Abstract
The rapid identification and management of air leak syndrome in the neonatal intensive care unit is critical to prevent and/or minimize short- and long-term complications. Traditionally, chest X-ray is used to diagnose pneumothorax or pneumomediastinum. However, point-of-care ultrasound is increasingly being used for procedural and diagnostic purposes. Current ultrasound guidelines recommend specific criteria to diagnose pneumothorax in newborns including sharp A-lines, absence of B-lines, lack of shimmering of the pleural line, and the presence of a lung point. Pneumomediastinum may have similar ultrasound characteristics. In this case report, we present two cases of pneumomediastinum in newborns, describe the associated ultrasound findings, and review some of the criteria to differentiate from pneumothorax, including the presence of a still lung point. A high index of suspicion for pneumomediastinum should be maintained when using ultrasound to diagnose air leak given the overlapping sonographic features with pneumothorax. This distinction is of particular importance if evacuation of air by needle thoracentesis or the placement of a chest tube is under consideration.
Keywords: POCUS, pneumothorax, pneumomediastinum, lung sliding, ultrasound, air leak syndrome, newborn, lung point
Air leak syndrome (ALS), such as pulmonary interstitial emphysema, pneumothorax, or pneumomediastinum, occurs with relative frequency in the neonatal population. 1 The underlying mechanism is rupture of the distal lung with subsequent air dissection into the pulmonary interstitium. Further dissection of the air into the pleural cavity can cause pneumothorax, whereas air migration to the hilum can rupture into the mediastinal space and induce pneumomediastinum. 2 ALS is associated with resuscitation maneuvers, extreme prematurity, respiratory distress syndrome (RDS), pneumonia, meconium aspiration syndrome, and assisted ventilation. 3 Air leaks can cause significant morbidity and mortality. Traditionally, the diagnosis is suspected by physical exam, indicated by decreased breath sounds and positive transillumination, and confirmed by X-ray.
Point-of-care ultrasound (POCUS) is increasingly used in the neonatal intensive care unit (NICU) as a rapid, nonradiating tool for bedside diagnosis. The presence of sharp A-lines, absence of B-lines, lack of pleural sliding or shimmering, and the presence of a lung point are considered ultrasound criteria for the diagnosis of pneumothorax. 4 Pneumomediastinum is present less frequently than pneumothorax and often does not require intervention. 2 However, ultrasound findings of pneumomediastinum may mimic those of pneumothorax. 5 In this study, we present two cases of pneumomediastinum and discuss the ultrasound findings to differentiate both entities.
Case 1
Supplementary Video S1 Lung ultrasound showing an extensive parasternal area of sharp A-lines, absence of B-lines and absence of lung sliding. A contiguous area of lung point is seen dividing these findings from an area of adjacent coalescent B-lines and minimal pleural sliding.
Supplementary Video S2 For comparison, a video with pneumothorax and pleura sliding at the lung point is shown.
A 1,790 g male infant was born at 33 weeks' gestation to a 27-year-old G3P1 mother via urgent cesarean section due to nonreassuring fetal heart tracing in the setting of preeclampsia. The infant's mother received a single dose of betamethasone for fetal lung maturity less than 12 hours prior to delivery. Membranes were ruptured at time of delivery revealing brown-colored fluid concerning for meconium, blood product, or chorioamnionitis. At birth, the infant was nonvigorous and apneic, requiring positive pressure ventilation and eventually intubation due to nonsustained respiratory effort and high FiO2 requirement on continuous positive airway pressure (CPAP). APGAR scores were 2, 4, and 7 at 1, 5, and 10 minutes, respectively. Upon admission to the NICU, the chest X-ray (CXR) revealed severe RDS with a “white-out” appearance of both lungs. His FiO2 requirement increased to 100% and did not immediately improve following intubation and surfactant administration. Due to refractory hypoxemia on high conventional ventilator settings and 100% FiO2, the infant was initially placed on high-frequency oscillatory ventilation. A blood culture was obtained, and he was started on ampicillin and gentamicin. Echocardiogram revealed a structurally normal heart. At 24 hours of life, a follow-up CXR revealed large volume pneumomediastinum without pneumothorax; air was seen tracking up into the neck, which corresponded with subcutaneous emphysema that was easily palpable on exam ( Fig. 1A ). The pneumomediastinum persisted over the next 5 days despite overall improvement in the infant's respiratory status and transition to high-frequency jet ventilation (HFJV). His blood culture remained negative, and he was treated with antibiotics for 7 days for presumed congenital pneumonia, given the severity of his lung disease and brown amniotic fluid color that had raised concern for intra-amniotic infection at the time of delivery, though no specific organism was identified. Of note, placental pathology revealed signs of malperfusion as well as placental abruption, raising the possibility of fetal blood aspiration versus meconium aspiration (though atypical in a premature infant) from fetal distress as possible etiologies of the infant's severe lung disease. Lung POCUS revealed an extensive parasternal area of sharp A-lines, absence of B-lines, and absence of lung sliding. A contiguous area of lung point is seen dividing these findings from an area of adjacent coalescent B-lines and minimal pleural sliding ( Supplementary Video S1 ). For comparison, a video with pneumothorax and pleura sliding at the lung point is shown ( Supplementary Video S2 ). Fig. 1B shows a picture of lung point for this patient. When using M-mode, the barcode sign was seen ( Fig. 1C ). In addition, hyperechoic A-lines were seen over the area of the thymus ( Fig. 1D ). The infant was extubated from HFJV on day of life (DOL) #7 to high flow nasal cannula (HFNC) and transitioned to room air on DOL #9. His course was further complicated by the development of bilateral grade 3 interventricular hemorrhage with ventricular dilation detected on his initial cranial ultrasound on DOL #4. He unfortunately went on to develop severe, worsening posthemorrhagic hydrocephalus that required a ventriculoperitoneal (VP) shunt prior to discharge. He otherwise did well and was discharged home on room air taking full feeds by mouth at 7 weeks of age. His post-NICU discharge course was complicated by VP shunt infection at age 3 months requiring pediatric intensive care unit admission, shunt removal, and external ventricular drain placement, and ultimately placement of a new VP shunt. He was unfortunately lost to follow-up after his initial outpatient appointment with our NICU follow-up clinic at age 4 months.
Fig. 1.
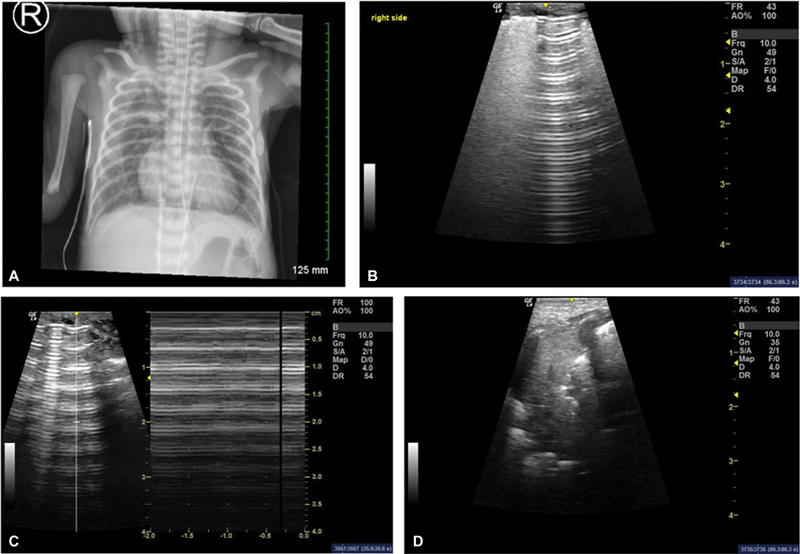
( A ) Case 1 chest X-ray at 24 hours of life showing large bilateral pneumomediastinum with air tracking into the neck. ( B ) Lung ultrasound of the same patient demonstrating a transition between sharp A-lines and confluent B-lines (lung point). ( C ) Picture depicting the barcode sign (right) when using M-mode over the A-lines area (left). ( D ) Lung ultrasound over the thymus area showing hyperechoic images (white) consistent with pneumomediastinum.
Case 2
Supplementary Video S3 Bedside POCUS video demonstrates area of sharp A-lines, absence of B-lines, and lack of lung sliding in the right parasternal area. Additionally, a vertically contiguous area of lung point without pleural sliding is observed.
A 710 g female infant was born at 24 weeks of gestation to a 29-year-old G1P0 mother in the setting of preterm labor. The mother presented to the emergency department with abdominal pain and was found to be fully dilated. She received one dose of betamethasone prior to delivery. The infant was born via vaginal delivery after rapid progression of preterm labor. Membranes were ruptured artificially at the time of delivery to clear fluid. CPAP was initially applied in the delivery room then transitioned to positive pressure ventilation via Neopuff prior to eventual intubation with a 2.0 endotracheal tube for poor respiratory effort and inaudible heart rate at 40 seconds of life. Patient's APGARs were 1, 2, 2, and 6 at 1, 5, 10, and 15 minutes, respectively. The patient was transported to the NICU and was started on HFJV. Initial CXR showed a “white-out” of the lungs, which improved after surfactant administration. On DOL 1 to 2, frank blood was noted in the mouth, along with new, diffuse opacities on CXR that were consistent with pulmonary hemorrhage. The patient was stabilized with packed red blood cells, and HFJV settings were adjusted to achieve tamponade. Unfortunately, cranial ultrasound obtained the following day revealed left grade IV intraventricular hemorrhage (IVH) and right grade I IVH. On DOL 4, an enteric tube was found to be malpositioned, projecting over the right upper quadrant, consistent with an esophageal perforation. Patient subsequently developed pneumomediastinum with no radiologic evidence of pneumothorax or pneumoperitoneum ( Fig. 2A ). Bedside POCUS was obtained with the following findings: area of sharp A-lines, absence of B-lines, and lack of lung sliding observed in the right parasternal area; additionally, a vertically contiguous area of lung point without pleural sliding was observed ( Supplementary Video S3 ). Fig. 2B shows sharp A-lines and absence of B-lines. Patient was maintained on HFJV and radiographic evidence of pneumomediastinum resolved by DOL 7. Over time, the patient was weaned from respiratory support, diagnosed with moderate bronchopulmonary dysplasia (BPD), and discharged with home O2, tolerating full feeds by mouth. She did require an Ommaya reservoir with serial ventricular taps at time of discharge and ultimately required a VP shunt shortly after discharge for posthemorrhagic hydrocephalus. At her NICU follow-up appointment at age 9 months (6 months corrected age), she was meeting all developmental milestones, doing well with feeding, no longer requiring supplemental oxygen, and other than mild truncal hypotonia was noted to have a normal neurologic exam.
Fig. 2.
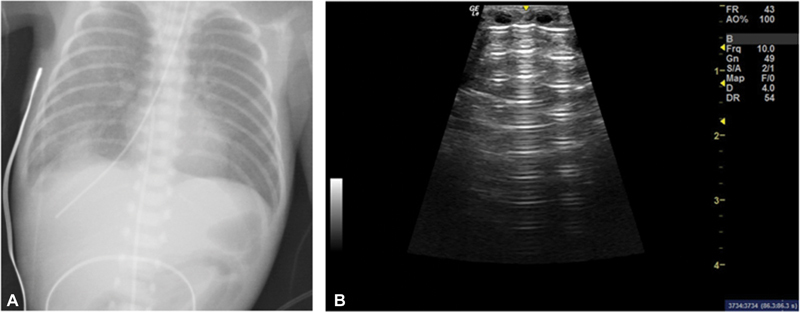
( A ) Case 2 chest X-ray on day of life (DOL) 4 demonstrating right side pneumomediastinum and malpositioned enteral feeding tube. ( B ) Lung ultrasound of the same patient showing sharp A-lines and absence of B-lines.
Discussion
Pneumomediastinum is usually a self-limiting condition. However, stretching of the mediastinal pleura can lead to its rupture and cause pneumothorax. Early detection and close follow-up of pneumomediastinum is therefore important to try and minimize this progression. The diagnosis of pneumomediastinum by CXR is characterized by elevation of the thymus by air, giving the typical “angel wing” or “spinnaker sail” signs. 2 By ultrasound, pneumomediastinum may appear as thick linear/curvilinear horizontal echogenic lines located between the anterior chest wall and the thymus, in the lateral margins of the thymus, between the thymus and the great vessels, or in the middle of the thymus. 6 7 A recent study 8 has also described the “stairway sign”; a stairway-like set of horizontal hyperechogenic reflections seen in the high parasternal view, indicating the presence of trapped air below the thymus. This is proposed to be an equivalent of “spinnaker sign” by X-ray.
Whereas the diagnosis of pneumomediastinum by ultrasound is straightforward when the air is accumulated between the heart and the thymus (as in our case 1, Fig. 1D ), the diagnosis becomes more difficult when the air is present in the parasternal areas of the heart. Saracino and Tessaro 5 described a patient with pneumomediastinum in which the ultrasound findings of absent lung sliding in the parasternal area and “bar code” sign by M-mode mimicked pneumothorax. Lung sliding was observed in most of the left anterior chest but was absent over the left parasternal area. Additionally, they noticed the impossibility to obtain parasternal or apical views of the heart due to air artifact; in contrast, there were no difficulties assessing cardiac function by subcostal view. The ultrasound features described by Saracino and Tessaro could be helpful in cases where the pneumomediastinum is localized to a narrow parasternal area with no associated parenchymal lung disease. However, if the pneumomediastinum is extensive (as in case 1) the absence of lung sliding may extend beyond the parasternal area and be confused with pneumothorax.
To differentiate pneumomediastinum from pneumothorax, Matsumoto and Matano 9 introduced a new ultrasound finding, the “still lung point.” In their report, lung sliding was present in both lungs along the mid-clavicular zones but absent in the parasternal areas. A lung point was observed at the transition between the air leak area and the unaffected area, as typically described for the diagnosis of pneumothorax. What makes this study interesting is the lack of pleural sliding at the lung point transition , an ultrasound finding that is not observed in pneumothorax. They speculated that this finding in the transition area is caused by air trapped in the mediastinum which displaces and compress the lungs laterally, causing the prevention of pleural sliding with each breath. We clearly observed this sign in our case 2 whereas lung sliding was significantly decreased in case 1.
Another sonographic evaluation of pneumomediastinum is the “air gap sign,” described as air artifacts appearing cyclically with the cardiac cycle; this is also seen in pneumopericardium. 10 However, with pneumomediastinum, and in contrast to pneumopericardium, the heart is well visualized in the subxiphoid view. Other investigators have identified other ultrasound findings to help delineate the diagnosis of pneumothorax versus pneumomediastinum. They observed a “fluorescent white” echogenic rim of air outlining the cardiac shadow on the left and the right at the subxiphoid view, along with a thin sliver of air outlining the left hemidiaphragm in pneumomediastinum. 11
The distinction between pneumothorax and pneumomediastinum by ultrasound may be difficult for several reasons including the potential presence of multiple forms of air leak simultaneously, the obscured view of the pleura by air in the mediastinum, and the absence of lung sliding in the mediastinum, even under normal conditions, due to the lack of pleura. To make things more complicated, Montero-Gato et al 12 showed that the presence of A-lines behind the sternum in the anterior transverse plane was highly sensitive and specific for the diagnosis of pneumothorax. The authors speculated that the suprasternal plane offers a good acoustic window to visualize the mediastinal structures and to rule out pneumomediastinum in children diagnosed with pneumothorax. Through this window, they observed a pneumomediastinum as a large artifact of A-lines that prevented visualization of the mediastinal structures, or as small hyperechoic lines in the anterior, lateral, or posterior margin of the thymus, or even inside the thymus. Therefore, the presence of A-lines in the anterior transverse plane without the ultrasound findings of pneumomediastinum from the suprasternal view was diagnostic of pneumothorax.
Even though no formal lung ultrasound description of neonatal pneumomediastinum exists, pneumomediastinum should be suspected in newborns with the absence of lung sliding on parasternal ultrasound with normal pleural shimmering in other more peripheral areas of the lung, by the parasternal “still” lung point, and by the inability to obtain a normal parasternal heart view due to air artifact. 13
In recent review manuscripts, 4 14 15 16 pneumomediastinum is not routinely included in the differential diagnosis for neonates with air leak. Interestingly, Raimondi et al 13 in their sonographic assessment of life-threatening emergencies (SAFE) algorithm for suddenly decompensating neonates protocol, indirectly addressed this issue, by suggesting moving the probe to a more lateral position and considering the diagnosis of pneumothorax and possible needle aspiration, if ultrasound findings of air leak detected in the parasternal region are also demonstrated in more peripheral areas of the lung.
In conclusion, with the proliferation of POCUS in newborn medicine, and to avoid unnecessary and potentially risky procedures, neonatologists should consider pneumomediastinum in the differential diagnosis when ultrasound findings are suggestive of pneumothorax. From the two ultrasound findings suggestive of pneumomediastinum, we speculate that the still lung point would be the most specific for this condition, but it will require confirmation in future studies applied to the neonatal population. In the meantime, in an emergency scenario, the approach recommended by Raimondi et al 13 seems to be reasonable.
Footnotes
Conflict of Interest None declared.
References
- 1.Carey B. Neonatal air leaks: pneumothorax, pneumomediastinum, pulmonary interstitial emphysema, pneumopericardium. Neonatal Netw. 1999;18(08):81–84. doi: 10.1891/0730-0832.18.8.81. [DOI] [PubMed] [Google Scholar]
- 2.Cagle K J. Pneumomediastinum in the neonate. Neonatal Netw. 2014;33(05):275–282. doi: 10.1891/0730-0832.33.5.275. [DOI] [PubMed] [Google Scholar]
- 3.Jhaveri V, Vali P, Giusto E, Singh Y, Lakshminrusimha S. Pneumothorax in a term newborn. J Perinatol. 2024;44(04):465–471. doi: 10.1038/s41372-024-01899-2. [DOI] [PubMed] [Google Scholar]
- 4.Liu J, Copetti R, Sorantin E et al. Protocol and guidelines for point-of-care lung ultrasound in diagnosing neonatal pulmonary diseases based on international expert consensus. J Vis Exp. 2019;(145) doi: 10.3791/58990. [DOI] [PubMed] [Google Scholar]
- 5.Saracino C, Tessaro M. Pneumomediastinum as a sonographic mimic of pneumothorax. J Ultrasound Med. 2015;34(08):1521–1522. doi: 10.7863/ultra.34.8.1521. [DOI] [PubMed] [Google Scholar]
- 6.Klavžar P, Plut D.Ultrasound as a problem solver to diagnose pneumomediastinum in an infant Acta Radiol Open 2021100820584601211030657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young Jung A, Yang I, Sun Go H et al. Imaging neonatal spontaneous pneumomediastinum using ultrasound. J Med Ultrason. 2014;41:45–49. [Google Scholar]
- 8.Küng E, Habrina L, Berger A, Werther T, Aichhorn L.Diagnosing pneumomediastinum in a neonate using a lung ultrasound Lancet 2021398(10303):e13. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto T, Matano H. The still lung point: new sonographic evidence for pneumomediastinum. Am J Emerg Med. 2016;34(02):3440–34400. doi: 10.1016/j.ajem.2015.06.029. [DOI] [PubMed] [Google Scholar]
- 10.Zachariah S, Gharahbaghian L, Perera P, Joshi N. Spontaneous pneumomediastinum on bedside ultrasound: case report and review of the literature. West J Emerg Med. 2015;16(02):321–324. doi: 10.5811/westjem.2015.1.24514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Gelderen W F. Ultrasound diagnosis of an atypical pneumomediastinum. Pediatr Radiol. 1992;22(06):469. doi: 10.1007/BF02013517. [DOI] [PubMed] [Google Scholar]
- 12.Montero-Gato J, Rodeño-Fernández L, Serna-Guerediaga I, Aguirre-Unceta-Barrenechea A, Aguirre-Conde A, Perez-Legorburu A. Ultrasound of pneumothorax in neonates: diagnostic value of the anterior transverse plane and of mirrored ribs. Pediatr Pulmonol. 2022;57(04):1008–1014. doi: 10.1002/ppul.25829. [DOI] [PubMed] [Google Scholar]
- 13.Raimondi F, Yousef N, Migliaro F, Capasso L, De Luca D. Point-of-care lung ultrasound in neonatology: classification into descriptive and functional applications. Pediatr Res. 2021;90(03):524–531. doi: 10.1038/s41390-018-0114-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aichhorn L, Küng E, Habrina L et al. The role of lung ultrasound in the management of the critically ill neonate-a narrative review and practical guide. Children (Basel) 2021;8(08):628. doi: 10.3390/children8080628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conlon T W, Nishisaki A, Singh Y et al. Moving beyond the stethoscope: diagnostic point-of-care ultrasound in pediatric practice. Pediatrics. 2019;144(04):e20191402. doi: 10.1542/peds.2019-1402. [DOI] [PubMed] [Google Scholar]
- 16.Kurepa D, Zaghloul N, Watkins L, Liu J. Neonatal lung ultrasound exam guidelines. J Perinatol. 2018;38(01):11–22. doi: 10.1038/jp.2017.140. [DOI] [PubMed] [Google Scholar]


