperature for 10 minutes (the antibodies were bought from BD Biosciences [San Jose, CA]: anti-CD3 [SK7 APC], anti-CD4 [SK3 AmCyan], anti-CD8 [SK1 PerCP], anti-CD16 [3G8 BV605], anti-CD56 [MY31 PE], and anti-CD19 [4G7 FITC]). Subsequently, the samples were centrifuged at 1200 rpm for 5 minutes at room temperature to remove the supernatant. Following that, 500 μL of PBS was added to the samples. Flow cytometry data were acquired using a BD FACSCanto instrument and analyzed using the BD FACSDiva flow cytometry analysis software. 10 mL of blood were drawn from the patient’s antecubital vein, and the whole blood sample was analyzed using a hematology analyzer to obtain neutrophil count, lymphocyte count, and platelet count. Additionally, another 10 mL of blood was drawn for centrifugation to obtain serum, which was then analyzed using a biochemical analyzer to determine the levels of LDH and albumin.
2.3. Statistical analysis
Data analysis was executed using IBM SPSS (version 25.0; IBM Corp, Armonk, NY). Data normality was assessed using the Shapiro–Wilk test. Categorical variables were described using numbers and percentages. Variance inflation factor (VIF) and tolerance measures were used to confirm the absence of multicollinearity among variables. The dependent variable PFS is a binary variable containing time information. We therefore used Cox regression analysis and Kaplan–Meier survival curves to explore the association between lymphocyte subpopulations and PFS of patients. After excluding tumor location, smoking status, alcohol use status, hypertension, hyperlipemia, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, LDH, and albumin (VIF > 10), the rest of variables list in Table 1 were included in univariate Cox regression analysis, and only those reaching a significance level of P < .05 were incorporated into multivariate Cox regression analysis. The dependent variable was a binary variable, so Logistic regression was used to analyze the relationship between CD4+/CD8+ ratio, CD16+CD56+ lymphocytes and baseline characteristics of patients. Significance was determined with a 2-tailed P-value < .05.
Table 1.
Baseline clinicopathological characteristics of patients.
| Characteristics | Number (%) (n = 70) | |
|---|---|---|
| Age, years | <70 | 47 (67.1) |
| >70 | 23 (32.9) | |
| Gender | Male | 56 (80.0) |
| Female | 14 (20.0) | |
| BMI | <18.5 | 5 (7.1) |
| 18.5–24 | 47 (67.2) | |
| >24 | 18 (25.7) | |
| Tumor location | Upper | 13 (18.6) |
| Middle | 28 (40.0) | |
| Lower | 29 (44.4) | |
| Visceral metastases | Yes | 12 (17.1) |
| No | 58 (82.9) | |
| Prior surgery | Yes | 9 (12.9) |
| No | 61 (87.1) | |
| Prior chemotherapy | Yes | 8 (11.4) |
| No | 62 (88.6) | |
| Combined targeted therapy | Yes | 9 (12.9) |
| No | 61 (87.1) | |
| Combined radiotherapy | Yes | 17 (24.3) |
| No | 53 (75.7) | |
| Smoking status | Yes | 25 (35.7) |
| No | 45 (64.3) | |
| Alcohol use status | Yes | 19 (27.1) |
| No | 51 (72.9) | |
| Hypertension | Yes | 26 (37.1) |
| No | 44 (62.9) | |
| Diabetes | Yes | 9 (12.9) |
| No | 61 (87.1) | |
| Hyperlipemia | Yes | 41 (58.6) |
| No | 29 (41.4) | |
| NLR (median 2.83) | High | 36 (51.5) |
| Low | 34 (48.5) | |
| PLR (median 141.35) | High | 36 (51.5) |
| Low | 34 (48.5) | |
| LDH (median 240) | High | 8 (11.4) |
| Low | 62 (88.6) | |
| Albumin (median 38.3) | High | 32 (45.9) |
| Low | 38 (54.1) | |
| CD3+ (median 68.75%) | High | 34 (48.6) |
| Low | 36 (51.4) | |
| CD3+CD4+ (median 41.06%) | High | 35 (50.0) |
| Low | 35 (50.0) | |
| CD3+CD8+ (median 21.89%) | High | 34 (48.6) |
| Low | 36 (51.4) | |
| CD16+CD56+ (median 17.88%) | High | 36 (51.4) |
| Low | 34 (48.6) | |
| CD19+ (median 9.38%) | High | 40 (57.1) |
| Low | 30 (42.9) | |
| CD4+/CD8+ ratio (median 1.89) | High | 36 (51.4) |
| Low | 34 (48.6) |
BMI = body mass index, LDH = lactic dehydrogenase, NLR = neutrophil-to-lymphocyte ratio, PLR = platelet-to-lymphocyte ratio.
3. Results
3.1. Patient characteristics
A total of 100 advanced ESCC patients receiving first-line PD-1 inhibitors were included. 30 patients were excluded because of lacking of data of lymphocyte subpopulation. Ultimately, 70 patients were included in the analysis. The median follow-up time was 10.2 months. Forty-two patients developed progression at the last follow-up. Each patient received PD-1 inhibitors in combination with chemotherapy. The type of PD-1 inhibitors in our study include camrelizumab (24.9%), tislelizumab (30.9%), sintilimab (37.5%), and pembrolizumab (6.7%). The baseline characteristics of patients are shown in Table 1.
3.2. Factors affecting PFS
Initially, we confirmed the absence of multicollinearity among variables list in Table 1 using VIF and tolerance measures. The preliminary results revealed a significant collinearity among tumor location, smoking status, alcohol use status, hypertension, hyperlipemia, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, LDH, and albumin. Consequently, we removed these variables and conducted another collinearity analysis. The final results indicated no collinearity among the remaining variables (all VIF < 10, Table 2). Univariate Cox regression analysis encompassed patient demographics and baseline lymphocyte subpopulations. In the univariate Cox regression, patients with prior surgery (hazard ratio [HR] 2.597, 95% confidence interval [CI] 1.186–5.185; P = .017, Table 3), those who had prior chemotherapy (HR 2.301, 95% CI 1.009–5.247; P = .048, Table 3), and those with visceral metastasis (HR 2.457, 95% CI 1.192–5.063; P = .015, Table 3) exhibited a higher likelihood of disease progression. In contrast, patients in the CD16+CD56+-high group (HR 0.478, 95% CI 0.249–0.921; P = .027, Table 3) and the CD4+/CD8+ ratio-high group (HR 0.571, 95% CI 0.274–0.977; P = .042, Table 3) demonstrated longer PFS. Subsequent multivariate Cox regression analysis that integrated these variables reaffirmed the statistical significance of the CD16+CD56+-high group (HR 0.359, 95% CI 0.174–0.739; P = .005, Table 3) and the presence of visceral metastasis (HR 3.056, 95% CI 1.334–7.005; P = .008, Table 3), thereby confirming them as independent factors affecting PFS in first-line PD-1 inhibitors-treated advanced ESCC patients.
Table 2.
Multicollinearity analysis.
| Characteristics | Tolerance | VIF | |
|---|---|---|---|
| Age, years | >70/<70 | 0.403 | 2.478 |
| Gender | Female/male | 0.479 | 2.089 |
| BMI | >24/18.5–24/<18.5 | 0.510 | 1.962 |
| Diabetes | Yes/no | 0.430 | 2.327 |
| Visceral metastasis | Yes/no | 0.378 | 2.643 |
| Prior surgery | Yes/no | 0.130 | 7.704 |
| Prior chemotherapy | Yes/no | 0.206 | 4.859 |
| Combined targeted therapy | Yes/no | 0.428 | 2.167 |
| Combined radiotherapy | Yes/no | 0.513 | 1.949 |
| Type of PD-1 inhibitor | 0.425 | 2.258 | |
| Camrelizumab | |||
| Tislelizumab | |||
| Sintilimab | |||
| Pembrolizumab | |||
| CD3+ | High/low | 0.239 | 4.183 |
| CD3+CD4+ | High/low | 0.298 | 3.356 |
| CD3+CD8+ | High/low | 0.164 | 6.090 |
| CD16+CD56+ | High/low | 0.156 | 6.420 |
| CD19+ | High/low | 0.304 | 3.288 |
| CD4+/CD8+ ratio | High/low | 0.154 | 6.501 |
BMI = body mass index, VIF = variance inflation factor.
Table 3.
Univariate and multivariate Cox regression analysis of patients.
| Characteristics | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | ||
| Age, years | >70/<70 | 1.006 | 0.532–1.901 | .98 | |||
| Gender | Female/male | 0.713 | 0.315–1.613 | .47 | |||
| BMI | >24/18.5–24/<18.5 | 1.32 | 0.699–2.491 | .93 | |||
| Diabetes | Yes/no | 1.246 | 0.521–2.98 | .62 | |||
| Visceral metastasis | Yes/no | 2.457 | 1.192–5.063 | .015 | 3.056 | 1.334–7.005 | .008 |
| Prior surgery | Yes/no | 2.597 | 1.186–5.685 | .017 | 2.599 | 0.484–9.945 | .26 |
| Prior chemotherapy | Yes/no | 2.301 | 1.009–5.247 | .048 | 1.17 | 0.206–6.646 | .85 |
| Combined targeted therapy | Yes/no | 2.058 | 0.932–4.544 | .074 | |||
| Combined radiotherapy | Yes/no | 1.085 | 0.526–2.238 | .82 | |||
| Type of PD-1 inhibitor | |||||||
| Camrelizumab | Reference | ||||||
| Tislelizumab | 0.551 | 0.238–1.279 | .16 | ||||
| Sintilimab | 0.723 | 0.336–1.558 | .40 | ||||
| Pembrolizumab | 1.527 | 0.489–4.711 | .46 | ||||
| CD3+ | High/low | 1.401 | 0.751–2.613 | .28 | |||
| CD3+CD4+ | High/low | 1.39 | 0.75–2.579 | .29 | |||
| CD3+CD8+ | High/low | 1.177 | 0.636–2.178 | .60 | |||
| CD16+CD56+ | High/low | 0.478 | 0.249–0.921 | .027 | 0.359 | 0.174–0.739 | .005 |
| CD19+ | High/low | 1.017 | 0.548–1.89 | .95 | |||
| CD4+/CD8+ ratio | High/low | 0.517 | 0.274–0.977 | .042 | 0.584 | 0.298–1.145 | .11 |
BMI = body mass index, CI = confidence interval, HR = hazard ratio.
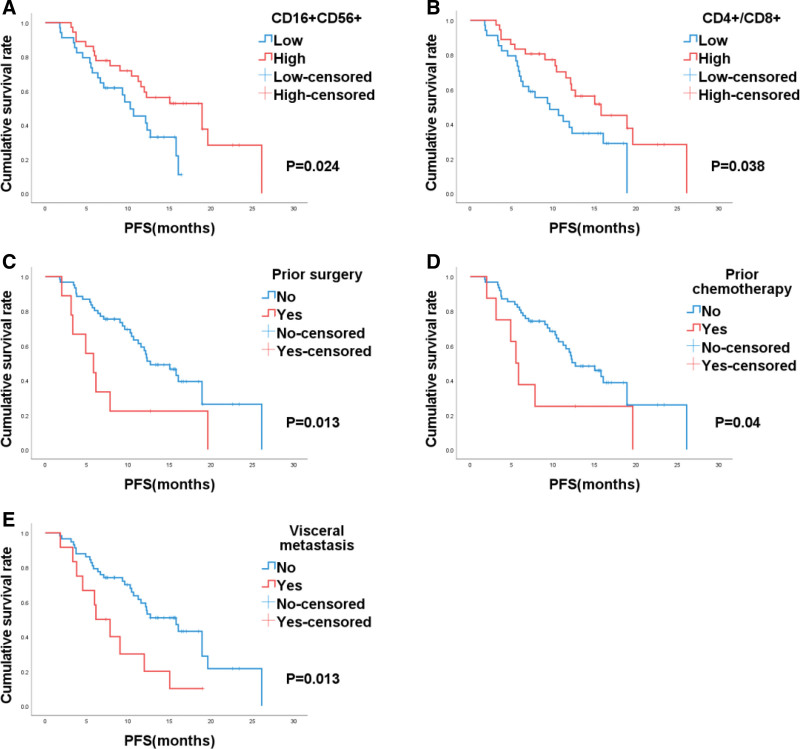
In the entire patient cohort, Kaplan–Meier survival curves illustrated that patients in the CD16+CD56+-high group (CD16+CD56+-high vs CD16+CD56+-low, 18.9 vs 10.3 months, P = .024, Fig. 2A) and those in the CD4+/CD8+ ratio-high group (CD4+/CD8+-high vs CD4+/CD8+-low, 15.8 vs 9.6 months, P = .038, Fig. 2B) experienced extended PFS. Conversely, patients who had prior surgery (yes versus no, 5.8 vs 12.7, P = .013, Fig. 2C), those who had prior chemotherapy (yes vs no, 5.5 vs 12.7, P = .004, Fig. 2D), and patients with visceral metastasis (yes vs no, 5.8 vs 12.7, P = .013, Fig. 2E) had shorter PFS.
Figure 2.
Kaplan–Meier analysis based on CD16+CD56+ (A), CD4+/CD8+ ratio (B), prior surgery (C), prior chemotherapy (D), and visceral metastasis (E) in patients with esophageal cancer.
3.3. Correlations between CD4+/CD8+ ratio, CD16+CD56+ lymphocytes and clinicopathological features
Additionally, we explored the relationship between the CD4+/CD8+ ratio and CD16+CD56+ lymphocytes and the baseline characteristics of patients. Logistic regression analysis indicated that there were no significant differences in the CD4+/CD8+ ratio and CD16+CD56+ lymphocytes concerning baseline characteristics, suggesting their strong alignment with all the considered baseline information (Table 4). This also means that these factors do not affect the final results through lymphocyte subtypes.
Table 4.
Correlations between CD4+/CD8+ ratio, CD16+CD56+ lymphocyte and clinicopathological variables.
| Characteristics | CD4+/CD8+ ratio | CD16+CD56+ lymphocyte | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | ||
| Age, years | >70/<70 | 1.768 | 0.641–4.876 | .27 | 1.723 | 0.63–4.07 | .22 |
| Gender | Female/male | 0.65 | 0.199–2.119 | .47 | 0.3 | 0.084–1.073 | .30 |
| BMI | >24/18.5–24/<18.5 | 0.957 | 0.377–2.433 | .92 | 0.69 | 0.267–1.783 | .44 |
| Smoking status | Yes/no | 0.808 | 0.303–2.150 | .66 | 0.722 | 0.216–2.014 | .65 |
| Drinking status | Yes/no | 0.8 | 0.279–2.298 | .67 | 1.43 | 0.494–4.142 | .51 |
| Hypertension | Yes/no | 1.494 | 0.562–3.969 | .42 | 0.913 | 0.346–2.409 | .85 |
| Diabetes | Yes/no | 2.067 | 0.473–9.025 | .33 | 2.012 | 0.425–5.265 | .35 |
| Hyperlipemia | Yes/no | 1.385 | 0.491–3.901 | .53 | 0.933 | 0.332–2.62 | .89 |
| Tumor location | Upper/middle/lower | 1.905 | 0.961–3.780 | .06 | 1.342 | 0.695–2.593 | .38 |
| Visceral metastasis | Yes/no | 0.253 | 0.062–1.030 | .05 | 2.143 | 0.58–7.911 | .25 |
| Prior surgery | Yes/no | 0.725 | 0.177–2.962 | .65 | 3.862 | 0.742–9.015 | .11 |
| Prior chemotherapy | Yes/no | 0.938 | 0.215–4.088 | .93 | 1.667 | 0.366–7.586 | .51 |
BMI = body mass index, CI = confidence interval, OR = odds ratio.
4. Discussion
The advent of ICIs has significantly improved the prognosis of patients with EC. Based on a series of clinical studies, ICIs have received approval from the Chinese Society of Clinical Oncology for first-line treatment in inoperable, locally advanced, recurrent, or metastatic EC patients.[6,7,13] However, due to various factors, the response to immunotherapy is suboptimal in the majority of patients.[14,15] Some patients receiving ICIs as a first-line treatment rapidly experience disease progression and develop resistance to immunotherapy.[16]
Microsatellite instability and tumor mutational burden are the primary predictive biomarkers for immunotherapy efficacy, but both rely on obtaining tumor tissue.[14] Therefore, it is imperative for EC patients to have a serological marker that can predict their prognosis.
Our analysis revealed that a high proportion of CD16+CD56+ lymphocytes in peripheral blood and an elevated CD4+/CD8+ ratio were associated with prolonged PFS in first-line PD-1 inhibitors-treated advanced ESCC patients.
Multiple clinical studies have indicated that the type of PD-1 inhibitor does not significantly impact the prognosis of advanced ESCC patients.[7,17] In our study, the efficacy of various PD-1 inhibitors was comparable, and there was no evidence of multicollinearity between these inhibitors and lymphocyte subpopulations. This implies that the type of PD-1 inhibitor does not confound the ultimate conclusion.
NK cells are a crucial component of the human innate immune system, playing a vital role in defending against viral infections, controlling cancer, and immune regulation.[18] CD16 and CD56 are standard molecular markers for NK cells.[19] They primarily eliminate infected or malignant cells by discharging pre cytolytic granules containing perforin and granzyme B through an immunological synapse, ultimately inducing apoptosis in target cells.[20] PD-1 is expressed on various immune cells, including T cells, B cells, monocytes, and NK cells, while its ligand, PD-L1 is typically expressed on the surface of tumor cells and upregulated within tumor cells.[21] The interaction between PD-L1 on the surface of tumor cells and PD-1 on immune cells suppresses the function of immune cells, consequently promoting tumor progression.[21] This implies that ICIs can enhance the direct or indirect anti effects of NK cells. Research by Cichocki et al concluded that NK cells induced from pluripotent stem cells can recruit T cells and enhance the effects of PD-1 inhibitors.[22] Nakamura et al demonstrated that NK cell stimulants can reduce resistance to anti-PD-1 therapy in a B16-F10 lung metastasis model and have a synergistic antitumor effect.[23] These findings are consistent with our conclusion that esophageal cancer patients with a higher proportion of CD16+CD56+ lymphocytes (NK cells) in peripheral blood have longer progression-free survival.
CD8+ T cells (cytotoxic T lymphocytes) can directly kill tumor cells, playing a pivotal role in the body’s antitumor response. Meanwhile, CD4+ T cells (helper T lymphocytes) can secrete cytokines and antibodies, activating NK cells and CD8+ T lymphocytes, thereby exerting their antitumor effects.[24] Dai et al found that lung cancer patients with higher CD4+/CD8+ ratios in peripheral blood have a better prognosis.[25] Marchi et al found a strong association between lower CD4+/CD8+ ratios and recurrence in head and neck squamous cell carcinoma patients.[11] Additionally, Yuan et al discovered that the serum CD4+/CD8+ ratio in patients with advanced, refractory solid tumors responding to anlotinib combined with PD-1 inhibitors was higher than in non-responding patients.[26] This is consistent with our finding that esophageal cancer patients with a higher CD4+/CD8+ ratio have longer progression-free survival. However, in the study by Xu et al, esophageal and gastric cancer patients receiving immunotherapy combined with chemotherapy who had lower CD4+/CD8+ ratios had better outcomes.[12] This discrepancy may be related to tumor type and the line of immunotherapy and requires further research to understand the relationship between CD4+/CD8+ ratio and prognosis in different cancers.
Furthermore, we conducted an analysis of the relationship between patients’ baseline information and CD16+CD56+ lymphocytes, as well as the CD4+/CD8+ ratio. The results demonstrate that there is no statistically significant association between them. Additionally, variance inflation factor and tolerance measures confirm the absence of multicollinearity among these variables. The study by Deng et al also indicates that chemotherapy has no long-term impact on T lymphocyte subpopulations and NK cells in cancer patients. In other words, frontline treatment and demographic characteristics of patients do not influence CD16+CD56+ lymphocytes and the CD4+/CD8+ ratio.[27] This suggests that the predictive value of frontline treatment and CD16+CD56+ lymphocytes, as well as the CD4+/CD8+ ratio, for patient prognosis is independent of each other. This further enhances the reliability of the conclusions drawn in our study.
However, there are still some limitations in this study. Firstly, the study’s small sample size and the nature of observational research, but we use statistical methods to reduce the impact of these factors as much as possible. Additionally, due to the absence of data, we were unable to explore changes in lymphocyte subpopulations after immunotherapy. So further investigation is necessary.
In summary, immunotherapy has become an indispensable approach in the treatment of esophageal cancer. Peripheral blood biomarkers, as a fast, convenient, and nondiagnostic method, can offer predictive value for the immunotherapy outcomes of advanced ESCC. Our research suggests that peripheral blood CD16+CD56+ lymphocytes and the CD4+/CD8+ ratio can serve as predictive markers for the effectiveness of PD-1 inhibitors in advanced ESCC patients. This study will provide a basis for further research into peripheral blood biomarkers for the efficacy of ICIs in EC.
Author contributions
Conceptualization: Jiukang Sun, Wenyuan Gan, Jialin Yao, Zhihang Han, Zhang Fang, Weili Xiong, Dongqing Li, Lei Cao, Lingjun Zhu.
Formal analysis: Jialin Yao, Lei Cao, Lingjun Zhu.
Investigation: Jiukang Sun, Lei Cao, Lingjun Zhu.
Methodology: Jialin Yao, Lei Cao, Lingjun Zhu.
Project administration: Lei Cao, Lingjun Zhu.
Resources: Lei Cao, Lingjun Zhu.
Software: Jiukang Sun, Wenyuan Gan, Jianhui Wu, Lei Cao, Lingjun Zhu.
Supervision: Lei Cao, Lingjun Zhu.
Validation: Weili Xiong, Dongqing Li.
Writing – original draft: Jiukang Sun, Lingjun Zhu.
Writing – review & editing: Jiukang Sun, Wenyuan Gan, Jialin Yao, Zhihang Han, Zhang Fang, Lei Cao, Lingjun Zhu.
Abbreviations:
- CI
- confidence interval
- EC
- esophageal cancer
- ESCC
- esophageal squamous cell carcinoma
- HR
- hazard ratio
- ICIs
- immune checkpoint inhibitors
- LDH
- lactate dehydrogenase
- NK cells
- natural killer cells
- PD-1
- programmed death 1
- PD-L1
- programmed death ligand-1
- PFS
- progression-free survival
- VIF
- variance inflation factor
This study is supported in part by the National Natural Science Foundation of China (grant numbers 82273407), the Natural Science Foundation of Jiangsu Province (grant numbers BK20201495), and Jiangsu Province Capability Improvement Project through Science, Technology and Education (grant numbers CXZX202204).
Patient consent was waived due to the nature of retrospective analysis.
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Review Committee of the First Affiliated Hospital of Nanjing Medical University.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
How to cite this article: Sun J, Gan W, Yao J, Han Z, Fang Z, Xiong W, Li D, Wu J, Cao L, Zhu L. Peripheral blood lymphocyte subpopulations as predictive biomarkers for first-line programmed death 1 inhibitors efficacy in esophageal squamous cell carcinoma: A retrospective study. Medicine 2024;103:40(e39967).
JS, WG, and JY contributed equally to this work.
Contributor Information
Jiukang Sun, Email: jksun7336@163.com.
Wenyuan Gan, Email: wenyuan@stu.njmu.edu.cn.
Jialin Yao, Email: Jialinyao1999@163.com.
Zhihang Han, Email: 1070367268@qq.com.
Zhang Fang, Email: zhangfang.yzu@outlook.com.
Weili Xiong, Email: xiong_weili123@163.com.
Dongqing Li, Email: sydongqing@163.com.
Jianhui Wu, Email: wujh.jeffrey@outlook.com.
Lei Cao, Email: BrawnyM80@163.com.
References
- [1].Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. [DOI] [PubMed] [Google Scholar]
- [2].Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J (Engl). 2021;134:783–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wang H, Xu Y, Zuo F, Liu J, Yang J. Immune-based combination therapy for esophageal cancer. Front Immunol. 2022;13:1020290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tungekar A, Mandarthi S, Mandaviya PR, et al. ESCC ATLAS: a population wide compendium of biomarkers for esophageal squamous cell carcinoma. Sci Rep. 2018;8:12715–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Valkema MJ, Mostert B, Lagarde SM, Wijnhoven BPL, van Lanschot JJB. The effectivity of targeted therapy and immunotherapy in patients with advanced metastatic and non-metastatic cancer of the esophagus and esophago-gastric junction. Updates Surg. 2022;75:313–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kojima T, Shah MA, Muro K, et al. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol. 2020;38:4138–48. [DOI] [PubMed] [Google Scholar]
- [7].Sun JM, Shen L, Shah MA, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet. 2021;398:759–71. [DOI] [PubMed] [Google Scholar]
- [8].Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Miao K, Zhang X, Wang H, et al. Peripheral blood lymphocyte subsets predict the efficacy of immune checkpoint inhibitors in non-small cell lung cancer. Front Immunol. 2022;13:1111230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Marchi F, Missale F, Incandela F, et al. Prognostic significance of peripheral T-cell subsets in laryngeal squamous cell carcinoma. Laryngoscope Investig Otolaryngol. 2019;4:513–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Xu S, Zhu Q, Wu L, et al. Association of the CD4+/CD8+ ratio with response to PD-1 inhibitor-based combination therapy and dermatological toxicities in patients with advanced gastric and esophageal cancer. Int Immunopharmacol. 2023;123:110642–53. [DOI] [PubMed] [Google Scholar]
- [13].Wang ZX, Cui C, Yao J, et al. Toripalimab plus chemotherapy in treatment-naïve, advanced esophageal squamous cell carcinoma (JUPITER-06): a multi-center phase 3 trial. Cancer Cell. 2022;40:277–88.e3. [DOI] [PubMed] [Google Scholar]
- [14].Rizzo A, Ricci AD, Brandi G. PD-L1, TMB, MSI, and other predictors of response to immune checkpoint inhibitors in biliary tract cancer. Cancers (Basel). 2021;13:558–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Petrillo A, Smyth EC. Immunotherapy for squamous esophageal cancer: a review. J Pers Med. 2022;12:862–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fang P, Zhou J, Liang Z, et al. Immunotherapy resistance in esophageal cancer: Possible mechanisms and clinical implications. Front Immunol. 2022;13:975986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Luo H, Lu J, Bai Y, et al. Effect of camrelizumab vs placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: the ESCORT-1st randomized clinical trial. JAMA. 2021;326:916–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Euchner J, Sprissler J, Cathomen T, et al. Natural killer cells generated from human induced pluripotent stem cells mature to CD56brightCD16+NKp80+/‐ in-vitro and express KIR2DL2/DL3 and KIR3DL1. Front Immunol. 2021;12:640672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Eguizabal C, Zenarruzabeitia O, Monge J, et al. Natural killer cells for cancer immunotherapy: pluripotent stem cells-derived NK cells as an immunotherapeutic perspective. Front Immunol. 2014;5:439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Myers JA, Miller JS. Exploring the NK cell platform for cancer immunotherapy. Nat Rev Clin Oncol. 2020;18:85–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Seliger B. Basis of PD1/PD-L1 therapies. J Clin Med. 2019;8:2168–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cichocki F, Bjordahl R, Gaidarova S, et al. iPSC-derived NK cells maintain high cytotoxicity and enhance in vivo tumor control in concert with T cells and anti–PD-1 therapy. Sci Transl Med. 2020;12:eaaz5618–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Nakamura T, Sato T, Endo R, et al. STING agonist loaded lipid nanoparticles overcome anti-PD-1 resistance in melanoma lung metastasis via NK cell activation. J Immunother Cancer. 2021;9:e002852–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Borst J, Ahrends T, Bąbała N, Melief CJM, Kastenmüller W. CD4+ T cell help in cancer immunology and immunotherapy. Nat Rev Immunol. 2018;18:635–47. [DOI] [PubMed] [Google Scholar]
- [25].Dai S, Ren P, Ren J, Yang L, Li W. The relationship between lymphocyte subsets and the prognosis and genomic features of lung cancer: a retrospective study. Int J Med Sci. 2021;18:2228–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Yuan M, Zhu Z, Mao W, et al. Anlotinib combined with anti-PD-1 antibodies therapy in patients with advanced refractory solid tumors: a single-center, observational, prospective study. Front Oncol. 2021;11:683502–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Deng H, Zhou J, Chen H, et al. Impact of lymphadenectomy extent on immunotherapy efficacy in post-resectional recurred non-small cell lung cancer: a multi-institutional retrospective cohort study. Int J Surg. 2023;110:238–52. [DOI] [PMC free article] [PubMed] [Google Scholar]