Abstract
Gellan gum (GG) is a soft, tractable, and natural polysaccharide substrate used for cell incubation. In this study, we examined the effects of GG on porcine oocyte maturation. Cumulus cells and oocyte complexes (COCs) were collected from slaughterhouse-derived porcine ovaries and cultured on plastic plates containing 0.05% or 0.1% GG gels. The 0.1% GG gel improved the maturation rate and quality of blastocysts, as determined by the total cell number and the rate of abnormally condensed nuclei. GG gels have antioxidant abilities and oocytes cultured on GG gels (0.05% and 0.1%) have reduced reactive oxygen species (ROS) content. Furthermore, GG gels (0.05% and 0.1%) increased F-actin formation, whereas treatment of oocytes with H2O2 reduced F-actin levels. GG gels increased the ATP content in oocytes but did not affect the mitochondrial DNA copy number or mitochondrial membrane potential. In addition, the medium cultured on 0.05% GG increased the glucose consumption of COCs. In conclusion, GG gel reduced ROS content, increased energy content, and improved subsequent embryonic development in pigs.
Keywords: Antioxidant, F-actin, Gellan gum, Oocyte maturation
In vitro embryos are widely used in human-assisted reproductive technologies and livestock reproduction. However, embryos produced in vitro have relatively higher rates of chromosomal abnormalities and altered gene expression, and are associated with higher rates of pregnancy failure than embryos developed in vivo [1,2,3]. In vitro maturation is the first step in in vitro embryo production, and in-vitro-matured embryos have a lower rate of development to the blastocyst stage than that of in-vivo-matured embryos [4]. In vivo, oocytes mature on soft cellular materials, whereas in vitro oocytes mature on rigid plastic plates. In our previous study, a soft gel culture substrate made of the polysaccharides, xanthan gum (XG) and locust bean gum (LBG), improved embryonic development, increased actin polymerization in oocytes, and affected the expression of mitochondria-associated genes in oocytes [5]. However, the formation and properties of XG-LBG gels have several disadvantages. To form a gel, XG and LBG were mixed in appropriate ratios, but XG-LBG was poorly dissolved in water and formed an opaque gel, which was difficult to observe under a microscope. Gellan gum (GG) is a polysaccharide produced by Sphingomonas elodia, which forms a soft gel. The main advantages of GG are its ability to produce steady, transparent, malleable, and versatile textures that allow for adjustable gel elasticity and non-toxicity [6]. There are two types of GG gels: low-acyl and high-acyl GG. Low-acyl GG is the most transparent gel compared with other polysaccharide gels [7]. Therefore, low-acyl GG easily form gels that are optimal for microscopic observations. Recently, GG has been used for stem cell incubation [8, 9]; however, low-acyl GG has not been used for in vitro embryo production.
Oxidative stress caused by reactive oxygen species (ROS) causes DNA damage and apoptosis, and impairs embryonic development [10]. The accumulation of ROS in oocytes and embryos inhibits actin polymerization and reduces mitochondrial function and quantity [11, 12]. Therefore, establishing anti-ROS conditions is essential for in vitro embryo production. Polysaccharides contain many hydroxyl groups and are potential antioxidants. GG has consistently been reported to have antioxidant properties [13].
In this study, we examined the effects of GG gels on porcine oocyte maturation, ROS content, actin polymerization, mitochondrial function, and subsequent embryonic development.
Material and Methods
Porcine ovaries and collected oocytes
Porcine ovaries were collected from the slaughterhouse. Ovaries were transported to the laboratory in phosphate-buffered saline (PBS) containing antibiotics at 25°C within 2 h. Cumulus cell and oocytes complexes (COCs) were collected from antral follicle (3–5 mm in diameter) using a 21 G needle (Terumo, Tokyo, Japan) connected to a 10 ml syringe (Terumo). This study was approved by the Ethics Committee for Animal Experiments of Tokyo University of Agriculture (2023009).
Chemicals and media
All drugs used in this study were purchased from Nacalai Tesque (Kyoto, Japan), unless stated otherwise. The medium for in vitro maturation (IVM) of oocytes was porcine oocyte medium [14] containing 10% porcine follicle fluid, 0.5 mM L-cysteine, 10 ng/ml epidermal growth factor (Sigma-Aldrich, St. Louis, MO, USA), 10 IU/ml equine chorionic gonadotropin (Aska Pharma Co., Ltd., Tokyo, Japan), 10 IU/ml human chorionic gonadotropin (Fuji Pharma Co., Ltd., Tokyo, Japan), and 1 mM dibutyryl cAMP (dbcAMP) [15]. dbcAMP was added to the IVM medium during the first half of IVM (0–24 h). The medium for in vitro culture (IVC) of embryos was porcine zygote medium 3 (PZM3) [16]. IVM was performed under 5% CO2 and 95% air at 38.5°C, and IVC was performed at 5% O2, 5% CO2, and 90% N2 at 38.5°C.
Preparation of Gel and IVM
In the preliminary experiment, over 0.5% GG reduced the cleavage rate following parthenogenetic activation (Con, Gel [0.5%]; 70.6 ± 2.0a, 50.5 ± 3.2b) and that lower than 0.2% did not affect the rate. Therefore, in the present study, we examined 0.05% and 0.1% gels. GG (Sansho Co., Ltd., Osaka, Japan) was mixed in PBS (0.05% or 0.1%) and heated in a microwave oven until boiling (125°C, 20 min). Dissolved GG (300 µl) was added to a four-well plate. After cooling on ice, the dissolved gel formed an elastic gel. The gels were incubated with IVM medium overnight and the medium was replaced with fresh IVM medium before incubation with COCs. Collected COCs (50 COCs/500 µl) were cultured in IVM medium containing hormones (equine chorionic gonadotropin and human chorionic gonadotropin) for 24 h (referred to as the first step of IVM) and then these COCs were washed and incubated in IVM medium without hormones for 24 h (the later step).
Parthenogenetic activation (PA) of oocytes and in vitro culture of embryos
In vitro fertilization of porcine oocytes results in highly polyspermic fertilization, which is used to evaluate oocyte quality. Here, we used PA to examine the developmental ability of oocytes. After IVM, oocytes were subjected to parthenogenetic activation. The oocytes were denuded from surrounding cumulus cells (CCs) and activated in a solution containing 280 mM mannitol, 0.05 mM CaCl2, and 0.1 mM MgSO4 using a single electrical pulse of 100 V for 0.1 msec and a CUY500P1 electrode (NEPA21; NepaGene Ltd., Chiba, Japan) followed by incubation in PZM3 containing 10 µM cytochalasin B and 10 µM cycloheximide for 4.5 h. The embryos were then cultured in IVC medium (10 embryos/50 µl) for 7 days. Cleavage (> four-cell stage embryos) was determined 2 days after activation, and the blastulation rate and total cell number (TCN) of the blastocysts were evaluated 7 days after activation. The TCN was measured and abnormal chromatin condensation of the blastocysts was detected under a microscope (Keyence, Osaka, Japan) following Hoechst 33342 (Sigma; 5 µg/ml) staining.
Measurement of the antioxidant ability of the gel
PBS (300 µl) or 0.05% gel (300 µl) were mixed with PBS (300 µl) following heating at 80°C for 5 min. The antioxidant ability of the samples was measured using a commercial kit (Potential Antioxidant; Japan Institute for the Control of Aging, NIKKEN SEIL Co., Ltd., Shizuoka, Japan).
Measurement of ROS in oocytes
After IVM, oocytes were stained with 5 μM CMH2DCFDA (Thermo Fisher Scientific, Waltham, MA, USA) for 30 min and then washed three times with polyvinyl alcohol (PVA)-PBS three times. The oocytes were mounted on a depression slide and observed under a Leica DMI 6000 B microscope using the LAS AF software (Leica, Wetzlar, Germany). The fluorescence intensities of the equatorial regions of the oocytes were quantified using the ImageJ software (National Institutes of Health [NIH], Bethesda, MD, USA).
Evaluation of F-actin and actin cap
After IVM, oocytes were fixed in 4% paraformaldehyde in PBS overnight. The oocytes were then incubated in a permeabilization solution (0.25% Triton X/PBS) for 30 min, followed by F-actin staining and staining with Actin-stain 555 phalloidin (Cytoskeleton, Denver, CO, USA) for 30 min. They were then mounted on a glass slide and observed under a Leica DMI 6000 B microscope using the LAS AF software (Leica). The fluorescence intensities of the equatorial region of oocytes and the actin-condensed region (actin cap) were quantified using ImageJ software (Ver. 1.53).
Measurement of mitochondrial membrane potential
After IVM, oocytes were denuded from CCs and stained with 0.5 µM Mitotracker Orange CMTMRos (indicator of active mitochondria; Molecular Probes, Eugene, OR, USA) and 0.5 µM Mitotracker Green FM (indicator of all mitochondria; Molecular Probes) for 30 min. The oocytes were then washed thrice with PVA-PBS and mounted on a glass slides. Fluorescence images of whole oocytes were obtained under a Leica DMI 6000 B microscope using LAS AF software (Ver. 1.53), and the fluorescence intensities of the equatorial regions of the oocytes were quantified using ImageJ software. The value for red (active mitochondria) was normalized to that for green (whole mitochondria).
Mitochondrial DNA copy number (Mt-cn)
Mt-cn expression in oocytes was measured using quantitative PCR (qPCR). DNA of the oocytes was extracted by incubation in 20 µl of lysis buffer (final concentration: 20 mM Tris, 0.4 mg/ml proteinase K, 0.9% Nonidet P-40, and 0.9% Tween-20) at 55°C for 30 min, followed by incubation at 98°C for 10 min. PCR was conducted using the CFX ConnectTM Real-Time PCR system (Bio-Rad, Hercules, CA, USA), KAPA SYBER FAST qPCR Kit (Roche, Indianapolis, IN, USA), and specific primers. The primers used to determine the Mt-cn were 5′-ATCCAAGCACTATCCATCACCA -3′ and 5′-CCGATGATTACGTGCAACCC -3′ (155 bp, NC_000845.1). The PCR program was 95°C for 3 min, followed by 39 cycles of 97°C for 6 sec and 60°C for 10 sec. Standard DNA was used as a plasmid vector, in which the PCR product was cloned and sequenced. The copy number of the standard was calculated using Avogadro’s constant and the vector DNA concentration. All PCR efficiencies were > 1.99.
Measurement of ATP content
After IVM, oocytes were denuded and individually transferred into distilled water (50 µl) The adenosine triphosphate (ATP) content in individual oocytes was measured using an ATP assay kit (Toyo-Inc., Tokyo, Japan). The luminescence generated by the ATP-dependent luciferin-luciferase reaction was measured using a luminometer (Spark 10 M; Tecan Japan Co., Ltd., Kanagawa, Japan).
Glucose consumption
Fifty COCs were incubated in IVM medium (500 µl) with 300 µl of 0.1% or 0.05% GG gel. For the control groups, 50 COCs were incubated in IVM medium (500 µl) without GG gel. The gel (300 µl) was added to control medium and incubated in IVM medium without COCs. For the experimental group, spent IVM medium (500 µl) and the gel was used for measurements. The total sample volume was adjusted to 800 µl using gels (300 µl), followed by heating to melt the gel (80°C, 5 min). After heating, the samples were cooled to 25°C and analyzed using the LaboassayTM Glucose kit (Fujifilm, Tokyo, Japan). Glucose consumptions was calculated as the amount of glucose substrate in the spent culture medium compared with that in the original medium.
Experimental design
Experiment 1: This study addressed whether the application of GG gel during IVM affects oocyte developmental competence. The effects of the GG gels (0.05% and 0.1%) on IVM, the nuclear maturation rate, and embryonic development were examined. Approximately 20 oocytes were used for each replicate, and the experiments were performed nine (nuclear maturation rate) or 11 times (cleavage and blastulation rate).
Experiment 2: The antioxidant abilities of the GG gels were examined. In addition, the effect of the gels on ROS content in oocytes was examined. Approximately 30 oocytes were used in this experiment.
Experiment 3: This experiment addressed whether GG gel affects the levels of F-actin and the actin cap because F-actin is a target of oxidative stress [17]. Approximately 20 oocytes were used in the experiments.
In addition, the effect of supplementation of the IVM medium with H2O2 (Kanto Kagaku, Tokyo, Japan: 100 µM, 200 µM, and 300 µM) on F-actin content was examined. After COCs collection, the oocytes were denuded from the CCs and cultured without GG gels in IVM medium containing H2O2 for 5 h. Approximately 20 oocytes were used in the experiment.
Experiment 4: GG gels affect ROS content and F-actin, therefore, this study addressed the effects of the GG gels on Mt-cn, ATP content, and mitochondrial membrane potential (MMP) in oocytes. COCs were matured on 0%, 0.05%, and 0.1% gels, and oocytes were measured. Approximately 20–30 oocytes were used for each subject (ATP, Mt-cn, and MMP).
Experiment 5: GG gel increased ATP content, but not MMP in oocytes, therefore, the present study examined whether GG gel affects the metabolism of granulosa cells. COCs were matured on 0%, 0.05%, and 0.1% GG gels, and the spent culture medium was used for measurements. The effect of GG on glucose consumption by COCs was then examined. This experiment was performed six times.
Statistical analysis
The measured data were analyzed using the Shapiro–Wilk test, followed by the Tukey–Kramer test for parametric data. Nonparametric data were analyzed using the Kruskal–Wallis test, followed by the Steel–Dwass test. Maturation (M2), cleavage, and blastulation rates were arcsine-transformed before analysis. The antioxidant ability of the GG gel was analyzed using the Shapiro–Wilk test, followed by the Student’s t-test. All data are presented as the means.
Results
Experiment 1
Compared with plastic plates, the gel culture system (0.1% GG) improved the rate of nuclear maturation (Control; 72.3 ± 2.0 vs. 0.1% Gel; 80.3 ± 1.1, Table1, P < 0.05). The rates of cleavage and blastulation did not differ between the plastic plate and gel culture systems. Blastocysts derived from the 0.1% gel culture system had significantly higher TCNs (Control; 50.2 ± 2.9 vs. 0.1% Gel; 59.6 ± 3.2, Table1, P < 0.05), whereas both the 0.05% and 0.1% gel culture systems significantly reduced the rate of abnormal nuclear condensation in blastocysts compared with those of the plastic plate culture system (Control, 0.05% Gel, and 0.1% Gel; 9.9 ± 0.9, 6.2 ± 0.8, and 5.8 ± 0.9, respectively, Table 1, P < 0.05).
Table 1. Effect of gel system on oocyte maturation and subsequent embryonic development.
| Group |
In vitro maturation |
In vitro culture |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of |
M2 (%) | No. of |
Rate of (%) |
Blastocysts |
||||||||
| Trials | Oocytes | Trials | Oocytes | Cleaved | Blastocysts | No. | TCN | AC (%) | ||||
| Control | 9 | 175 | 72.3 ± 2.0 a | 11 | 227 | 70.6 ± 3.2 | 35.1 ± 1.9 | 44 | 50.2 ± 2.9 a | 9.9 ± 0.9 a | ||
| Gel (0.05%) | 9 | 171 | 75.5 ± 2.4 ab | 11 | 215 | 71.0 ± 4.1 | 31.8 ± 2.7 | 59 | 56.9 ± 3.1 ab | 6.2 ± 0.8 b | ||
| Gel (0.1%) | 9 | 178 | 80.3 ± 1.1 b | 11 | 217 | 72.6 ± 3.0 | 38.7 ± 2.6 | 59 | 59.6 ± 3.2 b | 5.8 ± 0.9 b | ||
Data were analyzed using the Steel–Dwass test. AC, abnormal condensation; M2, maturation rate; TCN, total cell number of the blastocyst. Date are presented as mean ± SEM. a-b P < 0.05.
Experiment 2
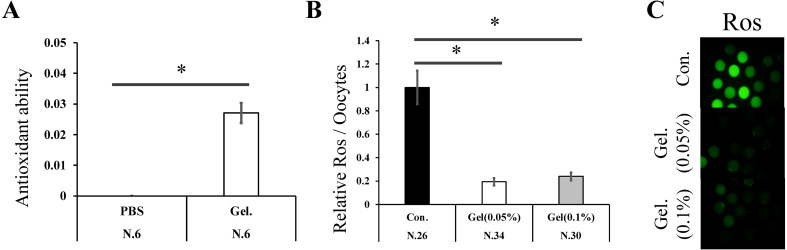
Compared to PBS, the gel culture system had a significantly higher antioxidant ability (PBS: 0 vs. Gel: 0.027, Fig. 1A, P < 0.05). Furthermore, oocytes matured on the gel culture system (0.05% and 0.1%) had lower ROS content than those matured on plastic plates (0.05% Gel; 0.19 fold, 0.1% Gel; 0.24 fold, Fig. 1B, P < 0.05).
Fig. 1.
The effect of a gellan gum gel (Gel) on the antioxidant ability and reactive oxygen species content of oocytes. The antioxidant abilities of the phosphate-buffered saline (PBS) and gellan gum (Gel) was measured. Y-axis: µM (A). Data were analyzed using Student’s t-test. Reactive oxygen species (ROS) levels in mature oocytes (B). Values for the control group (Con.) was defined as 1.0. Data were analyzed using the Steel–Dwass test. Representative images of ROS content in oocytes (C). Data are presented as the mean ± standard error of the mean (SEM), * P < 0.05. The number of samples is indicated in each figure.
Experiment 3
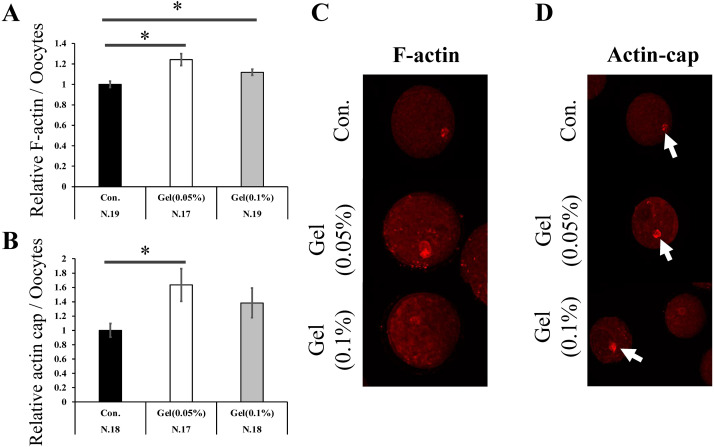
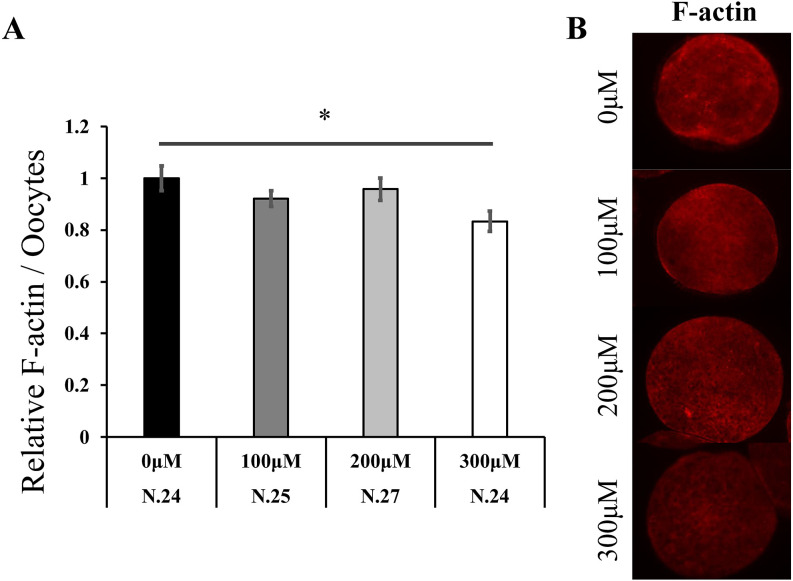
Oocytes matured on both 0.05% and 0.1% gel culture systems showed increased F-actin content compared to those matured on plastic plates (0.05% Gel; 1.24 fold, 0.1% Gel; 1.12 fold, Fig. 2A, P < 0.05). In addition, the 0.05% gel culture system increased actin cap formation (1.64 fold, Fig. 2B, P < 0.05). Immediately after collection, the oocytes were denuded from the surrounding CCs, treated with various concentrations of H2O2 for 5 h, and F-actin content in the oocytes was examined. High concentrations of H2O2 (300 µM) significantly reduced F-actin levels (0.83 fold, Fig. 3, P < 0.05).
Fig. 2.
The effect of the GG gel on actin polymerization in oocytes. F-actin (A) and actin cap (B) expression levels in mature oocytes. Data were analyzed using the Tukey–Kramer test. Values for the control group (Con.) was defined as 1.0. Representative images of F-actin (C) and actin cap (D) in oocytes. Arrow, actin cap. Data are presented as the mean ± SEM, * P < 0.05. The number of samples is indicated in each figure.
Fig. 3.
The effect of H2O2 on actin polymerization in oocytes. Denuded oocytes were cultured without (0 µM) or with H2O2 (100 µM, 200 µM, and 300 µM) for 5 h. F-actin expression in oocytes (A). Data were analyzed using the Tukey–Kramer test. The value for the 0 µM group was defined as 1.0. Representative images of F-actin expression in oocytes (B). Data are presented as the mean ± SEM, * P < 0.05. The number of samples is indicated in each figure.
Experiment 4
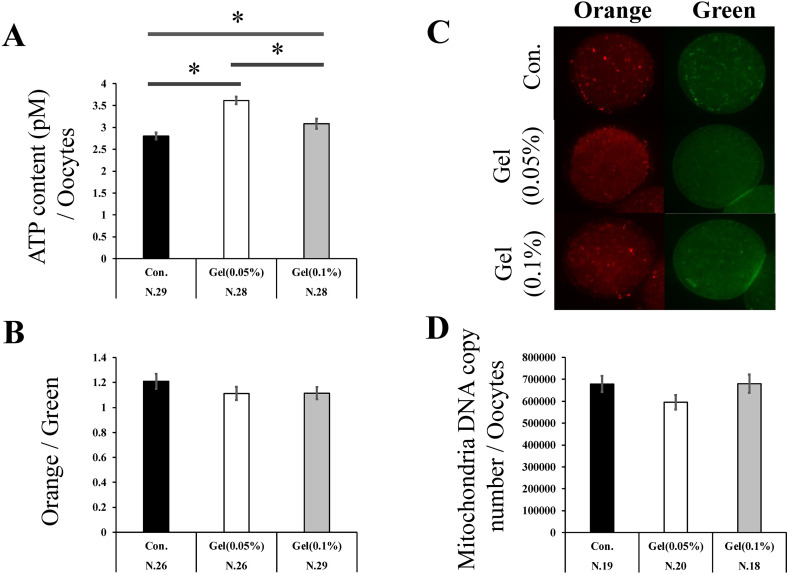
Oocytes matured on the gel culture system had significantly increased ATP content compared to those matured on the plastic plates (Control, 0.05% Gel, and 0.1% Gel; 2.81 ± 0.1, 3.62 ± 0.1, and 3.08 ± 0.1, respectively, Fig. 4A, P < 0.05), while the MMP did not differ among the groups (Figs. 4B, C). Mt-cn did not differ between the gel culture system and plastic plates (Fig. 4D).
Fig. 4.
The effect of the GG gel on mitochondrial functions in oocytes. Adenosine triphosphate (ATP) content of oocytes (A). The proportion of orange (active mitochondria)/green (whole mitochondria)-stained cells (B). Representative images of oocytes stained with MitoTracker Orange and Green (C). Mt-cn Oocytes (D). Mitochondrial membrane potential (MMP) and Mt-cn data were analyzed using the Steel–Dwass test. ATP data were analyzed using the Tukey–Kramer test. Data are presented as the mean ± SEM, * P < 0.05. The number of samples is indicated in each figure.
Experiment 5
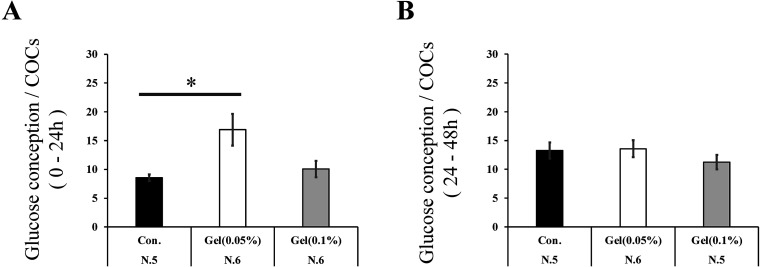
In the first step (0–24 h) of IVM, COCs matured on a 0.05% gel culture system showed significantly increased glucose consumption compared to those matured on plastic plates (1.98 fold, Fig. 5A, P < 0.05). During the latter step of IVM (24–48 h), glucose consumption did not differ between groups (Fig. 5B).
Fig. 5.
Effect of the gellan gum (GG) gel on glucose consumption. Glucose consumption (ratio of the initial concentration to the concentration of the spent culture medium) of COCs in the first (0–24 h: A) and later steps (24–48 h: B). Data were analyzed using the Tukey–Kramer test. Data are presented as the mean ± SEM, * P < 0.05. The number of samples is indicated in each figure. Y-axis: mg/dl.
Discussion
The present study showed that the application of GG for IVM improved the rate of maturation and subsequent development, with high TCN and low levels of abnormal chromatin condensation. GG has antioxidant ability, and oocytes matured on GG have low levels of ROS, which affects F-actin content. Furthermore, GG increased ATP content in oocytes and enhanced glucose consumption by COCs.
The effect of IVM on oocyte quality has generally been evaluated based on maturation rate and blastocyst quality. IVM rate and TCN are common markers, and condensed nuclei are markers of embryonic apoptosis [18]. The present study demonstrates the beneficial effects of GG gels on oocyte IVM. GG gels have previously been used for stem cell incubation [19]. However, the causal factors for the beneficial effects of GG have not yet been evaluated. In this study, we focused on the antioxidant properties of GG and their effects on oocyte mitochondria.
GG contains hydroxyl groups and is predicted to exhibit strong antioxidant properties. Our experiments also revealed that GG gels have antioxidant properties and reduce ROS content in oocytes. In embryos, ROS cause mitochondrial dysfunction, actin depolymerization, and apoptosis [11, 17, 20]. A variety of antioxidants have been used in IVM to avoid the harmful effects caused by excess ROS. For example, the supplementation of IVM medium with antioxidants, such as nobiletin, reduces ROS levels in oocytes and improves subsequent embryonic development [21, 22]. Hence, the GG gel is a useful culture system that reduces ROS levels and increases oocyte quality.
F-actin and actin caps play important roles in oocyte maturation [23]. They increase from the germinal vesicle stage to metaphase 2 [24]. The inhibition of actin polymerization reduces F-actin and actin cap formation in oocytes and subsequent nuclear maturation and embryonic development [25,26,27]. Actin polymerization occurs via ATP hydrolysis [28]. In addition, ROS decreases actin formation. High ATP and low ROS levels may support high actin levels in oocytes matured on the GG gel. The present study showed that GG increased ATP content, whereas no change was observed in Mt-cn or MMP, suggesting that the high ATP content was unlikely to be derived from high levels of ATP production in oocytes. Oocyte energy homeostasis depends not only on oocyte ATP production, but also on the provision of ATP from the surrounding CCs. The removal of CCs from COCs consistently reduces the ATP content and oocyte quality [29]. The 0.05% gel increased glucose consumption, the GG gel culture system may increase glycolytic activity and provide ATP to the oocytes.
Here, we focused on the effect of the GG gel on mitochondrial function; however, polysaccharide gels reportedly affect cellular metabolism and homeostasis [5, 30]. For example, the GG gel activates autophagy-related proteins in chondrocytes [31]. Autophagy is required for oocyte maturation [32]. Additionally, GG activates the extracellular matrix (ECM), and enhances integrin-mediated cell attachment [33]. CCs cultured on other polysaccharides (XG-LBG) affected the expression of genes associated with ECM interactions and improved subsequent embryonic development [5]. Therefore, other aspects of the beneficial effects of GG on oocytes and embryos should be examined in future studies.
The present study demonstrated the beneficial effects of GG. Compared to XG-LBG, the preparation of the GG gel is simple, and the GG gel is transparent, which is beneficial for observation. The results of the present study suggest that GG is useful materials which is applied for porcine embryo production.
Conflicts of interests
The authors declare that they have no conflict of interest.
Acknowledgments
This study was supported by the JSPS fellows (23KJ1954; S.A.).
References
- 1.Farin PW, Crosier AE, Farin CE. Influence of in vitro systems on embryo survival and fetal development in cattle. Theriogenology 2001; 55: 151–170. [DOI] [PubMed] [Google Scholar]
- 2.Leese HJ, Sturmey RG, Baumann CG, McEvoy TG. Embryo viability and metabolism: obeying the quiet rules. Hum Reprod 2007; 22: 3047–3050. [DOI] [PubMed] [Google Scholar]
- 3.Kassens A, Held E, Salilew-Wondim D, Sieme H, Wrenzycki C, Tesfaye D, Schellander K, Hoelker M. Intrafollicular oocyte transfer (IFOT) of abattoir-derived and in vitro-matured oocytes results in viable blastocysts and birth of healthy calves. Biol Reprod 2015; 92: 150. [DOI] [PubMed] [Google Scholar]
- 4.Rizos D, Ward F, Duffy P, Boland MP, Lonergan P. Consequences of bovine oocyte maturation, fertilization or early embryo development in vitro versus in vivo: implications for blastocyst yield and blastocyst quality. Mol Reprod Dev 2002; 61: 234–248. [DOI] [PubMed] [Google Scholar]
- 5.Hara S, Inoue Y, Aoki S, Tanaka K, Shirasuna K, Iwata H. Beneficial effect of polysaccharide gel made of xanthan gum and locust bean gum on bovine oocytes. Int J Mol Sci 2023; 24: 3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zia KM, Tabasum S, Khan MF, Akram N, Akhter N, Noreen A, Zuber M. Recent trends on gellan gum blends with natural and synthetic polymers: A review. Int J Biol Macromol 2018; 109: 1068–1087. [DOI] [PubMed] [Google Scholar]
- 7.Lorenzo G, Zaritzky N, Califano A. Mechanical and optical characterization of gelled matrices during storage. Carbohydr Polym 2015; 117: 825–835. [DOI] [PubMed] [Google Scholar]
- 8.Kapr J, Petersilie L, Distler T, Lauria I, Bendt F, Sauter CM, Boccaccini AR, Rose CR, Fritsche E. Human Induced pluripotent stem cell-derived neural progenitor cells produce distinct neural 3D in vitro models depending on alginate/gellan gum/laminin hydrogel blend properties. Adv Healthc Mater 2021; 10: e2100131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li W, Huang A, Zhong Y, Huang L, Yang J, Zhou C, Zhou L, Zhang Y, Fu G. Laminin-modified gellan gum hydrogels loaded with the nerve growth factor to enhance the proliferation and differentiation of neuronal stem cells. RSC Adv 2020; 10: 17114–17122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prasad S, Tiwari M, Pandey AN, Shrivastav TG, Chaube SK. Impact of stress on oocyte quality and reproductive outcome. J Biomed Sci 2016; 23: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kageyama M, Ito J, Shirasuna K, Kuwayama T, Iwata H. Mitochondrial reactive oxygen species regulate mitochondrial biogenesis in porcine embryos. J Reprod Dev 2021; 67: 141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou N, Liu Q, Qi X, Zhang X, Ru Z, Ma Y, Yu T, Zhang M, Li Y, Zhang Y, Cao Z. Paraquat exposure impairs porcine oocyte meiotic maturation. Theriogenology 2022; 179: 60–68. [DOI] [PubMed] [Google Scholar]
- 13.Dodi G, Sabau RE, Crețu BE, Gardikiotis I. Exploring the antioxidant potential of gellan and guar gums in wound healing. Pharmaceutics 2023; 15: 2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshioka K, Suzuki C, Onishi A. Defined system for in vitro production of porcine embryos using a single basic medium. J Reprod Dev 2008; 54: 208–213. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki C, Iwamura S, Yoshioka K. Birth of piglets through the non-surgical transfer of blastocysts produced in vitro. J Reprod Dev 2004; 50: 487–491. [DOI] [PubMed] [Google Scholar]
- 16.Yoshioka K, Suzuki C, Tanaka A, Anas IM, Iwamura S. Birth of piglets derived from porcine zygotes cultured in a chemically defined medium. Biol Reprod 2002; 66: 112–119. [DOI] [PubMed] [Google Scholar]
- 17.Zhou N, Liu Q, Qi X, Zhang X, Ru Z, Ma Y, Yu T, Zhang M, Li Y, Zhang Y, Cao Z. Paraquat exposure impairs porcine oocyte meiotic maturation. Theriogenology 2022; 179: 60–68. [DOI] [PubMed] [Google Scholar]
- 18.Matwee C, Betts DH, King WA. Apoptosis in the early bovine embryo. Zygote 2000; 8: 57–68. [DOI] [PubMed] [Google Scholar]
- 19.Pacelli S, Paolicelli P, Petralito S, Subham S, Gilmore D, Varani G, Yang G, Lin D, Casadei MA, Paul A. Investigating the role of polydopamine to modulate stem cell adhesion and proliferation on gellan gum-based hydrogels. ACS Appl Bio Mater 2020; 3: 945–951. [DOI] [PubMed] [Google Scholar]
- 20.Jeong PS, Lee S, Park SH, Kim MJ, Kang HG, Nanjidsuren T, Son HC, Song BS, Koo DB, Sim BW, Kim SU. Butylparaben is toxic to porcine oocyte maturation and subsequent embryonic development following in vitro fertilization. Int J Mol Sci 2020; 21: 3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sovernigo TC, Adona PR, Monzani PS, Guemra S, Barros F, Lopes FG, Leal C. Effects of supplementation of medium with different antioxidants during in vitro maturation of bovine oocytes on subsequent embryo production. Reprod Domest Anim 2017; 52: 561–569. [DOI] [PubMed] [Google Scholar]
- 22.Cajas YN, Cañón-Beltrán K, Ladrón de Guevara M, Millán de la Blanca MG, Ramos-Ibeas P, Gutiérrez-Adán A, Rizos D, González EM. Antioxidant nobiletin enhances oocyte maturation and subsequent embryo development and quality. Int J Mol Sci 2020; 21: 5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duan X, Sun SC. Actin cytoskeleton dynamics in mammalian oocyte meiosis. Biol Reprod 2019; 100: 15–24. [DOI] [PubMed] [Google Scholar]
- 24.Namgoong S, Kim NH. Roles of actin binding proteins in mammalian oocyte maturation and beyond. Cell Cycle 2016; 15: 1830–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang J, Ma R, Li L, Wang L, Hou X, Han L, Ge J, Li M, Wang Q. Intersectin 2 controls actin cap formation and meiotic division in mouse oocytes through the Cdc42 pathway. FASEB J 2017; 31: 4277–4285. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Wang QC, Liu J, Xiong B, Cui XS, Kim NH, Sun SC. The small GTPase CDC42 regulates actin dynamics during porcine oocyte maturation. J Reprod Dev 2017; 63: 505–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan MH, Xu R, Zhang Y, Yin L, Li R, Wen D, Lu S, Gao Y, Zhao X, Wei Q, Han B, Ma B. The impact of Arp2/3 complex inhibition on cytoskeleton dynamics and mitochondrial function during goat oocyte meiosis. Animals (Basel) 2023; 13: 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo K, Xiao W, Qiu D. Polymerization of actin filaments coupled with adenosine triphosphate hydrolysis: Brownian dynamics and theoretical analysis. J Chem Phys 2011; 135: 105101. [DOI] [PubMed] [Google Scholar]
- 29.Dalton CM, Szabadkai G, Carroll J. Measurement of ATP in single oocytes: impact of maturation and cumulus cells on levels and consumption. J Cell Physiol 2014; 229: 353–361. [DOI] [PubMed] [Google Scholar]
- 30.Sugimoto A, Inoue Y, Tanaka K, Sinozawa A, Shirasuna K, Iwata H. Effects of a gel culture system made of polysaccharides (xanthan gum and locust bean gum) on in vitro bovine oocyte development and gene expression of the granulosa cells. Mol Reprod Dev 2021; 88: 516–524. [DOI] [PubMed] [Google Scholar]
- 31.Heo DN, Kim HJ, Lee D, Kim H, Lee SJ, Lee HR, Kwon IK, Do SH. Comparison of polysaccharides in articular cartilage regeneration associated with chondrogenic and autophagy-related gene expression. Int J Biol Macromol 2020; 146: 922–930. [DOI] [PubMed] [Google Scholar]
- 32.Shen XH, Jin YX, Liang S, Kwon JW, Zhu JW, Lei L, Kim NH. Autophagy is required for proper meiosis of porcine oocytes maturing in vitro. Sci Rep 2018; 8: 12581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muthukumar T, Song JE, Khang G. Biological role of gellan gum in improving scaffold drug delivery, cell adhesion properties for tissue engineering applications. Molecules 2019; 24: 4514. [DOI] [PMC free article] [PubMed] [Google Scholar]