Abstract
Our previous studies have shown that targeting DNA vaccine-encoded major histocompatibility complex class I epitopes to the proteasome enhanced CD8+ T-cell induction and protection against lymphocytic choriomeningitis virus (LCMV) challenge. Here, we expand these studies to evaluate CD4+ T-cell responses induced by DNA immunization and describe a system for targeting proteins and minigenes to lysosomes. Full-length proteins can be targeted to the lysosomal compartment by covalent attachment to the 20-amino-acid C-terminal tail of lysosomal integral membrane protein-II (LIMP-II). Using minigenes encoding defined T-helper epitopes from lymphocytic choriomeningitis virus, we show that the CD4+ T-cell response induced by the NP309–328 epitope of LCMV was greatly enhanced by addition of the LIMP-II tail. However, the immunological consequence of lysosomal targeting is not invariably positive; the CD4+ T-cell response induced by the GP61–80 epitope was almost abolished when attached to the LIMP-II tail. We identify the mechanism which underlies this marked difference in outcome. The GP61–80 epitope is highly susceptible to cleavage by cathepsin D, an aspartic endopeptidase found almost exclusively in lysosomes. We show, using mass spectrometry, that the GP61–80 peptide is cleaved between residues F74 and K75 and that this destroys its ability to stimulate virus-specific CD4+ T cells. Thus, the immunological result of lysosomal targeting varies, depending upon the primary sequence of the encoded antigen. We analyze the effects of CD4+ T-cell priming on the virus-specific antibody and CD8+ T-cell responses which are mounted after virus infection and show that neither response appears to be accelerated or enhanced. Finally, we evaluate the protective benefits of CD4+ T-cell vaccination in the LCMV model system; in contrast to DNA vaccine-induced CD8+ T cells, which can confer solid protection against LCMV challenge, DNA vaccine-mediated priming of CD4+ T cells does not appear to enhance the vaccinee's ability to combat viral challenge.
The great majority of DNA vaccine studies published to date have focused on the induction of antibodies and/or CD8+ T cells; CD4+ T-cell responses have rarely been directly evaluated. Here, we have used the lymphocytic choriomeningitis virus (LCMV) model to analyze CD4+ T-cell induction by DNA vaccines. We have previously demonstrated that improving the degradation of endogenously expressed antigens in the proteasome enhanced the induction of CD8+ T-cell responses; covalent linkage of the antigen to the cellular protein ubiquitin marked the fusion protein for rapid hydrolysis in the proteasome, improved class I-antigen presentation and enhanced the protection induced by the DNA vaccines in mice, both against a virus (27, 29) and an invasive melanoma (48). In this report we present a parallel strategy aimed at improving the CD4+ responses induced by DNA immunization. Most CD4+ T-cell responses are induced by proteins endocytosed from the extracellular milieu by specialized antigen-presenting cells (APCs) such as dendritic cells (DC); these proteins are then degraded in the acidic endosomal and lysosomal compartments, where they encounter major histocompatibility complex (MHC) class II molecules, leading to the eventual cell surface presentation of their encoded epitopes. However, although the underlying mechanisms are not fully understood, it has been clearly demonstrated that some proteins synthesized within an APC can be presented by MHC class II molecules and can induce CD4+ T-cell responses (1, 4, 6, 21, 25, 32). This observation suggested that a DNA vaccine could be designed which should direct endogenously synthesized proteins to the lysosomal compartment of APCs, thus enhancing the induction of CD4+ T cells. To achieve our goal, we have used the lysosomal targeting signal of lysosomal integral membrane protein-II (LIMP-II) (41). Unlike other lysosomal proteins, which usually take an indirect route to the lysosome, LIMP-II moves directly from the endoplasmic reticulum to the lysosomal compartment, directed by residues in its C-terminal tail (22, 31, 40). Here, working mainly with the LCMV model, we report the cloning of the 20-amino-acid tail of LIMP-II in association with full-length proteins and with minigenes encoding LCMV MHC class II epitopes. We use these materials to ask the following questions. (i) Are the chimeric proteins directed to the lysosomes? (ii) What is the effect of this targeting on the induction of CD4+ T cells? (iii) Does lysosomal targeting of a viral protein enhance the induction of antiviral antibodies and/or CD8+ T cells? (iv) Do vaccine-induced virus-specific CD4+ T cells confer any advantage on the vaccinee after virus challenge? We find that lysosomal targeting by the LIMP-II tail is very effective. However, depending on the primary sequence of the antigen, improving its degradation in acidic lysosomes can enhance or inhibit the CD4+ T-cell responses induced. This observation has implications for vaccine design.
MATERIALS AND METHODS
Cell lines and viruses.
The MC57 cell line (H-2b) is maintained in RPMI medium, and HeLa cells are maintained in Dulbecco's modified Eagle's medium, each supplemented with 10% fetal calf serum, l-glutamine, and penicillin-streptomycin. The virus used was LCMV (Armstrong strain).
Mice.
C57BL/6 mice (H-2b) were obtained from the breeding colony at Scripps Research Institute and were used at 6 to 16 weeks of age.
Generation of plasmid constructs for efficient lysosomal delivery of the encoded proteins.
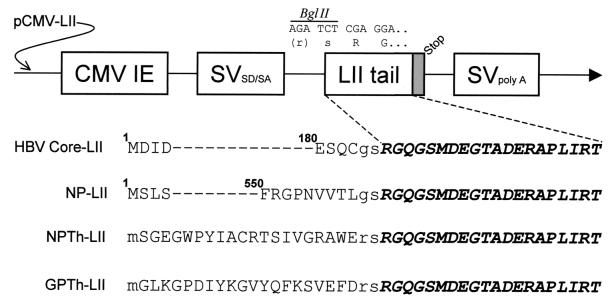
The 20-amino-acid C-terminal tail of LIMP-II has been shown to be sufficient for lysosomal targeting (22, 31, 40), and in an attempt to improve MHC class II presentation of plasmid-encoded endogenously expressed antigens, we constructed the five plasmids shown in Fig. 1. First, we constructed a parental vector, pCMV-LIMP-II, to act as the recipient for other open reading frames (ORFs) encoding proteins to be expressed as fusions with the LIMP-II tail. This plasmid was made by cloning complementary oligonucleotides encoding the 20-amino-acid LIMP-II tail, flanked by an upstream BglII site and a downstream stop codon, into the unique NotI site of the pCMV vector (Clontech, Palo Alto, Calif.). Four fusion constructs were made by cloning ORFs into the BglII site of pCMV-LIMP-II, upstream of and in-frame with the LIMP-II tail. To confirm that the LIMP-II tail directed proteins to the lysosomal compartment, two constructs were made by cloning the ORFs encoding LCMV NP and hepatitis B virus (HBV) core protein, into pCMV-LIMP-II, generating pCMV-HBVcore-LII and pCMV-NP-LII. In addition, to facilitate detailed analyses of the epitope-specific CD4+ T-cell responses induced by DNA vaccines, we constructed two plasmids containing minigenes encoding previously characterized LCMV epitopes which are presented by MHC class II I-Ab (24). pCMV-NPTh-LII encodes NP309–328 (SGEGWPYIACRTSIVGRAWE), and pCMV-GPTh-LII encodes GP61–80 (GLKGPDIYKGVYQFKSVEFD); in each case the minigene ORF is preceded by an ATG start codon, flanked by a good Kozak sequence (2, 16). In addition to these four plasmids encoding LIMP-II fusion proteins, four related plasmids were prepared, identical to the above but lacking the LIMP-II tail (pCMV-HBVcore, pCMV-NP, pCMV-NPTh, and pCMV-GPTh).
FIG. 1.
Sequences of the LIMP-II plasmids used in this study. The nucleic acid sequence and the related amino acids adjacent to the BglII cloning site are shown above a cartoon of the parental plasmid, pCMV-LIMP-II (pCMV-LII). CMV IE, human cytomegalovirus immediate-early promoter; SVSD/SA and SVpolyA, SV40 splice donor-acceptor and transcription terminator-polyadenylation signal, respectively; LII tail, 20 amino acids from the C terminus of LIMP-II. The stop codon which follows the LII tail is shown as a grey box. Partial or complete amino acid sequences are shown for each of the four LIMP-II constructs used in this study. For HBV core and LCMV NP, the superscripted numerals indicate the position in the full-length protein of the adjacent amino acid. In all cases, the native viral amino acids are shown in regular uppercase type, the 20-residue LIMP-II tail is shown in boldface italic uppercase type, and any additional amino acids inserted as a result of the cloning procedure are shown in regular lowercase type.
Protocol for DNA immunization.
DNA purification was carried out by standard techniques using Qiagen Megaprep columns with endotoxin removal buffer. DNA was dissolved in 1 N saline, at a concentration of 1 mg/ml, and C57BL/6 mice were immunized once with 50 μg of the specified plasmid injected into each anterior tibial muscle using a 28-gauge needle (thus, each mouse received 100 μg of DNA).
Evaluation of LIMP-II targeting by confocal microscopy.
HeLa cells were transfected (using Lipofectamine; GIBCO-BRL, Bethesda, Md.) with the plasmids pCMV-HBVcore, pCMV-HBVcore-LII, pCMV-NP, or pCMV-NP-LII and 48 h later were stained to evaluate protein expression and localization. The cells were incubated for 30 min at 37°C in phosphate-buffered saline–normal goat serum (1:100 dilution) to block nonspecific binding sites, and goat serum was maintained in all subsequent antibody incubations. To identify the lysosomal compartments, cells were incubated for 1 h at 37°C with a purified mouse monoclonal antibody (concentration, 5 μg/ml; CD107a [catalog no. 34201A]; Pharmingen, San Diego, Calif.) specific for the lysosome-associated membrane protein-I (LAMP-I), and after several washes in phosphate-buffered saline, cells were incubated for 30 min with a rhodamine-conjugated anti-mouse antibody at a 1:500 dilution (Boehringer Mannheim catalog no. 605-150). Next, cells were stained to detect expression of the plasmid-encoded viral protein. HBV core protein was detected using a rabbit polyclonal antiserum (DAKO catalog no. B0586) at a 1:5,000 dilution, followed by incubation for 30 min with a fluorescein isothiocyanate (FITC)-conjugated anti-rabbit antibody (Boehringer Mannheim catalog no. 1814-257) at a 1:1,000 dilution. LCMV NP was detected using a polyclonal anti-LCMV guinea pig serum (the generous gift of Michael Buchmeier, Scripps Research Institute), followed by a biotinylated anti-guinea pig antibody and then by streptavidin conjugated to FITC. Single-plane confocal microscope images were collected sequentially using a Bio-Rad MRC-1024 unit attached to a Zeiss Axiovert S100TV microscope with 43× or 60× objective lenses. A krypton-argon mixed-gas laser produced excitation wavelengths at 488 nm (FITC; for HBV core or LCMV NP) and 568 nm (rhodamine; for LAMP-I). Individual fluorophore images were merged and pseudocolored using Adobe Photoshop 5.5.
Detection of epitope-specific CD4+ and CD8+ T-cell responses by ICCS.
Mice were inoculated with the specified plasmids and, 3 weeks later, were infected with LCMV (2 × 105 PFU, intraperitoneally [i.p.]) to boost any DNA-primed CD4+ T-cell responses. Six days postinfection (a time point at which virus-specific CD4+ T cells are difficult to detect in previously nonimmune mice), the mice were sacrificed, and their spleens were harvested for analysis by intracellular cytokine staining (ICCS) assay. A total of 106 splenocytes were incubated in 96-well plates together with the indicated stimulator peptides (at 5 μg/ml). After a 6-h incubation in the presence of interleukin 2 (150 U/ml), β-mercaptoethanol, and Brefeldin A (at 1 μg/ml, to increase the accumulation of gamma interferon [IFN-γ] in the responding cells), cells were washed and labeled with cychrome-conjugated anti-CD4 antibody (0.25 mg/ml), for 30 min on ice. The cells were then washed, permeabilized with Cytofix/Cytoperm for 20 min on ice, and stained with a fluorescein-conjugated anti-IFN-γ antibody (0.4 mg/ml). Finally, the cells were washed, fixed in 2% formaldehyde, and analyzed by flow cytometry. Reagents were purchased from Pharmingen. For detection of CD8+ T cells, splenocytes were incubated for 5 h in the presence of Brefeldin A with the peptide NP396 (FQPQNGQFI), which is a dominant epitope in C57BL/6 mice, and then were stained with an antibody to CD8, followed by staining for IFN-γ as described above.
Digestion of peptides with cathepsin D and analyses of the reaction products.
The peptides (NP309–328 or GP61–80) (10 μg) were incubated at 37°C in the absence or in the presence of 1.48 μg (0.06 U) of cathepsin D (EC 3.4.23.5; Sigma, St. Louis, Mo.) in a final volume of 100 μl in 100 mM NaNH4 (pH 4) to mimic the acidic pH of the lysosomes (14). Four hours later, samples were lyophilized and resuspended in 10 μl of water to keep the peptides at 1 mg/ml. Treated peptides were used in two different experiments: as stimulators in an ICCS assay (see Fig. 5) and to analyze the peptide profile in a mass spectrometer (see Fig. 6). Matrix-assisted laser desorption-ionization–mass spectrometry spectra were obtained with a Voyager DE-RP MALDI-TOF mass spectrometer (PerSeptive Biosystems, Framingham, Mass.), using the reflector mode and alpha-cyano-4-hydroxycinnamic acid as the matrix. Fragments were assigned using the computer program PAWS (Proteometrics).
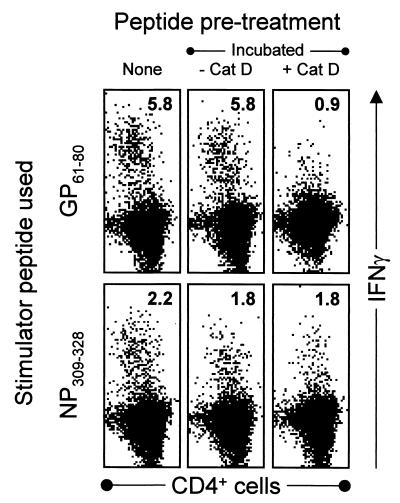
FIG. 5.
Incubation with cathepsin D diminishes the stimulatory activity of GP61–80 but not of NP309–328. Aliquots of the NP309–328 and GP61–80 peptides were pretreated by incubation in acidic medium, either without (−) or with (+) cathepsin D (Cat D) as indicated. After incubation, the stimulatory activities of the resulting materials were determined by incubating them with splenocytes from C57BL/6 mice taken 8 days after LCMV infection. For details, see Materials and Methods.
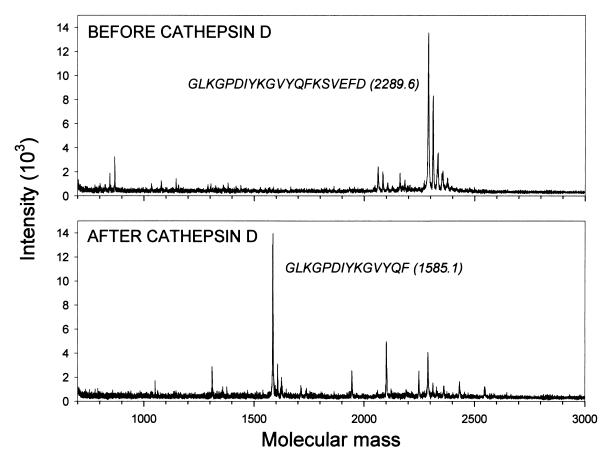
FIG. 6.
Cathepsin D specifically cleaves GP61–80. Peptide GP61–80 was incubated without or with cathepsin D as described in Materials and Methods, and the resulting products were analyzed by mass spectrometry. The data in the absence of enzyme cleavage appear in the top panel. The MW of the observed peak and the sequence GP61–80 are shown. As shown in the bottom panel, cathepsin D cleavage generated a major peak with an observed MW of 1,585.1 Da; the probable sequence of the peptide forming this peak is shown.
Evaluation of antibody induction following DNA vaccination.
LCMV-specific antibody levels were determined by enzyme-linked immunosorbent assay (ELISA), using plates coated with purified LCMV as targets.
Evaluation of the protective efficacy of plasmids containing the LIMP-II tail.
C57BL/6 mice were immunized as indicated in the text or figure legend and 6 weeks later were challenged with LCMV. Two modes of viral challenge were used. (i) For intracranial (i.c.) challenge, mice were inoculated with 20 50% lethal doses of LCMV i.c. and were observed daily for 21 days. All deaths occurred between days 7 and 10 postinfection. (ii) For peripheral challenge, mice received 2 × 105 PFU of LCMV i.p. At 4 days postchallenge, spleens were harvested. For each mouse, the virus titer in a known weight of spleen was determined by plaque assay on Vero 76 cells. At 4 days postinfection, nonimmune mice have a high load of residual virus, which is substantially reduced in immune animals.
RESULTS
The LIMP-II tail directs fusion proteins to the lysosomes.
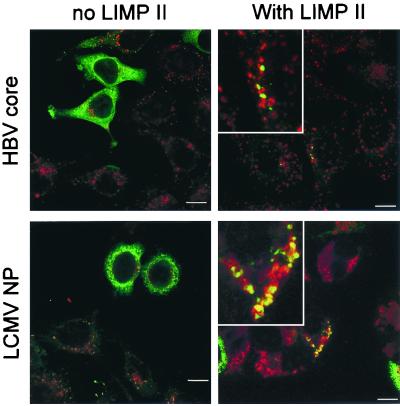
We first determined whether the LIMP-II tail could target two different fusion proteins to the lysosomal compartment. HeLa cells were transfected with pCMV-HBVcore, pCMV-HBVcore-LII, pCMV-NP, or pCMV-NP-LII and 48 h later were fixed in ice-cold methanol. After several washes, cells were stained for the viral proteins (green fluor) and for the lysosomal protein LAMP-I (red fluor), and staining was visualized by confocal microscopy (Fig. 2). Cells transfected with plasmids encoding the wild-type viral proteins showed strong cytosolic green fluorescence, which was clearly distinct from the punctate red signal indicating LAMP-I expression in lysosomes; essentially no colocalization (yellow signal) was visible. In contrast, after transfection with the LIMP-II fusion constructs, although some cells showed cytosolic expression, most of the signal from the viral proteins was punctate and colocalized with the LAMP-I (generating a yellow signal, magnified in insets in right-hand column). If the transfected cells were treated with chloroquine, which deacidifies the lysosomes and inhibits lysosomal protein degradation, the amount and the number of cells expressing the antigen increased dramatically (data not shown). Thus, the 20-amino-acid tail of LIMP-II is sufficient to direct a substantial proportion of endogenously expressed proteins to the lysosome, where they are rapidly degraded in the acid milieu.
FIG. 2.
The LIMP-II tail targets proteins to the lysosomal compartment. Cells were transfected with plasmids encoding the HBV core protein (top row) or the LCMV NP (bottom row), with or without the LIMP-II tail (left and right columns, respectively). After 48 h, cells were stained with FITC-labeled antibodies specific for the HBV core or for the LCMV NP (green signal) and were costained with rhodamine-labeled antibodies specific for the lysosomal protein LAMP-I (red signal). Fluorescence was evaluated using a confocal microscope. A bar representing 10 μm is shown in all panels. The right-hand panels include enlargements of regions showing a dual signal (yellow), which indicates colocalization of the viral antigen and LAMP-I.
The LIMP-II tail enhances the induction of the CD4+ T-cell responses to NP309–328.
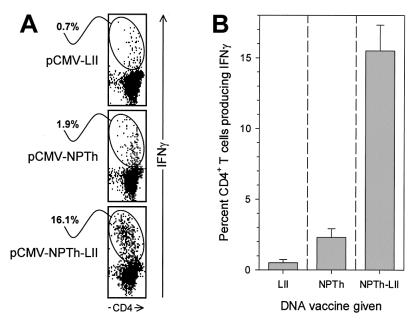
Having demonstrated successful targeting to the lysosome by LIMP-II fusion, we wished next to determine whether this would lead to enhanced induction of CD4+ T cells. We have recently shown that antigen-specific CD8+ T cells can be detected directly ex vivo after a single inoculation of DNA (2, 10); consequently, we thought that we might be able to identify epitope-specific CD4+ T cells directly ex vivo after immunizing mice with our various plasmids. Mice were immunized with the plasmids pCMV-NP, pCMV-NPTh, or pCMV-GPTh, with and without the LIMP-II tail; however, even with the LIMP-II fusion plasmids, we were unable to convincingly demonstrate plasmid-induced epitope-specific CD4+ T-cell responses directly ex vivo (data not shown). We conclude that, in response to DNA immunization, CD4+ T-cell responses appear to be weaker than CD8+ T-cell responses, rendering difficult their detection directly ex vivo. This parallels the findings during virus infection, in which virus-specific CD4+ T-cell responses are much less pronounced than the better-characterized CD8+ T-cell responses; virus-specific CD4+ T cells first become detectable around day 7 postinfection; they peak in frequency between days 8 to 10 postinfection and decline slightly thereafter (38, 39, 43). We reasoned that, if injection of plasmid DNA did prime antigen-specific CD4+ T cells, subsequent virus infection should result in an accelerated virus-specific CD4+ T-cell response, compared to that seen in previously naïve mice; detecting this accelerated response would, therefore, constitute evidence of successful DNA vaccination. Therefore, groups of eight C57BL/6 mice were immunized once with the plasmids pCMV-NPTh or pCMV-NPTh-LII, and four mice were inoculated with the negative control plasmid pCMV-LIMP-II. Three weeks later all mice were infected with LCMV, and 6 days later (a time point at which it remains difficult to detect virus-specific CD4+ T cells in previously naïve mice) the spleens were harvested and analyzed by ICCS after a 6-h incubation with or without the NP309–328 peptide. The results from a representative individual mouse from each vaccine group are shown (Fig. 3A); along with the mean response (± standard error of the mean [SEM]) in each group (Fig. 3B). At 6 days postinfection, ∼0.5% of all CD4+ T cells were specific for this epitope in mice which had been immunized with the negative control plasmid pCMV-LIMP-II. Mice immunized with pCMV-NPTh showed a slightly elevated percentage of epitope-specific CD4+ T cells (average, ∼2.2%), suggesting that the isolated Th epitope could prime a low number of CD4+ T cells. Strikingly, all mice immunized with pCMV-NPTh-LII showed a much stronger CD4+ T-cell response, at least sevenfold greater than that seen in mice immunized with the construct lacking the LIMP-II tail. From these results we conclude that endogenously expressed minigene antigens can be correctly presented by the MHC class II pathway and that targeting the antigen to the lysosomes can markedly enhance the induction of antigen-specific CD4+ T cells.
FIG. 3.
LIMP-II enhances induction of NP309–328-specific CD4+ T cells. C57BL/6 mice were immunized with pCMV-LII, pCMV-NPTh, or pCMV-NPTh-LII (four mice per group) and 3 weeks later were infected with LCMV. Six days later, spleens were harvested, cells were stimulated with peptide NP309–328, and an ICCS assay was carried out. (A) Representative data, gated on CD4+ T cells, from individual mice are shown. (B) The percentage of CD4+ T cells producing IFN-γ is shown for each group (mean + SEM [error bars]).
The induction of a T-helper response against the GP61–80 peptide is inhibited by targeting the epitope to the lysosomes.
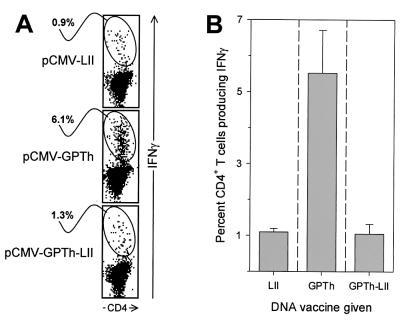
To determine if this enhancement of CD4+ T-cell induction applied to another MHC class II I-Ab-restricted epitope, we expressed the LCMV T helper epitope GP61–80 with and without the LIMP-II tail. Three groups of C57BL/6 mice (four mice per group) were injected with pCMV-GPTh, pCMV-GPTh-LII, or pCMV-LIMP-II, and 3 weeks later the mice were infected with LCMV. The GP-specific T-helper responses were evaluated at day 6 postinfection by ICCS as previously described. The results from a representative individual mouse from each vaccine group are shown (Fig. 4A) along with the mean response (± SEM) in each group (Fig. 4B). As expected, all mice immunized with pCMV-GPTh showed stronger responses to GP61–80 than did mice immunized with the negative control plasmid; on average, the CD4+ T-cell response was increased approximately sixfold. Surprisingly, the effect of LIMP-II fusion was the opposite of that seen with the NP epitope; none of the mice immunized with pCMV-GPTh-LII showed an elevated CD4+ T-cell response, indicating that lysosomal targeting of this sequence somehow inhibited its capacity to prime CD4+ T-cell responses. These results suggest that the GP61–80 peptide may be processed by a lysosome-independent pathway, since forcing the sequence into the lysosomal compartment (using the LIMP-II tail) abrogates its immunogenicity. Conversely, the NP309–328 epitope appears to be processed more conventionally. These data are consistent with studies of the CD4+ T-cell response to LCMV infection, which showed that these two epitopes are processed differently and that presentation of the GP61–80 epitope in a virus-infected cell was not disrupted by chloroquine treatment (24). However, the underlying mechanism was not identified.
FIG. 4.
LIMP-II inhibits induction of GP61–80-specific CD4+ T cells. C57BL/6 mice were immunized with pCMV-LII, pCMV-GPTh, or pCMV-GPTh-LII (four mice per group) and 3 weeks later were infected with LCMV. Six days later, spleens were harvested, cells were stimulated with peptide GP61–80, and an ICCS assay was carried out. (A) Representative data, gated on CD4+ T cells, from individual mice are shown. (B) The percentage of CD4+ T cells producing IFN-γ is shown for each group (mean + SEM [error bars]).
The GP61–80 peptide loses its stimulatory capacity after cathepsin D digestion.
The aspartic endopeptidase cathepsin D, which is found exclusively in the lysosomes (3, 18), is one of the most specific and abundant lysosomal enzymes implicated in protein hydrolysis, peptide generation, and MHC class II antigen presentation. We therefore determined whether cathepsin D could modify the stimulatory capacity of the NP309–328 and GP61–80 peptides. Each peptide was incubated for 4 h at pH 4 in the presence of cathepsin D, and the reaction products were evaluated for their ability to stimulate IFN-γ production by CD4+ T cells from a day 8 LCMV-infected mouse. To ensure that any changes in stimulatory capacity were attributable to the effects of cathepsin D, and not to the acidic environment, an aliquot of each peptide was incubated in the acidic medium in the absence of cathepsin D. As shown in Fig. 5 (upper row), the ability of peptide GP61–80 to stimulate a CD4+ T-cell response was dramatically (∼85%) reduced by cathepsin D digestion; in clear contrast, the stimulatory activity of the NP309–328 peptide was not diminished by the addition of enzyme. These results suggest that cathepsin D may degrade peptide GP61–80, which would provide a possible explanation for the abrogation of the CD4+ T-cell responses that occurs after DNA immunization in vivo with the plasmid pCMV-GPTh-LII (Fig. 4) and would explain why this epitope has to be presented in a lysosome-independent route during virus infection.
Cathepsin D specifically cleaves between the phenylalanine (F74) and the lysine (K75) amino acid residues of the GP61–80 peptide.
To determine if the GP61–80 peptide is being specifically cleaved by cathepsin D and to identify the cleavage site, 3 μg of the GP61–80 peptide was analyzed by mass spectrometry before and after cathepsin D treatment (Fig. 6). Before enzyme treatment (upper panel), a major peak with an observed molecular weight (MW) of 2,289.6 Da was detected, corresponding to the intact 20-amino-acid peptide (theoretical MW, 2,290.6 Da). After digestion with cathepsin D (Fig. 6, lower panel), most of the original signal is absent, and a new major peak with an MW 1,585.1 Da is observed, closely corresponding to the predicted MW of the peptide GLKGPDIYKGVYQF (predicted MW, 1584.8 Da). This result demonstrates that cathepsin D specifically cleaves the 20-mer GP61–80 peptide between residues F74 and K75, two amino acids that are commonly found flanking the cathepsin D cleavage sites (14), providing an explanation for the loss of stimulatory activity observed in Fig. 5.
Effects of CD4+ T-cell priming on the induction of virus-specific antibodies and CD8+ T cells.
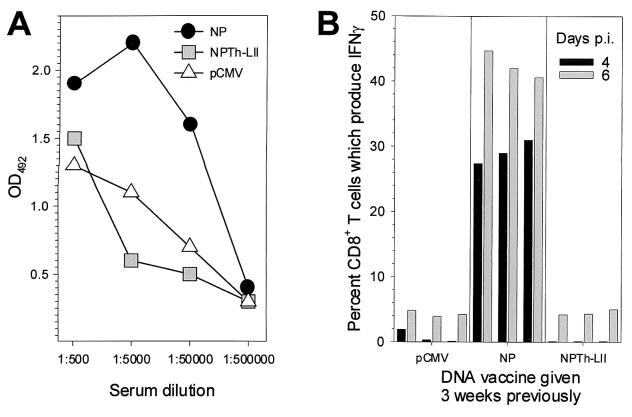
CD4+ T cells often play a critical role in providing help to B cells, facilitating antibody induction and immunoglobulin class switching, and also enhance the longevity of the memory CD8+ T-cell response (42). Therefore, we wished to determine if our plasmid constructs might accelerate or elevate the antibody and CD8+ T-cell responses to subsequent viral infection. C57BL/6 mice were immunized with pCMV, pCMV-NP, or pCMV-NPTh-LII and 3 weeks later were infected with virus. On days 0, 2, 4, 6, and 8 postinfection, mice were sacrificed, and antibody and CD8+ T-cell responses were measured by ELISA and ICCS, respectively. LCMV-specific immunoglobulin G (IgG) levels are displayed in Fig. 7A; for the sake of clarity, only responses at 8 days postinfection are shown. Previously naïve mice (those inoculated with pCMV) have, as expected, readily detectable antibody titers, and these are elevated in mice which have been immunized with pCMV-NP. However, antibody titers in mice immunized with pCMV-NPTh-LII are similar to those seen in control mice, indicating that the presence of virus-specific CD4+ T cells (which were shown in Fig. 3) does not increase the IgG response to virus infection. Furthermore, there is no indication that preexisting NP-specific CD4+ T cells can accelerate or elevate the CD8+ T-cell response to LCMV. As shown in Fig. 7B, at 4 and 6 days after infection of previously naïve mice, only low levels of virus-specific CD8+ T cells are detected. These levels are accelerated and greatly increased in pCMV-NP vaccinees, but no such results are seen in mice immunized with pCMV-NPTh-LII.
FIG. 7.
Immunization with pCMV-NPTh-LII has no apparent effect on subsequent induction of antibodies or CD8+ T cells. Mice were immunized with one of the three indicated plasmids and, 3 weeks later, were infected with LCMV. At various time points postinfection, mice were sacrificed (three mice per time point, for each vaccine group), and their LCMV-specific antibody titers (A) and CD8+ T-cell responses (B) were determined. (A) Antibody titers (total IgG) at 8 days after infection were determined by ELISA. (B) CD8+ T-cell responses were evaluated by ICCS after stimulating with peptide NP396; the data for days 4 and 6 postinfection are shown for each mouse.
Priming of CD4+ T cells alone does not enhance the vaccinee's ability to combat subsequent virus infection.
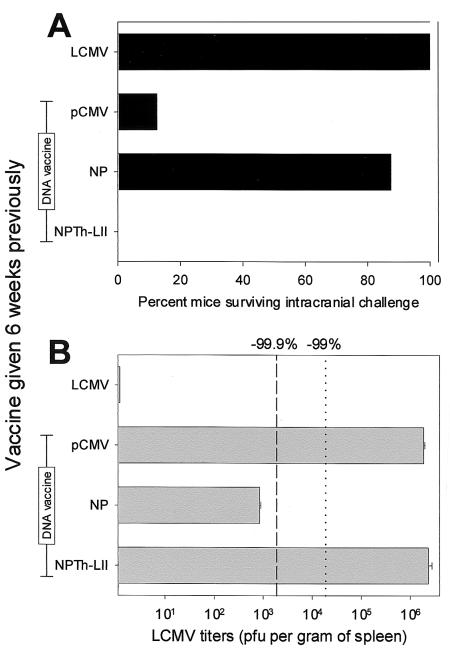
We have previously shown that vaccines which induce only CD8+ T cells can confer solid antiviral protection (2, 23, 27, 44), and we wished to determine whether a similar result would be observed after immunizing with a vaccine which induced only CD4+ T cells. Therefore, mice were immunized with LCMV (as a positive control for the assay), pCMV (negative control DNA), pCMV-NP (a DNA vaccine which we know to be protective [49]), or pCMV-NPTh-LII (which induces CD4+ T cells, as shown in Fig. 3). Six weeks later, mice were challenged with LCMV by either the i.c. or peripheral routes, as described in the figure legend. Following i.c. challenge (Fig. 8A), all mice immunized with LCMV survived, as did seven of eight mice which had been inoculated with pCMV-NP. However, no protective effect was noted in mice inoculated with pCMV-NPTh-LII. A similar lack of protective efficacy was noted following peripheral virus challenge (Fig. 8B). Mice inoculated with pCMV had LCMV titers of ∼2 × 106 PFU per g of tissue, and mice vaccinated with pCMV-NP showed a >3-log reduction, as we have previously found (29, 49). In contrast, mice immunized with pCMV-NPTh-LII showed titers indistinguishable from those of the pCMV control mice. We conclude that priming of CD4+ T cells alone does not enhance the vaccinee's ability to combat these two types of LCMV challenge.
FIG. 8.
Priming of CD4+ T cells alone does not confer protection against LCMV challenge. Groups of mice were immunized with one inoculation of the indicated plasmid DNA vaccine or, as a positive control, with LCMV (2 × 105 PFU, i.p.). Six weeks later, mice were challenged via the i.c. (A) or i.p. (B) routes. (A) Mice (eight per vaccine group) received 20 50% lethal doses of LCMV i.c. and were observed daily for 21 days. For each group, the percentage of mice which survived infection is shown. All deaths occurred between days 7 and 10 postchallenge. (B) Mice (four per vaccine group) were challenged with LCMV (2 × 105 PFU, i.p.), and 4 days postchallenge, spleens were harvested and virus titers were determined by plaque assay. For each vaccine group, the mean + SEM (in PFU per gram of spleen) (error bars) is shown. The vertical lines indicate percentage reductions in titer compared to titers in the negative control (pCMV) animals (dotted line, 99% reduction; dashed line, 99.9% reduction).
DISCUSSION
The rational manipulation of the immune response by targeting antigens (full-length proteins or individual epitopes) to different antigen-presenting pathways (28) and/or to specific antigen presenting cells (5) has proven to be an effective way to enhance DNA immunization. LCMV has provided much information about many aspects of T-cell immunity, but most of this has centered on CD8+ T cells. However, LCMV infection also induces a relatively strong T-helper response, which, in C57BL/6 mice, is focused almost entirely upon two epitopes, GP61–80 and NP309–328, both of which are presented by I-Ab (24); during acute infection of previously naïve mice, the response to GP61–80 is approximately threefold stronger than the response to NP309–328 (38). Because DC can take up the plasmid DNA and present encoded class II-restricted epitopes to CD4+ T cells (7), and since the lysosomal compartment plays a critical role in antigen presentation by MHC class II molecules, we reasoned that direct targeting to the lysosome by fusing the antigen to the LIMP-II tail might improve the T-helper responses induced by a DNA vaccine. Others have targeted proteins to the lysosome-endosome pathway, by using the tyrosine-dependent targeting signal of LAMP-I (see references 30, 34, and 47), and promising data have been reported in a tumor model (12, 17). However, LAMP-I transport is regulated by a tyrosine-dependent transport signal (26, 46) and is indirect, requiring the translocation of the chimeric protein from the endoplasmic reticulum to the cell membrane, whence it is endocytosed into lysosomes. We therefore chose to evaluate the effect of attaching proteins to the C-terminal tail of LIMP-II, which transports materials directly from the endoplasmic reticulum into the lysosome (40). Here we confirm that the addition of the LIMP-II tail drives a substantial proportion of the signal in transfected cells into the lysosomal compartment (Fig. 2). In order to maximize the specificities of vaccine-induced immunity, most of our subsequent studies employed minigenes encoding the above LCMV MHC class II epitopes.
Mice immunized with plasmids encoding the minigenes alone (pCMV-NPTh or pCMV-GPTh) showed a somewhat accelerated CD4+ T-cell response following LCMV infection (Fig. 3 and Fig. 4). In general, the response to the GP61–80 epitope was approximately threefold stronger than that mounted to the NP309–328 epitope (compare Fig. 3 to Fig. 4), consistent with others' finding that GP61–80 is the dominant epitope during LCMV infection of previously naïve mice (38). Addition of the LIMP-II tail to these minigenes had dramatically different outcomes. Mice immunized with the plasmid pCMV-NPTh-LII (expressing the NP309–328 epitope fused to the LII tail) had a markedly (approximately sevenfold) increased CD4+ T-cell response (Fig. 3), indicating that lysosomal targeting by the LIMP-II tail may be a useful addition to the DNA vaccine arsenal. Note that the increased CD4+ T-cell response implies that our plasmid DNAs are most probably taken up and expressed in APCs; their expression in normal somatic cells would be unlikely to result in an enhanced CD4+ T-cell response, since these cells express neither MHC class II nor the relevant costimulatory molecules. Others have shown that plasmid DNA vaccines can enter DC in vivo (8). In contrast to the positive data with the NP Th epitope, LIMP-II targeting not only failed to increase the response to GP61–80; it reduced the CD4+ T-cell response to background levels (Fig. 4). What could explain the detrimental effect of lysosomal targeting? Oxenius and coworkers have shown that these two Th epitopes differ in their sensitivity to chloroquine; this drug, which prevents acidification of the endosome-lysosome compartment, prevented presentation of the NP309–328 epitope but had little effect on presentation of the GP61–80 epitope (24), suggesting that the latter sequence may be presented by a lysosome-independent route. One could, therefore, argue that in being targeted to the lysosome, the GP61–80 sequence had been deflected from its normal route of presentation, and therefore the epitope was unable to induce a CD4+ T-cell response in the normal manner. This may be true, but we reasoned that lysosomal delivery of the epitope might nevertheless have been expected to result in MHC class II presentation and induction of CD4+ T cells; therefore, the absence of a CD4+ response to pCMV-GPTh-LII led us to hypothesize that the GP61–80 epitope might be actively degraded in the lysosome. Cathepsin D, an endopeptidase which cleaves preferentially after hydrophobic residues (3), is the most abundant lysosomal enzyme involved in the generation of MHC class II peptides and has a profound effect on antigen processing (11, 14, 18, 20, 36, 37, 45, 50). We investigated the effects of this enzyme on the GP61–80 peptide and found that the peptide was cleaved after amino acid F74 (Fig. 6) and that the reaction products were unable to stimulate LCMV-specific CD4+ T cells (Fig. 5). Thus, we conclude that the GP61–80 epitope cannot be successfully presented by MHC class II when delivered by the lysosomal pathway. The route taken by the GP61–80 peptide to encounter MHC class II during LCMV infection remains unclear and is currently under investigation. However, these results clearly demonstrate that lysosomal targeting can deleteriously affect the induction of CD4+ T-cell responses; this has obvious implications for vaccine design.
Next, we determined whether the induction of virus-specific CD4+ T cells could enhance the antibody and CD8+ T-cell responses to subsequent viral infection (Fig. 7). Mice were immunized with pCMV-NPTh-LII, which induces a strong CD4+ T-cell response (pCMV-GPTh-LII was not evaluated, since this plasmid fails to induce CD4+ T cells, for the reason described above). Six weeks later, the vaccinees were infected with LCMV, and antibody and CD8+ T-cell responses were measured at various times postinfection. The total IgG response measured at 8 days postinfection was not increased compared to that seen in previously naïve mice. Similar conclusions were drawn from comparison of antibody titers at all time points analyzed, and even when antibody isotypes were measured (IgG1 and IgG2a), no differences were revealed (data not shown). Furthermore, virus-specific CD8+ T-cell responses were not accelerated in mice primed with pCMV-NPTh-LII, in contrast to the marked enhancement seen in mice immunized with pCMV-NP.
We (15, 44) and others (9, 13, 33, 35) have shown that the induction of CD8+ T cells, in the absence of CD4+ T cells or antibodies, can confer a sufficient advantage upon vaccinees to allow them to survive a normally lethal challenge or to more rapidly clear virus following sublethal challenge. The failure of CD4+ priming to accelerate the virus-induced CD8+ T-cell response (Fig. 7B) suggested that pCMV-NPTh-LII vaccinees would not be protected against virus challenge, but we wished to determine experimentally the protective efficacy of a DNA vaccine which induced only CD4+ T cells. Six weeks after immunization with pCMV-NPTh-LII, mice were challenged with LCMV either i.c. or i.p.; as shown in Fig. 8, the immunized mice remained fully susceptible to both challenge regimens. In contrast, a plasmid encoding the full-length NP conferred good protection; this is expected, because the sequence FQPQNGQFI (NP396–404), present in pCMV-NP, is recognized as a dominant MHC class I epitope presented by Db. Thus, at least in this model system, the CD4+ T cells induced by a DNA vaccine do not confer a marked protective advantage upon a vaccinee. However, we cannot conclude that CD4+ T cells are generally insignificant in DNA vaccination, for at least two reasons. First, although the attachment of the LIMP-II tail clearly enhances the vaccine-induced CD4+ T-cell response, it is possible that the CD4+ T cells induced are insufficient in quantity or quality to exert the biological effects that we have sought. Perhaps further improvement of the CD4+ vaccine regimen would reveal a biological benefit in our LCMV model. Second, we have relied on the LCMV model, in which it is already known that virus-specific CD8+ T-cell induction can occur in the absence of CD4+ T cells. It is possible that the levels of CD4+ T cells induced by DNA vaccination might have measurable effects in other systems; and indeed such findings have been described (19). In conclusion, DNA vaccines can be designed to direct proteins, or epitopes, to specific intracellular organelles, thus permitting the rational manipulation of vaccine-induced immunity. We show here that lysosomal targeting can markedly increase CD4+ T-cell responses but also may diminish responses to antigens normally processed by other routes.
ACKNOWLEDGMENTS
We are grateful to Annette Lord for excellent secretarial support. The cDNA for HBV, from which we cloned the core protein, was the generous gift of Frank Chisari (TSRI).
This work was supported by NIH grant AI-37186 (J.L.W.) and by contractual funding to F.R. from the Fondo de Investigaciones Sanitarias (FIS) of the Ministerio de Sanidad y Consumo, Madrid, Spain.
Footnotes
This is manuscript number 13917-NP from The Scripps Research Institute.
REFERENCES
- 1.Adorini L, Moreno J, Momburg F, Hammerling G J, Guery J C, Valli A, Fuchs S. Exogenous peptides compete for the presentation of endogenous antigens to major histocompatibility complex class II-restricted T cells. J Exp Med. 1991;174:945–948. doi: 10.1084/jem.174.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.An L L, Rodriguez F, Harkins S, Zhang J, Whitton J L. Quantitative and qualitative analyses of the immune responses induced by a multivalent minigene DNA vaccine. Vaccine. 2000;18:2132–2141. doi: 10.1016/s0264-410x(99)00546-0. [DOI] [PubMed] [Google Scholar]
- 3.Barrett, A. J. 1979. Cathepsin D: the lysosomal aspartic proteinase. Ciba Found. Symp. 37–50. [DOI] [PubMed]
- 4.Bonifaz L C, Arzate S, Moreno J. Endogenous and exogenous forms of the same antigen are processed from different pools to bind MHC class II molecules in endocytic compartments. Eur J Immunol. 1999;29:119–131. doi: 10.1002/(SICI)1521-4141(199901)29:01<119::AID-IMMU119>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 5.Boyle J S, Brady J L, Lew A M. Enhanced responses to a DNA vaccine encoding a fusion antigen that is directed to sites of immune induction. Nature. 1998;392:408–411. doi: 10.1038/32932. [DOI] [PubMed] [Google Scholar]
- 6.Calin-Laurens V, Forquet F, Lombard-Platet S, Bertolino P, Chretien I, Trescol-Biemont M C, Gerlier D, Rabourdin-Combe C. High efficiency of endogenous antigen presentation by MHC class II molecules. Int Immunol. 1992;4:1113–1121. doi: 10.1093/intimm/4.10.1113. [DOI] [PubMed] [Google Scholar]
- 7.Casares S, Inaba K, Brumeanu T D, Steinman R M, Bona C A. Antigen presentation by dendritic cells after immunization with DNA encoding a major histocompatibility complex class II-restricted viral epitope. J Exp Med. 1997;186:1481–1486. doi: 10.1084/jem.186.9.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Condon C, Watkins S C, Celluzzi C M, Thompson K, Falo J L D. DNA-based immunization by in vivo transfection of dendritic cells. Nat Med. 1996;2:1122–1128. doi: 10.1038/nm1096-1122. [DOI] [PubMed] [Google Scholar]
- 9.del Val M, Schlicht H J, Volkmer H, Messerle M, Reddehase M J, Koszinowski U H. Protection against lethal cytomegalovirus infection by a recombinant vaccine containing a single nonameric T-cell epitope. J Virol. 1991;65:3641–3646. doi: 10.1128/jvi.65.7.3641-3646.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hassett D E, Slifka M K, Zhang J, Whitton J L. Direct ex vivo kinetic and phenotypic analyses of CD8+ T-cell responses induced by DNA immunization. J Virol. 2000;74:8286–8291. doi: 10.1128/jvi.74.18.8286-8291.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hewitt E W, Treumann A, Morrice N, Tatnell P J, Kay J, Watts C. Natural processing sites for human cathepsin E and cathepsin D in tetanus toxin: implications for T cell epitope generation. J Immunol. 1997;159:4693–4699. [PubMed] [Google Scholar]
- 12.Ji H, Wang T L, Chen C H, Pai S I, Hung C F, Lin K Y, Kurman R J, Pardoll D M, Wu T C. Targeting human papillomavirus type 16 E7 to the endosomal/lysosomal compartment enhances the antitumor immunity of DNA vaccines against murine human papillomavirus type 16 E7-expressing tumors. Hum Gene Ther. 1999;10:2727–2740. doi: 10.1089/10430349950016474. [DOI] [PubMed] [Google Scholar]
- 13.Jonjic S, del Val M, Keil G M, Reddehase M J, Koszinowski U H. A nonstructural viral protein expressed by a recombinant vaccinia virus protects against lethal cytomegalovirus infection. J Virol. 1988;62:1653–1658. doi: 10.1128/jvi.62.5.1653-1658.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kageyama T, Yonezawa S, Ichinose M, Miki K, Moriyama A. Potential sites for processing of the human invariant chain by cathepsins D and E. Biochem Biophys Res Commun. 1996;223:549–553. doi: 10.1006/bbrc.1996.0932. [DOI] [PubMed] [Google Scholar]
- 15.Klavinskis L S, Whitton J L, Oldstone M B A. Molecularly engineered vaccine which expresses an immunodominant T-cell epitope induces cytotoxic T lymphocytes that confer protection from lethal virus infection. J Virol. 1989;63:4311–4316. doi: 10.1128/jvi.63.10.4311-4316.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kozak M. Possible role of flanking nucleotides in recognition of the AUG initiator codon by eukaryotic ribosomes. Nucleic Acids Res. 1981;9:5233–5262. doi: 10.1093/nar/9.20.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin K Y, Guarnieri F G, Staveley-O'Carroll K F, Levitsky H I, August J T, Pardoll D M, Wu T C. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996;56:21–26. [PubMed] [Google Scholar]
- 18.Lutz M B, Rovere P, Kleijmeer M J, Rescigno M, Assmann C U, Oorschot V M, Geuze H J, Trucy J, Demandolx D, Davoust J, Ricciardi-Castagnoli P. Intracellular routes and selective retention of antigens in mildly acidic cathepsin D/lysosome-associated membrane protein-1/MHC class II-positive vesicles in immature dendritic cells. J Immunol. 1997;159:3707–3716. [PubMed] [Google Scholar]
- 19.Maecker H T, Umetsu D T, DeKruyff R H, Levy S. Cytotoxic T cell responses to DNA vaccination: dependence on antigen presentation via class II MHC. J Immunol. 1998;161:6532–6536. [PubMed] [Google Scholar]
- 20.Mizuochi T, Yee S T, Kasai M, Kakiuchi T, Muno D, Kominami E. Both cathepsin B and cathepsin D are necessary for processing of ovalbumin as well as for degradation of class II MHC invariant chain. Immunol Lett. 1994;43:189–193. doi: 10.1016/0165-2478(94)90221-6. [DOI] [PubMed] [Google Scholar]
- 21.Moreno J, Vignali D A, Nadimi F, Fuchs S, Adorini L, Hammerling G J. Processing of an endogenous protein can generate MHC class II-restricted T cell determinants distinct from those derived from exogenous antigen. J Immunol. 1991;147:3306–3313. [PubMed] [Google Scholar]
- 22.Ogata S, Fukuda M. Lysosomal targeting of Limp II membrane glycoprotein requires a novel Leu-Ile motif at a particular position in its cytoplasmic tail. J Biol Chem. 1994;269:5210–5217. [PubMed] [Google Scholar]
- 23.Oldstone M B A, Tishon A, Eddleston M, de la Torre J C, McKee T A, Whitton J L. Vaccination to prevent persistent viral infection. J Virol. 1993;67:4372–4378. doi: 10.1128/jvi.67.7.4372-4378.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oxenius A, Bachmann M F, Ashton-Rickardt P G, Tonegawa S, Zinkernagel R M, Hengartner H. Presentation of endogenous viral proteins in association with major histocompatibility complex class II: on the role of intracellular compartmentalization, invariant chain and the TAP transporter system. Eur J Immunol. 1995;25:3402–3411. doi: 10.1002/eji.1830251230. [DOI] [PubMed] [Google Scholar]
- 25.Parra-Lopez C A, Lindner R, Vidavsky I, Gross M, Unanue E R. Presentation on class II MHC molecules of endogenous lysozyme targeted to the endocytic pathway. J Immunol. 1997;158:2670–2679. [PubMed] [Google Scholar]
- 26.Peters C, Braun M, Weber B, Wendland M, Schmidt B, Pohlmann R, Waheed A, von Figura K. Targeting of a lysosomal membrane protein: a tyrosine-containing endocytosis signal in the cytoplasmic tail of lysosomal acid phosphatase is necessary and sufficient for targeting to lysosomes. EMBO J. 1990;9:3497–3506. doi: 10.1002/j.1460-2075.1990.tb07558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodriguez F, An L L, Harkins S, Zhang J, Yokoyama M, Widera G, Fuller J T, Kincaid C, Campbell I L, Whitton J L. DNA immunization with minigenes: low frequency of memory CTL and inefficient antiviral protection are rectified by ubiquitination. J Virol. 1998;72:5174–5181. doi: 10.1128/jvi.72.6.5174-5181.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez F, Whitton J L. Enhancing DNA Immunization. Virol. 2000;268:233–238. doi: 10.1006/viro.2000.0209. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez F, Zhang J, Whitton J L. DNA immunization: ubiquitination of a viral protein enhances CTL induction, and antiviral protection, but abrogates antibody induction. J Virol. 1997;71:8497–8503. doi: 10.1128/jvi.71.11.8497-8503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rowell J F, Ruff A L, Guarnieri F G, Staveley-O'Carroll K, Lin X, Tang J, August J T, Siliciano R F. Lysosome-associated membrane protein-1-mediated targeting of the HIV-1 envelope protein to an endosomal/lysosomal compartment enhances its presentation to MHC class II-restricted T cells. J Immunol. 1995;155:1818–1828. [PubMed] [Google Scholar]
- 31.Sandoval I V, Arredondo J J, Alcalde J, Gonzalez N A, Vandekerckhove J, Jimenez M A, Rico M. The residues Leu(Ile)475-Ile(Leu, Val, Ala)476, contained in the extended carboxyl cytoplasmic tail, are critical for targeting of the resident lysosomal membrane protein LIMP II to lysosomes. J Biol Chem. 1994;269:6622–6631. [PubMed] [Google Scholar]
- 32.Scheffner M, Werness B A, Huibregtse J M, Levine A J, Howley P M. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 33.Schulz M, Aichele P, Vollenweider M, Bobe F W, Cardinaux F, Hengartner H, Zinkernagel R M. Major histocompatibility complex-dependent T cell epitopes of lymphocytic choriomeningitis virus nucleoprotein and their protective capacity against viral disease. Eur J Immunol. 1989;19:1657–1668. doi: 10.1002/eji.1830190921. [DOI] [PubMed] [Google Scholar]
- 34.Thomson S A, Burrows S R, Misko I S, Moss D J, Coupar B E, Khanna R. Targeting a polyepitope protein incorporating multiple class II-restricted viral epitopes to the secretory/endocytic pathway facilitates immune recognition by CD4+ cytotoxic T lymphocytes: a novel approach to vaccine design. J Virol. 1998;72:2246–2252. doi: 10.1128/jvi.72.3.2246-2252.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ulmer J B, Donnelly J J, Parker S E, Rhodes G H, Felgner P L, Dwarki V J, Gromkowski S H, Deck R R, DeWitt C M, Friedman A, Hawe L A, Leander K R, Martinez D, Perry H C, Shiver J W, Montgomery D L, Liu M A. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 36.van Noort J M, Boon J, van der Drift A C, Wagenaar J P, Boots A M, Boog C J. Antigen processing by endosomal proteases determines which sites of sperm-whale myoglobin are eventually recognized by T cells. Eur J Immunol. 1991;21:1989–1996. doi: 10.1002/eji.1830210904. [DOI] [PubMed] [Google Scholar]
- 37.van Noort J M, Jacobs M J. Cathepsin D, but not cathepsin B, releases T cell stimulatory fragments from lysozyme that are functional in the context of multiple murine class II MHC molecules. Eur J Immunol. 1994;24:2175–2180. doi: 10.1002/eji.1830240936. [DOI] [PubMed] [Google Scholar]
- 38.Varga S M, Welsh R M. Detection of a high frequency of virus-specific CD4+ T cells during acute infection with lymphocytic choriomeningitis virus. J Immunol. 1998;161:3215–3218. [PubMed] [Google Scholar]
- 39.Varga S M, Welsh R M. Stability of virus-specific CD4+ T cell frequencies from acute infection into long term memory. J Immunol. 1998;161:367–374. [PubMed] [Google Scholar]
- 40.Vega M A, Rodriguez F, Segui B, Cales C, Alcalde J, Sandoval I V. Targeting of lysosomal integral membrane protein LIMP II. The tyrosine-lacking carboxyl cytoplasmic tail of LIMP II is sufficient for direct targeting to lysosomes. J Biol Chem. 1991;266:16269–16272. [PubMed] [Google Scholar]
- 41.Vega M A, Segui-Real B, Garcia J A, Cales C, Rodriguez F, Vanderkerckhove J, Sandoval I V. Cloning, sequencing, and expression of a cDNA encoding rat LIMP II, a novel 74-kDa lysosomal membrane protein related to the surface adhesion protein CD36. J Biol Chem. 1991;266:16818–16824. [PubMed] [Google Scholar]
- 42.von Herrath M G, Yokoyama M, Dockter J, Oldstone M B A, Whitton J L. CD4-deficient mice have reduced levels of memory cytotoxic T lymphocytes after immunization and show diminished resistance to subsequent virus challenge. J Virol. 1996;70:1072–1079. doi: 10.1128/jvi.70.2.1072-1079.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whitmire J K, Asano M S, Murali-Krishna K, Suresh M, Ahmed R. Long-term CD4 Th1 and Th2 memory following acute lymphocytic choriomeningitis virus infection. J Virol. 1998;72:8281–8288. doi: 10.1128/jvi.72.10.8281-8288.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whitton J L, Sheng N, Oldstone M B A, McKee T A. A “string-of-beads” vaccine, comprising linked minigenes, confers protection from lethal-dose virus challenge. J Virol. 1993;67:348–352. doi: 10.1128/jvi.67.1.348-352.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Williams K P, Smith J A. Isolation of a membrane-associated cathepsin D-like enzyme from the model antigen presenting cell, A20, and its ability to generate antigenic fragments from a protein antigen in a cell-free system. Arch Biochem Biophys. 1993;305:298–306. doi: 10.1006/abbi.1993.1426. [DOI] [PubMed] [Google Scholar]
- 46.Williams M A, Fukuda M. Accumulation of membrane glycoproteins in lysosomes requires a tyrosine residue at a particular position in the cytoplasmic tail. J Cell Biol. 1990;111:955–966. doi: 10.1083/jcb.111.3.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu T C, Guarnieri F G, Staveley-O'Carroll K F, Viscidi R P, Levitsky H I, Hedrick L, Cho K R, August J T, Pardoll D M. Engineering an intracellular pathway for major histocompatibility complex class II presentation of antigens. Proc Natl Acad Sci USA. 1995;92:11671–11675. doi: 10.1073/pnas.92.25.11671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiang R, Lode H N, Chao T H, Ruehlmann J M, Dolman C S, Rodriguez F, Whitton J L, Overwijk W W, Restifo N P, Reisfeld R A. An autologous oral DNA vaccine protects against murine melanoma. Proc Natl Acad Sci USA. 2000;97:5492–5497. doi: 10.1073/pnas.090097697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yokoyama M, Zhang J, Whitton J L. DNA immunization confers protection against lethal lymphocytic choriomeningitis virus infection. J Virol. 1995;69:2684–2688. doi: 10.1128/jvi.69.4.2684-2688.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang T, Maekawa Y, Yasutomo K, Ishikawa H, Fawzy N B, Dainichi T, Hisaeda H, Sakai T, Kasai M, Mizuochi T, Asao T, Katunuma N, Himeno K. Pepstatin A-sensitive aspartic proteases in lysosome are involved in degradation of the invariant chain and antigen-processing in antigen presenting cells of mice infected with leishmania major. Biochem Biophys Res Commun. 2000;276:693–701. doi: 10.1006/bbrc.2000.3538. [DOI] [PubMed] [Google Scholar]