Abstract
Background:
Despite the promise of oral immunotherapy (OIT) to treat food allergies, this procedure is associated with potential risk. There is no current agreement about what elements should be included in the preparatory or consent process.
Objective:
We developed consensus recommendations about the OIT process considerations and patient-specific factors that should be addressed before initiating OIT and developed a consensus OIT consent process and information form.
Methods:
We convened a 36-member Preparing Patients for Oral Immunotherapy (PPOINT) panel of allergy experts to develop a consensus OIT patient preparation, informed consent process, and framework form. Consensus for themes and statements was reached using Delphi methodology, and the consent information form was developed.
Results:
The expert panel reached consensus for 4 themes and 103 statements specific to OIT preparatory procedures, of which 76 statements reached consensus for inclusion specific to the following themes: general considerations for counseling patients about OIT; patient- and family-specific factors that should be addressed before initiating OIT and during OIT; indications for initiating OIT; and potential contraindications and precautions for OIT. The panel reached consensus on 9 OIT consent form themes: benefits, risks, outcomes, alternatives, risk mitigation, difficulties/challenges, discontinuation, office policies, and long-term management. From these themes, 219 statements were proposed, of which 189 reached consensus, and 71 were included on the consent information form.
Conclusion:
We developed consensus recommendations to prepare and counsel patients for safe and effective OIT in clinical practice with evidence-based risk mitigation. Adoption of these recommendations may help standardize clinical care and improve patient outcomes and quality of life.
Keywords: Allergy, anaphylaxis, Delphi, food allergy, oral immunotherapy, consent, patient preparation, shared decision making, risk mitigation
Food allergy is a significant public health issue, affecting up to 5% to 10% of the population.1,2 Reactions to accidental exposures are common and result in quality-of-life concerns, potential for social isolation, nutritional limitations, and progressive psychological burden for many families.3 Several immunotherapy options are emerging as reasonable food allergy risk mitigation strategies.4 On the basis of phase 3 clinical trials and real-world experience, guidelines now support implementing oral immunotherapy (OIT) into routine clinical practice, with varying global uptake.5–12
A shared decision-making (SDM) approach to OIT patient selection and preparation requires grounding in a robust discussion to explore the patient’s goals and preferences, as well as a thorough review of potential alternatives, outcomes, benefits, and risks.13,14 The majority of OIT dosing is performed at home, without immediate medical supervision, shifting a significant burden of responsibility for safety, adherence, and effectiveness to patients and caregivers.15 OIT is generally regarded as safe, but there is a well-recognized potential for severe dose-related reactions. Such reactions are often associated with complicating cofactors such as illness or exercise, and attempts have been made to mitigate these cofactors with safe-dosing rules.6,16 Nevertheless, patients require an adequate understanding of OIT procedures and how potential cofactors may complicate and affect dosing. There are few validated tools or published data for optimizing the key elements that should be included in SDM discussions and the formal consent and counseling process.17,18
We report the results of the first international Delphi consensus panel, the Preparing Patients for Oral Immunotherapy (PPOINT), study, assembled to help define recommended optimal components of an optimized OIT evaluation preparation, SDM, counseling, and informed consent process to best prepare patients and caregivers for this therapy.
METHODS
The full methods used to develop the OIT Delphi PPOINT expert consensus panel and the voting process are described in Fig E1 in the Online Repository available at www.jacionline.org. From October 2021 through July 2023, we convened a 36-member panel of allergy experts from 10 countries. Pediatric and adult allergists and immunologists were selected on the basis of their clinical expertise and prior published research. Briefly, after soliciting and iteratively developing themes and statements from participants, a modified Delphi methodology was used to determine whether there was consensus for candidate themes and statements. An anonymous electronic REDCap survey was sent to panelists, who were asked to rate each theme and statement on the level of agreement (1 = strongly disagree, 2 = agree, 3 = neutral, 4 = agree, 5 = strongly agree), or not applicable. “Strongly agree” and “agree” were grouped, and “strongly disagree” and “disagree” were grouped.19–21 Panelists were also encouraged to anonymously submit free-text comments.
Defining consensus
Consensus was defined as agreement or disagreement of ≥75% for themes and statements—a common Delphi prespecified threshold.22–24 Wording adjustment and revoting continued in additional rounds for each theme and statement until consensus was reached, or after 3 survey rounds. If the third round reached no consensus, the theme or statement was categorized as “consensus not reached.” OIT contraindications that reached consensus were further ranked by the participants as relative or absolute.
Prioritizing statements to include in template OIT consent form
An additional survey was conducted to prioritize statements for inclusion in a template that could be used to create a customizable OIT consent information form model, recognizing that consent forms will be modified and tailored according to contextual provider and practice differences, as well as local, regional, and national laws and regulations. Statements that reached “consensus agree” were incorporated into an anonymous REDCap survey. Using the clinical impact method, for each statement, panelists were asked to rate the importance of including the statement in an OIT consent form using a 0-to-10 scale (0 = not important, 5 = neutral, 10 = very important).25 Panelists could also select “not applicable.” The median importance score (0-10) with the corresponding interquartile range was reported for each statement, and a sample customizable template OIT consent form was developed incorporating statements that reached a median score of 9 or more. Where necessary, statements selected for inclusion were modified on the final form to enhance readability and formatting. This study was approved by the Cincinnati Children’s Hospital institutional review board.
RESULTS
OIT Delphi panel participants and overview of Delphi voting process
The PPOINT OIT Delphi panel consisted of 36 experts in food OIT. A description of the participants and their OIT experience is detailed in Table I. The expert panel proposed 322 separate statements, divided into 4 procedural themes (A-D; 103 statements) and 9 consent themes (E-M; 219 statements). Of these, 265 reached consensus for inclusion, 9 reached consensus for exclusion, and 49 did not meet consensus (see Table E1 in the Online Repository available at www.jacionline.org). Percentages in the text below are listed in parentheses as the proportion of participants who voted “agree/strongly agree” in the final voting round and the number of rounds (1 to 3) required to reach consensus—for example, (94.5%; 2).
TABLE I.
Characteristics of 36 members of expert panel
| Characteristic | No. (%) or median [IQR] |
|---|---|
| Practice type | |
| Academic | 20 (55.6) |
| Private practice | 9 (25.0) |
| Mixed/both | 7 (19.4) |
| Patient profile | |
| Child | 21 (58.3) |
| Adult | 0 |
| Mixed/both | 15 (41.7) |
| Years in practice | 15.0 [10.0, 25.0] |
| Have you published peer-reviewed articles on OIT? | |
| Yes | 36 (100) |
| Do you perform OIT in your practice? | |
| Yes | 34 (94.4) |
| How many years have you performed OIT? | 10.0 [7.0, 12.0] |
| Please estimate the number of patients you have managed with OIT. | 400.0 [150.0, 800.0] |
| What allergens do you perform OIT for? | |
| Peanut | 34 (94.4) |
| Tree nuts | 28 (77.8) |
| Milk | 31 (86.1) |
| Egg | 30 (83.3) |
| Sesame | 24 (66.7) |
| Wheat | 25 (69.4) |
| Other | 14 (38.9) |
| In what context do you perform OIT? | |
| Clinical practice | 15 (44.1) |
| Clinical trial | 2 (5.9) |
| Both | 17 (50.0) |
| Do you obtain written informed consent before initiating OIT? | |
| Yes | 32 (94.1) |
| How long (minutes) on average do you estimate you spend obtaining informed consent for OIT? | 30.0 [20.0, 60.0] |
| Who performs OIT consent discussion at your center? | |
| Attending physician | 30 (83.3) |
| Resident or fellow (physician in training) | 7 (19.4) |
| Physician assistant | 7 (19.4) |
| Nurse practitioner | 7 (19.4) |
| Nurse | 9 (25.0) |
| Other | 3 (8.3) |
| How is OIT funded at your center? | |
| Public insurance | 15 (41.7) |
| Private insurance | 18 (50.0) |
| Direct payment (out of pocket) | 13 (36.1) |
| Clinical trial | 14 (38.9) |
| Other | 3 (8.3) |
General considerations for counseling patients about OIT
The panel strongly agreed that the counseling process should include a detailed discussion about the steps in the OIT process (100%; 1), including the stages and timelines (97.2%; 1) (buildup, maintenance, and possible sustained unresponsiveness [SU] and remission) (see Table E2 in the Online Repository available at www.jacionline.org). There was consensus that oral food challenges be prioritized to confirm diagnosis, establish threshold, and assess desensitization and SU (88.9%; 1). Panelists prioritized clearly understanding the patients’ and caregivers’ goals (97.2%; 1). Panel members reached consensus for robust education of the patient and all relevant caregivers (100%; 1) through a detailed consent process (Fig 1, A, and Table E2).
FIG 1.

(A) Theme A key statements regarding general considerations for counseling patients about OIT. (B) Theme B key statements regarding general considerations for counseling patients about OIT. (C) Theme C key statements regarding general considerations for counseling patients about OIT. Statement number and statement are listed. Percentage of participants who voted for statement is represented by number and graphically as blue circle. Blue dots represent number of rounds to reach consensus. Full list of statements is provided in Tables E2–E4.
Patient- and family-specific factors to be addressed before and during OIT
Panelists highlighted control of comorbid allergic conditions, with all panelists agreeing on the importance of optimal asthma control (100%; 1). Addressing anxiety surrounding OIT with counseling support was also prioritized (94.4%; 1). Practical factors, including establishing adequate parental supervision for dosing (100%; 1) and assessing the feasibility of activity restriction to comply with safe dosing rules (100%; 1), were also agreed as important to address before initiating OIT. The panel reached consensus about the importance of ensuring divorced or separated parents agree on OIT treatment plans (86.1%; 1) (Fig 1, B, and see Table E3 in the Online Repository available at www.jacionline.org).
Indications for initiating OIT
There was consensus regarding OIT being indicated for patients up to 17 years of age (age under 1 year—77.1%; 3, age 1-4 years—83.3%; 1, age 4-17 years—88.9%; 1), but no consensus was reached regarding indication for patients over 18 (66.7%; 3) (Fig 1, C, and see Table E4 in the Online Repository available at www.jacionline.org). Having multiple food allergies was considered an indication for OIT (77.8%; 1). While consensus was reached regarding OIT being indicated for patients unlikely to outgrow their allergy spontaneously (91.7%; 1), there was no consensus for patients likely to outgrow their allergy (eg, milk, egg, soy) (63.9%; 3). The panel agreed that impairment in quality-of-life concerns about accidental exposure (80%; 3) and nutritional burden (80.6%; 3) were indications for OIT (Fig 1, C).
Potential contraindications and precautions for OIT
Active eosinophilic esophagitis (EoE) reached consensus as a contraindication (94.3%; 1), with 57.6% considering this an absolute contraindication, but no consensus was reached about EoE in remission’s being a contraindication (52.8%; 3) (Fig 2, and see Table E5 in the Online Repository available at www.jacionline.org). Uncontrolled asthma was unanimously considered a contraindication (100%; 1), with most participants considering it an absolute contraindication (88.9%). Uncontrolled psychological disorders (86.1%; 1) (including eating disorders—83.3%; 1, anxiety—83.3%; 1, and obsessive-compulsive disorder—86.1%; 2) were considered contraindications if poorly controlled but were not considered contraindications if controlled. Social factors, including parental discord (94.4%;1), poor parental communication (86.1%; 1), language barriers (77.8%; 1), and poor prior adherence (94.4%; 1), were all considered contraindications. Unwillingness to use epinephrine was a contraindication (97.2%; 1), with 94.3% considering this an absolute contraindication (Fig 2).
FIG 2.
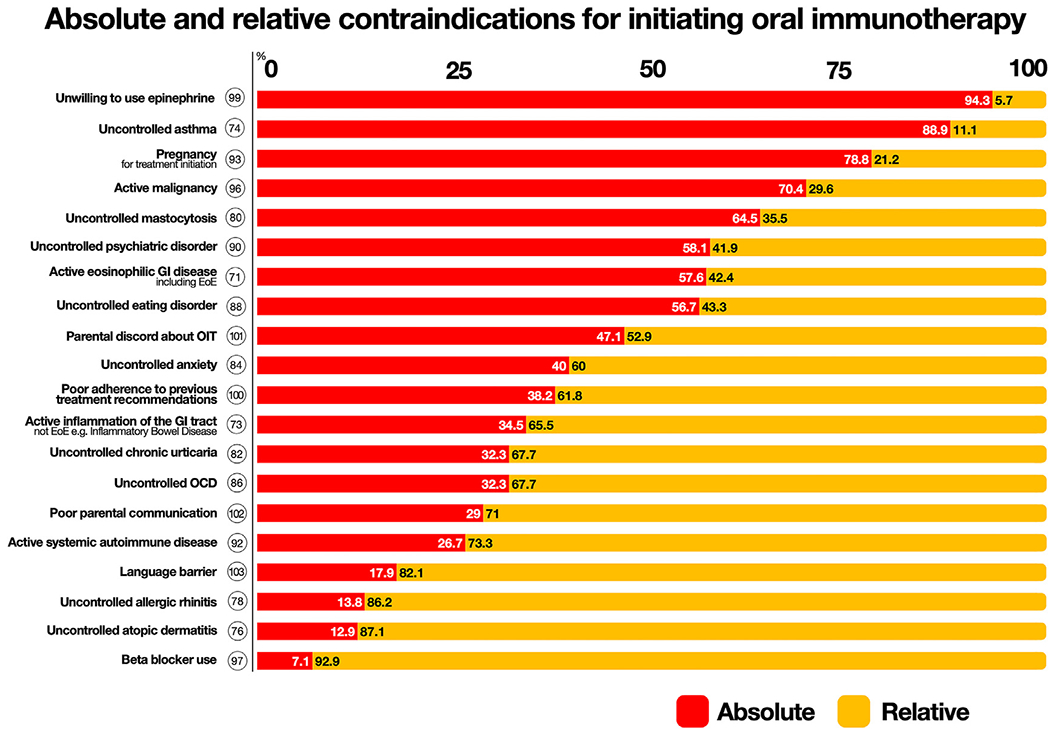
Theme D ranked absolute and relative contraindications that reached consensus. Circle represents statement number. Voting percentages for absolute (red) and relative (yellow) listed in bars. Full list of statements is provided in Table E5.
Defining potential benefits of OIT
The panel reached consensus regarding the benefits of a reduced risk of reacting to accidental exposures (94.4%; 1), reduced risk of a severe accidental reaction (88.9%; 1), an increased threshold required to elicit a reaction (97.1%; 1), and improved quality of life (88.9%; 1) and food-related anxiety among patients (83.3%; 1) and caregivers (88.9%; 1) (Fig 3, and see Table E6 in the Online Repository available at www.jacionline.org).
FIG 3.
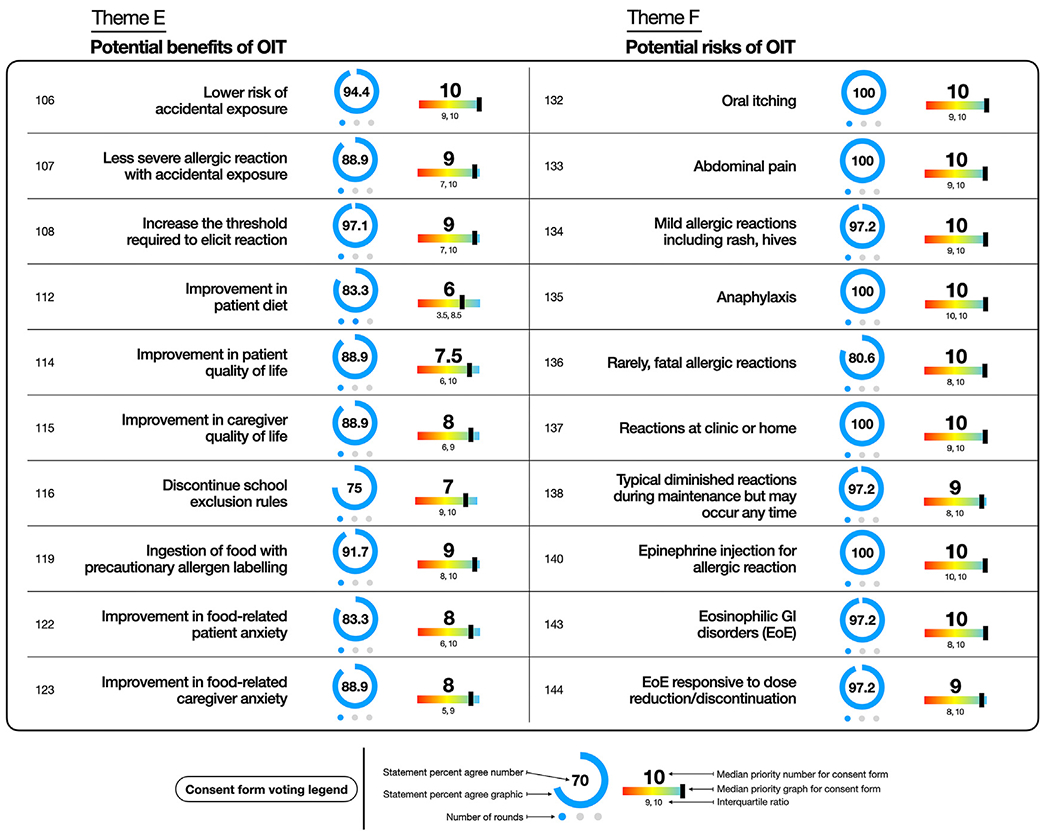
Theme E and F key statements for general considerations for counseling patients about OIT. Statement number and statement are listed. Percentage of participants who voted for statement represented by number and graphically as blue circle. Blue dots represent number of rounds to reach consensus. Median priority to include statement on consent form represented with number and rainbow lever graphic representation. Interquartile range listed below. Full list of statements is provided in Tables E6 and E7.
Defining potential risks associated with OIT
Foremost, mild (97.2%; 1), severe (100%; 1), and even fatal (80.6%; 1) reactions were identified as potential risks that should be clearly communicated to patients and caregivers (see Table E7 in the Online Repository available at www.jacionline.org). The panel also agreed (83.3%; 2) that there is an increased risk of dose-related allergic reactions requiring epinephrine during OIT if following strict allergen avoidance. In addition, EoE was recognized as a potential risk of therapy requiring disclosure to the patient (97.2%; 1), although this condition is typically reversible with OIT discontinuation or dose reduction (97.2%; 1) (Fig 3).
Defining potential outcomes of OIT
Panelists agreed that OIT outcomes are variable (100%; 1), poorly predictable (77.8%; 1), and may be allergen specific (94.4%; 1) (see Table E8 in the Online Repository available at www.jacionline.org). The panel further agreed (83.3%; 1) on defining the scope of desensitization, including limiting consumption to freely eating allergenic foods. While remission (SU) as a discrete potential outcome reached consensus (77.8%; 1), free-text comments tempered its inclusion, given concerns regarding the rarity of SU, inconsistency of SU, its unclear definition, and its potential age dependence (see Fig E2 in the Online Repository).
Alternative therapies and options to OIT to consider
The panel reached consensus that OIT alternatives include continued food avoidance (100%; 1), epicutaneous immunotherapy (if this were to become available) (82.9%; 1), and participation in a clinical trial for a potential therapy, if available (85.7%; 1) (see Table E9 in the Online Repository available at www.jacionline.org). There was unanimous agreement to discuss discontinuation of OIT at any time (100%; 1) (see Fig E3 in the Online Repository).
Practical risk mitigation strategies (including protocol modifications) for OIT
There was consensus for discussing that OIT should be supervised by an allergist (88.9%; 1) (see Table E10 in the Online Repository available at www.jacionline.org). Consensus was reached recognizing the importance of caution with cofactors that may trigger or worsen an allergic reaction during OIT (Fig 4), including active infection (88.9%; 1), uncontrolled allergic disease (91.7%; 1), asthma exacerbation (88.9%; 1), exercise before (88.9; 2) and after (88.9%; 1) the dose, hot showers or baths (83.3%; 1), tiredness or sleep deprivation (77.8%; 1), menstruation (88.9%; 1), dental work or oral trauma (77.8%; 1), nonsteroidal anti-inflammatory drugs (86.1%; 2), and alcohol (80.6%; 1) (Fig 4).
FIG 4.
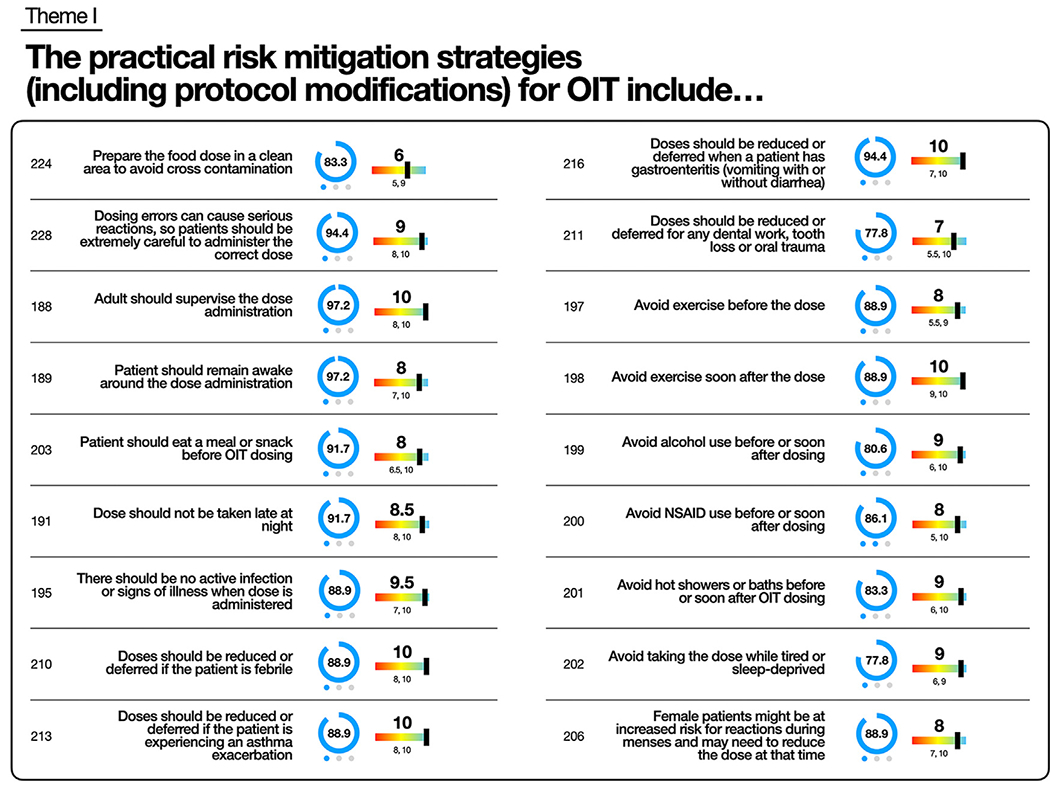
Theme I key statements for practical risk mitigation strategies for OIT. Statement number and statement are listed. Percentage of participants who voted for statement represented by number and graphically as blue circle. Blue dots represent number of rounds to reach consensus. Median priority to include statement on consent form represented with number and rainbow lever graphic representation. Interquartile range listed below. Full list of statements is provided in Table E10.
Difficulties and challenges during OIT
There were multiple statements regarding difficulties arising during OIT that reached consensus, including difficulty with adherence (97.2%; 1), dosing fatigue (91.7%; 1), food aversion (97.2%; 1), dose-related anxiety (91.7%; 1), extended time taking the dose (77.8%; 1), and exercise restrictions (86.1%; 1) (see Fig E4 and Table E11 in the Online Repository available at www.jacionline.org).
OIT discontinuation
Multiple indications for discontinuation met consensus, including recurrent dose-related systemic reactions (94.4%; 1), EoE (77.8%; 1), and uncontrolled asthma (94.4%; 1) (Fig 5, and see Table E12 in the Online Repository available at www.jacionline.org). Consensus was also reached surrounding poor protocol adherence (100%; 1), safety recommendations (including not administering epinephrine when necessary) (86.1%; 1), or asthma treatment (94.4%; 1) (Fig 5).
FIG 5.
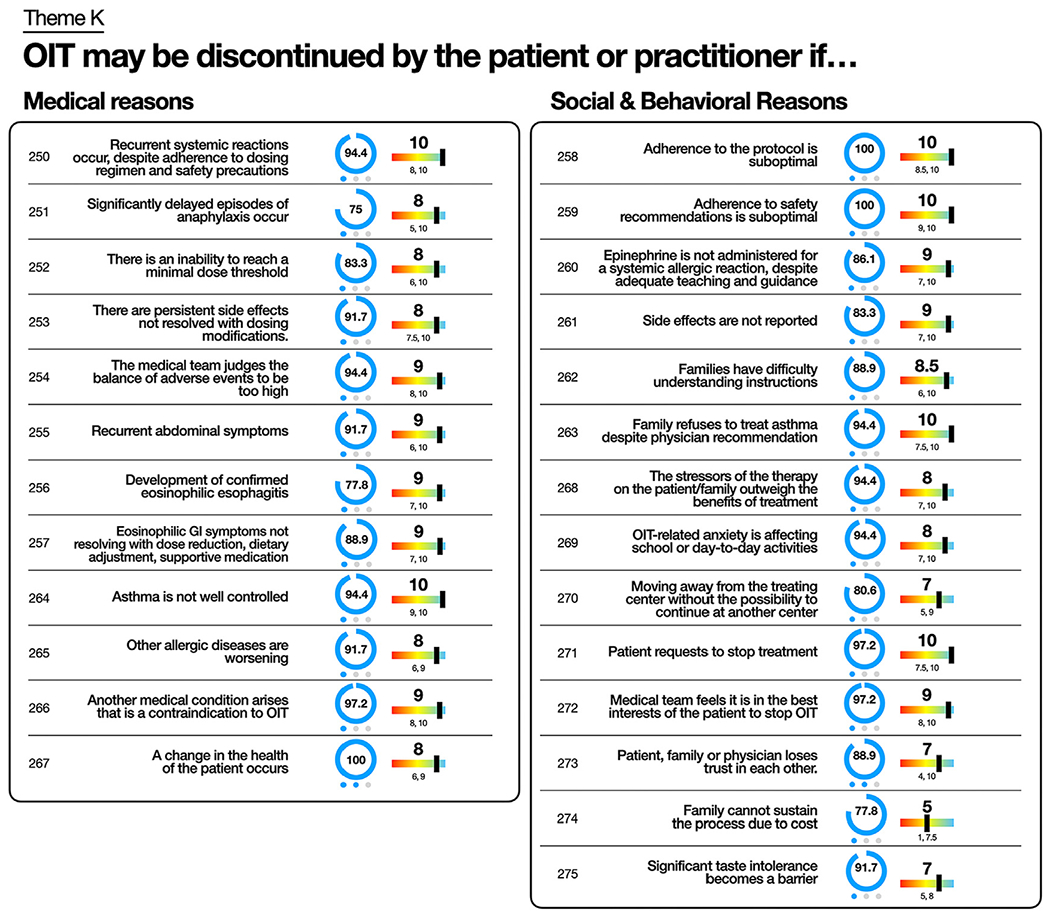
Theme K key statements regarding “OIT may be discontinued by patient or practitioner if…” Statement number and statement are listed. Percentage of participants who voted for statement represented by number and graphically as blue circle. Blue dots represent number of rounds to reach consensus. Median priority to include statement on consent form represented with number and rainbow lever graphic representation. Interquartile range listed below. Full list of statements is provided in Table E12.
Options for long-term management of OIT
Consensus was reached regarding the implications and options for long-term OIT management, specifically the importance of regular dose consumption (94.4%; 1) and that dose quantity and dosing frequency should not be modified without medical advice (86.1%; 1). Continued requirement for epinephrine carriage reached consensus (88.9%; 1), as did the potential for reactions even after years of maintenance dosing (91.7%; 1) (see Fig E6 and Table E14 in the Online Repository available at www.jacionline.org).
Ranked importance of OIT consent information
The final voting stage was used to determine which consensus statements should be included in the framework informed consent information form template. Of the 189 consent statements, 71 were prioritized for inclusion in the consent form, having reached a median importance score of ≥9 (Table II).
TABLE II.
Suggested statements with over 90% agreement for inclusion as part of model OIT consent information form
| Potential benefits |
| □ There is a lower risk of reacting to accidental exposures to the food allergen. □ Less severe allergic reactions occur with accidental exposure. □ An increased amount of food allergen (threshold) is required to elicit a reaction. □ Subjects may ingest foods with precautionary allergen (“may contain”) labeling. |
| Potential risks |
| □ Commonly occurring mild allergic reactions include oral symptoms (affecting mouth, tongue, lips, and/or throat), abdominal pain, rash, hives, and itchiness. □ Anaphylaxis reactions may include swelling, wheezing, cough, shortness of breath, vomiting, diarrhea, and, in severe cases, low blood pressure and loss of consciousness. □ Reactions can occur in the clinic or at home. □ Epinephrine injection may be required for allergic reaction. □ Emergency room/urgent care visit with or without hospitalization may be required for allergic reaction. □ Eosinophilic gastrointestinal disorders such as EoE may occur. □ Adverse reactions typically decrease during OIT maintenance phase but may occur at any time. |
| Potential outcomes |
| □ Patient response may be variable and poorly predictable, and may depend on patient and food being treated. □ OIT effectiveness may be lost if the food is not eaten regularly or is discontinued. □ Patients should expect to eat the allergenic food with some frequency indefinitely. |
| Alternatives and options |
| □ Continued food avoidance is a reasonable alternative. □ OIT may be discontinued at any time. |
| Practical risk mitigation strategies |
| □ Adults should supervise dose administration. □ OIT procedures should be supervised by allergists. □ OIT dose increases should be performed under medical supervision in a facility equipped to treat anaphylactic reactions. □ No active infection or signs of illness should be present when dose is administered or increased. □ Doses should be reduced or deferred if patient is febrile, has gastroenteritis, or is experiencing asthma exacerbation. □ Other allergic diseases should be controlled when dose is increased. □ Patients should avoid exercise, alcohol, and hot showers or baths before or soon after the dose. □ Patients should avoid taking the dose while tired or sleep deprived. □ Doses may be reduced and may need to be resumed in a medical facility if not provided for an extended period of time. □ Patients should have emergency medication with them at all times, and should treat any severe allergic reaction with epinephrine with potential activation of emergency medical services (eg, calling 911 or 999). □ Patients and caregivers (depending on age) should be trained to administer rescue medication, including epinephrine/adrenaline autoinjectors. □ Epinephrine-treated reactions should be reported to the treatment team immediately. □ Because dosing errors can cause serious reactions, caregivers should be careful to administer the correct dose. □ Doses may be reduced if there have been recurrent or severe reactions. |
| Difficulty encountered |
| □ Patients have trouble with adherence to daily dosing. |
| Reasons for discontinuation |
| □ Recurrent systemic reactions occur, despite adherence to dosing regimen and safety precautions. □ Recurrent abdominal symptoms or development of confirmed EoE occur. □ Eosinophilic gastrointestinal symptoms occur that do not resolve with dose reduction, dietary adjustment, and/or supportive medication. □ Asthma is not well controlled or family refuses to treat asthma. □ Another medical condition arises that is a contraindication to OIT. □ Adherence to therapy protocols or safety recommendations is suboptimal. □ Epinephrine is not administered by caregivers during a systemic allergic reaction. □ Adverse effects are not reported. □ Patient requests to stop treatment. □ Medical team judges the balance of adverse events to be too high or thinks it is in the patient’s best interest to stop OIT. |
| Office-specific policies, protocols, and procedures |
| □ Unscheduled clinic visits may be necessary for dose adjustments. □ Buildup phase may last 4 to 12 months, or even longer. □ Patients and caregivers are provided with guidance on how to contact health care providers with OIT-related questions, both during and outside of office hours and via phone, text, pager, or email. □ Treating physicians must be notified of cases of significant adverse effects. □ Patients and caregivers are expected to follow the allergist’s guidance in the event of an OIT-related reaction. □ Any prolonged OIT dose deferral should be promptly communicated to the health care provider. □ Patients are required to bring their epinephrine autoinjector to all dosing visits. □ At least one adult per child is required during OIT visits. □ Patients and caregivers agree not to share dosing protocols that may be used by individuals to attempt OIT without medical supervision. |
| Options for long-term management |
| □ Dose and frequency should not be modified without medical advice. □ Epinephrine autoinjector carriage may still be required even when receiving maintenance dosing. □ Adverse events might occur even after years of treatment and should be reported. □ Changes to patient health status may affect OIT safety and should be reported to the treatment team. □ Treatment decisions should be made in partnership and consultation with an allergist. □ Recommendations for OIT treatment may change as experience with OIT grows and as additional food allergy treatments become available. |
DISCUSSION
We convened a 36-member international expert PPOINT panel to develop a consensus for themes and elements important for OIT patient preparation and counseling. This is the first published study to develop a consensus-based sample template to assist in developing an OIT informed consent information form using Delphi methodology and clinical impact methods to define and prioritize topics for OIT consent, including detailed risk-mitigation procedures. Our expansive expert panel with vast clinical and research expertise will promote the dissemination and uptake of these findings into clinical care to harmonize patient care procedures and optimize clinical outcomes. This study is the first to systematically evaluate and attempt to standardize recommended elements for the OIT evaluation, preparation, and counseling and consent process.
More than 322 potential statements were initially proposed, many of which came from preexisting consent forms, processes, and standard operating procedures already in use. This breadth of input highlights the substantial variability in current approaches, mirrors the extensive number of issues that should be addressed, and represents the complexity of a complete approach to counseling patients.15 Moreover, this heterogeneity reinforces the need for more standardized recommendations of specific items to discuss during pre-OIT patient evaluation and candidate selection with specific inclusion of a detailed SDM framework to consider risks, benefits, and obligations inherent to participating in OIT.
Patient selection and preparation approach for OIT
A thorough OIT counseling and preparation process can help establish 2 critical goals: SDM along with voluntary informed consent; and patient evaluation and preparation. A proposed flow diagram of the OIT preparation process can be found in Fig 6. We recognize that practicing clinicians will develop their own individual approaches that may include a very different structure and order. We suggest this as a guidance document on the basis of the Delphi-based prioritization of this panel, and we emphasize the critical nature of a robust preparation process.
FIG 6.
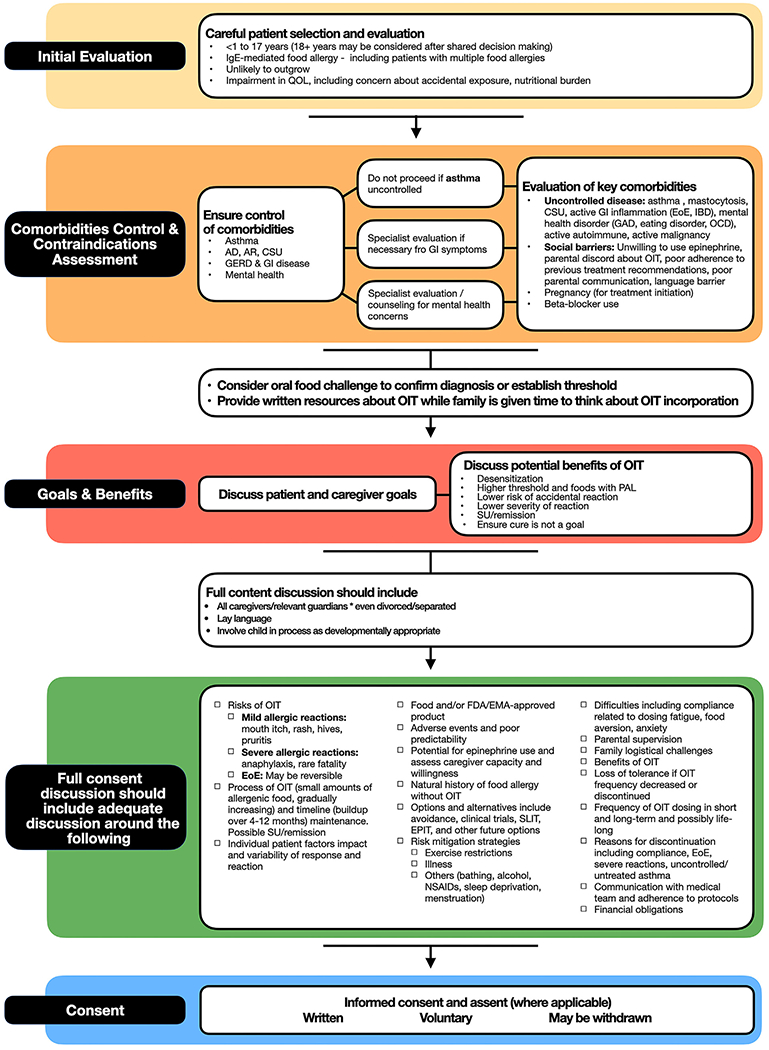
Proposed flow diagram resulting from procedural and consent elements of PPOINT study. AD, Atopic dermatitis; AR, allergic rhinitis; CSU, chronic spontaneous urticaria; EMA, European Medicines Agency; FDA, US Food and Drug Administration; GAD, general anxiety; GERD, gastroesophageal reflux disease; GI, gastrointestinal; IBD, inflammatory bowel disease; NSAID, nonsteroidal anti-inflammatory; OCD, obsessive-compulsive disorder; QOL, quality of life; SLIT, sublingual immunotherapy.
The panel recognized the importance of optimal control of comorbidities. In particular, poor asthma control has been highlighted as potentially increasing the risk of severe OIT reactions, and our panel repeatedly prioritized this important medical condition.16,19 However, other comorbidities, such as allergic rhinitis, eczema, gastrointestinal disease, and psychosocial factors, were also identified as important to assess for control, with psychological and behavioral barriers explicitly recognized as being potential threats to long-term OIT adherence and success.
Social and behavioral factors were also identified as priorities to address during the OIT preparatory process. Familial factors such as parental disagreement and divorced parental agreement were recognized as essential to address before beginning OIT. Parental discordance regarding OIT knowledge has been previously reported.18 Although one parent may be enthusiastic and knowledgeable about OIT, parental discord may lead to conflict and/or medicolegal risk; most importantly, however, it may affect patient safety if the process and safeguards are not well understood or accepted.
We also attempted to define the appropriate ages for initiating OIT. Currently, the only registered product has an age indication of 4 to 17 years.5 However, several studies have identified younger age groups as priority targets of OIT.12,20,21 Our group recognized that OIT could be considered in younger age groups, even under 1 year of age, although the level of agreement was highest for the approved indication. While our group did not reach a consensus on patients over the age of 18, we recognize that this group may be suitable if adequately informed and prepared. We note most participants were pediatric allergists, thus potentially biasing the consideration of adult patients.
The benefits of OIT have been evaluated in multiple studies and meta-analyses and include reduced risk of reaction and reaction severity and potentially improved quality of life and anxiety, which also have aligned with prior research defining patient preferences and goals of therapy.5–7,26 Our panel recognized and agreed that these outcomes may be variable and depend on patient characteristics, such as age, baseline degree of sensitization, and protocol. While patients may want to understand success rates, variability in baseline patient characteristics and protocols makes such determinations challenging to specify to patients.
Contraindications, risk mitigation, and OIT discontinuation
One of the major outcomes of this study was delineating OIT contraindications, as well as delineating whether experts considered contraindications to be absolute or relative. Performing proper clinical trials to specifically assess contraindications is potentially unethical, so contraindications can primarily be based on expert opinion or safety outcomes from trials and real-world data.4,20 Opinions regarding these designations vary, and a lack of clarity on such heterogeneity may affect OIT outcomes. Panelists agreed on a few absolute contraindications: unwillingness to use epinephrine, uncontrolled asthma, and pregnancy. However, there were differences in agreement regarding the degree of contraindication (relative vs absolute) with other potential concerns, such as active EoE, concurrent β-blocker receipt, control of other allergic comorbidities, and prior severity of reactions. While other groups have attempted to define absolute and relative contraindications for OIT, this is the first published data to add granularity to these contraindications.4,20
There was strong consensus regarding the recommended inclusion of multiple statements regarding risk and risk mitigation. While OIT remains a reasonably safe therapeutic process, it is well documented that reactions tend to occur in the setting of cofactors, such as illness, exercise, or uncontrolled asthma.4,27 This is the first panel using a Delphi methodology to not only define recommended discussion of suitable risk mitigation strategies during the consent process but also to rank each statement’s relative importance for inclusion in the discussion before agreeing to OIT. Many of these risk-mitigating procedures result in lifestyle limitations, including exercise restriction, and families must know about these potential limitations before signing consent. On the basis of this feedback, we have developed a practical list of strategies, in ranked order of perceived importance, that can be incorporated into the consent process and used when guiding families through OIT treatment (Fig 4).
There was strong consensus regarding discussion and disclosure of the risk of discontinuation and acknowledgment that it can result from voluntary patient/caregiver preference, medical necessity, or physician recommendation. Families and physicians make considerable investments to ensure the success of the OIT process and to avoid potential conflict down the road. A clear discussion about indications for discontinuing OIT, including noncompliance, severe reaction, or emerging contraindications, should occur before initiating OIT. Of note, while there are medical reasons for discontinuation, our findings are unique in that many of these reasons for discontinuation are also related to social and behavioral factors.
Informed consent
While the nature of informed consent necessitates that adequate information is provided to the patient and family, communicating this information can take several forms, and there is little standardized guidance regarding what topics and procedural information are necessary and sufficient to include. Alarmingly, recent surveys have suggested that up to one third of allergists offering OIT do not engage in informed consent.8,28 We present OIT consensus statements recommended for families to discuss, consider, and understand before providing written OIT consent (Table II). While these statements are recommended items to include in a consent discussion or potentially incorporate into a formal consent document, no individual statement or statements are intended to be substitute for a detailed discussion of the risks and benefits of OIT. These statements represent a guideline that may help clinicians create a tailored informed consent document, which would be used to supplement the aforementioned SDM process. It is recommended that prescribers work with their practice or institution to determine the final wording of any such document.
The dialogue between physician and patient represents the most critical element of the consent process. Handouts (such as Table II) are supplemental tools to assist such explanations and should ideally be provided before the consent discussion. Furthermore, the counseling clinician should document the discussion and provision of any supplemental materials and include the signed consent form in the patient’s medical record. Formal consent will also include a signed acknowledgment that these elements were adequately discussed with the patient, family, and/or legal guardian and understood.
Limitations
While we developed a list of statements to be communicated to families during the consent process, a limitation of our findings is that these statements are based on expert opinion, collective experience, and educated investigator perspective; they may not have a firm evidence base, and thus they serve as suggestions, not mandates. Additionally, the consensus statements are likely not applicable to all populations or clinical scenarios, so consent forms should be customized to one’s area of practice and the local medicolegal climate. As evidence grows and practice variation regarding risks and outcomes is better clarified, the consent process and elements included in the consent form will require modification. Another limitation of this process is the lack of patient and caregiver input, but this was designed to gather clinician-level consensus specific to support the safe provision of OIT. Future evaluation of these recommendations among patients and caregivers will be an important step for prospective validation and implementation. While we defined consensus as ≥75%, raising or lowering the consensus threshold would have affected the number of included statements, and we thus encourage clinicians to review the other statements in Tables E2–E14. Importantly, providers may choose to include or exclude statements as part of their counseling and consent process according to their own experience, patient population, and regional and institutional requirements.
Conclusion
OIT necessitates high levels of patient knowledge, involvement, and shared responsibility. To help support these needs, a thorough patient preparatory process is recommended to ensure that OIT candidates and their caregivers are adequately evaluated and prepared for the full range of potential risks and benefits, ideally through a SDM approach and informed consent. This Delphi approach has been used to establish specifically recommended fundamental elements of this preparatory consent process to optimize the safe and successful implementation of OIT. Clinicians may implement all or part of this informed consent process and consent form when evaluating and preparing families for their OIT treatment journey.
Supplementary Material
Clinical implications:
Implementation of these international consensus recommendations for the OIT preparation and consent process may standardize patient care, enhance education and communication, improve OIT safety, and optimize risk mitigation and outcomes.
DISCLOSURE STATEMENT
Institutionally supported in part by the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH; award UL1TR001425); and the National Center for Advancing Translational Sciences of the NIH (award KL2TR001426). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Disclosure of potential conflict of interest:
D. P. Mack has provided consultation and speaker services for DBV Technologies (DBV), ALK-Abelló, and Alladapt; and is an investigator for DBV and ALK-Abelló. P. J. Turner reports grants from UK Medical Research Council, NIHR/Imperial BRC, and J. M. Charitable Foundation; and personal fees from UK Food Standards Agency, Aimmune Therapeutics, Allergenis, and Aquestive Therapeutics outside the submitted work. R. L. Wasserman has provided consultation and advisory board services to Aimmune. M. A. Hanna is an investigator for DBV and ALK-Abelló. M. Shaker has participated in research funded by DBV; is an associate editor for Annals of Allergy, Asthma & Immunology; is a member of the Joint Task Force on Practice Parameters; and serves on the editorial boards of the Journal of Allergy and Clinical Immunology: In Practice and the Journal of Food Allergy. M. L. K. Tang reports consultant fees from Pfizer and Novartis; past employee (ended July 2022) and share interest/options in Prota Therapeutic; member of medical advisory board of Anaphylaxis & Anaphylaxis Australia; on the board of directors of Asia Pacific Association of Allergy Asthma and Clinical Immunology; past member of the board of directors of the World Allergy Organization (WAO; ended 2019); membership of expert committees of the American Academy of Allergy Asthma and Immunology (AAAAI), Asia Pacific Association of Allergy Asthma and Clinical Immunology (APAAACI), and Australasian Society of Clinical Immunology and Allergy (ASCIA); past membership of expert committees of WAO (ended 2019); and past membership of the International Union of Immunological Societies (ended 2019). P. Rodríguez del Río reports research grants from FAES and Aimmune Therapeutics; speaker honoraria from DBV, GSK, FAES, Novartis, ALK-Abelií, LETI Pharma, Aimmune Therapeutics, Sanofi Regeneron, and Stallergenes; and consultant fees from FAES and Miravo outside the submitted work. E. M. Abrams is a member of Joint Task Force on Practice Parameters; is an editorial board member of the Journal of Allergy and Clinical Immunology: In Practice; serves on the board of directors of the Canadian Society of Allergy and Clinical Immunology (CSACI); and is an employee of Public Health Agency of Canada (views expressed are her own and not those of PHAC). A. Anagnostou has received institutional funding from Aimmune and Novartis; consultation/speaker fees from ALK-Abelló, EPG Health, MJH, Adelphi, Aimmune Therapeutics, Genentech, and Food Allergy Research and Education (FARE); and served as an advisory board member for Ready,set,food and Novartis. S. Arasi has participated as advisory board member, consultant, and/or speaker for Novartis, Aimmune, DBV, Ferrero, and Ulrich outside the submitted work. S. Bajowala has served as consultant and advisory board member for Novartis; has served as a volunteer medical advisory board member of FPIES Foundation; is a shareholder of Solid Starts LLC; and owns WisePrince LLC (medical technology for oral immunotherapy [OIT]). P. Bégin reports grants from Novartis, Sanofi Regeneron, ALK-Abelló, and DBV; and personal fees from Bausch Health, Pfizer, AstraZeneca, DBV, Novartis, Sanofi Regeneron, and ALK-Abelló. S. B. Cameron reports membership on advisory boards for Medexus, Sanofi Regeneron, Bausch Health, Pfizer, and Alladapt; and served as a committee member for the CSACI OIT guidelines. E. S. Chan has received research support from DBV; has been a member of advisory boards for Pfizer, Miravo, Medexus, Leo Pharma, Kaleo, DBV, AllerGenis, Sanofi Genzyme, Bausch Health, Avir Pharma, AstraZeneca, and ALK-Abelló; and was colead of the CSACI OIT guidelines. S. Chinthrajah reports grants from National Institute of Allergy and Infectious Diseases (NIAID), Consortium of Food Allergy Research (CoFAR), FARE, Stanford Maternal and Child Health Research Institute, Genentech, and Regeneron; and personal fees from Alladapt Therapeutics, Novartis, Genentech, Allergenis, Intrommune Therapeutics, IgGenix, and Phylaxis. A. T. Clark is chief medical officer and stockholder in Camallergy Ltd (manufacturer of food OIT products). G. du Toit has received grants from NIAID, NIH, FARE, MRC & Asthma UK Centre, Action Medical Research, and National Peanut Board; was scientific advisory board member for Aimmune and Novartis; and was an investigator on pharma-sponsored allergy studies for Aimmune, DBV, and Novartis. J. Greiwe has provided speaker services for AbbVie, AstraZeneca, Incyte, Mylan, Regeneron, and Sanofi Genzyme; and has served on advisory boards for AbbVie, Aimmune, ALK-Abelló, AstraZeneca, DBV, Dermavant, Genentech, GSK, Regeneron, and Sanofi Genzyme. J. O’B Hourihane reports consultancy and research funding for Aimmune Therapeutics; research funding from DBV, Johnson & Johnson, Temple St Foundation, Ireland, City of Dublin Skin and Cancer Hospital Charity, and National Children’s Research Centre, Ireland; board membership for Clemens Von Pirquet Foundation Irish Food Allergy Network; and patent applications for Johnson & Johnson. D. H. Jones reports being consultant, speaker, and/or member of advisory boards for Genentech, Novartis, AstraZeneca, and Sanofi Regeneron. A. Muraro is a principal investigator (PI) for Aimmune, DBV, Novartis, Sanofi Regeneron; and has served on advisory boards and/or received speaker fees from Aimmune, DBV, Novartis, Sanofi Regeneron, Viatris, ALK-Abelló, Nestlé Heath Science, and Nutricia Danone. A. Nowak-Wegrzyn reports receipt of research support from NIAID, DBV, Alladapt, Danone and Nestle; receipt of consultancy fees from Regeneron, Novartis, Thermo Fisher Scientific, Aquestive, and Aimmune; and service as associate editor for Annals of Allergy, Asthma & Immunology; director of American Academy of Allergy, Asthma and Immunology board of directors; and chair of medical advisory board of International FPIES Association. N. B. Patel reports grants from UK Medical Research Council, NIHR/Imperial BRC, and J. M. Charitable Foundation; and personal fees from UK Food Standards Agency, Aimmune Therapeutics, Allergenis, Aquestive Therapeutics and Novartis outside of the submitted work. A. M. Scurlock receives grant funding from NIH/NIAID (CoFAR), FARE; and clinical trial funding from Aimmune Therapeutics, DBV, Genentech, Novartis, Siolta Therapeutics, and Regeneron Therapeutics. S. B. Sindher has received grant support to conduct trials from the National Institutes of Health, DBV, Regeneron, Aimmune, Novartis, CoFAR, and FARE; and is advisor/consultant for Genentech and DBV. S. Tilles is an employee of Aimmune Therapeutics. B. P. Vickery reports grants from Alladapt, AstraZeneca, Genentech, NIAID/NIH, and Siolta; personal fees from Allergenis, Aravax, Reacta Biosciences, and Sanofi Regeneron; both grants and personal fees from Aimmune, DBV, FARE, Novartis, and Regeneron; and stock options from Moonlight Therapeutics outside the submitted work. J. Wang receives research support from NIAID, Aimmune, DBV, and Siolta; and consultancy fees from ALK-Abelló, DBV, and Novartis. H. H. Windom serves as a PI in clinical trials with Sanofi Regeneron, Novartis, GSK, Areteia, Chiesi, and AstraZeneca. M. Greenhawt is a consultant for Aquestive; is a member of physician/medical advisory boards for DBV, Nutricia, Novartis, Aquestive, Allergy Therapeutics, AstraZeneca, ALK-Abelló, Bryn, Genentech, and Prota; is an unpaid member of the scientific advisory council for the National Peanut Board and the medical advisory board of the International Food Protein Induced Enterocolitis Syndrome Association; is a member of the Brighton Collaboration Criteria Vaccine Anaphylaxis 2.0 working group; is a senior associate editor for Annals of Allergy, Asthma & Immunology; is a member of the Joint Taskforce on Allergy Practice Parameters; and has received honoraria for lectures from ImSci, Red Nucleus, Medscape, Paradigm Medical Communications, and multiple state/local allergy societies. The rest of the authors declare that they have no relevant conflicts of interest.
Abbreviations used
- EoE
Eosinophilic esophagitis
- OIT
Oral immunotherapy
- PPOINT
Preparing Patients for Oral Immunotherapy
- SDM
Shared decision making
- SU
Sustained unresponsiveness
REFERENCES
- 1.Clarke AE, Elliott SJ, St Pierre Y, Soller L, La Vieille S, Ben-Shoshan M. Temporal trends in prevalence of food allergy in Canada. J Allergy Clin Immunol Pract 2020;8:1428–30.e5. [DOI] [PubMed] [Google Scholar]
- 2.Gupta RS, Warren CM, Smith BM, Jiang J, Blumenstock JA, Davis MM, et al. Prevalence and severity of food allergies among US adults. JAMA Netw Open 2019;2:e185630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lieberman JA, Gupta RS, Knibb RC, Haselkorn T, Tilles S, Mack DP, et al. The global burden of illness of peanut allergy: a comprehensive literature review. Allergy 2021;76:1367–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wasserman RL, Factor J, Windom HH, Abrams EM, Begin P, Chan ES, et al. An approach to the office-based practice of food oral immunotherapy. J Allergy Clin Immunol Pract 2021;9:1826–38.e8. [DOI] [PubMed] [Google Scholar]
- 5.PALISADE Group of Clinical Investigators, Vickery BP, Vereda A, Casale TB, Beyer K, du Toit G, et al. AR101 oral immunotherapy for peanut allergy. N Engl J Med 2018;379:1991–2001. [DOI] [PubMed] [Google Scholar]
- 6.Wasserman RL, Hague AR, Pence DM, Sugerman RW, Silvers SK, Rolen JG, et al. Real-world experience with peanut oral immunotherapy: lessons learned from 270 patients. J Allergy Clin Immunol Pract 2019;7:418–26.e4. [DOI] [PubMed] [Google Scholar]
- 7.Hourihane JOB, Beyer K, Abbas A, et al. Efficacy and safety of oral immunotherapy with AR101 in European children with a peanut allergy (ARTEMIS): a multicentre, double-blind, randomised, placebo-controlled phase 3 trial. Lancet Child Adolesc Health 2020;4:728–39. [DOI] [PubMed] [Google Scholar]
- 8.Mack DP, Soller L, Chan ES, Hanna MA, Terpstra C, Vander Leek TK, et al. A high proportion of Canadian allergists offer oral immunotherapy but barriers remain. J Allergy Clin Immunol Pract 2021;9:1902–8. [DOI] [PubMed] [Google Scholar]
- 9.Rodríguez Del Río P, Alvarez-Perea A, Blumchen K, Caimmi D, Caubet JC, Konstantinopoulos AP, et al. Food immunotherapy practice: nation differences across Europe, the FIND project. Allergy 2022;77:920–32. [DOI] [PubMed] [Google Scholar]
- 10.Begin P, Chan ES, Kim H, Wagner M, Cellier MS, Favron-Godbout C, et al. CSACI guidelines for the ethical, evidence-based and patient-oriented clinical practice of oral immunotherapy in IgE-mediated food allergy. Allergy Asthma Clin Immunol 2020;16:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pajno GB, Fernandez-Rivas M, Arasi S, Roberts G, Akdis CA, Alvaro-Lozano M, et al. EAACI guidelines on allergen immunotherapy: IgE-mediated food allergy. Allergy 2018;73:799–815. [DOI] [PubMed] [Google Scholar]
- 12.Muraro A, de Silva D, Halken S, Worm M, Khaleva E, Arasi S, et al. Managing food allergy: GA2LEN guideline, 2022. World Allergy Organ J 2022;15:100687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anagnostou A, Hourihane JO, Greenhawt M. The role of shared decision making in pediatric food allergy management. J Allergy Clin Immunol Pract 2020;8:46–51. [DOI] [PubMed] [Google Scholar]
- 14.Greenhawt M. Shared decision-making in the care of a patient with food allergy. Ann Allergy Asthma Immunol 2020;125:262–7. [DOI] [PubMed] [Google Scholar]
- 15.Mack DP, Greenhawt M, Turner PJ, Wasserman RL, Hanna MA, Shaker M, et al. Information needs of patients considering oral immunotherapy for food allergy. Clin Exp Allergy 2022;52:1391–402. [DOI] [PubMed] [Google Scholar]
- 16.Mack DP, Greenhawt M, Anagnostou A. Are there hidden dangers associated with milk and egg dietary advancement therapy? J Allergy Clin Immunol Pract 2023;11:1056–62. [DOI] [PubMed] [Google Scholar]
- 17.Greenhawt M, Shaker M, Winders T, Bukstein DA, Davis RS, Oppenheimer J, et al. Development and acceptability of a shared decision-making tool for commercial peanut allergy therapies. Ann Allergy Asthma Immunol 2020;125:90–6. [DOI] [PubMed] [Google Scholar]
- 18.Mack DP, Foster GA, Bouwers LM, Hanna MA. A counseling video with pre- and posttesting and checklist for oral immunotherapy consent improves participant knowledge. Ann Allergy Asthma Immunol 2020;125:468–74.e4. [DOI] [PubMed] [Google Scholar]
- 19.Anagnostou A, Sharma V, Herbert L, Turner PJ. Fatal food anaphylaxis: distinguishing fact from fiction. J Allergy Clin Immunol Pract 2022;10:11–7. [DOI] [PubMed] [Google Scholar]
- 20.de Silva D, Rodríguez Del Río P, de Jong NW, Khaleva E, Singh C, Nowak-Wegrzyn A, et al. Allergen immunotherapy and/or biologicals for IgE-mediated food allergy: a systematic review and meta-analysis. Allergy 2022;77:1852–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones SM, Kim EH, Nadeau KC, Nowak-Wegrzyn A, Wood RA, Sampson HA, et al. Efficacy and safety of oral immunotherapy in children aged 1-3 years with peanut allergy (the Immune Tolerance Network IMPACT trial): a randomised placebo-controlled study. Lancet 2022;399(10322):359–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shankar-Hari M, Phillips GS, Levy ML, Seymour CW, Liu VX, Deutschman CS, et al. Developing a new definition and assessing new clinical criteria for septic shock: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:775–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dribin TE, Schnadower D, Spergel JM, Campbell RL, Shaker M, Neuman MI, et al. Severity grading system for acute allergic reactions: a multidisciplinary Delphi study. J Allergy Clin Immunol 2021;148:173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenhawt M, Abrams EM, Shaker M, Chu DK, Khan D, Akin C, et al. The risk of allergic reaction to SARS-CoV-2 vaccines and recommended evaluation and management: a systematic review, meta-analysis, GRADE assessment, and international consensus approach. J Allergy Clin Immunol Pract 2021;9:3546–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Juniper EF, Guyatt GH, Streiner DL, King DR. Clinical impact versus factor analysis for quality of life questionnaire construction. J Clin Epidemiol 1997;50:233–8. [DOI] [PubMed] [Google Scholar]
- 26.Epstein-Rigbi N, Goldberg MR, Levy MB, Nachshon L, Elizur A. Quality of life of food-allergic patients before, during, and after oral immunotherapy. J Allergy Clin Immunol Pract 2019;7:429–36.e2. [DOI] [PubMed] [Google Scholar]
- 27.Afinogenova Y, Rubin TN, Patel SD, Powell RL, Gilo JM, Denno MN, et al. Community private practice clinical experience with peanut oral immunotherapy. J Allergy Clin Immunol Pract 2020;8:2727–35. [DOI] [PubMed] [Google Scholar]
- 28.Greenhawt MJ, Vickery BP. Allergist-reported trends in the practice of food allergen oral immunotherapy. J Allergy Clin Immunol Pract 2015;3:33–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


