Abstract
Quercetin, a major representative of the flavonol subclass found abundantly in almost all edible vegetables and fruits, showed remarkable therapeutic properties and was beneficial in numerous degenerative diseases by preventing lipid peroxidation. Quercetin is beneficial in different diseases, such as atherosclerosis and chronic inflammation. This study aims to find out the anticancer activities of quercetin and to determine different mechanisms and pathways which are responsible for the anticancer effect. It also revealed the biopharmaceutical, toxicological characteristics, and clinical utilization of quercetin to evaluate its suitability for further investigations as a reliable anticancer drug. All of the relevant data concerning this compound with cancer was collected using different scientific search engines, including PubMed, Springer Link, Wiley Online, Web of Science, SciFinder, ScienceDirect, and Google Scholar. This review demonstrated that quercetin showed strong anticancer properties, including apoptosis, inhibition of cell proliferation, autophagy, cell cycle arrest, inhibition of angiogenesis, and inhibition of invasion and migration against various types of cancer. Findings also revealed that quercetin could significantly moderate and regulate different pathways, including PI3K/AKT-mTORC1 pathway, JAK/STAT signaling system, MAPK signaling pathway, MMP signaling pathway, NF-κB pathway, and p-Camk2/p-DRP1 pathway. However, this study found that quercetin showed poor oral bioavailability due to reduced absorption; this limitation is overcome by applying nanotechnology (nanoformulation of quercetin). Moreover, different investigations revealed that quercetin expressed no toxic effect in the investigated subjects. Based on the view of these findings, it is demonstrated that quercetin might be considered a reliable chemotherapeutic drug candidate in the treatment of different cancers. However, more clinical studies are suggested to establish the proper therapeutic efficacy, safety, and human dose.
1. Introduction
Cancer refers to the condition when tumor cells can proliferate and invade other tissues around the tumor cell by halting tumor-suppressing genes and upregulating oncogenes, genomic instability, and intracellular signaling cascades [1]. Cancer is a significant global health burden. Cancer is the second leading cause of death in many nations, after heart disease. Every year, a significant number of people, approximately ten million, get cancer globally and almost fifty percent of these patients ultimately die from the disease [2]. According to the survey by the International Agency for Research on Cancer (IARC), 20 million new cancer patients will be diagnosed and almost 9.7 million patients died of cancer in 2022. By 2030, it is projected that there will be a significant increase in the current burden of cancer with an annual occurrence of 22 million new cancer cases and 13 million deaths due to cancer [3, 4]. In Bangladesh, the number of new cancer cases is 1,67,256 and the number of deaths is 1,16,598 due to cancer in 2022 [5].
In developing and low-income countries, multiple aspects of people's lifestyles are the major causes of cancer including smoking, low fruit and vegetable intake, sexual transmission of human papillomavirus, and alcohol drinking. On the other hand, in highly developed countries, significant causes of cancer are drinking alcohol, smoking, overweight, and obesity [6]. IARC also investigated several factors associated with cancer development such as salted fish, sunlight, pharmaceuticals, hormones, tobacco, parasites, bacteria, fungi, herbs, and wood dust. Different causes of cancer were also identified by the World Cancer Research Fund and the American Institute for Cancer Research which include red meat, beta carotene, low fiber diets, processed meats, not breastfeeding, increased adult height, and sedentary lifestyles [7]. Cancer's symptoms and risks can affect a patient's quality life. Treatment, disease acceptance, organ displacement, psychological distress, pain and duration of disease, etc., could affect a patient's quality of life [8]. Numerous transcription factors and proteins (NF-κB, AP-1 and STAT3, cyclooxygenase-2, interleukin-1, interleukin-6, chemokines, tumor necrosis factor, 5 lipoxygenase, matrix metalloproteases, vascular endothelial growth factor, and adhesion molecules) cause inflammation in cancer [9]. There are different conventional treatment strategies like chemotherapy, radiotherapy, and surgery, but they have several side effects including they also destroy the healthy cells, nausea, fatigue, hair loss, vomiting, etc., and they can also cause death in different cases [10]. Therefore, it is required to apply alternate concepts or approaches for the prevention of cancer [11]. In recent times, several advanced approaches have been made to avoid these side effects including ablation therapy, targeted therapy, nanoparticles, natural antioxidants, natural compounds, and ferroptosis-based therapy [12].
From early human history, natural products have been studied and used to treat disease [13]. The use of natural products, extracted from plants, fungi, and herbs as therapeutic compounds is considered as a significant precursor to modern medicine due to their limited side effects, reliable safety records, and cost-effectiveness. These natural compounds have two applications, being used as cancer therapeutics and chemopreventive chemical substances [14–16]. Besides, natural products have various significant biochemical properties and have shown remarkable anticancer activity for more than fifty years [17].
Quercetin, a unique bioflavonoid, chemically known as 3, 3′, 4′, 5, 7-pentahydroxyflvanone (synonym 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy- 4H-chromen-4-one), found widely in different food products including berries, apples, cauliflower, tea, cabbage, nuts, onions, broccoli, and berries, which has numerous pharmacological properties including anticancer activities, antiaging, anti-inflammatory [18, 19], antiviral activities, as well as reducing lipid peroxidation, platelet aggregation, and capillary permeability [19, 20]. It has more biological properties, including antioxidant [21], antifungal [22], anticarcinogenic, hepatoprotective [23], and cytotoxic activity [24]. It is demonstrated to accumulate in the lungs, liver, kidneys, and small intestines, and it is extracted through the renal, fecal, and respiratory systems [25].
Different in vitro and some animal models studies have shown that quercetin could diminish the growth of cancer cells such as breast, bladder, colon, colorectal, prostate, and lung cancer cells [26–29]. Moreover, quercetin has significant antioxidant properties which can prevent reactive oxygen species (ROS) and trigger DNA damage and also mutational changes [30]. Different clinical and preclinical studies showed that quercetin could alleviate cell division, the invasion, and migration of different cancer cells by various types of mechanisms and pathways like apoptosis, cytotoxicity, AKT pathways, JAK/STAT pathways, ferroptosis, angiogenesis, etc. [31–33].
The novelty of this study from other previous studies is that our study describes the pharmacokinetic properties and anticancer activity of quercetin in monotherapy and nanotherapy, along with different anticancer mechanisms in various types of cancer cells. This study also demonstrated the toxicological profile of quercetin and the role of quercetin in immunotherapy. We found that different in vivo and in silico studies indicated the synergistic potential of combination and nanotherapy. Complexities with other drugs or nanoparticles significantly enhanced the bioavailability, solubility, and potency of quercetin, which could improve the potentiality of quercetin to develop into a reliable drug for the treatment of cancer. The main aim of this review is to summarize the biopharmaceutical properties of quercetin. Our focus will be on discussing the anticancer activities of quercetin as well as its biological sources. Moreover, our objective is to determine different mechanisms and pathways which are significant for anticancer activities.
2. Methodology
2.1. Literature Search Stratagem
The data were collected (up to August 16, 2024) by searching electronic databases such as PubMed, ScienceDirect, Springer Link, Scopus, Wiley Online, Web of Science, ResearchGate, and Google Scholar with the terms “Quercetin,” then paired with “Cancer,” “Tumor,” “Pathophysiology of cancer,” “Anticancer activity,” “Antiproliferation activity,” “Apoptotic effect,” “Oxidative stress,” “Protective effect,” “Cytotoxic activity,” “Genotoxic activity,” “Carcinogenesis,” “Anti-angiogenic effect,” “Antitumor activity,” “Human cancer,” “Biological activities,” “Biological evaluation,” “Chemical features,” “Pharmacokinetics,” “Biopharmaceutics,” “Medicinal use,” “Pharmacology,” “Pharmacological effects,” “Pharmacological activities,” “In vivo studies,” or “In vitro studies.” No language restrictions were imposed. The studies were thoroughly assessed, with information on the sources, dose, concentration, test system, hypothesized anticancer effect mechanism, and overall conclusion provided. The following are the criteria for inclusion and exclusion.
2.2. Inclusion and Exclusion Criteria
Inclusion criteria were defined as follows: (a) studies performed in different laboratory animals, humans, and their derived tissues or cells; (b) studies of the anticancer activities of quercetin; (c) studies with quercetin in combination with other molecules; (d) studies with or without hypothesized mechanisms of action; (e) studies with the physical and chemical characteristics of quercetin; (f) studies with the biopharmaceutical profiles of quercetin or its preparations; (g) studies with the toxicological profile of quercetin; (h) studies of clinical investigation of quercetin; and (i) studies of the anticancer properties of quercetin investigated up to date 2024.
Exclusion criteria were defined as follows: (i) Studies exhibited duplicate data and/or titles and abstracts that did not meet the inclusion criteria; (ii) quercetin, in conjunction with other studies, sheds light on the current issue; (iii) papers written in languages other than English; (iv) studies do not have complete written content accessible; and (v) case reports, letters, editorials, and commentaries.
2.3. Biological Sources of Quercetin
Medicinal plants are globally recognized as valuable resources for the exploration of new pharmaceuticals [34, 35]. Traditional herbal remedies have long been an integral part of healthcare systems across the world, helping with a wide range of acute and chronic illnesses with little to no side effects. A wide range of medical conditions, including cancer, tuberculosis, diabetes, wound healing, heart disease, pharyngitis, asthma, hypertension, and many more, have traditionally been treated using herbal remedies [36, 37]. Seventy to ninety-five percent of people in underdeveloped nations use medicinal plants as their main source of treatment, according to the World Health Organization (WHO). Despite their extensive usage, only a few medicinal plants have had their phytochemical and phytopharmacological characteristics studied in order to determine their possible therapeutic effects [38].
One of the several flavonoid compounds found naturally in plants, quercetin, has many medicinal uses [39]. Several plant's aerial parts, seeds, roots, and leaves contain the chemical source, as described in published research. The botanical origins of this phytochemical are shown in Table 1.
Table 1.
Botanical sources of quercetin.
| Plant | Part (s) | References |
|---|---|---|
| Alchornea glandulosa | Leaves | [40] |
| Allium cepa L. | — | [41] |
| Bergia ammannioides | Aerial | [42] |
| Fraxinus angustifolia | Leaf and bark | [43] |
| Leonurus sibiricus | Whole plant | [44] |
| Melilotus officinalis | — | [45] |
| Sambucus ebulus L. | Leaves | [46] |
| Rubus niveus | Roots | [47] |
| Tephrosia purpurea | Aerial | [48] |
| Thea sinensis | — | [49] |
| Glycyrrhiza glabra | — | [49] |
| Hypericum perforatum | — | [49] |
| Ginkgo biloba | — | [49] |
| Styphnolobium japonicum L. Schott | Flower and flower bud | [50] |
| Lannea coromandelica | Stem bark | [51] |
| Solidago canadensis L. | Aerial part | [52] |
| Solidago gigantea Ait. | Aerial part | [52] |
| Pseudocydonia sinensis C. K. Schneid | Fruit | [53] |
| Hancornia speciosa | Leave | [54] |
| Waltheria indica L. | — | [55] |
| Quillaja saponaria Mol. | — | [56] |
| Physalis lagascae | Whole plant | [57] |
| Adiantum capillus-veneris | Leave | [58] |
| Clitoria ternatea | Flower | [58] |
| Holarrhena antidysenterica | Seed | [59] |
| Helichrysum armenium subsp. armenium | Aerial part | [60] |
2.4. Pharmacokinetics
Pharmacokinetics (PK) is a significant concept that determines the final clinical success or failure of a drug to treat a disease [61]. To determine the exact concentrations and the time course for a drug to act properly, different pharmacological characteristics like clearance, biological half-life, and bioavailability are need to be taken into consideration. These factors are important to characterize the effects of disease with respect to drug absorption, distribution, excretion, and metabolism (ADME) in each patient [62, 63]. Bioavailability is a major pharmacokinetic parameter which demonstrates the concentration of a nonvascular drug which can enter the systemic circulation through a nonvascular route. The adsorption rate is also another important PK parameter that is evaluated by calculating the value of Cmax and Tmax [64]. However, in new drug discovery and development, ADME effects of drugs are a significant consideration. Besides, inappropriate PK often creates challenges during the drug development process. Moreover, the main reasons for costly failures in developing medications during the later stages were the unfavorable pharmacokinetic characteristics [65, 66]. Different studies demonstrated that low PK and bioavailability are the major causes of medication failure, which represent approximately 40% of the causes [67, 68].
Quercetin (C15H10O7) is a lipophilic compound (yellow crystalline powder) with a high molecular mass, density, and melting point of 302.236 g/mol, 1.799 g/cm3, and 316°C, respectively [69]. The pharmacokinetic properties of quercetin are poor solubility, low bioavailability, poor permeability, and instability [70]. Moreover, quercetin has very poor water solubility. However, different studies demonstrated that the water solubility of quercetin could increase rapidly and significantly with temperature. The water solubility of quercetin varied from 0.00215 g/L at 25°C to 0.665 g/L at 140°C [25, 71]. Furthermore, due to the macronutrient absorption, the oral bioavailability of quercetin is very poor after a single oral dose [72].
So, to develop quercetin as a potential drug, it is necessary to improve its pharmacokinetic properties. In order to enhance the bioavailability of quercetin, various strategies have been employed, including the application of potential drug delivery methods such as inclusion complexes, liposomes, nanoparticles, or micelles, which have been demonstrated to increase efficacy, stability, solubility, and bioavailability [70, 73]. For instance, quercetin nanoparticles have shown numerous advantages including a high degree of encapsulation efficiency, high stability, prolonged release, circulation for a long time, accumulation effectively at tumor sites, and improved therapeutic efficacy [74].
Quercetin also has a low adsorption quality in the gut and stomach after oral intake [75]. The primary adsorption site of quercetin is the small intestine where it enters through the sodium-dependent glucose cotransporters (SGLTs) expressed on the apical membrane of intestinal epithelial cells [76, 77]. After intestinal absorption, quercetin undergoes phase II metabolism which includes different mechanisms catalyzed by sulfotransferases (SULTs), uridine-5′-diphosphate glucuronosyltransferases (UGTs), and catechol-O-methyl-transferases (COMTs) and ejected through bile [78]. Quercetin is highly unstable in alkaline conditions [75]. Quercetin remarkably accumulates in the lungs, liver, kidneys, and small intestines at high concentrations and in the brain, heart, and spleen at low concentrations. The brain contains a low amount of quercetin due to the presence of a specific brain transporter for quercetin. However, the excretion of quercetin occurs through the renal, fecal, and respiratory systems, and it has no carcinogenic effects [25, 79]. The pharmacokinetic activities and bioavailability of quercetin are depicted in Figure 1.
Figure 1.
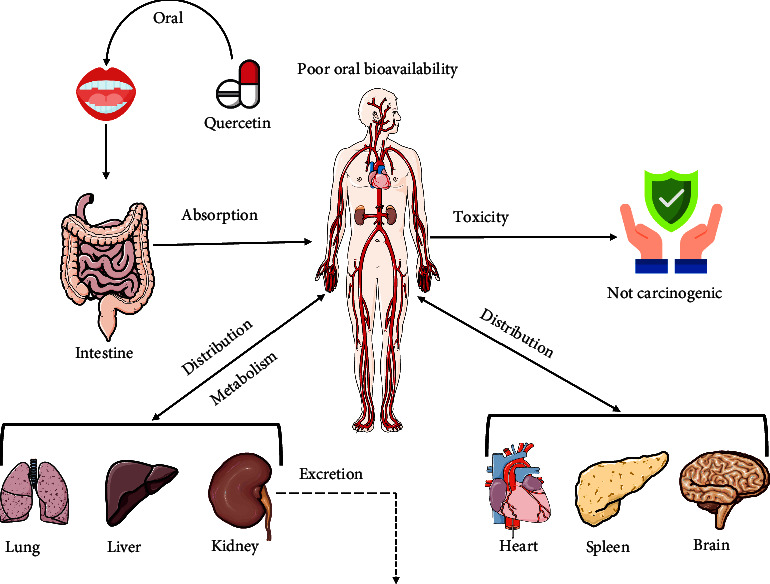
Pharmacokinetics and bioavailability of quercetin.
2.5. Anticancer Activity: Underlying Molecular Mechanistic Analysis
2.5.1. Induction of Oxidative Stress
The term “oxidative stress” refers to the relative abundance of reactive oxygen species (ROS) along with antioxidants, which have a remarkable role in determining the ultimate fate of cells. It has been associated with numerous conditions, including diabetes, cancer, heart disease, and neurological disorders [14, 80]. Oxidative stress is a key factor for the development and progression of tumors, but it is also commonly found during programmed cell death when cells are exposed to different anticancer drug treatments [81]. Reactive species are mainly of four types, including ROS, reactive nitrogen species (RNS), reactive sulfur species (RSS), and reactive chloride species (RCS) which are produced by oxidative metabolism [82]. From these types, ROS are produced in abundance. The extremely high ROS concentrations are cytotoxic, which can reduce cell defense mechanisms like antioxidants [83, 84]. High ROS levels can also activate tyrosine kinases to dissociate Nrf2: Keap1 complex which is responsible for inducing apoptosis [85].
A study conducted by Wu et al. [86] revealed that quercetin could induce oxidative stress in breast cancer cells (MCF-7 BC) by elevating the level of ROS in cellular mitochondria, which also induced apoptosis consequently at concentrations of 100 μM [86]. Another study carried out by Wang and his team members showed that quercetin could trigger oxidative stress in colorectal cancer cells (HepG2, Hep3B, MDA-MB-231, Atg7-WT, and Atg7-KO MEF) by activating lipid peroxidation and increasing ROS levels at a 50 μM concentration [87]. In gastric cancer cells (AGS and GES-1), quercetin could elevate the ROS levels in mitochondria and trigger cell death at 40 μM. It also decreased the expression of NRF2 and induced oxidative stress in cancer cells [88]. Another study also found that quercetin could remarkably elevate oxidative stress in lung cancer cells by increasing ROS concentrations and DNA damage to induce cell death [89]. Ward et al. [28] investigated the capability of quercetin to induce oxidative stress in prostate cancer cells with mutated p53 (LNCaP, DU-145) and PC-3 cells via increasing ROS levels and mitogen-activated extracellular signal-regulated kinase (MEK) at the dose of 40 μM [28]. Wang and his colleagues revealed that quercetin could cause oxidative stress in glioblastoma cancer cells (T98g) by increasing the expression of ROS [90]. So, the overall findings of different studies suggest that quercetin conducts anticancer activity by inducing oxidative stress in cancer cells. A brief overview of pathophysiological events including oxidative stress, apoptosis, antiangiogenesis, cell cycle arrest, autophagy, and the antiproliferative effects of quercetin are shown in Figure 2.
Figure 2.
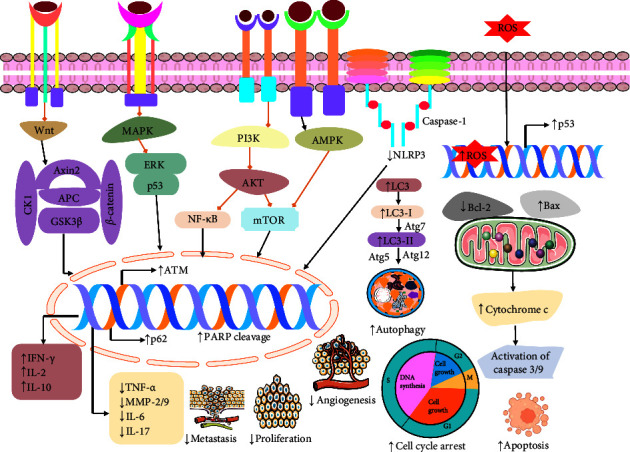
Estimated possible anticancer mechanism of quercetin involving various cellular pathways. ASC: caspase recruitment domain; NLRP3: NLR family pyrin domain containing 3; PARP: poly (ADP-ribose) polymerase; Caspase: cysteine-aspartic acid protease; MEK: mitogen-activated extracellular signal-regulated kinase; Bax: BcL2-associated X protein; Bcl-2: B-cell lymphoma; LC3-II: microtubule-associated protein 1A/1B-light chain 3; p62: ubiquitin-binding protein p62; AKT: protein kinase B; mTOR: mammalian (or mechanistic) target of rapamycin; MMP2 and MMP9: matrix metalloproteinase-2 and matrix metalloproteinase-9; PI3K: phosphoinositide 3-kinase; Beclin1: protein that regulated autophagy; IL-2: interleukin-2; IFN-γ: interferon-gamma; TNF-α: tumor necrosis factor alpha; TNF-β: tumor necrosis factor beta; Atg 5, 7, and 12: autophagy protein 5, 7, and 12; AMPK: AMP-activated protein kinase; NF- κB: nuclear factor-κB; EGRF: epidermal growth factor receptor.
2.5.2. Cell Cycle Arrest
Cell cycle arrest refers to a pause in the cell cycle when it declines to engage in activities related to replication and division. This cycle is one of the most crucial processes in a living cell which is strictly regulated and controlled by many mechanisms to prevent parent cell abnormalities from transferring to the daughter cell. Disrupting such systems of regulation is part of cancer pathogenesis [91, 92]. Cell cycle arrest commonly occurs at the G1/S or G2/M borders. When the regulation of checkpoint arrest is disrupted, cellular damage does not hinder the initiation of the S phase or mitosis. Consequently, inhibiting these stages leads to aberrant responses as a consequence of cellular damage [93, 94]. Retinoblastoma protein RB and the transcription factor p53 are key tumor-suppressing proteins that mainly contribute to inducing cell death and cell cycle arrest. Both proteins have essential functions in controlling the cell division cycle [95]. Through the selective targeting of specific proteins, several medications against cancer hinder the transition of cells from a single phase to another in the cell cycle, resulting in the accumulation of cancer cells at a given stage. The cell cycle is halted, hence impeding the proliferation of cancer cells into tumors and metastasizing to other regions of the body [96, 97].
Studies revealed that quercetin (10−80 μM) had an anticancer effect, including its ability to arrest the cell cycle in T24 bladder cancer cells [26]. Similar promising activity was reported in breast cancer MCF-7 cells which was arresting the cell cycle at the S phase by quercetin (100 μM) [86]. According to a study by García-Gutiérrez et al. [32], quercetin also showed anticancer effects in colon cancer HT-29 cells exposed to BPA, including the ability of cell cycle arrest in the G0/G1 [32]. Additionally, quercetin exhibited a cell cycle arrest in colon cancer cells (HCT 116, COLO 320, and COLO 205) [31]. A study showed that quercetin arrests the cell cycle in esophageal carcinoma cells and downregulates cell proliferation, invasion, and clonal formation [98]. Yang et al. [89] and his team investigated quercetin-induced cell cycle arrest, especially S-phase cell cycle arrest [89]. Another investigation demonstrated that quercetin is also able to arrest the G0/G1 phase and the G2/M phase in the cell cycle of breast cancer, hepatocellular carcinoma, cervical carcinoma, and human ovarian carcinoma in MCF-7, SMMC-7721, HeLa, and SKOV3 cell lines [39]. A study conducted by Hisaka et al. [99] revealed that quercetin (100 μM) showed cell cycle arresting properties in KIM1, KYN-2, KYN-3, HAK-1B, HAK-2, HAK-5, HAK-6, KMCH-1 and KMCH-2 cells [99]. Son and his colleagues demonstrated that quercetin-induced cell cycle arrest specially in G1 phase resulted in reduced cell proliferation in oral squamous cell carcinomas cells (YD10B and YD38) [100]. A study revealed that quercetin (50 μg/mL) suppressed malignant melanoma B16 murine melanoma cell proliferation and cell viability by arresting the cell cycle in the S and G2/M stages [101]. Another investigation demonstrated that quercetin has an anticancer effect in glioblastoma cancer cells (T98g) resulting in induced arrested cells in the S-phase cell cycle by decreasing the growth and migration of cells [90]. Song and his team exhibited that quercetin had an anticancer effect in intrahepatic cholangiocarcinoma ICC cell proliferation and survival, and invasion was suppressed through cell cycle arrest at the G1 phase [102]. All these findings demonstrated the ability of quercetin to block cell cycle progression (Figure 2).
2.6. Ferroptosis Cell Death
Unlike conventional apoptosis and necrosis, ferroptosis is a recently discovered sort of cell death associated with the formation of iron-dependent lipid peroxide. It is characterized by different cytological properties, including a reduction in cell volume and diminished mitochondrial cristae, a fractured outer mitochondrial membrane. It differs from apoptosis and necrosis on the basis of morphology, biochemistry, and genetics [103, 104]. Ferroptosis can be triggered by ROS accumulation and lipid peroxidation. Polyunsaturated fatty acid-containing phospholipids associated with lipid peroxidation are induced by ACSL4, LPCAT3, ALOXs, or POR enzymes. Different pathways related to autophagy including the mTOR/S6KP70 pathway and the NF-κB pathway also induce ferroptosis by increasing iron concentration [105].
A recent study carried out by Zhu and his colleagues revealed that quercetin could induce ferroptosis cell death in oral squamous cell carcinoma cells by triggering mTOR/S6KP70 and lipid peroxidation. Quercetin could also knockdown the levels of GSH and SLC7A11 [106]. Another study found the ferroptosis activity of quercetin in breast cancer cells (MCF-7 and MDA-231) via increasing the accumulation of iron, malondialdehyde (MDA), carbonylation protein (CFP), ferric ions, TFEB, and LAMP-1 at the concentrations of 0.1, 1, and 10 μM [107]. Wang and his team members have investigated that quercetin could stimulate ferroptosis in colorectal cancer cells (HepG2, Hep3B, MDA-MB-231, Atg7-WT, and Atg7-KO MEF) through triggering lysosomal activation, nuclear translocation of EB, TFEB, ferritin degradation, free iron release, ROS, lipid peroxidation, Bid, cleavage of PARP, and caspase-9. The study also showed that quercetin could halt mTOR pathways to stimulate ferroptosis [87]. The upregulation of ROS levels and downregulation of the expression of SLC1A5, NRF2, xCT, and GPX4 by quercetin result in ferroptosis in gastric cancer cell lines (GC cells, AGS, and GES-1) at the dose of 40 μM [88]. There is also another study that showed that quercetin could induce ferroptosis in gastric cancer cells (AGS and MKN45) by diminishing tumor volume, GSH, MDA, ROS, Beclin1, and LC3B which alternatively trigger apoptosis [108]. In intrahepatic cholangiocarcinoma cells, quercetin showed a remarkable role in inducing ferroptosis by diminishing NF-κB concentrations [102]. From these investigations, it is clear that ferroptosis plays an unignorable role in the treatment of different cancer cells by halting their growth and proliferation. The mechanism of action of quercetin in the ferroptosis signaling pathway is displayed in Figure 3.
Figure 3.
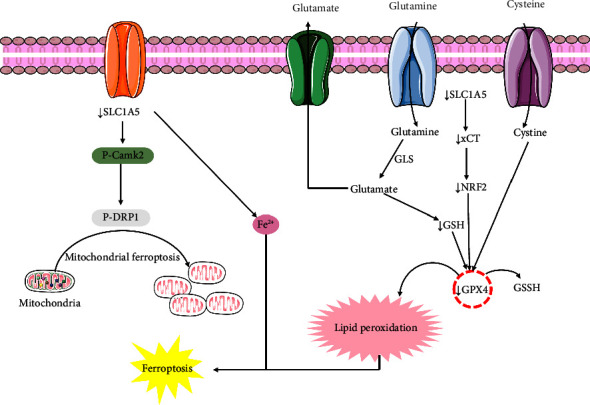
Mechanism of action of quercetin in the ferroptosis signaling pathway. SLC1A5: solute-linked carrier family A1 member 5; NRF2: nuclear factor erythroid 2 (NF-E2)-related factor 2; GSH: glutathione; GPX4: glutathione peroxidase 4; p-DRP1: dynamin-related protein 1.
2.7. Apoptotic Cell Death
Apoptosis, also called programmed cell death, defines different physiological characteristics, including cell shrinkage, membrane blebbing, chromatin condensation, and nuclear fragmentation [67, 109]. The process of programmed cell death depends on various proteins and pathways. Apoptotic proteins such as Bcl-2, Bax, MCL-1, Bcl-w, caspase-3, caspase-6, caspase-7, caspase-8, and caspase-9 and BFL-1/A1 induce apoptosis via different mechanisms [110]. Numerous pathways, including the MPK pathway, the EGFR and ERK pathway, the PI3K/AKT-mTORC1 pathway, and the NF-κB pathway, could stimulate apoptosis [111]. Moderation of apoptotic proteins and pathways leads to the proliferation and growth of cancer cells [112]. Different studies suggested that the moderation of the apoptotic protein and pathways might be a great target for therapeutic drugs to treat cancer by triggering programmed cell death [113].
Research findings showed that quercetin (10−80 μM) induced apoptosis in bladder cancer cells (T24) via increasing cytotoxicity and decreasing cytoplasmic retraction and membrane condensation [26]. Another study conducted by Wu et al. [114] revealed quercetin (100 μM) mediated apoptosis cell death in breast cancer (MCF-7 cells) by elevating cytotoxicity, ROS, and oxidative stress [86]. A study demonstrated that quercetin could induce apoptosis in breast cancer cells via stimulating the EGFR and ERK pathways [115]. Tezerji et al. [33] found quercetin (10 mg/kg) mediated apoptosis cell death in colon cancer by increasing caspase-3 and decreasing beta-catenin and Bcl-2 proteins [33]. A study conducted by García-Gutiérrez et al. [32] showed that quercetin (160.63 μM) induced apoptosis in colon cancer (HT-29 cells exposed to BPA) via upregulating ESR2 and GPR30 genes, cytotoxicity, and downregulating cell viability [32]. A study also demonstrated that quercetin in colon cancer (HCT 116, COLO 320, and COLO 205 cells) induced apoptosis by increasing Sirtuin-6 and Klotho, cell cycle arrest, and decreasing proteasome 20S [31]. Özsoy and his colleagues demonstrated that quercetin at a concentration of 25 μg/ml quercetin for 48 hours caused cell death in colon cancer (Colo-320 and Colo-741 cell lines) through upregulating p16, lamin B1 and cyclin B1, senescence, cytotoxicity, Bax, and cleaved caspase-3 and downregulating cell growth and Bcl-2 [116]. In the context of colorectal cancer in HepG2, Hep3B, MDA-MB-231, Atg7-WT, Atg7-KO MEF, and HCT116 cells, quercetin (50 μM for 24 h) was investigated and found to be responsible for the apoptotic cell death via upregulating lysosomal activation, nuclear translocation of EB and transcriptional activation of lysosomal genes, cytotoxicity, TFEB, ferritin degradation, free iron release, ROS, lipid peroxidation, Bid, cleavage of PARP, and caspase-9 and downregulating mTOR, p53, PI3K/AKT-mTORC1 pathway, and MMP [87]. A study revealed that quercetin induced apoptosis in gastric cancer (AGS and MKN45 cells) by increasing ferroptosis, autophagy, and decreasing tumor volume, GSH, MDA, and ROS, Beclin1, and LC3B [108]. Guo and his team investigated that quercetin is responsible for the apoptotic cell death in lung cancer cells (A549 and H1299) at different concentrations of 12.5, 25, 50, and 100 μM of for 24 hours via upregulating autophagy, LC3-II, Beclin1, Atg5, Atg7, and Atg12, SIRT1 protein, pAMPK-AMPK, cleaved caspase-3 protein, Bax, cytotoxicity, and downregulating Bcl-2 levels [27]. The apoptosis effect of quercetin was confirmed in lung cancer by several studies where quercetin showed a positive effect on apoptosis by upregulating ROS, caspase-2 and caspase-3, DNA damage, S-phase cell cycle arrest, and ATM [89]. Ward and his team revealed that quercetin (40 μM) induced apoptosis in prostate cancer cells (LNCaP, DU-145 with mutated p53, and PC-3) by increasing Raf, MEK, ROS, and Bax levels and decreasing cell viability, the AKT pathway, NF-κB pathway, and Bcl-2 [28]. Another study revealed that quercetin showed a positive effect on apoptosis in different cancers, including breast cancer, hepatocellular carcinoma, cervical carcinoma, and human ovarian carcinoma cell lines (MCF-7, SMMC-7721, HeLa, and SKOV3) via Bax and downregulating Bcl-2 [39]. Quercetin-induced apoptosis in lung cancer cells (BEAS-2B, A549, and H1299) through increasing caspase-3, Bax, DNA damage, p-CDK1, and ferroptosis as well as decreasing SIRT5/PI3K/AKT, HR, NHEJ, and Bcl-2 [117]. Son et al. [100] demonstrated that quercetin was responsible for apoptosis in oral squamous cell carcinoma (YD10B and YD38 cells) via activating G1 cell cycle arrest, CDK inhibitor, cleavage of PARP, and p38 MAPK [100]. In the context of malignant melanoma, cancer quercetin induced apoptosis in B16 murine melanoma cells at the concentrations of 50 μg/mL via decreasing the level of BCL-2 [101]. Furthermore, the apoptotic effect of quercetin was investigated in glioblastoma cancer cells where quercetin showed apoptotic cell death by increasing ROS, Bax, caspase-3 and caspase-9, PARP and decreasing Bcl-2, β-catenin, AKT, and NF-κB pathway [90]. All these findings suggest that quercetin has a great ability to induce apoptosis in different cancer cells (Figure 2).
2.7.1. Antiproliferative Effect
The development and progression of cancer cells increases when there is a disruption in the cell cycle checkpoints and multiple pathways that control the cell cycle progression [118, 119]. Numerous cells controlling pathways including the JAK-STAT, c-Myc, and Ras proto-oncoproteins are essential to control both cell proliferation and apoptosis under particular conditions where different growth factors are constricted [120]. Through the activation of different signal-transferring cascades by the mitogen-activated protein (MAP), kinase pathways stimulate different physiological activities including cell proliferation, differentiation, and apoptotic cell death [121]. The PI3K/AKT/mTOR system is the most significant signaling pathway, which can regulate cell growth, proliferation, and death [67]. Therefore, the modification of these pathways by different natural products can be used for cancer treatment.
A recent study conducted by Zhou and his team demonstrated the remarkable role of quercetin in the inhibition of cell proliferation in non-small-cell lung cancer cells (BEAS-2B, A549, and H1299) at 12.5, 50, and 200 μM concentrations by stimulating the levels of p-CDK1 and halting the PI3K/AKT pathway which reduced the concentrations of HR, NHEJ, and DDR to knockdown cell proliferation by inducing apoptosis [117]. Another study carried out by Hisaka and his colleagues revealed the antiproliferative activity of quercetin in oral squamous cell carcinoma cells (YD10B and YD38) by inducing apoptosis and V-EGFP at 100 μM dose [99]. In the oral squamous cell carcinoma cells (YD10B and YD38), quercetin could inhibit proliferation by activating PARP, p38, MAPK, CDK inhibitor, and apoptosis [100]. There is also a different study which has been investigated the antiproliferative effect of quercetin in B16 murine melanoma cells at 50 μg/mL [101]. In T24 bladder cancer cells, quercetin could halt cell proliferation at 10−80 µM concentrations [26]. Quercetin could block cell proliferation in breast cancer cell lines via increasing the sensitivity of BC to PTX and reducing the EGFR and ERK levels [115]. According to a study carried out by Li et al. [98], quercetin could diminish cell proliferation in esophageal cancer cells by inhibiting the NF-κB pathway [98]. A recent study conducted by Ding et al. [88] revealed that quercetin could also block cell proliferation in gastric cancer cells (GC cells, AGS, and GES-1) by inducing ferroptosis, p-Camk2/p-DRP1pathway and reducing SLC1A5, NRF2, xCT, and GPX4 expressions at the dose of 40 μM [88]. In lung cancer cells, quercetin could inhibit cell progression [89]. Quercetin could induce antiproliferative activity in both hepatocellular carcinoma and intrahepatic cholangiocarcinoma cells by halting the NF-κB pathway [102, 122]. These investigations suggested that quercetin has the unneglectable ability to block the proliferation of different cancer cells (Figure 2).
2.8. Inhibition of Invasion and Migration
The propagation of cancer from the primary tumor to distant sites is facilitated by two basic cellular mechanisms: invasion and migration [123]. In cancer cells, cellular invasion and migration become unregulated which consequently causes metastasis [124–126]. Different studies have demonstrated that the inhibition of invasion and migration of cancer cells is thought to be a significant therapeutic target [127, 128]. Different pathways and mechanisms could stimulate the inhibition of the invasion of cancer cells as revealed by different studies. Findings found that proteins involved in the processes of migration and invasion including matrix metalloproteinases (MMPs), along with disintegrin and metalloproteinases (ADAMs), and ADAM with thrombospondin motifs (ADAMTS), L1 cell adhesion molecule (L1CAM), nucleostemin, and nestin. SOX2 is also a key regulator of the process of invasion and migration of cancer cells [129, 130]. Different pathways including TNF-α/NF-κB/Snail, ERK pathway, AKT pathway, TGF-β, Wnt, and Notch pathways [131–133].
Numerous studies have revealed that quercetin could trigger the inhibition of invasion and migration of different cancer cells including breast, esophageal, lung, and prostate cancer cells. A study carried out by Zhu and his coworkers found that quercetin could stimulate cell migration by inducing IL-2, IFN-γ, and IL-10 levels while reducing ASC, NLRP3, 5-HT, DA, and NE concentrations in breast cancer cells [29]. Similarly, in esophageal cancer cells (Eca109 and CLR-1730), quercetin could suppress cell migration and invasion by lowering the concentrations of VEGF-A and MMP-2 and MMP-9 at the dose of 10 μg/mL [134]. Quercetin also blocked MAPK and NF-κB pathways in esophageal cancer cells [98]. Quercetin could downregulate the levels of cytoplasmic HuR, β-catenin (HuR-dependent), and CD44 in TNBC breast cancer cells (MDA-MB-231 and MDA-MB-468) at 20–200 µM where the IC50 was 90 μM and 98 μM, respectively [135]. Additionally, quercetin could diminish cell migration by suppressing MGMT, Wnt3a, β-catenin, AKT, and NF-κB in glioblastoma cancer cells [90]. Moreover, in hepatocellular carcinoma and intrahepatic cholangiocarcinoma cells, quercetin could halt the migration and invasion of cancer cells by increasing LC3 II/I and decreasing G-CSF, PD-L1, TNF-α, IL-6, and IL-17A and NF-κB [102, 122]. So, quercetin could halt the invasion of cancer cells and give potential therapeutic (Figure 2).
2.8.1. Inhibition of Angiogenesis
Cancer cells require oxygen and nutrients to survive and proliferate which can be supplied through the blood circulation system. These blood vessels are formed by a cellular process called angiogenesis which generates a new blood circulation system to provide nutrients and oxygen to the cancer cells [136, 137]. Numerous factors and pathways are involved in the process of angiogenesis. Different types of proteins like angiogenin, vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), transforming growth factor (TGF)-α, TGF-β, tumor necrosis factor (TNF)-α, platelet-derived endothelial growth factor, granulocyte colony-stimulating factor, placental growth factor, IL-8, hepatocyte growth factor, and epidermal growth factor could stimulate angiogenesis. On the other hand, interferon, angiostatin, endostatin, platelet factor 4, thrombospondin, prolactin 16 kd fragment, and tissue inhibitors of metalloproteinase-1, metalloproteinase-2, and metalloproteinase-3 could suppress angiogenesis to prevent different types of cancer [138]. Therefore, targeting this angiogenesis stimulatory or inhibitory factors became the focus of therapeutic interventions. Different natural products could inhibit angiogenesis in cancer cells [139, 140].
An investigation carried out by Liu and his team members demonstrated that quercetin could suppress angiogenesis in esophageal cancer cells (Eca109 and CLR-1730) by diminishing the expression of VEGF-A and MMP-2 and MMP-9 at 10 μg/mL concentrations [134]. Another study found that quercetin could significantly reduce angiogenesis to prevent cancer cells in the GBM xenograft mouse model [141]. These findings revealed the remarkable angiogenesis capability of quercetin in different cancer cells (Figure 2).
2.8.2. Autophagy
Autophagy is a natural cellular process that breaks down and removes misfolded proteins and damaged organelles. It plays a role in responding to starvation, growth, cell death, and preventing tumor growth [142]. Autophagy can have multiple impacts on cancer, acting as either neutral, tumor-suppressive, or tumor-promoting depending on the specific circumstances and phases of cancer progression [143, 144]. A chain reaction of proteins regulates the mechanism of the autophagic process. Research studies have shown that the serine/threonine kinases, Beclin1, microtubule-associated protein light chain, p62, mTOR, AMPK, and autophagy-ATG genes are strongly preserved across different species and have a vital function in tightly controlling the autophagy process [145–147]. Activation of mTORC1 is crucial in phosphorylating autophagy-related protein (ATG) and subsequently suppressing autophagy [148]. Moreover, the suppression of the PI3K/AKT/mTOR signaling mechanism and the initiation of endoplasmic reticulum (ER) stress enhance the autophagy process [149]. In certain conditions, pharmacological autophagy modulation offers great promise as a novel cancer treatment, expanding the present repertoire.
Numerous studies have demonstrated that quercetin increases autophagy in cancer cells. Quercetin at concentrations of 50 μM for 24 h in colorectal cancer cells (HepG2, Hep3B, MDA-MB-231, Atg7-WT, Atg7-KO MEF, and HCT116) resulted in the stimulation of autophagy [87]. Another study revealed that quercetin induced autophagy in AGS and MKN45 cells via increasing apoptosis and decreasing GSH, MDA, and ROS, Beclin1, and LC3B in gastric cancer [108]. Additionally, Guo et al. [27] showed that quercetin induced autophagy in A549 and H1299 cell lines, in vitro at different concentrations (12.5, 25, 50, and 100 μM) for 24 h in lung cancer via upregulating the LC3-II, Beclin1, Atg5, Atg7, and Atg12, SIRT1 protein, and the pAMPK-AMPK as well as downregulating p62 [27]. In the context of hepatocellular carcinoma, Wu et al. [122] reported that quercetin enhanced autophagy in H22 and HepG2 cells, in vitro and in vivo by increasing LC3 II/I, and decreasing GM-CSF, G-CSF, PD-L1, p62, TNF-α, IL-6, IL-17A, and NF-κB pathway [122] (Figure 2).
2.9. Anticancer Activity of Quercetin-Loaded Nanoformulations
Nanotechnology has completely transformed the field of cancer diagnosis and treatment. Nanoparticles within the size range of 1–100 nm have distinct characteristics that make them ideal for cancer treatment. These advantages include biocompatibility, lessened toxicity, higher stability, improved permeability, and absorption effect, as well as precision targeting capabilities [150, 151]. Nanotechnology-based transporters are highly effective for consecutive combination therapy because of their ability to encapsulate several cargos and deliver them precisely to cancer cells. Nanoformulations have demonstrated synergistic anticancer effects [152, 153]. Different studies suggested that natural product-based nanoformulations have more efficiency and eliminated side effects associated with monotherapy, including less aqueous solubility, poor bioavailability, multidrug resistance, and nonspecificity [154, 155].
In breast cancer cell lines, different quercetin-loaded nanoformulations showed higher anticancer efficacy compared to free quercetin therapy [155–157]. A recent study carried out by Sun and his colleagues demonstrated that the drug-carrying micelles (dHAD-QT), a combination of amphiphilic hyaluronic acid polymers (dHAD) with quercetin, showed strong cytotoxicity and apoptotic effect in breast cancer cell lines. dHAD-QT also diminished cell growth [155]. Another study conducted by Mohammed et al. [157] found that poly(d,l)-lactic-co-glycolic acid (PLGA)-encapsulated quercetin nanoparticles (Q-PLGA-NPs) could remarkably induce apoptosis, gene expression, cytotoxicity, Bax, and caspases-3 while reducing Bcl-2 and p53 expressions in breast cancer cells (MCF7 and CAL51cell) at different concentrations [157]. The findings of Liu et al. [156] also revealed the strong anticancer effects of quercetin (QC) coloaded and chondroitin sulfate (ChS)-coated mesoporous silica nanoparticles (MSNs) (MSNs-ChS@PQ) which could synergistically stimulate apoptotic cell death, G2/M phase arrest, and cytotoxicity in breast cancer cells at the dose of 1.5–45 μg/mL [156]. In colorectal cancer cells (SW48), quercetin-loaded nanoliposomes showed greater anticancer activities than free quercetin (3–50 μg/mL) by inducing cytotoxicity and apoptotic cell death and reducing EGFR gene expression [158]. A different study investigated the strong anticancer efficacy of quercetin-loaded chitosan in metastatic bone tumor cell (SH-SY5Y) at different concentrations (0.5, 1, 2, 4, and 8 μg/m) compared to free quercetin treatment. At 2 μg/mL dose, quercetin-loaded chitosan nanoparticles (NPs) reduced cell viability and significantly stimulated the expressions of 8-oxo-dG, cleaved caspase-3, Bax, cleaved PARP, oxidative stress, DNA damage, apoptotic cell death, and cytotoxicity (Figure 4) [159]. These investigations proved the greater efficiency of nanoformulated quercetin in contrast to free quercetin treatment in different cancer cells as it enhanced bioavailability and solubility of quercetin (Table 2). Although quercetin nanoformulations have been extensively studied for their anticancer properties in laboratory and animal experiments, there are still several obstacles that hinder their practical application in medical clinical applications, including high cost, safety concerns, and potential side effects. Moreover, liposomes exhibit certain drawbacks as a carrier for drugs, such as limited capacity for drug loading, reduced stability, high costs, and active targeting [160, 161]. The mechanism of action of quercetin-loaded nanoparticles is depicted in Figure 4.
Figure 4.
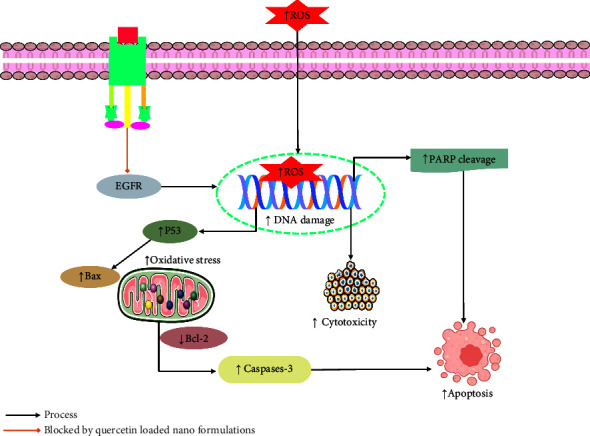
Mechanism of action of quercetin-loaded nanoparticles. EGRF: epidermal growth factor receptor; Bax: BcL2-associated X protein; p53: tumor protein p53; PARP: poly (ADP-ribose) polymerase; Bcl-2: B-cell lymphoma; ROS: reactive oxygen species.
Table 2.
Anticancer activity of different quercetin-loaded nanoformulations
| Types of cancer | Name of the compound | Experimental model | Tested concentration | Efficacy, IC50 (exposure time) | Anticancer effects and mechanisms | Ref. |
|---|---|---|---|---|---|---|
| Breast cancer | dHAD-QT micelles | BC cells, in vivo | — | — | ↑Cytotoxicity and apoptotic cell death, ↓cell growth | [155] |
| Poly(d,l)-lactic-co-glycolic acid (PLGA)-encapsulated quercetin nanoparticles (Q-PLGA-NPs) | MCF7 and CAL51cell lines, in vivo and in vivo | — | ↑Apoptosis, gene expression, and cytotoxicity, Bax, caspase-3, ↓Bcl-2, p53 | [157] | ||
| Quercetin (QC) coloaded and chondroitin sulfate (ChS)-coated mesoporous silica nanoparticles (MSNs) (MSNs-ChS@PQ) | MCF-7/ADR cells, in vivo and in vitro | 1.5−45 μg/mL | — | ↑Apoptotic cell death, G2/M phase arrest, cytotoxicity, ↓P-gp | [156] | |
|
| ||||||
| Colorectal cancer | Quercetin-loaded nanoliposomes | SW48 cell, in vitro | 3–50 μg/mL | 10.65 μg/mL | ↑Cytotoxicity, apoptotic cell death, ↓EGFR gene expression | [158] |
|
| ||||||
| Metastatic bone tumor | Quercetin-loaded chitosan nanoparticles (NPs) | SH-SY5Y cell, in vitro | 0.5, 1, 2, 4, and 8 μg/m | 2 μg/mL | ↑8-oxo-dG, cleaved caspase-3, Bax, cleaved PARP, and total oxidant, oxidative stress, DNA damage, apoptotic cell death, cytotoxicity, ↓cell viability | [159] |
Arrows (↑ and ↓) show an increase and decrease in the obtained variables, respectively. Bax: BcL2-associated X protein; P-gp: P-glycoprotein 1; EGRF: epidermal growth factor receptor; RAD51: RAD51 homolog 1; P53: tumor suppressor gene.
2.10. Anticancer Activity of Quercetin in Combination with Other Molecules
The process of combining two or more therapeutic compounds in order to increase the efficacy of a drug in contrast to the single therapy method is known as combination therapy. In combination therapy, each compound targets different key signaling pathways in a synergistic way which leads to reduce the required concentrations for individual drugs and consequently minimizes the cost of therapeutic treatment along with increasing the efficiency of the drug compared to monotherapy [162, 163]. It can also decrease the drug resistance possibility and, at the same time, induce different anticancer activities, including reducing cell growth, blocking cell cycle arrest, halting cell proliferation, and inducing apoptosis [164].
A current investigation carried out by Tanomrat et al. [165] demonstrated that quercetin and N-acetylcysteine (NAC) demonstrated strong anticancer activity in colorectal cancer cells (HT-29 and HCT-116) by increasing ROS levels and decreasing iNOS, ICAM-1, MMP-2, cell progression, migration, and invasion at the dose of 0.5 μg/ml of quercetin combined with 0.125 and 0.25 mM NAC in HT-29 cells; additionally, 10 μg/ml of quercetin combined with 2.5 and 5 mM NAC in HCT-116 cells [165]. Different studies revealed the efficacy of combination therapy in breast cancer cells compared to monotherapy [166–168]. Quercetin and curcumin combinedly induced BRCA1, and histone acetylation and reduced cell survival and migration at 50 μM dose in triple-negative breast cancer cells. These findings suggested that the combination of quercetin and curcumin demonstrated strong anticancer activities in breast cancer cells [167]. A study conducted by Hanikoglu and his team revealed the great anticancer activity of the combination of quercetin with curcumin and somatostatin could reduce p-S6-Ribosomal (Ser235/236), omega-3 acids, AKT 1 and p-AKT 1, EGFR, and MAPK in breast cancer cell lines (MCF-7 and MDA-MB231) [169]. Another study carried out by Rhman et al. [168] demonstrated the strong anticancer efficacy of quercetin and naringenin (CoQN) in breast cancer cells. They could stimulate oxidative stress, apoptosis, cytotoxicity, caspase 3/7, miR-1275, and lipid peroxidation. At the same time, they could diminish cell viability, Bcl-2, cell proliferation, MMP, and miR-27a-3p [166, 168]. A combination of quercetin and fisetin could also induce anticancer activities synergistically in breast cancer cells via upregulating apoptotic pathways and suppressing cell proliferation, migration, colony formation, and MMP [170]. In prostate cancer cells (PC3 and DU145), quercetin and vitamin C increase the anticancer activities synergistically through reducing CXCR and CXCR7; α4, α5, and β1 integrin subunits; VEGF; Ki-67; cell proliferation at the dose of quercetin (75 µM); and vitamin C (100 μM) [171]. Combination of quercetin and naringenin could stimulate cell cycle arrest at G0/G1 and S phases and apoptotic cell death, ROS, and cytotoxicity in hepatocellular carcinoma cell (HepG2). They also diminished cell migration and invasion simultaneously [172]. A different study carried out by Roy and his colleagues investigated that quercetin and ruthenium could induce p53, CAT, SOD, glutathione levels, Bax, and apoptotic cell death in colon cancer cells (HT-29). They also reduced the expression of VEGF and mTOR, Bcl-2, PCNA, and cell proliferation at different concentrations (10, 15, 20, 30, and 40 mM) [173]. In thyroid cancer cell, quercetin and sorafenib could remarkably increase E-cadherin and decrease cell proliferation, cell adhesion and migration properties, N-cadherin, and cell growth [174]. Besides, in human colorectal adenocarcinoma cells, a combination of quercetin, luteolin, and 5-FU could stimulate apoptosis and unneglectable knockdown VEGF, cell growth, Bcl-2, mTOR protein, and AKT gene at 50–1000 mg/ml concentrations [175]. The combination of quercetin and chrysin could downregulate the levels of TLR4/NF-κB, invasion and migration, p65, cytokines, IL-1β, IL-6, TNF-α and IL-10, TLR4, Myd88, phosphorylation of IKKβ, IκB, and MMP-9 in lung cancer cells (H1975 and A549) at 2 or 5 μM [114]. A study conducted by Li and his team revealed that quercetin and cisplatin increased apoptotic cell death and caspase-8 and caspase-9 while decreasing NF-κB, AKT, IKKβ, xIAP, and cell growth in oral cancer cells (Tca-8113 and SCC-15) at 3−6 mg/kg doses (Figure 5) [176]. According to a study conducted by Karimian et al. [177], quercetin and thymoquinone significantly enhanced the anticancer activities in breast cancer cells (MCF-7), lung cancer cells (A549), and prostate cancer cells (PC3) through stimulating DNA damage markers, H2AX, 8-OH-dG, DNA damage, and cytotoxicity. Additionally, the study demonstrated that quercetin and thymoquinone could suppress the levels of DNA repair mediators, RAD51, Ku70, XRCC1, cell proliferation, and P53 [177]. All these findings demonstrated the strong synergetic anticancer activities of different combination therapies over monotherapy in different cancer cells at low concentrations of individual drugs (Table 3). The mechanism of action of quercetin in combination therapy is depicted in Figure 5.
Figure 5.
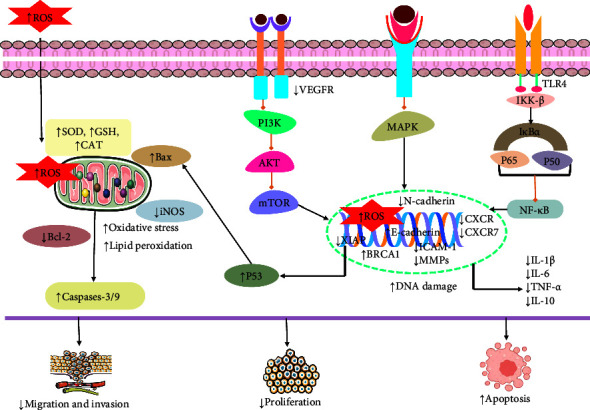
Possible anticancer mechanism of quercetin in combination therapy. XIAP: X-linked inhibitor of apoptosis protein; N-cadherin: neural cadherin (protein); p38: the serine/threonine kinase p38; iNOS: inducible nitric oxide synthase; ICAM-1: intercellular adhesion molecule 1; MMP-2: matrix metalloproteinase-2; CXCR: chemokine receptors; CAT: catalase; SOD: superoxide dismutase; PCNA: proliferating cell nuclear antigen; VEGF: vascular endothelial growth factor; IKKβ: inhibitor of nuclear factor kappa-B kinase subunit beta; xIAP: X-linked inhibitor of apoptosis protein; AKT: protein kinase B; IL-1β: cytokine interleukin-1β; ROS: reactive oxygen species; Bax: BcL2-associated X protein; Bcl-2: B-cell lymphoma; p53; MAPK: mitogen-activated protein kinases; p65: transcription factor p65; PI3K: phosphoinositide 3-kinase; IFN-γ: interferon-gamma; E-cadherin: epithelial cadherin; NF-κB: nuclear factor-κB.
Table 3.
Anticancer activity of quercetin with other molecules in combination therapy.
| Types of cancer | Name of the compound | Experimental model | Tested concentration | Efficacy, IC50 (exposure time) | Anticancer effects and mechanisms | Ref. |
|---|---|---|---|---|---|---|
| Colorectal cancer | Combination of quercetin and N-acetylcysteine (NAC) | HT-29 and HCT-116 cells | The HT-29 cells were treated with 0.5 μg/ml of quercetin combined with 0.125- and 0.25 mM NAC. The HCT-116 cells were treated with 10 μg/ml of quercetin combined with 2.5 and 5 mM NAC | — | ↑ROS, ↓cell progression, migration and invasion, iNOS, ICAM-1, and MMP-2 | [165] |
|
| ||||||
| Triple-negative breast cancer (TNBC) | Combination of quercetin and curcumin | MDA-MB-231, MDA-MB-468 cells , induced by BRCA1 siRNA-induced, in vitro | 50 μM | — | ↑BRCA1, histone acetylation, ↓cell survival and migration | [167] |
|
| ||||||
| Breast cancer | Combination of quercetin with curcumin and somatostatin | MCF-7 and MDA-MB231 cells, in vitro | MCF7 cells with the EC50 concentrations of somatostatin combination of quercetin (38.58 μM), curcumin (29.65 μM) and quercetin (73.63 μM) for 24 h. For MDA-MB-231 cells, EC50 concentrations of somatostatin was 96.75 μM, curcumin was 10.46 μM, and quercetin was 92.98 μM | — | ↓p-S6-Ribozomal (Ser235/236),omega-3 acids, AKT 1 and p- AKT 1, EGFR and MAPK | [169] |
| Combination of quercetin with naringenin (CoQN) | MCF-7 BC cells, in vitro | 50 μg/mL of quercetin with naringenin | 44.31 μg/mL | ↑Oxidative stress and apoptosis, cytotoxicity, caspase 3/7, lipid peroxidation, ↓cell viability, Bcl-2 ,cell proliferation, MMP | [168] | |
| Combination of quercetin and naringenin (CoQN) | MCF7 and MDA-MB-231 breast cancer cell lines, in vitro | — | — | ↑Apoptotic cell death, miR-1275, ↓cell growth, migration, miR-27a-3p. | [166] | |
| Combination of quercetin and fisetin | MCF7, MDA-MB-231, BT549, T47D, and 4T1, in vivo and in vitro | — | — | ↑Apoptotic pathways, ↓cell proliferation, migration, and colony formation, MMP | [170] | |
|
| ||||||
| Prostate cancer | Combination of quercetin and vitamin C | PC3 and DU145 cell lines, in vitro | quercetin (75 μM) and vitamin C (100 μM) | — | ↓ CXCR and CXCR7, α4, α5 and β1 integrin subunits, VEGF, Ki-67, proliferation | [171] |
|
| ||||||
| Hepatocellular carcinoma | Combination of quercetin and naringenin | HepG2 cell, in vitro | naringenin and quercetin 200 and 100 μM respectively | — | ↑Cell cycle arrest at G0/G1 and S phases and apoptotic cell death, ROS, cytotoxicity, ↓ cell migration, invasion | [172] |
|
| ||||||
| Colon cancer | Combination of quercetin and ruthenium | HT-29 cells, in vitro and in vivo | 10, 15, 20, 30, 40 mM | — | ↑p53, CAT, SOD and glutathione levels, Bax, apoptotic cell death, ↓VEGF and mTOR, Bcl-2, cell proliferation, PCNA | [173] |
|
| ||||||
| Thyroid cancer cells | Combination of quercetin and sorafenib | K1 and BCPAP cells, in vivo. | sorafenib 0.1 μM, quercetin 25 μM | — | ↑E-cadherin, ↓cell proliferation, cell adhesion and migration properties, N-cadherin, cell growth | [174] |
|
| ||||||
| Human colorectal adenocarcinoma | Combination of quercetin and luteolin and 5-FU | HT 29 cells, in vitro | (50–1000 mg/ml) for 24 h | — | ↑Apoptotic cell death, p53, Bax, p38 MAPK, and PTEN, ↓VEGF, growth , Bcl-2, mTOR, and AKT gene | [175] |
|
| ||||||
| Lung cancer | Combination of quercetin and chrysin | H1975 and A549cells, in vitro | 2 or 5 μM | — | ↓TLR4/ NF-κB, invasion and migration, p65, cytokines, IL-1β, IL-6, TNF-α and IL-10, TLR4 ,Myd88, phosphorylation of IKKβ and IκB, MMP-9 | [114] |
|
| ||||||
| Oral cancer | Combination of quercetin and cisplatin | Tca-8113 and SCC-15 cells, in vitro and in vivo | quercetin and cisplatin (3−6 mg/kg), cisplatin (2 mg/kg) , quercetin (50 mg/kg) | — | ↑Apoptotic cell death,caspase-8 and -9, ↓NF-κB, AKT, IKKβ, xIAP, growth | [176] |
|
| ||||||
| Breast cancer | Combination of quercetin and thymoquinone | MCF-7 cells, in vitro | — | 50 μM | ↑DNA damage markers, H2AX, and 8-OH-dG, DNA damage, cytotoxicity, ↓DNA repair mediators, RAD51, Ku70, XRCC1, cell proliferation, P53 | [177] |
| Lung cancer | A549 cells, in vitro | — | 200 μM | |||
| Prostate cancer | PC3 cells, in vitro | — | 400 μM | |||
Arrows (↑ and ↓) show an increase and decrease in the obtained variables, respectively. XIAP: X-linked inhibitor of apoptosis protein; N-cadherin: neural cadherin (protein); p38: the serine/threonine kinase p38; iNOS: inducible nitric oxide synthase; ICAM-1: intercellular adhesion molecule 1; MMP-2: matrix metalloproteinase-2; CXCR: chemokine receptors; CAT: catalase; SOD: superoxide dismutase; PCNA: proliferating cell nuclear antigen; VEGF: vascular endothelial growth factor; IKKβ: inhibitor of nuclear factor kappa-B kinase subunit beta; xIAP: X-linked inhibitor of apoptosis protein; AKT: protein kinase B; IL-1β: cytokine interleukin-1β; ROS: reactive oxygen species.
2.11. Role on Immunotherapy
The immune system plays a significant role in immune defense, immune surveillance, and immune homeostasis. The development of cancer is an outcome of reduced immunity because when the immune response is decreased, aging-damaged mutant cells or invasive pathogens cannot be eradicated in a timely manner. Therefore, enhancing immune response is very important to prevent cancer cell growth [178, 179]. The field of cancer immunotherapy (neoadjuvant and adjuvant immunotherapies) has been significantly revolutionized by immune checkpoint blockade therapy [180, 181]. Different signaling proteins such as TNF-α, PD-L1, IL-6, NRF2IL-2, and IFN-γ as well as immune cells (CD4+ and CD8+ T) regulate the immune response which can be the target for anticancer drugs to prevent cancer cell proliferation and cell growth [29, 182, 183]. By understanding the mechanism of tumor immune response and its evasion by tumors, the field of immunotherapy can manipulate this interaction and elucidate the therapeutic function of immunity in cancer. This improved understanding of immunotherapy and the mechanisms underlying immunity in cancer has opened a new door to developing a wide range of therapeutic agents for treating a variety of cancers [184]. Quercetin not only has anti-inflammatory, antitumor, antiplatelet aggregation, endothelial cell protection, and antioxidant effects but also has a regulatory effect on immune cells to control immune responses [179].
Different studies revealed that quercetin remarkably showed an immune response to prevent tumor growth in different cancer cells. In LPS-treated 4T1 cells, quercetin significantly stimulated immune response and upregulated the levels of IL-2, IFN-γ, and IL-10 [29]. NRF2 played an important role in inducing oxidative stress and immune response in gastric cancer cells at 40 μM [88]. A study conducted by Wu and his team revealed that quercetin notably reduced TNF-α, IL-6, PD-L1, and IL-17A to enhance the immune response to resist cancer cell proliferation in hepatocellular carcinoma cells (H22 and HepG2) [185] (Table 4). Another study conducted by Qiu and his team found the immune therapeutic activity of quercetin in breast cancer cells (MCF-10A, MCF-10AT, MCF-7, and MDA-MB-231). Quercetin stimulated apoptosis and differentiation of γδ T cells into the Vδ2 T-cell subpopulation. It also induced the level of IFNγ-R, p-JAK2, and p-STAT1 and decreased PD-L1 level [179]. On the other hand, nanoformulated quercetin displayed a synergistic immune response compared to free quercetin treatment. According to a study of Sun and his coworkers demonstrated that a cyclodextrin-based nanoformulation of ginsenoside Rg3 and quercetin (CD-PEG-FA.Rg3.QTN) and found that CD-PEG-FA.Rg3.QTN significantly induced immunogenic cell death (ICD) by stimulating effector T cells and inhibiting CD4+ or CD8+ T cells in colorectal cancer cells (CT26 and HCT116). CD-PEG-FA.Rg3.QTN also reduced immunosuppressive tumor microenvironment (TME) and increased blood circulation and antiproliferative effects with IC50 of 32 μmol/L and 30 μmol/L, respectively [181]. The tumor microenvironment could suppress the immune response [186]. In microsatellite-stable colorectal cancer, the micellar delivery of quercetin and alantolactone (QA-M) potentially enhanced ICD, toxicity, immune response, and memory tumor surveillance as well as diminished tumor growth and immunosuppressive TME [187] (Table 5). These findings suggested that quercetin has the potential ability to treat cancer through immunotherapy.
Table 4.
Anticancer activity of quercetin against different cancers
| Cancer type | Test system | Dose/concentration (R/A) | IC50 | Results/possible mechanism | References |
|---|---|---|---|---|---|
| Bladder cancer | T24 bladder cancer cells, MTT assay and trypan blue exclusion test, in vitro | 10−80 μM | — | ↑Apoptotic and senescence effect, cell death, cell cycle arrest, cytotoxicity, ↓proliferation, cell body, cytoplasmic retraction, and membrane condensation | [26] |
|
| |||||
| Breast cancer | LPS-treated 4T1 cells, in vivo and in vitro | — | — | ↑Immune response, IL-2, IFN-γ, and IL-10, ↓PYD ,ASC, NLRP3, and caspase-1, 5-HT, DA, and NE | [29] |
| MCF-7 cell, in vitro | 100 μM | 23.1 μM | ↑Cell cycle arrest at S phase, apoptosis, cytotoxicity, ROS, oxidative stress | [86] | |
| BC cell, in vitro | — | — | ↑Apoptotic cell death, sensitivity of BC to PTX, ↓cell proliferation, EGFR, ERK | [115] | |
| MCF-7 and MDA-231 cells, in vitro | 0.1, 1, and 10 μM | — | ↑Ferroptosis, iron, MDA, and carbonyl protein, LAMP-1, TFEB, malondialdehyde (MDA), carbonylation protein (CFP), ↓cell viability | [107] | |
|
| |||||
| C26 tumor (mice) | C26 cells, in vitro in vivo | 4 mg/mL | — | ↑Antioxidant, protein expression of mitochondrial complexes V, III, and II, cytochrome c expression, MFN1, BNIP3, ↓FIS1, TFAM protein | [208] |
|
| |||||
| Colon cancer | In vitro and in vivo (n =15) | 10 mg/kg | ↑Caspase-3, apoptotic cell death, ↓beta-catenin, and Bcl-2 proteins | [33] | |
| HT-29 cells exposed to BPA, in vitro and in silico | — | 160.63 μM | ↑Antioxidant, cell cycle arrest in the G0/G1, ESR2 and GPR30 genes, apoptosis, cell death, cytotoxicity, ↓cell viability | [32] | |
| HCT 116, COLO 320, and COLO 205 cells, in vitro | — | 80 μM in L132 cells and 120 μM in all the colon cancer cell lines | ↑Apoptosis, sirtuin-6 and klotho, cell cycle arrest, ↓cell proliferation, cell growth, proteasome 20S | [31] | |
| Colo-320 and Colo-741 cell lines, MTT assay, in vitro | 25 μg/ml quercetin for 48 hours | - | ↑Apoptotic cell death , p16, lamin B1 and cyclin B1, senescence, cytotoxicity, Bax, cleaved caspase-3, ↓cell growth, Bcl-2, cell viability | [116] | |
|
| |||||
| Colorectal cancer cells | HepG2 cells, Hep3B, MDA-MB-231, Atg7-WT and Atg7-KO MEF cells, HCT116 cells, MTT assays, in vitro | 50 μM for 24 h | — | ↑Cell death, autophagy, lysosomal activation, nuclear translocation of EB and transcriptional activation of lysosomal genes, cytotoxicity, cell death, TFEB, ferroptosis, ferritin degradation, free iron release, ROS, lipid peroxidation, Bid, cleavage of PARP and caspase-9, ↓mTOR, p53 , PI3K/AKT-mTORC1 pathway, MMP | [87] |
|
| |||||
| Esophageal cancer | Eca109, CLR-1730 cells, in vitro | 10 μg/mL | — | ↓Cell migration, invasion, angiogenesis, VEGF-A, MMP-2, and -9 | [134] |
| Esophageal carcinoma cells, in vitro | — | ↑Cell cycle arrest, ↓cell proliferation, invasion, and clonal formation, MAPK, NF-κB | [98] | ||
|
| |||||
| Gastric cancer | GC cells, AGS cells, GES-1 cells, in vitro and in vivo | 40 μM | In AGS cells 38.78 μM | ↑Ferroptosis, p-Camk2/p-DRP1, cytotoxicity, ROS levels, ↓SLC1A5, NRF2, xCT, GPX4, cell progression | [88] |
| AGS and MKN45 cells, in vitro BALB/c mice, in vivo | ↑Ferroptosis, autophagy, and apoptosis, ↓cell viability and tumor volume, GSH, MDA, ROS, Beclin1, and LC3B | [108] | |||
|
| |||||
| Lung cancer | A549 and H1299 cell lines, in vitro | 12.5, 25, 50, and 100 μM of quercetin for 24 hours | — | ↑Apoptotic effect, autophagy, LC3-II, Beclin1, Atg5, Atg7, and Atg12 , SIRT1 protein and the pAMPK-AMPK, autophagy, cleaved caspase-3 protein, Bax, cytotoxicity, ↓cell viability, p62, Bcl-2 | [27] |
| Lung cancer cells, in vitro | — | — | ↑ROS, caspase -2 and-3, DNA damage, S-phase cell cycle arrest, ATM, cell death, ↓cell proliferation | [89] | |
|
| |||||
| Prostate cancer | LNCaP, DU-145 with mutated p53 , and PC-3 cells, in vitro and in vivo | 40 μM | — | ↑Apoptotic cell death, Raf, MEK, ROS, Bax, ↓cell viability, AKT pathway, NF-κB pathways, Bcl-2 | [28] |
|
| |||||
| TNBC breast cancer | TNBC Cells (MDA-MB-231 and MDA-MB-468), in vitro | 20–200 μM | 90 μM and 98vM | ↑Cytotoxicity, ↓cytoplasmic HuR, adhesion and migration, β-catenin (HuR-dependent) and CD44 | [135] |
|
| |||||
| Glioblastoma | GBM xenograft mouse model, in vivo | — | — | ↑Apoptosis, ↓cell proliferation, cell growth, glycolytic metabolism, angiogenesis | [141] |
|
| |||||
| Breast cancer, hepatocellular carcinoma, cervical carcinoma, and human ovarian carcinoma | MCF-7, SMMC-7721, HeLa, SKOV3 cell lines, in vitro | — | — | ↑Apoptosis, G0/G1 phase and G2/M phase arrest, Bax, ↓cell proliferation, Bcl-2 | [39] |
|
| |||||
| Non-small cell lung cancer (NSCLC) | BEAS-2B, A549 and H1299 cells, in vitro | (12.5, 50, and 200 μM) of quercetin for 24 h | — | ↑Apoptotic cell death , caspase-3, Bax, DNA damage, p-CDK1, ferroptosis, ↓SIRT5/PI3K/AKT, HR, NHEJ, proliferation, Bcl-2, DDR | [117] |
|
| |||||
| Liver cancer | KIM1, KYN-2, KYN-3, HAK-1B, HAK-2, HAK-5, HAK-6, KMCH-1, KMCH-2 cells, in vitro | 100 μM | — | ↑Cell cycle arrest, apoptosis, V-EGFP, ↓cell proliferation | [99] |
|
| |||||
| Oral squamous cell carcinoma (OSCC) | YD10B and YD38 cells, in vitro | — | — | ↑Cytotoxic, G1 cell cycle arrest, CDK inhibitor, cleavage of PARP, p38 MAPK, apoptotic cell death, ↓cell Proliferation | [100] |
| OSCC cell lines, in vitro | — | — | ↑Ferroptosis, mTOR/S6KP70, lipid peroxidation, ↑tumor growth, GSH, SLC7A11 | [106] | |
|
| |||||
| Malignant melanoma | B16 murine melanoma cells, in vitro | 50 μg/mL | — | ↑Cell cycle arrest in the S and G2/M stages, apoptotic cell death, ↓cell viability, proliferation, BCL-2 | [101] |
|
| |||||
| Glioblastoma cancer | T98g cells, in vitro | — | — | ↑Apoptotic cell death, arrested cells in the S-phase cell cycle, ROS, Bax, caspase-3 and caspase-9, PARP, ↓growth and migration, Bcl-2, MGMT, Wnt3a, β-catenin, AKT, NF-κB | [90] |
|
| |||||
| Hepatocellular carcinoma | H22 and HepG2 cells, in vitro and in vivo | — | — | ↑LC3 II/I, autophagy, ↓cell proliferation, migration, and invasion, GM-CSF and G-CSF, PD-L1, p62, TNF-α, IL-6, and IL-17A, NF-κB | [122] |
|
| |||||
| Intrahepatic cholangiocarcinoma | ICC cells, in vitro and in vivo | ↑Cell cycle arrest at G1 phase, ferroptosis, apoptosis, ↓cell proliferation and survival, invasion, and NF-κB | [102] | ||
Arrows (↑ and ↓) show an increase and decrease in the obtained variables, respectively. PYD: PYRIN domain; ASC: caspase recruitment domain; NLRP3: NLR family pyrin domain containing 3; 5-HT: 5-hydroxytryptamine; DA: dopamine; NE: neutrophil elastase; EGRF: epidermal growth factor receptor; ERF: ETS2 repressor factor; PARP: poly(ADP-ribose) polymerase; Caspase: cysteine-aspartic acid protease; MEK: mitogen-activated extracellular signal-regulated kinase; Bax: BcL2-associated X protein; Bcl-2: B-cell lymphoma; p53: tumor protein p53; p65: transcription factor p65; ER stress: endoplasmic reticulum stress; LC3-II: microtubule-associated protein 1A/1B-light chain 3; p62: ubiquitin-binding protein p62; AKT: protein kinase B; mTOR: mammalian (or mechanistic) target of rapamycin; p38: the serine/threonine kinase p38; MAPK: mitogen-activated protein kinases; ERK: extracellular signal-regulated kinase; MMP2 and MMP9: matrix metalloproteinase-2 and metalloproteinase-9; PI3K: phosphoinositide 3-kinase; Beclin1: protein which regulated autophagy; IL-2: interleukin-2; MFN1: mitofusin-1; Bcl-XL: B-cell lymphoma-extra-large; JAK/STAT: Janus kinase/signal transducers and activators of transcription; ROS: reactive oxygen species; NGFR: nerve growth factor receptor; pRb1: proline rich protein BstNI subfamily 1; CDK4: cyclin-dependent kinase 4; IFN-γ: interferon-gamma;; E-cadherin: epithelial cadherin; EMT: epithelial-mesenchymal transition; Bid: biotin acceptor domain, BH3 interacting domain death agonist; VEGF: vascular endothelial growth factor; TNF-α: tumor necrosis factor alpha; TNF-β: tumor necrosis factor beta; CSC: cancer stem cell; CAT level: chloramphenicol acetyltransferase level; BNIP3: BCL2 interacting protein 3; FIS1: mitochondrial fission 1 protein; TFAM: mitochondrial transcription factor A; BI6C9: bid inhibitor; SLC1A5: solute-linked carrier family A1 member 5; NRF2: nuclear factor erythroid 2 (NF-E2)-related factor 2; GPX4: glutathione peroxidase 4; Atg 5, 7, and 12: autophagy protein 5, 7, and 12; AMPK: AMP-activated protein kinase; ATM: ataxia telangiectasia mutated; HR: hormone receptor; NHEJ: nonhomologous end-joining; DDR: DNA damage response; NF-κB: nuclear factor-κB; GSH: glutathione.
Table 5.
Immunotherapeutic role of quercetin against different cancers
| Cancer type | Name of the compound | Test system | Dose/concentration (R/A) | IC50 | Results/possible mechanism | References |
|---|---|---|---|---|---|---|
| Colorectal cancer | CD-PEG-FA.Rg3.QTN | CT26, HCT116, in vitro female BALB/c and nude mice (six to eight-week), in vivo | — | 32 and 30 μmol/L, respectively | ↑ICD, ROS, blood circulation, cytotoxicity, apoptosis, BAX, caspase 9, and caspase 3, effector T cells, antiproliferative, ↓immunosuppressive TME, Bcl-2, CD4+ or CD8+ T cells | [181] |
|
| ||||||
| Microsatellite-stable colorectal cancer | QA-M | Colorectal cancer cell, in vitro | — | — | ↑ICD, toxicity, immune response, memory tumor surveillance, ↓tumor growth; immunosuppressive TME | [187] |
|
| ||||||
| Breast cancer | Quercetin | MCF-10A, MCF-10AT, MCF-7 and MDA-MB-231, in vitro | 5 μM | ↑Apoptosis, differentiation of γδ T cells into the Vδ2 T cell subpopulation, IFNγ-R, p-JAK2 and p-STAT1, ↓PD-L1 | [179] | |
Arrows (↑ and ↓) show an increase and decrease in the obtained variables, respectively. ICD; immunogenic cell death; TME: tumor microenvironment; Q: quercetin; A: alantolactone.
2.12. Clinical Evidence
Polyphenolic substances called flavonoids have the potential to be used as therapeutics. Numerous investigations have demonstrated the stronger anticancer potential of these substances. Quercetin is one of the most significant flavonoids among them found in the human diet [188]. Different studies revealed that quercetin has shown remarkable anticancer and anti-inflammatory activities, mainly due to the potential for antioxidants [189–191].
Quercetin lacks sufficient clinical evidence to support it as a significant anticancer drug. However, there is a few numbers of clinical studies of quercetin in cancer and other diseases. A clinical study carried out by Henning and his coworkers found the great anticancer activity of quercetin in prostate cancer cells. They used 31 male subjects to investigate, and they combined 1 gram of green tea extract (GTE) with 800 mg of quercetin. They revealed that quercetin could induce bioavailability, epigallocatechin (EGC) levels in urine, plasma epigallocatechin, and the accumulation rate in plasma, urine, and prostate tissue. Quercetin also reduced methylation activity and liver toxicity [192]. Another study demonstrated that quercetin could decrease lymphocyte tyrosine kinase activity and the serum alpha-fetoprotein where t(1/2) alpha of 6 min and median t(1/2) beta of 43 min in 11 patients with ovarian cancer. Quercetin was infusion at escalating doses initially at 3-week intervals. The first dose level was 60 mg/m2, and the 10th dose level was 1700 mg/m2 [193]. In the United Kingdom, Morrow et al. [194] conducted a clinical study that revealed the strong antitumor activity of quercetin in 4 healthy males which could decrease the level of TIMP-1 in the cell at the dose of 30 mg per day for 14 days. But it has some side effects as it could cause aggressive disease and poor prognosis in patients with certain malignancies [194]. Apart from cancer treatment, quercetin is also investigated to treat different diseases. The combination of quercetin and ascorbic acid (97 mg quercetin and 16 mg ascorbic acid a day) could upregulate plasma concentrations of quercetin and ascorbic acid and Trolox equivalent antioxidant capacity (TEAC) and oxidative DNA damage in 114 females and 54 males (aged 18–45 years) [195]. Besides, in polycystic ovary syndrome, quercetin could decrease the number and size of ileal and rectal adenomas in 72 women at concentrations of 500 mg for 40 days [196]. Cruz-Correa and his team revealed that quercetin and curcumin could suppress the number and size of ileal and rectal adenomas in five FAP patients with prior colectomy (4 with retained rectum and 1 with an ileal anal pouch) in Weston, Florida, USA. However, there were some limitations like it caused nausea and the taste is sour [197] (Table 6). Though a significant number of in vivo and in vitro studies demonstrated the anticancer activities of quercetin in different cancer cells, more clinical studies should be conducted to support quercetin as an anticancer drug.
Table 6.
Data of clinical trials in various cancers
| Diseases/related effects | Gender (number of samples) | Dose and treatment duration | Biological activities/mechanisms | Side effects | References |
|---|---|---|---|---|---|
| Prostate cancer | Male, n = 31 | 1 gram of GTE (830 mg of GTP) with 800 mg of Q (GT + Q) for four weeks before prostatectomy | ↑Bioavailability, EGC levels in urine and plasma, accumulation in plasma, urine, and prostate tissue, ↓methylation activity, liver toxicity | — | [192] |
|
| |||||
| Ovarian cancer, hepatoma | 11 patients (23–78 years) | Infusion at escalating doses initially at 3-week intervals. The first dose level was 60 mg/m2; at the 10th dose level of 1700 mg/m2 | ↓Lymphocyte tyrosine kinase activity, the serum alpha-fetoprotein | — | [193] |
|
| |||||
| Cancer | Healthy male subjects aged between 33 and 64 years, n = 4 | 30 mg per day for 14 days | ↑Antitumor activity, ↓TIMP-1 | Aggressive disease and poor prognosis in patients with certain malignancies | [194] |
|
| |||||
| Oxidative DNA damage. | 114 females and 54 males, aged 18–45 years. | 97 mg quercetin and 16 mg ascorbic acid a day | ↑Plasma concentrations of quercetin, ascorbic acid, and TEAC, oxidative DNA damage | — | [195] |
|
| |||||
| Polycystic ovary syndrome | Women, (n = 72) | 500 mg of Quercetin for 40 days | ↓LH, TNF-α, IL-6, inflammatory | — | [196] |
|
| |||||
| Familial adenomatous polyposis | Five FAP patients with prior colectomy (4 with retained rectum and 1 with an ileal anal pouch) | Curcumin 480 mg and quercetin 20 mg orally 3 times a day. | ↓The number and size of ileal and rectal adenomas | Nausea and sour taste | [197] |
Arrows (↑ and ↓) show an increase and decrease in the obtained variables, respectively. EGC: epigallocatechin; TIMP-1: tissue inhibitor of metalloproteinases 1; TEAC: Trolox equivalent antioxidant capacity; IL-6: interleukin-6; GTE: green tea extract; GTP: green tea polyphenol.
2.12.1. Toxicological Profile
It has been demonstrated that numerous drugs can undergo conversion into different metabolites within the body, which elicit both therapeutic and toxicological effects [198, 199]. One of the major challenges in drug discovery and development is the toxicity of natural products and isolated chemicals. So, an in-depth analysis is always necessary in search of safer natural medications [200]. Toxicological biomarkers of natural products can be found and evaluated using metabolomics-based toxicology, which helps to prevent adverse drug reactions and guide clinical medication. Numerous metabolomic studies have been conducted over the past few decades to evaluate toxicity, identify toxicological biomarkers, and investigate the primary pathways of nephrotoxicity, hepatotoxicity, cardiotoxicity, and central nervous system toxicity stimulated by different natural compounds [35, 201]. The process of toxicity testing is a significant step in the drug manufacturing process. The toxicity of an investigational substance is highly specific based on the species, organ, and dose which are investigated through different preclinical toxicity studies. The toxicity test can be conducted in different ways, including by studying accidental exposures to a compound, in vitro studies using cells and animal studies [202]. The median lethal dosage (LD50) is used to determine the short-term toxic effect of the compound [203].
Different investigations carried out by researchers demonstrated that quercetin had no adverse effect on the survival of the animal and did not show any toxicity. A toxicological study revealed no toxicity of quercetin which was conducted in male and female F344/N rats at the dose of 40–1900 mg/kg/day for 2 years. However, a high dose could reduce the body weight of the mice [204]. A combination study of 4-chloromercuribenzoic (pCMB) acid and quercetin showed no remarkable changes in physiological, behavioral, and serum biochemical properties in Swiss albino mice in the oral administration where the LC50 was 91.57 ± 0.35 mg/L and 448.45 ± 0.46 mg/L, respectively, though they showed a minor toxicity in high dose [205]. An in vivo and in vitro study carried out by Han and his team members found that quercetin could suppress LPS-induced microglial toxicity and neurodegeneration in PD mouse models. It also halted neuronal injury through the blocking of mtROS-mediated NLRP3 inflammasome activation in microglia by mitophagy stimulations [206]. Another subchronic toxicity demonstrated the safety of quercetin in male and female CD2F1 mice at several concentrations (62, 125, and 250 mg/kg of diet) for 98 days [207]. Quercetin could prevent Cd-induced toxicity via moderating energy and lipid metabolism, stimulating the antioxidant protective mechanism, and protecting liver and kidney function in sixty male Sprague Dawley rats at different concentrations (10–50 mg/kg bw/day) [156].
These findings demonstrated that quercetin exerts significant anticancer activity while it has no toxic effect at low doses. Therefore, further investigation is required to investigate the potentially toxic effects of quercetin following chronic ingestion.
3. Conclusion and Future Perspectives
In spite of significant advancements in the treatment approach that have been established in recent years, cancer still remains a most fearsome disease which causes a significant number of deaths around the world each year. Though chemotherapy was thought to be the most useful treatment for cancer, it has numerous side effects on human health. In recent years, several natural products have been used in the drug discovery process for cancer treatment as they have shown strong anticancer activities. Quercetin, a flavonoid compound, is used in the medications of different diseases, including cancer, inflammation, and neurological disorders. This study has unveiled the remarkable anticancer activities of quercetin in different cells, including bladder, breast, colon, colorectal, esophageal, gastric, lung, prostate, liver, and oral squamous cell carcinoma (OSCC) cells. Quercetin showed anticancer properties like apoptosis, cell cycle arrest, oxidative stress, inhibition of angiogenesis, inhibition of cell proliferation and migration, ferroptosis, and cytotoxicity through different mechanisms and pathways including suppression of EGFR and ERK, PI3K/AKT/mTORC1 pathway, regulating the JAK/STAT signaling system, diminishing the MAPK signaling pathway, modulation of the MMP signaling pathway, suppression of NF-κB pathways, upregulation of p-Camk2/p-DRP1, and SIRT5/PI3K/AKT pathway. Different studies investigated that quercetin significantly accumulated in the lungs, liver, kidneys, and small intestines at high concentrations and in the brain, heart, and spleen at low concentrations while excreted through the renal, fecal, and respiratory systems. The oral bioavailability of quercetin is very low, and it shows low water solubility as well as a short biological half-life. So, it requires alternative ways of administration. In this case, combination with other molecules or nanoformulation treatment could give potential effects of quercetin to overcome these limitations. This study also described that quercetin showed synergistic effects in both combination therapy and nanotherapy which will be helpful in the future to develop innovative therapeutics. The updated nanosystems discussed in the literature demonstrated promising methods for effectively encapsulating quercetin and releasing it in a controlled way. Nanotechnology provides an opportunity to produce nanoparticles loaded with phytochemicals, which can be used to prevent and treat cancer. In addition, several techniques for manipulating nanoparticles have enabled the precise delivery of quercetin to specific tissues in tumor microenvironments, as well as enhancing its ability to cross the blood-brain barrier and penetrate within skin layers. But it has different side effects and it is a costly process. So, future research should be conducted to develop more effective nanoformulation of quercetin with low cost and minimal side effects. Addressing the challenge of quercetin′s poor bioavailability, different studies revealed that complexation with other molecules or nanoparticles notably enhanced the bioavailability of quercetin. These findings indicated that future research on the discovery of novel pharmaceutics by combination or nanoformulation process indicating a wide spectrum of potential medical applications. These recommended future investigations will determine the potential of quercetin to be used as a potential drug in the field of oncology. This study also found that quercetin demonstrated safety in the animal clinical trial and no toxicity was observed. However, there is a lack of clinical studies to support quercetin as an effective drug in cancer treatment. So, in conclusion, this study suggested that more clinical trials should be conducted to find out the strong anticancer activities of quercetin and to support quercetin as a significant anticancer drug.
Data Availability
No data were used to support this study.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Authors' Contributions
T.A.E. and M.S.B. conceptualized the study; M.S.B. and S.S. were involved in methodology; T.A.E. and M.S.B. performed data curation; T.A.E., M.S.B., and S.A. wrote the original draft of the manuscript; M.S.B. and R.C. reviewed and edited the manuscript; R.C. performed mechanism draw; M.T.I. supervised the study; M.S.B. and M.T.I. were involved in project administration. All authors have read and agreed to the published version of the manuscript.
References
- 1.Brown J. S., Amend S. R., Austin R. H., Gatenby R. A., Hammarlund E. U., Pienta K. J. Updating the definition of cancer. Molecular Cancer Research: MCR . 2023;21:1142–1147. doi: 10.1158/1541-7786.MCR-23-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma X., Yu H. Global burden of cancer. Yale Journal of Biology & Medicine . 2006;79(3-4):85–94. [PMC free article] [PubMed] [Google Scholar]
- 3.Fidler M. M., Bray F., Soerjomataram I. The global cancer burden and human development: a review. Scandinavian Journal of Public Health . 2018;46(1):27–36. doi: 10.1177/1403494817715400. [DOI] [PubMed] [Google Scholar]
- 4.Thun M. J., DeLancey J. O., Center M. M., Jemal A., Ward E. M. The global burden of cancer: priorities for prevention. Carcinogenesis . 2010;31(1):100–110. doi: 10.1093/carcin/bgp263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bray F., Laversanne M., Sung H., et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians . 2024;74(3):229–263. doi: 10.3322/caac.21834. [DOI] [PubMed] [Google Scholar]
- 6.Danaei G., Vander Hoorn S., Lopez A. D., Murray C. J., Ezzati M. Causes of cancer in the world: comparative risk assessment of nine behavioural and environmental risk factors. The Lancet . 2005;366(9499):1784–1793. doi: 10.1016/s0140-6736(05)67725-2. [DOI] [PubMed] [Google Scholar]
- 7.Blackadar C. B. Historical review of the causes of cancer. World Journal of Clinical Oncology . 2016;7(1):54–86. doi: 10.5306/wjco.v7.i1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isikhan V., Güner P., Kömürcü S., Ozet A., Arpaci F., Oztürk B. The relationship between disease features and quality of life in patients with cancer--I. Cancer Nursing . 2001;24(6):490–495. doi: 10.1097/00002820-200112000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Aggarwal B. B., Gehlot P. Inflammation and cancer: how friendly is the relationship for cancer patients? Current Opinion in Pharmacology . 2009;9(4):351–369. doi: 10.1016/j.coph.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aslam M. S., Naveed S., Ahmed A., Abbas Z., Gull I., Athar M. A. Side effects of chemotherapy in cancer patients and evaluation of patients opinion about starvation based differential chemotherapy. Journal of Cancer Therapy . 2014;05(08):817–822. doi: 10.4236/jct.2014.58089. [DOI] [Google Scholar]
- 11.Reddy L., Odhav B., Bhoola K. D. Natural products for cancer prevention: a global perspective. Pharmacology & therapeutics . 2003;99:1–13. doi: 10.1016/s0163-7258(03)00042-1. [DOI] [PubMed] [Google Scholar]
- 12.Debela D. T., Muzazu S. G., Heraro K. D., et al. New Approaches and Procedures for Cancer Treatment: Current Perspectives . SAGE open medicine; 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koehn F. E., Carter G. T. The evolving role of natural products in drug discovery. Nature reviews Drug discovery . 2005;4(3):206–220. doi: 10.1038/nrd1657. [DOI] [PubMed] [Google Scholar]
- 14.Bhuia M. S., Aktar M. A., Chowdhury R., et al. Therapeutic potentials of ononin with mechanistic insights: A comprehensive review. Food Bioscience . 2023;56 doi: 10.1016/j.fbio.2023.103302.103302 [DOI] [Google Scholar]
- 15.Nobili S., Lippi D., Witort E., et al. Natural compounds for cancer treatment and prevention. Pharmacological research . 2009;59(6):365–378. doi: 10.1016/j.phrs.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 16.Seyhan A. A. Lost in translation: the valley of death across preclinical and clinical divide – identification of problems and overcoming obstacles. Translational Medicine Communications . 2019;4(1):p. 18. doi: 10.1186/s41231-019-0050-7. [DOI] [Google Scholar]
- 17.Huang M., Lu J. J., Ding J. Natural products in cancer therapy: past, present and future. Natural products and bioprospecting . 2021;11(1):5–13. doi: 10.1007/s13659-020-00293-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chowdhury R., Bhuia M. S., Rakib A. I., et al. Assessment of quercetin antiemetic properties: in vivo and in silico investigations on receptor binding affinity and synergistic effects. Plants (Basel) . 2023;12(24):p. 4189. doi: 10.3390/plants12244189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y., Yao J., Han C., et al. Quercetin, inflammation and immunity. Nutrients . 2016;8(3):p. 167. doi: 10.3390/nu8030167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lakhanpal P, Rai D. Quercetin: a versatile flavonoid. Internet Journal of Medical Update - EJOURNAL . 2007;2:22–37. doi: 10.4314/ijmu.v2i2.39851. [DOI] [Google Scholar]
- 21.Xu D., Hu M. J., Wang Y. Q., Cui Y. L. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules (Basel, Switzerland) . 2019;24(6):p. 1123. doi: 10.3390/molecules24061123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rocha M. F. G., Sales J. A., da Rocha M. G., et al. Antifungal effects of the flavonoids kaempferol and quercetin: a possible alternative for the control of fungal biofilms. Biofouling . 2019;35(3):320–328. doi: 10.1080/08927014.2019.1604948. [DOI] [PubMed] [Google Scholar]
- 23.Costa A. C. F., de Sousa L. M. Anti-inflammatory and hepatoprotective effects of quercetin in an experimental model of rheumatoid arthritis. Inflammation . 2021;44(5):2033–2043. doi: 10.1007/s10753-021-01479-y. [DOI] [PubMed] [Google Scholar]
- 24.Pereira G. S., Percebom I., Mendes S., et al. Quercetin inhibits neutrophil extracellular traps release and their cytotoxic effects on A549 cells, as well the release and enzymatic activity of elastase and myeloperoxidase. Brazilian Journal of Biology . 2022;84 doi: 10.1590/1519-6984.252936.e252936 [DOI] [PubMed] [Google Scholar]
- 25.Batiha G. E., Beshbishy A. M., Ikram M., Mulla Z. S. The Pharmacological Activity. Biochemical Properties, and Pharmacokinetics of the Major Natural Polyphenolic Flavonoid: Quercetin . 2020 doi: 10.3390/foods9030374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adami B. S., Diz F. M., Oliveira Gonçalves G. P. Morphological and mechanical changes induced by quercetin in human T24 bladder cancer cells. Micron . 2021;151 doi: 10.1016/j.micron.2021.103152.103152 [DOI] [PubMed] [Google Scholar]
- 27.Guo H., Ding H., Tang X., et al. Quercetin induces pro-apoptotic autophagy via SIRT1/AMPK signaling pathway in human lung cancer cell lines A549 and H1299 in vitro. Thoracic cancer . 2021;12(9):1415–1422. doi: 10.1111/1759-7714.13925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ward A. B., Mir H., Kapur N., Gales D. N., Carriere P. P., Singh S. Quercetin inhibits prostate cancer by attenuating cell survival and inhibiting anti-apoptotic pathways. World journal of surgical oncology . 2018;16(1):p. 108. doi: 10.1186/s12957-018-1400-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu Q., Yang L., Yang H., Han Y., Chen Y., He Y. Quercetin alleviates the progression of breast cancer-related depression via inhibiting the pyroptosis and promoting the immune response. Mediators of inflammation . 2022;2022:1–14. doi: 10.1155/2022/8011988.8011988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plakas S. M., Lee T. C., Wolke R. E. Absence of overt toxicity from feeding the flavonol, quercetin, to rainbow trout (Salmo gairdneri) Food and Chemical Toxicology . 1985;23(12):1077–1080. doi: 10.1016/0278-6915(85)90055-9. [DOI] [PubMed] [Google Scholar]
- 31.Bhatiya M., Pathak S., Jothimani G., Duttaroy A. K., Banerjee A. A comprehensive study on the anti-cancer effects of quercetin and its epigenetic modifications in arresting progression of colon cancer cell proliferation. Archivum immunologiae et therapiae experimentalis . 2023;71(1):p. 6. doi: 10.1007/s00005-023-00669-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.García-Gutiérrez N., Luna-Bárcenas G. Quercetin and its fermented extract as a potential inhibitor of bisphenol a-exposed HT-29 colon cancer cells’ viability. International journal of molecular sciences . 2023;24(6):p. 5604. doi: 10.3390/ijms24065604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tezerji S., Nazari Robati F. Quercetin’s effects on colon cancer cells apoptosis and proliferation in a rat model of disease. Clinical nutrition ESPEN . 2022;48:441–445. doi: 10.1016/j.clnesp.2022.01.004. [DOI] [PubMed] [Google Scholar]
- 34.Aktar M. A., Bhuia M. S., Chowdhury R. An insight of plant source, toxicological profile, and pharmacological activities of iridoid loganic acid: a comprehensive review. Chemistry & Biodiversity . 2024 doi: 10.1002/cbdv.202400874.e202400874 [DOI] [PubMed] [Google Scholar]
- 35.Chen D. Q., Chen H., Chen L., Tang D. D., Miao H., Zhao Y. Y. Metabolomic application in toxicity evaluation and toxicological biomarker identification of natural product. Chemico-biological interactions . 2016;252:114–130. doi: 10.1016/j.cbi.2016.03.028. [DOI] [PubMed] [Google Scholar]
- 36.Ali Khan M., El-Kersh D. M. Mikania micrantha kunth: an ethnopharmacological treasure trove of therapeutic potential. Chemistry & Biodiversity . 2023;20(11) doi: 10.1002/cbdv.202300392.e202300392 [DOI] [PubMed] [Google Scholar]
- 37.Prasathkumar M., George A., Sadhasivam S. Influence of chitosan and hydroxyethyl cellulose modifications towards the design of cross-linked double networks hydrogel for diabetic wound healing. International Journal of Biological Macromolecules . 2024;265 doi: 10.1016/j.ijbiomac.2024.130851.130851 [DOI] [PubMed] [Google Scholar]
- 38.De Luca V., Salim V., Atsumi S. M., Yu F. Mining the biodiversity of plants: a revolution in the making. Science (New York, NY) . 2012;336(6089):1658–1661. doi: 10.1126/science.1217410. [DOI] [PubMed] [Google Scholar]
- 39.Wang L., Du X., Yue D., Chen X. Catechin, rutin and quercetin in Quercus mongolica Fisch leaves exert inhibitory effects on multiple cancer cells. Journal of Food Biochemistry . 2022;46(12) doi: 10.1111/jfbc.14486.e14486 [DOI] [PubMed] [Google Scholar]
- 40.Calvo T. R., Lima Z. P. Constituents and antiulcer effect of Alchornea glandulosa: activation of cell proliferation in gastric mucosa during the healing process. Biological & pharmaceutical bulletin . 2007;30(3):451–459. doi: 10.1248/bpb.30.451. [DOI] [PubMed] [Google Scholar]
- 41.Hwang I. K., Lee C. H. Neuroprotective effects of onion extract and quercetin against ischemic neuronal damage in the gerbil hippocampus. Journal of medicinal food . 2009;12(5):990–995. doi: 10.1089/jmf.2008.1400. [DOI] [PubMed] [Google Scholar]
- 42.Ezzat S. M., Choucry M. A., Kandil Z. A. Antibacterial, antioxidant, and topical anti-inflammatory activities of Bergia ammannioides: A wound-healing plant. Pharmaceutical biology . 2016;54(2):215–224. doi: 10.3109/13880209.2015.1028079. [DOI] [PubMed] [Google Scholar]
- 43.Moulaoui K., Caddeo C. Identification and nanoentrapment of polyphenolic phytocomplex from Fraxinus angustifolia: in vitro and in vivo wound healing potential. European Journal of Medicinal Chemistry . 2015;89:179–188. doi: 10.1016/j.ejmech.2014.10.047. [DOI] [PubMed] [Google Scholar]
- 44.Sitarek P., Skała E., Wysokińska H., et al. The Effect of Leonurus sibiricus Plant Extracts on Stimulating Repair and Protective Activity against Oxidative DNA Damage in CHO Cells and Content of Phenolic Compounds . 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pastorino G. Biological activities of the legume crops Melilotus officinalis and Lespedeza capitata for skin care and pharmaceutical applications. Industrial Crops and Products . 2017;96:158–164. doi: 10.1016/j.indcrop.2016.11.047. [DOI] [Google Scholar]
- 46.Süntar I. P. Wound healing potential of Sambucus ebulus L. leaves and isolation of an active component, quercetin 3-O-glucoside. Journal of Ethnopharmacology . 2010;129(1):106–114. doi: 10.1016/j.jep.2010.01.051. [DOI] [PubMed] [Google Scholar]
- 47.George B. P., Parimelazhagan T., Sajeesh T., Saravanan S. Antitumor and wound healing properties of Rubus niveus Thunb. root. Journal of Environmental Pathology, Toxicology and Oncology . 2014;33(2):145–158. doi: 10.1615/jenvironpatholtoxicoloncol.2014010949. [DOI] [PubMed] [Google Scholar]
- 48.Lodhi S., Jain A., Jain A. P., Pawar R. S., Singhai A. K. Effects of flavonoids from Martynia annua and Tephrosia purpurea on cutaneous wound healing. Avicenna Journal of Phytomedicine . 2016;6(5):578–591. [PMC free article] [PubMed] [Google Scholar]
- 49.Borrelli F., Izzo A. A. The plant kingdom as a source of anti-ulcer remedies. Phytotherapy Research . 2000;14(8):581–591. doi: 10.1002/1099-1573(200012)14:8<581::aid-ptr776>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 50.Madden E., McLachlan C., Oketch-Rabah H., Calderón A. I. United States Pharmacopeia comprehensive safety review of Styphnolobium japonicum flower and flower bud. Phytotherapy Research . 2022;36(5):2061–2071. doi: 10.1002/ptr.7438. [DOI] [PubMed] [Google Scholar]
- 51.Malú Q., Caldeira G. I. Ethnomedicinal, chemical, and biological aspects of lannea species-a review. Plants (Basel, Switzerland) . 2024;13(5):p. 690. doi: 10.3390/plants13050690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Woźniak D., Ślusarczyk S. Comparison of polyphenol profile and antimutagenic and antioxidant activities in two species used as source of solidaginis herba - goldenrod. Chemistry & biodiversity . 2018;15(4) doi: 10.1002/cbdv.201800023.e1800023 [DOI] [PubMed] [Google Scholar]
- 53.Grygorieva O, Klymenko S. Evaluation of the antioxidant activity and phenolic content of Chinese quince (Pseudocydonia sinensisSchneid.) fruit. Acta scientiarum polonorum Technologia alimentaria . 2020;19(1):25–36. doi: 10.17306/j.afs.0738. [DOI] [PubMed] [Google Scholar]
- 54.Bastos K. X., Dias C. N. Identification of phenolic compounds from Hancornia speciosa (Apocynaceae) Leaves by UHPLC Orbitrap-HRMS. Molecules (Basel, Switzerland) . 2017;22(1):p. 143. doi: 10.3390/molecules22010143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zongo F., Ribuot C. Botany, traditional uses, phytochemistry and pharmacology of Waltheria indica L. (syn. Waltheria americana): a review. Journal of Ethnopharmacology . 2013;148(1):14–26. doi: 10.1016/j.jep.2013.03.080. [DOI] [PubMed] [Google Scholar]
- 56.Montenegro G., Díaz-Forestier J. Phenolic profiles of nectar and honey of Quillaja saponaria Mol. (Quillajaceae) as potential chemical markers. Biological research . 2013;46(2):177–182. doi: 10.4067/s0716-97602013000200009. [DOI] [PubMed] [Google Scholar]
- 57.Alexander J., Parthiban S., Boopathi T. Isolation of Quercetin from Physalis Lagascae and Its In-Vitro Anticancer Activity. 2021;10:1196–1209. [Google Scholar]
- 58.Al-Snafi. Phenolics and flavonoids contents of medicinal plants, as natural ingredients for many therapeutic purposes-a review. 2020. pp. 42–81.
- 59.Mehesare S. S., Waghmare S. P. Quantification of gallic acid. Rutin and Quercetin in Hydro-Ethanolic Extract of Holarrhena Antidysenterica using High Performance Thin Layer Chromatography (HPTLC) . 2022;4 [Google Scholar]
- 60.Kekeç N. Phytochemical studies of Helichrysum armenium subsp. armenium and its antioxidant, acetylcholinesterase, and antimicrobial activities . 2024;28 [Google Scholar]
- 61.Jang G. R., Harris R. Z., Lau D. T. Pharmacokinetics and its role in small molecule drug discovery research. Medicinal research reviews . 2001;21(5):382–396. doi: 10.1002/med.1015. [DOI] [PubMed] [Google Scholar]
- 62.Afroz M. Anti-diarrheal effect of piperine possibly through the interaction with inflammation inducing enzymes: In vivo and in silico studies. European Journal of Pharmacology . 2024;965 doi: 10.1016/j.ejphar.2023.176289.176289 [DOI] [PubMed] [Google Scholar]
- 63.Gibaldi M., Levy G. Pharmacokinetics in clinical practice. I. Concepts. Jama . 1976;235(17):1864–1867. doi: 10.1001/jama.1976.03260430034020. [DOI] [PubMed] [Google Scholar]
- 64.Toutain P. L., Bousquet-Mélou A. Bioavailability and its assessment. Journal of veterinary pharmacology and therapeutics . 2004;27(6):455–466. doi: 10.1111/j.1365-2885.2004.00604.x. [DOI] [PubMed] [Google Scholar]
- 65.Hasan R. Antiemetic activity of abietic acid possibly through the 5HT(3) and muscarinic receptors interaction pathways. Science Reports . 2024;14(1):p. 6642. doi: 10.1038/s41598-024-57173-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ru J., Li P. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. Journal of Cheminformatics . 2014;6(1):p. 13. doi: 10.1186/1758-2946-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bhuia M. S. Anticancer potential of the plant‐derived Saponin Gracillin: a comprehensive review of mechanistic approaches. Chemistry & Biodiversity . 2023;20(9) doi: 10.1002/cbdv.202300847.e202300847 [DOI] [PubMed] [Google Scholar]
- 68.Ruiz-Garcia A., Bermejo M., Moss A., Casabo V. G. Pharmacokinetics in drug discovery. Journal of Pharmaceutical Sciences . 2008;97(2):654–690. doi: 10.1002/jps.21009. [DOI] [PubMed] [Google Scholar]
- 69.Mukhopadhyay P., Prajapati A. K. Quercetin in anti-diabetic research and strategies for improved quercetin bioavailability using polymer-based carriers – a review. RSC Advances . 2015;5(118):97547–97562. doi: 10.1039/c5ra18896b. [DOI] [Google Scholar]
- 70.Cai X. Bioavailability of quercetin: problems and promises. Current medicinal chemistry . 2013;20:2572–2582. doi: 10.2174/09298673113209990120. [DOI] [PubMed] [Google Scholar]
- 71.Srinivas K. Solubility and solution thermodynamic properties of quercetin and quercetin dihydrate in subcritical water. Journal of Food Engineering . 2010;100(2):208–218. doi: 10.1016/j.jfoodeng.2010.04.001. [DOI] [Google Scholar]
- 72.Reinboth M. Oral bioavailability of quercetin from different quercetin glycosides in dogs. British Journal of Nutrition . 2010;104(2):198–203. doi: 10.1017/s000711451000053x. [DOI] [PubMed] [Google Scholar]
- 73.Wang W. The biological activities, chemical stability, metabolism and delivery systems of quercetin: A review. Trends in Food Science & Technology . 2016;56:21–38. doi: 10.1016/j.tifs.2016.07.004. [DOI] [Google Scholar]
- 74.Zang X. Quercetin nanoformulations: a promising strategy for tumor therapy. Food & function . 2021;12(15):6664–6681. doi: 10.1039/d1fo00851j. [DOI] [PubMed] [Google Scholar]
- 75.Graefe E. U. Pharmacokinetics and bioavailability of the flavonol quercetin in humans. International journal of clinical pharmacology and therapeutics . 1999;37(5):219–233. [PubMed] [Google Scholar]
- 76.Ader P., Wessmann A., Wolffram S. Bioavailability and metabolism of the flavonol quercetin in the pig. Free radical biology & medicine . 2000;28(7):1056–1067. doi: 10.1016/s0891-5849(00)00195-7. [DOI] [PubMed] [Google Scholar]
- 77.Röder P. V. The role of SGLT1 and GLUT2 in intestinal glucose transport and sensing. PloS one . 2014;9(2) doi: 10.1371/journal.pone.0089977.e89977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hai Y. Advance on the absorption, metabolism, and efficacy exertion of quercetin and its important derivatives. 2020. pp. 420–434.
- 79.Youdim K. A. Flavonoid permeability across an in situ model of the blood-brain barrier. Free Radical Biology and Medicine . 2004;36(5):592–604. doi: 10.1016/j.freeradbiomed.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 80.Hayes J. D. Oxidative stress in cancer. Cancer cell . 2020;38(2):167–197. doi: 10.1016/j.ccell.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bhuia M. S. Anticancer potentials of the lignan magnolin: a systematic review. Molecules (Basel, Switzerland) . 2023;28(9):p. 3671. doi: 10.3390/molecules28093671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhou X., Joshi S. Reactive oxygen, nitrogen, carbonyl and sulfur species and their roles in plant abiotic stress responses and tolerance . 2021. [Google Scholar]
- 83.Reczek C. R. A CRISPR screen identifies a pathway required for paraquat-induced cell death. Nature chemical biology . 2017;13(12):1274–1279. doi: 10.1038/nchembio.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sosa V. Oxidative stress and cancer: an overview. Ageing research reviews . 2013;12(1):376–390. doi: 10.1016/j.arr.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 85.Sajadimajd S., Khazaei M. Oxidative stress and cancer: the role of Nrf2. Current cancer drug targets . 2018;18(6):538–557. doi: 10.2174/1568009617666171002144228. [DOI] [PubMed] [Google Scholar]
- 86.Wu Q. Different antitumor effects of quercetin, quercetin-3’-sulfate and quercetin-3-glucuronide in human breast cancer MCF-7 cells. Food & function . 2018;9(3):1736–1746. doi: 10.1039/c7fo01964e. [DOI] [PubMed] [Google Scholar]
- 87.Wang Z. X. Quercetin induces p53-independent cancer cell death through lysosome activation by the transcription factor EB and Reactive Oxygen Species-dependent ferroptosis. British journal of pharmacology . 2021;178(5):1133–1148. doi: 10.1111/bph.15350. [DOI] [PubMed] [Google Scholar]
- 88.Ding L. Quercetin induces ferroptosis in gastric cancer cells by targeting SLC1A5 and regulating the p-Camk2/p-DRP1 and NRF2/GPX4 Axes. Free Radical Biology and Medicine . 2024;213:150–163. doi: 10.1016/j.freeradbiomed.2024.01.002. [DOI] [PubMed] [Google Scholar]
- 89.Yang P. Quercetin attenuates the proliferation of arsenic-related lung cancer cells via a caspase-dependent DNA damage signaling. Molecular carcinogenesis . 2022;61(7):655–663. doi: 10.1002/mc.23408. [DOI] [PubMed] [Google Scholar]
- 90.Wang W. Quercetin induces MGMT+ glioblastoma cells apoptosis via dual inhibition of Wnt3a/β-Catenin and Akt/NF-κB signaling pathways. Phytomedicine . 2023;118 doi: 10.1016/j.phymed.2023.154933.154933 [DOI] [PubMed] [Google Scholar]
- 91.Bhuia M. S. Bioactivities of morroniside: A comprehensive review of pharmacological properties and molecular mechanisms. Fitoterapia . 2024;175 doi: 10.1016/j.fitote.2024.105896.105896 [DOI] [PubMed] [Google Scholar]
- 92.Zhang L. Xanthatin induces cell cycle arrest at G2/M checkpoint and apoptosis via disrupting NF-κB pathway in A549 non-small-cell lung cancer cells. Molecules (Basel, Switzerland) . 2012;17(4):3736–3750. doi: 10.3390/molecules17043736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bhuia M. S. Efficacy of Rotundic Acid and Its Derivatives as Promising Natural Anticancer Triterpenoids: A Literature-Based Study. Chemistry & Biodiversity . 2024;21(2) doi: 10.1002/cbdv.202301492.e202301492 [DOI] [PubMed] [Google Scholar]
- 94.Shapiro G. I., Harper J. W. Anticancer drug targets: cell cycle and checkpoint control. Journal of Clinical Investigation . 1999;104(12):1645–1653. doi: 10.1172/jci9054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Otto T., Sicinski P. Cell cycle proteins as promising targets in cancer therapy. Nature reviews Cancer . 2017;17(2):93–115. doi: 10.1038/nrc.2016.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brown J. M., Attardi L. D. The role of apoptosis in cancer development and treatment response. Nature reviews Cancer . 2005;5(3):231–237. doi: 10.1038/nrc1560. [DOI] [PubMed] [Google Scholar]
- 97.Waldman T. Cell-cycle arrest versus cell death in cancer therapy. Nature medicine . 1997;3(9):1034–1036. doi: 10.1038/nm0997-1034. [DOI] [PubMed] [Google Scholar]
- 98.Li J. Network pharmacology and in vitro experimental verification on intervention of quercetin, present in chinese medicine yishen qutong granules, on esophageal cancer. Chinese journal of integrative medicine . 2023;29(3):233–243. doi: 10.1007/s11655-022-3677-6. [DOI] [PubMed] [Google Scholar]
- 99.Hisaka T. Quercetin suppresses proliferation of liver cancer cell lines in vitro. Anticancer research . 2020;40(8):4695–4700. doi: 10.21873/anticanres.14469. [DOI] [PubMed] [Google Scholar]
- 100.Son H. K., Kim D. Quercetin induces cell cycle arrest and apoptosis in yd10b and yd38 oral squamous cell carcinoma cells. Asian Pacific Journal of Cancer Prevention . 2023;24(1):283–289. doi: 10.31557/apjcp.2023.24.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Soll F. Quercetin inhibits proliferation and induces apoptosis of B16 melanoma cells in vitro. Assay and drug development technologies . 2020;18(6):261–268. doi: 10.1089/adt.2020.993. [DOI] [PubMed] [Google Scholar]
- 102.Song Y. Quercetin inhibits intrahepatic cholangiocarcinoma by inducing ferroptosis and inhibiting invasion via the NF-κB pathway. The American Journal of Chinese Medicine . 2023;51(03):701–721. doi: 10.1142/s0192415x23500337. [DOI] [PubMed] [Google Scholar]
- 103.Mou Y. Ferroptosis, a new form of cell death: opportunities and challenges in cancer. Journal of hematology & oncology . 2019;12(1):p. 34. doi: 10.1186/s13045-019-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yu H. Ferroptosis, a new form of cell death, and its relationships with tumourous diseases. Journal of cellular and molecular medicine . 2017;21(4):648–657. doi: 10.1111/jcmm.13008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu J. Signaling pathways and defense mechanisms of ferroptosis. The FEBS journal . 2022;289(22):7038–7050. doi: 10.1111/febs.16059. [DOI] [PubMed] [Google Scholar]
- 106.Zhu Y. W. Quercetin induces ferroptosis by inactivating mTOR/S6KP70 pathway in oral squamous cell carcinoma. Toxicology Mechanisms and Methods . 2024;34(6):669–675. doi: 10.1080/15376516.2024.2325989. [DOI] [PubMed] [Google Scholar]
- 107.An S., Hu M. Quercetin promotes tfeb nuclear translocation and activates lysosomal degradation of ferritin to induce ferroptosis in breast cancer cells. Computational intelligence and neuroscience . 2022;2022:6. doi: 10.1155/2022/5299218.5299218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Huang J., Chen J., Li J. Quercetin promotes ATG5-mediating autophagy-dependent ferroptosis in gastric cancer. Journal of molecular histology . 2024;55(2):211–225. doi: 10.1007/s10735-024-10186-5. [DOI] [PubMed] [Google Scholar]
- 109.Lowe S. W., Lin A. W. Apoptosis in cancer. Carcinogenesis . 2000;21(3):485–495. doi: 10.1093/carcin/21.3.485. [DOI] [PubMed] [Google Scholar]
- 110.Letai A. Apoptosis and cancer. Annual Review of Cancer Biology . 2017;1:275–294. doi: 10.1146/annurev-cancerbio-050216-121933. [DOI] [Google Scholar]
- 111.Jan R., Chaudhry G. E. Understanding apoptosis and apoptotic pathways targeted cancer therapeutics. Advanced pharmaceutical bulletin . 2019;9(2):205–218. doi: 10.15171/apb.2019.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ghobrial I. M., Witzig T. E., Adjei A. A. Targeting apoptosis pathways in cancer therapy. CA: A Cancer Journal for Clinicians . 2005;55(3):178–194. doi: 10.3322/canjclin.55.3.178. [DOI] [PubMed] [Google Scholar]
- 113.Wong R. S. Apoptosis in cancer: from pathogenesis to treatment. Journal of Experimental & Clinical Cancer Research . 2011;30(1):p. 87. doi: 10.1186/1756-9966-30-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wu T. C. Quercetin and chrysin inhibit nickel-induced invasion and migration by downregulation of TLR4/NF-κB signaling in A549 cells. Chemico-biological interactions . 2018;292:101–109. doi: 10.1016/j.cbi.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 115.Yang Y. Exploring the mechanism by which quercetin re-sensitizes breast cancer to paclitaxel: network pharmacology, molecular docking, and experimental verification. Naunyn-Schmiedeberg2019s archives of pharmacology . 2023;396(11):3045–3059. doi: 10.1007/s00210-023-02510-9. [DOI] [PubMed] [Google Scholar]
- 116.Özsoy S. Quercetin-Mediated apoptosis and cellular senescence in human colon cancer. Anti-cancer agents in medicinal chemistry . 2020;20(11):1387–1396. doi: 10.2174/1871520620666200408082026. [DOI] [PubMed] [Google Scholar]
- 117.Zhou B. Quercetin inhibits DNA damage responses to induce apoptosis via SIRT5/PI3K/AKT pathway in non-small cell lung cancer. Biomedicine & Pharmacotherapy . 2023;165 doi: 10.1016/j.biopha.2023.115071.115071 [DOI] [PubMed] [Google Scholar]
- 118.Bhuia M. S. Hirsutine, an Emerging Natural Product with Promising Therapeutic Benefits: A Systematic Review. Molecules . 2023;28(16):p. 6141. doi: 10.3390/molecules28166141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Golias C. H. Cell proliferation and cell cycle control: a mini review. International journal of clinical practice . 2004;58(12):1134–1141. doi: 10.1111/j.1742-1241.2004.00284.x. [DOI] [PubMed] [Google Scholar]
- 120.Sears R. C., Nevins J. R. Signaling networks that link cell proliferation and cell fate. Journal of Biological Chemistry . 2002;277(14):11617–11620. doi: 10.1074/jbc.r100063200. [DOI] [PubMed] [Google Scholar]
- 121.Shapiro P. Ras-MAP kinase signaling pathways and control of cell proliferation: relevance to cancer therapy. Critical reviews in clinical laboratory sciences . 2002;39(4-5):285–330. doi: 10.1080/10408360290795538. [DOI] [PubMed] [Google Scholar]
- 122.Wu R. Quercetin, the Ingredient of Xihuang Pills, Inhibits Hepatocellular Carcinoma by Regulating Autophagy and Macrophage Polarization. Frontiers in Bioscience-Landmark . 2022;27(12):p. 323. doi: 10.31083/j.fbl2712323. [DOI] [PubMed] [Google Scholar]
- 123.Bhuia M. S. Anticancer Potential of the Plant-Derived Saponin Gracillin: A Comprehensive Review of Mechanistic Approaches. Chemistry & biodiversity . 2023;20(9) doi: 10.1002/cbdv.202300847.e202300847 [DOI] [PubMed] [Google Scholar]
- 124.Huang W. J. The β-catenin-LINC00183-miR-371b-5p-Smad2/LEF1 axis promotes adult T-cell lymphoblastic lymphoma progression and chemoresistance. Journal of Experimental & Clinical Cancer Research . 2023;42(1):p. 105. doi: 10.1186/s13046-023-02670-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jiang L. J. PHB promotes bladder cancer cell epithelial-mesenchymal transition via the Wnt/β-catenin signaling pathway. Pathology, research and practice . 2023;247 doi: 10.1016/j.prp.2023.154536.154536 [DOI] [PubMed] [Google Scholar]
- 126.Jiang L. J. Snai2-mediated upregulation of NADSYN1 promotes bladder cancer progression by interacting with PHB. Clinical and translational medicine . 2024;14(1) doi: 10.1002/ctm2.1555.e1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lu L., Yuan X. LMTK3 knockdown retards cell growth and invasion and promotes apoptosis in thyroid cancer. Molecular medicine reports . 2017;15(4):2015–2022. doi: 10.3892/mmr.2017.6262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Veiseh O. Cancer cell invasion: treatment and monitoring opportunities in nanomedicine. Advanced drug delivery reviews . 2011;63(8):582–596. doi: 10.1016/j.addr.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chowdhury R. An insight into the anticancer potentials of lignan arctiin: A comprehensive review of molecular mechanisms. Heliyon . 2024;10(12) doi: 10.1016/j.heliyon.2024.e32899.e32899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liu C. A. Migration/Invasion of Malignant Gliomas and Implications for Therapeutic Treatment. International journal of molecular sciences . 2018;19(4):p. 1115. doi: 10.3390/ijms19041115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cohen D. P. Mathematical modelling of molecular pathways enabling tumour cell invasion and migration. PLoS computational biology . 2015;11 doi: 10.1371/journal.pcbi.1004571.e1004571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Brown J. S. Updating the definition of cancer. Molecular Cancer Research . 2023;21(11):1142–1147. doi: 10.1158/1541-7786.mcr-23-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wu Y., Zhou B. P. TNF-α/NF-κB/Snail pathway in cancer cell migration and invasion. British journal of cancer . 2010;102(4):639–644. doi: 10.1038/sj.bjc.6605530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liu Y. Quercetin inhibits invasion and angiogenesis of esophageal cancer cells. Pathology, research and practice . 2021;222 doi: 10.1016/j.prp.2021.153455.153455 [DOI] [PubMed] [Google Scholar]
- 135.Umar S. M. Quercetin impairs hur-driven progression and migration of triple negative breast cancer (TNBC) cells. Nutrition and cancer . 2022;74(4):1497–1510. doi: 10.1080/01635581.2021.1952628. [DOI] [PubMed] [Google Scholar]
- 136.Ferdous J. Pharmacological activities of plant-derived fraxin with molecular mechanisms: a comprehensive review. Chemistry & Biodiversity . 2024;21(5) doi: 10.1002/cbdv.202301615.e202301615 [DOI] [PubMed] [Google Scholar]
- 137.Khan R. A. Anticancer effects of acteoside: Mechanistic insights and therapeutic status. European journal of pharmacology . 2022;916 doi: 10.1016/j.ejphar.2021.174699.174699 [DOI] [PubMed] [Google Scholar]
- 138.Nishida N. Angiogenesis in cancer. Vascular health and risk management . 2006;2(3):213–219. doi: 10.2147/vhrm.2006.2.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Khalid E. B. Natural products against cancer angiogenesis. Tumor Biology . 2016;37(11):14513–14536. doi: 10.1007/s13277-016-5364-8. [DOI] [PubMed] [Google Scholar]
- 140.Prager G. W., Poettler M. Angiogenesis in cancer. Basic mechanisms and therapeutic advances. Hamostaseologie . 2012;32(2):105–114. doi: 10.5482/ha-1163. [DOI] [PubMed] [Google Scholar]
- 141.Wang L., Ji S., Liu Z., Zhao J. Quercetin inhibits glioblastoma growth and prolongs survival rate through inhibiting glycolytic metabolism. Chemotherapy . 2022;67(3):132–141. doi: 10.1159/000523905. [DOI] [PubMed] [Google Scholar]
- 142.Mizushima N. Autophagy: process and function. Genes & development . 2007;21(22):2861–2873. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- 143.Mathew R. Role of autophagy in cancer. Nature reviews Cancer . 2007;7(12):961–967. doi: 10.1038/nrc2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.White E. The role for autophagy in cancer. Journal of Clinical Investigation . 2015;125(1):42–46. doi: 10.1172/jci73941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Debnath J., Gammoh N., Ryan K. M. Autophagy and autophagy-related pathways in cancer. Nature reviews Molecular cell biology . 2023;24(8):560–575. doi: 10.1038/s41580-023-00585-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Vega-Rubín-de-Celis S. The role of beclin 1-dependent autophagy in cancer. Biology . 2019;9(1):p. 4. doi: 10.3390/biology9010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zou L. Autophagy and beyond: Unraveling the complexity of UNC-51-like kinase 1 (ULK1) from biological functions to therapeutic implications. Acta pharmaceutica Sinica B . 2022;12(10):3743–3782. doi: 10.1016/j.apsb.2022.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Li X. Autophagy and autophagy-related proteins in cancer. Molecular Cancer . 2020;19(1):p. 12. doi: 10.1186/s12943-020-1138-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhao G. Beta carotene protects H9c2 cardiomyocytes from advanced glycation end product-induced endoplasmic reticulum stress, apoptosis, and autophagy via the PI3K/Akt/mTOR signaling pathway. Annals of translational medicine . 2020;8(10):p. 647. doi: 10.21037/atm-20-3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bhuia M. S. A mechanistic insight into the anticancer potentials of resveratrol: Current perspectives. Phytotherapy Research . 2024;38(8):3877–3898. doi: 10.1002/ptr.8239. [DOI] [PubMed] [Google Scholar]
- 151.Gavas S. Nanoparticles for cancer therapy: current progress and challenges. Nanoscale research letters . 2021;16(1):p. 173. doi: 10.1186/s11671-021-03628-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Miao L. Nanoformulations for combination or cascade anticancer therapy. Advanced drug delivery reviews . 2017;115:3–22. doi: 10.1016/j.addr.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shim G. Nanoformulation-based sequential combination cancer therapy. Advanced drug delivery reviews . 2017;115:57–81. doi: 10.1016/j.addr.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 154.Kashyap D. Natural product-based nanoformulations for cancer therapy: Opportunities and challenges. Seminars in cancer biology . 2021;69:5–23. doi: 10.1016/j.semcancer.2019.08.014. [DOI] [PubMed] [Google Scholar]
- 155.Sun J. Delivery of quercetin for breast cancer and targeting potentiation via hyaluronic nano-micelles. International journal of biological macromolecules . 2023;242 doi: 10.1016/j.ijbiomac.2023.124736.124736 [DOI] [PubMed] [Google Scholar]
- 156.Liu M. Paclitaxel and quercetin co-loaded functional mesoporous silica nanoparticles overcoming multidrug resistance in breast cancer. Colloids and surfaces B, Biointerfaces . 2020;196 doi: 10.1016/j.colsurfb.2020.111284.111284 [DOI] [PubMed] [Google Scholar]
- 157.Mohammed H. A. Quercetin against MCF7 and CAL51 breast cancer cell lines: apoptosis, gene expression and cytotoxicity of nano-quercetin. Nanomedicine (London, England) . 2021;16(22):1937–1961. doi: 10.2217/nnm-2021-0070. [DOI] [PubMed] [Google Scholar]
- 158.Keshavarz F. Quercetin-loaded liposomes effectively induced apoptosis and decreased the epidermal growth factor receptor expression in colorectal cancer cells: an in vitro study. Iranian journal of medical sciences . 2023;48(3):321–328. doi: 10.30476/IJMS.2022.95272.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Dogan M. Assessment of mechanism involved in the apoptotic and anti-cancer activity of Quercetin and Quercetin-loaded chitosan nanoparticles. Medical oncology (Northwood, London, England) . 2022;39(11):p. 176. doi: 10.1007/s12032-022-01820-x. [DOI] [PubMed] [Google Scholar]
- 160.Li C. Biocompatible and biodegradable nanoparticles for enhancement of anti-cancer activities of phytochemicals. Chinese journal of natural medicines . 2015;13(9):641–652. doi: 10.1016/s1875-5364(15)30061-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nam J. S. Application of bioactive quercetin in oncotherapy: from nutrition to nanomedicine. Molecules (Basel, Switzerland) . 2016;21(1):p. 108. doi: 10.3390/molecules21010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bhuia M. S. Anxiolytic-like effects by trans-ferulic acid possibly occur through gabaergic interaction pathways. Pharmaceuticals (Basel) . 2023;16(9):p. 1271. doi: 10.3390/ph16091271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sun W., Sanderson P. E., Zheng W. Drug combination therapy increases successful drug repositioning. Drug discovery today . 2016;21(7):1189–1195. doi: 10.1016/j.drudis.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mokhtari R. B. Combination therapy in combating cancer. Oncotarget . 2017;8(23):38022–38043. doi: 10.18632/oncotarget.16723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tanomrat R. N-acetylcysteine improves the inhibitory effect of Quercetin-rich onion extract on HT-29 and HCT-116 colorectal cancer migration and invasion through iNOS suppression. International journal of medical sciences . 2023;20(9):1123–1134. doi: 10.7150/ijms.86573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jalalpour Choupanan M. Naringenin in combination with quercetin/fisetin shows synergistic anti-proliferative and migration reduction effects in breast cancer cell lines. Molecular biology reports . 2023;50(9):7489–7500. doi: 10.1007/s11033-023-08664-2. [DOI] [PubMed] [Google Scholar]
- 167.Kundur S. Synergistic anticancer action of quercetin and curcumin against triple-negative breast cancer cell lines. Journal of cellular physiology . 2019;234(7):11103–11118. doi: 10.1002/jcp.27761. [DOI] [PubMed] [Google Scholar]
- 168.Rhman M. A. Synergism potentiates oxidative antiproliferative effects of naringenin and quercetin in MCF-7 breast cancer cells. Nutrients . 2022;14(16):p. 3437. doi: 10.3390/nu14163437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hanikoglu A. Effects of somatostatin, curcumin, and quercetin on the fatty acid profile of breast cancer cell membranes. Canadian journal of physiology and pharmacology . 2020;98(3):131–138. doi: 10.1139/cjpp-2019-0352. [DOI] [PubMed] [Google Scholar]
- 170.Hosseini S. S. Study of quercetin and fisetin synergistic effect on breast cancer and potentially involved signaling pathways. Cell biology international . 2023;47(1):98–109. doi: 10.1002/cbin.11942. [DOI] [PubMed] [Google Scholar]
- 171.Amiri A. New perspectives of quercetin and vitamin C effects on fibronectin-binding integrins and chemokine receptors in prostate cancer cell lines. Bratislava Medical Journal . 2021;122(07):507–512. doi: 10.4149/bll_2021_082. [DOI] [PubMed] [Google Scholar]
- 172.Xu Z. Naringenin and quercetin exert contradictory cytoprotective and cytotoxic effects on tamoxifen-induced apoptosis in HepG2 Cells. Nutrients . 2022;14(24):p. 5394. doi: 10.3390/nu14245394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Roy S. Deciphering the biochemical and molecular mechanism underlying the in vitro and in vivo chemotherapeutic efficacy of ruthenium quercetin complex in colon cancer. Molecular carcinogenesis . 2018;57(6):700–721. doi: 10.1002/mc.22792. [DOI] [PubMed] [Google Scholar]
- 174.Celano M. Quercetin improves the effects of sorafenib on growth and migration of thyroid cancer cells. Endocrine . 2020;67(2):496–498. doi: 10.1007/s12020-019-02140-3. [DOI] [PubMed] [Google Scholar]
- 175.Erdoğan M. K. Quercetin and luteolin improve the anticancer effects of 5-fluorouracil in human colorectal adenocarcinoma in vitro model: a mechanistic insight. Nutrition and cancer . 2022;74(2):660–676. doi: 10.1080/01635581.2021.1900301. [DOI] [PubMed] [Google Scholar]
- 176.Li X., Guo S. Combination of quercetin and cisplatin enhances apoptosis in OSCC cells by downregulating xIAP through the NF-κB pathway. Journal of Cancer . 2019;10(19):4509–4521. doi: 10.7150/jca.31045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Karimian A. The modulatory effects of two bioflavonoids, quercetin and thymoquinone on the expression levels of DNA damage and repair genes in human breast, lung and prostate cancer cell lines. Pathology, research and practice . 2022;240 doi: 10.1016/j.prp.2022.154143.154143 [DOI] [PubMed] [Google Scholar]
- 178.Mellman I. Cancer immunotherapy comes of age. Nature . 2011;480(7378):480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Qiu D. To explore immune synergistic function of Quercetin in inhibiting. breast cancer cells . 2021;21:1–14. doi: 10.1186/s12935-021-02345-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Guo S. B. large-sample informatics study . London, England: International journal of surgery; 2024. Comparative investigation of neoadjuvant immunotherapy versus adjuvant immunotherapy in perioperative patients with cancer: a global-scale, cross-sectional. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sun D. A cyclodextrin-based nanoformulation achieves co-delivery of ginsenoside Rg3 and quercetin for chemo-immunotherapy in colorectal cancer. Acta pharmaceutica Sinica B . 2022;12(1):378–393. doi: 10.1016/j.apsb.2021.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Guo S. B. A scientometrics and visualization analysis of oxidative stress modulator Nrf2 in cancer profiles its characteristics and reveals its association with immune response. Heliyon . 2023;9(6) doi: 10.1016/j.heliyon.2023.e17075.e17075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sun D. A cyclodextrin-based nanoformulation achieves co-delivery of ginsenoside Rg3 and quercetin for chemo-immunotherapy in colorectal cancer. Acta Pharmaceutica Sinica B . 2022;12(1):378–393. doi: 10.1016/j.apsb.2021.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kirkwood J. M. Immunotherapy of cancer in 2012. CA: A Cancer Journal for Clinicians . 2012;62(5):309–335. doi: 10.3322/caac.20132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wu R. Quercetin, the ingredient of xihuang pills, inhibits hepatocellular carcinoma by regulating autophagy and macrophage polarization. Front Biosci . 2022;27(12):p. 323. doi: 10.31083/j.fbl2712323. [DOI] [PubMed] [Google Scholar]
- 186.Guo S. B. Global research hotspots, development trends and prospect discoveries of phase separation in cancer: a decade-long informatics investigation. Biomarker research . 2024;12(1):p. 39. doi: 10.1186/s40364-024-00587-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zhang J. Nanoformulated codelivery of quercetin and alantolactone promotes an antitumor response through synergistic immunogenic cell death for microsatellite-stable colorectal cancer. ACS nano . 2019;13(11):12511–12524. doi: 10.1021/acsnano.9b02875. [DOI] [PubMed] [Google Scholar]
- 188.Brito A. F. Quercetin in cancer treatment, alone or in combination with conventional therapeutics? Current medicinal chemistry . 2015;22(26):3025–3039. doi: 10.2174/0929867322666150812145435. [DOI] [PubMed] [Google Scholar]
- 189.Iacopetta D. New insights for the use of quercetin analogs in cancer treatment. Future medicinal chemistry . 2017;9(17):2011–2028. doi: 10.4155/fmc-2017-0118. [DOI] [PubMed] [Google Scholar]
- 190.Kashyap D. Molecular mechanisms of action of quercetin in cancer: recent advances. Tumor Biology . 2016;37(10):12927–12939. doi: 10.1007/s13277-016-5184-x. [DOI] [PubMed] [Google Scholar]
- 191.Vafadar A. Quercetin and cancer: new insights into its therapeutic effects on ovarian cancer cells. Cell & bioscience . 2020;10(1):p. 32. doi: 10.1186/s13578-020-00397-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Henning S. M. Prospective randomized trial evaluating blood and prostate tissue concentrations of green tea polyphenols and quercetin in men with prostate cancer. Food & function . 2020;11(5):4114–4122. doi: 10.1039/d0fo00565g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ferry D. R. Phase I clinical trial of the flavonoid quercetin: pharmacokinetics and evidence for in vivo tyrosine kinase inhibition. Clinical cancer research: an official journal of the American Association for Cancer Research . 1996;2(4):659–668. [PubMed] [Google Scholar]
- 194.Morrow D. M. Dietary supplementation with the anti-tumour promoter quercetin: its effects on matrix metalloproteinase gene regulation. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis . 2001;480-481:269–276. doi: 10.1016/s0027-5107(01)00184-1. [DOI] [PubMed] [Google Scholar]
- 195.Wilms L. C. Impact of multiple genetic polymorphisms on effects of a 4-week blueberry juice intervention on ex vivo induced lymphocytic DNA damage in human volunteers. Carcinogenesis . 2007;28(8):1800–1806. doi: 10.1093/carcin/bgm145. [DOI] [PubMed] [Google Scholar]
- 196.Vaez S. Quercetin and polycystic ovary syndrome; inflammation, hormonal parameters and pregnancy outcome: A randomized clinical trial. American journal of reproductive immunology (New York, NY: 1989) . 2023;89(3) doi: 10.1111/aji.13644.e13644 [DOI] [PubMed] [Google Scholar]
- 197.Cruz-Correa M. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clinical Gastroenterology and Hepatology . 2006;4(8):1035–1038. doi: 10.1016/j.cgh.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 198.Gillette J. R. Biochemical Mechanisms of Drug Toxicity . 1974;14:271–288. [Google Scholar]
- 199.Khatun M. M., Bhuia M. S. Potential utilization of ferulic acid and its derivatives in the management of metabolic diseases and disorders: An insight into mechanisms. Cellular Signalling . 2024;121 doi: 10.1016/j.cellsig.2024.111291.111291 [DOI] [PubMed] [Google Scholar]
- 200.Ali Abdalla Y. O. Natural Products for Cancer Therapy: A Review of Their Mechanism of Actions and Toxicity in the Past Decade. Journal of tropical medicine . 2022;2022:1–20. doi: 10.1155/2022/5794350.5794350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Bhuia M. S., Chowdhury R. Therapeutic Promises of Ferulic Acid and its Derivatives on Hepatic damage Related with Oxidative Stress and Inflammation: A Review with Mechanisms. Chemistry & Biodiversity . 2024;21(7) doi: 10.1002/cbdv.202400443.e202400443 [DOI] [PubMed] [Google Scholar]
- 202.Parasuraman S. Toxicological screening. Journal of pharmacology & pharmacotherapeutics . 2011;2:74–79. doi: 10.4103/0976-500X.81895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Saganuwan. Toxicity studies of drugs and chemicals in animals: an overview. 2017.
- 204.Dunnick J. K., Hailey J. R. Toxicity and carcinogenicity studies of quercetin, a natural component of foods. Fundamental and applied toxicology: official journal of the Society of Toxicology . 1992;19(3):423–431. doi: 10.1016/0272-0590(92)90181-g. [DOI] [PubMed] [Google Scholar]
- 205.Pal A., Tripathi A. Toxicological and behavioral study of two potential antibacterial agents:4-chloromercuribenzoic acid and quercetin on Swiss-albino mice. Drug and chemical toxicology . 2020;43(6):645–655. doi: 10.1080/01480545.2018.1517774. [DOI] [PubMed] [Google Scholar]
- 206.Han X. Quercetin hinders microglial activation to alleviate neurotoxicity via the interplay between NLRP3 inflammasome and mitophagy. Redox biology . 2021;44 doi: 10.1016/j.redox.2021.102010.102010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Cunningham P., Patton E. Sub-chronic oral toxicity screening of quercetin in mice. BMC complementary medicine and therapies . 2022;22(1):p. 279. doi: 10.1186/s12906-022-03758-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.VanderVeen B. N., Cardaci T. D. Quercetin improved muscle mass and mitochondrial content in a murine model of cancer and chemotherapy-induced cachexia. Nutrients . 2022;15(1):p. 102. doi: 10.3390/nu15010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were used to support this study.


