Abstract
Cryopreservation adversely affects embryo quality and viability in vitro. We investigated the effects of cryopreservation solutions supplemented with the antioxidant carnosine on frozen-thawed bovine embryo viability. Bovine blastocysts were produced in vitro and cryopreserved using slow freezing. The rates of re-expanded and hatched blastocysts in the 50 μg/ml carnosine-supplemented group at 4, 24, and 48 h after thawing were higher than those in the control (P < 0.05) group. In frozen-thawed embryos, cryopreservation solution supplemented with carnosine (50 μg/ml) significantly reduced reactive oxygen species (ROS) production (P < 0.05), decreased TUNEL-positive apoptotic cells (P < 0.05), and increased the mRNA expression of BCL2 (P < 0.05), an apoptosis suppressor gene. The expression of translocase of outer mitochondrial membrane 20 (TOMM20), which is involved in protein mitochondrial transport, in the carnosine (50 μg/ml)-treated embryos was significantly higher than that in the control group (P < 0.05). ATP production in frozen-thawed embryos in the 50 μg/ml carnosine-supplemented group was significantly higher than that in the control group (P < 0.05), however no significant difference in the total number of cells per embryo among the groups was observed. These results suggest that supplementing the cryopreservation solution with carnosine can improve the viability of frozen-thawed bovine embryos by reducing oxidative damage.
Keywords: Carnosine, Cattle, Embryo, ROS, Slow-freezing
Slow freezing is the most widely used method for bovine embryo cryopreservation. The application of freeze-thaw processes in vitro-produced embryos has grown exponentially over the past decade owing to increased consumer demand [1]. Freeze-thaw processes reduce the quality of embryos and contribute to low conception rates in cows [2]. Bovine embryos produced in vitro are more susceptible to chilling injury and have a lower viability than those produced in vivo [3]. Freeze thawing increases reactive oxygen species (ROS) production in murine embryos [4], and the associated oxidative stress reduces mitochondrial function and inhibits embryonic development [5]. Mitochondria, which are subcellular organelles crucial for cell death or survival, are involved in many cellular pathways such as metabolic synthesis and apoptosis. In particular, mitochondria play a critical role in apoptosis, which is tightly regulated by the Bcl-2 family of proteins [6]. Bcl-2–mitochondrial interactions are controlled by translocase of outer mitochondrial membrane 20 (TOMM20), which belongs to the protein import machinery of the mitochondrial outer membrane [7]. Several strategies have been proposed to improve the quality of frozen-thawed embryos produced in vitro by reducing ROS production using antioxidants [8, 9], such as glutathione [10], L-ascorbic acid [11], β-mercaptoethanol [12], and vitamin E [13]. Most studies have evaluated the effects of antioxidant supplementation during in vitro oocyte maturation and embryo culture. Recently, Carrascal‐Triana et al. [14] demonstrated that ascorbate supplementation in the cryopreservation solution (CPS) improved the cryotolerance of bovine embryos produced in vitro, and controlled-rate freezing reduced oxidative stress and cell death. However, the efficacy of adding other antioxidants to CPS to promote the recovery and maintenance of embryo quality after freeze-thawing remains unclear.
Carnosine is a dipeptide synthesized from the precursors, β-alanine and L-histidine, through the activity of carnosine synthase and is degraded by carnosinase, which regulates it at cellular level [15]. Carnosine acts as a hydroxyl radical scavenger and exhibits antioxidant activity through various mechanisms involving chelation of metal ions and inactivation of ROS [16]. Removing carnosine during seminal cryopreservation promotes oxidative imbalance by increasing lipid peroxidation products, thereby reducing sperm quality after thawing [17]. Thus, we hypothesized that carnosine improves the viability and quality of frozen-thawed bovine embryos by reducing ROS levels. We evaluated the effects of CPS supplementation with carnosine on post-thaw embryo quality by evaluating re-expansion and hatching rates, ROS content, and intracellular mitochondrial functions. This is the first study to report the effects of carnosine supplementation in CPS on the viability of frozen-thawed bovine embryos.
Materials and Methods
Materials and ethical considerations
Ovaries were procured from a commercial slaughterhouse (Mie Matsusaka Meat Corporation Co., Ltd., Mie, Japan). Therefore, no prior ethical approval was required for this study. All reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise stated. All the experiments were performed independently using different samples.
In vitro embryo production
Embryo production was performed in vitro as previously described [18]. Briefly, ovaries were obtained from Japanese Black beef cows at a local slaughterhouse and transported to the laboratory within 2 h, where they were stored in physiological saline containing 500 ng/ml kanamycin sulfate at 25°C. Cumulus-oocyte complexes (COCs) were collected from 2–6-mm-wide follicles using 18-G needles containing HEPES-buffered TCM199 (Medium 199, HEPES; Thermo Fisher Scientific, Waltham, MA, USA) with 10 ng/ml gentamicin sulfate. The in vitro maturation (IVM) medium consisted of Medium 199, HEPES supplemented with 10% newborn calf serum (NBCS, heat-inactivated; Thermo Fisher Scientific), 0.02 AU/ml follicle-stimulating hormone (Antrin-R10; Kyoritsu Seiyaku, Tokyo, Japan), 50 ng/ml epidermal growth factor, 5.0 μg/ml dibutyryl cAMP, and 10 ng/ml gentamicin sulfate. COCs with two or more layers were washed three times with the IVM medium. Groups of 30–50 COCs were cultured in 4-well dishes in 500-μl droplets of IVM medium, covered with mineral oil, and incubated for 21 h at 38.5°C in 5% CO2 and saturated humidity. Frozen semen from Japanese Black bulls (stored in straws; Livestock Improvement Association of Japan, Tokyo, Japan) was thawed in water at 37°C for 30 sec and washed twice with 6 ml IVF100 (Research Institute for the Functional Peptides, Yamagata, Japan) by centrifugation (600 × g, 5 min). Spermatozoa were removed from the pellets and added to IVF100 to obtain a suspension with a final sperm concentration of 5.0 × 106 cells/ml, which served as the in vitro fertilization (IVF) medium. After IVM, COCs were removed from the maturation medium and washed three times with IVF100. Up to 30 COCs were incubated in 35-mm dishes containing 100-µl droplets of IVF medium covered with mineral oil for 6 h at 38.5°C in 5% CO2 and saturated humidity. Potassium simplex optimization medium (KSOM) [19] with amino acids containing 5% NBCS and 10 ng/ml gentamicin sulfate was used as an in vitro culturing (IVC) medium. After IVF, cumulus cells and spermatozoa were removed from the surface of the zona pellucida by high-speed vortexing, and the putative zygotes were washed three times with the IVC medium. Up to 50 zygotes were cultured in 4-well dishes in 500-μl droplets of IVC medium covered with mineral oil for 7 days at 38.5°C in 5% CO2, 5% O2, and 90% N2 with saturated humidity.
Effects of carnosine supplementation in CPS
CPS supplemented with different concentrations (0, 5, and 50 μg/ml for experiment 1, 0, and 50 μg/ml for experiment 2, 3, 4, 5, 6) of carnosine was used for embryo cryopreservation. Each embryo was transferred to wells of ultra-low attachment U-bottomed 96-well plates (PrimeSurface; Sumitomo Bakelite, Tokyo, Japan) containing 100-μl blastocyst culture medium (KSOM with 10% NBCS and 10 ng/ml gentamicin sulfate) and cultured at 38.5°C in 5% CO2 with saturated humidity.
Freezing and thawing embryos
Blastocysts were randomly selected, cryopreserved, and thawed as previously described [20]. Briefly, blastocysts obtained 7 days after IVF were collected, and their quality was determined according to the methods specified in the International Embryo Transfer Society (IETS) manual [21]. The CPS comprised M199 supplemented with 20% heat-inactivated NBCS (Thermo Fisher Scientific), 5% ethylene glycol, 6% propylene glycol, 0.1 M sucrose, and 10 ng/ml gentamicin sulfate. Blastocysts classified as code 1 or 2 were transferred to the CPS and packed in 0.25 ml straws (IVM Technologies, L’Aigle, France) within 15 min. The straws were placed into an alcohol bath of a programmable freezer (ET-1N; Fujihira Industry, Tokyo, Japan) and precooled to –7°C. After 2 min, the straws were seeded with ice using forceps (precooled in liquid nitrogen), incubated at –7°C for 10 min, cooled to –30°C at a rate of –0.3°C/min, and immersed into liquid nitrogen. The straws were stored in liquid nitrogen for at least one week. The straws were removed from liquid nitrogen and thawed at room temperature for 10 sec. Post which, they were placed in water maintained at 30°C for 20 sec, after which they were completely thawed. In experiment 1, the survival rates of embryos were determined by observing the cumulative number of re-expanded and hatched blastocysts at 4, 24, and 48 h after thawing. In experiments 2, 3, 4, 5, and 6, the expanded blastocysts were collected 4 h after thawing. ROS generation in frozen-thawed blastocysts was quantified using CM-H2DCFDA (Experiment 2). The expression of the apoptotic genes BCL2 and BAX in frozen-thawed blastocysts was quantified by qRT-PCR (Experiment 3). The proportion of apoptotic DNA fragmentation in frozen-thawed blastocysts was measured using the TUNEL assay (Experiment 4). Expanded frozen–thawed blastocysts were subjected to immunostaining for TOMM20 (Experiment 5). ATP production in frozen-thawed blastocysts was quantified using the luciferin-luciferase assay (Experiment 6).
Measurement of ROS levels
Blastocysts classified as codes 1 or 2 were cryopreserved. Four hours after thawing, the expanded blastocysts were incubated in KSOM with amino acids containing 10 μM 2′,7′-dichlorodihydrofluorescein diacetate (DCHFDA; CM-H2DCFDA, Thermo Fisher Scientific) for 20 min at 38.5°C, 5% CO2, and saturated humidity. After incubation, the embryos were washed twice with phosphate-buffered saline (PBS), and their intracellular ROS levels were examined by fluorescence microscopy (IX71; Olympus, Tokyo, Japan) using a U-MWIB3 fluorescent filter unit (excitation range 460–495 nm; emission at 510 nm). Fluorescence images were acquired using a digital camera (AdvanCam-E3R; Advan-Vision Co., Ltd., Tokyo, Japan) in a darkened room. Fluorescence images were captured at the same exposure times (302.8 msec). The fluorescence intensity of whole embryos was measured using ImageJ v. 1.53 (National Institutes of Health, Bethesda, MD, USA).
RNA extraction and quantitative real-time reverse transcription polymerase chain reaction
Blastocysts classified as codes 1 or 2 were cryopreserved. Four hours after thawing, expanded blastocysts were pooled and immediately immersed in liquid nitrogen. RNA was extracted from the pooled blastocysts (each pool comprised 10 blastocysts) using a Nucleospin RNA XS Kit (Macherey-Nagel, Düren, Germany), following the manufacturer’s instructions. Isolated RNA was reverse-transcribed into cDNA using ReverTra Ace qPCR RT Master Mix with Genomic DNA Remover (TOYOBO, Osaka, Japan), following the manufacturer’s instructions. The cDNA samples were stored at –20°C until further use. Real-time PCR was performed using an Applied Biosystems QuantStudio 5 Real-Time PCR System (Thermo Fisher Scientific) in a 20 µl reaction mixture containing 10 pmol of each primer, diluted cDNA as a template, and THUNDERBIRD SYBR qPCR Mix (TOYOBO). The primer pair was designed based on a similarity-based search using BLAST, and the sequences for each gene are as follows: BCL2 forward, 5′-GATGACCGAGTACCTGAACC-3′; BCL2 reverse, 5′-AGGAGAAATCAAACAGGGGC-3′; BAX forward, 5′-CGCCCTTTTCTACTTTGCCAG-3′; BAX reverse, 5′-GGCCGTCCCAACCACC-3′; ACTB forward, 5′-ACCTAACTTGCGCAGAAAACG-3′; and ACTB reverse, 5′- TGTCACCTTCACCGTTCCAG-3′. The cDNA sequences were amplified using a 40-cycle reaction of denaturation (15 sec, 95°C) and annealing/extension (60 sec, 58°C). The formation of a single product was determined by melting curve analysis. The mRNA quantification was performed using the comparative Ct method and β-actin mRNA was used as an endogenous reference. Fold increase above control levels was determined through the 2ΔΔCt method, where ΔΔCt = ΔCt treatment – ΔCt control.
Detection of apoptosis by the TUNEL assay
The mitochondrial membrane potential of the blastocysts was measured using an In Situ Apoptosis Detection Kit (Takara Bio Inc., Shiga, Japan) following the manufacturer’s instructions. Blastocysts classified as codes 1 or 2 were cryopreserved. Four hours after thawing, the expanded blastocysts were fixed in 4% paraformaldehyde in PBS at 25°C for 20 min. After washing three times with PBS containing 0.3% polyvinylpyrrolidone, the fixed embryos were treated with permeabilization buffer (Takara Bio Inc.) at 0°C for 5 min. After incubation, the embryos were washed twice with PBS containing 0.3% polyvinylpyrrolidone and TUNEL-positive nuclei were examined by fluorescence microscopy (IX71; Olympus) using a U-MWIB3 fluorescent filter unit. Fluorescent images were acquired using a digital camera (Advan Vision Co.). After staining with Hoechst 33345 (Thermo Fisher Scientific), the embryos were mounted on slides using an antifade reagent (VECTASHIELD Mounting Medium; Vector Laboratories, Newark, CA, USA) and the signals were recorded using a fluorescence microscope (IX71; Olympus). The proportion of apoptotic cells was calculated as the ratio of terminal deoxynucleotidyl transferase dUTP nick end labeling-positive nuclei to the total number of nuclei.
Immunostaining and total cell count
Blastocysts classified as codes 1 or 2 were cryopreserved. Four hours after thawing, the expanded blastocysts were fixed in 4% paraformaldehyde in PBS at 25°C for 20 min. After washing three times with PBS containing 0.3% polyvinylpyrrolidone, fixed embryos were treated with 0.5% Triton X-100 in PBS at 25°C for 60 min and blocked in PBS containing 5 mg/ml bovine serum albumin and 0.02% Tween-20 (PBST) at 4°C overnight, followed by staining with a mouse anti-translocase of outer mitochondrial membrane 20 (TOMM20) antibody (Anti TOM20, #sc-17764, 1:200; Santa Cruz Biotechnology Inc., Dallas, TX, USA). Embryos were washed thrice in PBST before incubation with the secondary antibody (Alexa Fluor 488-conjugated goat anti-mouse IgG, 1:1,000; Thermo Fisher Scientific) in PBST at 25°C for 1 h. After staining with Hoechst 33345 (Thermo Fisher Scientific), the embryos were mounted on slides in an antifade reagent (Vector Laboratories, Burlingame, CA, USA) and staining signals were recorded using a fluorescence microscope (IX71; Olympus). The fluorescence intensity of whole embryos was measured using the ImageJ v. 1.53 software (National Institutes of Health).
ATP assay
Mitochondrial ATP production in frozen-thawed blastocysts was measured using an ATP assay kit (Dojindo Laboratories, Kumamoto, Japan) according to the manufacturer’s instructions. Blastocysts classified as codes 1 or 2 were cryopreserved. After thawing, the blastocysts were incubated in KSOM supplemented with 10% FCS for 4 h. Expanded blastocysts were collected and washed three times with PBS before incubation. The embryos were incubated with 100 μl working solution on a microplate mixer and incubated in dark conditions for 10 min at 25°C. The luminescent signal was detected using a luminometer (Infinite 200 PRO microplate plate reader; Tecan Group Ltd., Zurich, Switzerland) and it was calculated using a calibration curve generated by the serial dilution of ATP standard solutions with PBS.
Statistical analysis
Statistical differences (P < 0.05) in the rates of blastocyst re-expansion and hatched among the different carnosine-supplemented groups (0, 5, and 50 μg/ml) were analyzed via chi-squared testing with Bonferroni correction. Other results are expressed as mean ± SEM. For the frozen-thawed blastocysts, Student’s t-tests were used to determine the statistical differences (P < 0.05) in the fluorescence intensity of 2′,7′-dichlorofluoroscein (DCF), the proportion of TUNEL-positive nuclei, BCL2 and BAX mRNA expression, the fluorescence intensity of TOMM20, and the ATP concentrations by comparing the carnosine-supplemented (50 μg/ml) and control groups.
Results
Experiment 1
The rates of blastocyst re-expansion in the 50 μg/ml carnosine-supplemented group at 4, 24, and 48 h after thawing were higher than those in the control and 5 μg/ml carnosine-supplemented groups (P < 0.05, Table 1). The rates of hatched blastocyst in the 50 μg/ml carnosine-supplemented group at 4, 24, and 48 h after thawing were higher than those in the control.
Table 1. Effects of different concentrations of carnosine on the number of re-expanded and hatched blastocysts.
| Treatment groups | Number of blastocysts cultured | Number of blastocysts re-expanded (%) |
Number of hatched blastocysts (%) |
||||
|---|---|---|---|---|---|---|---|
| 4 h | 24 h | 48 h | 4 h | 24 h | 48 h | ||
| Control | 67 | 31(46.3) a | 40 (59.7) a | 40 (59.7) a | 2 (3.0) a | 7 (10.4) a | 9 (13.4) a |
| Carnosine, 5 μg/ml | 54 | 23 (42.6) a | 29 (53.7) a | 29 (53.7) a | 4 (7.4) | 6 (11.1) | 6 (11.1) a |
| Carnosine, 50 μg/ml | 54 | 39 (72.2) b | 45 (83.3) b | 45 (83.3) b | 14 (25.9) b | 15 (27.8) b | 18 (33.3) b |
a, b P < 0.05, based on five independent experiments. Different lowercase letters indicate significant differences between the treatment groups.
Experiment 2
The blastocysts stained with DCF exhibited green fluorescence (Fig. 1A), indicating the presence of intracellular ROS. The intensity of the green fluorescence signal in the 50 μg/ml carnosine-supplemented group was lower than that in the control (P < 0.05, Fig. 1B).
Fig. 1.
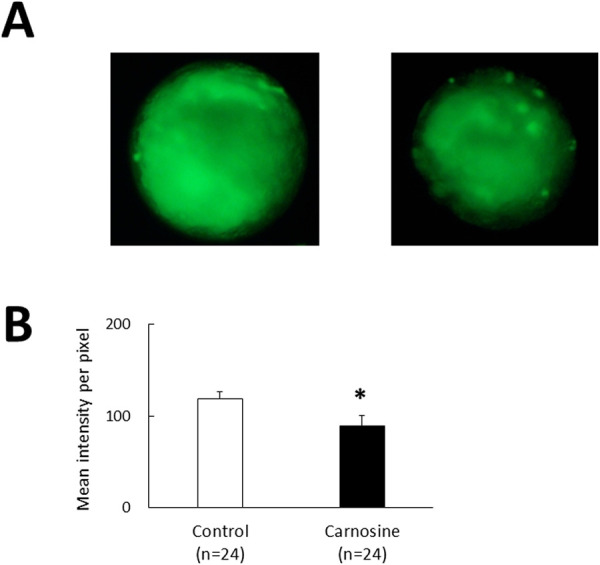
Effects of CPS supplemented with carnosine on intracellular ROS levels determined by DCF staining. (A) Representative images of bovine blastocysts in the control (left) and carnosine-supplemented (right) groups after DCF staining. (B) DCF fluorescence intensity of the control and carnosine-supplemented group. Data are presented as the mean ± SEM. * P < 0.05.
Experiment 3
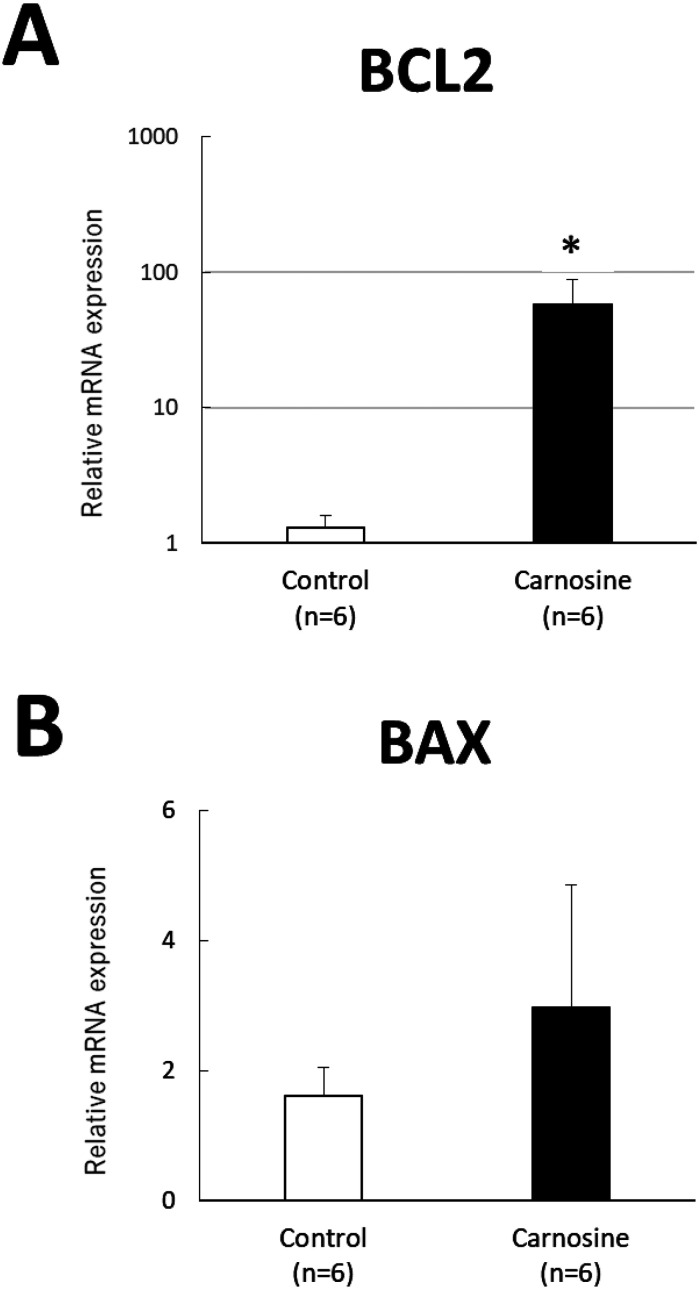
The expression of BCL2 mRNA in the 50 μg/ml carnosine-supplemented group was higher than that in the control group (P < 0.05, Fig. 2A). No significant difference was observed in the expression of BAX mRNA between the groups (Fig. 2B).
Fig. 2.
Quantitative real-time PCR analysis of mRNA levels of BCL2 (A) and BAX (B), normalized to ACTB levels, in bovine blastocysts frozen and thawed. Data are presented as mean ± SEM. * P < 0.05.
Experiment 4
Blastocysts stained with TUNEL reagent and Hoechst stain exhibited green and blue fluorescence signals, respectively. The ratio of TUNEL-positive cells in the 50 μg/ml carnosine-supplemented group was lower than that in the control (P < 0.05, Fig. 3).
Fig. 3.
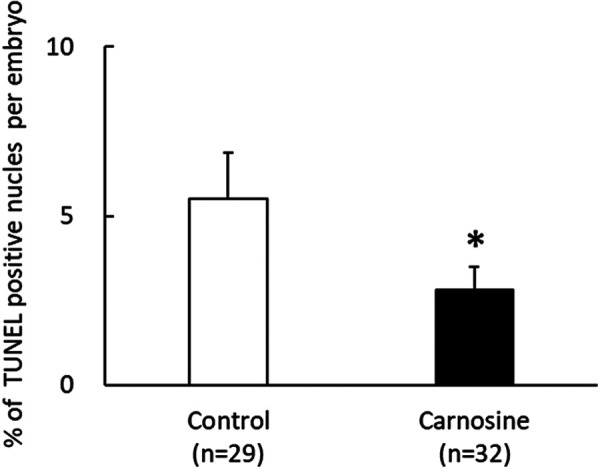
Effects of CPS supplemented with carnosine on apoptosis in bovine blastocysts frozen and thawed. Percentage of TUNEL-positive cells per embryo in the control and carnosine-supplemented groups. TUNEL is a marker of the fragmented DNA in apoptotic cells. Data are presented as mean ± SEM. * P < 0.05.
Experiment 5
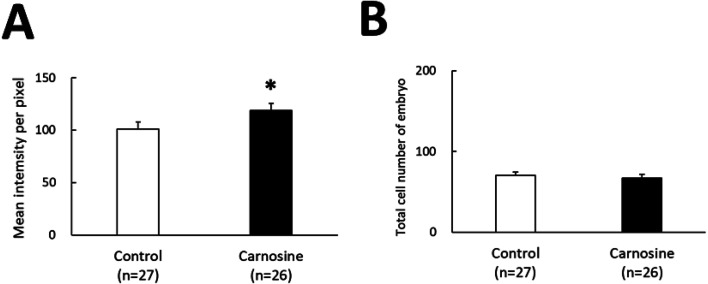
Blastocysts stained with TOMM20 and Hoechst stain exhibited green and blue fluorescence, respectively. The intensity of green fluorescence, indicating the mitochondrial activity, was found to be higher in the 50 μg/ml carnosine-supplemented group compared to that in the control (P < 0.05, Fig. 4A). The numbers of cells exhibiting blue fluorescence, indicating the number of cells, were similar in the 50 μg/ml carnosine-supplemented and control groups (Fig. 4B).
Fig. 4.
Effects of CPS supplemented with carnosine on translocase of outer membrane 20 (TOMM20) expression. (A) TOMM20 fluorescence intensity of the control and carnosine-supplemented groups. Data are presented as mean ± SEM. * P < 0.05. (B) Total number of cells per bovine embryo.
Experiment 6
The ATP concentration per embryo in the 50 μg/ml carnosine-supplemented group was significantly higher than that in the control group (P < 0.05, Fig. 5).
Fig. 5.
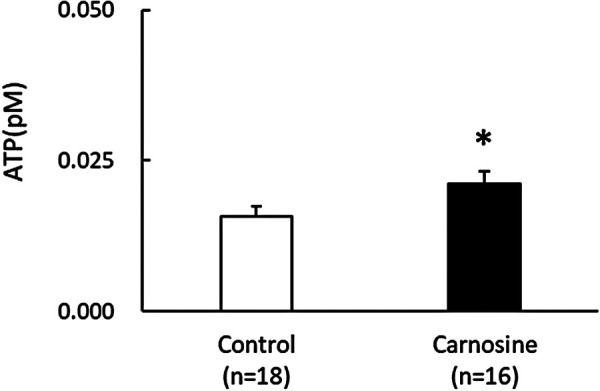
Effects of CPS supplemented with carnosine on the ATP production in embryo. The ATP level per embryo in the control and carnosine-supplemented groups. Data are presented as mean ± SEM. * P < 0.05.
Discussion
Bovine blastocysts frozen and thawed in CPS supplemented with carnosine exhibited greater competence for re-expansion and hatched after thawing than those without carnosine supplementation. Carnosine supplementation reduced intracellular ROS levels and increased BCL2 mRNA expression in frozen-thawed embryos. In addition, the intensity of TOMM20 and ATP levels were higher in embryos frozen-thawed using CPS with carnosine. These results suggest that carnosine improves cell viability by reducing oxidative damage. This is the first study to demonstrate that carnosine supplementation with CPS promotes the recovery and maintenance of embryo viability after cryopreservation of bovine embryos.
We showed that bovine blastocysts cryopreserved with carnosine-supplemented CPS had higher re-expansion and hatching rates than those cryopreserved with control CPS. The process of freezing and thawing has deleterious effects on mammalian embryos, causing acute shrinkage and trophectoderm damage [22, 23]. Failure of blastocyst re-expansion and cavity collapse are the most common adverse effects of freeze-thaw [24]. Sefid et al. [25] demonstrated that the addition of VK2, an antioxidant, to the culture medium before and after vitrification significantly reduced ROS production and improved the rates of embryo survival, re-expansion, and hatching post-vitrification. In mammalian embryos, ATP production significantly increases during the blastocyst stage [26, 27]. During the blastocyst stage, ATP production by mitochondrial oxidative phosphorylation is activated [28], and this oxidative phosphorylation enhances ROS production. Oxidative stress is caused due to an imbalance between ROS and antioxidants [29, 30] and can lead to cell injury or even cell death [31, 32]. In embryos, high intracellular ROS levels cause various types of cellular damage, including DNA fragmentation and apoptosis, resulting in developmental retardation and embryo fragmentation [33, 34]. In the present study, the green fluorescence signal of DCF in carnosine-supplemented embryos was significantly lower than that in the control group, indicating that carnosine reduced ROS production in frozen-thawed embryos. Therefore, in this study, carnosine-induced ROS reduction may have resulted in higher re-expansion and hatching rates of frozen-thawed embryos.
The ratio of TUNEL-positive cells per embryo in the 50 μg/ml carnosine-supplemented group was significantly lower than that in the control. BCL2 mRNA in the carnosine-supplemented group was significantly higher than in the control group. BCL2 is involved in apoptosis [35]. These findings suggest that carnosine inhibits apoptosis. The fluorescent signals of TOMM20 in the carnosine-supplemented blastocysts were stronger than those in the control group. TOMM20 regulates the BCL2–mitochondria interaction [36]. TOMM20 overexpression in cells leads to upregulation of the anti-apoptotic protein BCL2, thereby promoting growth in vivo and in vitro [37]. Park et al. [38] demonstrated that siTOMM20-treated cells stalled in the S phase owing to a delay in DNA synthesis caused by reduced ATP synthesis. In addition to cell cycle dysregulation, TOMM20 downregulation induces apoptosis, resulting in reduced cell proliferation [38]. Accordingly, reducing ROS levels in frozen-thawed bovine blastocysts through carnosine supplementation could inhibit apoptosis caused by cryodamage in embryos.
TOMM20, located on the mitochondrial outer membrane, has been used as a marker of mitochondrial mass, metabolic activity, and oxidative phosphorylation [39, 40]. Cryopreservation can cause extensive damage to membranes by reducing cell metabolism and disrupting cell bioenergetics by damaging the mitochondria. Cryopreservation of porcine and murine germinal vesicle oocytes reduces their TOMM20 expression, inhibiting cell development [41, 42]. Sustained mitochondrial damage disrupts energy metabolism, thereby reducing ATP production [43]. In the present study, the TOMM20 fluorescent signal in carnosine-treated blastocysts was not only stronger but also had higher ATP levels, suggesting enhanced intracellular mitochondrial function. Considering that the amount of ATP affects oocyte and embryo quality and viability [44], the carnosine-induced increase in mitochondrial activity in CPS may improve frozen-thawed bovine blastocyst viability, thereby increasing re-expansion and hatching rates. Spontaneously hatched blastocysts have greater potential for implantation and development into a positive pregnancy [45]. The hatching rate of the cryopreserved embryos is a reliable indicator of embryonic development.
As shown in this experiment, addition of carnosine to CPS increased the re-expansion and hatching rates of frozen-thawed bovine blastocysts, whereas carnosine supplementation alone in IVM medium has been reported to have no effect on the developmental kinetics of frozen-thawed bovine blastocysts [46]. This discrepancy may be due to the presence of serum in the medium [46], as an antioxidant is generally added to culture media to counter the oxidative effects of long-chain fatty acids in serum [47]. Moreover, carnosine is more effective at inhibiting lipid peroxidation than other antioxidants such as ascorbic acid [48]. Lipid peroxidation mediates ROS production in phospholipid bilayers and promotes mitochondrial apoptosis [49]. Therefore, carnosine might be a useful antioxidant that can be added to serum-containing culture media.
Thus, carnosine supplementation with CPS can protect the mitochondrial membrane from oxidative damage by reducing ROS levels, thereby improving the viability of frozen-thawed blastocysts. Our study provides a novel approach to improve the viability of slow-frozen cryopreserved bovine embryos.
Conflict of interests
The authors declare no conflict of interest.
Acknowledgments
We thank Mr. T. Shimoda (Mie Prefectural Southern Livestock Hygiene Service Center), Mr. F. Satoh, and Ms. M. Totsuka (Mie Prefectural Central Livestock Hygiene Service Center) for their technical support with the qRT-PCR. We thank Mr. I. Matsufuji (Nagoya University) for technical support with the luciferin-luciferase assay. This study was supported by funding from the Mie Prefectural Livestock Research Center, Japan.
References
- 1.Viana JHM. 2020 Statistics of embryo production and transfer in domestic farm animals. Embryo Technology Newsletter 2021; 39: 1–15. [Google Scholar]
- 2.Leibo SP, Loskutoff NM. Cryobiology of in vitro-derived bovine embryos. Theriogenology 1993; 39: 81–94. [Google Scholar]
- 3.Pontes JH, Nonato-Junior I, Sanches BV, Ereno-Junior JC, Uvo S, Barreiros TR, Oliveira JA, Hasler JF, Seneda MM. Comparison of embryo yield and pregnancy rate between in vivo and in vitro methods in the same Nelore (Bos indicus) donor cows. Theriogenology 2009; 71: 690–697. [DOI] [PubMed] [Google Scholar]
- 4.Lane M, Maybach JM, Gardner DK. Addition of ascorbate during cryopreservation stimulates subsequent embryo development. Hum Reprod 2002; 17: 2686–2693. [DOI] [PubMed] [Google Scholar]
- 5.Tatone C, Di Emidio G, Vento M, Ciriminna R, Artini PG. Cryopreservation and oxidative stress in reproductive cells. Gynecol Endocrinol 2010; 26: 563–567. [DOI] [PubMed] [Google Scholar]
- 6.Gillies LA, Kuwana T. Apoptosis regulation at the mitochondrial outer membrane. J Cell Biochem 2014; 115: 632–640. [DOI] [PubMed] [Google Scholar]
- 7.Lalier L, Mignard V, Joalland MP, Lanoé D, Cartron PF, Manon S, Vallette FM. TOM20-mediated transfer of Bcl2 from ER to MAM and mitochondria upon induction of apoptosis. Cell Death Dis 2021; 12: 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Matos DG, Gasparrini B, Pasqualini SR, Thompson JG. Effect of glutathione synthesis stimulation during in vitro maturation of ovine oocytes on embryo development and intracellular peroxide content. Theriogenology 2002; 57: 1443–1451. [DOI] [PubMed] [Google Scholar]
- 9.Hosseini SM, Forouzanfar M, Hajian M, Asgari V, Abedi P, Hosseini L, Ostadhosseini S, Moulavi F, Safahani Langrroodi M, Sadeghi H, Bahramian H, Eghbalsaied S, Nasr-Esfahani MH. Antioxidant supplementation of culture medium during embryo development and/or after vitrification-warming; which is the most important? J Assist Reprod Genet 2009; 26: 355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takahashi M, Nagai T, Hamano S, Kuwayama M, Okamura N, Okano A. Effect of thiol compounds on in vitro development and intracellular glutathione content of bovine embryos. Biol Reprod 1993; 49: 228–232. [DOI] [PubMed] [Google Scholar]
- 11.Sovernigo TC, Adona PR, Monzani PS, Guemra S, Barros F, Lopes FG, Leal C. Effects of supplementation of medium with different antioxidants during in vitro maturation of bovine oocytes on subsequent embryo production. Reprod Domest Anim 2017; 52: 561–569. [DOI] [PubMed] [Google Scholar]
- 12.Castillo-Martín M, Bonet S, Morató R, Yeste M. Comparative effects of adding β-mercaptoethanol or L-ascorbic acid to culture or vitrification-warming media on IVF porcine embryos. Reprod Fertil Dev 2014; 26: 875–882. [DOI] [PubMed] [Google Scholar]
- 13.Yashiro I, Tagiri M, Ogawa H, Tashima K, Takashima S, Hara H, Hirabayashi M, Hochi S. High revivability of vitrified-warmed bovine mature oocytes after recovery culture with α-tocopherol. Reproduction 2015; 149: 347–355. [DOI] [PubMed] [Google Scholar]
- 14.Carrascal-Triana EL, Zolini AM, de King AR, Penitente-Filho JM, Hansen PJ, Torres CAA, Block J. Effect of addition of ascorbate, dithiothreitol or a caspase-3 inhibitor to cryopreservation medium on post-thaw survival of bovine embryos produced in vitro. Reprod Domest Anim 2022; 57: 1074–1081. [DOI] [PubMed] [Google Scholar]
- 15.di Pierro F, Bertuccioli A, Bressan A, Rapacioli G. Carnosine-based supplement. Nutrafoods 2011; 10: 43–47. [Google Scholar]
- 16.Boldyrev AA, Aldini G, Derave W. Physiology and pathophysiology of carnosine. Physiol Rev 2013; 93: 1803–1845. [DOI] [PubMed] [Google Scholar]
- 17.Ogata K, Sasaki A, Kato Y, Takeda A, Wakabayashi M, Sarentonglaga B, Yamaguchi M, Hara A, Fukumori R, Nagao Y. Glutathione supplementation to semen extender improves the quality of frozen-thawed canine spermatozoa for transcervical insemination. J Reprod Dev 2015; 61: 116–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishii T, Kawano K, Tanaka N, Tomita K, Saito N, Yamada M. Mild hypothermia promotes the viability of in vitro-produced bovine blastocysts and their transcriptional expression of the cold-inducible transcription factor Rbm3 during in vitro culture. J Reprod Dev 2019; 65: 275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erbach GT, Lawitts JA, Papaioannou VE, Biggers JD. Differential growth of the mouse preimplantation embryo in chemically defined media. Biol Reprod 1994; 50: 1027–1033. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi T, Ueda S, Mori M, Baba T, Abe T, Iwata H. Influence of resveratrol pretreatment on thawed bovine embryo quality and mitochondrial DNA copy number. Theriogenology 2018; 106: 271–278. [DOI] [PubMed] [Google Scholar]
- 21.Robertson I, Nelson RE. Certification and identification of embryos. In: Stringfellow DA, Givens MD (eds.), Manual of The International Embryo Transfer Society. 4th ed., Champaign: International Embryo Transfer Society; 2010: 86–105. [Google Scholar]
- 22.Kaidi S, Bernard S, Lambert P, Massip A, Dessy F, Donnay I. Effect of conventional controlled-rate freezing and vitrification on morphology and metabolism of bovine blastocysts produced in vitro. Biol Reprod 2001; 65: 1127–1134. [DOI] [PubMed] [Google Scholar]
- 23.Camargo LS, Boite MC, Wohlres-Viana S, Mota GB, Serapiao RV, Sa WF, Viana JH, Nogueira LA. Osmotic challenge and expression of aquaporin 3 and Na/K ATPase genes in bovine embryos produced in vitro. Cryobiology 2011; 63: 256–262. [DOI] [PubMed] [Google Scholar]
- 24.Moussa M, Shu J, Zhang X, Zeng F. Cryopreservation of mammalian oocytes and embryos: current problems and future perspectives. Sci China Life Sci 2014; 57: 903–914. [DOI] [PubMed] [Google Scholar]
- 25.Sefid F, Ostadhosseini S, Hosseini SM, Ghazvini Zadegan F, Pezhman M, Nasr Esfahani MH. Vitamin K2 improves developmental competency and cryo-tolerance of in vitro derived ovine blastocyst. Cryobiology 2017; 77: 34–40. [DOI] [PubMed] [Google Scholar]
- 26.Leese HJ. Metabolism of the preimplantation mammalian embryo. Oxf Rev Reprod Biol 1991; 13: 35–72. [PubMed] [Google Scholar]
- 27.Leese HJ. Metabolic control during preimplantation mammalian development. Hum Reprod Update 1995; 1: 63–72. [DOI] [PubMed] [Google Scholar]
- 28.Guérin P, El Mouatassim S, Ménézo Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum Reprod Update 2001; 7: 175–189. [DOI] [PubMed] [Google Scholar]
- 29.Dumollard R, Duchen M, Carroll J. The role of mitochondrial function in the oocyte and embryo. Curr Top Dev Biol 2007; 77: 21–49. [DOI] [PubMed] [Google Scholar]
- 30.Banihani SA. Role of uric acid in semen. Biomolecules 2018; 8: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Banihani SA. Effect of captopril on semen quality. Andrologia 2017; 49: e12641. [DOI] [PubMed] [Google Scholar]
- 32.Bisht S, Dada R. Oxidative stress: Major executioner in disease pathology, role in sperm DNA damage and preventive strategies. Front Biosci (Schol Ed) 2017; 9: 420–447. [DOI] [PubMed] [Google Scholar]
- 33.Banihani SA. Vitamin B12 and semen quality. Biomolecules 2017; 7: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kitagawa Y, Suzuki K, Yoneda A, Watanabe T. Effects of oxygen concentration and antioxidants on the in vitro developmental ability, production of reactive oxygen species (ROS), and DNA fragmentation in porcine embryos. Theriogenology 2004; 62: 1186–1197. [DOI] [PubMed] [Google Scholar]
- 35.Ruvolo PP, Deng X, May WS. Phosphorylation of Bcl2 and regulation of apoptosis. Leukemia 2001; 15: 515–522. [DOI] [PubMed] [Google Scholar]
- 36.Lalier L, Mignard V, Joalland MP, Lanoé D, Cartron PF, Manon S, Vallette FM. TOM20-mediated transfer of Bcl2 from ER to MAM and mitochondria upon induction of apoptosis. Cell Death Dis 2021; 12: 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roche ME, Lin Z, Whitaker-Menezes D, Zhan T, Szuhai K, Bovee JVMG, Abraham JA, Jiang W, Martinez-Outschoorn U, Basu-Mallick A. Translocase of the outer mitochondrial membrane complex subunit 20 (TOMM20) facilitates cancer aggressiveness and therapeutic resistance in chondrosarcoma. Biochim Biophys Acta Mol Basis Dis 2020; 1866: 165962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park SH, Lee AR, Choi K, Joung S, Yoon JB, Kim S. TOMM20 as a potential therapeutic target of colorectal cancer. BMB Rep 2019; 52: 712–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Curry JM, Tuluc M, Whitaker-Menezes D, Ames JA, Anantharaman A, Butera A, Leiby B, Cognetti DM, Sotgia F, Lisanti MP, Martinez-Outschoorn UE. Cancer metabolism, stemness and tumor recurrence: MCT1 and MCT4 are functional biomarkers of metabolic symbiosis in head and neck cancer. Cell Cycle 2013; 12: 1371–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wurm CA, Neumann D, Lauterbach MA, Harke B, Egner A, Hell SW, Jakobs S. Nanoscale distribution of mitochondrial import receptor Tom20 is adjusted to cellular conditions and exhibits an inner-cellular gradient. Proc Natl Acad Sci USA 2011; 108: 13546–13551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ito J, Shirasuna K, Kuwayama T, Iwata H. Resveratrol treatment increases mitochondrial biogenesis and improves viability of porcine germinal-vesicle stage vitrified-warmed oocytes. Cryobiology 2020; 93: 37–43. [DOI] [PubMed] [Google Scholar]
- 42.Qin J, Guo S, Yang J, Qazi IH, Pan B, Lv T, Zang S, Fang Y, Zhou G. Melatonin promotes in vitro development of vitrified-warmed mouse GV oocytes, potentially by modulating phosphorylation of drp1. Front Vet Sci 2021; 8: 752001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Norat P, Soldozy S, Sokolowski JD, Gorick CM, Kumar JS, Chae Y, Yağmurlu K, Prada F, Walker M, Levitt MR, Price RJ, Tvrdik P, Kalani MYS. Mitochondrial dysfunction in neurological disorders: Exploring mitochondrial transplantation. NPJ Regen Med 2020; 5: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krisher RL, Prather RS. A role for the Warburg effect in preimplantation embryo development: metabolic modification to support rapid cell proliferation. Mol Reprod Dev 2012; 79: 311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chimote NM, Chimote NN, Nath NM, Mehta BN. Transfer of spontaneously hatching or hatched blastocyst yields better pregnancy rates than expanded blastocyst transfer. J Hum Reprod Sci 2013; 6: 183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Janati Idrissi S, Slezec-Frick V, Le Bourhis D, Le Berre L, Joly T, Buff S, Desmarchais A, Schibler L, Salvetti P, Elis S. Effect of DHA on the quality of in vitro produced bovine embryos. Theriogenology 2022; 187: 102–111. [DOI] [PubMed] [Google Scholar]
- 47.Nikoloff N, Campagna A, Luchetti C, Carranza-Martín AC, Pascua AM, Anchordoquy JM, Anchordoquy JP, Lombardo DM, Seoane A, Furnus CC. Effects of EPA on bovine oocytes matured in vitro with antioxidants: Impact on the lipid content of oocytes and early embryo development. Theriogenology 2020; 146: 152–161. [DOI] [PubMed] [Google Scholar]
- 48.Jun Lee B, G Hendricks D, P Cornforth D. A comparison of carnosine and ascorbic acid on color and lipid stability in a ground beef pattie model system. Meat Sci 1999; 51: 245–253. [DOI] [PubMed] [Google Scholar]
- 49.Wang B, Wang Y, Zhang J, Hu C, Jiang J, Li Y, Peng Z. ROS-induced lipid peroxidation modulates cell death outcome: mechanisms behind apoptosis, autophagy, and ferroptosis. Arch Toxicol 2023; 97: 1439–1451. [DOI] [PubMed] [Google Scholar]