Abstract
Background
Primary care clinicians have key responsibilities in obesity prevention and weight management.
Aims
We aimed to identify risk factors for developing obesity among people aged ≥45 years.
Methods
We conducted a record linkage longitudinal study of residents of metropolitan Sydney, Australia using data from the: (1) 45 and Up Study at baseline (2005–2009) and first follow-up (2012–2015); (2) Medicare claims; (3) Pharmaceutical Benefits Scheme; and (4) deaths registry. We examined risk factors for developing obesity (body mass index [BMI]: 30–40) at follow-up, separately for people within the: (1) healthy weight range (BMI 18.5–<25) and (2) overweight range (BMI 25–<30) at baseline. Covariates included demographics, modifiable behaviours, health status, allied health use, and medication use. Crude and adjusted relative risks were estimated using Poisson regression modelling.
Results
At follow-up, 1.1% (180/16,205) of those in the healthy weight range group, and 12.7% (1,939/15,266) of those in the overweight range group developed obesity. In both groups, the following were associated with developing obesity: current smoking at baseline, physical functioning limitations, and allied health service use through team care planning, while any alcohol consumption and adequate physical activity were found to be associated with a lower risk of developing obesity. In the healthy weight group, high psychological distress and the use of antiepileptics were associated with developing obesity. In the overweight group, female sex and full-time work were associated with developing obesity, while older age was found to be associated with a lower risk of developing obesity.
Conclusions
These findings may inform the targeting of preventive interventions for obesity in clinical practice and broader public health programs.
Keywords: general practice, obesity, preventive care, risk factors
Key messages.
A targeted multidisciplinary approach is required for obesity prevention.
Identifying priority at-risk groups will help to target interventions.
Smoking and physical inactivity are associated with developing obesity.
Chronic conditions or their treatment are also associated with developing obesity.
Background
The World Health Organisation estimated obesity (Body mass index [BMI]; ≥30) increased 3-fold globally between 1975 and 2016.1 Obesity is the greatest contributing factor to the chronic disease burden, and is associated with higher healthcare utilisation and mortality.2–4 A meta-analysis of 28 international studies found obesity is associated with a 36% median healthcare cost increase compared to healthy weight.5 Australia has one of the world’s highest rates of adult obesity; there were 31% of Australians with obesity in 2017–2018, and the prevalence is rising.6 If this trend continues, the Australian Bureau of Statistics predicts there will be 18 million Australians with obesity or overweight by 2030.7
Primary care clinicians, with their focus on longitudinal comprehensive person-centred care, have important responsibilities in obesity prevention and weight management.8 The Australian Government, through the Medicare universal health insurance scheme, provides rebates for general practice consultations and team care arrangements.9 Most general practitioners (GPs) work together in small (2–5 doctors) group practices that are privately-owned, with 76% of GPs working with other healthcare professionals within the same practice.10,11 The majority of Australians (85%; 21 million approximately) will see their GP at least once per year10; and 90% of these presentations are managed entirely within primary care. Patients prefer to receive weight management support from their GP rather than other healthcare professionals.12 Early intervention requires a targeted multidisciplinary approach to improve diet, increase physical activity, and change behaviour.13 However, capacity to provide this within primary care is limited and Medicare currently funds only 5 consultations with allied health professionals each year.9 There is a need to identify priority at-risk groups to help target such interventions to those in most need.14
Further research is required to assist primary care clinicians to identify people at risk of developing obesity for targeting prevention interventions.15 However, most epidemiological studies assessing this have been based on cross-sectional designs.16,17 We aimed to identify risk factors for developing obesity among people aged ≥45 years for those in the healthy and overweight weight range over a 5–7-year period with a focus on the role of primary care.
Methods
Design, setting, and participants
We conducted a prospective population-based cohort study of people aged ≥ 45 years residing in Sydney metropolitan area using the Central and Eastern Sydney Primary and Community Health Cohort (CES-P&CH)18 derived from the Sax Institute’s 45 and Up Study.19 The 45 and Up Study collected information from 267,357 people living in New South Wales (NSW) between 2005 and 2009. Prospective study participants aged ≥ 45 years and living in NSW were randomly sampled from the Services Australia Medicare enrolment database, with oversampling of people aged 80 years and over, and those living in rural and remote areas. About 19% of those invited joined the study (participants included ≈ 11% of the NSW population aged ≥ 45 years) by completing the baseline questionnaire and providing consent for long-term follow-up, including linkage of their questionnaire data to routinely collected records. Participants living in metropolitan Sydney who completed both the baseline and first follow-up questionnaires (2012–2015) with BMIs between 18.5 and < 30 at baseline were included in this analysis. We divided our analytic cohort into 2 groups to assess any variation in factors associated with developing obesity (BMI [calculated as weight in kilograms divided by height in metres squared] ≥ 30) among those within the: (1) healthy weight range (BMI 18.5 to <25.0) and (2) overweight range (BMI 25.0 to <30.0) at baseline. Participants with a self-reported BMI of >40 were excluded because management usually does not occur in primary care alone.
Data linkage
The Sax Institute facilitated linkage of the 45 and Up Study questionnaire data with Medicare claims and Pharmaceutical Benefits Scheme (PBS) data provided by Services Australia using a unique identifier and deterministic matching. The NSW Centre for Health Record Linkage (CHeReL) linked death notifications from the NSW Registry of Births Deaths and Marriages with the 45 and Up Study questionnaire data using probabilistic matching.
Outcome and covariates
Outcome
The study outcome was obesity (BMI ≥ 30) based on self-reported weight and height recorded in the first follow-up questionnaire between 2012 and 2015.
Covariates
Table 1 shows participant demographics, health behaviours, and health status at baseline (2005–2009). Primary care usage (including allied health usage as part of chronic care team planning) and medication use were ascertained for each participant during the time period between baseline and follow-up (2012–2015) surveys.
Table 1.
Baseline participant characteristics and descriptions.
| Characteristic | Question | Categorisation for analysis |
|---|---|---|
| Age group | Self-reported age | 45–59 years 60–74 years 75 years and over |
| Sex | Male questionnaire Female questionnaire |
Male Female |
| Highest qualification | Self-reported highest level of educational qualification—categorised as | No school certificate or other qualification School or intermediate certificate Higher school or leaving certificate Trade or apprenticeship Certificate or diploma University degree or higher |
| Work status | Working status | Not working Working part-time Working full-time |
| Household income | Self-reported household income category (in Australian dollars [AUD]) | <$20,000 $20,000–39,999 $40,000–69,999 $70,000 or more Won’t disclose |
| Born in Australia | In which country where you born? | Yes: born in Australia No: otherwise |
| Speaks language other than English at home | Whether a language other than English is spoken at home? | Yes: speaks language other than English at home No: speaks only English at home |
| Currently married/partnered | Current marital status | Yes: currently married/partnered No: not currently married/partnered |
| Smoking status | Smoking status | Never smoked Ex-smoker Current smoker |
| Adequate vegetable intake (5 or more serves a day) | How many serves of vegetables do you usually eat each day? | Yes: 5 or more serves No: none or less than 5 serves |
| Adequate fruit intake (2 or more serves a day) | How many serves of fruit or glass of fruit juice do you usually have each day | Yes: 2 or more serves No: none or less than 2 serves |
| Alcohol consumption | Based on self-reported number of standard drinks each week- | Yes: 1 or more drinks per week No: zero |
| Adequate physical activity | Based on amount of self-reported physical activity20 | Yes: adequate physical activity No: inadequate physical activity |
| Good quality of life | Self-reported quality of life on a Likert scale | Yes: excellent, very good or good quality of life No: fair or poor quality of life |
| Chronic conditions | Has a doctor EVER told you that you have: cancer, heart disease, high blood pressure, stroke, diabetes, blood clot, asthma, hay fever, depression, anxiety, or Parkinson’s disease | None One Two or more |
| Psychological distress- Index calculated based on 10 indicators | During the past 4 weeks about how often did you feel: Tired out for no good reason? Nervous? So nervous that nothing could calm you down? Hopeless? Restless or fidgety? So restless that you could not sit still? Depressed? That everything was an effort? So sad that nothing could cheer you up? Worthless? |
Responses for each indicator 1 = none of the time 2 = a little of the time 3 = some of the time 4 = most of the time 5 = all the time Low = total score < 22 High = total score >=22 |
| Physical functioning | Based on scores calculated from 10 items of physical functioning in the 36-item short-form (SF-36) tool (23) | No limitation = score of 100 Minor limitation = score of 90 to <100 Moderate limitation = score of 60 to <90 Severe limitation = score of 0 to <60 |
| Needing help with daily activity | Do you regularly need help with daily tasks because of long-term illness or disability | Yes No |
| Allied health service use through team care arrangement* | If any of the following MBS item numbers were recorded: 10954, 10953, 10962, 10964, and 10960 | Yes: any of them recorded No: none of them recorded |
| Use of psychotropic medications# | If any of the following ATC codes are recorded: N06AF03 (phenelzine), N06AF04 (tranylcypromine), N06AA09 (amitriptyline), N06AA04 (clomipramine), N06AA16 (dosulepin), N06AA12 (doxepin), N06AA02 (imipramine), N06AA10 (nortriptyline), N06AX11 (mirtazapine), N05AL05 (amisulpride), N05AX12 (aripiprazole), N05AH05 (asenapine), N05AX16 (brexpiprazole), N05AX15 (cariprazine), N05AA01 (chlorpromazine), N05AH02 (clozapine), N05AD08 (droperidol), N05AF01 (flupentixol), N05AD01 (haloperidol), N05AE05 (lurasidone), N05AH03 (olanzapine), N05AX13 (paliperidone), N05AC01 (periciazine), N05AH04 (quetiapine), N05AX08 (risperidone), N05AB06 (trifluoperazine), N05AE04 (ziprasidone), N05AF05 (zuclopenthixol) and N05AN01 (lithium). |
Yes: any of them recorded No: none of them recorded |
| Use of diabetic medications # | If any of the following ATC codes are recorded: A10BB01 (glibenclamide), A10BB09 (gliclazide), A10BB12 (glimepiride), A10BB07 (glipizide), A10AB06 (insulin) and A10BG03 (pioglitazone). |
Yes: any of them recorded No: none of them recorded |
| Use of antiepileptic medications# | If any of the following ATC codes are recorded: N03AX12 (gabapentin), N03AX22 (perampanel), N03AX16 (pregabalin), N03AG01 (valproate) and N03AG04 (vigabatrin). |
Yes: any of them recorded No: none of them recorded |
| Body mass index (BMI) | Calculation based on 2 questions: How tall are you without shoes (in centimetres or feet and inches)? How much do you weigh (in kilograms or stones and pounds)? | Underweight: <18.5 Normal weight: 18.5–24.9 Overweight: 25.0–29.9 Obesity: ≥30 |
Data was sourced from the 45 and Up Study at baseline questionnaire (2005–2009),21 except for *allied healthcare use which was sourced from Medicare claims data and #medication use which was sourced from Pharmaceutical Benefits Scheme data between baseline and follow-up surveys (2005–2011; prior to change in weight).
Physical activity was measured using the Australian Physical Activity and Sedentary Behaviour Guidelines.20 Study participants self-reported their physical activity in the week prior to completion of the baseline questionnaire. This was calculated by adding minutes spent walking continuously for at least 10 minutes, minutes spent on moderate physical activity (e.g. gardening or housework), and minutes spent on vigorous physical activity (e.g. jogging or cycling), where time spent on vigorous exercise only was multiplied by 2 as it is more beneficial. Adequate physical activity was defined as undertaking physical activity for a total of at least 150 minutes per week over 5 separate occasions within that week.20
Self-reported quality of life was measured on a 5-point Likert scale (excellent, very good, good, fair, or poor) in the baseline questionnaire. Good quality of life was defined by excellent, very good, or good responses.
Psychological distress was measured using 10 items in the Kessler Psychological Distress Score (K10) in the baseline questionnaire.22 K10 scores range from 10 to 50. High psychological distress was defined by a score of 22 or greater.
Physical functioning scores were calculated using 10 items of physical functioning in the 36-item short-form (SF-36) tool in the baseline questionnaire.23 Physical functioning scores range between 0 and 100. Physical function was categorized as: “no limitation” for scores of 100, “minor limitation” for scores of 90 to <100, “moderate limitation” for scores of 60 to <90, and “severe limitation” for scores of 0 to <60.
Self-reported past personal history of chronic health conditions included up to 11 conditions (cancer, heart disease, high blood pressure, stroke, diabetes, blood clot, asthma, hay fever, depression, anxiety, or Parkinson’s disease) self-reported in the baseline questionnaire.
Allied health usage as part of chronic care team planning was ascertained from Medicare claims data, which contains date of the consultations, Medicare Benefits Schedule (MBS) item numbers, provider charge, and benefit paid for the service. Allied health usage included consultations with dieticians, exercise physiologists, podiatrists, chiropractors and physiotherapists (MBS item numbers 10954, 10953, 10962, 10964, and 10960 respectively).9 If any of these 5 item numbers were recorded in Medicare claims data at any time between baseline and first follow-up questionnaire, then the person was considered as using allied health services through chronic care team planning.
Use of psychotrophic, diabetic, and antiepileptic medications associated with weight gain24 as provided in Table 1 were ascertained from the PBS data, which consists of Anatomical Therapeutic Chemical (ATC) classification of drugs, date of supply and prescription, net benefit, and patient’s contribution. If any of these ATC codes were recorded in PBS data at any time between baseline and first follow-up questionnaire, then the person was considered as using these medications.
Statistical analyses
We created 3 age categories (45–59 years, 60–74 years, and ≥75 years) to assess any differential effect of age on the risk of developing obesity. We calculated values for health status variables (e.g. BMI and psychological distress22). Missing data were excluded; except where tool calculation allows substitution of averages (e.g. psychological distress22). We calculated frequencies and proportions for participant characteristics and the cumulative incidence of obesity at the first follow-up questionnaire by each category of covariates. Relative risk (RR) was used to estimate the association of baseline characteristics, the use of allied health services, and different types of medication with obesity at follow-up. We used simple and multiple Poisson regression models to estimate crude and adjusted RRs and their 95% confidence intervals (CIs). In the multiple Poisson model, we included all the variables in Table 1. We set P = 0.05 as a cut-off for all statistical significance. A variance inflation factor (VIF) was used to check for multicollinearity with the cut-off set at greater than 10. We used R 4.1.3 software (R Foundation, Vienna, Austria) for data analysis and SAS 9.4 (SAS Institute, Cary, NC) for data management. We used “glm” command with Poisson family and log link function to fit the Poisson model and used “vif” command from “car” package in R.
Results
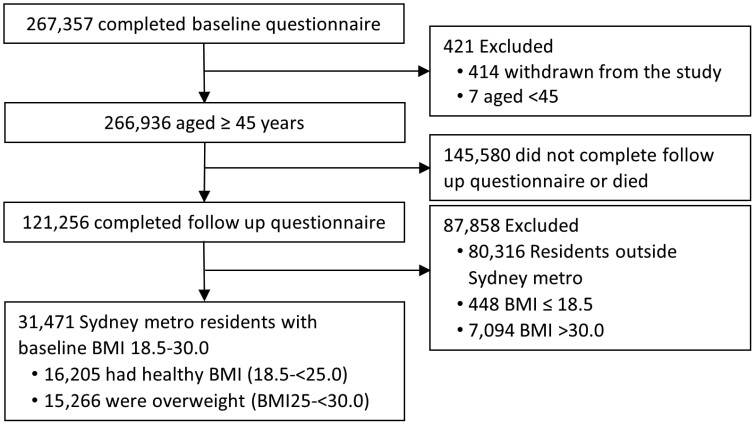
There were 31,471 participants with BMIs between 18.5 and < 30.0 at baseline who were alive up until the follow-up survey and completed both the baseline and follow-up questionnaires (Figure 1). The healthy weight group (BMI 18.5 to <25.0 at baseline) comprised 16,205 participants, and the overweight group (BMI 25.0 to <30.0 at baseline) comprised 15,266 participants; and the mean follow-up time for both groups was 6.3 years. At follow-up, incidence of obesity was 1.1% (180/16,205) in the healthy weight group and 12.7% (1,939/15,266) in the overweight group.
Figure 1.
Assembly of the analytic cohort. This diagram summarises the assembly of the analytic cohort.
Factors associated with developing obesity in the healthy weight group (BMI 18.5 to <25.0)
Table 2 shows the cumulative incidence of obesity at follow-up among people within the healthy weight range at baseline, with crude and adjusted RRs by covariate categories. While obesity incidence was slightly higher among older age groups, adjusted analysis did not show any significant association between age group and developing obesity. We did not find any statistically significant associations between other socio-demographic variables and developing obesity at follow-up.
Table 2.
Risk factors for obesity at follow-up among people in the healthy weight range (BMI 18.5 to <25)
| Characteristics | Number | Without obesity n (%) |
Obesity n (%) |
Crude relative risk (95% confidence interval) | Adjusted* relative risk (95% confidence interval) | P-value |
|---|---|---|---|---|---|---|
| Age at baseline | ||||||
| 45–59 years | 9,098 | 9,000 (98.9) | 98 (1.1) | 1 | 1 | |
| 60–74 years | 5,269 | 5,211 (98.9) | 58 (1.1) | 1.02 (0.73, 1.41) | 0.83 (0.52, 1.29) | 0.412 |
| 75 years and over | 1,838 | 1,814 (98.7) | 24 (1.3) | 1.21 (0.76, 1.86) | 0.63 (0.30, 1.28) | 0.215 |
| Sex | ||||||
| Male | 6,254 | 6,188 (98.9) | 66 (1.1) | 1 | 1 | |
| Female | 9,951 | 9,837 (98.9) | 114 (1.1) | 1.09 (0.80, 1.48) | 0.82 (0.56, 1.21) | 0.317 |
| Highest qualification | ||||||
| No school certificate or other qualification | 599 | 585 (97.7) | 14 (2.3) | 1 | 1 | |
| School or intermediate certificate | 2,173 | 2142 (98.6) | 31 (1.4) | 0.61 (0.33, 1.18) | 0.99 (0.49, 2.19) | 0.987 |
| Higher school or leaving certificate | 1,611 | 1,586 (98.4) | 25 (1.6) | 0.66 (0.35, 1.31) | 1.17 (0.55, 2.68) | 0.689 |
| Trade or apprenticeship | 947 | 939 (99.2) | 8 (0.8) | 0.36 (0.14, 0.84) | 0.53 (0.18, 1.46) | 0.226 |
| Certificate or diploma | 3,625 | 3,586 (98.9) | 39 (1.1) | 0.46 (0.26, 0.88) | 0.85 (0.42, 1.87) | 0.665 |
| University degree or higher | 7,132 | 7,072 (99.2) | 60 (0.8) | 0.36 (0.21, 0.67) | 0.65 (0.32, 1.43) | 0.251 |
| Work status | ||||||
| Not working | 5,750 | 5,678 (98.7) | 72 (1.3) | 1 | 1 | |
| Part time | 4,219 | 4,182 (99.1) | 37 (0.9) | 0.70 (0.47, 1.03) | 1.10 (0.66, 1.84) | 0.713 |
| Full time | 5,972 | 5,904 (98.9) | 68 (1.1) | 0.91 (0.65, 1.27) | 1.22 (0.72, 2.10) | 0.460 |
| Household income (AUD) | ||||||
| <$20,000 | 1,415 | 1,391 (98.3) | 24 (1.7) | 1 | 1 | |
| $20,000–39,999 | 1,923 | 1,899 (98.8) | 24 (1.2) | 0.74 (0.42, 1.30) | 1.03 (0.51, 2.03) | 0.969 |
| $40,000-69,999 | 3,064 | 3034 (99.0) | 30 (1.0) | 0.58 (0.34, 1.00) | 0.93 (0.47, 1.86) | 0.825 |
| $70,000 or more | 6,887 | 6827 (99.1) | 60 (0.9) | 0.51 (0.32, 0.84) | 1.12 (0.56, 2.30) | 0.754 |
| Won’t disclose | 2,916 | 2,874 (98.6) | 42 (1.4) | 0.85 (0.52, 1.42) | 1.20 (0.63, 2.34) | 0.583 |
| Born in Australia | ||||||
| No | 5,452 | 5,404 (99.1) | 48 (0.9) | 1 | 1 | |
| Yes | 10,674 | 10,544 (98.8) | 130 (1.2) | 1.38 (1.00, 1.94) | 1.50 (0.98, 2.34) | 0.069 |
| Speaks language other than English at home | ||||||
| No | 14,100 | 13,944 (98.9) | 156 (1.1) | 1 | 1 | |
| Yes | 2,105 | 2,081 (98.9) | 24 (1.1) | 1.03 (0.65, 1.55) | 1.02 (0.56, 1.78) | 0.945 |
| Currently married/partnered | ||||||
| No | 3,798 | 3,745 (98.6) | 53 (1.4) | 1 | 1 | |
| Yes | 12,348 | 12,224 (99.0) | 124 (1.0) | 0.72 (0.52, 1.00) | 0.85 (0.57, 1.28) | 0.434 |
| Smoking status | ||||||
| Never smoker | 10,674 | 10,566 (99.0) | 108 (1.0) | 1 | 1 | |
| Ex-smoker | 4,758 | 4,712 (99.0) | 46 (1.0) | 0.96 (0.67, 1.34) | 1.12 (0.74, 1.65) | 0.585 |
| Current smoker | 773 | 747 (96.6) | 26 (3.4) | 3.32 (2.12, 5.02) | 2.96 (1.73, 4.81) | <0.001 |
| Adequate vegetable intake (5 or more serves a day) | ||||||
| No | 11,739 | 11,614 (98.9) | 125 (1.1) | 1 | 1 | |
| Yes | 4,466 | 4411 (98.8) | 55 (1.2) | 1.16 (0.84, 1.58) | 1.20 (0.82, 1.74) | 0.331 |
| Adequate fruit intake (2 or more serves a day) | ||||||
| No | 6,008 | 5,940 (98.9) | 68 (1.1) | 1 | 1 | |
| Yes | 10,197 | 10,085 (98.9) | 112 (1.1) | 0.97 (0.72, 1.32) | 1.06 (0.74, 1.52) | 0.763 |
| Alcohol consumption | ||||||
| No | 4,175 | 4,105 (98.3) | 70 (1.7) | 1 | 1 | |
| Yes | 11,866 | 11,757 (99.1) | 109 (0.9) | 0.55 (0.41, 0.74) | 0.59 (0.41, 0.85) | 0.005 |
| Adequate physical activitya | ||||||
| No | 3,424 | 3,351 (97.9) | 73 (2.1) | 1 | 1 | |
| Yes | 12,781 | 12,674 (99.2) | 107 (0.8) | 0.39 (0.29, 0.53) | 0.44 (0.31, 0.63) | <0.001 |
| Self-reported good quality of lifeb | ||||||
| No | 710 | 696 (98.0) | 14 (2.0) | 1 | 1 | |
| Yes | 14,940 | 14,778 (98.9) | 162 (1.1) | 0.55 (0.33, 0.99) | 1.69 (0.85, 3.68) | 0.157 |
| Number of self-reported chronic conditions | ||||||
| None | 6,189 | 6,135 (99.1) | 54 (0.9) | 1 | 1 | |
| One | 5,809 | 5,738 (98.8) | 71 (1.2) | 1.40 (0.99, 2.00) | 1.13 (0.76, 1.69) | 0.545 |
| Two or more | 4,207 | 4,152 (98.7) | 55 (1.3) | 1.50 (1.03, 2.18) | 0.77 (0.47, 1.24) | 0.283 |
| Psychological distressc | ||||||
| Low | 14,527 | 14,381 (99.0) | 146 (1.0) | 1 | 1 | |
| High | 786 | 764 (97.2) | 22 (2.8) | 2.78 (1.73, 4.26) | 2.01 (1.13, 3.38) | 0.013 |
| Physical functioningd | ||||||
| No limitations | 7,483 | 7,419 (99.1) | 64 (0.9) | 1 | 1 | |
| Minor limitations | 4,454 | 4,412 (99.1) | 42 (0.9) | 1.10 (0.74, 1.62) | 0.92 (0.59, 1.41) | 0.691 |
| Moderate limitations | 2,422 | 2,386 (98.5) | 36 (1.5) | 1.74 (1.14, 2.60) | 1.35 (0.82, 2.16) | 0.227 |
| Severe limitations | 661 | 639 (96.7) | 22 (3.3) | 3.89 (2.35, 6.21) | 2.93 (1.50, 5.45) | 0.001 |
| Needing help with daily activity | ||||||
| No | 15,460 | 15,291 (98.9) | 169 (1.1) | 1 | 1 | |
| Yes | 253 | 248 (98.0) | 5 (2.0) | 1.81 (0.64, 3.95) | 0.56 (0.18, 1.40) | 0.254 |
| Allied health service use through team care arrangementse | ||||||
| No | 13,467 | 13,341 (99.1) | 126 (0.9) | 1 | 1 | |
| Yes | 2,738 | 2,684 (98.0) | 54 (2.0) | 2.11 (1.52, 2.88) | 1.62 (1.06, 2.42) | 0.021 |
| Use of psychotropic medicationsf | ||||||
| No | 14,145 | 14,005 (99.0) | 140 (1.0) | 1 | 1 | |
| Yes | 2,060 | 2,020 (98.1) | 40 (1.9) | 1.96 (1.36, 2.76) | 1.46 (0.92, 2.26) | 0.095 |
| Use of diabetic medicationsg | ||||||
| No | 15,894 | 15,720 (98.9) | 174 (1.1) | 1 | 1 | |
| Yes | 311 | 305 (98.1) | 6 (1.9) | 1.76 (0.69, 3.63) | 1.38 (0.47, 3.20) | 0.502 |
| Use of antiepilepticsh | ||||||
| No | 15,834 | 15,668 (99.0) | 166 (1.0) | 1 | 1 | |
| Yes | 371 | 357 (96.2) | 14 (3.8) | 3.60 (1.99, 5.98) | 2.08 (1.03, 3.86) | 0.029 |
*Relative risks were adjusted for all other variables in the table.
aAdequate physical activity was defined as at least 150 minutes of activity (walking, or moderate and vigorous exercise) in the week prior to the completion of the baseline survey.
bSelf-reported good quality of life was defined if people reported their quality of life was good, very good or excellent in response to the self-rated quality of life question.
cPsychological distress was measured using the Kessler Psychological Distress Scale (K10)22 and categorised as either low and moderate (<22) or high (>22) based on the K10 score that ranges between 10 and 50.
dPhysical functioning score was measured using the RAND Health Medical Outcomes Study Physical Functioning (MOS-PF) scale23 and was classified as no limitations (≥81), minor limitations (61–81), moderate limitations (41–60), or severe limitations (≤40).
eServices delivered by accredited dieticians, exercise physiologists, podiatrists, chiropractors, and physiotherapists through team care arrangements.
fPsychotrophic medications included: antidepressants (phenelzine, tranylcypromine, amitriptyline, clomipramine, dosulepin, doxepin, imipramine, nortriptyline, mirtazapine), antipsychotics (amisulpride, aripiprazole, asenapine, brexpiprazole, cariprazine, chlorpromazine, clozapine, droperidol, flupentixol, haloperidol, lurasidone, olanzapine, paliperidone, periciazine, quetiapine, risperidone, trifluoperazine, ziprasidone, and zuclopenthixol), and drugs for bipolar disorder (lithium).
gAntiepileptics included: gabapentin, perampanel, pregabalin, valproate, and vigabatrin.
hDiabetic medications included: sulfonylureas (glibenclamide, gliclazide, glimepiride, glipizide) and other drugs for diabetes (insulin and pioglitazone).
Current smokers at baseline were at higher risk of developing obesity compared to those who had never smoked (adjusted RR: 2.96; 95%CI: 1.73–4.81), while any alcohol consumption (adjusted RR: 0.59; 95%CI: 0.41–0.85) and adequate physical activity (adjusted RR: 0.44; 95%CI: 0.31–0.63) were found to be associated with a lower risk of developing obesity. People reporting high levels of psychological distress were at higher risk of developing obesity compared with those reporting no or low level of psychological distress (adjusted RR: 2.01; 95%CI: 1.13–3.38). People reporting severe physical functional limitations were at higher risk of developing obesity compared to those with none (adjusted RR: 2.93; 95%CI: 1.50–5.45). People who used allied health services through team care planning were 62% more likely to develop obesity compared to people who did not (adjusted RR: 1.62, 95%CI: 1.06–2.42). People who used antiepileptics were 2.08-fold more likely to develop obesity (adjusted RR: 2.08; 95%CI: 1.03–3.86) compared with people who did not, while the use of psychotropic and diabetic medications had no significant effect (adjusted RR: 1.46; 95%CI: 0.92–2.26 and adjusted RR 1.38; 95%CI: 0.47–3.20, respectively). There was no multicollinearity demonstrated among covariates in the multivariable Poisson model; the VIFs for all covariates were <1.20 (data not shown).
Factors associated with developing obesity in the overweight group (BMI 25.0 to <30.0)
Table 3 shows the cumulative incidence of obesity at follow-up among people who were overweight at baseline, with crude and adjusted RRs by covariate categories. The risk of developing obesity at follow-up significantly decreased with increasing age. Compared to people aged 45–59 years, the risk of developing obesity was 17% less for those aged 60–74 years (adjusted RR: 0.83, 95%CI: 0.73–0.94) and 56% less for those aged ≥75 years (adjusted RR: 0.44, 95%CI: 0.34–0.56), respectively. Females were 34% more likely to develop obesity compared with males (adjusted RR: 1.34; 95%CI: 1.20–1.50). Full-time workers were 26% more likely to develop obesity compared to those who did not work (adjusted RR: 1.26; 95%CI: 1.08–1.47).
Table 3.
Risk factors for obesity at follow-up among people in the overweight range (BMI 25-<30)
| Characteristics | Number | Without obesity n (%) |
Obesity n (%) |
Crude relative risk (95% confidence interval) | Adjusted* relative risk (95% confidence interval) | P-value |
|---|---|---|---|---|---|---|
| Age at baseline | ||||||
| 45–59 years | 7,913 | 6,816 (86.1) | 1097 (13.9) | 1 | 1 | |
| 60–74 years | 5,769 | 5,053 (87.6) | 716 (12.4) | 0.90 (0.81, 0.98) | 0.83 (0.73, 0.94) | 0.005 |
| 75 years and over | 1,584 | 1,458 (92.0) | 126 (8.0) | 0.57 (0.47, 0.69) | 0.44 (0.34, 0.56) | <0.001 |
| Sex | ||||||
| Male | 8,878 | 7,922 (89.2) | 956 (10.8) | 1 | 1 | |
| Female | 6,388 | 5,405 (84.6) | 983 (15.4) | 1.43 (1.31, 1.56) | 1.34 (1.20, 1.50) | <0.001 |
| Highest qualification | ||||||
| No school certificate or other qualification | 727 | 618 (85.0) | 109 (15.0) | 1 | 1 | |
| School or intermediate certificate | 2,252 | 1919 (85.2) | 333 (14.8) | 0.99 (0.80, 1.23) | 1.06 (0.83, 1.38) | 0.651 |
| Higher school or leaving certificate | 1,543 | 1,327 (86.0) | 216 (14.0) | 0.93 (0.74, 1.18) | 1.04 (0.79, 1.37) | 0.784 |
| Trade or apprenticeship | 1,336 | 1139 (85.3) | 197 (14.7) | 0.98 (0.78, 1.25) | 1.13 (0.86, 1.50) | 0.384 |
| Certificate or diploma | 3,350 | 2,897 (86.5) | 453 (13.5) | 0.90 (0.73, 1.12) | 0.99 (0.78, 1.28) | 0.951 |
| University degree or higher | 5,936 | 5,323 (89.7) | 613 (10.3) | 0.69 (0.56, 0.85) | 0.81 (0.63, 1.05) | 0.104 |
| Work status | ||||||
| Not working | 5,465 | 4,823 (88.3) | 642 (11.7) | 1 | 1 | |
| Part time | 3,243 | 2,827 (87.2) | 416 (12.8) | 1.09 (0.96, 1.23) | 1.09 (0.94, 1.27) | 0.259 |
| Full time | 6,400 | 5,541 (86.6) | 859 (13.4) | 1.14 (1.03, 1.27) | 1.26 (1.08, 1.47) | 0.004 |
| Household income (AUD) | ||||||
| <$20,000 | 1,415 | 1,212 (85.7) | 203 (14.3) | 1 | 1 | |
| $20,000–39,999 | 1,833 | 1,579 (86.1) | 254 (13.9) | 0.97 (0.80, 1.16) | 1.03 (0.83, 1.28) | 0.783 |
| $40,000–69,999 | 2,858 | 2,446 (85.6) | 412 (14.4) | 1.00 (0.85, 1.19) | 1.03 (0.84, 1.28) | 0.764 |
| $70,000 or more | 6,599 | 5,859 (88.8) | 740 (11.2) | 0.78 (0.67, 0.92) | 0.90 (0.72, 1.13) | 0.374 |
| Won’t disclose | 2,561 | 2,231 (87.1) | 330 (12.9) | 0.90 (0.75, 1.07) | 0.94 (0.76, 1.17) | 0.574 |
| Born in Australia | ||||||
| No | 4,489 | 3,952 (88.0) | 537 (12.0) | 1 | 1 | |
| Yes | 10,694 | 9,305 (87.0) | 1389 (13.0) | 1.09 (0.98, 1.20) | 1.10 (0.97, 1.24) | 0.137 |
| Speaks language other than English at home | ||||||
| No | 13,617 | 11,889 (87.3) | 1728 (12.7) | 1 | 1 | |
| Yes | 1,649 | 1,438 (87.2) | 211 (12.8) | 1.01 (0.87, 1.16) | 0.98 (0.82, 1.17) | 0.835 |
| Currently married/partnered | ||||||
| No | 3,010 | 2,537 (84.3) | 473 (15.7) | 1 | 1 | |
| Yes | 12,171 | 10,711 (88.0) | 1460 (12.0) | 0.76 (0.69, 0.85) | 0.90 (0.80, 1.02) | 0.103 |
| Smoking status | ||||||
| Never smoker | 9,084 | 8,006 (88.1) | 1078 (11.9) | 1 | 1 | |
| Ex-smoker | 5,524 | 4,796 (86.8) | 728 (13.2) | 1.11 (1.01, 1.22) | 1.09 (0.98, 1.21) | 0.132 |
| Current smoker | 658 | 525 (79.8) | 133 (20.2) | 1.70 (1.42, 2.03) | 1.47 (1.20, 1.79) | <0.001 |
| Adequate vegetable intake (5 or more serves a day) | ||||||
| No | 11,292 | 9,889 (87.6) | 1403 (12.4) | 1 | 1 | |
| Yes | 3,974 | 3,438 (86.5) | 536 (13.5) | 1.09 (0.98, 1.20) | 1.01 (0.90, 1.13) | 0.879 |
| Adequate fruit intake (2 or more serves a day) | ||||||
| No | 6,398 | 5,581 (87.2) | 817 (12.8) | 1 | 1 | |
| Yes | 8,868 | 7,746 (87.3) | 1122 (12.7) | 0.99 (0.91, 1.08) | 0.99 (0.89, 1.10) | 0.807 |
| Alcohol consumption | ||||||
| No | 3,568 | 2,998 (84.0) | 570 (16.0) | 1 | 1 | |
| Yes | 11,539 | 10,190 (88.3) | 1349 (11.7) | 0.73 (0.66, 0.81) | 0.81 (0.72, 0.90) | <0.001 |
| Adequate physical activitya | ||||||
| No | 3,959 | 3,375 (85.2) | 584 (14.8) | 1 | 1 | |
| Yes | 11,307 | 9,952 (88.0) | 1355 (12.0) | 0.81 (0.74, 0.90) | 0.88 (0.79, 0.99) | 0.026 |
| Self-reported good quality of lifeb | ||||||
| No | 737 | 606 (82.2) | 131 (17.8) | 1 | 1 | |
| Yes | 14,016 | 12,275 (87.6) | 1741 (12.4) | 0.70 (0.59, 0.84) | 1.09 (0.87, 1.39) | 0.465 |
| Number of self-reported chronic conditions | ||||||
| None | 4,753 | 4,183 (88.0) | 570 (12.0) | 1 | 1 | |
| One | 5,498 | 4,842 (88.1) | 656 (11.9) | 0.99 (0.89, 1.11) | 1.01 (0.89, 1.14) | 0.876 |
| Two or more | 5,015 | 4,302 (85.8) | 713 (14.2) | 1.19 (1.06, 1.32) | 1.14 (1.00, 1.30) | 0.054 |
| Psychological distressc | ||||||
| Low | 13,705 | 12,009 (87.6) | 1,696 (12.4) | 1 | 1 | |
| High | 799 | 649 (81.2) | 150 (18.8) | 1.52 (1.28, 1.79) | 1.18 (0.97, 1.44) | 0.095 |
| Physical functioningd | ||||||
| No limitations | 5,611 | 5,003 (89.2) | 608 (10.8) | 1 | 1 | |
| Minor limitations | 4,782 | 4,206 (88.0) | 576 (12.0) | 1.11 (0.99, 1.25) | 1.12 (1.00, 1.27) | 0.060 |
| Moderate limitations | 2,853 | 2,413 (84.6) | 440 (15.4) | 1.42 (1.26, 1.61) | 1.36 (1.18, 1.56) | <0.001 |
| Severe limitations | 869 | 708 (81.5) | 161 (18.5) | 1.71 (1.43, 2.03) | 1.50 (1.19, 1.88) | 0.001 |
| Needing help with daily activity | ||||||
| No | 14,548 | 12,712 (87.4) | 1,836 (12.6) | 1 | 1 | |
| Yes | 248 | 201 (81.0) | 47 (19.0) | 1.50 (1.11, 1.98) | 1.11 (0.77, 1.57) | 0.559 |
| Allied health service use through team care arrangementse | ||||||
| No | 11,944 | 10,554 (88.4) | 1,390 (11.6) | 1 | 1 | |
| Yes | 3,322 | 2,773 (83.5) | 549 (16.5) | 1.42 (1.29, 1.57) | 1.28 (1.13, 1.44) | <0.001 |
| Use of psychotropic medications f | ||||||
| No | 13,215 | 11,582 (87.6) | 1,633 (12.4) | 1 | 1 | |
| Yes | 2,051 | 1,745 (85.1) | 306 (14.9) | 1.21 (1.07, 1.36) | 1.02 (0.88, 1.18) | 0.779 |
| Use of diabetic medicationsg | ||||||
| No | 14,630 | 12,801 (87.5) | 1,829 (12.5) | 1 | 1 | |
| Yes | 636 | 526 (82.7) | 110 (17.3) | 1.38 (1.13, 1.67) | 1.23 (0.97, 1.54) | 0.070 |
| Use of antiepileptic medicationsh | ||||||
| No | 14,850 | 12,985 (87.4) | 1,865 (12.6) | 1 | 1 | |
| Yes | 416 | 342 (82.2) | 74 (17.8) | 1.42 (1.11, 1.77) | 1.07 (0.80, 1.40) | 0.625 |
*Relative risks were adjusted for all other variables in the table.
aAdequate physical activity was defined as at least 150 minutes of activity (walking, or moderate and vigorous exercise) in the week prior to the completion of the baseline survey.
bSelf-reported good quality of life was defined if people reported their quality of life was good, very good or excellent in response to the self-rated quality of life question.
cPsychological distress was measured using the Kessler Psychological Distress Scale (K10)22 and categorised as either low and moderate (<22) or high (>22) based on the K10 score that ranges between 10 and 50.
dPhysical functioning score was measured using the RAND Health Medical Outcomes Study Physical Functioning (MOS-PF) scale23 and was classified as no limitations (≥81), minor limitations (61–81), moderate limitations (41–60), or severe limitations (≤40).
eServices delivered by accredited dieticians, exercise physiologists, podiatrists, chiropractors and physiotherapists through team care arrangements.
fPsychotrophic medications included: antidepressants (phenelzine, tranylcypromine, amitriptyline, clomipramine, dosulepin, doxepin, imipramine, nortriptyline, mirtazapine), antipsychotics (amisulpride, aripiprazole, asenapine, brexpiprazole, cariprazine, chlorpromazine, clozapine, droperidol, flupentixol, haloperidol, lurasidone, olanzapine, paliperidone, periciazine, quetiapine, risperidone, trifluoperazine, ziprasidone and zuclopenthixol) and drugs for bipolar disorder (lithium).
gDiabetic medications included: sulfonylureas (glibenclamide, gliclazide, glimepiride, glipizide) and other drugs for diabetes (insulin and pioglitazone).
hAntiepileptics included: gabapentin, perampanel, pregabalin, valproate, and vigabatrin.
Current smokers at baseline were at higher risk of developing obesity compared to those who had never smoked (adjusted RR: 1.47; 95%CI: 1.20–1.79), while any alcohol consumption (adjusted RR: 0.81; 95%CI: 0.72–0.90) and adequate physical activity (adjusted RR: 0.88; 95%CI: 0.79–0.99) were found to be associated with a lower risk of developing obesity. People reporting physical functional limitations were at higher risk of developing obesity: for people reporting minor limitations compared to none (adjusted RR: 1.12; 95%CI: 1.18–1.27), for people reporting moderate limitations compared to none (adjusted RR: 1.36; 95%CI: 1.18–1.56), and for people reporting severe limitations compared to none (adjusted RR: 1.50; 95%CI: 1.19–1.88). People who had 2 or more chronic conditions were 14% more likely to develop obesity compared to those with none (adjusted RR: 1.14; 95%CI: 1.00–1.30) and those who used allied health services during follow-up time through team care planning were 28% more like to develop obesity compared to those who did not use these services (adjusted RR: 1.28; 95%CI: 1.13–1.44). We did not find any significant association between different types of medication use during follow-up and obesity. There was no multicollinearity observed among covariates in the multivariable Poisson model; the VIFs for all covariates were <1.20.
Discussion
This population-based community-dwelling cohort study has identified risk factors for developing obesity in people of healthy weight and those who are overweight. Transition to obesity was common in this cohort. We have identified characteristics associated with that transition which can be used by primary care clinicians to identify those most at risk so they can be offered interventions to support obesity prevention and weight management in primary care. In this study, 1.1% of people within the healthy weight range experienced weight gain to develop obesity at follow-up; while 12.7% of people within the overweight range experienced weight gain to develop obesity at follow-up. In both groups, the following were associated with an increased risk of developing obesity: current smoking, physical functioning limitations, and allied health service use through team care planning, while any alcohol consumption and adequate physical activity were found to be associated with a lower risk of developing obesity. Additionally, in the healthy weight group, high psychological distress and the use of antiepileptics were associated with developing obesity; and in the overweight group, being female sex, working full-time, and self-report of 2 or more chronic conditions were associated with developing obesity, while older age was found to be associated with a lower risk of developing obesity.
Our study was different from published cross-sectional epidemiological studies because we were able to use a community-dwelling cohort to explore associations between developing obesity and an extended range of covariates relevant to primary care; including diet, smoking, alcohol consumption, physical activity, physical functioning, self-reported quality of life, psychological distress, allied health use in primary care, and use of 3 classes of medications. A longitudinal cohort study using electronic health records from primary care practices in England found a lower risk of developing obesity with increasing age in people who were a healthy weight and overweight range at baseline,25 while we found a lower risk of developing obesity with increasing age among people who were overweight at baseline only. The English study included people aged from 18 to 74 years,25 while ours included an older population who are more likely to be experiencing physical functioning limitations as well as age-related physiological changes such as sarcopenia.26 Unlike ours, the English study did not include smoking status, allied health use, or medication use (except for diuretics) in their analyses.25 There is growing evidence that low to moderate alcohol consumption is associated with health benefits as people age; however, it is unclear if this is related to its social benefits or ethanol.27
The association between smoking and obesity is complex. The belief that smoking is an effective way to manage body weight and frequent weight gain following smoking cessation is underpinned by the acute energy expenditure and appetite reductions associated with nicotine.28–30 However, smoking is associated with central obesity and insulin resistance,31 as well as other poor health habits, such as poor diet and physical inactivity,32 leading to weight gain and obesity. Our finding that smoking was associated with the transition to obesity builds on the evidence of harms associated with smoking and counteracts the perception some people may have that smoking assists with weight management. GPs have skills and training that enable them to assist their patients with behaviour change regarding smoking and alcohol use, and these skills can also be applied in weight management and obesity care.33 The risk factors we have identified that could be potentially modifiable after GP and allied health intervention include smoking, physical functioning, physical activity, psychological distress, and use of specific medications. GPs have a central role to play in obesity prevention and management as patients prefer to receive that from GPs rather than other healthcare professionals,12 and GPs can provide personalised patient-centred care encompassing the condition being treated, other co-morbidities and the personal circumstances of each patient.33 While we have not differentiated between specific medications within drug classes in our analysis, it is clear from previous research that medications within drug classes differ regarding the risk of weight gain associated with their use.34–36 The risk of weight gain associated with specific medications within a drug class would be one of the factors GPs discuss with their patients when initiating therapy, especially for those with other risk factors for obesity. However, switching medication to assist with weight management would require very careful consideration and consultation with the patient and other healthcare professionals involved regarding potential risks and benefits, including the risk of relapse of the indicator condition.34
There is a large and growing proportion of Australians with obesity.6 For the majority of patients requiring obesity prevention and management, this is best provided by the general practice and primary care teams which are more accessible than specialist care.12,33,37 Though Medicare funds bariatric surgery for severe obesity and its use is increasing in Australia,38,39 there is limited access for those who have not reached that stage of severity and do not have private health insurance cover. While there is evidence on the beneficial effects of dietetic consultations and regular exercise on weight management40,41; the availability of dietetic and exercise physiology consultations may be limited by the need for other allied health consultations for other chronic conditions under team care arrangements.11 Notably, allied health consultations regarding obesity are subsidised by Medicare only when the patient has other chronic conditions (e.g. diabetes) under team care arrangements.12, 33 Unfortunately, “uncomplicated” obesity does not qualify for Medicare subsidised allied health consultations, making access difficult for patients on low incomes.12,33 While our study found an association between allied health service use through team care planning and developing obesity, this service use was likely for the management of other chronic conditions not weight.
We agree with the World Health Organisation, numerous health professional organisations and other authors that obesity should be classified as a chronic disease, rather than a chronic disease risk factor, as failing to do so prohibits many patients with obesity from qualifying for structured funded management plans.42,43 We recommend that patients with obesity should have access to funded chronic disease management plans as do other patients with chronic disease.11,42 We also recommend implementation of comprehensive public health campaigns that seek to de-stigmatise discussing obesity, address the obesogenic environment, highlight the benefits of returning to a healthy weight, and urge those with obesity to see their primary care clinician to access the care they need.12,33
Strengths and limitations
To the best of our knowledge, this is the first study to include past personal history of chronic health conditions, allied health service use through team care planning, and medication usage when assessing risk factors for developing obesity. The major strength of this study was our use of a large community-dwelling cohort of older adults, which was not restricted only to those engaged with health services, thus providing a more realistic denominator. Recruitment of individuals across the age spectrum from 45 to 90 years to the 45 and Up Study at baseline enabled us to assess the impacts on older people in the community.
Our study also has several potential limitations. While the 45 and Up Study is the largest study of its kind in the southern hemisphere, it was not designed to be representative of the general population.19 Though non-response at baseline may mean the cohort varies slightly from the broader population, comparison of these rates over time and between subgroups is still valid and should be applicable to those groups within the broader population.19, 44 Another potential limitation is using the baseline survey to identify the number of chronic health conditions through self-report. However, a recent Australian study exploring the concordance between the 45 and Up Study baseline survey and administrative healthcare datasets, found that over 70% of individuals classified as having multimorbidity were identified in the baseline survey.45 Since our study involved comparing BMI at baseline and the first follow-up, the study needed to be limited to those who had completed both surveys with 65% of the cohort responding to the first follow-up. While this could potentially introduce bias, Wang and colleagues found the participants’ nonresponse did not result in substantial bias and did not alter the interpretation of results in general.46 Our study was restricted to metropolitan Sydney, which limits its generalisability. We made that decision because of differences between rural and urban areas and because of our close working relationship with service providers in metropolitan Sydney.
We found an association between allied health service use through team care planning and developing obesity and, despite including multiple variables known to be associated with obesity to adjust for confounding in our analysis, there may be residual confounding. This may also occur if variables used to control for confounding are subject to misclassification and/or response bias. We also cannot be sure of the temporality of developing obesity and allied health service use—it is possible that developing obesity may have preceded allied health service use between the baseline and follow-up surveys.
The British Association for Psychopharmacology (BAP) classifies several antipsychotic drugs (amisulpride, aripiprazole, ziprasidone, haloperidol, lurasidone, and asenapine) as being low risk for weight gain.34 However, we used the Australian Medicines Handbook, an independent clinical resource that is readily available in primary care, to identify and categorise medications that are associated with weight gain for inclusion in our analyses.24 In the Australian Medicine Handbook, weight gain is listed as a common adverse side effect for all antipsychotic drugs.24 That said, we used the list of antipsychotics classified as low risk for weight gain by BAP to check for any impact of misclassification on our findings. The number of people taking antipsychotic drugs with low risk for weight gain was small (<50 in each group in our cohort) and recategorising them had little impact on the effect size and confidence intervals regarding whether psychotropic drugs as a class (which includes drugs other than antipsychotics) were associated with developing obesity (data not shown). The classification of medications can be difficult. Furthermore, some medications have multiple indications. For example, the tricyclic antidepressant, amitriptyline was analysed as a psychotropic but is also used to manage neuropathic pain.47 Hence, there is a possibility of non-differential misclassification and thus more conservative relative risk estimates.
Implications for research and practice
Effective obesity prevention strategies are an urgent priority. This study identified key characteristics of older patients who are at risk of developing obesity, including risk behaviours (smoking and physical inactivity) and chronic conditions or their treatment (self-report of two or more chronic conditions, physical function, psychological distress, and use of anti-epileptic medications). These findings may help alert clinicians to the need for preventive interventions in selected cases as well as informing the targeting of public health programs.
Supplementary material
Supplementary material is available at Family Practice online.
Acknowledgements
The research was completed using data collected through the 45 and Up Study (www.saxinstitute.org.au/our-work/45-up-study/). The 45 and Up Study is managed by the Sax Institute in collaboration with major partner Cancer Council NSW; and partners the Heart Foundation and NSW Ministry of Health. We thank the many thousands of people participating in the 45 and Up Study. We acknowledge the NSW Centre for Health Record Linkage (CHeReL) for probabilistic linkage and provision of the deaths data (http://www.cherel.org.au/); and the Sax Institute for facilitating linkage with Medicare claims data and Pharmaceutical Benefits Scheme (PBS) data supplied by Services Australia using a unique identifier and deterministic matching. We also acknowledge the Sax Institute’s Secure Unified Research Environment (SURE) for the provision of secure data access.
Contributor Information
Kylie Vuong, School of Medicine and Dentistry, Griffith University, Queensland, Australia; School of Population Health, University of New South Wales, New South Wales, Australia.
Alamgir Kabir, Centre for Primary Health Care and Equity, University of New South Wales, New South Wales, Australia.
Damian P Conway, Population and Community Health Directorate, South Eastern Sydney Local Health District, New South Wales, Australia; The Kirby Institute, University of New South Wales, New South Wales, Australia.
Margaret Williamson, Centre for Primary Health Care and Equity, University of New South Wales, New South Wales, Australia.
Mark F Harris, Centre for Primary Health Care and Equity, University of New South Wales, New South Wales, Australia.
Margo L Barr, Centre for Primary Health Care and Equity, University of New South Wales, New South Wales, Australia.
Ethical Approvals
This research was approved by the New South Wales Population and Health Services Research Ethics Committee (2016/06/642) and the 45 and Up Study overall was approved by the University of New South Wales Human Research Ethics Committee.
Funding
This research was funded by Sydney Local Health District, South Eastern Sydney Local Health District and the Central and Eastern Sydney Primary Health Network. MB, AK and MW’s positions were supported by the funding partners. The Central and Eastern Sydney Primary and Community Health Cohort/Linkage Resource management group includes representatives from each of the funding partners. The management group oversees which projects are conducted using the Resource and provides input into the overall design, interpretation of the results and knowledge translation opportunities.
Conflict of interest
The authors declare no potential conflicts of interest.
Data Availability
Data that support the findings of this study are available from the Sax Institute, but restrictions apply to the availability of these data, which were used under licence for the current study and so are not publicly available. The data, however, are available from the authors upon reasonable request and with permission from the Sax Institute.
References
- 1. World Health Organisation. Obesity and overweight. World Health Organisation [accessed 2022 Aug 31]. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
- 2. Di Angelantonio E, Bhupathiraju SN, Wormser D, et al. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. The Lancet. 2016:388(10046):776–786. doi: 10.1016/S0140-6736(16)30175-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nørtoft E, Chubb B, Borglykke A.. Obesity and healthcare resource utilization: comparative results from the UK and the USA. Obes Sci Pract. 2018:4(1):41–45. doi: 10.1002/osp4.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. GBD. 2015 Obesity Collaborators. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017:377(1):13–27. doi: 10.1056/NEJMoa1614362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kent S, Fusco F, Gray A, Jebb SA, Cairns BJ, Mihaylova B.. Body mass index and healthcare costs: a systematic literature review of individual participant data studies. Obes Rev. 2017:18(8):869–879. doi: 10.1111/obr.12560 [DOI] [PubMed] [Google Scholar]
- 6. Australian Institute of Health and Welfare. Overweight and obesity [accessed 2023 Dec 11]. https://www.aihw.gov.au/reports/overweight-obesity/overweight-and-obesity/contents/about
- 7. Department of Health and Aged Care. Australia’s first National Obesity Strategy launched on World Obesity Day [accessed 2022 Aug 31]. https://www.health.gov.au/ministers/the-hon-greg-hunt-mp/media/australias-first-national-obesity-strategy-launched-on-world-obesity-day
- 8. Murtagh J, Rosenblatt J, Coleman J, Murtagh C.. The nature, scope and content of general practice. John Murtagh’s General Practice. 8th Edition. McGraw-Hill Education, 2022. [accessed 2023 Dec 12]. https://murtagh.mhmedical.com/content.aspx?bookid=3133§ionid=262289286 [Google Scholar]
- 9. Australian Government Department of Health and Aged Care. MBS Online Medicare Benefits Schedule. Australian Government. Updated 16 January 2022 [accessed 2022 Sept 1]. http://www.health.gov.au/internet/mbsonline/publishing.nsf/Content/Medicare-Benefits-Schedule-MBS-1
- 10. RACGP. General Practice: Health of the Nation 2020 [accessed 2023 Dec 11]. https://www.racgp.org.au/getmedia/c2c12dae-21ed-445f-8e50-530305b0520a/Health-of-the-Nation-2020-WEB.pdf.aspx
- 11. Services Australia. Chronic disease GP Management Plans and Team Care Arrangements [accessed 2023 Mar 23]. https://www.servicesaustralia.gov.au/chronic-disease-gp-management-plans-and-team-care-arrangements?context=20
- 12. Jansen S, Desbrow B, Ball L.. Obesity management by general practitioners: the unavoidable necessity. Aust J Prim Health. 2015:21(4):366–368. doi: 10.1071/py15018 [DOI] [PubMed] [Google Scholar]
- 13. Semlitsch TA-O, Stigler FL, Jeitler K, Horvath K, Siebenhofer A.. Management of overweight and obesity in primary care—a systematic overview of international evidence-based guidelines. Obes Rev. 2019:20(9) (1467-789X (Electronic):1218–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brauer P, Gorber SC, Shaw E, Singh H, Bell N, Shane ARE, Jaramillo A, Tonelli M; Canadian Task Force on Preventive Health Care. Recommendations for prevention of weight gain and use of behavioural and pharmacologic interventions to manage overweight and obesity in adults in primary care. Can Med Assoc J. 2015:187(3):184–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lemmens VEPP, Oenema A, Klepp KI, Henriksen HB, Brug J.. A systematic review of the evidence regarding efficacy of obesity prevention interventions among adults. Obes Rev. 2008:9(5):446–455. doi: 10.1111/j.1467-789X.2008.00468.x [DOI] [PubMed] [Google Scholar]
- 16. Thurber KA, Joshy G, Korda R, Eades SJ, Wade V, Bambrick H, Liu B, Banks E.. Obesity and its association with sociodemographic factors, health behaviours and health status among Aboriginal and non-Aboriginal adults in New South Wales, Australia. J Epidemiol Community Health. 2018:72(6):491–498. doi: 10.1136/jech-2017-210064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Biddle SJH, Bengoechea García E, Pedisic Z, Bennie J, Vergeer I, Wiesner G.. Screen time, other sedentary behaviours, and obesity risk in adults: a review of reviews. Curr Obes Rep. 2017:6(2):134–147. doi: 10.1007/s13679-017-0256-9 [DOI] [PubMed] [Google Scholar]
- 18. Comino EJ, Harris E, Page J, McDonald J, Harris MF.. The 45 and Up Study: a tool for local population health and health service planning to improve integration of healthcare. Public Health Res Pract. 2016:26(3):2631629–2631629. doi: 10.17061/phrp2631629 [DOI] [PubMed] [Google Scholar]
- 19. Bleicher K, Summerhayes R, Baynes S, Swarbrick M, Navin Cristina T, Luc H, Dawson G, Cowle A, Dolja-Gore X, McNamara M.. Cohort profile update: the 45 and up study. Int J Epidemiol. 2022:52(1):dyac104. doi: 10.1093/ije/dyac104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Australian Institute of Health and Welfare. Physical activity [accessed 2023 Dec 11]. 2023. https://www.aihw.gov.au/reports/physical-activity/physical-activity
- 21. Sax Institute. 45 and Up Study [accessed 2023 Dec. 11]. https://www.saxinstitute.org.au/solutions/45-and-up-study/use-the-45-and-up-study/data-and-technical-information/
- 22. Kessler RC, Barker PR, Colpe LJ, Epstein JF, Gfroerer JC, Hiripi E, Howes MJ, Normand ST, Manderscheid RW, Walters EE, et al. Screening for serious mental illness in the general population. Arch Gen Psychiatry. 2003:60(2):184–189. doi: 10.1001/archpsyc.60.2.184 [DOI] [PubMed] [Google Scholar]
- 23. Ware JE Jr, Sherbourne CD.. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992:30(6):473–483. [PubMed] [Google Scholar]
- 24. Australian Medicines Handbook Pty Ltd. Australian Medicines Handbook (online). Updated 14 Nov 2022 [accessed 2022 Nov 14]. https://amhonline.amh.net.au/
- 25. Katsoulis M, Lai AG, Diaz-Ordaz K, Gomes M, Pasea L, Banerjee A, Denaxas S, Tsilidis K, Lagiou P, Misirli G, et al. Identifying adults at high-risk for change in weight and BMI in England: a longitudinal, large-scale, population-based cohort study using electronic health records. Lancet Diabetes Endocrinol. 2021:9(10):681–694. doi: 10.1016/s2213-8587(21)00207-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Walston JD. Sarcopenia in older adults. Curr Opin Rheumatol. 2014:24(6):623–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Byles J, Young A, Furuya H, Parkinson L.. A drink to healthy aging: The association between older women’s use of alcohol and their health-related quality of life. J Am Geriatr Soc. 2006:54(9):1341–1347. doi: 10.1111/j.1532-5415.2006.00837.x [DOI] [PubMed] [Google Scholar]
- 28. Potter BK, Pederson LL, Chan SS, Aubut J-AL, Koval JJ.. Does a relationship exist between body weight, concerns about weight, and smoking among adolescents? An integration of the literature with an emphasis on gender. Nicotine Tob Res. 2004:6(3):397–425. [DOI] [PubMed] [Google Scholar]
- 29. Hofstetter A, Schutz Y, Jéquier E, Wahren J.. Increased 24-hour energy expenditure in cigarette smokers. N Engl J Med. 1986:314(2):79–82. [DOI] [PubMed] [Google Scholar]
- 30. Ward KD, Klesges RC, Vander Weg MW.. Cessation of smoking and body weight. In: Björntop P, editor. International Textbook of Obesity. Chichester, UK: Wiley & Sons Ltd; 2001:323–336. [Google Scholar]
- 31. Houston TK, Person SD, Pletcher MJ, Liu K, Iribarren C, Kiefe CI.. Active and passive smoking and development of glucose intolerance among young adults in a prospective cohort: CARDIA study. Bmj. 2006:332(7549):1064–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kaufman A, Augustson EM, Patrick H.. Unraveling the relationship between smoking and weight: the role of sedentary behavior. J Obes. 2012:2012:735465. doi: 10.1155/2012/735465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sturgiss EA, van Weel C, Ball L, Jansen S, Douglas K.. Obesity management in Australian primary care: where has the general practitioner gone? Aust J Prim Health. 2016:22(6):473–476. doi: 10.1071/py16074 [DOI] [PubMed] [Google Scholar]
- 34. Cooper SJ, Reynolds GP, Barnes T, England E, Haddad PM, Heald A, Holt R, Lingford-Hughes A, Osborn D, McGowan O, et al. ; With expert co-authors (in alphabetical order):. BAP guidelines on the management of weight gain, metabolic disturbances and cardiovascular risk associated with psychosis and antipsychotic drug treatment. J Psychopharmacol. 2016:30(8)(1461-7285 (Electronic)):717–748. doi: 10.1177/0269881116645254 [DOI] [PubMed] [Google Scholar]
- 35. Bak M, Fransen A, Janssen J, van Os J, Drukker M.. Almost all antipsychotics result in weight gain: a meta-analysis. PLoS One. 2014:9(4):e94112. doi: 10.1371/journal.pone.0094112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ben-Menachem E. Weight issues for people with epilepsy—a review. Epilepsia. 2007:48(Suppl 9):42–45. doi: 10.1111/j.1528-1167.2007.01402.x [DOI] [PubMed] [Google Scholar]
- 37. The Royal Australian College of General Practitioners. General Practice: Health of the Nation 2023. East Melbourne, VIC: RACGP; 2023. [Google Scholar]
- 38. Department of Health and Aged Care. Medicare Benefits Schedule- Item 31575. [accessed 2023 December 11]. https://www9.health.gov.au/mbs/fullDisplay.cfm?type=item&q=31575&qt=ItemID
- 39. Australian Institute of Health and Welfare. Weight loss surgery in Australia 2014–15: Australian hospital statistics [accessed 2023 Dec 11]. https://www.aihw.gov.au/reports/hospitals/ahs-2014-15-weight-loss-surgery/summary
- 40. Mitchell LJ, Ball LE, Ross LJ, Barnes KA, Williams LT.. Effectiveness of dietetic consultations in primary health care: a systematic review of randomized controlled trials. J Acad Nutr Diet. 2017:117(12):1941–1962. doi: 10.1016/j.jand.2017.06.364 [DOI] [PubMed] [Google Scholar]
- 41. Petridou A, Siopi A, Mougios V.. Exercise in the management of obesity. Metabolism. 2019:92:163–169. doi: 10.1016/j.metabol.2018.10.009 [DOI] [PubMed] [Google Scholar]
- 42. Opie CA, Haines HM, Ervin KE, Glenister K, Pierce D.. Why Australia needs to define obesity as a chronic condition. BMC Public Health. 2017:17(1):500. doi: 10.1186/s12889-017-4434-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bray GA, Kim KK, Wilding JPH; World Obesity Federation. Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes Rev. 2017:18(7):715–723. doi: 10.1111/obr.12551 [DOI] [PubMed] [Google Scholar]
- 44. Mealing NM, Banks E, Jorm LR, Steel DG, Clements MS, Rogers KD.. Investigation of relative risk estimates from studies of the same population with contrasting response rates and designs. BMC Med Res Methodol. 2010:10(1):26. doi: 10.1186/1471-2288-10-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lujic S, Simpson JM, Zwar N, Hosseinzadeh H, Jorm L.. Multimorbidity in Australia: comparing estimates derived using administrative data sources and survey data. PLoS One. 2017:12(8):e0183817. doi: 10.1371/journal.pone.0183817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang JJJ, Bartlett M, Ryan L.. On the impact of nonresponse in logistic regression: application to the 45 and Up study. BMC Med Res Methodol. 2017:17(1):80. doi: 10.1186/s12874-017-0355-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Moore RA, Derry S, Aldington D, Cole P, Wiffen PJ.. Amitriptyline for neuropathic pain in adults. Cochrane Database Syst Rev. 2015:2015(7):CD008242. doi: 10.1002/14651858.CD008242.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data that support the findings of this study are available from the Sax Institute, but restrictions apply to the availability of these data, which were used under licence for the current study and so are not publicly available. The data, however, are available from the authors upon reasonable request and with permission from the Sax Institute.