ABSTRACT
Helicobacter pylori (H. pylori) is closely associated with the diseases such as gastric sinusitis, peptic ulcers, and gastric adenocarcinoma. Its drug resistance is very severe, and new antibiotics are urgently needed. Nine comfrey compounds were screened by antimicrobial susceptibility testing, among which deoxyshikonin had the best inhibitory effect, with a minimum inhibitory concentration (MIC) of 0.5–1 µg/mL. In addition, deoxyshikonin also has a good antibacterial effect in an acidic environment, it is highly safe, and H. pylori does not readily develop drug resistance. Through in vivo experiments, it was proven that deoxyshikonin (7 mg/kg) had a beneficial therapeutic effect on acute gastritis in mice infected with the multidrug-resistant H. pylori BS001 strain. After treatment with desoxyshikonin, colonization of H. pylori in the gastric mucosa of mice was significantly reduced, gastric mucosal damage was repaired, inflammatory factors were reduced, and the treatment effect was better than that of standard triple therapy. Therefore, deoxyshikonin is a promising lead drug to solve the difficulty of drug resistance in H. pylori, and its antibacterial mechanism may be to destroy the biofilm and cause an oxidation reaction.
KEYWORDS: Helicobacter pylori, deoxyshikonin, inhibitory action, lead drug, acidic environment
INTRODUCTION
Helicobacter pylori is a Gram-negative, spiral bacterium that causes a variety of gastrointestinal diseases such as gastritis, chronic gastritis, peptic ulcers, and gastric adenocarcinoma (1). The prevalence of H. pylori infection varies by age, ethnicity, and diet (2). The infection rate of H. pylori among young Chinese individuals aged 20–40 years is 40%–70%, accounting for half of the total infected population, with those aged over 70 years being at high risk of infection (3). The current treatment options for H. pylori infections include standard triple therapy or bismuth-containing quadruple therapy (two antibiotics). Long-term antibiotic use leads to increased antibiotic resistance in H. pylori and reduced eradication rates (4–7). Therefore, for the health of the global population, there is an urgent need to develop new drugs to eradicate drug-resistant H. pylori.
Radix lithospermi is a herbal medicine included in the Chinese pharmacopoeia made with the dried roots of Radix lithospermi or Arnebia gutlata Bunge, all from the comfrey plant as used for medicinal purposes. It promotes lymphangiogenesis and wound healing and has antibacterial and anticancer effects and other pharmacological actions (8, 9). Deoxyshikonin as a chemical component of Radix lithospermi inhibits drug resistance of non-small cells causing lung carcinoma to cisplatin by suppressing the ABCB1 expression in Akt signaling (10). In addition, deoxyshikonin exerted a better inhibitory effect on the formation of Staphylococcus aureus biofilms than on that of Escherichia coli and Pseudomonas aeruginosa (11). Vukic et al. performed antibacterial tests using components of Radix lithospermi on five Gram-positive bacteria (Bacillus megaterium, Enterococcus faecalis, Microbacterium arborescens, micrococcus luteus, and Staphylococcus epidermidis) and five Gram-negative bacteria (Citrobacter koseri, Hafnia alvei, proteolytic Pseudomonas strains, Stenotrophomonas maltophilia, and Yersinia intermedia) (12). α-methylbutyrylshikonin and acetylshikonin demonstrated good bacteriostatic activity against the aforementioned Gram-positive and Gram-negative bacteria (13–16). However, no reports on the inhibitory effects of Radix lithospermi and deoxyshikonin on H. pylori are available. Since H. pylori is a special bacterium that grows in the acidic environment, many drugs do not work well in the acidic environment, so it is necessary to study the antimicrobial effect of deoxyshikonin on H. pylori therein.
RESULTS
The relationship between chemical structure and antibacterial effect
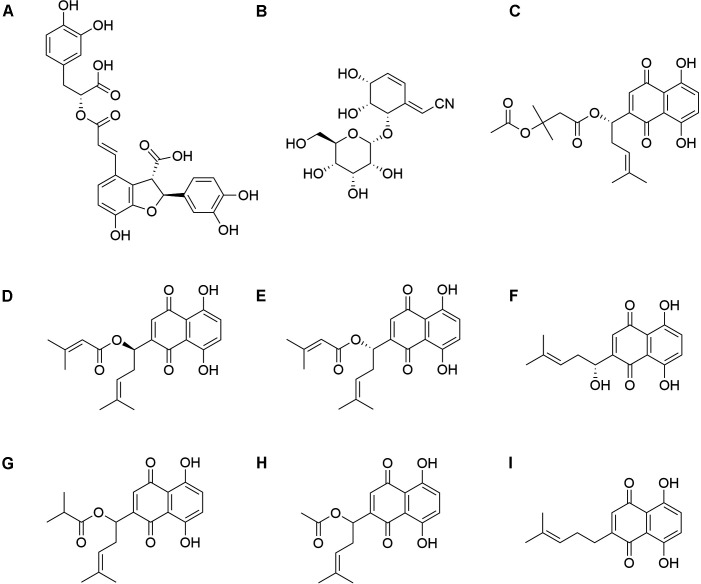
Different functional groups and their different positions can lead to differences in antibacterial activity (Fig. 1). Lithospermic acid contains two parts: phenylpropanoid and shikonin. β-acetoxyisovaleryl akanin contains multiple different functional groups such as acetoxy and isoprene groups. β, β-dimethylacryloyl akanin contains a dimethylacryloyl functional group. Shikonin has a complex multiring structure. These complex structures may complicate its metabolic pathways in vivo, reduce its solubility in aqueous solutions, limit its absorption and distribution in vivo, reduce its bioavailability and antibacterial activity, and may also affect its binding mode or affinity with target molecules.
Fig 1.
Chemical structure of nine components of Radix lithospermi. (A) Lithospermic acid; (B) cyanoside; (C) β-acetoxyisovaleryl akanin; (D) β,β-dimethylacryloyl shikonin; (E) β,β-dimethylacryloyl akanin; (F) shikonin; (G) isobutyryl shikonin; (H) acetylshikonin; (I) deoxyshikonin.
The isobutyryl functional group in isobutyryl shikonin replaces other functional groups in shikonin, which may alter the electron density and spatial configuration of the molecule. Acetylshikonin has an acetyl functional group substitution. The presence of cyanide groups in cyanoside may lead to unstable reactions of the compound under certain conditions. The structure of β, β-dimethylacryloyl shikonin contains many double bonds, which may cause it to undergo photo-oxidation or other unstable reactions under certain conditions, leading to decomposition or deactivation. These instabilities may reduce the persistence and efficacy of their antibacterial activity. Due to the lack of an oxygen atom in deoxyshikonin, its structure may be more compact and stable, increasing its binding efficiency with target molecules in organisms and reducing its susceptibility to degradation by metabolic enzymes. This may be beneficial for enhancing its antibacterial activity in vivo.
Screening of antibacterial active components of agrimonin in vitro
The antibacterial activities of nine components found in Radix lithospermi were detected using the broth dilution method, including shikosin, lithospermoside, β,β-dimethylacryl shikonin, β,β-dimethyl-acryl-alkannin, lithospermic acid, β-acetoxyisovalerylalkannin, isobutyrylshikonin, acetylshikonin, and deoxyshikonin. The results indicate that deoxyshikonin exerted best antibacterial effects on H. pylori among them (MIC90, 0.5 µg/mL); however, it was found that deoxyshikonin had no inhibitory effects on Klebsiella pneumoniae, P. aeruginosa, Acinetobacter baumannii, S. aureus, and other bacteria, with all MIC90 values exceeding 128 µg/mL (Table 1). The present study clarifies that the main component of lithospermum, which inhibits H. pylori, is deoxyshikonin and thus the focus of subsequent research.
TABLE 1.
MIC of related components of Radix lithospermi (μg/mL)
| Component | HPBS001 | Hp26695 |
Klebsiella
pneumoniae |
Pseudomonas
aeruginosa |
Acinetobacter
baumannii |
Staphylococcus
aureus |
|---|---|---|---|---|---|---|
| Shikonin | 2 | 2 | > 128 | > 128 | > 128 | > 128 |
| Lithosprmoside | > 128 | > 128 | > 128 | > 128 | > 128 | > 128 |
| β,β-Dimethylacrylshikonin | 32 | 32 | > 128 | > 128 | > 128 | > 128 |
| β,β-Dimethylacrylalkannin | 32 | 32 | > 128 | > 128 | > 128 | > 128 |
| Lithospermic acid | > 128 | 128 | > 128 | > 128 | > 128 | > 128 |
| β-Acetoxyisovalerylshikonin | 8 | 16 | > 128 | > 128 | > 128 | > 128 |
| Isobutyrylshikonin | 8 | 8 | > 128 | > 128 | > 128 | > 128 |
| Acetylshikonin | 4 | 2 | > 128 | > 128 | > 128 | > 128 |
| Deoxyshikonin | 1 | 0.5 | > 128 | > 128 | > 128 | > 128 |
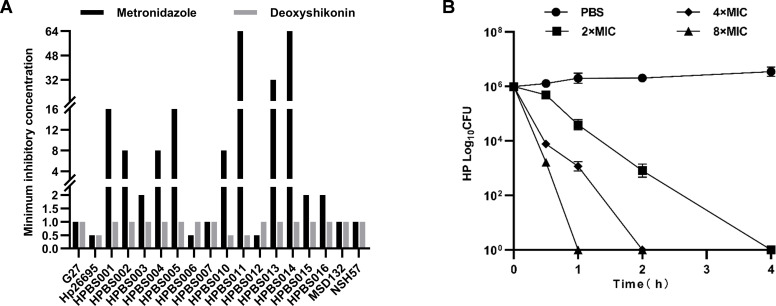
The antibacterial effects of deoxyshikonin on 18 H. pylori strains from different sources were detected using a microdilution method. It was found that deoxyshikonin had good inhibitory effects on all H. pylori strains, both seven sensitive strains and eleven resistant strains, with an MIC of 0.5–1 µg/mL (Table 2), and the minimum bacterial concentration (MBC) 99.9% was 2 µg/mL at 2 hours (Fig. 2).
TABLE 2.
MIC of metronidazole and deoxyshikonin against the different H. pylori strains
| Strains | Drug resistance | MIC (μg/mL) | |
|---|---|---|---|
| Metronidazole | Deoxyshikonin | ||
| G27 | Sensitive | 1 | 1 |
| Hp26695 | Sensitive | 0.5 | 0.5 |
| HPBS001 | Resistant to LEV, CLA, and MET | 16 | 1 |
| HPBS002 | Resistant to MET | 8 | 1 |
| HPBS003 | Resistant to CLA | 2 | 1 |
| HPBS004 | Resistant to LEV | 8 | 1 |
| HPBS005 | Resistant to LEV and MET | 16 | 1 |
| HPBS006 | Resistant to CLA | 0.5 | 1 |
| HPBS007 | Resistant to CLA | 1 | 1 |
| HPBS010 | Resistant to LEV, CLA, and MET | 8 | 0.5 |
| HPBS011 | Resistant to CLA and MET | 64 | 0.5 |
| HPBS012 | Sensitive | 0.5 | 1 |
| HPBS013 | Resistant to LEV, CLA, and MET | 32 | 1 |
| HPBS014 | Resistant to LEV, CLA, AMO, and MET | 64 | 1 |
| HPBS015 | Sensitive | 2 | 1 |
| HPBS016 | Sensitive | 2 | 1 |
| MSD132 | Sensitive | 1 | 1 |
| NSH57 | Sensitive | 1 | 1 |
Fig 2.
Antimicrobial activity of deoxyshikonin. (A) The minimum inhibitory concentration (MIC) of deoxyshikonin; (B) the minimum bactericidal concentration (MBC) of deoxyshikonin.
Safety of deoxyshikonin in vivo
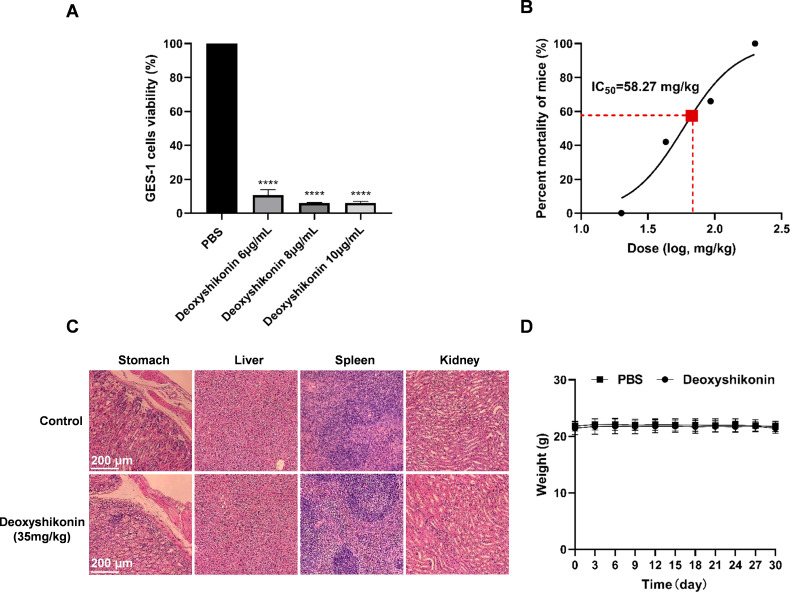
In vitro cytotoxicity experiments showed that deoxyshikonin has certain toxicity to normal gastric epithelial cells GES-1 (Fig. 3A). The LD50 of deoxyshikonin determined by intraperitoneal injection was 58.27 mg/kg, indicating low toxicity and high safety of deoxyshikonin in mice (Fig. 3B). The safety test was conducted via intragastric administration of five times the therapeutic dose of deoxyshikonin (35 mg/kg) to SPF C57BL/6 mice, and no evident pathological damage occurred in the stomach, liver, spleen, and kidneys (Fig. 3C). The mice were weighed for 30 days, consecutively, and there was no significant change in their weights (Fig. 3D).
Fig 3.
Safety of deoxyshikonin in vivo. (A) Cytotoxicity of deoxyshikonin to GES-1 cells; (B) dosage response mortality curves of deoxyshikonin; (C) effects of deoxyshikonin on the organs of mice; (D) effects of deoxyshikonin on the weights of mice.
Antibacterial activity of deoxyshikonin in vivo
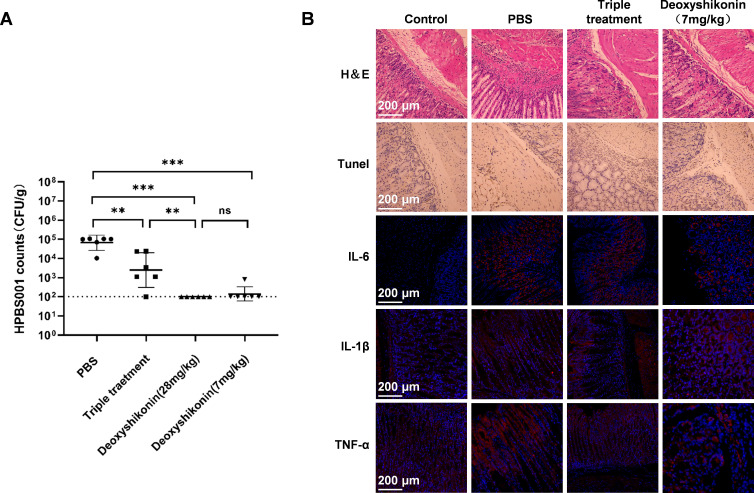
The clinical H. pylori BS001 strain was induced by adaptive colonization and inoculated into C57BL/6 mice to establish model mice with acute gastritis following the process as previously described.The antibacterial activity in vivo of deoxyshikonin was evaluated. It was found that the deoxyshikonin group could produce therapeutic effects significantly better than the triple therapy group. However, a dosage of 7 mg/kg could almost inhibit all H. pylori adhesion and colonization in the stomach (Fig. 4A). HE staining and TUNEL staining were performed on tissues of gastric mucosae. The results showed few apoptotic and inflammatory cells and light tissue damage in the deoxyshikonin treatment group, with no significant difference compared with the normal group (Fig. 4B). The expressions of IL-6, TNF-α, and IL-1β detected through immunofluorescence staining were all significantly reduced (Fig. 4B), indicating that deoxyshikonin inhibited the adhesion and colonization of H. pylori in the gastric mucosa of mice, reduced the inflammatory response, and repaired the damage thereto.
Fig 4.
Antibacterial activity of deoxyshikonin in vivo. (A) Colonization of H. pylori in mice with acute gastritis after treatment with deoxyshikonin; (B) repair of gastric mucosa of mice with acute gastritis after treatment with deoxyshikonin (10×).
Deoxyshikonin inhibits flagellar movement of H. pylori and enhances its membrane permeability and oxidation
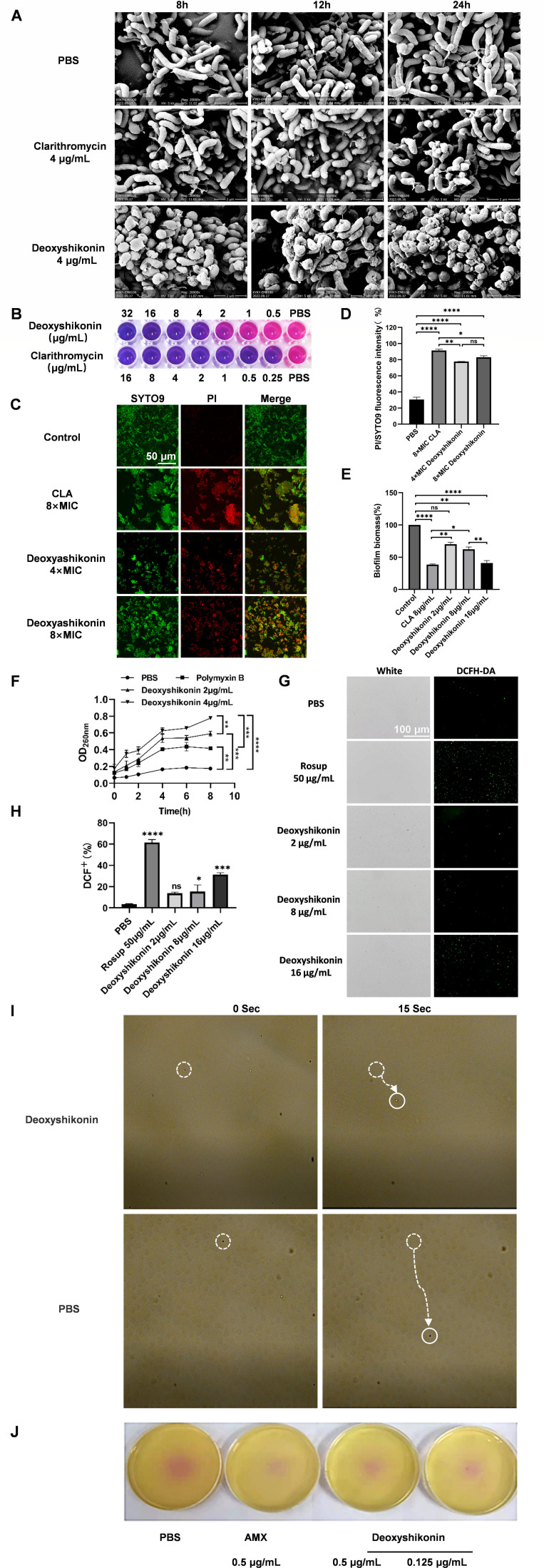
In the present study, the effects of deoxyshikonin on the morphology and structure of H. pylori were detected using an SEM. After treatment with four times the MIC of deoxyshikonin on H. pylori for 24 hours, spherical changes and roughness were detected on the cell surface (Fig. 5A). When detecting the permeability of the H. pylori membrane, it was found that deoxyshikonin destroyed the biofilm (Fig. 5B through E) and enhanced the permeability of the cell membrane in a concentration-dependent manner, and a dose of 2 µg/mL could exert better effects on enhancing cell membrane permeability than did polymyxin B (Fig. 5F). When detecting the oxidation of deoxyshikonin on H. pylori, it was found that the oxidation reaction occurred in a concentration-dependent manner and became stronger at 16 µg/mL (Fig. 5G and H). In soft agar and exercise experiments, deoxyshikonin can effectively inhibit the movement of H. pylori flagella (Fig. 5I and J).
Fig 5.
Deoxyshikonin inhibits the growth of H. pylori biofilms and movement of flagella and enhances the permeability of cell membranes as well as oxidation. (A) Morphological and structural damage caused by H. pylori; (B) the effect of deoxyshikonin on H. pylori biofilm detected using Alamar staining; (C) the effect of deoxyshikonin on the H. pylori biofilm detected using a confocal microscope after staining with SYTO9; (D) the effect of deoxyshikonin on the H. pylori biofilm detected quantitatively after I/SYTO9 staining; (E) the effect of deoxyshikonin on the H. pylori biofilm detected through crystal violet staining; (F) the osmotic damage of the membrane of H. pylori cells caused by deoxyshikonin; (G) the oxidation effect of deoxyshikonin on H. pylori as detected quantitatively; (H) the oxidation effect of deoxyshikonin on H. pylori detected using fluorescence staining; (I) the suspension drop method to observe the inhibitory effect of deoxyshikonin on the movement of H. pylori (40 x); (J) detection of the inhibitory effect of deoxyshikonin on H.pylori flagella by soft agar assay.
Advantages and characteristics
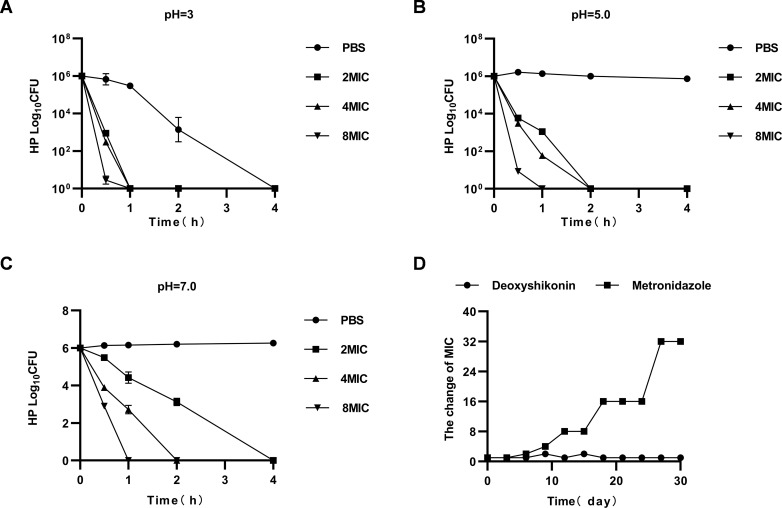
After deoxyshikonin was co-incubated with H. pylori in the culture medium at pH 3.0, pH 5.0, and pH 7.0 for 1 hour, its inhibitory effects were assessed. The results suggest that the number of viable bacteria of pH 3.0 and 5.0 in the PBS group were significantly decreased over time, especially at pH 3.0 when the number of viable bacteria was less than 10, whereas no significant change was observed in the PBS group (Fig. 6A through C). This finding indicates that deoxyshikonin demonstrated better antibacterial effects at a faster rate in an acidic environment. H. pylori was not easily resistant to deoxyshikonin (Fig. 6D).
Fig 6.
Acid resistance testing for deoxyshikonin and detection of H. pylori resistance. (A) MBC of deoxyshikonin at pH = 3.0; (B) MBC of deoxyshikonin at pH = 5.0; (C) MBC of deoxyshikonin at pH = 7.0; (D) detection of drug resistance of H. pylori to deoxyshikonin and metronidazole.
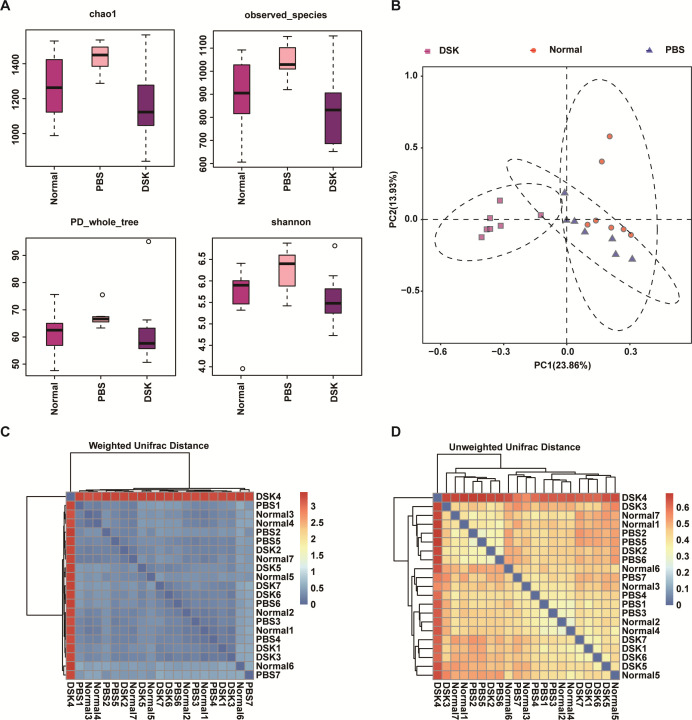
In addition, the inhibitory effect of deoxyshikonin on 17 non-H. pylori species was examined. The MIC of deoxyshikonin against 17 non-H. pylori species, including Escherichia coli, exceeded 128 µg/mL, except for the MIC of 8 µg/mL against Bacillus subtilis, indicating that deoxyshikonin has good specificity for H. pylori (Table 3) and shows no effect on the intestinal microbiota (Fig. 7). Deoxyshikonin reduced the difference in gut microbiota abundance caused by H. pylori modeling. Moreover, the distance between the gut microbiota in each group was roughly equal, indicating that the treatment with deoxyshikonin did not increase the difference in gut microbiota.
TABLE 3.
MIC of deoxyshikonin against different non-H. pylori species
| Species | MIC (μg/mL) |
|---|---|
| Lactobacillus campylosus | > 128 |
| Morganella morganii | > 128 |
| Enterobacter hormaechei | > 128 |
| Staphylococcus haemolyticus | > 128 |
| Proteus mirabilis | > 128 |
| Escherichia coli | > 128 |
| Staphylococcus aureus | > 128 |
| Pseudomonas aeruginosa | > 128 |
| Klebsiella pneumoniae | > 128 |
| Acinetobacter baumannii | > 128 |
| Oligotrophic bacterium maltophilia | > 128 |
| Acetobacter pasteurianus | > 128 |
| Saccharomyces cerevisiae | > 128 |
| Bifidobacterium longum | > 128 |
| Enterococcus faecalis | > 128 |
| Bacillus subtilis | 8 |
| Campylobacter jejuni | > 128 |
Fig 7.
Diversity of intestinal flora in mice treated with deoxyshikonin. (A) Chao, observed species, PD whole tree, and Shannon indices reflect the α-diversity; (B) principal component analysis (PCA) to evaluate the intestinal flora β-diversity; (C) weighted analysis to evaluate intestinal microflora β-diversity; (D) unweighted analysis and evaluation of intestinal flora β-diversity.
DISCUSSION
The plants have antibacterial properties, but it is difficult to determine their antibacterial components (17, 18). The antibacterial activity of nine components was analyzed in purple grass. The results showed that deoxyshikonin had the best antimicrobial effect against H. pylori, with an MIC of 0.5 µg/mL, and was also effective against strains resistant to metronidazole, comparable to clinical drugs such as levofloxacin (19, 20). Whether a compound can be used as an ideal drug depends on their antibacterial effects and safety in vivo and in vitro (18). In our animal experimental studies, 7 mg/kg of deoxyshikonin reduced the colonization of H. pylori in mice, repaired the damage to gastric mucosa, and significantly decreased the release of inflammatory factors. The treatment effect in vivo was significantly better than that of the standard triple therapy; a large dose of deoxyshikonin (35 mg/kg) had no toxic effect on mice, thus meeting the requirements of the lead drug for safety and effectiveness. In addition, deoxyshikonin shows advantages such as good acid resistance, strong specificity, and less resistance to H. pylori, which can survive in the acidic environment of the stomach, whereas some antibacterial drugs are degraded by gastric acid or pepsin. This leads to a significant decrease in efficacy (21), making it necessary to combine them with antacids to achieve therapeutic effects. After co-culturing deoxyshikonin with H. pylori in media at different pH, deoxyshikonin exerted better antibacterial effects at a faster rate in acidic environments, exhibiting excellent acid resistance. Furthermore, this also indicated the reduced need for acid suppressants, reduced medical costs, and enhanced patient compliance. In recent years, it has been reported that the primary resistance rate of H. pylori to clarithromycin is 20%–50%, to levofloxacin 20%–50%, and metronidazole is 40%–70%. However, H. pylori may develop double, triple, or multiple resistance to these antibiotics, and the dual resistance rate of clarithromycin and metronidazole exceeds 25%. This finding implies that H. pylori is prone to develop resistance to these antibiotics, leading to very severe resistance. We induced the resistance of H. pylori to deoxyshikonin, but after 30 days of induction, no significant resistance was found, indicating that H. pylori is not easily resistant to deoxyshikonin, which is a rare advantage. However, the MIC of metronidazole increased 32-fold. In addition, our antibacterial spectrum and gut microbiota detection analyses found that the antibacterial spectrum of deoxyshikonin is relatively narrow and will not cause an imbalance in the intestinal microbiota, which is in agreement with the current characteristics of precise medication. Meanwhile, the drug sensitivity tests we conducted on other non-H. pylori strains are reasonable based on the differences in results caused by strains, experimental methods, and compound sources. This result indicates that deoxyshikonin has good antibacterial effects and safety in vitro and in vivo and the advantages of acid resistance, specificity, and less resistance to H. pylori. Therefore, it is a promising lead drug for research and development.
Conclusion
The present study confirms that the main active ingredient found in comfrey which inhibits H. pylori is deoxyshikonin, which has good antibacterial effects both in vivo and in vitro with the advantages such as low toxicity, strong specificity, strong acid resistance, and difficulty in rendering H. pylori drug resistance. Therefore, it is an ideal, potential lead drug to alleviate the severe problem of H. pylori resistance.
MATERIALS AND METHODS
Culture and collection of standard and clinically isolated H. pylori strains
H. pylori strains (Standard 26695, NSH57, MSD132, and G27, all provided by Professor Bi Hongkai of Nanjing Medical University; H. pylori BS001-H. pylori BS016 isolated in our laboratory) and S. aureus were used (Table 4). S. aureus and other bacteria were cultured on nutrient agar plates (Baisi, BS1101) at 37°C for 1–2 days. The colonies were scraped from the plate, placed in the nutrient broth liquid medium (Baise, BS1002), and shaken for 1–2 days to obtain the S. aureus suspension. H. pylori strains were removed from the refrigerator at −80°C and centrifuged to remove the preservation solution, and thereafter, the precipitate was evenly coated on a Columbia blood agar base (OXOID, Lot 3484622) medium (OXOID, Lot 2179850) containing 10% calf serum (Pingrui, Lot 20220922) and cultured at 37°C (85% nitrogen [N2], 5% oxygen [O2], and 10% carbon dioxide [CO2]) for 3–4 days. The colonies were scraped from the medium, inoculated in a brain heart infusion (BHI) medium (OXOID, Lot 3555372) containing 10% calf serum, and placed under microaerobic conditions for shaking culture for 2–3 days.
TABLE 4.
Bacterial information
| Species | Origin | Medium composition |
|---|---|---|
| Lactobacillus campylosus | Guangdong Microbial Culture Collection Centre | Peptones, beef extract, yeast extract, glucose, sodium acetate, ammonium citrate dibasic, Tween-80, dipotassium hydrogenphosphate, magnesium sulfate heptahydrate, manganese sulfate heptahydrate, calcium carbonate, distilled water, and agar (no need for liquid medium) |
| Morganella morganii | Guangdong Microbial Culture Collection Center | Nutrient agar/broth |
| Enterobacter hormaechei | Clinical separation | Nutrient agar/broth |
| Staphylococcus haemolyticus | Clinical separation | Nutrient agar/broth |
| Proteus mirabilis | Clinical separation | Nutrient agar/broth |
| Escherichia coli | Clinical separation | Nutrient agar/broth |
| Staphylococcus aureus | Clinical separation | Nutrient agar/broth |
| Pseudomonas aeruginosa | Clinical separation | Nutrient agar/broth |
| Klebsiella pneumoniae | Clinical separation | Nutrient agar/broth |
| Acinetobacter baumannii | Clinical separation | Nutrient agar/broth |
| Oligotrophic bacterium maltophilia | Clinical separation | Nutrient agar/broth |
| Acetobacter pasteurianus | Guangdong Microbial Culture Collection Centre | Glucose, Radix scutellariate, calcium carbonate, absolute ethanol, distilled water, and agar (no need for liquid medium) |
| Saccharomyces cerevisiae | Guangdong Microbial Culture Collection Centre | Peptone, glucose, yeast extract, malt extract, distilled water, and agar |
| Bifidobacterium longum | Guangdong Microbial Culture Collection Centre | Bacto Soytone, typtone, yeast extract, glucose, saline solution, L-cysteine, 0.1% resazurin, distilled water, and agar (no need for liquid medium) |
| Enterococcus faecalis | Clinical separation | Nutrient agar/broth |
| Bacillus subtilis | Clinical separation | Sabourauds |
| Campylobacter jejuni | Clinical separation | Columbia agar base/BHI broth (with 10% serum) |
Cell culture
MGC803, BGC823 (both purchased from KeyGEN Biotech, Nanjing, China), and Ges-1 gastric epithelial cells (RRID: CVCL_EQ22) were inoculated on an RPMI medium 1640 (KeyGEN Biotech, KGM31800-500) and dosed with 10% fetal bovine serum (FBS; OriCell, FBSST-01033–500). The cells were cultured in a cell incubator containing 5% CO2 at 37°C.
Determination of the MIC
The first well of a 96-well plate was added with 173.6 µL medium, then 6.4 µL shikosin (Chengdu Herbpurify Biological Co., Ltd, CAS:517–89-5), lithospermoside (Chengdu Herbpurify Biological Co., Ltd, CAS: 63492–69-3), β,β-dimethylacrylshikonin (Chengdu Herbpurify Biological Co., Ltd, CAS: 24502–79-2); β,β-dimethyl-acryl-alkannin (Chengdu Herbpurify Biological Co., Ltd, CAS:34539–65-6), lithospermic acid (Chengdu Herbpurify Biological Co., Ltd, CAS:28831–65-4), β-acetoxyisovalerylalkannin (Chengdu Herbpurify Biological Co., Ltd, CAS: 69091–17-4); isobutyrylshikonin (Chengdu Herbpurify Biological Co., Ltd, CAS:52438–12-7), acetylshikonin (Chengdu Herbpurify Biological Co., Ltd, CAS: 24502–78-1); and deoxyshikonin (Chengdu Herbpurify Biological Co., Ltd, CAS: 43043–74-9). Diluting it to the ninth well, the concentrations of the drug were 128, 64, 32, 16, 8, 4, 2, 1 and 0.5 µg/mL from the first well to the eighth, respectively. Negative well (sterile, medium, and drug only) and positive well (no drug, only medium, and bacteria) were used as the control. H. pylori were diluted tenfold with OD600 = 0.3 (1.0 × 108 culture-forming units [CFU]/mL). The bacterial solution (10 µL) was distributed across the first to the ninth wells and the positive well (working concentration, 1 × 106 CFU/mL) and placed under microaerobic conditions and shaking culture for 72 hours, after which the results were noted. The final working concentration of other bacteria was 1 × 105 CFU/mL and shaking culture for 16–18 hours. Metronidazole was the positive control drug, and PBS was the negative control drug. An enzyme-linked immunosorbent assay was used to measure absorbance interpretation results. Each experiment was repeated three times.
Determination of the MBC and acid resistance of deoxyshikonin
Prepare the brain–heart culture medium (pH 3.0, pH 5.0, and pH 7.0), and use the MIC detection method to double dilute so as to make the final concentration of deoxyshikonin 2, 4, and 8 µg/mL PBS as the positive control, put the 96-well plate under the micro-aerobic condition for oscillatory culture, dilute the H. pylori G27 solution (100 times, 1,000 times, etc.) after a certain time of drug action (such as 2 hours), and then coat it on the Columbia agar plate, and place it in a three-gas incubator for 4–5 days. Calculate the number of bacteria growing on the agar plate. The minimum bactericidal concentration (MBC) was the drug concentration with 99.9% inhibition of bacteria.
Antimicrobial resistance testing
H. pylori G27 to the logarithmic phase, adjust the concentration of OD600 to 0.3 (1 × 108 CFU/mL), dilute 100 times to 1 × 106 CFU/mL, take 5 mL of the bacterial suspension, and add in a 50-mL sealed membrane centrifuge tube. Multiply one-fourth of the MIC concentration of deoxynivalenol by 5 mL as the dose. Check MIC every 3 days for a total of 30 days. The drug concentration was adjusted with changes in the MIC. The positive drug control group was metronidazole.
Experimental animal modeling, detection of the amount of colonization, and pathological analyses
Thirty specific pathogen-free (SPF) C57BL/6 mice aged 6 weeks were purchased from Changsha Tianqin Biological Co., Ltd; SPF animal license number: SCXK Gui 2017–0004; animal experiment ethics number: 2019112501. Mice were administered 0.5 mL H. pylori suspension (1 × 109 CFU/mL) prepared by BHI (the acclimated bacterium H. pylori BS001 after domestication and validation in mice using strain H. pylori BS001), we administered by gavage once a day, on 5 consecutive days. After 14 days of observation, the mice models were established. Thereafter, the mice were divided into the omeprazole (138.2 mg/kg) (Shanxi Jinhua Bright Star Pharmaceutical Co., Ltd, 20200901) +amoxicillin (28.5 mg/kg) (Hainan Simcere Pharmaceutical Group Co., Ltd, 02–170404) +clarithromycin (14.3 mg/kg) (Wanglintang Pharmaceutical Co., Ltd, 220601) group (the standard triple therapy group); the omeprazole (138.2 mg/kg) +deoxyshikonin (28 mg/kg) high-concentration group; the omeprazole (138.2 mg/kg) +deoxyshikonin (7 mg/kg) low-concentration group; the phosphate-buffered saline (PBS) group (mice infected with H. pylori but untreated); the negative control group (mice not infected with H. pylori). We administered medication by gavage once a day for 3 consecutive days. On the second day after drug withdrawal, the mice were euthanized, and half of the tissue from their stomach was excised. The tissue specimens were weighed, ground, diluted, coated on a Columbia plate (containing calf serum and antibiotics) under microaerobic conditions (85% N2, 5% O2, and 10% CO2), and cultured for 3–4 days. Thereafter, the amount of H. pylori colonized was calculated, with pathological analyses performed on the other half of the collected stomach tissue. This method is found in Huang’s article (22).
Pathological safety assessment in vivo in mice
SPF C57BL/6 mice aged 6 weeks (the same mice as described in Section 2.4) were administered a dose of 35 mg/kg (five times the aforementioned treatment dose of 7 mg/kg) for 30 days, consecutively, and weighed. They were also weighed the day before and after gavage with tissues collected from the liver, spleen, and kidneys for hematoxylin and eosin (HE), TUNEL, IL-6, IL-1β, and TNF-α staining.
Detection of the effect of deoxyshikonin on the morphological structure of H. pylori using scanning electron microscopy (SEM)
H. pylori G27 solution (10 mL) of cells in the logarithmic phase (1.0 × 108 CFU/mL) was treated with deoxyshikonin and clarithromycin at concentrations adjusted to 4 µg/mL; incubated for 8, 12, and 24 hours, and centrifuged to remove the preservation solution. Low-speed centrifugation at 1,000 rpm prevented damage to the morphology of H. pylori. The resulting precipitate was fixed at 4°C overnight using the electron microscope fixative, dehydrated for 10 minutes using ethyl alcohol (30, 50, 70, 90, and 100%) respectively, dehydrated twice using 100% anhydrous ethanol, and centrifuged to obtain precipitate for freeze-drying for 2 days. The samples were coated with gold and observed by using an SEM.
Biofilm inhibition experiments
The H. pylori G27 solution in the growth phase was distributed across a 96-well plate (LABSELECT, 11510), 200 µL/well at OD600 = 0.1, cultured under microaerobic conditions for 3 days, treated with clarithromycin and deoxyshillin both at concentrations as shown in the results, and co-incubated for 24 hours. Thereafter, the samples were treated with 10 uL Alamar blue (Solarbio A7631), and photos were taken after 4 hours. The inoculations were repeated with 0.1% crystal violet staining (McLean, CAS: 548–62-9); quantitative tests using a multifunctional enzyme marker (BioTek, America) and confocal experiments (Olympus, FV3000) using a Thermo Fisher L-7012 kit were performed on the samples. Three compound holes were established per group. Fluorescence quantification by PI/Syto9 was performed using Image J’s Split Channel feature, which splits the image to be quantified into red, green, and blue channels, and then automatically counts the fluorescence of the red and green channels, respectively.
Effect of deoxynivalenol on the permeability of H. pylori cell membranes
H. pylori G27 in the logarithmic stage were centrifuged at 5,000 rpm for 10 minutes, collected, washed thrice using PBS, and resuspended. Thereafter, the concentration of the bacterial suspension A was adjusted to OD600 = 0.3 (1 × 108 CFU/mL). Drug solutions and the diluted bacterial solutions were mixed according to different volumes with the final concentrations of deoxyshikonin being twice the MIC and four times the MIC, respectively, and then placed under microaerobic conditions for shaking culture for 0, 1, 2, 4, 6, and 8 hours. The light absorption values of the bacterial solutions were measured at 260 nm by using an ultraviolet spectrophotometer, with the untreated bacterial suspension used as the blank control. These procedures were repeated three times for each sample.
Reactive oxygen species (ROS) experiments
The H. pylori G27 could be cultured until they reached the logarithmic stage with their concentration adjusted to 1 × 107 CFU/mL, treated with 10 µM of DCFDA (Beyotime, S0033S), co-inoculated in a three-gas incubator for 1 hour, centrifuged to obtain the precipitate, and washed twice using the PBS solution. Thereafter, the samples were treated with 2, 8, and 16 µg/mL deoxyshikonin, with PBS and Rosup in the kit used as negative and positive controls, respectively. After incubation for 2 hours, the samples were observed by using a fluorescence microscope (Olympus, BX53).
Flagella movement soft agar experiment
The standard G27 strains that had grown in BHI were collected with the concentration adjusted to OD600 = 3. A pipette was used to inoculate 1 µL bacterial solution into the semi-solid medium containing 0.3% agar. The medium was a drug plate containing 10% calf serum, 0.3% agar, and drugs at specified concentrations (0.125 and 0.5 µg/mL deoxyshikonin; 0.5 µg/mL amoxicillin). After 5 days, the diameters of H. pylori colonies growing on the medium were measured.
The suspension drop method to observe the movement of shikonin against H. pylori
The logarithmic growth period G27 was taken, and the OD of the bacterial suspension was adjusted to 0.3 (1 × 108 CFU/mL); a six-hole plate was used where the bacterial solution and deoxyshikonin 2 µg/mL were placed under micro-aerobic conditions and cultured for 8 hours. The bacterial solution was resuspended with PBS, and 1 µL was placed on a concave slide and observed using an ordinary optical microscope.
Statistical analysis
The data in the bar chart were expressed as mean standard error (SEM: standard error of mean). All statistical analyses were performed using Graphpad PRISM software, Version 7.0. All data were subjected to Student’s t-tests and single-factor analysis of variance (ANOVA).
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (32060018); Guangxi Science and Technology Major Project Guike (GUIKEAA23073012); and Changzhou Science and Technology Project Fund (No. CJ20210012).
The authors declare they have no competing financial interests or personal relationships that could have influenced the work reported in this paper.
Contributor Information
Liang Huang, Email: youyihuangl@163.com.
Ai-xing Guan, Email: 425117899@qq.com.
Yan-Qiang Huang, Email: hyq77615@163.com.
Anne-Catrin Uhlemann, Columbia University Irving Medical Center, New York, New York, USA.
REFERENCES
- 1. Wenzhong L. 2016. Strive to improve the eradication rate of Helicobacter pylori (I). Gastroenterology 21:450–454. doi: 10.3969/j.issn.1008-7125.2016.08.002 [DOI] [Google Scholar]
- 2. Kotilea K, Bontems P, Touati E. 2019. Epidemiology, diagnosis and risk factors of Helicobacter pylori infection. Adv Exp Med Biol 1149:17–33. doi: 10.1007/5584_2019_357 [DOI] [PubMed] [Google Scholar]
- 3. Yu L, Jun M, Liwen S, Jian L. 2021. Research progress in the treatment of gastric Helicobacter pylori infection with traditional Chinese and western medicine. Mil Med J 64:786–788. doi: 10.3969/j.issn.1000-9736.2021.08.028 [DOI] [Google Scholar]
- 4. Hongzhang L, Xuehua X, Yunhui L, e CJ, Wanli Z, Qiang C. 2020. Study on drug resistance and eradication treatment of refractory Helicobacter pylori gastritis. Zhejiang Med J 42. doi: 10.12056/j.issn.1006-2785.2020.42.12.2018-3188 [DOI] [Google Scholar]
- 5. Wandai Z, Fulian H, Shudong X, Zhimin X. 2010. Epidemiological investigation of Helicobacter pylori infection in natural populations in China. Mod Dig Interv Diagn Treat 5:265–270. doi: 10.3969/j.issn.1672-2159.2010.05.001 [DOI] [Google Scholar]
- 6. Shumei Z, Qi L. 2020. Selection of antibiotic resistance in Helicobacter pylori eradication program. Fam Dr 06:196–199. [Google Scholar]
- 7. Shanshan Y, Hui Y, Xuezhi Z. 2020. Research progress in the treatment of drug-resistant Helicobacter pylori with integrated traditional Chinese and western medicine. Tradit Chin Med 39:178–181. doi: 10.16025/j.1674-1307.2002.022 [DOI] [Google Scholar]
- 8. National Pharmacopoeia Commission . 2020. Pharmacopoeia of the people’s republic of China: 2020 edition, p 355–356. Beijing: China Medical Science and Technology Press. [Google Scholar]
- 9. Jie H, Xinchu W, Kaishun B. 2007. In vitro anticancer effects of active ingredients in comfrey. Fine Chem 05:473–476. doi: 10.3969/j.issn.1006-2084.2020.02.021 [DOI] [Google Scholar]
- 10. Zhang S, Wang Y. 2020. Deoxyshikonin inhibits cisplatin resistance of non-small-cell lung cancer cells by repressing Akt-mediated ABCB1 expression and function. J Biochem Mol Toxicol 34:e22560. doi: 10.1002/jbt.22560 [DOI] [PubMed] [Google Scholar]
- 11. Gilbert-Girard S, Reigada I, Savijoki K, Yli-Kauhaluoma J, Fallarero A. 2021. Screening of natural compounds identifies ferutinin as an antibacterial and anti-biofilm compound. Biofouling 37:791–807. doi: 10.1080/08927014.2021.1971655 [DOI] [PubMed] [Google Scholar]
- 12. Vukic MD, Vukovic NL, Djelic GT, Popovic SL, Zaric MM, Baskic DD, Krstic GB, Tesevic VV, Kacaniova MM. 2017. Antibacterial and cytotoxic activities of naphthoquinone pigments from Onosma visianii Clem. EXCLI J 16:73–88. doi: 10.17179/excli2016-762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brigham LA, Michaels PJ, Flores HE. 1999. Cell-specific production and antimicrobial activity of naphthoquinones in roots of lithospermum erythrorhizon. Plant Physiol 119:417–428. doi: 10.1104/pp.119.2.417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rajbhandari M, Schoepke TH, Mentel R, Lindequist U. 2007. Antibacterial and antiviral naphthazarins from maharanga bicolor. Pharm 62:633–635. [PubMed] [Google Scholar]
- 15. Damianakos H, Kretschmer N, Sykłowska-Baranek K, Pietrosiuk A, Bauer R, Chinou I. 2012. Antimicrobial and cytotoxic isohexenylnaphthazarins from arnebia euchroma (royle) jonst. (Boraginaceae) callus and cell suspension culture. Molecules 17:14310–14322. doi: 10.3390/molecules171214310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang S, Wang J, Xu W, Liu Y, Wang W, Wu K, Wang Z, Zhang X. 2015. Antibacterial effects of traditional Chinese medicine monomers against Streptococcus pneumoniae via inhibiting pneumococcal histidine kinase (VicK). Front Microbiol 6:479. doi: 10.3389/fmicb.2015.00479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jun Z, Xinxin L, Zhaoxu Y, Lei Z. 2003. Evaluation of the antibacterial activity of levofloxacin against Helicobacter pylori in vitro. Chin J Antibiotics 05:292–294. doi: 10.14053/j.cnki.ppcr.202104004 [DOI] [Google Scholar]
- 18. Weijia H. 2018. Some problems and countermeasures in the clinical application of antibacterial drugs at present. Electron J Cardiovasc Dis Integr Tradit Chin West Med 6:26–27. doi: 10.16282/j.cnki.cn11-9336/r.2018.08.08.017 [DOI] [Google Scholar]
- 19. Yamaoka Y. 2008. Increasing evidence of the role of Helicobacter pylori SabA in the pathogenesis of gastroduodenal disease. J Infect Dev Ctries 2:174–181. doi: 10.3855/jidc.259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hanyi S, Xinjie Y, Yuqi Z, Cong G. 2019. Li Yan comparison of drug sensitivity detection methods for Helicobacter pylori and clinical analysis of individualized treatment in 200 cases. Chin J Pract Intern Med 39:813–816. doi: 10.19538/j.nk2019090117 [DOI] [Google Scholar]
- 21. Huiqin Y, Li M. 1995. Some antibacterial drugs susceptible to acid and alkali. Int Med J 06:48–49. [Google Scholar]
- 22. Huang Y, Hang X, Jiang X, Zeng L, Jia J, Xie Y, Li F, Bi H. 2019. In vitro and in vivo activities of zinc linolenate, a selective antibacterial agent against Helicobacter pylori. Antimicrob Agents Chemother 63:e00004-19. doi: 10.1128/AAC.00004-19 [DOI] [PMC free article] [PubMed] [Google Scholar]