Abstract
In mammals, secretion of tonic (pulsatile) gonadotropin-releasing hormone (GnRH)/luteinizing hormone (LH) is often suppressed during lactation. Suppression of GnRH/LH pulses in lactating dams is assumed to be caused by suckling stimuli and a chronic negative energy balance due to milk production. The present study aimed to investigate whether the central enkephalin-δ opioid receptor (DOR) signaling mediated the suppression of LH secretion by acute suckling stimuli and/or chronic negative energy balance due to milk production in rats during late lactation when dams were under a heavy energy demand. On postpartum day 16, the number of Penk (enkephalin mRNA)-expressing cells in the arcuate nucleus was significantly higher in lactating rats than in non-lactating control rats. Pulsatile LH secretion was suppressed in rats with chronic suckling or acute 1-h suckling stimuli 6 h after pup removal on day 16 of lactation. Central DOR antagonism significantly increased the mean LH concentrations and the baseline of LH pulses in rats with chronic suckling but not with acute suckling stimuli on day 16 of lactation. Besides, central κ opioid receptor (KOR) antagonism increased the amplitude of LH pulses in rats with the acute suckling stimuli on day 16 of lactation. These results suggest that central DOR signaling mediates the suppression of LH secretion caused by a negative energy balance in rats receiving chronic suckling during late lactation. On the other hand, central KOR signaling likely mediates acute suckling stimuli-induced suppression of LH secretion in rats during late lactation.
Keywords: Arcuate nucleus, δ opioid receptor, Enkephalin, κ opioid receptor, Luteinizing hormone
During lactation, ovarian functions, such as follicular development and subsequent ovulation, are often inhibited in mammals, such as rodents [1,2,3,4], ruminants [5, 6], and primates including humans [7,8,9,10,11]. Lactational inhibition of ovarian function has been suggested to be mainly caused by the suppression of tonic (pulsatile) gonadotropin-releasing hormone (GnRH)/luteinizing hormone (LH) secretion in rodents [12,13,14,15,16,17,18,19,20,21,22], ruminants [23,24,25], and primates [26,27,28,29,30]. The suppression of pulsatile GnRH/LH secretion is presumed to be primarily triggered by the suckling stimulus of their pups because the discontinuation of suckling by the removal of pups restores LH pulses within a few hours after the removal in lactating rats [14, 18, 31]. In addition, a chronic negative energy balance from milk production may be involved in suppressing GnRH/LH secretion in rats during late lactation [2, 32]. Indeed, food intake levels of lactating rats increase progressively throughout the lactation period to 3–4 times that during pregnancy [33], suggesting that energy demand increases as lactation progresses. Furthermore, our previous study showed that the blockade of milk production by peripheral administration of bromocriptine, which blocks lactogenic prolactin secretion from the anterior pituitary gland, further increased plasma LH levels in ovariectomized (OVX) rats during the late, but not early, lactation period [15]. These findings suggest that suckling stimuli are the key determinant of lactational suppression of LH secretion in rats throughout the lactation period and that the negative energy balance due to milk production may be largely responsible for lactational suppression of LH secretion in rats during the late lactation period. To the best of our knowledge, no previous study has demonstrated the neural pathways underlying the suppression of GnRH/LH secretion caused by acute suckling stimuli or chronic suckling during late lactation in rats.
Previous studies have demonstrated that LH suppression in lactating rats and mice is caused by the suppression of Kiss1/kisspeptin expression in the hypothalamic arcuate nucleus (ARC) [34,35,36,37,38,39,40,41,42,43], in which kisspeptin neurons act as the GnRH pulse generator in mammals, including rodents and ruminants [44,45,46,47,48,49]. Notably, the central mechanism underlying the lactational suppression of ARC Kiss1/kisspeptin expression and LH secretion appears to differ in terms of estrogen dependence between the early (estrogen-independent) and late (estrogen-dependent) lactation periods in rats [34, 35]. Estrogen dependency appears to be a common characteristic of the suppression of LH secretion in female rats during late lactation and malnutrition. Indeed, 48-h fasting suppressed pulsatile LH secretion in diestrous levels of estradiol-17β-treated OVX (OVX + E2) rats, but not in OVX rats [50, 51]. We previously demonstrated that central opioids and their three receptor signaling pathways mediate lactational and/or malnutritional suppression of LH secretion in female rats [52,53,54,55,56,57]. Specifically, a central administration of nor-binaltorphimine (nor-BNI, a selective κ opioid receptor [KOR] antagonist) blocked suppression of LH pulses in OVX + E2 rats during late lactation [54] and in OVX + E2 rats bearing glucoprivation by a peripheral administration of 2-deoxy-D-glucose (2DG), an inhibitor of glucose utilization [53]. Importantly, selective μ opioid receptor (MOR) antagonism blocked the 2DG-induced suppression of LH pulses in OVX + E2 female rats [52], but the same MOR antagonism failed to block the suppression of LH pulses in OVX + E2 rats during late lactation [54], suggesting that the mechanisms underlying the suppression of LH secretion during late lactation and malnutrition might not be identical. Besides, central administration of naltrindole (NTI, a selective δ opioid receptor [DOR] antagonist) blocked the suppression of LH pulses in ovariectomized (OVX) lactating rats during early lactation [56] and in 2DG-treated glucoprived OVX + E2 rats [55]. Therefore, we hypothesized that DOR and/or KOR signaling mediates the suppression of GnRH/LH secretion triggered by acute suckling stimuli and/or chronic suckling causing a negative energy balance due to milk production during late lactation. Our previous study showed that the number of Penk (enkephalin mRNA)-expressing cells in the ARC was significantly higher in OVX lactating rats during early lactation than in non-lactating control rats that were deprived of their pups a day after parturition [56]. Furthermore, 1-h of re-suckling 24 h after pup removal increased the number of activated (fos-expressing) Pdyn (dynorphin mRNA)-expressing neurons in the hypothalamic paraventricular nucleus (PVN) and supraoptic nucleus (SON) in OVX + E2 lactating rats during late lactation [54].
This study aimed to reveal the opioidergic pathways underlying the suppression of GnRH/LH secretion by acute suckling stimuli and/or chronic suckling in rats during late lactation. We first investigated Penk expression in the ARC, PVN, and hypothalamic ventromedial nucleus (VMH) of OVX + E2 rats with chronic suckling during late lactation. Next, we investigated whether central administration of NTI, a DOR antagonist, blocked the suppression of LH secretion in OVX + E2 rats with chronic suckling during late lactation. In addition, we investigated whether acute suckling suppressed LH secretion and activated ARC Penk-expressing cells in OVX + E2 rats during late lactation. Finally, we investigated whether central administration of NTI or nor-BNI, a KOR antagonist, blocked acute suckling-induced suppression of LH secretion in OVX + E2 lactating rats during late lactation.
Materials and Methods
Animals
Wistar-Imamichi strain female and male rats were kept at 22 ± 2°C under a 14-h light and 10-h dark cycle (lights on at 0500 h) with free access to food (CE-2; CLEA Japan, Tokyo, Japan) and water. Female rats (8–10 weeks old) with at least two consecutive regular 4-day estrus cycles were mated overnight with stud males on the day of proestrus, and pregnant females were housed individually. The day a newborn litter was found at noon was designated day 0 of lactation. The litter size of dams assigned to the lactating group was adjusted to eight on day 1, whereas dams assigned to the non-lactating group were deprived of their pups from day 1. All animals were OVX and subcutaneously implanted with Silastic tubing (inner diameter 1.5 mm; outer diameter 3.0 mm; length 25 mm; Dow Corning, Midland, MI, USA) containing E2 dissolved in peanut oil (20 µg/ml; Sigma-Aldrich, St Louis, MO, USA) on day 2 under anesthesia with an intraperitoneal injection of a mixture of ketamine (26.7 mg/kg; Fujita Pharmaceutical, Tokyo, Japan) and xylazine (5.3 mg/kg; Bayer, Leverkusen, Germany), and then inhalant 1–3% isoflurane (Pfizer Japan, Tokyo, Japan). The E2 tube was replaced with a new tube on day 9 under anesthesia with isoflurane inhalation (without brain surgery) or with an intraperitoneal injection of a mixture of medetomidine (0.375 mg/kg; ZENOAQ, Koriyama, Japan), midazolam (2 mg/kg; SANDOZ, Tokyo, Japan), and butorphanol (2.5 mg/kg; Meiji Animal Health, Kumamoto, Japan) (with brain surgery; see below for details). The E2 treatment has been confirmed to suppress LH secretion during lactation, as shown in intact lactating rats [34]. OVX + E2 lactating rats were assigned to the groups of pup-removal, acute suckling, or chronic suckling. In the pup-removal group, OVX + E2 lactating rats were deprived of their pups for 6 h immediately before blood or brain sampling (see below for details) on day 16 of lactation. In the acute suckling group, OVX + E2 lactating rats were deprived of their pups for 6 h and received them for 1 h immediately before blood or brain sampling on day 16 of lactation. In the chronic suckling group, OVX + E2 lactating rats were housed with their pups throughout the experimental period, except during surgery. This study was approved by the Committee on Animal Experiments of the Graduate School of Bioagricultural Sciences, Nagoya University.
Brain sampling to determine the effect of suckling stimuli on hypothalamic Penk expression
OVX + E2 rats in the lactating (n = 3) and non-lactating (n = 3) groups were subjected to brain sampling on postpartum day 16, as previously described [34]. Briefly, the rats were deeply anesthetized with sodium pentobarbital (40 mg/kg; Tokyo Chemical Industry, Tokyo, Japan) and perfused with 4% paraformaldehyde (Sigma-Aldrich) in 0.05 M phosphate buffer. The brains were immediately removed, post-fixed overnight at 4°C in the same fixative, and immersed in 30% sucrose in 0.05 M phosphate buffer at 4°C until the brains sank. Frozen frontal sections (50 μm thickness) containing the ARC, PVN, and VMH were prepared using a cryostat (CM1800, Leica Biosystems, Wetzlar, Germany). Sections were subjected to in situ hybridization (ISH) for Penk mRNA.
Brain sampling to determine the effect of acute suckling stimuli on ARC Penk expression and activation of ARC Penk-expressing neurons
OVX + E2 rats in the pup-removal (n = 3) and acute suckling (n = 4) groups were subjected to brain sampling on postpartum day 16, as described above. Sections containing the ARC were subjected to double ISH for Penk and fos (a marker of neuronal activation) mRNA.
ISH for Penk in the ARC, PVN, and VMH and Penk/fos in the ARC
Free-floating ISH for Penk mRNA was performed using every fourth frontal section (including the ARC, PVN, and VMH) obtained from OVX + E2 rats in the lactating and non-lactating groups, as described previously [56]. A Penk-specific digoxigenin (DIG)-labeled complementary RNA (cRNA) probe (position 447-1256; GenBank accession number NM_017139) was designed and synthesized from whole rat hypothalamic cDNA using a DIG-labeling kit (Roche Diagnostics, Basel, Switzerland). The brain sections were hybridized with the antisense Penk cRNA probe (1 µg/ml) overnight at 60°C. The hybridized DIG-labeled probe was detected with an alkaline phosphatase-conjugated sheep anti-DIG antibody (1:1000, RRID: AB_2734716; Roche Diagnostics) and a chromogen solution (338 µg/ml 4-nitroblue tetrazolium chloride and 175 µg/ml 5-bromo-4-chloro-3-indoyl-phosphate; Roche Diagnostics). The sections were mounted on glass slides, and the number of Penk-expressing cells in the ARC (every 200 µm, from 1.72 to 4.36 mm posterior to the bregma, 13 sections in total) and PVN (every 200 µm, from 0.96 to 1.92 mm posterior to the bregma, four sections in total) was unilaterally counted twice by one of the authors under an optical microscope (BX53; Olympus, Tokyo, Japan), and the average was calculated. The examined areas were identified based on a rat brain atlas [58]. Note that the number of Penk-expressing cells in the VMH (every 200 µm, from 2.04 to 3.36 mm posterior to the bregma, six sections in total) could not be counted because Penk-expressing cells overlapped each other in the VMH; thus, each Penk-expressing cell was not distinguishable. Consequently, the intensity of Penk mRNA signals in digital photographs of the ARC, PVN, and VMH was also quantitatively analyzed using Fiji software (ImageJ2, version 2.3.0/1.53f), as previously described [56].
Free-floating double ISH for Penk and fos mRNA was performed using every fourth ARC section obtained from OVX + E2 rats in the pup-removal and acute suckling groups, as previously described [55]. The fos-specific DIG-labeled cRNA probe (position 573-1193; GenBank accession number NM_022197) and Penk-specific fluorescein isothiocyanate (FITC)-labeled cRNA probe were designed and synthesized from whole rat hypothalamic cDNA using DIG- and FITC-labeling kits (Roche Diagnostics), respectively. The brain sections were hybridized with the antisense Penk and fos cRNA probes (1 µg/ml each) overnight at 60°C. The hybridized FITC-labeled Penk probe was detected using a peroxidase (POD)-conjugated sheep anti-FITC antibody (1:1000, RRID: AB_840257; Roche Diagnostics) and a tyramide signal amplification (TSA) Plus FITC Kit (1:100; Perkin Elmer, Waltham, MA). After the inactivation of POD on the anti-FITC antibody by incubating the sections in 0.1 N hydrochloric acid for 30 min, the hybridized DIG-labeled fos probe was detected using a POD-conjugated sheep anti-DIG antibody (1:1000, RRID: AB_514500; Roche Diagnostics), a TSA plus Biotin kit (1:100; Perkin Elmer), and DyLight 594-conjugated streptavidin (Thermo Fisher Scientific, Waltham, MA, USA). Fluorescence images were obtained on an ApoTome.2 fluorescence microscope (Carl Zeiss, Oberkochen, Germany), and Penk-expressing and fos-positive Penk-expressing cells were counted using Fiji software unilaterally in the ARC (every 200 µm, from 1.72 to 4.36 mm posterior to the bregma, 13 sections in total).
Brain surgery and blood sampling to determine the effects of central DOR and KOR antagonism on the suppression of LH secretion in dams with chronic and acute suckling during late lactation
A stainless-steel guide cannula (22G; P1 Technologies, Roanoke, VA, USA) was stereotaxically implanted into the third cerebroventricle (3V) of OVX + E2 lactating rats, according to the rat brain atlas [58]. The coordinates were as follows: 0.8 mm posterior and 7.5 mm ventral to the bregma and at the midline. Surgery was performed on day 9 of lactation under anesthesia with an intraperitoneal injection of a mixture of medetomidine, midazolam, and butorphanol. After surgery, anesthesia was immediately reversed with an intraperitoneal injection of atipamezole (0.75 mg/kg; ZENOAQ).
Free-moving conscious rats in the chronic (n = 4) and acute (n = 6) suckling groups were administered NTI (a selective DOR antagonist, 22.175 nmol/µl; Sigma-Aldrich) into the 3V at a flow rate of 1 μl/min for 2 min using a microsyringe pump (EICOM, Kyoto, Japan) through an internal cannula (28G; P1 Technologies) immediately after the first blood collection at 1300 h on day 16 of lactation. Blood samples (100 µl) were collected at 6-min intervals for 3 h via the atrial silicon cannula (inner diameter, 0.5 mm; outer diameter, 1.0 mm; Shin-Etsu Polymer, Tokyo, Japan), which was inserted through the jugular vein on the day before blood sampling (the surgery was performed under anesthesia with the mixture of ketamine and xylazine and then inhalant 1–3% isoflurane). Red blood cells, taken from donor rats and washed with heparinized saline, were replaced at each blood collection to maintain a constant hematocrit level. The dose of NTI (22.175 nmol/µl) was selected according to our previous studies, which showed that the dose of NTI blocked lactational suppression of LH pulses in OVX rats during early lactation [56] and malnutritional suppression of LH pulses in OVX + E2 rats treated with 2DG [55]. In addition, other free-moving conscious rats in the acute suckling group (n = 6) were administered nor-BNI (a selective KOR antagonist, 13.61 nmol/µl; Sigma-Aldrich) in the same manner. The dose of nor-BNI was selected according to our previous studies, which showed that it blocked lactational suppression of LH pulses in OVX + E2 rats during late lactation [54], malnutritional suppression of LH pulses in OVX + E2 rats treated with 2DG [53], and negative feedback action of E2 on LH pulses in OVX rats [59]. Ultrapure water was injected into the 3V of vehicle-treated control rats in the acute (n = 7) and chronic (n = 8) suckling groups. Plasma samples (50 µl) were obtained by immediate centrifugation and stored at –20°C until assayed for plasma LH concentrations.
At the end of blood sampling, the animals were deeply anesthetized with sodium pentobarbital and injected with 2 µl of 3% brilliant blue solution at the same flow rate as NTI/nor-BNI injection. The brain was removed, and the placement of the 3V cannula was verified by visual inspection of the brilliant blue solution. All animals were confirmed to have the right cannula placement.
Blood sampling to determine the effects of acute suckling stimuli on pulsatile LH secretion in OVX + E2 dams during late lactation
Blood samples (100 µl) were collected from free-moving conscious rats via the atrial silicon cannula, which was inserted on the day before blood sampling, in the acute suckling (n = 4) and control (pup removal, n = 5) groups at 6-min intervals for 3 h, starting at 1300 h on day 16 of lactation. Plasma samples (50 µl) were obtained and stored at –20°C until assayed for plasma LH concentrations.
Radioimmunoassay for plasma LH concentrations and LH pulse analysis
Plasma LH concentrations were measured by double-antibody radioimmunoassay (RIA) using a rat LH-RIA kit, including rabbit anti-rat LH antiserum (AFP240580; RRID: AB_2665533), provided by the National Hormone and Peptide Program (Harbor-UCLA Medical Center, Torrance, CA, USA), as previously described [56]. The concentrations were expressed in terms of rat LH-RP-3 (National Institute of Diabetes and Digestive and Kidney Diseases). The least detectable level was 0.078 ng/ml in 50 µl plasma samples. The intra- and inter-assay coefficients of variation were 6.1% and 4.8% at 1.61 ng/ml, respectively. LH pulses were identified using the PULSAR computer program [60], as described previously [52]. The mean LH concentrations and the baseline, frequency, and amplitude of LH pulses were calculated for each individual and then for the groups.
Statistical analysis
Statistical differences in the number of Penk-expressing cells throughout the ARC between lactating and non-lactating rats were determined using two-way repeated measures ANOVA (groups and localization as main effects). Statistical differences in the total number of Penk-expressing cells and/or the intensity of Penk mRNA signals in the ARC, PVN, and VMH between lactating and non-lactating rats; the number of ARC Penk-expressing cells and fos-positive Penk-expressing cells between the pup-removal and acute suckling groups; and the LH pulse parameters (the mean LH concentrations and the baseline, frequency, and amplitude of LH pulses) between NTI- and vehicle-injected lactating rats with chronic suckling or between the pup-removal and acute suckling groups were determined using Student’s t-test. Statistical differences in the LH pulse parameters between NTI-, nor-BNI-, and vehicle-injected lactating rats with acute suckling stimuli were determined using a one-way ANOVA followed by Dunnett’s test. All analyses were performed using SAS OnDemand for Academics (https://welcome.oda.sas.com).
Results
Chronic suckling increased the number of ARC Penk-expressing cells in OVX + E2 rats during late lactation
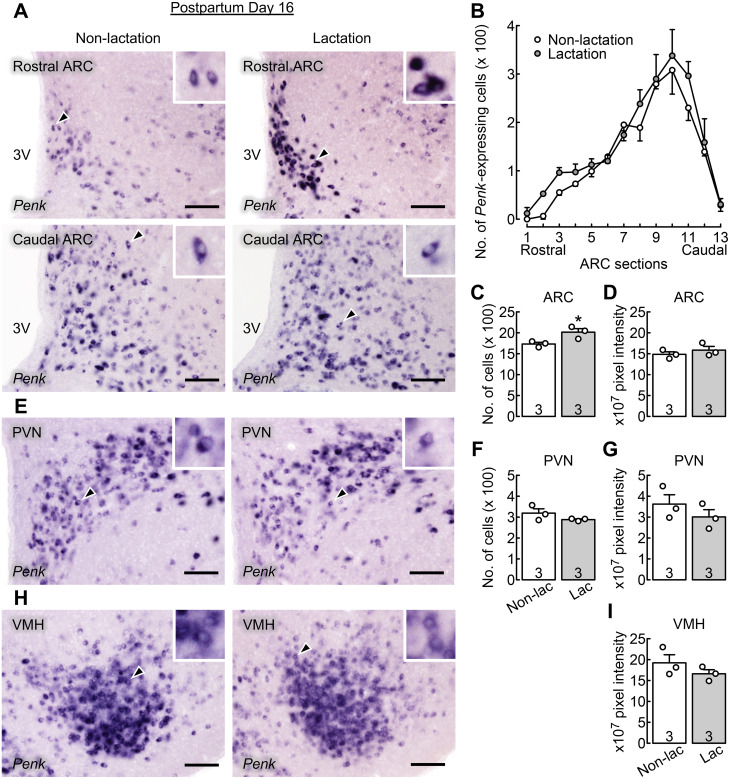
Figure 1A shows Penk-expressing cells in the rostral (upper images) and caudal (lower images) parts of the ARC of representative OVX + E2 lactating rats with chronic suckling (right images) and non-lactating control rats (deprived of their pups from day 1; left images) on postpartum day 16. Many Penk-expressing cells were found throughout the ARC and abundantly localized in the caudal ARC in lactating and non-lactating rats (n = 3 per group, Fig. 1B). Two-way repeated-measures ANOVA revealed significant main effects (group, F[1,4] = 9.14, P = 0.039; and localization, F[12,48] = 32.66, P < 0.001) on the number of ARC Penk-expressing cells. No significant interaction effect was observed between the main effects (F[12,48] = 0.46, P = 0.927). The total number of Penk-expressing cells throughout the ARC was significantly higher in lactating rats than in non-lactating control rats (*, P = 0.039, Student’s t-test; Fig. 1C). The intensity of Penk mRNA signals in the ARC was comparable between groups (Fig. 1D).
Fig. 1.
Chronic suckling increased the number of Penk (enkephalin gene)-expressing cells in the arcuate nucleus (ARC) of estradiol-17β-treated ovariectomized (OVX + E2) lactating rats during late lactation. (A) Penk-expressing cells in the rostral (upper images) and caudal (lower images) parts of ARC of representative OVX + E2 lactating and non-lactating control rats. The insets indicate magnified images of Penk-expressing cells (arrowheads). 3V, third cerebroventricle. Scale bars, 100 µm. (B) Distribution of ARC Penk-expressing cells throughout the ARC (from the rostral to caudal region) of OVX + E2 lactating and non-lactating control rats. The x-axis represents brain sections 1 through 13 (corresponding to 1.72 to 4.36 mm [every 200 µm] posterior to the bregma). Many Penk-expressing cells were found throughout the ARC and abundantly localized in the caudal part of ARC in lactating and non-lactating rats. The number of Penk-expressing cells was higher in OVX + E2 lactating rats than in OVX + E2 non-lactating rats (P < 0.05, two-way repeated measures ANOVA). (C) The total number of Penk-expressing cells throughout the ARC was significantly higher in OVX + E2 lactating rats than in OVX + E2 non-lactating rats (*, P < 0.05, Student’s t-test). (D) The intensity of Penk mRNA signals in the ARC was comparable between groups. (E) Penk-expressing cells in the hypothalamic paraventricular nucleus (PVN) of representative OVX + E2 lactating and non-lactating control rats. The number of Penk-expressing cells (F) and the intensity of Penk mRNA signals (G) in the PVN were comparable between groups. (H) Penk-expressing cells in the hypothalamic ventromedial nucleus (VMH) of representative OVX + E2 lactating and non-lactating control rats. (I) The intensity of VMH Penk mRNA signals was comparable between groups. Values in charts are means ± SEMs. Open circles on the bar chart indicate the individual values. The number in each column indicates the number of animals used. Non-lac, non-lactation; Lac, lactation.
Figure 1E shows Penk-expressing cells in the PVN of representative OVX + E2 lactating rats with chronic suckling and non-lactating control rats on postpartum day 16. Many Penk-expressing cells were found in the PVN. The number of Penk-expressing cells (Fig. 1F) and the intensity of Penk mRNA signals (Fig. 1G) in the PVN were comparable between groups. Many Penk-expressing cells were also found in the VMH of OVX + E2 lactating and non-lactating rats on postpartum day 16 (Fig. 1H). The intensity of VMH Penk mRNA signals was comparable between the lactating and non-lactating groups (Fig. 1I).
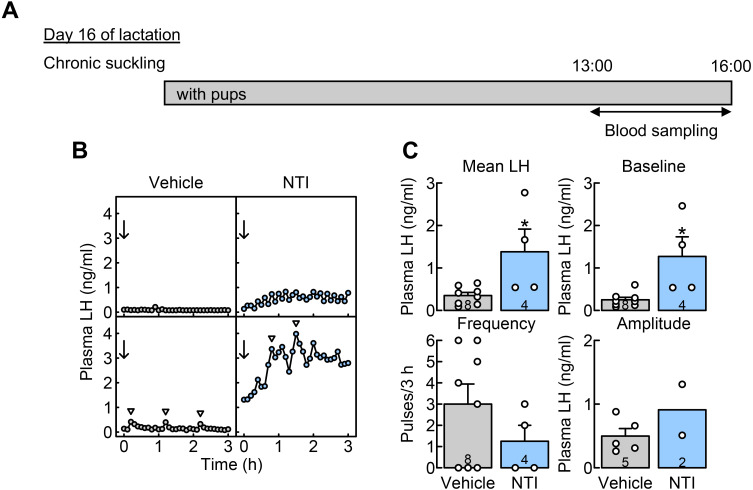
Central DOR antagonism blocked the suppression of LH secretion in OVX + E2 rats in the chronic suckling group during late lactation
The effects of central DOR antagonism on the suppression of LH secretion during late lactation were investigated in OVX + E2 rats with chronic suckling, where the animals were housed with their pups throughout the lactation period (Fig. 2A). Figure 2B shows the plasma LH profiles of representative chronically suckled OVX + E2 rats administered NTI (a selective DOR antagonist) or vehicle into the 3V on day 16 of lactation. Pulsatile LH secretion was suppressed in most vehicle-injected lactating dams (n = 8), whereas plasma LH levels increased in all NTI-injected lactating dams (n = 4). The mean LH concentrations (*, P = 0.020, Student’s t-test) and baseline of LH pulses (*, P = 0.010, Student’s t-test) in NTI-injected dams with chronic suckling were significantly higher than those in vehicle-injected control dams with chronic suckling (Fig. 2C). No significant difference was observed in the frequency of LH pulses between the groups. Statistical analysis did not apply to the amplitude of LH pulses because of the small sample size (only two NTI-injected rats showed LH pulses).
Fig. 2.
Central administration of naltrindole (NTI), a selective δ opioid receptor (DOR) antagonist, blocked the suppression of luteinizing hormone (LH) secretion in OVX + E2 lactating rats with chronic suckling during late lactation. (A) Schematic illustration of experimental schedule: Blood samples were collected from free-moving conscious rats in the chronic suckling group (animals always housed with their pups throughout the experimental period) on day 16 of lactation. (B) Plasma LH profiles of representative OVX + E2 lactating rats that were administered with NTI or a vehicle on day 16 of lactation. Blood samples were collected every 6 min for 3 h. Immediately after the first blood collection, NTI or vehicle (arrows) was injected into the 3V. Arrowheads indicate the peaks of LH pulses identified by the PULSAR computer program. (C) The mean LH concentrations and the baseline of LH pulses were significantly higher in NTI-injected rats than in vehicle-injected control rats on day 16 of lactation (*, P < 0.05, Student’s t-test). No significant difference was observed in the frequency of LH pulses between the groups. Statistical analysis did not apply to the amplitude of LH pulses because of the small sample size. LH pulses were detected in two of four NTI-injected rats and five of eight vehicle-injected control rats. Values in bar charts are means ± SEMs or means. Open circles on the bar chart indicate the individual values. The number in each column indicates the number of animals used.
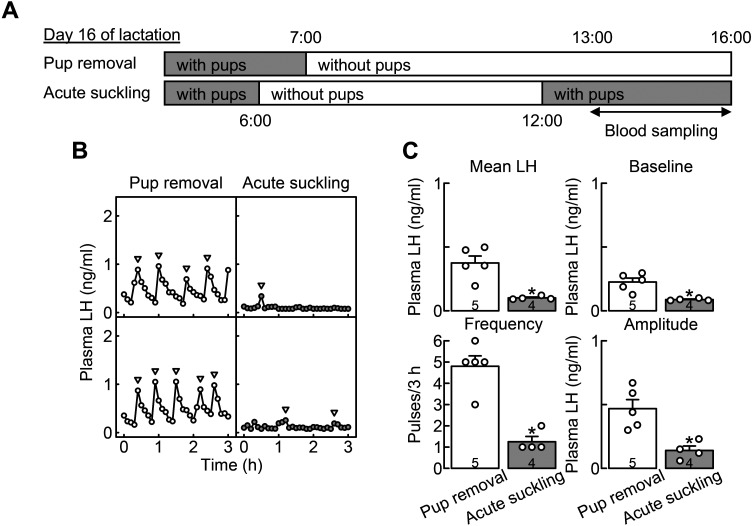
Acute 1-h suckling stimuli suppressed pulsatile LH secretion in OVX + E2 rats deprived of their pups for 6 h during late lactation
The effects of pup removal and acute suckling on pulsatile LH secretion were investigated in OVX + E2 rats during late lactation. Blood samples were collected from free-moving conscious rats in the pup-removal (animals were deprived of their pups for 6 h immediately before blood sampling, n = 5) and acute suckling groups (animals were deprived of their pups for 6 h and then received their pups for 1 h immediately before blood sampling, n = 4) on day 16 of lactation (Fig. 3A). Figure 3B shows the plasma LH profiles of representative OVX + E2 rats in the pup-removal and acute suckling groups on day 16 of lactation. Apparent LH pulses were observed in OVX + E2 rats in the pup-removal group, whereas LH pulses were suppressed in OVX + E2 rats in the acute suckling group. The mean LH concentrations (*, P = 0.003, Student’s t-test) and the baseline (*, P = 0.006, Student’s t-test), frequency (*, P < 0.001, Student’s t-test), and amplitude (*, P = 0.007, Student’s t-test) of LH pulses were significantly lower in the acute suckling group than in the pup-removal group (Fig. 3C).
Fig. 3.
Acute 1-h suckling stimuli suppressed pulsatile LH secretion in OVX + E2 dams deprived of their pups for 6 h during late lactation. (A) Schematic illustration of experimental schedule to investigate effects of pup removal and acute suckling on pulsatile LH secretion in OVX + E2 dams on day 16 of lactation. Blood samples were collected from free-moving conscious rats in the pup removal and acute suckling groups. Animals assigned to the pup removal group were deprived of their pups for 6 h immediately before the blood sampling on day 16 of lactation, and animals assigned to the acute suckling group received their pups for 1 h after the 6-h pup removal period immediately before the blood sampling on day 16 of lactation. (B) Plasma LH profiles of representative OVX + E2 dams in the pup removal and acute suckling groups. Blood samples were collected every 6 min for 3 h. Arrowheads indicate the peaks of LH pulses identified by the PULSAR computer program. (C) The mean LH concentrations, and the baseline, frequency, and amplitude of LH pulses were significantly lower in the acute suckling group than in the pup removal group (*, P < 0.05, Student’s t-test). Values in bar charts are means ± SEMs. Open circles on the bar chart indicate the individual values. The number in each column indicates the number of animals used.
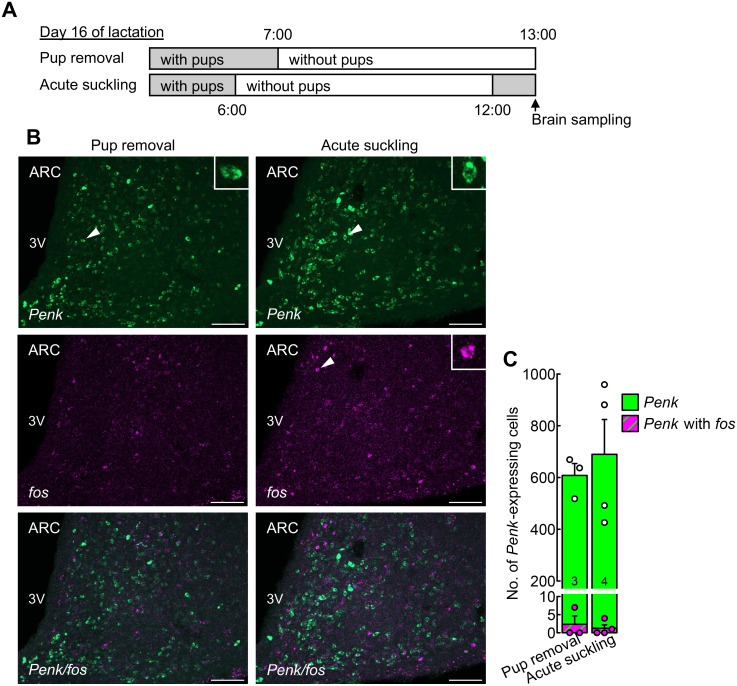
Acute 1-h suckling stimuli failed to activate ARC enkephalin neurons in OVX + E2 rats deprived of their pups for 6 h during late lactation
The effects of acute 1-h suckling stimuli on fos (a marker of neuronal activation) expression in ARC enkephalin neurons were investigated in OVX + E2 lactating rats that were deprived of their pups for 6 h on day 16 of lactation (Fig. 4A). Many fos-positive cells were found in the ARC of the acute suckling group (right images, Fig. 4B). In contrast, few fos-positive Penk-expressing cells were found in the ARC in both the pup-removal (left images) and acute suckling (right images) groups. No significant differences were observed in the number of ARC Penk-expressing and fos-positive Penk-expressing cells between the pup-removal (n = 3) and acute suckling (n = 4) groups (Fig. 4C).
Fig. 4.
Acute 1-h suckling stimuli failed to activate ARC enkephalin neurons in OVX + E2 rats deprived of their pups for 6 h during late lactation. (A) Schematic illustration of experimental schedule to investigate effects of acute suckling on ARC enkephalin neurons in OVX + E2 dams on day 16 of lactation. Animals assigned to the pup removal group were deprived of their pups for 6 h immediately before the brain sampling on day 16 of lactation, and animals assigned to the acute suckling group received their pups for 1 h after the 6-h pup removal period immediately before brain sampling on day 16 of lactation. (B) Penk (green)- and fos (magenta)-expressing cells in the ARC of representative OVX + E2 dams in the pup removal and acute suckling groups. The insets indicate magnified images of Penk- or fos-expressing cells (arrowheads). 3V, third cerebroventricle. Scale bars, 100 µm. (C) The number of ARC Penk-expressing cells (green) and fos-positive Penk-expressing cells (green and magenta striped) were comparable between the groups. Values in bar charts are means ± SEMs. Circles on the bar chart indicate the individual values of Penk-expressing cells (white) and fos-positive Penk-expressing cells (magenta). The number in each column indicates the number of animals used.
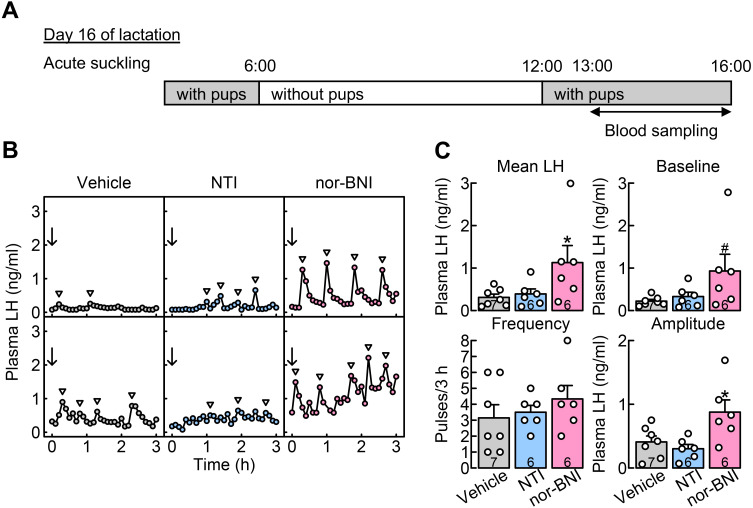
Central KOR, but not DOR, antagonism blocked the suppression of LH secretion in OVX + E2 rats in the acute suckling group during late lactation
The effects of central DOR or KOR antagonism on the suppression of LH secretion during late lactation were investigated in OVX + E2 rats, in which animals received acute suckling stimuli from their pups for 1 h after 6 h of pup removal on day 16 of lactation (Fig. 5A). Figure 5B shows the plasma LH profiles in representative OVX + E2 rats in the acute suckling group administered NTI (a selective DOR antagonist), nor-BNI (a selective KOR antagonist), or vehicle into the 3V on day 16 of lactation. Pulsatile LH secretion was suppressed in most vehicle- (n = 7) and NTI- (n = 6) injected dams exposed to acute suckling stimuli. In contrast, regular LH pulses were observed in most nor-BNI-injected dams exposed to acute suckling stimuli (n = 6). One-way ANOVA revealed that antagonist treatment significantly increased the mean LH concentrations (F[2,16] = 3.69, P = 0.048) and the amplitude of LH pulses (F[2,16] = 5.67, P = 0.014), and tended to increase the baseline of LH pulses (F[2,16] = 2.89, P = 0.085) in dams with acute suckling stimuli. Specifically, the mean LH concentrations (*, P = 0.042, Dunnett’s test) and the amplitude of LH pulses (*, P = 0.032, Dunnett’s test) in nor-BNI-injected dams with acute suckling stimuli were significantly higher than those in vehicle-injected dams with acute suckling stimuli (Fig. 5C). In addition, the baseline of LH pulses in nor-BNI-injected dams with acute suckling stimuli tended to be higher than those in vehicle-injected dams with acute suckling stimuli (#, P = 0.068, Dunnett’s test; Fig. 5C). No significant difference was observed in the frequency of LH pulses between nor-BNI-injected dams and vehicle-injected control dams in the acute suckling group. Besides, no significant differences were observed in the mean LH concentrations and the baseline, frequency, and amplitude of LH pulses between NTI- and vehicle-injected dams in the acute suckling group (Fig. 5C).
Fig. 5.
Central administration of nor-binaltorphimine (nor-BNI, a selective κ opioid receptor (KOR) antagonist), but not NTI (a selective DOR antagonist) blocked the suppression of LH secretion in OVX + E2 lactating rats with acute suckling stimuli during late lactation. (A) Schematic illustration of experimental schedule: Blood samples were collected from free-moving conscious rats in the acute suckling group, in which animals received their pups for 1 h after the 6-h pup removal period immediately before blood sampling on day 16 of lactation. (B) Plasma LH profiles of representative OVX + E2 lactating rats administered with NTI, nor-BNI, or vehicle on day 16 of lactation. Blood samples were collected every 6 min for 3 h. Immediately after the first blood collection, NTI, nor-BNI, or vehicle (arrows) was injected into the 3V. Arrowheads indicate the peaks of LH pulses identified by the PULSAR computer program. (C) The mean LH concentrations and the amplitude of LH pulses were significantly higher in nor-BNI-injected rats than in vehicle-injected rats with acute suckling stimuli on day 16 of lactation (*, P < 0.05, one-way ANOVA followed by Dunnett’s test). In addition, the baseline of LH pulses tended to be higher in nor-BNI-injected rats than in vehicle-injected control rats (#, P = 0.068, one-way ANOVA followed by Dunnett’s test). No significant difference was observed in the frequency of LH pulses between nor-BNI- and vehicle-injected lactating rats. Besides, no significant differences were observed in the mean LH concentrations and the baseline, frequency, and amplitude of LH pulses between the NTI- and vehicle-injected dams. Values in bar charts are means ± SEMs. Open circles on the bar chart indicate the individual values. The number in each column indicates the number of animals used.
Discussion
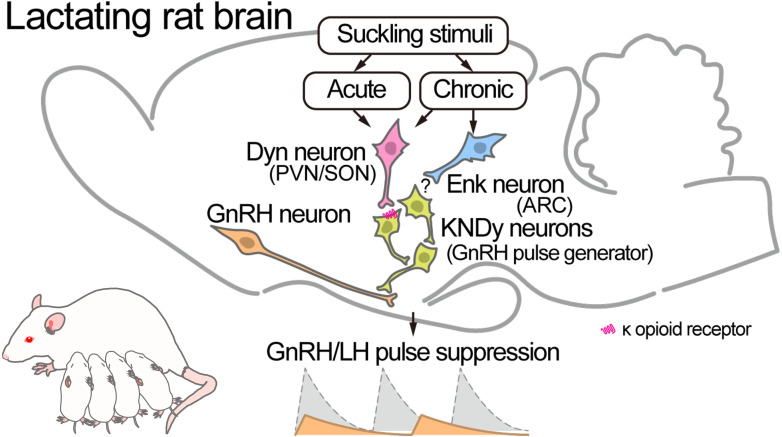
The present study suggests that enkephalin-DOR signaling at least partially mediates the suppression of LH secretion in lactating rats during late lactation because central DOR antagonism significantly increased the mean LH concentrations and the baseline of LH pulses in OVX + E2 lactating rats with chronic suckling on day 16 of lactation (late lactation). Furthermore, the present study showed an increase in the number of Penk-expressing cells in the ARC, but not in the PVN or VMH, of OVX + E2 lactating rats compared to OVX + E2 non-lactating control rats on postpartum day 16. These findings suggest that chronic suckling enhances ARC enkephalin expression, consequently causing chronic suckling-induced suppression of LH secretion in female rats during late lactation. The involvement of enkephalin-DOR signaling in chronic suckling-induced LH suppression is largely consistent with the results of our previous study, which showed that DOR signaling mediates LH suppression during early lactation (day 8 of lactation) in rats [56]. Notably, the current study showed that treatment with the same central DOR antagonist failed to recover LH pulse parameters in lactating rats exposed to acute suckling stimuli on day 16 of lactation. In addition, acute suckling stimuli failed to increase or activate ARC enkephalin neurons in OVX + E2 dams that were deprived of their pups for 6 h during late lactation. Therefore, enkephalin-DOR signaling is unlikely to be involved in acute suckling-induced LH suppression in lactating rats during late lactation. In contrast, central KOR antagonism increased the mean LH concentrations and the amplitude of LH pulses and tended to increase the baseline of LH pulses in lactating rats bearing acute suckling stimuli. Collectively, as shown in Fig. 6, these results suggest that enkephalin-DOR signaling mediates chronic suckling-induced LH suppression throughout the lactating period in rats and that dynorphin-KOR signaling, but not enkephalin-DOR signaling, mediates acute suckling-induced LH suppression in rats during late lactation. To the best of our knowledge, this is the first study that shows that the KOR and DOR signaling pathways separately mediate the suppression of LH pulses caused by acute and chronic suckling stimuli, respectively, in rats during late lactation.
Fig. 6.
Schematic illustration showing the involvement of opioidergic pathways suppressing gonadotropin-releasing hormone (GnRH)/LH pulses in lactating rats based on our current and previous studies [54]. Enkephalin (Enk) neurons in the ARC may, at least partially, mediate chronic suckling stimuli-induced GnRH/LH suppression during late lactation, in which the dams suffer from chronic negative energy balance due to milk production. Further studies are needed to clarify the action site of Enk to suppress GnRH/LH pulses. Dynorphin (Dyn) neurons in the PVN and supraoptic nucleus (SON) may, at least partially, mediate acute and chronic suckling stimuli-induced GnRH/LH suppression via direct suppression of KNDy neurons, namely the GnRH pulse generator, through KOR in the neurons.
The current study showed that the number of Penk-expressing cells in the ARC was higher in dams receiving chronic suckling than in non-lactating control rats, which were deprived of their pups from day 1 of lactation, on day 16 (late lactation). This result is largely consistent with that of our previous study [56], which showed that the number of Penk-expressing cells in the ARC was significantly higher in OVX lactating rats with chronic suckling than in OVX non-lactating control rats on postpartum day 8 (early lactation). The lactational increase in the number of ARC enkephalin neurons might be caused by the functional alteration of a sub-population of tuberoinfundibular dopamine (TIDA) neurons; Yip et al. [61] showed that TIDA neurons altered the synthesis and secretion of enkephalin rather than dopamine, an endogenous inhibitor of lactogenic prolactin secretion, in mice during lactation. Regarding dynorphin-KOR signaling, our previous study showed that acute 1-h suckling increased the number of activated (fos-expressing) Pdyn-expressing neurons in the PVN and SON, but not in the ARC, in lactating rats 24 h after pup removal on day 16 of lactation [54]. These results suggest that PVN and SON dynorphin neurons primarily mediate acute suckling stimuli-induced LH suppression in lactating rats during late lactation. Notably, our previous studies showed that central KOR antagonism blocked the suppression of LH secretion in lactating rats with chronic suckling on day 16 of lactation [54] and in glucoprived female rats [53], suggesting that KOR signaling also mediates chronic suckling-induced LH suppression during late lactation in rats facing a severely negative energy balance due to milk production (Fig. 6).
The present study also suggests the possibility that the targets of inhibitory dynorphin-KOR and enkephalin-DOR signaling pathways on GnRH/LH pulse generation differed from each other. Specifically, the target of dynorphin-KOR signaling appears to be the amplitude of LH pulses because KOR antagonism mainly increased the amplitude of LH pulses in lactating rats exposed to acute suckling stimuli. In contrast, the target of enkephalin-DOR signaling appears to be the baseline of LH pulses because DOR antagonism mainly increased the baseline LH pulses and then mean LH levels in lactating rats undergoing chronic suckling. We envision that the lactational suppression of GnRH/LH pulses is triggered by the suppression of the amplitude of LH pulses caused by acute suckling stimuli-induced dynorphin-KOR signaling and then enhanced by the suppression of the baseline of LH pulses caused by chronic suckling-induced enkephalin-DOR signaling in female rats during late lactation. On the other hand, DOR and KOR antagonists failed to restore the frequency of LH pulses in lactating rats to levels observed in non-lactating control rats. Further studies are needed to clarify the mechanism underlying the suppression of the frequency of GnRH/LH pulses in lactating rats during late lactation.
Dynorphin possibly directly suppresses ARC kisspeptin neurons, which serve as the GnRH pulse generator [45,46,47], during lactation (Fig. 6) because KOR was found in most ARC kisspeptin neurons in female rats [53, 62] and mice [63]. Supporting this notion, de Croft et al. [64] and Ruka et al. [65] showed that dynorphin or a KOR agonist decreased the firing frequency of GFP-tagged ARC kisspeptin neurons in male mice. In addition, conditional Kiss1 deletion in KOR-expressing kisspeptin neurons caused LH pulse disruption in OVX rats in an estrogen-dependent manner [62]. In contrast, the inhibitory action site(s) of enkephalin in suppressing pulsatile LH secretion in lactating rats remains unclear, as a previous study reported that DOR was rarely expressed in the ARC of rats [66]. Further studies are needed to clarify the enkephalin action site(s) involved in lactational suppression of LH secretion in rats.
In conclusion, the present study suggests that central DOR signaling partly mediates the suppression of LH secretion during the late lactation period, during which dams suffer from a chronic negative energy balance due to milk production. Furthermore, the present study suggests that central KOR signaling, but not DOR signaling, mediates the suppression of LH secretion induced by acute suckling stimuli in lactating rats during late lactation.
Conflict of interests
None of the authors has any potential conflicts of interest associated with this research.
Acknowledgments
The authors are grateful to the National Hormone and Peptide Program for the rat LH assay kit and to Drs. G.R. Merriam and K.W. Wachter for the PULSAR computer program. RIA was performed at the Radioisotope Research Center of Nagoya University. This study was supported in part by the Japan Society for the Promotion of Science KAKENHI (Grant Numbers: 21H05031 and 21K19186 to H. Tsukamura, 23H02362 to N. I., and 20H03127, 22K19245 and 24K01905 to Y. U.).
References
- 1.Tsukamura H. Kobayashi Award 2019: The neuroendocrine regulation of the mammalian reproduction. Gen Comp Endocrinol 2022; 315: 113755. [DOI] [PubMed] [Google Scholar]
- 2.Tsukamura H, Maeda K. Non-metabolic and metabolic factors causing lactational anestrus: rat models uncovering the neuroendocrine mechanism underlying the suckling-induced changes in the mother. Prog Brain Res 2001; 133: 187–205. [DOI] [PubMed] [Google Scholar]
- 3.Smith MS, True C, Grove KL. The neuroendocrine basis of lactation-induced suppression of GnRH: role of kisspeptin and leptin. Brain Res 2010; 1364: 139–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith MS, Grove KL. Integration of the regulation of reproductive function and energy balance: lactation as a model. Front Neuroendocrinol 2002; 23: 225–256. [DOI] [PubMed] [Google Scholar]
- 5.Morales-Terán G, Herrera-Corredor CA, Pérez-Hernández P, Salazar-Ortiz J, Sánchez JG. Influence of controlled suckling and the male effect on the resumption of postpartum ovarian activity in Pelibuey sheep. Trop Subtrop Agroecosystems 2011; 13: 493–500. [Google Scholar]
- 6.Peter AT, Vos PL, Ambrose DJ. Postpartum anestrus in dairy cattle. Theriogenology 2009; 71: 1333–1342. [DOI] [PubMed] [Google Scholar]
- 7.Van Ginneken JK. Prolonged breastfeeding as a birth spacing method. Stud Fam Plann 1974; 5: 201–206. [PubMed] [Google Scholar]
- 8.McNeilly AS. Lactation and fertility. J Mammary Gland Biol Neoplasia 1997; 2: 291–298. [DOI] [PubMed] [Google Scholar]
- 9.Stewart KJ. Suckling and lactational anoestrus in wild gorillas (Gorilla gorilla). J Reprod Fertil 1988; 83: 627–634. [DOI] [PubMed] [Google Scholar]
- 10.Maeda K-I, Tsukamura H, Ohkura S, Kanaizuka T, Suzuki J. Suppression of ovarian activity during the breeding season in suckling Japanese monkey (Macaca fuscata). J Reprod Fertil 1991; 92: 371–375. [DOI] [PubMed] [Google Scholar]
- 11.Howie PW, McNeilly AS. Effect of breast-feeding patterns on human birth intervals. J Reprod Fertil 1982; 65: 545–557. [DOI] [PubMed] [Google Scholar]
- 12.Smith MS, Neill JD. Inhibition of gonadotropin secretion during lactation in the rat: relative contribution of suckling and ovarian steroids. Biol Reprod 1977; 17: 255–261. [DOI] [PubMed] [Google Scholar]
- 13.Maeda K, Tsukamura H, Yokoyama A. Suppression of luteinizing hormone secretion is removed at late lactation in ovariectomized lactating rats. Endocrinol Jpn 1987; 34: 709–716. [DOI] [PubMed] [Google Scholar]
- 14.Maeda K-I, Tsukamura H, Uchida E, Ohkura N, Ohkura S, Yokoyama A. Changes in the pulsatile secretion of LH after the removal of and subsequent resuckling by pups in ovariectomized lactating rats. J Endocrinol 1989; 121: 277–283. [DOI] [PubMed] [Google Scholar]
- 15.Maeda K, Uchida E, Tsukamura H, Ohkura N, Ohkura S, Yokoyama A. Prolactin does not mediate the suppressive effect of the suckling stimulus on luteinizing hormone secretion in ovariectomized lactating rats. Endocrinol Jpn 1990; 37: 405–411. [DOI] [PubMed] [Google Scholar]
- 16.Tsukamura H, Maeda K, Ohkura S, Yokoyama A. Effect of hypothalamic deafferentation on the pulsatile secretion of luteinizing hormone in ovariectomized lactating rats. J Neuroendocrinol 1990; 2: 59–63. [DOI] [PubMed] [Google Scholar]
- 17.Tsukamura H, Ohkura S, Coen CW, Maeda K-I. The paraventricular nucleus and corticotrophin-releasing hormone are not critical in suppressing pulsatile LH secretion in ovariectomized lactating rats. J Endocrinol 1993; 137: 291–297. [DOI] [PubMed] [Google Scholar]
- 18.Fox SR, Smith MS. The suppression of pulsatile luteinizing hormone secretion during lactation in the rat. Endocrinology 1984; 115: 2045–2051. [DOI] [PubMed] [Google Scholar]
- 19.Smith MS. The relative contribution of suckling and prolactin to the inhibition of gonadotropin secretion during lactation in the rat. Biol Reprod 1978; 19: 77–83. [DOI] [PubMed] [Google Scholar]
- 20.Smith MS. A comparison of pituitary responsiveness to luteinizing hormone-releasing hormone during lactation and the estrous cycle of the rat. Endocrinology 1978; 102: 114–120. [DOI] [PubMed] [Google Scholar]
- 21.Pohl CR, Lee LR, Smith MS. Qualitative changes in luteinizing hormone and prolactin responses to N-methyl-aspartic acid during lactation in the rat. Endocrinology 1989; 124: 1905–1911. [DOI] [PubMed] [Google Scholar]
- 22.Naik SI, Young LS, Charlton HM, Clayton RN. Pituitary gonadotropin-releasing hormone receptor regulation in mice. II: Females. Endocrinology 1984; 115: 114–120. [DOI] [PubMed] [Google Scholar]
- 23.Williams GL, Talavera F, Petersen BJ, Kirsch JD, Tilton JE. Coincident secretion of follicle-stimulating hormone and luteinizing hormone in early postpartum beef cows: effects of suckling and low-level increases of systemic progesterone. Biol Reprod 1983; 29: 362–373. [DOI] [PubMed] [Google Scholar]
- 24.Carruthers TD, Hafs HD. Suckling and four-times daily milking: influence on ovulation, estrus and serum luteinizing hormone, glucocorticoids and prolactin in postpartum holsteins. J Anim Sci 1980; 50: 919–925. [DOI] [PubMed] [Google Scholar]
- 25.Carruthers TD, Convey EM, Kesner JS, Hafs HD, Cheng KW. The hypothalamo-pituitary gonadotrophic axis of suckled and nonsuckled dairy cows postpartum. J Anim Sci 1980; 51: 949–957. [DOI] [PubMed] [Google Scholar]
- 26.Ordög T, Chen MD, O’Byrne KT, Goldsmith JR, Connaughton MA, Hotchkiss J, Knobil E. On the mechanism of lactational anovulation in the rhesus monkey. Am J Physiol 1998; 274: E665–E676. [DOI] [PubMed] [Google Scholar]
- 27.Weiss G, Butler WR, Dierschke DJ, Knobil E. Influence of suckling on gonadotropin secretion in the postpartum rhesus monkey. Proc Soc Exp Biol Med 1976; 153: 330–331. [DOI] [PubMed] [Google Scholar]
- 28.Weiss G, Butler WR, Hotchkiss J, Dierschke DJ, Knobil E. Periparturitional serum concentrations of prolactin, the gonadotropins, and the gonadal hormones in the rhesus monkey. Proc Soc Exp Biol Med 1976; 151: 113–116. [DOI] [PubMed] [Google Scholar]
- 29.Tay CC, Glasier AF, McNeilly AS. The 24 h pattern of pulsatile luteinizing hormone, follicle stimulating hormone and prolactin release during the first 8 weeks of lactational amenorrhoea in breastfeeding women. Hum Reprod 1992; 7: 951–958. [DOI] [PubMed] [Google Scholar]
- 30.Glasier A, McNeilly AS, Howie PW. Fertility after childbirth: changes in serum gonadotrophin levels in bottle and breast feeding women. Clin Endocrinol (Oxf) 1983; 19: 493–501. [DOI] [PubMed] [Google Scholar]
- 31.Lee LR, Haisenleder DJ, Marshall JC, Smith MS. Expression of alpha-subunit and luteinizing hormone (LH) beta messenger ribonucleic acid in the rat during lactation and after pup removal: relationship to pituitary gonadotropin-releasing hormone receptors and pulsatile LH secretion. Endocrinology 1989; 124: 776–782. [DOI] [PubMed] [Google Scholar]
- 32.Uenoyama Y, Inoue N, Tsukamura H. Kisspeptin and lactational anestrus: Current understanding and future prospects. Peptides 2023; 166: 171026. [DOI] [PubMed] [Google Scholar]
- 33.Ota K, Yokoyama A. Body weight and food consumption of lactating rats: effects of ovariectomy and of arrest and resumption of suckling. J Endocrinol 1967; 38: 251–261. [DOI] [PubMed] [Google Scholar]
- 34.Yamada S, Uenoyama Y, Deura C, Minabe S, Naniwa Y, Iwata K, Kawata M, Maeda K-I, Tsukamura H. Oestrogen-dependent suppression of pulsatile luteinising hormone secretion and Kiss1 mRNA expression in the arcuate nucleus during late lactation in rats. J Neuroendocrinol 2012; 24: 1234–1242. [DOI] [PubMed] [Google Scholar]
- 35.Yamada S, Uenoyama Y, Kinoshita M, Iwata K, Takase K, Matsui H, Adachi S, Inoue K, Maeda K-I, Tsukamura H. Inhibition of metastin (kisspeptin-54)-GPR54 signaling in the arcuate nucleus-median eminence region during lactation in rats. Endocrinology 2007; 148: 2226–2232. [DOI] [PubMed] [Google Scholar]
- 36.Araujo-Lopes R, Crampton JR, Aquino NS, Miranda RM, Kokay IC, Reis AM, Franci CR, Grattan DR, Szawka RE. Prolactin regulates kisspeptin neurons in the arcuate nucleus to suppress LH secretion in female rats. Endocrinology 2014; 155: 1010–1020. [DOI] [PubMed] [Google Scholar]
- 37.Brown RS, Herbison AE, Grattan DR. Prolactin regulation of kisspeptin neurones in the mouse brain and its role in the lactation-induced suppression of kisspeptin expression. J Neuroendocrinol 2014; 26: 898–908. [DOI] [PubMed] [Google Scholar]
- 38.Hackwell ECR, Ladyman SR, Brown RSE, Grattan DR. Mechanisms of Lactation-induced Infertility in Female Mice. Endocrinology 2023; 164: bqad049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Higo S, Aikawa S, Iijima N, Ozawa H. Rapid modulation of hypothalamic Kiss1 levels by the suckling stimulus in the lactating rat. J Endocrinol 2015; 227: 105–115. [DOI] [PubMed] [Google Scholar]
- 40.Liu X, Brown RS, Herbison AE, Grattan DR. Lactational anovulation in mice results from a selective loss of kisspeptin input to GnRH neurons. Endocrinology 2014; 155: 193–203. [DOI] [PubMed] [Google Scholar]
- 41.Ladyman SR, Woodside B. Food restriction during lactation suppresses Kiss1 mRNA expression and kisspeptin-stimulated LH release in rats. Reproduction 2014; 147: 743–751. [DOI] [PubMed] [Google Scholar]
- 42.True C, Kirigiti M, Ciofi P, Grove KL, Smith MS. Characterisation of arcuate nucleus kisspeptin/neurokinin B neuronal projections and regulation during lactation in the rat. J Neuroendocrinol 2011; 23: 52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ozawa H. Kisspeptin neurons as an integration center of reproductive regulation: Observation of reproductive function based on a new concept of reproductive regulatory nervous system. Reprod Med Biol 2021; 21: e12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagae M, Uenoyama Y, Okamoto S, Tsuchida H, Ikegami K, Goto T, Majarune S, Nakamura S, Sanbo M, Hirabayashi M, Kobayashi K, Inoue N, Tsukamura H. Direct evidence that KNDy neurons maintain gonadotropin pulses and folliculogenesis as the GnRH pulse generator. Proc Natl Acad Sci USA 2021; 118: e2009156118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uenoyama Y, Nagae M, Tsuchida H, Inoue N, Tsukamura H. Role of KNDy neurons expressing kisspeptin, neurokinin B, and dynorphin A as a GnRH pulse generator controlling mammalian reproduction. Front Endocrinol (Lausanne) 2021; 12: 724632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goodman RL, Herbison AE, Lehman MN, Navarro VM. Neuroendocrine control of gonadotropin-releasing hormone: Pulsatile and surge modes of secretion. J Neuroendocrinol 2022; 34: e13094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sobrino V, Avendaño MS, Perdices-López C, Jimenez-Puyer M, Tena-Sempere M. Kisspeptins and the neuroendocrine control of reproduction: Recent progress and new frontiers in kisspeptin research. Front Neuroendocrinol 2022; 65: 100977. [DOI] [PubMed] [Google Scholar]
- 48.Moore AM, Novak AG, Lehman MN. KNDy neurons of the hypothalamus and their role in GnRH pulse generation: An update. Endocrinology 2023; 165: bqad194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herbison AE. The gonadotropin-releasing hormone pulse generator. Endocrinology 2018; 159: 3723–3736. [DOI] [PubMed] [Google Scholar]
- 50.Cagampang FR, Maeda K-I, Tsukamura H, Ohkura S, Ota K. Involvement of ovarian steroids and endogenous opioids in the fasting-induced suppression of pulsatile LH release in ovariectomized rats. J Endocrinol 1991; 129: 321–328. [DOI] [PubMed] [Google Scholar]
- 51.Nagatani S, Tsukamura H, Maeda K. Estrogen feedback needed at the paraventricular nucleus or A2 to suppress pulsatile luteinizing hormone release in fasting female rats. Endocrinology 1994; 135: 870–875. [DOI] [PubMed] [Google Scholar]
- 52.Tsuchida H, Kawai N, Yamada K, Takizawa M, Inoue N, Uenoyama Y, Tsukamura H. Central μ-opioid receptor antagonism blocks glucoprivic LH pulse suppression and gluconeogenesis/feeding in female rats. Endocrinology 2021; 162: bqab140. [DOI] [PubMed] [Google Scholar]
- 53.Tsuchida H, Mostari P, Yamada K, Miyazaki S, Enomoto Y, Inoue N, Uenoyama Y, Tsukamura H. Paraventricular dynorphin A neurons mediate LH pulse suppression induced by hindbrain glucoprivation in female rats. Endocrinology 2020; 161: bqaa161. [DOI] [PubMed] [Google Scholar]
- 54.Tsuchida H, Nonogaki M, Inoue N, Uenoyama Y, Tsukamura H. Dynorphin-κ-opioid receptor signaling, but not µ-opioid receptor signaling, partly mediates the suppression of luteinizing hormone release during late lactation in rats. Neurosci Lett 2022; 791: 136920. [DOI] [PubMed] [Google Scholar]
- 55.Tsuchida H, Nonogaki M, Takizawa M, Inoue N, Uenoyama Y, Tsukamura H. Enkephalin-δ opioid receptor signaling mediates glucoprivic suppression of LH pulse and gluconeogenesis in female rats. Endocrinology 2023; 164: bqac216. [DOI] [PubMed] [Google Scholar]
- 56.Tsuchida H, Takizawa M, Nonogaki M, Inoue N, Uenoyama Y, Tsukamura H. Enkephalin-δ opioid receptor signaling partly mediates suppression of LH release during early lactation in rats. J Reprod Dev 2023; 69: 192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Uenoyama Y, Tsuchida H, Nagae M, Inoue N, Tsukamura H. Opioidergic pathways and kisspeptin in the regulation of female reproduction in mammals. Front Neurosci 2022; 16: 958377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates, 6th Edition. San Diego: Academic Press; 2008. [Google Scholar]
- 59.Mostari P, Ieda N, Deura C, Minabe S, Yamada S, Uenoyama Y, Maeda K, Tsukamura H. dynorphin-kappa opioid receptor signaling partly mediates estrogen negative feedback effect on LH pulses in female rats. J Reprod Dev 2013; 59: 266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Merriam GR, Wachter KW. Algorithms for the study of episodic hormone secretion. Am J Physiol 1982; 243: E310–E318. [DOI] [PubMed] [Google Scholar]
- 61.Yip SH, Romanò N, Gustafson P, Hodson DJ, Williams EJ, Kokay IC, Martin AO, Mollard P, Grattan DR, Bunn SJ. Elevated prolactin during pregnancy drives a phenotypic switch in mouse hypothalamic dopaminergic neurons. Cell Rep 2019; 26: 1787–1799.e5. [DOI] [PubMed] [Google Scholar]
- 62.Nagae M, Yamada K, Enomoto Y, Kometani M, Tsuchida H, Panthee A, Nonogaki M, Matsunaga N, Takizawa M, Matsuzaki S, Hirabayashi M, Inoue N, Tsukamura H, Uenoyama Y. Conditional Oprk1-dependent Kiss1 deletion in kisspeptin neurons caused estrogen-dependent LH pulse disruption and LH surge attenuation in female rats. Sci Rep 2023; 13: 20495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Coutinho EA, Esparza LA, Hudson AD, Rizo N, Steffen P, Kauffman AS. Conditional deletion of KOR (Oprk1) in kisspeptin cells does not alter LH pulses, puberty, or fertility in mice. Endocrinology 2022; 163: bqac175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Croft S, Boehm U, Herbison AE. Neurokinin B activates arcuate kisspeptin neurons through multiple tachykinin receptors in the male mouse. Endocrinology 2013; 154: 2750–2760. [DOI] [PubMed] [Google Scholar]
- 65.Ruka KA, Burger LL, Moenter SM. Regulation of arcuate neurons coexpressing kisspeptin, neurokinin B, and dynorphin by modulators of neurokinin 3 and κ-opioid receptors in adult male mice. Endocrinology 2013; 154: 2761–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Anatomy of CNS opioid receptors. Trends Neurosci 1988; 11: 308–314. [DOI] [PubMed] [Google Scholar]