Abstract
Retained placenta (RP) adversely affects postpartum productivity and reproduction in dairy cattle. Thus, methods to predict the occurrence of RP before calving would be desirable. Herein, we assessed whether vaginal temperature measurements (which have already been applied to detect calving) could be used to predict the occurrence of RP in cattle. A vaginal temperature recording device was inserted into the vagina of 49 pregnant Holstein-Friesian heifers (n = 16) and cows (n = 33); this device recorded the vaginal temperature every 5 min until the device dropped out at calving. Serum was collected 10 days before the expected calving date. The time points of calving and placental expulsion were identified via video recordings. We further calculated calving duration (temperature decrease to calving) and placenta expulsion time (PE time = calving to placenta expulsion). The PE times were divided into four categories (0–4 h, 4–8 h, 8–12 h, and RP at >12 h), while subsequent analysis revealed that an extension of the PE time dependent on the shortening of the calving duration (P < 0.05). The vaginal temperature patterns also differed in a PE time-dependent manner, and cows with RP did not show any re-elevation of vaginal temperature. Serum analyses indicated an energy deficiency in RP cattle. These results suggest that RP may be detected early as a specific change in the vaginal temperature associated with reproductive hormone secretion.
Keywords: Calving, Dairy cattle, Placenta expulsion, Vaginal temperature
In pregnant dairy cattle, fetal membranes are expelled after delivery. During pregnancy, the maternal body recognizes the placenta as ‘self’ and rejection does not occur, but as the fetus matures and delivery begins, the placenta begins to be recognized as non-self, after which detachment occurs, and the placenta is finally expelled following delivery of the fetus. Placenta expulsion is regulated by multiple factors involving the physical, endocrine, and cellular (immune) systems [1,2,3]. Once the gestational period expires and the placental tissue matures sufficiently, the levels of maternal major histocompatibility complex (MHC) class I rapidly increase. The number of inter-uterine macrophages increases concurrent with the allo-immune response; cytokines and neutrophils migrate, and placental abruption occurs [1, 2]. As the fetus matures, the placental adrenal glands secrete cortisol, which increases the secretion of prostaglandin F2α (PGF2α), decreases the synthesis of progesterone (P4), and increases the synthesis of estrogen (E2) in placental tissues [2]. The increases in PGF2α and E2 lead to contraction of the myometrium, which promotes the delivery of the fetus and expulsion of the placenta. PGF2α further increases the secretion of relaxin, which relaxes the pelvic ligaments and contributes to the delivery of the fetus and expulsion of the placenta.
Normal placental expulsion in dairy cattle is complete within approximately 8 h of parturition [4, 5]. The condition in which the placenta is not expelled within 12 h of delivery is termed ‘retained placenta (RP)’, and is an important issue in postpartum diseases [6, 7]. Although many factors affecting the development of RP remain unknown, it is thought to be related to premature birth, multiple pregnancies, dystocia, calving induction, maternal overweight, and negative energy balance [3, 8]. RP due to the breakdown of the cotyledon-caruncle attachment can also be caused by abnormal immune reactions, such as a decrease in neutrophil migration [1, 9, 10]. The secretion of prepartum sexual hormones, such as P4 and E2, also differs between cattle with and without RP [4, 11,12,13]. Retention of the placenta can cause intrauterine infections such as metritis and endometritis, resulting in decreased milk yield, a delayed recovery of reproductive function, and induced infertility [9, 14,15,16]. RP also leads to decreased productivity, increased culling rates and treatment costs, and significant economic losses.
The estimated incidence of RP in dairy cattle is approximately 10% [1, 9, 17]. It has been speculated that failing to control the occurrence of RP negatively affects cattle productivity. Most cases of RP are detected through simple visual observation of the failure of the placenta to expel after delivery, and are difficult to predict. Early postpartum administration of oxytocin to assist calving cows was reported to promote postpartum placental expulsion and suppress both postpartum retardation and low postpartum conception rate [18]. If the occurrence of RP could be predicted prior to delivery, appropriate treatment could be implemented early to suppress its occurrence, and thereby reduce its subsequent adverse effects on reproductive function. Although prepartum blood analysis can detect differences between RP and normal-calving cattle [3, 5, 19], it is very difficult to predict RP in a less or noninvasive manner, and methods that can predict RP at the field level have not yet been established.
Vaginal temperature measurement devices for cattle have been used for calving detection, and their effectiveness has been previously described [20,21,22]. Changes in cattle vaginal temperature closely reflect changes in hormonal secretions, such as those of P4 [23,24,25]. The body temperature of cows is high during the luteal phase and pregnancy, when the blood P4 levels are high, and low during the luteal regression phase [23,24,25]. A decrease in vaginal temperature before calving reflects the regression of the pregnant corpus luteum. Our research group demonstrated that the time from the start of the decrease in vaginal temperature (which is associated with the regression of the corpus luteum during pregnancy to delivery) is prolonged in a manner that is dependent on the ease of calving. For example, the time required for assisted and dystocia calving is longer than that for spontaneous calving [22]. The changes in vaginal temperature patterns during calving, which involves dystocia, also appear to differ from the patterns observed during natural calving [26]. We hypothesized that, compared to cows with normal births, cows with RP may show different patterns of vaginal temperature changes reflecting hormone changes prior to parturition. If such vaginal temperature pattern differences are consistent, it may be possible to predict RP before it occurs, thus reducing its negative impacts on postpartum productivity. We conducted the present study to (i) evaluate the relationship between prepartum vaginal temperature changes and placental expulsion time in Holstein dairy cattle and (ii) examine the possibility of predicting the occurrence of RP by measuring the cattle’s vaginal temperature.
Materials and Methods
Animals
All the animal experimental procedures were approved by the NARO Animal Care and Use Committee (approval no. 21B098ILGS). This study was conducted at the Institute of Livestock and Grassland Science farm, NARO in Nasushiobara, Tochigi, Japan (lat. 36° 91' 79" N, long. 139° 92' 27" E) from May 2021 to December 2022. A total of 49 pregnant Holstein-Friesian heifers (n = 16) and cows (n = 33, parity 1–4) were used in the experiment. All cattle were artificially inseminated with conventional or sex-sorted semen from Holstein-Friesian sires, and were moved to a maternity pen approximately three weeks before the expected calving date. They were fed forage and concentrate twice a day (0900 h and 1600 h) and water ad libitum until calving.
Measurement of vaginal temperature
A vaginal temperature measurement sensor (Gyuonkei, Remote Inc., Oita, Japan) was inserted into individual cattle 10 days before the expected calving date. In brief, the vulva was washed with inverted soap and disinfected with 70% ethanol, after which the disinfected sensor was inserted into the vagina via an applicator [26]. This sensor then measured the vaginal temperature every 5 min, and stored the measurements on a server via a Wi-Fi connection. Raw data were downloaded in the CSV format and stored on a personal computer (PC) for further analysis. This measurement system has two types of alerts for calving prediction: we set the system to automatically calculate a 4-h moving average value, and to issue a temperature-decreasing alert (Alert 1) if the value dropped by the threshold temperature (−0.4°C) or more from the same time value over the previous two days. The threshold value of –0.4°C, which is considered the most likely value for calving 24 h after Alert 1 in Holsteins in this sensor, was used in this study. When the temperature sensor dropped out of the vagina followed rupture of the allantois membrane and the recorded temperature value reached ambient temperature (i.e., < 37°C), another alert was issued (Alert 2).
The hourly average values of vaginal temperature for each cattle were calculated. We also calculated the relative temperature change from 48 h before Alert 2 to the time of Alert 2, using the 48-h period prior to Alert 2 as the reference value. Lammoglia et al. reported that vaginal temperature decline starts –40 h before calving [23]. Furthermore, diurnal variation in vaginal temperature was taken into account, because some cattle in the 0–4 h PE time group showed a mild decrease in vaginal temperature from –48 h.
Determination of calving ease, calving time, and placenta expulsion time
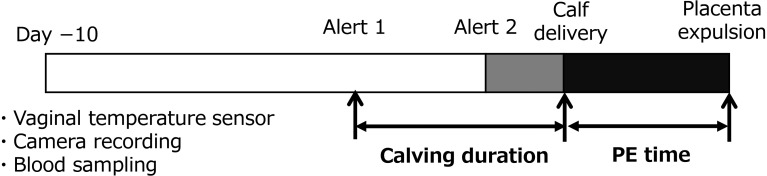
A video camera (Bohan-Kobo, Tokyo, Japan) was installed to cover the entire maternity pen, and images were stored on a hard disk. The recorded images were used to determine the exact time points of both calving, that is, when the entire calf’s body was outside the maternal body, and placental expulsion had occurred. Some studies have defined vaginal temperature reduction as the “onset of calving” [21,22,23]. Therefore, we defined and calculated the length of time from alert 1 to calving as the calving duration (Fig. 1). In addition, the length of time from calving to placental expulsion (i.e., placenta expulsion [PE] time) was calculated (Fig. 1). If the PE time exceeded 12 hours, the case was classified as RP. We further calculated the gestational period from the artificial insemination (AI) date to calving date.
Fig. 1.
Schematic of the experimental design. The vaginal temperature sensor installation, live image recording, and blood collection were performed 10 days before the expected calving date (Day −10).
Calving ease was determined from video recordings and classified into three levels: (1) natural calving, (2) assisted calving (assisted by one or two farm staff members), and (3) dystocia (assisted by more than three farm staff members or a veterinarian). Calving assistance was provided when the amnion ruptured and no progress was made for more than one hour, despite the leg being exposed. The sex and body weight of each calf was subsequently recorded.
Blood sampling and analysis
At the timepoint of the vaginal temperature sensor insertion 10 days before the expected calving date (average days −9.68), 10 ml of blood was collected from the jugular vein using a plain vacuum blood collection tube using a 21-gauge blood collection needle (Terumo, Tokyo, Japan). Blood samples were stored at ambient temperature, after which serum was collected by centrifugation (1,300 g for 15 min at room temperature) and stored at −30°C until analysis. Serum analysis was outsourced (Sanritu Co., Tochigi, Japan), and a total of 26 items were analyzed, including non-esterified fatty acids (NEFA), β-hydroxybutyric acid (BHBA), total fat, and total cholesterol (TCHO).
Statistical analysis
Statistical analyses were performed using EZR (Ver.1.64, Jichi-ikadai, Tochigi, Japan) [27]. The effects of each indicator (parity, season, gestation period, sex, and calf body weight) on calving duration and PE time were analyzed using regression analysis. The calving duration between heifers and cows was analyzed using Student’s t-test, and the ratio of calving conditions between heifers and cows was analyzed using the Fisher’s exact test. To clarify the relationship between calving duration and PE time in normal calving (PE time < 8 h), as previously reported [4, 5], a correlation analysis was performed between calving duration and PE time point. The influence of the PE time categories on calving duration and serum analyses was evaluated using a one-way analysis of variance (ANOVA). The influence of PE time categories on calving conditions was further analyzed using Fisher’s exact test. The changes in vaginal temperature were analyzed by repeated measures ANOVA at every 6 h (–48, –42, –36, –30, –24, –18, –12, –6 and 0 h from Alert 2). Results were considered significant if the probability was < 0.05.
Results
Calving status between the heifers and cows
Three cattle did not show Alert 1 because they calved within 2 days after the temperature sensor was inserted, and the data of these three animals were therefore excluded from the analyses. The remaining cattle showed both Alert 1 and Alert 2; thus, data from 46 cattle (15 heifers and 31 cows) were finally analyzed. Although Alert 2 was originally assumed to occur simultaneously with the rupture of the allantois membrane, this study found events in which Alert 2 occurred simultaneously with calving in 13 cows, regardless of PE time and calving difficulty. The cause of this event was unclear, and the time from Alert 2 to delivery could not be evaluated properly. Therefore, an analysis of Alert 2 with respect to the calving time was not performed in this study. The average calving duration for all 46 cattle was 23.66 ± 1.25 h, which means that Alert 1 detected the calving approx. 24 h before the delivery. There were no significant differences in calving duration or conditions between heifers and cows (Table 1). The percentage of RP cases among the 46 cattle was 15.2%, and the incidence was higher in cows (6/31) than in heifers (1/15), although this did not reach statistical significance (Table 1). Parity, season, gestation period, sex, and body weight of the calves did not affect calving duration or PE time (P > 0.05). In addition, no differences were found in the calving duration, PE time, or temperature change patterns between singleton and twin deliveries. As such, all data were analyzed together in the following results, regardless of the number of calves.
Table 1. Calving duration (h) and calving status.
| Heifers | Cows | Total | |
|---|---|---|---|
| Calving duration, h | 25.13 ± 1.98 | 22.94 ± 1.59 | 23.66 ± 1.25 |
| Total cattle, n | 15 | 31 | 46 |
| No. of natural calvings, % | 11 (73.3) | 25 (80.6) | 36 (78.2) |
| No. of assisted calvings, % | 2 (13.3) | 6 (19.4) | 8 (17.3) |
| No. of dystocia, % | 2 (13.3) | 0 (0.0) | 2 (4.3) |
| No. of RP, % | 1 (6.67) | 6 (19.3) | 7 (15.2) |
Data are by LSmean ±SE.
The association between the calving duration and the placenta expulsion (PE) time
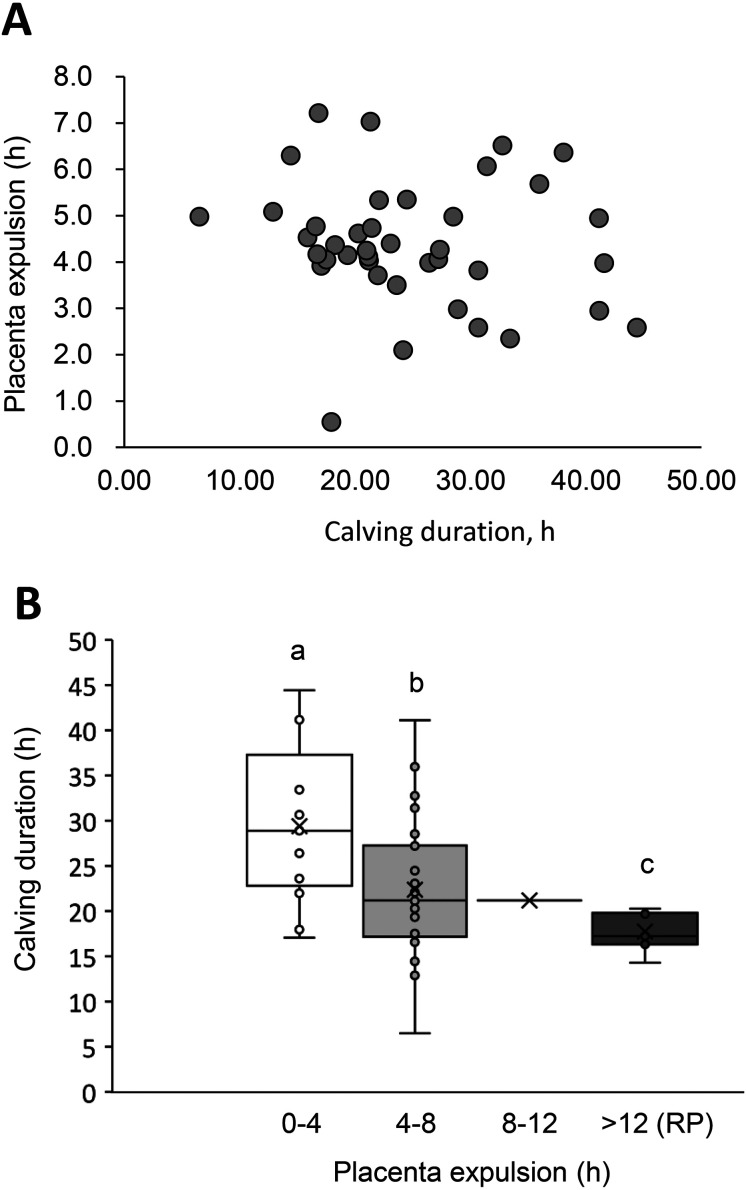
To clarify the characteristics of normal calving, we analyzed the relationship between calving duration and PE time in normal placenta-expulsion cattle (i.e., those with a PE time < 8 h, n = 38). As illustrated in Fig. 2A, our analysis revealed no correlation between the calving duration and PE time (R = –0.100, P = 0.536).
Fig. 2.
The association between the calving duration and the normal placenta expulsion (PE) time. The association between calving duration (h) and normal PE time (< 8 h) in the total cattle (n = 38, gray circles). A) The PE times were categorized into four categories: 0–4 h (n = 12), 4–8 h (n = 26), 8–12 h (n = 1), and RP (> 12 h, n = 7). The results are displayed in a box-and-whisker diagram. B) The linear approximation curve, its equation, and R2 values are also shown.
We next compared the calving durations among the four PE time categories (0–4 h, 4–8 h, 8–12 h, and RP), but excluded the 8–12-h category from the analysis as there was only one cow in this category and normal expulsion of the placenta usually occurs within 8 h after calving [4, 5]. More than half of the cattle (n = 26, 56.5%) expelled their placenta between 4 and 8 h after calving (Table 2). Parity, gestation period, calf body weight, and calving status (natural, assisted, or dystocia) were not significantly different among the four categories (Table 2). None of the cattle with RP exhibited dystocia.
Table 2. Relationships between calving conditions and placenta explusion (PE) time.
| PE time, h |
Total | ||||
|---|---|---|---|---|---|
| 0–4 | 4–8 | 8–12 | RP (> 12) | ||
| n (%) | 12 (26.1) | 26 (56.5) | 1 (2.2) | 7 (15.2) | 46 (100.0) |
| Calving duration, h | 29.05 ± 2.64 a | 22.84 ± 1.59 b | 21.20 | 17.75 ± 0.84 c | 23.66 ± 1.25 |
| Parities | 0.75 ± 0.28 | 1.12 ± 0.19 | 4 | 1.29 ± 0.29 | 1.11 ± 0.15 |
| Gestation period, d | 279.2 ± 0.9 | 278.9 ± 0.6 | 279 | 278.1 ± 1.2 | 279.0 ± 0.4 |
| No. of dystocia (%) | 1 (8.3) | 1 (3.8) | 0 (0.0) | 0 (0.0) | 2 (4.3) |
| No. of twin births (%) | 0 (0.0) | 1 (3.8) | 0 (0.0) | 2 (28.5) | 3 (6.5) |
| No. of female calves (%) | 9 (75.0) | 17 (65.4) | 0 (0.0) | 5 (71.4) | 31 (67.4) |
| Calf body weight, kg | 41.6 ± 1.4 | 42.7 ± 0.6 | 49.0 | 43.7 ± 2.2 | 42.4 ± 0.6 |
Data are LSmean ± SE. Different superscripts abc P < 0.05.
Calving duration showed a difference in accordance with PE time. Thus, as calving duration decreased, the PE time increased (Table 2, Fig. 2B). The calving duration of the PE category 0–4 h group was longer than that of the 4–8-h and RP groups (P < 0.05). The RP group further exhibited the shortest calving duration (Fig. 2B).
Association between vaginal temperature changes and the PE times
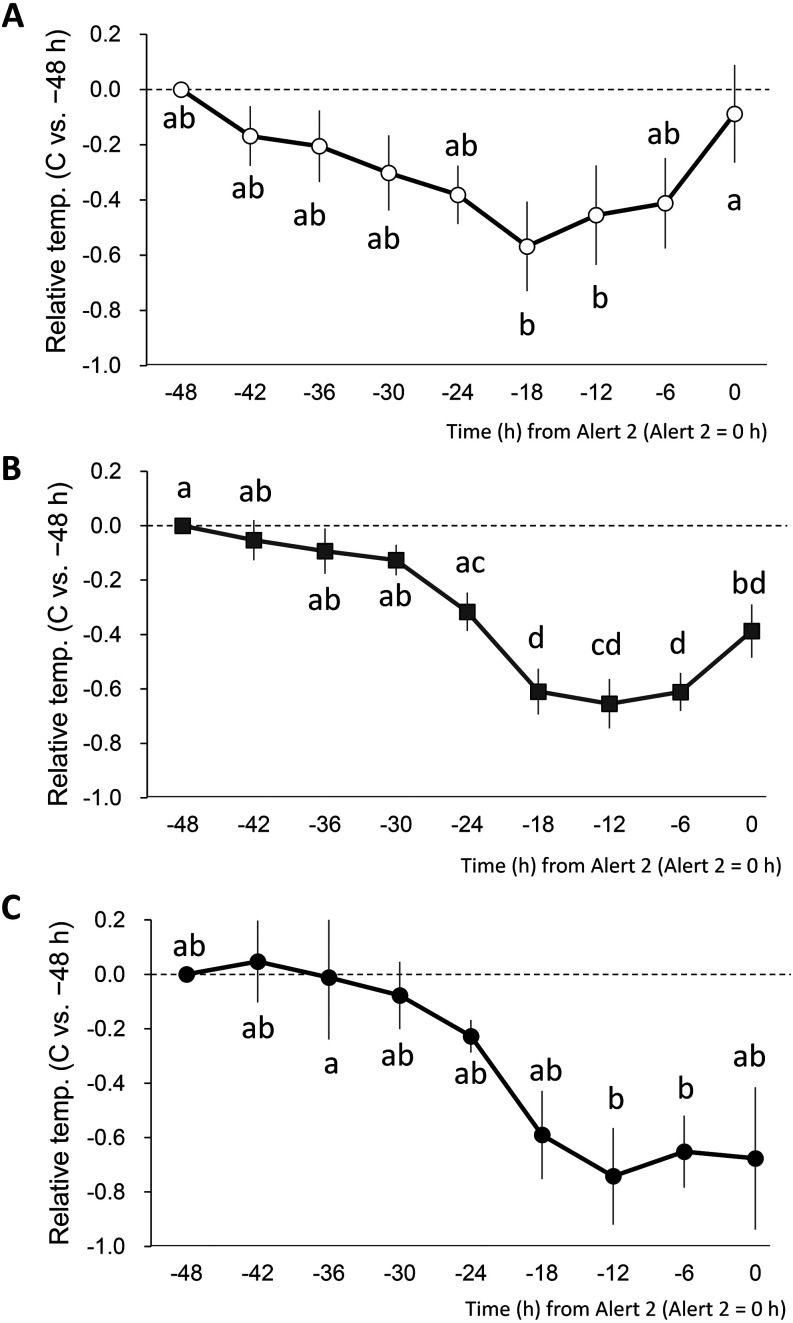
The relative vaginal temperature changes from 48 h before Alert 2 (−48 h) to Alert 2 (0 h) were analyzed in the PE time categories 0–4 h, 4–8 h, and RP. As shown in Figs. 3A, B, and C, vaginal temperature changes showed different patterns depending on PE time. The vaginal temperature began to drop at –42 h, but began to rise again from –12 h, and returned to its original level at 0 h in the PE time category of 0–4 h (Fig. 3A). Vaginal temperatures were the lowest at –18 and –12 h, and were significantly lower than those at 0 h (Fig. 3A, P < 0.05). Conversely, in the 4–8 h and RP categories, the onset of temperature decrease was observed at –30 and –24 h, respectively. In category 4–8 h, the relative temperatures at –18, –12, and –6 were significantly lower than those at –48, –42, –36 and –30 h (P < 0.05), but not at 0 h (Fig. 3B). Further, the relative temperature at –12 and –6 were significantly lower than that at –36 h (P < 0.05), but not at 0 h in RP cattle (Fig. 3C).
Fig. 3.
The association between the calving duration and PE time and the relative vaginal temperature changes from 48 h before Alert 2. PE times were categorized into four categories: 0–4 h (n = 12), 4–8 h (n = 26), 8–12 h (n = 1), and RP (> 12 h, n = 7). The relative vaginal temperature for each PE category, with −48 h (0 h = Alert 2, the time of sensor dropout) as the reference (= 0), is shown as every 6 h mean ± standard error. The PE time = 0–4 h (n = 12, A), 4–8 h (n = 26, B), and RP (> 12 h, n = 7, C). Different letters indicate P < 0.05. No significant differences were observed when the common letters were included.
Serum composition and placenta expulsion times
Table 3 provides the blood serum analysis results for the PE categories of 0–4 h, 4–8 h, and RP. Total fat, TCHO, and ALP levels were significantly lower in the RP group than in the non-RP group (P < 0.05), and total ketone, BHBA, aspartate aminotransferase (AST), and Mg levels were significantly higher in the RP group than in the non-RP group (Table 3, P < 0.05). The NEFA level was higher in the RP group, albeit not significantly.
Table 3. Serum compositions on 10 days before calving.
| Placenta expulsion time, h |
p-value | ||||
|---|---|---|---|---|---|
| 0–4 (n = 12) | 4–8 (n = 26) | RP (> 12) (n = 6) | non-RP 1vs. RP | ||
| Total fat | mg/dl | 259.08 ± 12.49 | 247.46 ± 5.61 | 217.00 ± 8.90 | 0.010 * |
| TCHO | mg/dl | 90.33 ± 4.75 a | 86.12 ± 2.21 ab | 76.50 ± 3.20 b | 0.026 * |
| TG | mg/dl | 16.42 ± 1.13 | 16.31 ± 1.14 | 13.83 ± 1.56 | 0.131 |
| Total ketone | umol/l | 346.17 ± 20.24 ab | 348.69 ± 17.71 a | 434.67 ± 53.37 b | 0.016 * |
| Acetoacetic acid | umol/l | 0.83 ± 0.75 | 1.12 ± 0.35 | 1.67 ± 0.76 | 0.235 |
| BHBA | umol/l | 345.33 ± 20.08 a | 347.58 ± 17.72 ab | 433.00 ± 53.40 b | 0.017 * |
| NEFA | mEq/l | 0.53 ± 0.05 | 0.55 ± 0.04 | 0.63 ± 0.09 | 0.140 |
| Glu | mg/dl | 60.08 ± 3.75 | 53.12 ± 2.79 | 51.17 ± 2.73 | 0.240 |
| AST | U/l | 55.42 ± 1.27 | 55.77 ± 1.38 | 63.33 ± 7.56 | 0.025 * |
| ALT | U/l | 8.42 ± 1.79 | 10.65 ± 1.05 | 10.33 ± 2.44 | 0.438 |
| ALP | U/l | 71.17 ± 10.14 | 62.54 ± 5.50 | 43.17 ± 7.75 | 0.044 * |
| LDH | U/l | 777.50 ± 26.99 | 754.69 ± 27.97 | 730.50 ± 65.17 | 0.294 |
| cGTP | U/l | 22.08 ± 1.23 | 21.88 ± 0.90 | 20.00 ± 1.57 | 0.155 |
| LAP | U/l | 12.08 ± 0.42 | 12.38 ± 0.26 | 11.83 ± 0.60 | 0.223 |
| TP | g/dl | 6.48 ± 0.15 | 6.39 ± 0.09 | 6.35 ± 0.18 | 0.368 |
| Alb | g/dl | 3.69 ± 0.06 | 3.75 ± 0.06 | 3.62 ± 0.08 | 0.153 |
| CRE | mg/dl | 1.08 ± 0.02 | 1.13 ± 0.03 | 1.05 ± 0.05 | 0.110 |
| BUN | mg/dl | 8.08 ± 0.67 | 8.92 ± 0.74 | 9.75 ± 1.72 | 0.236 |
| Na | mmol/l | 143.83 ± 0.53 | 144.42 ± 0.22 | 142.83 ± 1.72 | 0.054 |
| K | mmol/l | 4.36 ± 0.12 a | 4.14 ± 0.05 b | 4.08 ± 0.09 ab | 0.172 |
| Cl | mmol/l | 104.92 ± 1.10 | 104.42 ± 0.43 | 103.67 ± 1.31 | 0.231 |
| Ca | mg/dl | 9.64 ± 0.06 | 9.55 ± 0.08 | 9.35 ± 0.18 | 0.072 |
| IP | mg/dl | 5.07 ± 0.29 | 4.93 ± 0.12 | 4.85 ± 0.35 | 0.359 |
| Mg | mg/dl | 2.18 ± 0.04 ab | 2.23 ± 0.04 a | 1.98 ± 0.14 b | 0.010 * |
| Fe | ug/dl | 172.42 ± 9.63 | 166.77 ± 7.14 | 168.67 ± 8.86 | 0.497 |
| Amylase | U/l | 91.83 ± 5.46 | 92.73 ± 4.40 | 79.50 ± 5.03 | 0.074 |
| Lipase | U/l | 3.17 ± 0.59 | 3.52 ± 0.36 | 3.28 ± 0.62 | 0.439 |
Data are shown as the LSmean ± SE. The average serum collection date was −9.86 d. Different superscripts ab P < 0.05, * P < 0.05. 1 Non-RP: PE time < 8 h (0–4 h and 4–8 h). Alb, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BHBA, β-hydroxybutylic acid; BUN, blood urea nitrogen; CRE, creatinine; Glu, glucose; GTP, guanosine triphosphate; IP, inorganic phosphorus; LAP, leucine aminopeptidase; LDH, lactate dehydrogenase; NEFA, non-esterified fatty acid; TCHO, total cholesterol; TG, triglyceride; TP, total protein.
Discussion
Previous studies have shown that the measurement of vaginal temperature can effectively detect calving onset in dairy cattle. Calving accidents and the resulting diseases often have a major impact on milk production and reproductive recovery, and delays in addressing these problems can have a significant economic impact. Thermometry has the potential to detect prenatal accidents and diseases more precisely than behavioral measurements or evaluations of other signs of parturition, as it reflects the physiological state of individual cattle. This study is the first to assess the relationship between changes in prepartum vaginal temperature and placental expulsion in dairy cattle.
Our analyses of 46 deliveries revealed that calving factors including the rate of natural calving and the occurrence of retained placenta were not significantly different based on parity, while the average calving duration was similar between heifers and cows; therefore, all of our subsequent analyses were performed with heifers and cows together. In our analysis, we first evaluated the relationship between calving duration and PE time in individuals with normal (< 8 h) placenta expulsion, identifying no correlation between these parameters.
We further divided the PE times among the non-RP cases into two categories, and compared them with the RP cases, demonstrating an extension of PE time dependent on shortening of the calving duration. A decrease in vaginal temperature before parturition is due to decreased P4 concentration associated with the regression of the pregnant corpus luteus [21, 23]. Although we did not measure P4 concentrations in this study, our results suggest that endocrine factors may be involved in placental expulsion. Earlier studies comparing prepartum P4 levels in non-RP and RP cows have shown that (i) the RP group had higher levels of P4 the day of and the day before calving than the non-RP group, and (ii) the decline in P4 was slower in the RP group [4, 11]. This suggests that the regression of the pregnant luteal corpus is delayed in RP cattle, which is consistent with our present observation that the onset of temperature reduction occurs later in RP cattle; i.e., the calving duration is shorter in RP-calvings than in non-RP calvings. However, the total calving duration and PE time varied greatly among the individuals in this study, with no particular trend toward a constant value. This suggests that the time from calving onset to placental expulsion is not fixed; however, in RP cows, there is a delay in vaginal temperature reduction due to inadequate hormone secretion and other factors, resulting in a longer calving duration.
We observed different patterns not only for calving duration but also in the changes in vaginal temperature in a time-dependent manner for placental expulsion (Fig. 3). Although the vaginal temperatures in the 0–4-h group returned to pre-decline levels at calving, the intermediate 4–8-h group showed only half the pre-decline return, and the RP group showed no increase in vaginal temperature. Several investigations have revealed that the prepartum vaginal temperature decreases with the regression of the pregnant corpus luteum, begins to rise during the second stage of parturition, and subsequently returns to approximately pre-decline levels at the time of calving [23, 26, 28]. The increase in vaginal temperature that occurs after the decrease coincides with the onset of an increase in activity associated with calving labor, suggesting that the increase in vaginal temperature just prior to calving is caused by labor [28]. Oxytocin is a hormone that induces labor [29], and low oxytocin levels have been suggested to be involved in the development of RP [30]. Oxytocin levels in the placenta after parturition are significantly lower in RP cows than in non-RP cows [31].
It has further been reported that estrogen concentrations on the day of calving are lower in RP cows compared to normal non-RP cows [4, 13]. The E2:P4 ratio is important for the expression of oxytocin receptors and the induction of labor, reaching a maximum level during labor [29]. However, it is also known that RP is suppressed by an increase in exogenous or endogenous estrogen concentration following induced parturition treatment, which often results in RP [32, 33]. These observations suggest that RP cattle have lower estrogen and higher P4 levels than non-RP cattle, resulting in inadequate placental maturation. Low oxytocin levels cause weak labor, while cows with low oxytocin levels may experience inadequate uterine contractions and poor placental abruption. As such, we speculate that the lack of a vaginal temperature increase in the second stage of parturition in RP cattle in the present study was likely due to low oxytocin-induced labor weakness. However, we did not measure the hormonal concentrations or activity of the cattle using pedometers during calving in this study, and the relationship between these indicators and vaginal temperature changes could not be verified. As such, further research is required to confirm this hypothesis.
Multiple risk factors for RP have previously been reported, including dystocia [15, 34,35,36], stillbirth [36], short gestation period [15, 35] and multiple births [35, 36]. Interestingly, our present analyses demonstrated no significant differences in the gestation period, rate of twins, or sex ratio or body weight of calves between RP and non-RP cattle (Table 2). Furthermore, due to the small number of cases in this study, it was not possible to analyze the relationship between twin delivery, calving duration, and PE time. In addition, none of the cows that developed RP developed dystocia. These results indicate that the RP that occurred in this study may have occurred in mild deliveries that were not accompanied by severe labor pain. As such, the placenta was delivered in a shorter time than expected, resulting in insufficient placental maturation, detachment, and expulsion. Further studies are required to investigate the effects of twin delivery and dystocia on calving duration and PE time.
The significantly higher TCHO and BHBA values in the serum of RP cattle (Table 3) suggests the possibility of energy deficiency prior to calving in RP cattle. This result is consistent with reports that BHBA and NEFA levels are high in RP cows, indicating a negative energy balance (NEB), and that NEB cows are prone to immunodeficiency [5, 9, 10, 37]. Nutritional stress increases the secretion of cortisol in prepartum cows suffering from NEB compared with healthy cows [10]. Decreased immune function associated with increased cortisol levels is thought to decrease neutrophil migration, resulting in inadequate placental abruption and RP. Differences in liver enzymes were also observed between the non-RP and RP cows, indicating that there may have been between-group differences in steroid metabolism in the liver, which may have affected hormone secretion and led to calving progress.
In conclusion, in the present study, we observed that the extension of PE time was dependent on the shortening of the calving duration. The pattern of vaginal temperature changes varied in a time-dependent manner with placental expulsion, particularly following the onset of labor. Our findings indicate that measuring vaginal temperature before calving is likely to help predict the occurrence of RP. Body temperature changes reflect changes in hormone concentrations and metabolic heat, indicating that the characteristic vaginal temperature changes in cases of RP may be related to the abnormal secretion of sex hormones that are involved in the onset of labor. Although further studies are needed (such as investigations of the relationship between behavior during labor and the secretion of sex hormones), the results of the present study suggest the possibility of developing technology to reduce calving accidents by predicting the occurrence of a retained placenta.
Conflicts of interests
The authors declare no conflicts of interest.
Acknowledgments
We would like to thank the Animal Care Staff of the Institute of Livestock and Grassland Science for their assistance with sampling and data collection. This research was financially supported by a Research Grant from the Japan Association for Livestock New Technology (no. 2021-10), as well as a grant from the Japan Society for the Promotion of Science (JSPS) KAKENHI (no. JP21H02349).
References
- 1.Kimura K, Goff JP, Kehrli ME, Jr, Reinhardt TA. Decreased neutrophil function as a cause of retained placenta in dairy cattle. J Dairy Sci 2002; 85: 544–550. [DOI] [PubMed] [Google Scholar]
- 2.Attupuram NM, Kumaresan A, Narayanan K, Kumar H. Cellular and molecular mechanisms involved in placental separation in the bovine: A review. Mol Reprod Dev 2016; 83: 287–297. [DOI] [PubMed] [Google Scholar]
- 3.Chebel RC. Predicting the risk of retained fetal membranes and metritis in dairy cows according to prepartum hemogram and immune and metabolic status. Prev Vet Med 2021; 187: 105204. [DOI] [PubMed] [Google Scholar]
- 4.Grunert E, Ahlers D, Heuwieser W. The role of endogenous estrogens in the maturation process of the bovine placenta. Theriogenology 1989; 31: 1081–1091. [DOI] [PubMed] [Google Scholar]
- 5.Qu Y, Fadden AN, Traber MG, Bobe G. Potential risk indicators of retained placenta and other diseases in multiparous cows. J Dairy Sci 2014; 97: 4151–4165. [DOI] [PubMed] [Google Scholar]
- 6.Domecq JJ, Skidmore AL, Lloyd JW, Kaneene JB. Relationship between body condition scores and conception at first artificial insemination in a large dairy herd of high yielding Holstein cows. J Dairy Sci 1997; 80: 113–120. [DOI] [PubMed] [Google Scholar]
- 7.Drillich M, Mahlstedt M, Reichert U, Tenhagen BA, Heuwieser W. Strategies to improve the therapy of retained fetal membranes in dairy cows. J Dairy Sci 2006; 89: 627–635. [DOI] [PubMed] [Google Scholar]
- 8.Amin YA, Hussein HA. Latest update on predictive indicators, risk factors and ‘Omic’ technologies research of retained placenta in dairy cattle - A review. Reprod Domest Anim 2022; 57: 687–700. [DOI] [PubMed] [Google Scholar]
- 9.LeBlanc SJ. Postpartum uterine disease and dairy herd reproductive performance: a review. Vet J 2008; 176: 102–114. [DOI] [PubMed] [Google Scholar]
- 10.Mordak R, Stewart PA. Periparturient stress and immune suppression as a potential cause of retained placenta in highly productive dairy cows: examples of prevention. Acta Vet Scand 2015; 57: 84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agthe O, Kolm HP. Oestrogen and progesterone levels in the blood plasma of cows with normal parturition or with a retained placenta. J Reprod Fertil 1975; 43: 163–166. [DOI] [PubMed] [Google Scholar]
- 12.Peter AT, Bosu WTK. Peripartal endocrine changes associated with retained placenta in dairy cows. Theriogenology 1987; 28: 383–394. [DOI] [PubMed] [Google Scholar]
- 13.Moradi M, Zhandi M, Sharafi M, Akbari A, Atrabi MJ, Totonchi M. Gene expression profile of placentomes and clinical parameters in the cows with retained placenta. BMC Genomics 2022; 23: 760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fourichon C, Seegers H, Malher X. Effect of disease on reproduction in the dairy cow: a meta-analysis. Theriogenology 2000; 53: 1729–1759. [DOI] [PubMed] [Google Scholar]
- 15.Han IK, Kim IH. Risk factors for retained placenta and the effect of retained placenta on the occurrence of postpartum diseases and subsequent reproductive performance in dairy cows. J Vet Sci 2005; 6: 53–59. [PubMed] [Google Scholar]
- 16.Dubuc J, Duffield TF, Leslie KE, Walton JS, Leblanc SJ. Effects of postpartum uterine diseases on milk production and culling in dairy cows. J Dairy Sci 2011; 94: 1339–1346. [DOI] [PubMed] [Google Scholar]
- 17.Pinedo P, Santos JEP, Chebel RC, Galvão KN, Schuenemann GM, Bicalho RC, Gilbert RO, Rodriguez-Zas SL, Seabury CM, Rosa G, Thatcher W. Associations of reproductive indices with fertility outcomes, milk yield, and survival in Holstein cows. J Dairy Sci 2020; 103: 6647–6660. [DOI] [PubMed] [Google Scholar]
- 18.Magata F, Sone A, Watanabe Y, Deguchi Y, Aoki T, Haneda S, Ishii M. Prevention of retained fetal membranes and improvement in subsequent fertility with oxytocin administration in cows with assisted calving. Theriogenology 2021; 176: 200–205. [DOI] [PubMed] [Google Scholar]
- 19.Dervishi E, Zhang G, Hailemariam D, Dunn SM, Ametaj BN. Occurrence of retained placenta is preceded by an inflammatory state and alterations of energy metabolism in transition dairy cows. J Anim Sci Biotechnol 2016; 7: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aoki M, Kimura K, Suzuki O. Predicting time of parturition from changing vaginal temperature measured by data-logging apparatus in beef cows with twin fetuses. Anim Reprod Sci 2005; 86: 1–12. [DOI] [PubMed] [Google Scholar]
- 21.Burfeind O, Suthar VS, Voigtsberger R, Bonk S, Heuwieser W. Validity of prepartum changes in vaginal and rectal temperature to predict calving in dairy cows. J Dairy Sci 2011; 94: 5053–5061. [DOI] [PubMed] [Google Scholar]
- 22.Sakatani M, Sugano T, Higo A, Naotsuka K, Hojo T, Gessei S, Uehara H, Takenouchi N. Vaginal temperature measurement by a wireless sensor for predicting the onset of calving in Japanese Black cows. Theriogenology 2018; 111: 19–24. [DOI] [PubMed] [Google Scholar]
- 23.Lammoglia MA, Bellows RA, Short RE, Bellows SE, Bighorn EG, Stevenson JS, Randel RD. Body temperature and endocrine interactions before and after calving in beef cows. J Anim Sci 1997; 75: 2526–2534. [DOI] [PubMed] [Google Scholar]
- 24.Suthar VS, Burfeind O, Patel JS, Dhami AJ, Heuwieser W. Body temperature around induced estrus in dairy cows. J Dairy Sci 2011; 94: 2368–2373. [DOI] [PubMed] [Google Scholar]
- 25.Sakatani M, Takahashi M, Takenouchi N. The efficiency of vaginal temperature measurement for detection of estrus in Japanese Black cows. J Reprod Dev 2016; 62: 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakatani M, Sawado R, Miwa M, Hojo T, Tanaka M, Takenouchi N. Vaginal temperature before calving assessed with wireless vaginal temperature sensor in dairy and beef cattle. Theriogenology 2021; 172: 230–238. [DOI] [PubMed] [Google Scholar]
- 27.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 2013; 48: 452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miwa M, Matsuyama S, Nakamura S, Noda K, Sakatani M. Prepartum change in ventral tail base surface temperature in beef cattle: comparison with vaginal temperature and behavior indices, and effect of ambient temperature. J Reprod Dev 2019; 65: 515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fuchs AR, Helmer H, Behrens O, Liu HC, Antonian L, Chang SM, Fields MJ. Oxytocin and bovine parturition: a steep rise in endometrial oxytocin receptors precedes onset of labor. Biol Reprod 1992; 47: 937–944. [DOI] [PubMed] [Google Scholar]
- 30.Kotwica G, Janowski T, Zdunczyk S, Ras A. Oxytocin plasma levels in cows with normal parturition or dystocia and with placental retention. Exp Clin Endocrinol 1990; 95: 203–209. [DOI] [PubMed] [Google Scholar]
- 31.Takagi M, Fujimoto S, Ohtani M, Miyamoto A, Wijagunawardane MPB, Acosta TJ, Miyazawa K, Sato K. Bovine retained placenta: hormonal concentrations in fetal and maternal placenta. Placenta 2002; 23: 429–437. [DOI] [PubMed] [Google Scholar]
- 32.Garverick HA, Day BN, Mather EC, Gomez L, Thompson GB. Use of estrogen with dexamethasone for inducing parturition in beef cattle. J Anim Sci 1974; 38: 584–590. [DOI] [PubMed] [Google Scholar]
- 33.LaVoie VA, Moody EL. Estrogen pre-treatment of corticoid induced parturition in cattle. J Anim Sci 1973; 37: 770–775. [DOI] [PubMed] [Google Scholar]
- 34.Correa MT, Curtis CR, Erb HN, Scarlett JM, Smith RD. An ecological analysis of risk factors for postpartum disorders of Holstein-Friesian cows from thirty-two New York farms. J Dairy Sci 1990; 73: 1515–1524. [DOI] [PubMed] [Google Scholar]
- 35.Echternkamp SE, Gregory KE. Effects of twinning on gestation length, retained placenta, and dystocia. J Anim Sci 1999; 77: 39–47. [DOI] [PubMed] [Google Scholar]
- 36.Ghavi Hossein-Zadeh N, Ardalan M. Cow-specific risk factors for retained placenta, metritis and clinical mastitis in Holstein cows. Vet Res Commun 2011; 35: 345–354. [DOI] [PubMed] [Google Scholar]
- 37.Esposito G, Irons PC, Webb EC, Chapwanya A. Interactions between negative energy balance, metabolic diseases, uterine health and immune response in transition dairy cows. Anim Reprod Sci 2014; 144: 60–71. [DOI] [PubMed] [Google Scholar]