Abstract
Although it has been demonstrated that the adenovirus IVa2 protein binds to the packaging domains on the viral chromosome and interacts with the viral L1 52/55-kDa protein, which is required for viral DNA packaging, there has been no direct evidence demonstrating that the IVa2 protein is involved in DNA packaging. To understand in greater detail the DNA packaging mechanisms of adenovirus, we have asked whether DNA packaging is serotype or subgroup specific. We found that Ad7 (subgroup B), Ad12 (subgroup A), and Ad17 (subgroup D) cannot complement the defect of an Ad5 (subgroup C) mutant, pm8001, which does not package its DNA due to a mutation in the L1 52/55-kDa gene. This indicates that the DNA packaging systems of different serotypes cannot interact productively with Ad5 DNA. Based on this, a chimeric virus containing the Ad7 genome except for the inverted terminal repeats and packaging sequence from Ad5 was constructed. This chimeric virus replicates its DNA and synthesizes Ad7 proteins, but it cannot package its DNA in 293 cells or 293 cells expressing the Ad5 L1 52/55-kDa protein. However, this chimeric virus packages its DNA in 293 cells expressing the Ad5 IVa2 protein. These results indicate that the IVa2 protein plays a role in viral DNA packaging and that its function is serotype specific. Since this chimeric virus cannot package its own DNA, but produces all the components for packaging Ad7 DNA, it may be a more suitable helper virus for the growth of Ad7 gutted vectors for gene transfer.
Adenovirus is assembled in a multistep process (reviewed in reference 12). Hexon proteins polymerize to form capsomers, which join with other structural proteins, including the penton base and fiber protein, to form empty capsids. The viral genome, a linear double-stranded DNA molecule with preterminal protein attached to both ends, is then thought to be inserted into the capsid along with core proteins, followed by a final maturation step mediated by the viral protease. Specific packaging of adenovirus DNA requires the packaging sequence located at the left end of the viral genome (nucleotides 194 to 358 in Ad5) (25, 32). This region contains at least five functionally redundant domains, the A repeats, with AI, II, V, and VI as the most important elements (19, 20). Each of the A repeats has a consensus motif and can function independently (45). How the packaging sequence mediates DNA packaging is not known, and the viral proteins that are involved in DNA packaging have not been fully identified. A temperature-sensitive mutant in the L1 52/55-kDa protein, ts369, accumulates intermediate particles associated with only the left end of the viral genome (30). This indicates that a functional 52/55-kDa protein is required for transferring the complete viral genome into capsids. By constructing an Ad5-derived mutant virus, pm8001, which does not express any L1 52/55-kDa protein, our laboratory has previously demonstrated that the L1 52/55-kDa protein is required for viral DNA encapsidation: viral particles isolated from pm8001-infected cells are empty capsids that contain no viral DNA (23). In addition, it has been shown that the 52/55-kDa protein is present in empty capsids but not in mature virions (29). Furthermore, we have shown that the 52/55-kDa protein interacts with the viral IVa2 protein (24). The only previously identified function of IVa2 protein is as a transcriptional activator of the major late promoter (39, 41, 53). It is a component of DEF-A and DEF-B, two complexes that bind to the downstream element of the major late promoter (39, 41, 53). Based on an observation that the same conserved motifs are present in both the downstream element and the packaging sequence, it was demonstrated that the IVa2 protein binds to these motifs in the packaging sequence (57). This indicates a possible role for the IVa2 protein in DNA encapsidation through its interaction with the 52/55-kDa protein. Cellular proteins have also been shown to bind to the A repeats (46), but their role in packaging has not been determined.
In addition to increasing our understanding of the basic mechanisms by which adenovirus encapsidates its DNA, studies on the possible roles of the 52/55-kDa and IVa2 proteins in this process would be useful for developing helper-dependent adenovirus vectors for gene transfer. In so-called first-generation adenovirus vectors, the E1 region is deleted and replaced with a transgene. The E1 region is essential for effective viral replication and E1-deleted vectors are replication defective (34) but can be grown on cells such as 293 cells that express the E1 proteins (22). However, low levels of replication of E1-deleted viral vectors can still occur in other cells, resulting in expression of viral antigens (16, 33). These induce host immune responses that either make the vector toxic to the host or reduce the duration of transgene expression (10, 55, 56). Various attempts have been made to reduce the replication of the vector by deleting or mutating other early regions, such as E2 or E4, with mixed results (1, 2, 4, 5, 8, 11, 15, 17, 18, 38, 54, 58). Most recently, investigators have attempted to completely eliminate viral gene expression and the ensuing host immune response by developing gutted, or helper-dependent, adenoviral vectors. These vectors contain only the viral inverted terminal repeats (ITRs) and the packaging sequence and have deletions of all the viral protein coding genes (7, 9, 36), resulting in improved duration of expression of therapeutic genes in vivo (14, 35, 36, 42, 50, 51, 59, 60). However, the growth of these vectors requires a helper virus that provides all the replication and structural proteins required for completion of the viral life cycle in trans, and it is difficult to purify the therapeutic virus from the helper virus. One approach to prevent contamination with helper virus is to develop a system in which the vector DNA is specifically packaged and the helper virus DNA is not packaged. To date, investigators have used helper viruses containing mutated packaging signals (35) or viruses in which the packaging signal is removed during growth by Cre-lox recombination (26, 44), but significant contamination still occurs.
With this report, we explore the specificity of DNA packaging between Ad5, a member of virus subgroup C, and other serotypes. By coinfecting cells with the Ad5 mutant virus pm8001 and other adenovirus serotypes, Ad7 (subgroup B), Ad12 (subgroup A), or Ad17 (subgroup D), we have found that these serotypes cannot complement the packaging defect of the pm8001 mutant virus, indicating that packaging requires serotype-specific interactions. To study the restriction to DNA packaging between Ad5 and Ad7 further, we constructed an Ad7/Ad5 chimeric virus containing the Ad7 genome except for the ITRs and packaging sequence, which are from Ad5. This virus can replicate its DNA and express its genes in 293 cells, but no infectious viruses are produced. 293 cells expressing the Ad5 L1 52/55-kDa protein cannot support the growth of the virus, whereas 293 cells expressing the Ad5 IVa2 protein can, indicating that the IVa2 protein plays a role in viral DNA packaging and that a functional interaction between the IVa2 protein and the adenovirus packaging machinery is serotype specific.
MATERIALS AND METHODS
Plasmid constructs.
The entire open reading frame of the Ad5 IVa2 protein was amplified from a previously described cDNA clone, E53 (24), using primers 5′-GCGCGGATCCAAGATGGAAACCAGAGGGCGAAG-3′ and 5′-GCGCCTCGAGTTATTTAGGGGTTTTGCG-3′. The PCR product was cloned into the BamHI and XhoI sites of pBK-CMV (Stratagene, La Jolla, Calif.) to generate pBK-IVa2. A cDNA of the Ad5 tripartite leader was cloned into the PstI and BamHI sites of pBK-CMV to generate pBK-Tripld. pBK-TripIVa2 was constructed by cloning the BamHI-plus-XhoI-digested IVa2 fragment from pBK-IVa2 into the same sites of pBK-Tripld. pcDNA-TripIVa2 was generated by cloning the NheI-plus-XbaI-digested IVa2 fragment from pBK-TripIVa2 into the same sites of pcDNA3.1/Hygro (Invitrogen).
Cells and viruses.
293 cells are human embryonic kidney cells expressing adenovirus type 5 E1A and E1B proteins (22). 293-L1 cells are 293 cells that stably express the Ad5 52/55-kDa protein and are used as a helper cell line for growing the 52/55-kDa mutant virus pm8001 (23). Both these cell lines were maintained in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS). For 293-L1 cells, 0.5 mg of G418 per ml was added to the medium. A cell line that expresses the IVa2 protein was generated by cotransfecting 293 cells with pBK-TripIVa2 and pcDNA-TripIVa2. Ten micrograms of each plasmid was calcium phosphate precipitated (23). Precipitate (0.6 ml) was added to 50% confluent 293 cells in a 60-mm-diameter dish. Fresh DMEM with 10% FBS, 500 μg of G418 per ml, and 100 μg of hygromycin per ml was added to the transfected cells 16 h later. When the cells became confluent, they were trypsinized, seeded at different cell concentrations per dish, and fed with medium containing 500 μg of G418 per ml and 100 μg of hygromycin per ml. Individual colonies were selected and expanded. The expression of the IVa2 protein was detected by immunoblotting. A cell line which expressed the highest amount of the IVa2 protein was designated 293-IVa2.
Wild-type Ad5, Ad7, Ad12, and Ad17 viruses (from the American Type Culture Collection) were propagated on 293 cells as described previously (21). All infections were performed at a multiplicity of infection (MOI) of 5 PFU/cell. The virus was allowed to adsorb for 2 h in DMEM with 2% FBS with gentle mixing every 15 min followed by addition of DMEM with 10% FBS, and infected cells were harvested 48 h postinfection unless otherwise indicated.
Construction of chimeric virus Ad7/5ITRψ-GFP.
An Ad5 left-end 384-bp DNA fragment containing the left ITR and packaging sequence (left ITR-ψ) was PCR-amplified using primers 5′-GCGCATGCATGTTTAAACATCATCAATAATATACCTTA-3′ and 5′-GGCGGAGCTCACCTGGGCGAGTCTCCACGTA-3′. An Ad5 right-end 200-bp fragment was amplified using primers 5′-GCGCGGGCCCGTTTAAACATCATCAATAATATACCTTA-3′ and 5′-GCGCGCATGCACAACTTCCTCAAATCGTCAC-3′. The PCR product of the left ITR-ψ was cloned into the NsiI and SacI sites, and the right-end ITR was cloned into the ApaI and SphI sites, of the pGEM-7Zf(+) vector (Promega) to generate pGEM-Ad5GV. Next, a green fluorescent protein (GFP) expression cassette containing a cytomegalovirus (CMV) promoter, GFP open reading frame, and simian virus 40 poly(A) site was amplified from pEGFP-C1 (Clontech) and cloned into the SacI and BamHI sites of pGEM-Ad5GV to generate pGEM-Ad5GV-GFP. The fragment containing the left ITR-ψ, the right ITR, and the GFP cassette in pGEM-Ad5GV-GFP can be released from the vector by PmeI digestion. The PmeI sites were added to the left and right ends of the left and right ITRs, respectively, during the PCR amplification.
Cloning of the Ad7 genome without the leftmost 2711 bp and rightmost 153 bp was accomplished by standard cloning and homologous recombination in Escherichia coli. First, the Ad7 HindIII E fragment (nucleotides 2712 to 6135 from the left end) was cloned into the HindIII site of pGEM-Ad5GV-GFP to generate pW111000. Then, the PmeI fragment in pW111000 containing the Ad5 ITRs and packaging sequence, the GFP expression cassette, and the Ad7 HindIII E fragment was cloned into the cosmid vector pWE15 (Clontech) to generate pW112700. An Ad7 right-end 2.6-kbp fragment without the 153-bp right ITR was amplified by PCR using a primer from the 3′ end of the Ad7 fiber gene (5′-GCCCGGTACCTACACCAATCTCTCCCCACG-3′) and a primer just inside the Ad7 right ITR (5′-CGCGTCTAGATGACGTACCGTGAGAAA-3′). This fragment was cloned into the KpnI and XbaI sites of pW112700 to generate pW120100. The remainder of the Ad7 genome between these two fragments was introduced by recombination in E. coli (see Fig. 5). KpnI-digested pW120100 (10 ng) and 144 ng of purified Ad7 viral DNA were cotransformed into E. coli BJ5183 cells as described previously (23). Colonies were screened by colony hybridization using 32P-labeled probes from the Ad7 sequence, which are not present in the parental pW120100. A positive clone was named pW120700, and it contains the Ad5 ITRs and packaging sequence, a GFP expression cassette, and the entire Ad7 genome except for the leftmost 2711 bp and the rightmost 153 bp.
FIG. 5.
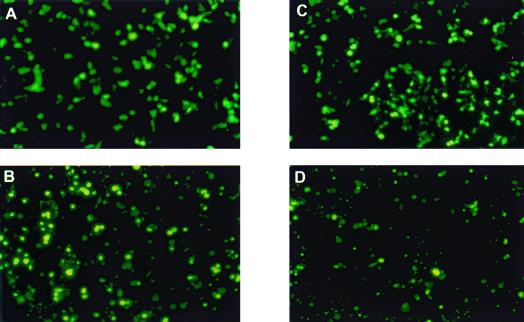
Ad5 52/55-kDa protein does not support the growth of Ad7/5ITRψ-GFP. 293 (A and B) or 293-L1 (C and D) cells were transfected with 10 μg of PmeI-digested pW120700. Expression of GFP was examined using a fluorescent microscope at 2 (A and C) and 11 (B and D) days posttransfection. Magnification, ×200.
Viral DNA isolation.
Viral DNA was extracted from infected cells as previously described (23). DNA from CsCl gradient-purified virions was extracted by adding an equal volume of predigested pronase (2 mg/ml in 50 mM Tris, 1 mM EDTA, 0.5% sodium dodecyl sulfate [SDS], pH 7.5) and incubating the mixture for 1 h at 37°C followed by phenol extraction and ethanol precipitation. The DNAs were dissolved in Tris-EDTA.
Southern blot and immunoblot analyses.
For southern blots, DNA samples from 2.5 × 109 viral particles were digested with KpnI and SpeI, loaded onto a 0.8% agarose gel for electrophoresis, and then transferred to GeneScreen Plus hybridization membranes (NEN Life Science Products, Inc., Boston, Mass.). The membranes were prehybridized in hybridization buffer (1% SDS, 10% dextran sulfate, 1 M NaCl, and 0.25 mg of denatured sheared salmon sperm DNA/ml) at 65°C for 6 h before a labeled probe was added. The 32P-labeled probe was generated by the Random Primer Labeling kit (Life Technologies, Inc., Gaithersburg, Md.) using both pTG3602, which contains the whole genome of Ad5 (6), and purified Ad7, Ad12, or Ad17 genomic DNA as templates. The probe (1 × 106 cpm/ml) was added to the prehybridization buffer and incubated overnight at 65°C. The membrane was washed twice with 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) at room temperature, and then it was washed twice with 2× SSC and 1% SDS at 65°C for 30 min. The membrane was dried and exposed to film. The intensities of the bands were measured by a PhosphorImager system (Molecular Dynamics, Inc., Sunnyvale, Calif.). The limit of detection in this assay was 25 pg of viral DNA, the equivalent of 106 mature virions. Immunoblotting was performed as previously described (27).
RESULTS
Ad7 cannot complement the packaging defect of pm8001.
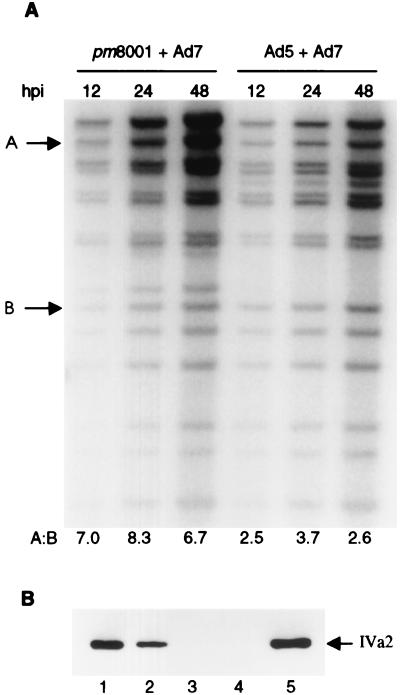
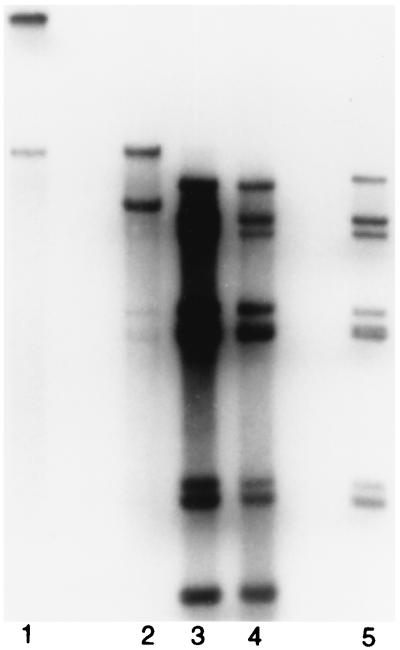
Since pm8001 requires the Ad5 52/55-kDa protein provided in trans to package its DNA, it allows us to investigate whether the 52/55-kDa proteins from other serotypes can complement the pm8001 mutation. This was examined using coinfection experiments. Cells were infected with pm8001 alone or coinfected with pm8001 and wild-type Ad7 or Ad5 at an MOI of 5 PFU/cell for each of the viruses. Forty-eight hours postinfection, progeny virions were purified by CsCl density centrifugation. All infections except for pm8001 alone in 293 cells yielded particles that sedimented at 1.34 g/cm3, corresponding to mature virions. In addition, the Ad7 plus pm8001 infection and the infection of pm8001 alone gave strong bands at 1.29 g/cm3, the density of empty capsids. When pm8001 was grown in 293-L1 cells, which stably express the Ad5 52/55-kDa protein, mature virions were produced. DNA was prepared from the virions isolated from the various infections, and Southern blotting was performed to determine if pm8001 DNA was packaged. DNAs from Ad7 and wild-type Ad5 can be distinguished by their KpnI and SpeI restriction enzyme digestion patterns (Fig. 1B, lanes 6 and 7). In addition, the mutation in pm8001 generates an extra SpeI recognition site (Fig. 1A); therefore, the mutant virus DNA can be distinguished from wild-type Ad5 (Fig. 1B, lanes 2 and 6). As reported previously, pm8001-infected 293 cells yield undetectable packaged DNA (Fig. 1B, lane 1), whereas DNA is packaged into virions when the virus is grown in 293-L1 cells (Fig. 1B, lane 2). Coinfection of 293 cells with wild-type Ad5 allowed pm8001 DNA to be packaged (Fig. 1B, lane 3). However, there was no detectable packaged pm8001 DNA in virions isolated from 293 cells coinfected with Ad7 (Fig. 1B, lane 4). Quantification of the results from multiple experiments indicates that the level of pm8001 is significantly less than 0.1% and often undetectable, as shown in Fig. 1. Mature viral particles from the coinfection with wild-type Ad5 and Ad7 contained both Ad5 and Ad7 DNA, indicating that Ad7 does not inhibit wild-type Ad5.
FIG. 1.
Other serotypes cannot complement the packaging defect of pm8001. (A) Restriction maps of Ad5 and pm8001. KpnI and SpeI sites are indicated by lines and arrowheads, respectively. (B and C) 293 or 293-L1 (lane 2 in both B and C) cells were infected with the indicated viruses at an MOI of 5 PFU/cell. Forty-eight hours postinfection, DNAs were extracted from purified virions and digested with KpnI and SpeI. Southern blotting was performed to determine the serotypes of the DNAs in the virions. In panel B, the arrow points to the Ad5-specific band containing the L1 gene, which is digested by SpeI in pm8001 DNA to yield the two bands indicated by the arrowheads.
We also tested whether two other serotypes, Ad12 and Ad17, could complement pm8001 in a coinfection. The results were similar to the Ad7 coinfection; pm8001 viral DNA was not detected in the purified virions from the coinfected cells (Fig. 1C). However, when wild-type Ad5 was coinfected with Ad17, the level of packaged Ad5 DNA was low, indicating that Ad17 may inhibit Ad5. These results indicate that packaging of adenovirus DNA may be serotype or subgroup specific.
Ad7 does not inhibit pm8001 DNA replication and capsid assembly in the coinfected cells.
The absence of pm8001 DNA in virions isolated from 293 cells coinfected with other serotypes indicated that the Ad7, Ad12, or Ad17 52/55-kDa proteins could not complement the mutation in pm8001. However, inhibition of pm8001 DNA replication or capsid assembly by the other serotypes would also yield the same result. Since Ad7 did not inhibit wild-type Ad5, in the following experiments we examined whether Ad7 inhibits pm8001 DNA replication and capsid assembly. Viral DNA was extracted from Ad7- and pm8001-coinfected 293 cells at 12, 24, and 48 h postinfection, and Southern blotting was performed to determine the extent of DNA replication at different time points. A coinfection with the two wild-type viruses was performed as a control. The amount of DNA from both pm8001 and Ad7 increased as the infection proceeded, indicating that the mutant virus could replicate in the coinfected cells (Fig. 2A). To determine whether the two viruses replicated in parallel, the amount of radioactivity in bands A and B shown in Fig. 2A, which are Ad7 and Ad5/pm8001 specific, was measured using a PhosphorImager. The ratio of the bands represents the ratio of replication of the two viruses at each time point. In the coinfections of Ad7 with either pm8001 or Ad5, similar ratios were found at all three time points. These data indicate that one virus in the coinfected cells did not affect the rate of replication of the other. The absolute amount of replication of pm8001 was twofold lower than that of Ad5, as previously described (23).
FIG. 2.
pm8001 replicates its DNA and assembles capsids in cells coinfected with Ad7. (A) 293 cells were coinfected with the indicated viruses at an MOI of 5 PFU/cell. At the indicated number of hours postinfection (hpi), low-molecular-weight DNAs were extracted from the cells and digested with KpnI and SpeI. Southern blotting was performed to determine the amounts of viral DNA in the infected cells. Bands A and B are Ad7 and Ad5/pm8001 specific, respectively, and the amount of radioactivity in each band was measured using a PhosphorImager to determine whether the two viruses replicated in parallel. The ratio of band A to band B represents the ratio of replication of the two viruses at each time point and is shown below each lane. (B) Empty capsids or mature virions were isolated from infected cells. Particles (5 × 1010) were boiled in sample buffer and loaded onto an SDS-polyacrylamide gel electrophoresis gel. Immunoblotting was performed to detect pm8001 empty capsids using an Ad5 IVa2 protein-specific monoclonal antibody. Lanes: 1, empty capsids from pm8001-infected cells; 2, empty capsids from pm8001- and Ad7-coinfected cells; 3, mature virions from Ad7-infected cells; 4, 1 μg of Ad7-infected whole-cell lysate; 5, 1 μg of Ad5-infected whole-cell lysate.
To test whether Ad7 blocks the assembly of pm8001 capsids, viral particles were isolated from the infected cells on CsCl gradients, and immunoblot analysis was performed to detect the Ad5 IVa2 protein, which is present in both empty capsids and mature virions (23), using an Ad5 IVa2 protein-specific monoclonal antibody. As shown in Fig. 2B, this antibody recognizes the Ad5 IVa2 protein but not the Ad7 IVa2 protein (lanes 4 and 5). The presence of Ad5 IVa2 protein in the empty capsids isolated from cells coinfected with pm8001 and Ad7 (lane 2) indicates that pm8001 empty capsids were assembled in the coinfected cells. Thus, the mutant virus replicated its DNA and capsids were formed in the coinfected cells, but the DNA was not encapsidated into progeny virions, demonstrating that Ad7 does not complement the packaging defect of pm8001.
Ad7 and Ad5 chimeric virus.
These coinfection experiments indicate that the Ad7 52/55-kDa protein cannot interact productively with the Ad5 packaging system in pm8001. To study this further, we constructed a chimeric virus, Ad7/5ITRψ-GFP, containing an Ad7 genome except for the ITRs and packaging sequence, which were derived from Ad5. In addition, the E1 region of Ad7 was replaced with a GFP expression cassette. The structure of this genome was confirmed by extensive restriction mapping (data not shown) and is shown in Fig. 3. Furthermore, the region containing the left ITR-ψ was sequenced, and it matched the Ad5 left ITR-ψ (data not shown).
FIG. 3.
Genomic structure of Ad7/5ITRψ-GFP.
Based on the coinfection data, we predicted that the chimeric virus would not be able to package itself since it has an Ad5 packaging sequence and the Ad7 52/55-kDa protein. We first wished to ensure, however, that it could express its early genes and replicate its DNA since it would need to rely on the Ad5 E1A proteins in 293 cells to transactivate the Ad7 early genes, and the ITRs and E2 proteins were derived from different serotypes. Five micrograms of PmeI-digested pW120700 was used to transfect 293 cells. Viral DNA extracted from the transfected cells at various time points was digested with BclI. The input cosmid DNA produced in bacteria is dam-methylated, and it should not be digested by BclI. BclI-digested replicated DNA was first detected at 1 day posttransfection, and it increased at 3 and 7 days posttransfection (Fig. 4), indicating that the Ad7 E2 proteins were expressed in the transfected cells and that these proteins functioned together with the Ad5 ITR to replicate the viral DNA.
FIG. 4.
Ad7/5ITRψ-GFP virus replicates its DNA in 293 cells. 293 cells were transfected with 5 μg of PmeI-digested pW120700. Viral DNAs were isolated at 1, 3, and 7 days posttransfection (lanes 2 to 4, respectively) and digested with BclI. Southern blotting was performed to detect viral DNA. Lane 1, BclI-digested pW120700; lane 5, BclI-digested wild-type Ad7 DNA.
Since wild-type Ad7 does not complement the packaging defect of pm8001, we assumed that the L1 52/55-kDa protein from Ad5 would be the key protein for the growth of the chimeric virus. To examine this, 293 cells or 293-L1 cells, which express the Ad5 L1 52/55-kDa protein, were transfected with PmeI-digested pW120700. Figure 5 shows GFP expression in the transfected cells at 2 and 11 days posttransfection. Only single GFP-positive cells were detected at both time points, indicating that although viral DNA was replicating, the virus might not be spreading. Fourteen days posttransfection, cytopathic effect (CPE) was not observed in either cell type. To confirm that viable virus was not being produced, viral lysates were made from the transfected cells at 14 days posttransfection and used to infect fresh 293 cells. No GFP expression or CPE was detected in these cells (data not shown), indicating that infectious virus was not produced in the initial transfection of 293 or 293-L1 cells and that the Ad5 52/55-kDa protein alone does not allow packaging of the chimeric virus to occur.
Given this result, we decided to test whether the restriction to packaging could be reversed by the addition of the Ad5 IVa2 protein, which has previously been demonstrated to interact with both the 52/55-kDa protein and the packaging sequence (24, 55). We cotransfected 293 or 293-L1 cells with PmeI-digested pW120700 and pBK-TripIVa2, which expresses the Ad5 IVa2 protein. Figure 6 shows GFP expression in the cotransfected cells. In this experiment, in addition to single GFP-positive cells, clusters of GFP-positive cells were seen surrounding areas of CPE at 9 to 14 days posttransfection. This indicated that viruses were spreading from the area of the CPE to the surrounding cells. To confirm that infectious viruses were produced from the cotransfected cells, viral lysates were prepared 11 to 14 days after cotransfection and used to infect fresh 293 cells. GFP-expressing cells were found in these freshly infected 293 cells (Fig. 7A). Based on this result, we generated a 293 cell line that stably expresses the Ad5 IVa2 protein. The chimeric virus was able to spread on these cells (Fig. 7B, D, and F). The virus did not grow any further in 293 cells (Fig. 7C and E), however, indicating that the Ad5 IVa2 protein is required for the continuous growth of the chimeric virus.
FIG. 6.
Ad5 IVa2 protein supports the growth of Ad7/5ITRψ-GFP. 293 (A to E) or 293 L1 (F to J) cells were cotransfected with 10 μg of pW120700 and 10 μg of pBK-TripIVa2. GFP expression was examined using a fluorescent microscope at 2 (A), 3 (F), 11 (B, C, G, and H), and 14 (D, E, I, and J) days posttransfection. Panels C, E, H, and J show combination fluorescent light-visible light micrographs of panels B, D, G, and I, respectively.
FIG. 7.
Ad5 IVa2-expressing 293 cells produce infectious Ad7/5ITRψ-GFP virus. Viral lysates made from 293 cells 14 days after cotransfection with PmeI-digested pW120700 and pBK-TripIVa2 were used to infect fresh 293 (A, C, and E) or 293-IVa2 (B, D, and F) cells. Expression of GFP was examined using a fluorescent microscope at 3 (A and B) and 6 (C and D) days postinfection. Panels E and F are combination fluorescent light-visible light micrographs of panels C and D, respectively.
DISCUSSION
The assembly of adenovirus virions starts with polymerization of hexons to form the capsomers that are the basic structural unit of the empty capsid (37). Empty capsids, with a density of 1.29 g/cm3, can be distinguished from mature virions (density, 1.34 g/cm3) by CsCl equilibrium centrifugation (40), and they are formed using the major structural proteins including the hexon, penton base, and fiber proteins as well as precursors of proteins VI and VIII. It is thought that adenovirus DNA and core proteins are inserted into preformed empty capsids (13), and it has been shown that this process starts from the left end of the viral genome (52). A packaging sequence located on the left end of the genome has been demonstrated to mediate the specific packaging of adenovirus DNA (25, 32). Proteins that can interact functionally with the packaging sequence have not been defined previously. Schmid and Hearing have detected cellular proteins binding to the packaging sequence (46). Gustin et al. have previously demonstrated that the Ad5 52/55-kDa protein is required for viral DNA encapsidation and that it interacts with the viral IVa2 protein (23, 24). The IVa2 protein binds to critical DNA motifs in the packaging sequence (57), suggesting that the interaction of the 52/55-kDa protein with the IVa2 protein may be involved in viral DNA encapsidation. Our present results indicate that the IVa2 protein does indeed play a role in this process.
Based on our coinfection data, we initially assumed that the Ad5 52/55-kDa protein would be needed for the growth of the chimeric virus. However, we demonstrated that the Ad5 52/55-kDa protein is not required for the growth of the chimeric virus, since the Ad5 IVa2 protein alone in 293 cells supports its growth. These results indicate that the Ad7 packaging system, including its 52/55-kDa protein, can use the Ad5 IVa2 protein to package DNAs that contain the Ad5 packaging sequence. It is difficult to quantify the degree of complementation due to the chimeric nature of the virus. We believe the isolation of an IVa2 mutant virus in a completely Ad5 background, as well as additional experiments to determine the precise step at which packaging is blocked, will allow such an assessment.
The ability to package the chimeric chromosome appears to be restricted at the interaction of the IVa2 protein with some other component(s) of the packaging machinery. What is puzzling, however, is why the presence of the Ad5 IVa2 protein in the context of the coinfection could not produce the same result as that obtained with the chimeric virus. There are at least two possible explanations for this disparity. First, in the coinfection, both the Ad5 and Ad7 packaging signals are present. If the Ad7 signal out-competed the Ad5 signal for trans-acting factors, then perhaps the packaging machinery would target the Ad7 chromosome for encapsidation. Such competition does not occur when the two wild-type viruses are used, however, making this scenario seem unlikely. Second, all the other Ad5 viral proteins are present in the coinfection but not in the chimeric virus experiments. Thus, it is possible that one or more of these proteins competes with its Ad7 counterpart for interactions with the Ad5 IVa2 protein and therefore prevents it from functioning with the rest of the packaging machinery. A more detailed understanding of the packaging mechanism will be required in order to fully explain this apparent inconsistency.
The chimeric virus replicates its DNA in 293 cells, indicating that the Ad5 E1 proteins expressed in 293 cells activate Ad7 early gene expression and that the Ad7 replication machinery can replicate DNA using the Ad5 ITRs. The human adenovirus ITRs are about 100 to 200 bp long. However, full replication requires only the first 45 to 70 bp of the ITR (3, 31). The ITR contains a 10-bp functional domain that is identical in all human adenovirus ITRs (3, 49). It has been shown previously that Ad2-infected cell extracts can replicate viral DNA from subgroups A to E in vitro (47, 48) and that Ad4-infected cell extracts can replicate DNA containing the Ad2 ITR in vitro (28). Our result that Ad7 proteins can drive replication from the Ad5 ITRs is consistent with these findings.
Ad7 and Ad5 are members of adenovirus subtypes B and C, respectively, whose sequences diverge significantly. The packaging sequences of Ad7 and Ad5 are only 68% identical overall, although the motifs previously demonstrated to be important for IVa2 binding in Ad5 are present in the Ad7 DNA packaging domains. In addition, the two 52/55-kDa proteins show only 70% identity, and the IVa2 proteins show 83% identity. Similar levels of sequence identity exist between Ad5 and Ad12 or Ad17. Therefore, it would not be surprising if there were serotype or subgroup specificity in the interactions between the components of the DNA packaging machinery. Although in the case of Ad12 and Ad17 we have not ruled out a block to earlier steps in the Ad5 life cycle, it has been shown that an Ad5 temperature-sensitive mutant in the hexon gene, ts147, can replicate its DNA well and express its penton protein when it is used to coinfect cells with Ad3 (subgroup A), Ad4 (subgroup E), and Ad9 (subgroup D) (43). These results indicate that earlier steps in the Ad5 life cycle are not blocked by these subgroups.
The demonstration of serotype-specific viral DNA packaging has important implications for the development of improved adenovirus gene transfer vectors. Gutted or helper-dependent adenovirus vectors have demonstrated great promise for prolonged therapeutic gene expression and reduced host immune responses. Presently, the utility of these vectors is limited by the fact that their growth requires a helper virus, and contamination by the helper virus cannot be totally eliminated by physical separation techniques or other manipulations. To date, investigators have either made the gutted chromosome smaller than that of the helper virus chromosome, resulting in slight differences in density that can be resolved somewhat in CsCl gradients (35, 36), used a mutant packaging signal on the helper virus chromosome that reduces its ability to be packaged (35), or introduced loxP sites on either side of the packaging sequence of the helper virus and grown the vector in cells that express Cre recombinase (26, 44). Such approaches still leave significant levels of helper virus contamination, however. Based on the data presented in this report, we propose that a system can be established in which vector DNA is specifically packaged without the packaging of the helper virus. In this system, our chimeric virus can be used as a helper virus, which can be grown in 293 cells expressing the Ad5 IVa2 protein. The helper-dependent vector will be derived from Ad7. When coinfecting both the helper virus and the vector in 293 cells in the absence of the Ad5 IVa2 gene, only the vector DNA should be packaged. If this were to work, it would be a hallmark in the effort to develop helper-dependent adenovirus vectors by greatly facilitating their production and purification.
ACKNOWLEDGMENTS
We thank the members of the Imperiale laboratory for help with this work, Claude Kedinger for anti-IVa2 monoclonal antibodies, and Jeff Chamberlain, Erle Robertson, and Hamish Young for useful discussions and suggestions.
This work was supported by NIH grants GM34902 and HL64762.
REFERENCES
- 1.Amalfitano A, Hauser M A, Hu H, Serra D, Begy C R, Chamberlain J S. Production and characterization of improved adenovirus vectors with the E1, E2b, and E3 genes deleted. J Virol. 1998;72:926–933. doi: 10.1128/jvi.72.2.926-933.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armentano D, Zabner J, Sacks C, Sookdeo C C, Smith M P, St. George J A, Wadsworth S C, Smith A E, Gregory R J. Effect of the E4 region on the persistence of transgene expression from adenovirus vectors. J Virol. 1997;71:2408–2416. doi: 10.1128/jvi.71.3.2408-2416.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernstein J A, Porter J M, Challberg M D. Template requirements for in vivo replication of adenovirus DNA. Mol Cell Biol. 1986;6:2115–2124. doi: 10.1128/mcb.6.6.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brough D E, Hsu C, Kulesa V A, Lee G M, Cantolupo L J, Lizonova A, Kovesdi I. Activation of transgene expression by early region 4 is responsible for a high level of persistent transgene expression from adenovirus vectors in vivo. J Virol. 1997;71:9206–9213. doi: 10.1128/jvi.71.12.9206-9213.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brough D E, Lizonova A, Hsu C, Kulesa V A, Kovesdi I. A gene transfer vector-cell line system for complete functional complementation of adenovirus early regions E1 and E4. J Virol. 1996;70:6497–6501. doi: 10.1128/jvi.70.9.6497-6501.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chartier C, Degryse E, Gantzer M, Dieterle A, Pavirani A, Mehtali M. Efficient generation of recombinant adenovirus vectors by homologous recombination in Escherichia coli. J Virol. 1996;70:4805–4810. doi: 10.1128/jvi.70.7.4805-4810.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen H H, Mack L M, Kelly R, Ontell M, Kochanek S, Clemens P R. Persistence in muscle of an adenoviral vector that lacks all viral genes. Proc Natl Acad Sci USA. 1997;94:1645–1650. doi: 10.1073/pnas.94.5.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chirmule N, Hughes J V, Gao G P, Raper S E, Wilson J M. Role of E4 in eliciting CD4 T-cell and B-cell responses to adenovirus vectors delivered to murine and nonhuman primate lungs. J Virol. 1998;72:6138–6145. doi: 10.1128/jvi.72.7.6138-6145.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clemens P R, Kochanek S, Sunada Y, Chan S, Chen H H, Campbell K P, Caskey C T. In vivo muscle gene transfer of full-length dystrophin with an adenoviral vector that lacks all viral genes. Gene Ther. 1996;3:965–972. [PubMed] [Google Scholar]
- 10.Dai Y, Schwarz E M, Gu D, Zhang W W, Sarvetnick N, Verma I M. Cellular and humoral immune responses to adenoviral vectors containing factor IX gene: tolerization of factor IX and vector antigens allows for long-term expression. Proc Natl Acad Sci USA. 1995;92:1401–1405. doi: 10.1073/pnas.92.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dedieu J F, Vigne E, Torrent C, Jullien C, Mahfouz I, Caillaud J M, Aubailly N, Orsini C, Guillaume J M, Opolon P, Delaere P, Perricaudet M, Yeh P. Long-term gene delivery into the livers of immunocompetent mice with E1/E4-defective adenoviruses. J Virol. 1997;71:4626–4637. doi: 10.1128/jvi.71.6.4626-4637.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D'Halluin J C. Virus assembly. Curr Top Microbiol Immunol. 1995;199(Pt. 1):47–66. [PubMed] [Google Scholar]
- 13.Everitt E, Sundquist B, Pettersson U, Philipson L. Structural proteins of adenoviruses. X. Isolation and topography of low molecular weight antigens from the virion of adenovirus type 2. Virology. 1973;52:130–147. doi: 10.1016/0042-6822(73)90404-2. [DOI] [PubMed] [Google Scholar]
- 14.Fisher K J, Choi H, Burda J, Chen S J, Wilson J M. Recombinant adenovirus deleted of all viral genes for gene therapy of cystic fibrosis. Virology. 1996;217:11–22. doi: 10.1006/viro.1996.0088. [DOI] [PubMed] [Google Scholar]
- 15.Gao G P, Yang Y, Wilson J M. Biology of adenovirus vectors with E1 and E4 deletions for liver-directed gene therapy. J Virol. 1996;70:8934–8943. doi: 10.1128/jvi.70.12.8934-8943.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaynor R B, Berk A J. Cis-acting induction of adenovirus transcription. Cell. 1983;33:683–693. doi: 10.1016/0092-8674(83)90011-9. [DOI] [PubMed] [Google Scholar]
- 17.Gorziglia M I, Kadan M J, Yei S, Lim J, Lee G M, Luthra R, Trapnell B C. Elimination of both E1 and E2 from adenovirus vectors further improves prospects for in vivo human gene therapy. J Virol. 1996;70:4173–4178. doi: 10.1128/jvi.70.6.4173-4178.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorziglia M I, Lapcevich C, Roy S, Kang Q, Kadan M, Wu V, Pechan P, Kaleko M. Generation of an adenovirus vector lacking E1, E2a, E3, and all of E4 except open reading frame 3. J Virol. 1999;73:6048–6055. doi: 10.1128/jvi.73.7.6048-6055.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grable M, Hearing P. Adenovirus type 5 packaging domain is composed of a repeated element that is functionally redundant. J Virol. 1990;64:2047–2056. doi: 10.1128/jvi.64.5.2047-2056.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grable M, Hearing P. cis and trans requirements for the selective packaging of adenovirus type 5 DNA. J Virol. 1992;66:723–731. doi: 10.1128/jvi.66.2.723-731.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graham F L, Prevec L. Manipulation of adenovirus vectors. Methods Mol Biol. 1991;7:109–128. doi: 10.1385/0-89603-178-0:109. [DOI] [PubMed] [Google Scholar]
- 22.Graham F L, Smiley J, Russell W C, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36:59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 23.Gustin K E, Imperiale M J. Encapsidation of viral DNA requires the adenovirus L1 52/55-kilodalton protein. J Virol. 1998;72:7860–7870. doi: 10.1128/jvi.72.10.7860-7870.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gustin K E, Lutz P, Imperiale M J. Interaction of the adenovirus L1 52/55-kilodalton protein with the IVa2 gene product during infection. J Virol. 1996;70:6463–6467. doi: 10.1128/jvi.70.9.6463-6467.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hammarskjold M L, Winberg G. Encapsidation of adenovirus 16 DNA is directed by a small DNA sequence at the left end of the genome. Cell. 1980;20:787–795. doi: 10.1016/0092-8674(80)90325-6. [DOI] [PubMed] [Google Scholar]
- 26.Hardy S, Kitamura M, Harris-Stansil T, Dai Y, Phipps M L. Construction of adenovirus vectors through Cre-lox recombination. J Virol. 1997;71:1842–1849. doi: 10.1128/jvi.71.3.1842-1849.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris K F, Christensen J B, Imperiale M J. BK virus large T antigen: interactions with the retinoblastoma family of tumor suppressor proteins and effects on cellular growth control. J Virol. 1996;70:2378–2386. doi: 10.1128/jvi.70.4.2378-2386.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris M P, Hay R T. DNA sequences required for the initiation of adenovirus type 4 DNA replication in vitro. J Mol Biol. 1988;201:57–67. doi: 10.1016/0022-2836(88)90438-x. [DOI] [PubMed] [Google Scholar]
- 29.Hasson T B, Ornelles D A, Shenk T. Adenovirus L1 52- and 55-kilodalton proteins are present within assembling virions and colocalize with nuclear structures distinct from replication centers. J Virol. 1992;66:6133–6142. doi: 10.1128/jvi.66.10.6133-6142.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hasson T B, Soloway P D, Ornelles D A, Doerfler W, Shenk T. Adenovirus L1 52- and 55-kilodalton proteins are required for assembly of virions. J Virol. 1989;63:3612–3621. doi: 10.1128/jvi.63.9.3612-3621.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hay R T, McDougall I M. Viable viruses with deletions in the left inverted terminal repeat define the adenovirus origin of DNA replication. J Gen Virol. 1986;67:321–332. doi: 10.1099/0022-1317-67-2-321. [DOI] [PubMed] [Google Scholar]
- 32.Hearing P, Shenk T. The adenovirus type 5 E1A transcriptional control region contains a duplicated enhancer element. Cell. 1983;33:695–703. doi: 10.1016/0092-8674(83)90012-0. [DOI] [PubMed] [Google Scholar]
- 33.Imperiale M J, Kao H T, Feldman L T, Nevins J R, Strickland S. Common control of the heat shock gene and early adenovirus genes: evidence for a cellular E1A-like activity. Mol Cell Biol. 1984;4:867–874. doi: 10.1128/mcb.4.5.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones N. Transcriptional modulation by the adenovirus E1A gene. Curr Top Microbiol Immunol. 1995;199:59–80. doi: 10.1007/978-3-642-79586-2_4. [DOI] [PubMed] [Google Scholar]
- 35.Kochanek S, Clemens P R, Mitani K, Chen H H, Chan S, Caskey C T. A new adenoviral vector: replacement of all viral coding sequences with 28 kb of DNA independently expressing both full-length dystrophin and beta-galactosidase. Proc Natl Acad Sci USA. 1996;93:5731–5736. doi: 10.1073/pnas.93.12.5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar-Singh R, Chamberlain J S. Encapsidated adenovirus minichromosomes allow delivery and expression of a 14 kb dystrophin cDNA to muscle cells. Hum Mol Genet. 1996;5:913–921. doi: 10.1093/hmg/5.7.913. [DOI] [PubMed] [Google Scholar]
- 37.Leibowitz J, Horwitz M S. Synthesis and assembly of adenovirus polypeptides. III. Reversible inhibition of hexon assembly in adenovirus type 5 temperature-sensitive mutants. Virology. 1975;66:10–24. doi: 10.1016/0042-6822(75)90175-0. [DOI] [PubMed] [Google Scholar]
- 38.Lusky M, Christ M, Rittner K, Dieterle A, Dreyer D, Mourot B, Schultz H, Stoeckel F, Pavirani A, Mehtali M. In vitro and in vivo biology of recombinant adenovirus vectors with E1, E1/E2A, or E1/E4 deleted. J Virol. 1998;72:2022–2032. doi: 10.1128/jvi.72.3.2022-2032.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lutz P, Kedinger C. Properties of the adenovirus IVa2 gene product, an effector of late-phase-dependent activation of the major late promoter. J Virol. 1996;70:1396–1405. doi: 10.1128/jvi.70.3.1396-1405.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maizel J V, Jr, White D O, Scharff M D. The polypeptides of adenovirus. II. Soluble proteins, cores, top components and the structure of the virion. Virology. 1968;36:126–136. doi: 10.1016/0042-6822(68)90122-0. [DOI] [PubMed] [Google Scholar]
- 41.Mondesert G, Tribouley C, Kedinger C. Identification of a novel downstream binding protein implicated in late-phase-specific activation of the adenovirus major late promoter. Nucleic Acids Res. 1992;20:3881–3889. doi: 10.1093/nar/20.15.3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Neal W K, Zhou H, Morral N, Langston C, Parks R J, Graham F L, Kochanek S, Beaudet A L. Toxicity associated with repeated administration of first-generation adenovirus vectors does not occur with a helper-dependent vector. Mol Med. 2000;6:179–195. [PMC free article] [PubMed] [Google Scholar]
- 43.Ostapchuk P, Hearing P. Pseudopackaging of adenovirus type 5 genomes into capsids containing the hexon proteins of adenovirus serotypes B, D, or E. J Virol. 2001;75:45–51. doi: 10.1128/JVI.75.1.45-51.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parks R J, Chen L, Anton M, Sankar U, Rudnicki M A, Graham F L. A helper-dependent adenovirus vector system: removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc Natl Acad Sci USA. 1996;93:13565–13570. doi: 10.1073/pnas.93.24.13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmid S I, Hearing P. Bipartite structure and functional independence of adenovirus type 5 packaging elements. J Virol. 1997;71:3375–3384. doi: 10.1128/jvi.71.5.3375-3384.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmid S I, Hearing P. Cellular components interact with adenovirus type 5 minimal DNA packaging domains. J Virol. 1998;72:6339–6347. doi: 10.1128/jvi.72.8.6339-6347.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stillman B W, Topp W C, Engler J A. Conserved sequences at the origin of adenovirus DNA replication. J Virol. 1982;44:530–537. doi: 10.1128/jvi.44.2.530-537.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stillman B W. Adenovirus DNA replication in vitro: a protein linked to the 5′ end of nascent DNA strands. J Virol. 1981;37:139–147. doi: 10.1128/jvi.37.1.139-147.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tamanoi F, Stillman B W. Initiation of adenovirus DNA replication in vitro requires a specific DNA sequence. Proc Natl Acad Sci USA. 1983;80:6446–6450. doi: 10.1073/pnas.80.21.6446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomas C E, Schiedner G, Kochanek S, Castro M G, Löwenstein P R. Peripheral infection with adenovirus causes unexpected long-term brain inflammation in animals injected intracranially with first-generation, but not with high-capacity, adenovirus vectors: toward realistic long-term neurological gene therapy for chronic diseases. Proc Natl Acad Sci USA. 2000;97:7482–7487. doi: 10.1073/pnas.120474397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thomas C E, Schiedner G, Kochanek S, Castro M G, Löwenstein P R. Preexisting antiadenoviral immunity is not a barrier to efficient and stable transduction of the brain, mediated by novel high-capacity adenovirus vectors. Hum Gene Ther. 2001;12:839–846. doi: 10.1089/104303401750148829. [DOI] [PubMed] [Google Scholar]
- 52.Tibbetts C. Viral DNA sequences from incomplete particles of human adenovirus type 7. Cell. 1977;12:243–249. doi: 10.1016/0092-8674(77)90202-1. [DOI] [PubMed] [Google Scholar]
- 53.Tribouley C, Lutz P, Staub A, Kedinger C. The product of the adenovirus intermediate gene IVa2 is a transcriptional activator of the major late promoter. J Virol. 1994;68:4450–4457. doi: 10.1128/jvi.68.7.4450-4457.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Q, Greenburg G, Bunch D, Farson D, Finer M H. Persistent transgene expression in mouse liver following in vivo gene transfer with a delta E1/delta E4 adenovirus vector. Gene Ther. 1997;4:393–400. doi: 10.1038/sj.gt.3300404. [DOI] [PubMed] [Google Scholar]
- 55.Yang Y, Ertl H C, Wilson J M. MHC class I-restricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenoviruses. Immunity. 1994;1:433–442. doi: 10.1016/1074-7613(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 56.Yang Y, Li Q, Ertl H C, Wilson J M. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J Virol. 1995;69:2004–2015. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang W, Imperiale M J. Interaction of the adenovirus IVa2 protein with viral packaging sequences. J Virol. 2000;74:2687–2693. doi: 10.1128/jvi.74.6.2687-2693.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou H, O'Neal W, Morral N, Beaudet A L. Development of a complementing cell line and a system for construction of adenovirus vectors with E1 and E2a deleted. J Virol. 1996;70:7030–7038. doi: 10.1128/jvi.70.10.7030-7038.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zou L, Yuan X, Zhou H, Lu H, Yang K. Helper-dependent adenoviral vector-mediated gene transfer in aged rat brain. Hum Gene Ther. 2001;12:181–191. doi: 10.1089/104303401750061249. [DOI] [PubMed] [Google Scholar]
- 60.Zou L, Zhou H, Pastore L, Yang K. Prolonged transgene expression mediated by a helper-dependent adenoviral vector (hdAd) in the central nervous system. Mol Ther. 2000;2:105–113. doi: 10.1006/mthe.2000.0104. [DOI] [PubMed] [Google Scholar]