Abstract
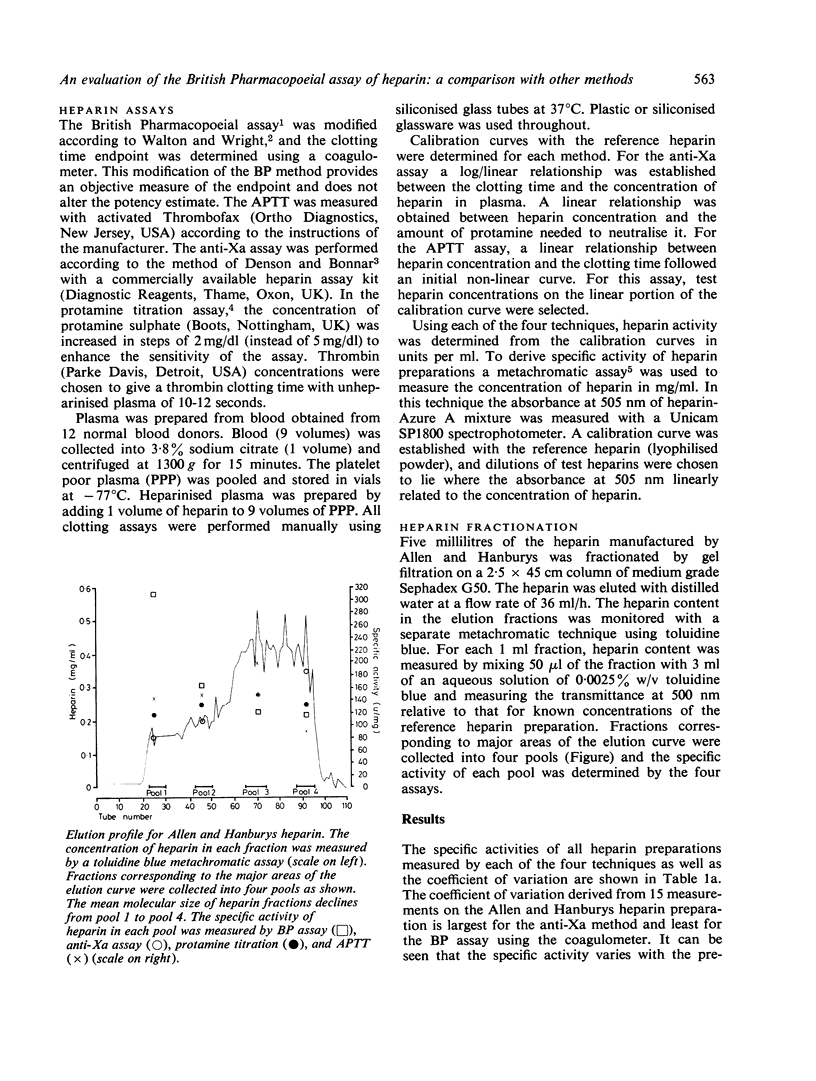
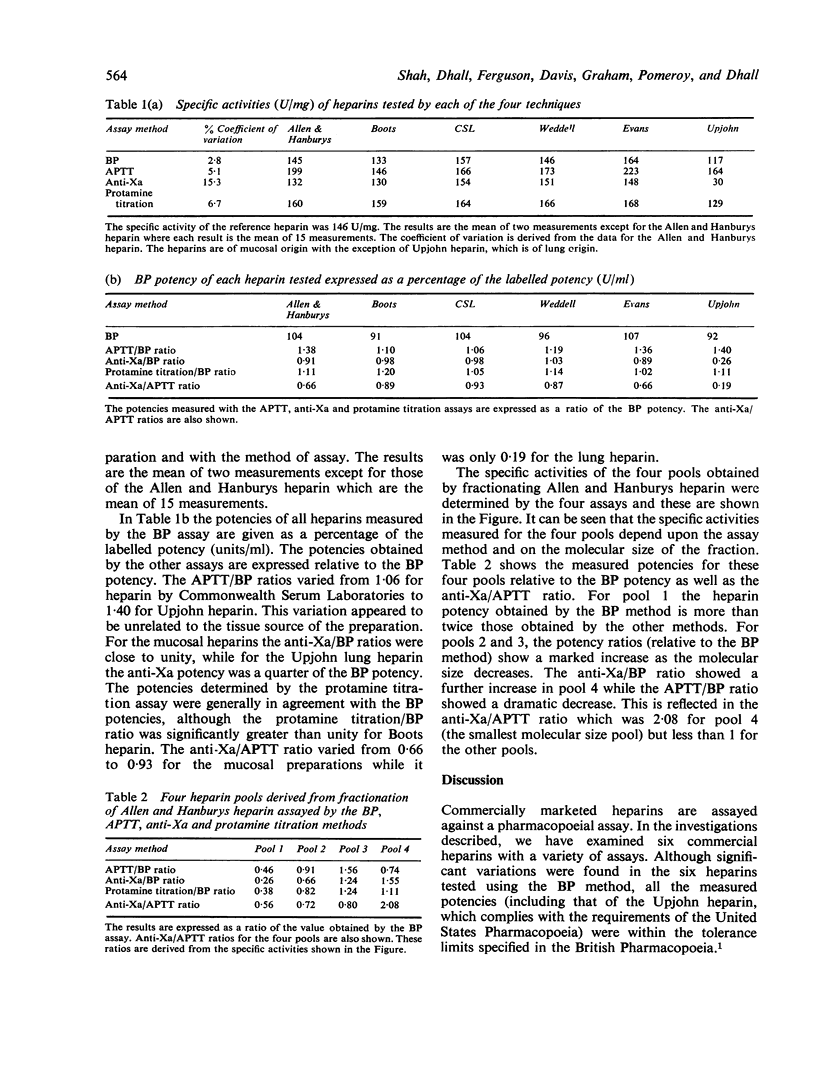
The potencies of six commercially manufactured heparins have been measured by the British Pharmacopoeial (BP) assay and activated partial thromboplastin time (APTT), protamine sulphate, and anti-Xa assays. The APTT/BP potency ratios were found to vary with the preparation but this was not dependent on the tissue source of heparin. For mucosal heparins, the anti-Xa/BP potency ratios were close to unity, but for heparin of lung origin the anti-Xa potency was approximately one-quarter of the BP potency. Four heparin fractions prepared by column gel chromatography of a commercial heparin were similarly examined by all four assays, and there was a wide divergence between the BP potency estimates and those obtained with the other methods. The degree of divergence was found to depend on the molecular size of the fraction.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott W. M., Warnock D. F., Austen W. G. The relationship of heparin source to the incidence of delayed hemorrhage. J Surg Res. 1977 Jun;22(6):593–597. doi: 10.1016/0022-4804(77)90095-6. [DOI] [PubMed] [Google Scholar]
- Baltes B. J., Diamond S., D'Agostino R. J. Comparison of anticoagulant activity of two preparations of purified heparin. Clin Pharmacol Ther. 1973 Mar-Apr;14(2):287–290. doi: 10.1002/cpt1973142287. [DOI] [PubMed] [Google Scholar]
- Barrowcliffe T. W., Johnson E. A., Eggleton C. A., Thomas D. P. Anticoagulant activities of lung and mucous heparins. Thromb Res. 1978 Jan;12(1):27–36. doi: 10.1016/0049-3848(78)90082-8. [DOI] [PubMed] [Google Scholar]
- Denson K. W., Bonnar J. The measurement of heparin. A method based on the potentiation of anti-factor Xa. Thromb Diath Haemorrh. 1973 Dec 31;30(3):471–479. [PubMed] [Google Scholar]
- Gomez-Perez F. Anticoagulant activity of two commercially available heparin preparations. A controlled study. J Clin Pharmacol New Drugs. 1972 Oct;12(10):413–416. doi: 10.1002/j.1552-4604.1972.tb00142.x. [DOI] [PubMed] [Google Scholar]
- Jaques L. B., Wollin A. A modified method for the colorimetric determination of heparin. Can J Physiol Pharmacol. 1967 Sep;45(5):787–794. doi: 10.1139/y67-093. [DOI] [PubMed] [Google Scholar]
- Michalski R., Lane D. A., Pepper D. S., Kakkar V. V. Neutralization of heparin in plasma by platelet factor 4 and protamine sulphate. Br J Haematol. 1978 Apr;38(4):561–571. doi: 10.1111/j.1365-2141.1978.tb01081.x. [DOI] [PubMed] [Google Scholar]
- Shen L. L., Barlow G. H., Holleman W. H. Differential activities of heparins in human plasma and in sheep plasma. Effects of heparin molecular sizes and sources. Thromb Res. 1978 Oct;13(4):671–679. doi: 10.1016/0049-3848(78)90156-1. [DOI] [PubMed] [Google Scholar]
- Silverglade A. Biological equivalence of beef lung and hog musosal heparins. Curr Ther Res Clin Exp. 1975 Jul;18(1 PT1):91–103. [PubMed] [Google Scholar]


