Abstract
The adenohypophysis is composed of the anterior and intermediate lobes (AL and IL, respectively), and secretes hormones that play an important role in reproduction. CD9- and SOX2-double (CD9/SOX2) positive cells located in the marginal cell layer (MCL) facing the Rathke’s cleft in the AL and IL form the primary stem cell niche in the adult adenohypophysis of rats. In this study, we successfully obtained 3-dimensional (3D) cell aggregates that closely resembled the primary niche of MCL in vivo. After incubation in a Matrigel containing several growth factors, approximately 20% of the cells in the CD9/SOX2-positive cell aggregates were differentiated into hormone-producing cells. The cell aggregates generated in this study may provide insight into the regulation of the pituitary stem/progenitor cell niche and the turnover of hormone-producing cells.
Keywords: CD9, Hanging drop three-dimensional cell culture, Intermediate lobe, Pituitary gland, Stem cells
The pituitary gland secretes hormones that play important roles in maintaining homeostasis, including growth, metabolism, stress response, and reproduction. The mammalian pituitary gland is composed of two anatomically distinct regions, the adenohypophysis and neurohypophysis. The adenohypophysis is composed of the anterior and intermediate lobes (AL and IL, respectively; note that primate IL is rudimentary). AL secretes six hormones, namely growth hormone (GH), prolactin (PRL), thyroid-stimulating hormone (TSH), luteinizing hormone (LH), follicle-stimulating hormone (FSH), and adrenocorticotrophic hormone (ACTH). In contrast, the IL only secretes alpha-melanocyte-stimulating hormone (αMSH). AL and IL contain not only hormone-producing cells but also non-hormone secreting cells, such as S100β-positive cells.
The interaction of stem cells with their particular microenvironment, called “niche,” is crucial for regulating their activity. Niches provide growth factors, cell-surface proteins, and extracellular matrix molecules to sustain stem and progenitor cell stemness [1]. The stem cell marker sex-determining region Y-box 2 (SOX2) is expressed in cells located along the marginal cell layer (MCL) facing the Rathke’s cleft in the AL and IL, forming the primary niche in the adult adenohypophysis [2,3,4,5]. The SOX2-positive cell clusters scattered throughout the parenchyma are proposed to act as secondary stem/progenitor cell niches [2,3,4, 6]. Previously, we found that the tetraspanin superfamily protein CD9 is a novel specific marker for a subpopulation of SOX2-positive cells in the MCL [7] and successfully isolated SOX2-positive cells from the MCL of the IL using a combination of an anti-CD9 antibody and the pluriBead-cascade cell isolation system. We found that a subset of the isolated CD9-, CD81-, S100β-, and SOX2-quadruple positive (CD9/CD81/S100β/SOX2-positive) cells showed sphere formation capacity and had the potential to differentiate into hormone-producing cells of the AL [8]. Pituisphere formation capacity was higher in cells derived from the IL than in those derived from the AL. Thus, CD9-positive cell clusters in the MCL of the IL exhibited higher stemness than those in the AL [8,9,10,11].
Sphere-forming assays have been used to analyze the characteristics of stem/progenitor cells in AL and IL [8, 12]. Because spheres are formed by activating a single proliferative stem/progenitor cell [13, 14], this assay is useful for evaluating single stem/progenitor cells. However, this assay does not resemble the in vivo-like microenvironment of the MCL in the anterior pituitary, where heterogeneous stem/progenitor cells, including quiescent stem cells, exist. Additionally, it takes 5–7 days to obtain pituitary spheres. Cell aggregates that preserve the fine structural features of the niche are required to understand the regulation of differentiation of SOX2-positive stem/progenitor cells into hormone producing cells. Tsukada et al. (2013) used the hanging drop 3D cell culture method to demonstrate that aggregates of isolated anterior pituitary cells closely resemble the cellular and subcellular organization of the rat anterior pituitary in vivo [15]. This spherical cell aggregate is formed in a droplet under gravity within 3–5 days [15]. Therefore, in the present study, we applied hanging drop 3D cell culture to CD9-positive cells from the IL side of MCL and examined whether they differentiated into hormone-producing cells in an in vivo-like primary niche.
Fig. 1A shows the pituitary gland cryosections from male rats (8 weeks old) stained with hematoxylin and eosin. The MCL faces Rathke’s cleft between the AL and IL (Fig. 1A). In the adult anterior pituitary, CD9/SOX2-positive cells have been identified in primary (MCL) and secondary (parenchyma) stem/progenitor cell niches [7]. Therefore, we characterized the cells in the MCL of the IL using immunohistochemistry. We observed that CD9-positive cells in the MCL of the IL did not colocalize with pro-opiomelanocortin (POMC) (Fig. 1B; in the IL, POMC gives rise to a bioactive peptide via a series of enzymatic steps, yielding αMSH). We found that the proportions of CD9- and SOX2-positive cells in MCL of the IL were 96.2% and 90.7%, respectively, and the proportion of CD9- and SOX2-double (CD9/SOX2)-positive cells in CD9-positive cells of the IL was 97.3% (Fig. 1B). CD9-positive cells were absent from the PL (Fig. 1B). Immunopositive cells were not observed when the pre-absorbed antibody was used (data not shown).
Fig. 1.
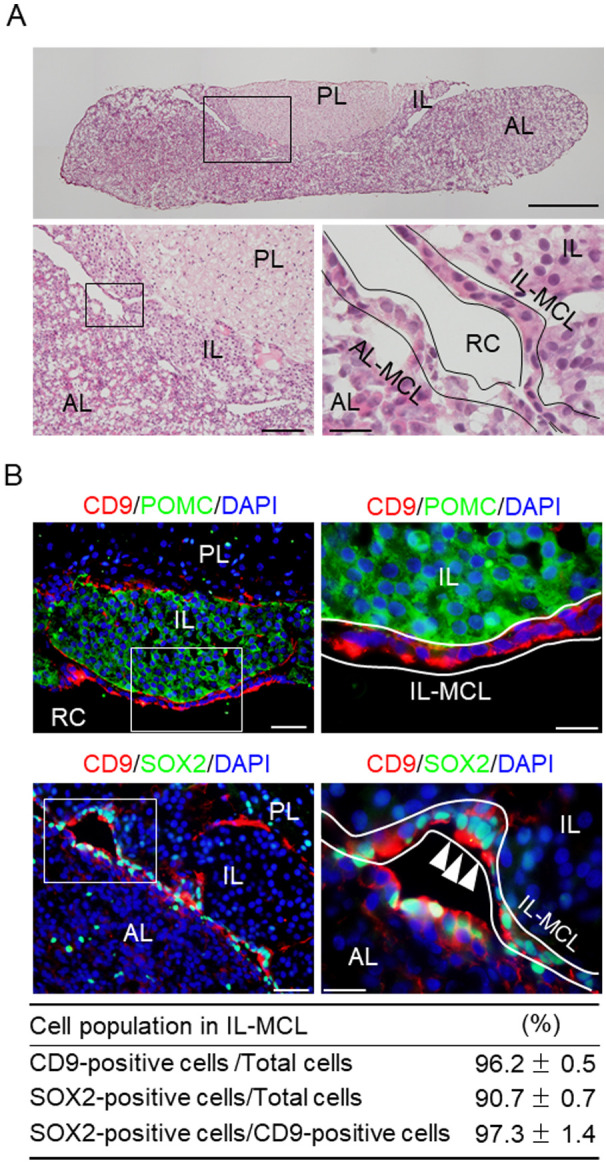
CD9-positive cells in the marginal cell layer (MCL) of the intermediated lobe (IL) in the rat pituitary gland. (A) Hematoxylin and eosin staining of a cryosection of the pituitary gland of a male adult rat. Lower panels: Higher magnification of the boxed area in the upper (left) and left panels (right). (B) Merged images of 4’,6-diamidino-2-phenylindole (DAPI) staining (blue) and double immunofluorescence staining for CD9 (red) and POMC (upper panels, green) or SOX2 (lower panels, green). The right panels show high-magnification images of the boxed areas in the left panels. White arrowheads indicate CD9/SOX2-positive cells. Proportions of CD9-or SOX2-positive cells among DAPI-positive cells or SOX2-positive cells among CD9-positive cells in a rectangular area (157.5 × 210 μm) in the MCL of the IL are shown in the bottom table (mean ± SEM, n = 5). PL, posterior lobe. AL-MCL, MCL of AL. IL-MCL, MCL of IL. RC, Rathke’s cleft. Scale bars: 500 μm (upper panel of A), 100 μm (lower left panel of A), 50 μm (lower right panel of A and upper and lower left panels of B), and 20 μm (lower right panel of A, upper and lower right panels of B).
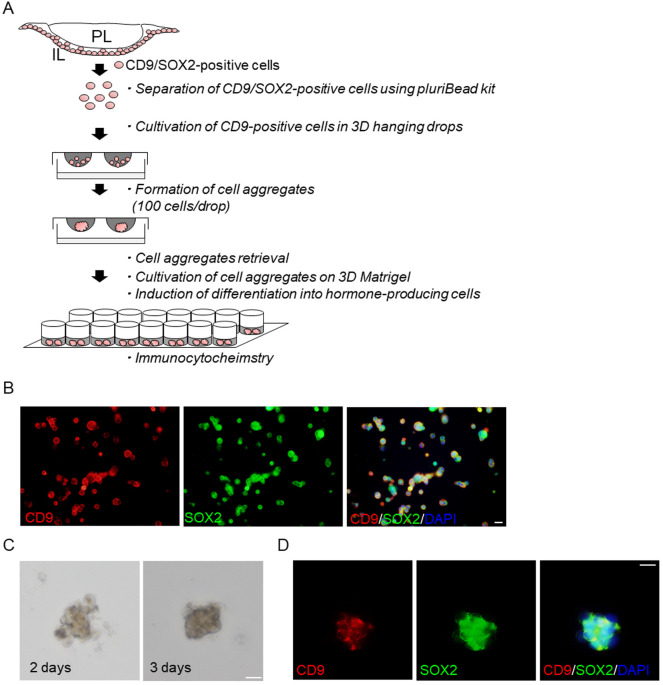
Next, we purified CD9-positive cells from the MCL of the IL using a monoclonal anti-rat CD9 antibody combined with a Pluribead cascade cell isolation system. A schematic of the experimental protocol for the isolation of CD9-positive cells and hanging-drop cultures is shown in Fig. 2A. Smears of the isolated CD9-positive cells were prepared for CD9 and SOX2 double-immunofluorescence staining (Fig. 2B). Most cells in the CD9-positive fraction were immunopositive for both CD9 and SOX2 (Fig. 2B). Previously, we showed that the proportion of CD9-positive cells in the isolated CD9-positive cell fraction was over 90% [8]. Purified CD9-positive cells settled at the center of the drop under gravity (1 drop = 100 cells) and formed a spherical cell aggregate approximately 50–100 μm in diameter within 2–3 days (Fig. 2C). After three days, the aggregates formed a round/oval shape and were fixed for subsequent staining (Fig. 2C). Most cells in the aggregates were immunopositive for CD9 and SOX2 (Fig. 2D). Tsukada et al. (2013) applied the hanging drop 3D technique to a conventional primary monolayer cell culture and showed that this in vitro 3D model of the AL successfully displayed in vivo characteristics, such as the fine structural features of hormone-producing cells, S100β-positive cells, and extracellular matrix deposition; all of which are observed in the anterior pituitary in vivo [15]. The present study demonstrated that cell aggregates were formed by heterogeneous IL-MCL cells in a shorter culture period compared to the former sphere-forming assay, in which homogeneous cell aggregates were formed within 5–7 days. Thus, the 3D culture method can expeditiously analyze CD9-positive cells in a microenvironment resembling the cellular and subcellular organization of the MCL of the pituitary IL.
Fig. 2.
Isolation, cell aggregate formation, and induction of CD9-positive cells from the marginal cell layer of the intermediate lobe (IL) in the pituitary gland of adult male rats. (A) Schematic of hanging drop 3D culture using isolated CD9-positive cells and subsequent induction procedures. (B) Double immunofluorescence staining for CD9 (left panels) and SOX2 (middle panel), and a merged image with DAPI staining (right panel) in smears of CD9-positive cells. (C) Bright fields images after 2 and 3 days of hanging drop 3D culture. (D) Double immunofluorescence staining for CD9 (left panels) and SOX2 (middle panel), and a merged image with DAPI staining (right panel) of cell aggregates obtained from hanging drop 3D cell culture. PL, posterior lobe. Scale bars: 20 μm (B, C and D).
We examined the differentiation of CD9-positive cells from the MCL of IL using an in vitro 3D model. Fig. 2A shows a schematic representation of the differentiation assay, which was performed after hanging drop 3D cell culture of CD9-positive cells from the MCL of the IL. The cell aggregates were manually collected using pipettes. The collected cell aggregates consisted of approximately 10–30 4’,6-diamidino-2-phenylindole (DAPI)-stained cells. Differentiation assays for cell aggregates were carried out using a method established for pituispheres using Matrigel-coated glass slides and serum-free medium containing basic fibroblast growth factor (bFGF), epidermal growth factor (EGF), KnockOut Serum Replacement (KSR), and glycogen synthase kinase 3β (GSK3β) inhibitor [8, 16]. After induction, we observed CD9-negative/hormone-positive cells in CD9-positive cell aggregates (Fig. 3). These findings indicate that isolated CD9/SOX2-positive cells in aggregates derived from the primary niche of MCL in the IL can differentiate into hormone-producing cells. Even after induction, some CD9/SOX2-positive cells remained undifferentiated. Thus, a primary niche-like environment may assist CD9/SOX2-positive cells in maintaining stemness. Although it has been reported that 3D aggregates using isolated IL cells, named pituispheres, can differentiate into hormone-producing cells, they require skill in isolating the pituisphere and a longer culture period (5–7 days) in serum-free conditions and do not form a primary niche-like environment. Thus, this study provides an easier and more efficient method for characterizing stem/progenitor cells isolated from MCL in a 3D context.
Fig. 3.
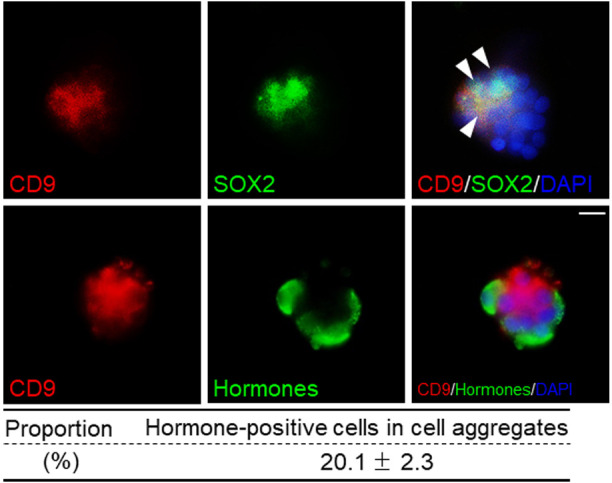
Differentiation capacity of CD9-positive cells isolated from the marginal cell layer of the intermediate lobe in 3D culture. Double immunofluorescence staining for CD9 (red) and SOX2 (upper panels, green) in CD9-positive cell aggregates after induction of differentiation. Arrowheads indicate CD9/SOX2-positive cells. Double immunofluorescence staining for CD9 (red) and hormones (GH, PRL, TSHβ, LHβ, and ACTH, stained with a mixture of antibodies) (bottom panels, green) in CD9-positive cell aggregates after induction of differentiation. Proportion of hormone-positive cells in cell aggregate is shown in the bottom table (mean ± SEM, n = 12). Scale bar: 20 μm.
To compare the differentiation capacities of cell aggregates and pituitary spheres, immunocytochemistry for AL hormones was performed after 14 days of incubation in a differentiation medium. The average proportion of hormone-positive cells was 20.1 ± 2.3% in the cell aggregates (Fig. 3), while the proportion of hormone-producing cells with respect to total cells in IL-derived pituispheres was 24.2 ± 2.7% [8]. Thus, the differentiation capacity was slightly lower in the cell aggregates than in the pituitary spheres. Yoshida et al. (2016) successfully isolated parenchymal stem/progenitor cell (PS) clusters, but not MCL clusters. PS-clusters cultured using the 3D culture method with a serum-free overlay maintained their stemness, and treatment with bFGF and EGF induced cyst formation [16]. The average proportion of hormone-positive cells found in the PS-cluster was 16 ± 6% [16]. However, the differences between cell aggregates, pituitary spheres, and PS clusters remain unclear. A possible explanation for these differences is the presence of quiescent SOX2-positive cells. Most SOX2-positive cells are quiescent in adult pituitary [17]. The proportion of quiescent SOX2-positive cells may differ between the MCL of the IL and AL and the parenchyma of the AL. Another explanation could be the heterogeneity of CD9/SOX2-positive cells and their differentiation capacities. Immunocytochemistry using S100β antibody demonstrated that CD9-positive cells were immunopositive for S100β in the cell aggregates after the differentiation assay (Supplementary Fig. 1). Undifferentiated CD9-positive cells sustained S100β expression in cell aggregates derived from the IL (Supplementary Fig. 1). Further studies involving comparative analyses of the pituisphere and cell aggregates may elucidate their hierarchy in the differentiation of niche cells.
In conclusion, we demonstrated a simple method for isolating stem cell aggregates of CD9/SOX2-positive pituitary stem/progenitor cells originating from the MCL of IL. CD9/SOX2-positive cells were capable of differentiation upon incubation with Matrigel containing the GSK3β-inhibitor, bFGF, and EGF. Further analysis of CD9/SOX2-positive cells in these cell aggregates can provide insights into the regulation of the pituitary stem/progenitor cell niche as well as the turnover of hormone-producing cells. For instance, this isolation method can be used to investigate the stability and changes in CD9-positive cell populations during development and between the sexes. Single-cell RNA-seq of these samples can identify factors influencing cell fate determination. Several groups have reported protocols for generating hormone-producing cells from induced pluripotent stem (iPS) cells in vitro [18,19,20]. However, the induction of iPS cells requires more than 5 weeks in both 2D and 3D cultures. Comparing the cellular characteristics of CD9/SOX2-positive cells with those of iPS cells may provide important insights for improving the induction rate of iPS cells in the future.
Materials and Methods
Animals
Adult Wistar rats were purchased from Japan SLC, Inc. (Shizuoka, Japan). Eight- to ten-week-old male rats weighing 180–220 g were provided ad libitum access to food and water and housed under a 12-h light/dark cycle. Rats were anesthetized with a combination of medetomidine (0.15 mg/kg; Zenyaku Kogyo, Tokyo, Japan), midazolam (2.0 mg/kg; Sandoz, Tokyo, Japan), and butorphanol (2.5 mg/kg; Meiji Seika Pharma, Tokyo, Japan), and euthanized by exsanguination from the right atrium. The rats were then perfused with Hanks’ balanced salt solution (Thermo Fisher Scientific, Waltham, CA, USA) in order to isolate CD9-positive cells from the IL or with 4% paraformaldehyde in 0.05 M phosphate buffer (PB; pH 7.4) for immunohistochemistry. All experimental protocols involving animals were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and approved by the Committee on Animal Experiments of Kyorin University.
Immunohistochemistry and immunocytochemistry
Cryosection: Tissue specimens were prepared for sectioning as described previously [7]. The pituitary glands of rats were immediately immersed in a fixative consisting of 4% paraformaldehyde in 0.05 M PB (pH 7.4) for 20–24 h at 4°C. Specimens were incubated in 0.05 M PB (pH 7.2) containing 30% sucrose at 4°C for more than two days. They were then embedded in Tissue-Tek O.C.T. compound (Sakura Finetek Japan, Tokyo, Japan) and frozen rapidly. Frozen frontal sections (8 μm in thickness) were obtained using a cryostat (Tissue-Tek Polar DM, Sakura Finetek Japan) and mounted on glass slides (Matsunami, Osaka, Japan).
Immunohistochemistry and immunocytochemistry: Immunohistochemistry was performed as previously described [7]. The primary and secondary antibodies used are listed in Supplementary Table 1. The absence of an observable nonspecific reaction was confirmed using normal mouse, rabbit, or goat serum (Vector Laboratories, Burlingame, CA, USA). The sections were scanned using an epifluorescence microscope (BX61; Olympus, Tokyo, Japan) with a CellSens Dimension system (Olympus). For double immunofluorescence, sections were incubated with phosphate-buffered saline (PBS; pH 7.2) containing 2% normal goat or donkey serum (Vector Laboratories) for 20 min at 30°C. Nuclei were counterstained with VECTASHIELD Mounting Medium containing DAPI (Vector Laboratories). The absence of an observable nonspecific immunoreaction against CD9 was confirmed using a pre-absorption control. Briefly, the anti-CD9 antibody was pre-incubated with a CD9 peptide (ORB217044; Biobyt, Cambridge, U.K.) at a molar ratio for 2 days at 4°C and then centrifuged, and the resultant supernatant was used as the pre-absorbed antibody. For immunocytochemistry, cultured cells were fixed with 4% paraformaldehyde in 0.025 M PB for 20 min at 22–23°C and then permeabilized with 0.5% Triton X-100 (Sigma-Aldrich, St. Louis, MO, USA) for 2 min at room temperature. Cells were incubated with PBS containing 2% normal goat or donkey serum for 20 min at 30°C, followed by incubation with primary and secondary antibodies (Supplementary Table 1). After immunocytochemistry, six random fields (157.5 × 210 μm rectangle) were imaged per section using a 40X objective lens. The number of hormone-secreting cells and the total number of cells (DAPI-stained) per area were counted using the CellSens Dimension system. Cell counts were performed in triplicate for each experimental group.
Isolation of CD9-positive cells
CD9-positive cells were isolated from IL of the pituitary gland as previously described [8]. The pituitary glands of male Wistar rats were dissected, and the AL was manually separated from the pituitary gland using tweezers. IL/PL tissue specimens were enzymatically digested to disperse the cells, as previously described [10]. Dispersed cells were counted using a hemocytometer and CD9-positive cells were specifically isolated using a Universal Mouse pluriBeads kit (pluriSelect, San Diego, CA, USA) and a monoclonal anti-rat CD9 antibody (BD Biosciences, San Jose, CA, USA) [21, 22]. S-pluriBeads® (32 µm diameter) uses non-magnetic monodispersed microparticles (beads) for the separation of cell mixtures. The surfaces were coated with CD9 monoclonal antibodies directed against specific structures on the target cell surface. During incubation, CD9/SOX2-positive cells in suspension bind to the pluriBeads® and can be separated afterwards by an S-pluriStrainer® (30 µm pore size) from the suspension. CD9/SOX2-positive cells were detached from the beads using a detachment buffer directly on the strainer. The CD9/SOX2-positive cells were then washed in a fresh tube, while the depleted beads remained in the strainer.
Hanging drop three-dimensional (3D) cell culture
CD9-positive cells isolated from the MCL in the IL were suspended in M199 (Thermo Fisher Scientific) supplemented with 0.1% bovine serum albumin, 100 units/ml penicillin, and 100 μg/ml of streptomycin (Thermo Fisher Scientific). For 3D culture, a 25 µl-drop containing 100 cells was placed on the undersurface of plastic Petri dish lids (90 mm; Asahi Glass, Tokyo, Japan). The cells were then cultured in sterile PBS (hanging drop) for 3 days at 37°C in a humidified incubator with 5% CO2.
Differentiation of CD9-positive cell aggregation into hormone-producing cells in vitro
After 2–3 days of 3D cell culture, cell aggregates were collected manually using a pipette under a microscope. Differentiation of cell aggregates was performed using the overlay 3D culture method on Matrigel-coated 16-well chamber slides (0.4 cm2/well) (Thermo Fisher Scientific) with 20 ng/ml each of bFGF and EGF and 20% KSR (Thermo Fisher Scientific) for 4 days, followed by replacement with medium containing 6-bromoindirubin-3’-oxime (GSK3β-inhibitor, 250 nM; FUJIFILM Wako Pure Chemical Co., Osaka, Japan) and incubation for another 10 days.
Conflicts of interests
The authors have nothing to declare.
Supplementary
Acknowledgments
We thank the “Joint Usage/Research Centre for Endocrine/Metabolism, Institute for Molecular and Cellular Regulation, Gunma University” (www.imcr.gunma-u.ac.jp/activity/activity3) for providing the antibodies. This work was supported by a JSPS KAKENHI Grant (Nos. 19K07255 and 22K06798 to K.H.) and the Kyorin University Individual Investigator Award from the Faculty of Health Sciences, Kyorin University. We would like to thank Editage (www.editage.jp) for their English language editing services.
References
- 1.Chen CC, Chuong CM. Multi-layered environmental regulation on the homeostasis of stem cells: the saga of hair growth and alopecia. J Dermatol Sci 2012; 66: 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen J, Hersmus N, Van Duppen V, Caesens P, Denef C, Vankelecom H. The adult pituitary contains a cell population displaying stem/progenitor cell and early embryonic characteristics. Endocrinology 2005; 146: 3985–3998. [DOI] [PubMed] [Google Scholar]
- 3.Gremeaux L, Fu Q, Chen J, Vankelecom H. Activated phenotype of the pituitary stem/progenitor cell compartment during the early-postnatal maturation phase of the gland. Stem Cells Dev 2012; 21: 801–813. [DOI] [PubMed] [Google Scholar]
- 4.Vankelecom H, Chen J. Pituitary stem cells: where do we stand? Mol Cell Endocrinol 2014; 385: 2–17. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida S, Kato T, Kato Y. Regulatory system for stem/progenitor cell niches in the adult rodent pituitary. Int J Mol Sci 2016; 17: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshida S, Kato T, Susa T, Cai LY, Nakayama M, Kato Y. PROP1 coexists with SOX2 and induces PIT1-commitment cells. Biochem Biophys Res Commun 2009; 385: 11–15. [DOI] [PubMed] [Google Scholar]
- 7.Horiguchi K, Fujiwara K, Yoshida S, Nakakura T, Arae K, Tsukada T, Hasegawa R, Takigami S, Ohsako S, Yashiro T, Kato T, Kato Y. Isolation and characterisation of CD9-positive pituitary adult stem/progenitor cells in rats. Sci Rep 2018; 8: 5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horiguchi K, Fujiwara K, Takeda Y, Nakakura T, Tsukada T, Yoshida S, Hasegawa R, Takigami S, Ohsako S. CD9-positive cells in the intermediate lobe of the pituitary gland are important supplier for prolactin-producing cells in the anterior lobe. Cell Tissue Res 2021; 385: 713–726. [DOI] [PubMed] [Google Scholar]
- 9.Horiguchi K, Fujiwara K, Tsukada T, Nakakura T, Yoshida S, Hasegawa R, Takigami S. Differentiation of stem progenitor CD9/SOX2-positive cells is promoted with increased prolactin-producing and endothelial cells in the pituitary. J Reprod Dev 2022; 68: 278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horiguchi K, Fujiwara K, Tsukada T, Nakakura T, Yoshida S, Hasegawa R, Takigami S, Ohsako S. CD9-positive cells in the intermediate lobe migrate into the anterior lobe to supply endocrine cells. Histochem Cell Biol 2021; 156: 301–313. [DOI] [PubMed] [Google Scholar]
- 11.Horiguchi K, Tsutsui Y, Fujiwara K, Tsukada T, Nakakura T, Yoshida S, Hasegawa R, Takigami S. Fluctuation of CD9/SOX2-positive cell populations during the turnover of GH- and TSH-producing cells in the adult anterior pituitary gland. J Reprod Dev 2023; 69: 308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fauquier T, Rizzoti K, Dattani M, Lovell-Badge R, Robinson IC. SOX2-expressing progenitor cells generate all of the major cell types in the adult mouse pituitary gland. Proc Natl Acad Sci USA 2008; 105: 2907–2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gritti A, Parati EA, Cova L, Frolichsthal P, Galli R, Wanke E, Faravelli L, Morassutti DJ, Roisen F, Nickel DD, Vescovi AL. Multipotential stem cells from the adult mouse brain proliferate and self-renew in response to basic fibroblast growth factor. J Neurosci 1996; 16: 1091–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pastrana E, Silva-Vargas V, Doetsch F. Eyes wide open: a critical review of sphere-formation as an assay for stem cells. Cell Stem Cell 2011; 8: 486–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsukada T, Kouki T, Fujiwara K, Ramadhani D, Horiguchi K, Kikuchi M, Yashiro T. Reassembly of anterior pituitary organization by hanging drop three-dimensional cell culture. Acta Histochem Cytochem 2013; 46: 121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshida S, Nishimura N, Ueharu H, Kanno N, Higuchi M, Horiguchi K, Kato T, Kato Y. Isolation of adult pituitary stem/progenitor cell clusters located in the parenchyma of the rat anterior lobe. Stem Cell Res 2016; 17: 318–329. [DOI] [PubMed] [Google Scholar]
- 17.Andoniadou CL, Matsushima D, Mousavy Gharavy SN, Signore M, Mackintosh AI, Schaeffer M, Gaston-Massuet C, Mollard P, Jacques TS, Le Tissier P, Dattani MT, Pevny LH, Martinez-Barbera JP. Sox2+ stem/progenitor cells in the adult mouse pituitary support organ homeostasis and have tumor-inducing potential. Cell Stem Cell 2013; 13: 433–445. [DOI] [PubMed] [Google Scholar]
- 18.Dincer Z, Piao J, Niu L, Ganat Y, Kriks S, Zimmer B, Shi SH, Tabar V, Studer L. Specification of functional cranial placode derivatives from human pluripotent stem cells. Cell Rep 2013; 5: 1387–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zimmer B, Piao J, Ramnarine K, Tomishima MJ, Tabar V, Studer L. Derivation of diverse hormone-releasing pituitary cells from human pluripotent stem cells. Stem Cell Reports 2016; 6: 858–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kasai T, Suga H, Sakakibara M, Ozone C, Matsumoto R, Kano M, Mitsumoto K, Ogawa K, Kodani Y, Nagasaki H, Inoshita N, Sugiyama M, Onoue T, Tsunekawa T, Ito Y, Takagi H, Hagiwara D, Iwama S, Goto M, Banno R, Takahashi J, Arima H. Hypothalamic contribution to pituitary functions is recapitulated in vitro using 3D-cultured human iPS Cells. Cell Rep 2020; 30: 18–24.e5. [DOI] [PubMed] [Google Scholar]
- 21.Horiguchi K, Nakakura T, Yoshida S, Tsukada T, Kanno N, Hasegawa R, Takigami S, Ohsako S, Kato T, Kato Y. Identification of THY1 as a novel thyrotrope marker and THY1 antibody-mediated thyrotrope isolation in the rat anterior pituitary gland. Biochem Biophys Res Commun 2016; 480: 273–279. [DOI] [PubMed] [Google Scholar]
- 22.Horiguchi K, Yoshida S, Tsukada T, Nakakura T, Fujiwara K, Hasegawa R, Takigami S, Ohsako S. Expression and functions of cluster of differentiation 9 and 81 in rat mammary epithelial cells. J Reprod Dev 2020; 66: 515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.