Abstract
Treatment satisfaction is a person’s rating of his or her treatment experience, including processes and outcomes. It is directly related to treatment adherence, which may be predictive of treatment effectiveness in clinical and real-world research. Consequently, patient-reported outcome (PRO) instruments have been developed to incorporate patient experience throughout various stages of drug development and routine care. PRO instruments enable clinicians and researchers to evaluate and compare treatment satisfaction data in different clinical settings. It is important to select fit-for-purpose PRO instruments that have demonstrated adequate levels of reliability, validity, and sensitivity to change to support their use. Some of these instruments are unidimensional while some are multidimensional; some are generic and can be applied across different therapeutic areas, while others have been developed for use in a specific treatment modality or condition. This article describes the role of treatment satisfaction in drug development as well as regulatory and Health Technology Assessment (HTA) decision making and calls for more widespread use of carefully selected treatment satisfaction PRO instruments in early- and late-phase drug development.
Key Points for Decision Makers
| This paper provides an overview of the role of treatment satisfaction in drug development, regulatory and HTA decision making. |
| The main goal is to call for more extensive use of fit-for-purpose PRO instruments to assess treatment satisfaction in all phases of drug development. |
Introduction
In the era of patient-centered drug development, it is critical for drug developers, regulators, payers, and researchers to collect and understand the patients’ perspectives on drugs (and other treatments) during their development [1, 2]. When a treatment is approved and made available for clinical use, data on how patients feel whilst taking the treatment provides healthcare professionals and patients with valuable insights, enabling the delivery of evidence-based medicine. Evidence-based medicine refers to the application of the best available research to clinical care, which requires the integration of evidence with clinical expertise and patient values [3]. The measurement of treatment satisfaction using a PRO instrument offers a standardized way of generating such data during treatment development.
Treatment Satisfaction Definition
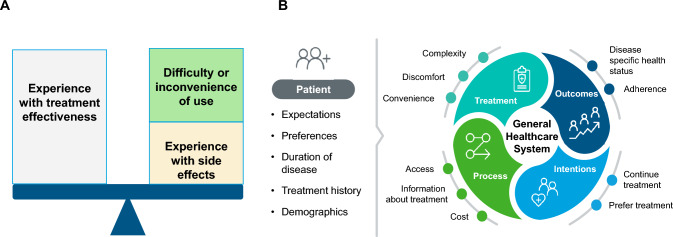
Treatment satisfaction is defined as the individual’s rating of important attributes of the process and outcomes of their treatment experience [4, 5]. An individual’s satisfaction with a treatment will be influenced by their knowledge and experience of the treatment. Specifically, perceived or experienced treatment effectiveness, administration complexity and convenience, discomfort and side effects (see the Decisional Balance Model of Treatment Satisfaction [6]; Fig. 1a), as well as cost of the treatment will inform how satisfied or dissatisfied an individual is with a treatment [7]. Patient expectations, demographic characteristics (age and education), and personal preferences can also affect treatment satisfaction, as can prior experience with disease and with treatment [8].
Fig. 1.
Treatment satisfaction framework: a Decision balance model of treatment satisfaction. b Adaptation of Weaver and colleagues' conceptual model of treatment satisfaction [9]
Treatment satisfaction can be a useful concept for researchers, intervention developers, and healthcare professionals wishing to understand the patient experience with treatment, and to differentiate among alternative treatments. Understanding treatment satisfaction can also help with understanding the likelihood of adherence and persistence to treatment [4]. This can ultimately lead to improved health status as depicted in the conceptual framework of treatment satisfaction developed by Weaver and colleagues [9] (Fig. 1b).
The association between treatment satisfaction, adherence, and persistence is clinically intuitive. If a patient is not satisfied with treatment, this feeling may negatively affect his or her behavior in terms of regimen execution as well as his or her willingness to persist with the treatment [6]. The connection between treatment satisfaction and persistence is even more important in chronic diseases where up to one half of patients make medication-related decisions without seeking medical advice [10]. Indeed, in chronic diseases, patient dissatisfaction (rather than clinical consultation and decision making) is one of the main drivers of treatment discontinuation [6, 11–13], which in turn can lead to an increased rate of complications, deterioration in health, and ultimately death [6, 14, 15].
Understanding treatment satisfaction across multiple treatments can also help to predict patient preferences for alternative treatments—an important consideration when there are several options for treatment that involve alternate routes of administration, types of medication, or drug regimens [16] [17]. Research in oncology has shown, for instance, that treatment satisfaction and adherence are highest when people are offered treatment that is in line with their own preferences [18].
Treatment Satisfaction Measurement
Treatment satisfaction is a highly individual and personal experience. To understand this concept, researchers as well as healthcare providers must rely on patients’ reports [4]. Patient reports can be generated in two ways: through narrative exploration (i.e., by talking to patients to qualitatively understand their experiences) or through PRO instruments (i.e., using standardized questionnaires to generate quantitative data).
Qualitative research offers the opportunity to explore satisfaction in depth, including drivers of satisfaction and implications of being satisfied/dissatisfied in terms of feelings and behaviors. Qualitative research can, however, be intrusive; reactive to personalities, moods and interpersonal dynamics between interviewer and interviewee; expensive; and time consuming [19].
PRO instruments are measures of a patient’s perspective as reported directly from the patient without added interpretation by a healthcare worker or anyone else [20]. PRO instruments offer a way to collect patient information quickly and in a standardized manner and are thus frequently used to evaluate the impact of disease and treatments on the patient’s functioning, well-being, and everyday life in clinical trials [4].
There are a large number of PRO instruments measuring treatment satisfaction [21]. They differ on a number of parameters, including number of items, measurement properties, and targeted use.
Number of Items
Some treatment satisfaction PRO instruments consist of a single item measuring global treatment satisfaction [22]. Other intruments include multiple items, some of which may contribute to one overall rating of satisfaction, or they may measure different dimensions of satisfaction (efficacy, side effects, convenience) [23]. Single-item measures offer simplicity and speed. However, use of a single item can mean the loss of important information about how patients view a treatment. Most of the patients that answer single-item questionnaires, for example, report high levels of satisfaction regardless of other negative information [24].
Measurement Properties
PRO instruments need to demonstrate that they measure what they were designed to measure in a reliable, valid, and an interpretable way in order to be considered ‘fit for purpose’ to support regulatory, payer, and healthcare decision making. A ‘fit for purpose’ PRO instrument demonstrates the following measurement properties: reliability (internal consistency and test re-test), validity (content and construct), and responsiveness (sensitivity to change) [25]. Sound measurement properties are not just critical for PRO instruments but rather applicable to all measurement methodologies for data collection [26]. Without evidence of reliability, validity, and sensitivity to change, the PRO instrument may produce inconsistent results that cannot be replicated or compared across studies, leading to inaccurate or misleading study results and a risk of misattribution of outcomes to the treatment under investigation [26].
Targeted Use
Treatment satisfaction PRO instruments can be generic (i.e., designed for use across different disease/therapeutic populations) or disease/context-specific (i.e., built to address those aspects of satisfaction that are important for a particular and specific group of patients) [27] [28]. Generic instruments allow for comparisons between diseases, across different populations, or across medication types and patient conditions [29]. Whereas disease/context-specific instruments arguably possess greater potential for showing differences between competing therapies, they cannot be applied across populations [30]. Examples of generic and disease-specific questionnaires developed for use in routine care and drug development to assess treatment satisfaction from patients are presented in Tables 1 and 2, respectively [31–48].
Table 1.
Examples of generic treatment satisfaction patient-reported outcome (PRO) instruments
| Name | Objective | Population | Recall period | Items | Domains | Psychometric properties* |
|---|---|---|---|---|---|---|
| The Treatment Satisfaction with Medicines Questionnaire (SATMED-Q) [31, 32] | Measure patient satisfaction with chronic diseases (T2DM, arterial hypertension, arthrosis, BPH, COPD/asthma, depression, and migraine) subjected to any type of prolonged pharmacological treatment | Adult | The past month | 17 |
Treatment effectiveness (3 items) Convenience of use (3 items) Impact on daily activities (3 items) Medical care (2 items) Undesirable side effects (3 items) Global satisfaction (3 items) |
Reliability: Internal consistency reliability: Cronbach's alpha coefficient Treatment effectiveness: 0.813; Convenience of use 0.861; Impact on daily activities 0.851; Medical care 0.885; Undesirable side effects 0.912; Global satisfaction 0.855 Test-retest reliability (reproducibility) Pearson correlation coefficient: 0.945 and Intraclass Correlation Coefficient (ICC): 0.943 Validity: Clinical validity The assessments of the clinicians were used to establish four groups of effectiveness (poor, acceptable, good, excellent). The groups of effectiveness formed with the assessments of the clinicians differ in the total composite score and for all the subscales (p < 0.0005 in all cases) except Convenience (p = 0.315) and Undesirable side effects (p = 0.220) |
| The Treatment Satisfaction Questionnaire for Medication TSQM-1.4 [6] | To measure patients’ satisfaction with medication in chronic diseases such as coronary diseases [27], cystic fibrosis [35], hypertension [36], COPD [37], multiple sclerosis [38], diabetes [39], and autoimmune diseases such as psoriasis [40] and angiodema [41] | Adult | 2–3 weeks, or since the last medication use | 14 |
Side effects (5 items) Effectiveness (3 items) Convenience (3 items) Global satisfaction (3 items) |
Reliability: Internal consistency reliability: Cronbach's alpha coefficient Patients with arthritis, asthma, major depression, T1DM, high cholesterol, hypertension, migraine, n = 280 Effectiveness: 0.88; Side effects: 0.88; Convenience: 0.90; Global satisfaction: 0.86 Validity: Clinical validity Comparisons of mean side effects scores and mean effectiveness scores between individuals on medication for < 2 months and individuals on medication for a longer period. Mean effectiveness scores were (F(df) = 8.57, p = 0.004) lower in the < 2-months group (68.3 ± 18.8) than the longer period group (74.4 ± 17.2). Mean side effects scores were significantly (F(df) = 4.76, p = 0.03) lower in the < 2-months group (84.6 ± 16.4) than the longer period group (88.4 ± 14.2) Known-groups validity Comparisons of TSQM 1.4 scores between groups of patients classified by their route of medication administration: oral (n = 357), topical (n = 53), injection (n = 53) and inhaler (n = 62). Effectiveness by route F(3552) = 11.98, p < 0.0001; Side effects by route F(3552) = 5.87, p < 0.001; Convenience by route F(3552) = 58.92, p < 0.0001; Global by route F(3552) = 4.89, p < 0.01 |
| The Treatment Satisfaction Questionnaire for Medication TSQM-II [33] | To measure patients’ satisfaction with medication in chronic diseases such as coronary diseases [27], cystic fibrosis [35], hypertension [36], chronic obstructive pulmonary disease (COPD) [37], multiple sclerosis [38], diabetes [39], and autoimmune diseases such as psoriasis [40] and angiodema [41] | Adult | 2– 3 weeks, or since the last medication use | 11 |
Side effects (4 items) Effectiveness (2 items) Convenience (3 items) Global satisfaction scale (2 items) |
Reliability: Internal consistency reliability: Cronbach's alpha coefficient: Patients who started new medication (n = 342) Side effects: 0.91; Convenience: 0.91 Spearman's correlation coefficients (because these scales contain only 2 items): Effectiveness: 0.94 Overall satisfaction: 0.88 |
| The Treatment Satisfaction Questionnaire for Medication TSQM-9 [34] | 9 |
Effectiveness (3 items) Convenience (3 items) Global satisfaction scale (3 items) |
Reliability: Internal consistency reliability: Cronbach's alpha coefficient: Patients with hypertension and taking prescription medication for their hypertension (n = 396) Effectiveness: 0.935 (at day 1); 0.924 (between days 7–17) Convenience: 0.911 (at day 1); 0.915 (between days 7–17) Global satisfaction: 0.837 (at day 1); 0.848 (between days 7–17) Test-retest reliability (reproducibility) Patients with hypertension and taking prescription medication for their hypertension (n = 396) Intraclass Correlation Coefficient (ICC): Effectiveness: 0.78 Convenience: 0.74 Global satisfaction: 0.76 Validity: Known-groups validity Compliers of medication measured with the Modified Morisky Scale Low compliers (Modified Morisky Scale < 6); n= 200 Medium compliers (Modified Morisky Scale ≥6 but < 7); n = 195 High compliers (Modified Morisky Scale = 7); n = 1 Concurrent validity Patients with hypertension and taking prescription medication for their hypertension (n = 396). A correlation was found between the Modified Morisky Scale and the medication adherence domain scores (r = 0.46), the effectiveness domain scores (r = 0.38) and the global satisfaction domain scores (r = 0.34) |
|||
| The Functional Assessment of Chronic Illness Therapy Patient Satisfaction (FACIT-TS-PS) [42] | To assess patient satisfaction with treatment for chronic illnesses such as cancer and HIV/AIDS | Adult | No information provided | 29 |
Physician communication (12 items) Treatment staff communication (4 items) Technical competence (3 items) Nurse communication (3 items) Confidence and trust (4 items) Overall (3 items) + 1 open-ended question for comments |
Reliability: Internal consistency reliability: Cronbach's alpha coefficient: Physician communication (n = 293): 0.95 Treatment staff communication (n = 414): 0.89 Technical competence (n = 49): 0.86 Confidence and trust (n = 58): 0.72 Nurse communication (n = 289): 0.93 Validity: Clinical validity Patients with lung, breast or other types of cancer: n = 288 Response of FACIT-TS-PS overall item 39 “would you choose this clinic or office again ?”: Yes, Maybe or No FACIT-TS-PS subscales were able to discriminate between patients according to their overall treatment satisfaction, with significantly higher mean scores observed in patients reporting higher general satisfaction Patients with lung, breast or other types of cancer: n = 291 Response of FACIT-TS-PS overall item 40 “How do you rate the care you received ?”: Excellent, Very good, Good, or Fair FACIT-TS-PS subscales were able to discriminate between patients according to their overall treatment satisfaction, with significantly higher mean scores observed in patients reporting higher general satisfaction Concurrent validity Patients with various types of cancer or HIV: n = 58 Correlations between FACIT-TS-PS and PSQ III subscales ranged from 0.40 to 0.70, with all p < 0.001, except correlations of FACIT-TS-PS Nurse communication with PSQ III general (0.42; p < 0.01) and with PSQ III communication (0.30; p < 0.01) |
BPH benign prostate hyperplasia, COPD chronic obstructive pulmonary disease, T1DM type 1 diabetes mellitus, T2DM type 2 diabetes mellitus
*Psychometric properties data sourced from ePROVIDE’s PROQOLID Database [43]
Table 2.
Examples of disease-specific treatment satisfaction patient-reported outcome (PRO) instruments
| Name | Objective | Population | Recall period | Items | Domains | Psychometric properties* |
|---|---|---|---|---|---|---|
|
The Diabetes Treatment Satisfaction Questionnaire (DTSQ): |
To measure satisfaction with diabetes treatment regimens in people with diabetes and changes in satisfaction with treatment | Adult |
DTSQs: over the past few weeks DTSQc: 6 months ago (before you changed to the medication you are using now) |
8 |
Satisfaction with treatment (6 items) Perceived hyperglycaemia (1 item) Perceived hypoglycaemia (1 item) |
Reliability: Internal consistency reliability: Cronbach's alpha coefficient Insulin-dependent diabetes; n = 128 0.76 (For the Satisfaction with treatment scale) Type 2 diabetes; n = 181 Cronbach's alpha coefficient 0.79 Validity: Clinical validity Type 2 diabetes; n = 181 According to the percent of ideal body weight, HbA1 levels, subjective estimates of diabetic control Greater treatment satisfaction was associated with being less overweight (r = −0.19; p < 0.01); better blood glucose control as indicated by lower HbA1 levels (r = −0.28; p < 0.001); optimistic patient estimates of recent diabetic control (r = −0.56; p < 0.001) |
| Cancer Therapy Satisfaction Questionnaire (CTSQ) [46, 47] | To measure satisfaction with and preference for chemotherapy treatment, and for biological therapy in either pill or intravenous administration form | Adult | In the last 4 weeks | 16 |
Expectations of Therapy (ET): 5 items Feelings about side effects (FSE): 4 items Satisfaction with therapy (SWT): 7 items |
Reliability: Internal consistency reliability; Cronbach's alpha coefficients: Oncology patients; n = 361 Expectations of therapy: 0.87; Feelings about side effects: 0.77; Satisfaction with therapy: 0.82; Convenience: 0.60 Test-retest reliability (reproducibility): Intraclass correlation coefficient: Oncology patients; n = 53 Expectations of therapy: 0.68; Feelings about side effects: 0.82; Satisfaction with therapy: 0.732 Validity: Clinical validity Oncology patients; n = 361. Cancer stage: ANOVA. ET domain (p = 0.005). Effect size 0.67 Stages I and IV. ECOG performance status. ET domain. Grades 0, 1, and 2 (p = 0.0007). Effect size 0.46 for Grades 0 vs 1. Correlation coefficient: one-way ANOVA Ability to detect change: Minimal Important Difference (MID): ET: 9.59 (0.5 SD of baseline scores); FSE 11.00 (0.5 SD of baseline scores); SWT: 6.88 (0.5 SD of baseline scores) |
| The Erectile Dysfunction Index of Treatment Satisfaction (EDITS) [48] | To assess satisfaction with medical treatments for erectile dysfunction | Adult | In the past 4 weeks |
Patient: 11 Partner: 5 |
Treatment satisfaction |
Reliability: Internal consistency reliability Couples with men having erectile dysfunction: n = 28 couples for the Patient EDITS version, and n = 29 couples for the Partner EDITS version Cronbach's alpha coefficient: 0.90 for the patients; 0.76 for the partners Test-retest reliability (reproducibility) Couples with men having erectile dysfunction: n = 28 couples for the Patient EDITS version, and n = 29 couples for the Partner EDITS version Spearman rank-order correlations: 0.98 for the Patient EDITS and 0.83 for the Partner EDITS |
| Pain Treatment Satisfaction Scale (PTSS) [48] | To measure patient satisfaction for patients receiving treatment for either acute or chronic pain | Adult | Present time, last week or last 24 hours | 39 + 22 not scored |
Information (5 items) Medical care (8 items) Impact of current pain medication (8 items) Satisfaction with pain medication (2 subscales: medication characteristics [3 items] + efficacy [3 items]) Side effects (12 items) + general health items (6 items) and stand-alone questions (not scored—providing complementary information) |
Reliability: Internal consistency reliability: Cronbach's alpha coefficient: Patients with acute pain (n = 111) and chronic pain (n = 89), n = 208 Satisfaction with current pain medication: 0.90; Efficacy subscale: 0.90; Medication characteristics subscale: 0.85; Side effects of medication: 0.83; Impact of current pain medication: 0.92; Medical care: 0.86; Information about pain and its treatment: 0.89 Test-retest reliability (reproducibility) a) Intraclass Correlation Coefficient (ICC) Satisfaction with current pain medication: 0.974; Efficacy subscale: 0.76; Medication characteristics subscale: 0.55; Side effects of medication: 0.67; Impact of current pain medication: 0.68; Medical care: 0.81; Information about pain and its treatment: 0.76 b) Wilcoxon signed rank test Patients with chronic pain; n = 87. All dimensions except information, mean scores were not significantly different between baseline and week 2; p > 0.05 Validity: Clinical validity Patients with acute pain (n = 111) and chronic pain (n = 89), n = 208. Spearman's correlation coefficient Satisfaction with current pain medication: −0.48; Efficacy subscale: −0.53; Medication characteristics subscale: −0.35; Side effects of medication: −0.17; Impact of current pain medication: −0.25; Medical care: −0.32; Information about pain and its treatment: −0.29 Known-groups validity Patients with acute pain (n = 111) and chronic pain (n = 89), n = 208 Pain severity in the last week: Scores were significantly lower (except medical care) in patients with severe pain. Pain severity after treatment: Scores were significantly lower in all scales (p < 0.05) in patients with severe pain. Pain severity in the last week, in the last 24 h and right now: PTSS scores were systematically lower in patients reporting more severe pain. The differences were significant for medication characteristics and side effects Ability to detect change: Patients with acute pain: n = 104. Mean ABLE change scores differed significantly for the improved group of patients based on the change in pain (p < 0.05), Wilcoxon signed rank test |
*Psychometric properties data sourced from ePROVIDE’s PROQOLID Database [43]
Treatment Satisfaction in Drug Development
The measurement of treatment satisfaction should not be prioritized over efficacy, safety, or survival data (which have been frequently used as primary indicators for drug development [52]). However, as barriers to developing new products increase, and the number of markets with generic competition or at least multiple alternative treatments grow, satisfaction can be an important secondary endpoint to provide information about how people feel about the treatment they took in the trial and provide evidence of the value (or concerns) of certain treatments. This can support key efficacy, safety, and survival endpoints [53].
Thus, treatment satisfaction has become an important outcome for drug development [54], particularly in trials (1) comparing treatments that present differences in terms of efficacy or side effects; (2) comparing treatments that are similar in terms of efficacy but have different routes of administration or dosing schedules; or (3) where demonstration of satisfaction with a medication relative to a comparator is considered to indicate adherence benefits [16] and/or treatment effectiveness [55]. Generic and disease-specific, multidimensional, and single-item PRO instruments can be useful to measure treatment satisfaction in clinical trials for novel drugs in development. But to do so, they must have demonstrated evidence of reliability, validity, and responsiveness for the intended use.
The use of PRO treatment satisfaction instruments in clinical research has increased in recent years, in line with various initiatives focusing on increasing the patient perspective in drug development [56]. From the authors’ recently completed review of clinicaltrials.gov data, it was found that 4978 clinical studies assessed a treatment satisfaction endpoint between 2004 and 2015, and 8488 clinical studies assessed a treatment satisfaction endpoint between 2016 and 2023 (data on file). The evaluation of treatment satisfaction as an outcome in drug development, however, only represents a small fraction of the total studies undertaken during this time (3.3%). The recent development of clear guidelines from regulators for the use of PRO instruments to support clinical trial evidence (e.g., the FDA Patient-Focused Drug Development [PFDD] [20] guidance), an increased concern towards patient centricity throughout the product evidence lifecycle, and an increase in the development of drugs that differentiate through non-efficacy parameters (e.g., by frequency or modality of administration, side-effect profiles, etc.) suggests that treatment satisfaction endpoints in clinical trials are likely to increase in coming years.
Where treatment satisfaction has been measured in clinical trials, it has tended to be in the later phases of drug development. An analysis of clinicaltrials.gov data on the use of the Treatment Satisfaction Questionnaire for Medication (TSQM) over the 5-year period between 2016–2021 demonstrates that TSQM has been more frequently used in phase III interventional studies than in phase II or phase I trials [54]. Its use in later phase trials makes sense. Once the safety and efficacy of a drug have been explored in an early phase study, measuring domains of satisfaction helps researchers and sponsors understand why one compound, dose, or method of administration may be preferred over another, predict adherence, and support messages regarding the value of the product to patients. However, treatment satisfaction may also have an important role to play in earlier phases of drug development. Treatment satisfaction in dose finding research (phase I/II) can inform the selection of doses for later trials, especially for products used for the treatment of chronic conditions that require adherence to medication over long periods of time. In such trials, an understanding of satisfaction with treatment can offer some insight and hypotheses [24]. For example, treatment satisfaction data can evaluate medical treatment in clinical trials, contributes to quality assurance, and facilitates product differentiation [57]. Specifically, in the field of cancer clinical trials, reported levels of treatment satisfaction added a unique view for the evaluation of treatment efficacy [58].
Treatment satisfaction data is also important in post-registration (phase IIIb/IV) real-world settings because it can provide valuable insight into the economic valuations and cost-effectiveness assessments of medical products, such as whether or not a treatment is worthy of reimbursement [59]. Real-world evidence (RWE) studies involve a greater number of diverse patients and in general a more representative population [60], which can further help inform regulatory decisions, reimbursement, and health policy-making. There are several measures of treatment satisfaction that have been used in RWE studies. For example, the TSQM has been used to measure treatment satisfaction in amyotrophic lateral sclerosis [61] [62], the Treatment Satisfaction with Medicines Questionnaire (SATMED-Q) in acromegalia patients [63], the Diabetes Treatment Satisfaction Questionnaire (DTSQ) in patients with type 2 diabetes [64], and the Cancer Therapy Satisfaction Questionnaire (CTSQ) in metastatic squamous cell carcinoma of the head and neck [65].
Patient-centered drug development is a shift in the way that drugs are developed, involving patients in all phases of drug development. In patient-centered research, patients are considered co-researchers informing the decisions about unmet needs, trial endpoints, trial design, and execution. Drug development companies that incorporate patient voice through treatment satisfaction PRO instruments are more likely to ensure a fit of their product to the patients’ needs in routine practice and provide the benefits patients are seeking. Specifically, treatment satisfaction measures allow for treatment comparison in clinical trials or the identification of the need to switch a patient's treatment in clinical practice. Additionally, these measures can address, among other outcomes, the willingness of patients to accept the negative effects of their treatment, adherence to the prescribed medication, and can be related to the overall effectiveness of their treatment [23]. Therefore, we highly recommend assessing treatment satisfaction in the different stages of drug development: during the initial development and validation, as well as at the point of implementation and communication of the results. Furthermore, it is more probable that this data can be proactively utilized to aid in regulatory decision making.
Treatment Satisfaction in Regulatory Decision Making
The regulatory environment is primed to consider data on treatment satisfaction from drug development. Both the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have noted the critical importance of involving patients in the identification of health priorities and the outcomes desired from health interventions and in understanding the patient experience with these interventions [66]. Data from reliable, valid, and responsive PRO instruments can be considered as ‘fit for purpose’ and help regulators make approval decisions [49–51].
The EMA has a long history of working with patients and patient data. In 2005, a reflection paper was developed as a framework for interaction between EMA and patients, consumers, and consumer organizations to encourage the collection of PRO data [67]. In EMA’s ‘Regulatory Science Strategy to 2025,’ one core recommendation is to “ensure the patient voice is incorporated all along the regulatory lifecycle of a medicine”, reflecting the importance the Agency places on such engagement [67]. The FDA also has a long history of patient engagement, starting from 1988 with the formation of an office to work with patient advocates [66]. In 2009, the FDA developed the PRO Guidance that outlines the rigor used by regulators to review and evaluate existing, modified, or newly developed PRO instruments to support label claims [68]. More recently, the FDA launched its PFDD initiative as a commitment to capture and submit patient experience data and other relevant information from patients for drug development and regulatory decision making more systematically [20].
At the FDA and EMA, evidence supporting efficacy and safety of the medication being developed is included in the ‘label’ at the point of approval (FDA ‘label’ is the US Prescribing Information; EMA label is the Summary of Product Characteristics). The primary purpose of drug labeling is to give healthcare professionals the information they need to prescribe the medicine appropriately [69]. The label cannot include promotional, false, or misleading statements [69]. It can, however, include other information deemed to be relevant and important in understanding the medication, assuming that the data is derived from fit-for-purpose measurement in adequate and well-controlled clinical investigations. The EMA considers both single and multidimensional domains—such as health status and satisfaction with treatment—for inclusion in labelling [70]. While traditionally more focused on core signs and symptoms of disease, recent PFDD guidance and workshop discussion from FDA proposes satisfaction as one component of a benefit–risk appraisal [20] [71].
Data extracted from 2010 until 2023 indicates that 57 drugs or medical products have included treatment satisfaction claims in their label, all using PRO instruments [43]. The EMA has approved 19 drugs (33.3%) and 38 (66.6%) have been approved by the FDA. Various PRO instruments have been used to support these claims, including the aforementioned TSQM which meets the evidence needed by regulators to support label decisions in certain contexts of use [57]. The TSQM supported six of the aforementioned treatment satisfaction label claims (5/19 drugs the EMA approved with treatment satisfaction claims in their label and 1/38 drugs the FDA approved with treatment satisfaction claims in their label) [72–77]. However, this represents only a small fraction of drugs approved in this timescale.
Therefore, treatment satisfaction is appealing to agencies because of its utility as a well-known patient-reported endpoint that captures patient experience [54, 57]. The assessment of treatment satisfaction plays an increasingly important role in regulatory decision making which ultimately improves the quality and value of health care [78] [79].
Treatment Satisfaction in Health Technology Assessment (HTA)
HTA agencies play a vital role in assessing the safety, efficacy, cost, and benefits of new treatments [80], which requires consideration of the patient experience with the given treatment. Patients are going to be the first beneficiaries of health innovation and are best suited to evaluate treatment satisfaction. Therefore, some HTA agencies have been utilizing PRO instruments to capture the patient's voice when evaluating pharmacotherapies or medical technologies.
PRO instruments are a key component of decision making during the benefit–risk appraisal of new drugs or biologic products across different therapeutic areas [81]. Data from reliable, valid, and responsive (i.e., ‘fit for purpose’) PRO instruments can help HTA bodies make access decisions [49–51]. For example, when assessing the effectiveness of a drug, not only are the clinical outcomes significant to regulatory and reimbursement agencies, but also the drug's influence on patients’ daily lives, functional status, treatment satisfaction, preferences, and adherence [82]. The inclusion of treatment satisfaction measures is an effective way to assess and evaluate patient experience with the new treatment by HTA agencies. For example, treatment satisfaction measures can help HTA bodies choose between two treatments that have similar biomedical effects but present differences in terms of side effects, convenience, and mode of administration. Moreover, HTAs look for evidence to help inform formulary decisions, both at launch and during post-launch reviews. They may find that treatment satisfaction data can support and complement the traditional efficacy and safety data available from classical clinical endpoints [82]. However, there are substantial differences in HTA reimbursement decisions that could be explained by the different processes and policies in place at different HTA agencies, such as criteria for the extent of added value versus cost effectiveness [83]. Such discrepancies across countries make it challenging for sponsors not only to identify and utilize appropriate PRO instruments to capture the patient experience but also to develop appropriate methodologies for capturing these data within both clinical trial and real-world settings. However, HTA bodies have recognized treatment satisfaction can confirm clinical benefits and support reimbursement recommendations, and thus it is essential to continue to include treatment satisfaction as a key assessment throughout the drug development and commercialization process.
A Call to Action
Patients are in a unique position to provide treatment satisfaction assessment as they are the ones who experience the effectiveness and side effects of the therapy. Several PRO instruments offer robust fit-for-purpose (reliable, valid, sensitive) measurement of treatment satisfaction, and research has shown these can predict the likelihood of patients continuing to use their medication, the correct usage of the medication, and adherence to the treatment. It is also known that treatment satisfaction can support drug development and needs to be considered by most of the stakeholders involved in the healthcare system, from development to launch of a product and within routine clinical practice use. Moreover, the FDA and EMA have approved treatment satisfaction in label claims of certain medications. Measuring treatment satisfaction more frequently in clinical trials and studies will give us a comprehensive understanding of patient health status, facilitating appropriate and optimal treatment decisions and improving future drug development.
We encourage measuring treatment satisfaction across the phases of interventional studies and RWE studies as doing so can be beneficial for the different stakeholders involved in drug development and regulatory decision making: (1) for pharmaceutical companies, satisfaction with a specific type of medication should lead to a differential advantage in the marketplace, product success, manufacturer profitability, and better market access; (2) for healthcare systems, understanding patient satisfaction is a critical pillar to develop more efficient and effective care models; (3) for patients, higher treatment satisfaction can lead to increased treatment adherence and better clinical outcomes.
Acknowledgments
We would like to extend our sincere gratitude to Dr Matthew Reaney, Dr David Bard, and Jodi Andrews for useful discussions and insightful comments.
Declarations
Funding
No external funding was received to assist with the preparation of this manuscript. All authors are IQVIA employees.
Data availability
Not applicable.
Author Contributions
All authors contributed to the study conception and design. The first draft of the manuscript was written by Carolina Navas and all authors commented on previous versions of the manuscript. All authors read, edited, and approved the final manuscript.
References
- 1.Algorri M, Cauchon NS, Christian T, O’Connell C, Vaidya P. Patient-centric product development: a summary of select regulatory CMC and device considerations. J Pharm Sci. 2023;112(4):922–36. 10.1016/j.xphs.2023.01.029. (Epub 2023 Feb 3). [DOI] [PubMed] [Google Scholar]
- 2.van Overbeeke E, Vanbinst I, Jimenez-Moreno AC, Huys I. Patient centricity in patient preference studies: the patient perspective. Front Med. 2020;7:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reena Pattani, Sharon E. Straus. What is EBM?.BMJ Best Practice. 2023.https://bestpractice.bmj.com/info/toolkit/learn-ebm/what-is-ebm/. Accessed 10 Oct 2023.
- 4.Revicki D. Patient assessment of treatment satisfaction: methods and practical issue. Gut. 2004. 10.1136/gut.2003.0343225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salame N, Perez-Chada LM, Singh S, CallisDuffin K, Garg A, Gottlieb AB, et al. Are your patients satisfied a systematic review of treatment satisfaction measures in psoriasis. Dermatology. 2018;234(5–6):157–65. [DOI] [PubMed] [Google Scholar]
- 6.Atkinson MJ, Sinha A, Hass SL, Colman SS, Kumar RN, Brod M, et al. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes. 2004;2(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barbosa CD, Balp MM, Kulich K, Germain N, Rofail D. A literature review to explore the link between treatment satisfaction and adherence, compliance, and persistence. Patient Prefer Adherence. 2012;6:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kravitz RL. Patients’ expectations for medical care: an expanded formulation based on review of the literature. Med Care Res Rev MCRR. 1996;53(1):3–27. [DOI] [PubMed] [Google Scholar]
- 9.Weaver M, Patrick DL, Markson LE, Martin D, Frederic I, Berger M. Issues in the measurement of satisfaction with treatment. Am J Manag Care. 1997;3(4):579–94 (PMID: 10169526). [PubMed] [Google Scholar]
- 10.Lemay J, Waheedi M, Al-Sharqawi S, Bayoud T. Medication adherence in chronic illness: do beliefs about medications play a role? Patient Prefer Adherence. 2018;12:1687–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandez-Lazaro CI, García-González JM, Adams DP, Fernandez-Lazaro D, Mielgo-Ayuso J, Caballero-Garcia A, et al. Adherence to treatment and related factors among patients with chronic conditions in primary care: a cross-sectional study. BMC Fam Pract. 2019;20(1):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schoemaker JH, Vingerhoets AJJM, Emsley RA. Factors associated with poor satisfaction with treatment and trial discontinuation in chronic schizophrenia. CNS Spectr. 2019;24(4):380–9. [DOI] [PubMed] [Google Scholar]
- 13.Baryakova TH, Pogostin BH, Langer R, McHugh KJ. Overcoming barriers to patient adherence: the case for developing innovative drug delivery systems. Nat Rev Drug Discov. 2023;22(5):387–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fenton JJ, Jerant AF, Bertakis KD, Franks P. The cost of satisfaction: a national study of patient satisfaction, health care utilization, expenditures, and mortality. Arch Intern Med. 2012;172(5):405–11. [DOI] [PubMed] [Google Scholar]
- 15.Hamine S, Gerth-Guyette E, Faulx D, Green BB, Ginsburg AS. Impact of mHealth chronic disease management on treatment adherence and patient outcomes: a systematic review. J Med Internet Res. 2015;17(2): e3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shikiar R, Rentz AM. Satisfaction with medication: an overview of conceptual, methodologic, and regulatory issues. Value Health J Int Soc Pharmacoecon Outcomes Res. 2004;7(2):204–15. [DOI] [PubMed] [Google Scholar]
- 17.Lindhiem O, Bennett CB, Trentacosta CJ, McLear C. Client preferences affect treatment satisfaction, completion, and clinical outcome: a meta-analysis. Clin Psychol Rev. 2014;34(6):506–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fallowfield L, Osborne S, Langridge C, Monson K, Kilkerr J, Jenkins V. Implications of subcutaneous or intravenous delivery of trastuzumab; further insight from patient interviews in the PrefHer study. Breast. 2015;24(2):166–70. 10.1016/j.breast.2015.01.002. (Epub 2015 Jan 24 PMID: 25623753). [DOI] [PubMed] [Google Scholar]
- 19.Matrisch L, Rau Y, Karsten H, Graßhoff H, Riemekasten G. The Lübeck medication satisfaction questionnaire—a novel measurement tool for therapy satisfaction. J Pers Med. 2023;13(3):505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Research C for DE and. FDA Patient-Focused Drug Development Guidance Series for Enhancing the Incorporation of the Patient’s Voice in Medical Product Development and Regulatory Decision Making. FDA [Internet]. 2023; https://www.fda.gov/drugs/development-approval-process-drugs/fda-patient-focused-drug-development-guidance-series-enhancing-incorporation-patients-voice-medical. Accessed 10 Oct 2023
- 21.Churruca K, Pomare C, Ellis LA, Long JC, Henderson SB, Murphy LED, et al. Patient-reported outcome measures (PROMs): A review of generic and condition-specific measures and a discussion of trends and issues. Health Expect Int J Public Particip Health Care Health Policy. 2021;24(4):1015–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waltz TJ, Campbell DG, Kirchner JE, Lombardero A, Bolkan C, Zivin K, et al. Veterans with depression in primary care: provider preferences, matching, and care satisfaction. Fam Syst Health. 2014;32(4):367–77. [DOI] [PubMed] [Google Scholar]
- 23.Speight J. Assessing patient satisfaction: concepts, applications, and measurement. Value Health. 2005;8:S6-8. [DOI] [PubMed] [Google Scholar]
- 24.Hareendran A, Abraham L. Using a treatment satisfaction measure in an early trial to inform the evaluation of a new treatment for benign prostatic hyperplasia. Value Health. 2005;8(Suppl 1):S35-40. 10.1111/j.1524-4733.2005.00074.x. (PMID: 16336487). [DOI] [PubMed] [Google Scholar]
- 25.Ahmed I, Ishtiaq S. Reliability and validity importance in medical research. J Pak Med Assoc. 2021;8(71):2403. [DOI] [PubMed] [Google Scholar]
- 26.Clinton-McHarg T, Yoong SL, Tzelepis F, Regan T, Fielding A, Skelton E, et al. Psychometric properties of implementation measures for public health and community settings and mapping of constructs against the Consolidated Framework for Implementation Research: a systematic review. Implement Sci. 2016;11(1):148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liberato ACS, Rodrigues RCM, São-João TM, Alexandre NMC, Gallani MCBJ. Satisfaction with medication in coronary disease treatment: psychometrics of the Treatment Satisfaction Questionnaire for Medication. Rev Lat Am Enfermagem. 2016;24(e2705):S0104-11692016000100334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Usmani SZ, Mateos MV, Hungria V, Iida S, Bahlis NJ, Nahi H, et al. Greater treatment satisfaction in patients receiving daratumumab subcutaneous vs. intravenous for relapsed or refractory multiple myeloma: COLUMBA clinical trial results. J Cancer Res Clin Oncol. 2021;147(2):619–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delestras S, Roustit M, Bedouch P, Minoves M, Dobremez V, Mazet R, et al. Comparison between two generic questionnaires to assess satisfaction with medication in chronic diseases. PLoS ONE. 2013;8(2): e56247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKenna SP. Measuring patient-reported outcomes: moving beyond misplaced common sense to hard science. BMC Med. 2011;14(9):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruiz MA, Pardo A, Rejas J, Soto J, Villasante F, Aranguren JL. Development and validation of the “Treatment Satisfaction with Medicines Questionnaire” (SATMED-Q). Value Health J Int Soc Pharmacoecon Outcomes Res. 2008;11(5):913–26. [DOI] [PubMed] [Google Scholar]
- 32.Rejas J, Ruiz M, Pardo A, Soto J. Detecting changes in patient treatment satisfaction with medicines: the SATMED-Q. Value Health. 2013;16(1):88–96. [DOI] [PubMed] [Google Scholar]
- 33.Atkinson MJ, Kumar R, Cappelleri JC, Hass SL. Hierarchical construct validity of the treatment satisfaction questionnaire for medication (TSQM Version II) among Outpatient Pharmacy Consumers. Value Health. 2005;8:S9-24. [DOI] [PubMed] [Google Scholar]
- 34.Bharmal M, Payne K, Atkinson MJ, Desrosiers MP, Morisky DE, Gemmen E. Validation of an abbreviated Treatment Satisfaction Questionnaire for Medication (TSQM-9) among patients on antihypertensive medications. Health Qual Life Outcomes. 2009;7(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Regnault A, Balp MM, Kulich K, Viala-Danten M. Validation of the treatment satisfaction questionnaire for medication in patients with cystic fibrosis. J Cyst Fibros Off J Eur Cyst Fibros Soc. 2012;11(6):494–501. [DOI] [PubMed] [Google Scholar]
- 36.Zyoud SH, Al-Jabi SW, Sweileh WM, Morisky DE. Relationship of treatment satisfaction to medication adherence: findings from a cross-sectional survey among hypertensive patients in Palestine. Health Qual Life Outcomes. 2013;11(1):191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Contoli M, Rogliani P, Di Marco F, Braido F, Corsico AG, Amici CA, et al. Satisfaction with chronic obstructive pulmonary disease treatment: results from a multicenter, observational study. Ther Adv Respir Dis. 2019;13:1753466619888128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hao J, Pitcavage J, Jones JB, Hoegerl C, Graham J. Measuring adherence and outcomes in the treatment of patients with multiple sclerosis. J Osteopath Med. 2017;117(12):737–47. [DOI] [PubMed] [Google Scholar]
- 39.Khdour MR, Awadallah HB, Al-Hamed DH. Treatment satisfaction and quality of life among type 2 diabetes patients: a cross-sectional study in West Bank, Palestine. J Diabetes Res. 2020;25(2020):1834534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abdshah A, Parsaeian M, Nasimi M, Ghiasi M. Validating the “Treatment Satisfaction Questionnaire for Medication” in Persian and Evaluating Treatment Satisfaction Among Patients With Psoriasis. Value Health Reg Issues. 2022;29:16–20. [DOI] [PubMed] [Google Scholar]
- 41.Fijen LM, Klein PCG, Cohn DM, Kanters TA. The disease burden and societal costs of hereditary angioedema. J Allergy Clin Immunol Pract. 2023;11(8):2468-2475.e2. [DOI] [PubMed] [Google Scholar]
- 42.Peipert JD, Beaumont JL, Bode R, Cella D, Garcia SF, Hahn EA. Development and validation of the functional assessment of chronic illness therapy treatment satisfaction (FACIT TS) measures. Qual Life Res. 2014;23(3):815–24. [DOI] [PubMed] [Google Scholar]
- 43.ePROVIDETM-Online Support for Clinical Outcome Assessments [Internet]. ePROVIDE - Mapi Research Trust. https://eprovide.mapi-trust.org/. Accessed 10 Oct 2023
- 44.Bradley C, Plowright R, Stewart J, Valentine J, Witthaus E. The Diabetes Treatment Satisfaction Questionnaire change version (DTSQc) evaluated in insulin glargine trials shows greater responsiveness to improvements than the original DTSQ. Health Qual Life Outcomes. 2007;10(5):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DTSQ - Diabetes Treatment Satisfaction Questionnaire [Internet]. Health Psychology Research Ltd. [cited 2023 Dec 18]. Available from: https://healthpsychologyresearch.com/guidelines/dtsq-diabetes-treatment-satisfaction-questionnaire/.
- 46.Abetz L, Coombs JH, Keininger DL, Earle CC, Wade C, Bury-Maynard D, et al. Development of the cancer therapy satisfaction questionnaire: item generation and content validity testing. Value Health J Int Soc Pharmacoeconomics Outcomes Res. 2005;8(Suppl 1):S41-53. [DOI] [PubMed] [Google Scholar]
- 47.Trask PC, Tellefsen C, Espindle D, Getter C, Hsu MA. Psychometric validation of the cancer therapy satisfaction questionnaire. Value Health J Int Soc Pharmacoecon Outcomes Res. 2008;11(4):669–79. [DOI] [PubMed] [Google Scholar]
- 48.Althof SE, Corty EW, Levine SB, Levine F, Burnett AL, McVary K, et al. EDITS: development of questionnaires for evaluating satisfaction with treatments for erectile dysfunction 1. Urology. 1999;53(4):793–9. [DOI] [PubMed] [Google Scholar]
- 49.Gilbride CJ, Wilson A, Bradley-Gilbride A, Bayfield J, Gibson K, Gohel M, et al. Design of a treatment satisfaction measure for patients undergoing varicose vein treatment: Venous Treatment Satisfaction Questionnaire (VenousTSQ). Br J Surg. 2023;110(2):200–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Friedel AL, Siegel S, Kirstein CF, Gerigk M, Bingel U, Diehl A, et al. Measuring patient experience and patient satisfaction—how are we doing it and why does it matter? A comparison of European and U.S. American Approaches. Healthcare. 2023;11(6):797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khanna PP, Shiozawa A, Walker V, Bancroft T, Essoi B, Akhras KS, et al. Health-related quality of life and treatment satisfaction in patients with gout: results from a cross-sectional study in a managed care setting. Patient Prefer Adherence. 2015;9(9):971–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lenderking WR. Brief reflections on treatment satisfaction. Value Health. 2005;8(s1):s2-5. [DOI] [PubMed] [Google Scholar]
- 53.Doward LC, Gnanasakthy A, Baker MG. Patient reported outcomes: looking beyond the label claim. Health Qual Life Outcomes. 2010;20(8):89. 10.1186/1477-7525-8-89.PMID:20727176;PMCID:PMC2936442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Using Patient Reported Treatment Satisfaction in Clinical Research and Beyond [Internet]. 2023. https://www.iqvia.com/events/2023/08/using-patient-reported-treatment-satisfaction-in-clinical-research-and-beyond. Accessed 10 Oct 2023.
- 55.Mehari EA, Muche EA, Gonete KA, Shiferaw KB. Treatment satisfaction and its associated factors of dolutegravir based regimen in a resource limited setting. Patient Prefer Adherence. 2021;15:1177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosenberg S. Trial Participants Are Heroes, Let’s Treat Them That Way. Appl Clin Trials [Internet]. 2023 Sep 8 [cited 2023 Sep 24];31(5). Available from: https://www.appliedclinicaltrialsonline.com/view/measuring-patient-satisfaction-as-a-primary-outcome-for-patient-centric-initiatives. Accessed 10 Oct 2023.
- 57.Rodriguez AM, Gemmen E, Minton AP, Parmenter L. Satisfaction With Treatment. [Internet]. https://www.iqvia.com/-/media/iqvia/pdfs/library/white-papers/satisfaction-with-treatment.pdf. Accessed 10 Oct 2023.
- 58.Brédart A, Bottomley A. Treatment satisfaction as an outcome measure in cancer clinical treatment trials. Expert Rev Pharmacoecon Outcomes Res. 2002;2(6):597–606. [DOI] [PubMed] [Google Scholar]
- 59.Naidoo P, Bouharati C, Rambiritch V, Jose N, Karamchand S, Chilton R, et al. Real-world evidence and product development: opportunities, challenges and risk mitigation. Wien Klin Wochenschr. 2021;133(15–16):840–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ziemssen T, Richter S, Mäurer M, Buttmann M, Kreusel B, Poehler AM, et al. OzEAN study to collect real-world evidence of persistent use, effectiveness, and safety of ozanimod over 5 years in patients with relapsing-remitting multiple sclerosis in Germany. Front Neurol [Internet]. 2022. 10.3389/fneur.2022.913616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Witzel S, Maier A, Steinbach R, Grosskreutz J, Koch JC, Sarikidi A, et al. Safety and effectiveness of long-term intravenous administration of edaravone for treatment of patients with amyotrophic lateral sclerosis. JAMA Neurol. 2022;79(2):121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meyer T. Real world experience of patients with amyotrophic lateral sclerosis (ALS) in the treatment of spasticity using tetrahydrocannabinol:cannabidiol (THC:CBD). 2019; [DOI] [PMC free article] [PubMed]
- 63.Cámara R, Venegas E, García-Arnés JA, Cordido F, Aller J, Samaniego ML, Mir N, Sánchez-Cenizo L. Treatment adherence to pegvisomant in patients with acromegaly in Spain: PEGASO study. Pituitary. 2019;22(2):137–45. 10.1007/s11102-019-00943-1. (PMID: 30756345). [DOI] [PubMed] [Google Scholar]
- 64.Yale JF, Bodholdt U, Catarig AM, Catrina S, Clark A, Ekberg NR, et al. Real-world use of once-weekly semaglutide in patients with type 2 diabetes: pooled analysis of data from four SURE studies by baseline characteristic subgroups. BMJ Open Diabetes Res Care. 2022;10(2): e002619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gogate A, Bennett B, Poonja Z, Stewart G, Medina Colmenero A, Szturz P, et al. Phase 4 multinational multicenter retrospective and prospective real-world study of nivolumab in recurrent and metastatic squamous cell carcinoma of the head and neck. Cancers. 2023;15(14):3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boutin M, Dewulf L, Hoos A, Geissler J, Todaro V, Schneider RF, et al. Culture and process change as a priority for patient engagement in medicines development. Ther Innov Regul Sci. 2017;51(1):29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Committee for medicinal products human use. Reflection paper on the regulatory guidance for the use of health-related quality of life (HRQOL) measures in the evaluation of medicinal products. EMA. 2023; https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-regulatory-guidance-use-health-related-quality-life-hrql-measures-evaluation_en.pdf. Accessed 10 Oct 2023.
- 68.Reaney M, Whitsett J. Our Perspectives on the US FDA Patient-Focused Drug Development (PFDD) Guidance 3 and 4 Integrating patient experience data into endpoints to inform a COA endpoint strategy. IQVIA. https://www.iqvia.com/-/media/iqvia/pdfs/library/white-papers/our-perspectives-on-the-us-fda-patient-focused-drug-development-pfdd-guidance-3-and-4.pdf. Accessed 10 Oct 2023
- 69.Fang H, Harris S, Liu Z, Thakkar S, Yang J, Ingle T, et al. FDALabel for drug repurposing studies and beyond. Nat Biotechnol. 2020;38(12):1378–9. [DOI] [PubMed] [Google Scholar]
- 70.Jarosławski S, Auquier P, Borissov B, Dussart C, Toumi M. Patient-reported outcome claims in European and United States orphan drug approvals. J Mark Access Health Policy. 2018;6(1):1542920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Methods to Identify What is Important to Patients & Select, Develop or Modify Fit-for-Purpose Clinical Outcomes Assessments. [Internet]. 2018. https://www.fda.gov/media/116276/download. Accessed 8 Apr 2024.
- 72.Afinitor : EPAR - Summary for the public [Internet]. 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/afinitor. Accessed 8 Apr 2024.
- 73.Humira : EPAR - Medicine overview.pdf.. [Internet]. 2020. https://www.ema.europa.eu/en/medicines/human/EPAR/humira Accessed 8 Apr 2024.
- 74.Assessment report for paediatric studies submitted according to Article 46 of the Regulation (EC) No 1901/2006 .pdf.. [Internet]. 2006. https://www.ema.europa.eu/en/documents/variation-report/novoeight-h-c-2719-p46-0111-epar-assessment-report_en.pdf. Accessed 8 Apr 2024.
- 75.Tysabri : EPAR - Medicine overview.pdf. [Internet]. 2020. https://www.ema.europa.eu/en/documents/product-information/tysabri-epar-product-information_en.pdf. Accessed 8 Apr 2024.
- 76.Picato : EPAR - Summary for the public.pdf [Internet]. 2020. https://www.ema.europa.eu/en/medicines/human/EPAR/picato. Accessed 8 Apr 2024.
- 77.Package Insert - CUVITRU.pdf. [Internet]. 2016. https://www.fda.gov/media/100531/download. Accessed 8 Apr 2024.
- 78.HTA and Evaluation Methods Qualitative: 1. Introduction | EUPATI Open Classroom [Internet]. 2024. https://learning.eupati.eu/mod/page/view.php?id=492. Accessed 8 Apr 2024.
- 79.Cizek J. How payers can use outcomes data to enhance care and member experience [Internet]. 2023. https://clarifyhealth.com/insights/blog/how-payers-can-use-outcomes-data-to-enhance-care-and-member-experience/ Accessed 8 Apr 2024.
- 80.Wale JL, Thomas S, Hamerlijnck D, Hollander R. Patients and public are important stakeholders in health technology assessment but the level of involvement is low - a call to action. Res Involv Engagem. 2021;7(1):1. 10.1186/s40900-020-00248-9. (PMID:33402216;PMCID:PMC7783693). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brettschneider C, Lühmann D, Raspe H. Informative value of Patient Reported Outcomes (PRO) in Health Technology Assessment (HTA). GMS Health Technol Assess. 2011;7:Doc01. 10.3205/hta000092. (PMID: 21468289; PMCID: PMC3070434). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chassany O, Engen AV, Lai L, Borhade K, Ravi M, Harnett J, Chen CI, Quek RG. A call to action to harmonize patient-reported outcomes evidence requirements across key European HTA bodies in oncology. Future Oncol. 2022;18(29):3323–34. 10.2217/fon-2022-0374. (Epub 2022 Sep 2 PMID: 36053168). [DOI] [PubMed] [Google Scholar]
- 83.Oderda G, Brixner D, Biskupiak J, Burgoyne D, Arondekar B, Deal LS, et al. Payer perceptions on the use of patient-reported outcomes in oncology decision making. J Manag Care Spec Pharm. 2022;28(2):188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.