Abstract
The mechanisms underlying myopia pathogenesis are not well understood. Using publicly-available human and animal datasets, we expound on the roles of known, implicated proteins, and new myopia-related signaling pathways were hypothesized. Proteins identified from human serum or ocular fluids, and from ocular tissues in myopic animal models, were uploaded and analyzed with the QIAGEN Ingenuity Pathway Analysis (IPA) software (March 2023). With each IPA database update, more potentially-relevant proteins and signaling pathways previously unavailable during data acquisition are added, allowing extraction of novel conclusions from existing data. Canonical pathway analysis was used to analyze these data and calculate an IPA activation z-score—which indicates not only whether an association is significant, but also whether the pathway is likely activated or inhibited. Cellular immune response and cytokine signaling were frequently found to be affected in both human and animal myopia studies. Analysis of two publicly-available proteomic datasets highlighted a potential role of the innate immune system and inflammation in myopia development, detailing specific signaling pathways involved such as Granzyme A (GzmA) and S100 family signaling in the retina, and activation of myofibroblast trans-differentiation in the sclera. This perspective in myopia research may facilitate development of more effective and targeted therapeutic agents.
Keywords: Myopia, Proteomics, Signaling pathways, Inflammation, Innate immunity
Subject terms: Refractive errors, Proteomics
Introduction
Human myopia is commonly defined as having a spherical equivalent (SE) refraction of ≤ − 0.50 diopter (D)1, and SE ≤ − 5.00 D is designated as high myopia (HM)2 with axial length (AL) typically > 26 mm3. The global prevalence of myopia has increased remarkably in recent decades, especially in East Asian countries such as China, Japan, and Singapore, where its prevalence can reach up to 80–90% in young people4–7. Individuals with high myopia are at significantly increased risk of developing a range of secondary pathologies8, such as cataracts9, glaucoma10, retinal detachment11, and myopic macular degeneration (MMD)12. Moreover, high myopia can progress to pathologic myopia (PM)—often accompanied by MMD and/or myopic traction maculopathy (MTM)—which is a major cause of low vision and permanent vision loss13. It is crucial to improve the understanding of myopia pathogenesis in order to develop novel treatments to limit the excessive ocular growth that is characteristic of myopia. Translated functional proteins are likely to be the best indicators of cellular processes because post-translational modifications can produce proteins that differ functionally despite their being encoded by the same gene. Therefore, proteomics is uniquely suited for identifying specific proteins that drive emmetropization and myopization, and thus to reveal proteins and functions that have high potential for testing as targets for new pharmaceuticals to treat myopia14.
Proteomic analysis is an approach that allows the identification and quantification of thousands of proteins, in both healthy and diseased conditions15. Additionally, it can elucidate functional aspects such as the relationships between proteins, resolving their function, and suggesting specific protein–protein interactions in functional networks16. Therefore, proteomic analysis is a powerful tool that provides a comprehensive assessment of molecular activities and has proven useful in human clinical research on a range of diseases, including studies of eye diseases17. For example, a proteomic study of human vitreous from non-diseased human donor eyes established the first human vitreous proteome18. This novel study provided insights into understanding the underlying mechanism of pathologic myopia, and evidence suggesting that the retina is a source of inflammatory mediators. This is particularly important since clinically, patients with pathologic myopia are typically identified only in the later stages of the myopia pathology spectrum; therefore, only late-stage tissue samples are available for proteomic analysis.
Novel approaches toward prevention of myopia development and/or progression require a better understanding of fundamental changes that occur in the initial stages of myopia formation—in order to identify causal factors, elucidate novel therapeutic targets, and allow for early intervention. Therefore, attempts to unravel the causal mechanisms of myopia development have led to the extension of myopia proteomic studies beyond late-stage human studies, into well-controlled animal models of experimental myopia, which allow for study at times from the initiation, through early-stage development, and ending in late-stage myopia. Animal studies have contributed greatly to our knowledge of myopia development, as they have identified myopia-associated proteins in other ocular signaling pathways and systemic functions19,20, suggesting that previously overlooked biological processes might also be involved in the onset of myopia. Many differentially abundant proteins (DAPs) are shared widely amongst species and across different myopia-inducing methods; for example, the discovery of DAPs common to both chick and guinea pig retinas indicates cross-species conservation of signaling pathways associated with these DAPs21,22.
To the authors’ knowledge, no existing studies have used updated bioinformatics software to reanalyze DAPs, for identifying previously unrecognized key signaling pathways in myopia development. The purpose of the present study is to better elucidate the roles in myopia pathogenesis of proteins identified in both human myopia and animal models, and to discover new myopiagenic signaling pathways (biological processes), through proteomics analysis with the QIAGEN Ingenuity Pathway Analysis (IPA) software. Leveraging on the wealth of existing and published data could enhance our understanding of the physiological roles of detected proteins. In previous publications, typically only the false discovery rate (FDR) and p-value of the signaling pathways have been reported. Thus, readers could only determine that there is an association between particular signaling pathways and myopia—without any indication as to whether the signaling pathway is activated or inhibited, and even less whether it is causal, in myopiagenesis.
Our approach was to reanalyze DAPs by merging high-quality, publicly-available datasets; this allows for the identification of associated signaling pathways that were previously unknown. QIAGEN Ingenuity Pathway Analysis software makes possible analyses of DAPs, by comparing them to the QIAGEN Biomedical Knowledge Base. With every database update, more proteins and signaling pathways that have been found to be associated with myopia are added; therefore, new analyses of existing data have the potential to reveal previously unrecognized mechanisms underlying the development of myopia. Here, publicly-available datasets were also analyzed in greater detail, which allowed for the calculation of an IPA activation z-score for the first time and use it for identifying the activity changes of the statistically significant pathways (p < 0.05) before and after atropine application or removing minus lens induction. This metric indicates not only an association with, but also whether the pathway is activated or inhibited by myopiagenesis. Thus, the results of the present study enhance our understanding of the physiological roles of the detected proteins and the molecular pathways in which they might be involved, as well as their association with myopia progression, potentially leading to clinical applications by targeting these pathways pharmacologically.
Results
Analysis of data from human myopia studies identified inflammation-related proteins
As summarized in Table 1, data from two existing human studies served to establish associations between serum and eye fluid protein profiles, while others focused mainly on the vitreous humor (VH) or aqueous humor (AH).
Table 1.
Core information (columns A–E) from 7 selected human studies on myopia, including the proteomics techniques used for identifying differentially abundant proteins (DAPs) from ocular tissue(s) and the signaling pathways identified, as well as significant signaling pathways affected in myopia (column F) confirmed or newly-predicted through the use of IPA analysis in the present study.
| (A) Tissue | (B) Subjects’ information | (C) Proteomics technique | (D) Identified proteins | (E) Pathways reported as in publications | (F)a Top speculated signaling pathways from IPA |
|---|---|---|---|---|---|
| (1) Serum and VH |
Serum 1: n = 16 HM and n = 4 controls23 Serum 2: n = 116 HM and n = 86 controls23 Vitreous: n = 40 PM and n = 8 controls23 Serum 3: n = 30 PM and n = 30 control24 Note: control cases are emmetropic |
Serum 1&3: analysed by LTQ-MASS23,24, serum 2: tested by WB and ELISA23, Vitreous: analysed by ELISA23 |
Serum: HPX, APO B-100, TTR, HP/HPr23,24 VH: TTR23 |
Associated with ocular pathologies, not myopia Serum TTR could be a biomarker for high myopia in patients with ocular pathologies |
Nuclear receptor signaling pathways (LXR/RXR, FXR/RXR, TR/RXR) |
| (2) VH | n = 66 PM (AL > 29.0 mm) and n = 66 non-PM (AL: 26.5–29.0 mm) patients before surgery25 | LC–MS, ELISA | 13 proteins were identified and their levels were lower in PM patients | No signaling pathways were identified | Cellular immune responses, humoral immune responses |
| (3) AH | n = 5 high myopic and n = 5 non-myopic patients before cataract surgery26 | Bradford method and 2D-GE | Total protein levels in myopic AH were significantly greater than those in controls | DBP and TTR were confirmed; others were albumins (either phosphorylated or hydrolysed) | Cellular immune response and nuclear receptor signaling (FXR/RXR, LXR/RXR) |
| (4) AH | n = 9 myopic and n = 9 control patients before cataract surgery27 | LC–MS/MS analysis | HPX, TTR, DKK3, APOH, APOA-II, APOA-IV, HRG, CST3 | Identified exosome profiles in high-myopic eyes | Nuclear receptor (LXR/RXR) signaling pathways |
| (5) AH | n = 20 PM and n = 20 controls before cataract surgery28 | LC–MS/MS analysis, ELISA |
63 proteins increased and 38 decreased Apolipoprotein A-I (APOA-I) was enriched as the hub protein of the network with the highest score, degree and centrality |
DAPs were closely associated with immunity and inflammation interactions, and remodeling of extracellular matrix | PPAR signaling cascade |
| (6) AH | HM patients with cataract vs cataract only, n = 7 for each group30 | iTRAQ, LC–MS/MS |
32 proteins increased and 26 decreased Validated protein level of plasminogen |
Coagulation and complement cascades | Cytokine signaling, nuclear receptor signaling, cellular immune response, and cellular stress and injury pathways |
aTop canonical pathways were listed in the column.
The results of two proteomics studies that included samples of human serum and VH are shown in Table 1, row 1. Analysis of these studies indicated that of the 4 identified proteins found in the serum—hemopexin (HPX), apolipoprotein B100 (APO B-100), transthyretin (TTR), and haptoglobin (HP)—only TTR was found concurrently in VH and serum, and all were involved in retinoid X receptor (RXR) signaling pathways (known to be involved in the immune response)23,24. In another study that focused on the VH (Table 1, row 2), analysis of PM samples identified 13 proteins, all of which were found less in amount in emmetropic control eyes25. IPA analysis indicated that most of these proteins are known to be involved in cellular and humoral immune responses.
Other reviewed proteomic studies focused mainly on AH (Table 1, rows 3–6). An early study of the AH of myopic eyes (compared to emmetropic controls) found significantly higher levels of vitamin D-binding protein (DBP), TTR, and albumins (Table 1, row 3)26. IPA analysis indicated that these three proteins are known mainly to be involved in the cellular immune response and nuclear receptor signaling. Recently, myopia-specific exosomal proteins were identified in the AH by comparing the profile of cell-secreted microvesicles obtained from patients undergoing cataract surgery, with or without pre-existing myopia (Table 1, row 4)27. Amounts of a total of 25 proteins were significantly different in these two groups; but functional pathways could be associated with only 8 of them: HPX, beta-2-glycoprotein 1 (APOH), apolipoprotein A-IV (APOA-IV), histidine-rich glycoprotein (HRG), TTR, apolipoprotein A-II (APOA-II), dickkopf-related protein 3 (DDK3), and cystatin-C (CST3). Analysis of these proteins showed that these 8 proteins are involved in nuclear receptor signaling pathways. Xue et al. compared proteomic profiles of AH from pathologic myopia compared to non-PM cataract patients; they identified 38 proteins lower in amount and 63 proteins greater in amount in PM than in non-PM controls—among which the levels of APOA-I appeared to be significantly greater in PM patients (Table 1, row 5)28. On the basis of these DAPs and bioinformatics analysis, the authors suggested that PM is closely associated with immune responses and inflammatory interactions. Consistent with this, IPA analysis indicated that PM is associated with the PPAR signaling cascade, which is known to play a key role in the modulation of immune and inflammatory reactions29. Wen et al. used iTRAQ and identified DAPs from HM eyes (compared to non-HM eyes) (Table 1, row 6); these DAPs were highly enriched in the coagulation and complement signaling cascades. Among the identified DAPs, plasminogen (PLG) protein was validated and suggested to be a candidate biomarker for HM30. Following extraction of data belonging to the 58 DAPs identified from the studies, IPA analysis identified cytokine signaling, nuclear receptor signaling, cellular immune response, and cellular stress and injury pathways as the signaling pathways with which the identified DAPs are involved.
Analysis of data from animal studies identified proteins related to oxidation, calcium transport and cellular immunity
Selected animal proteomic studies are listed in Table 2, which includes data from five studies on the retina, one study on the retina-RPE-choroid combination, and one study on the sclera. The data on proteins identified in the treated eyes versus the internal-control eyes in those publications were also uploaded to IPA for analysis. The most significant signaling pathways identified are listed in Table 2, column E. In the retinal proteome, more energy-producing oxidation reactions were found to be affected in myopic than in non-myopic eyes. Other affected signaling pathways include cellular immune responses (after a short period of myopia induction) and nuclear receptor pathways (after prolonged form deprivation). The key proteins in the retina/RPE/choroid complex of the tilapia myopia model31 are involved in calcium transport and osteoclast-related signaling pathways (Table 2, row 6, column E). Osteoclasts are known to be multifunctional; in addition to degrading and resorbing mineralized bone matrix, they also play a role in remodeling of cartilage, which is an important component of teleostean and avian sclerae. They are also involved in phagocytosis of apoptotic cells and immune modulation32,33, and this role is consistent with data from studies on the role of choroidal macrophages (which also play a key phagocytic function in innate immunity and tissue homeostasis34) in the development of myopia in mice35. In the sclera, during myopia induction, inflammation-related responses are consistently activated, and cholesterol biosynthesis is noted to be altered, but the direction of change (i.e. whether there is an activation or inhibition) remains unclear due to a paucity of relevant publications on cholesterol biosynthesis, which hinders the ability to perform proper bioinformatic analysis.
Table 2.
Details of included animal studies with induced myopia, such as animal model used (column A), proteomics technique (column B), identified DAPs (column C), and signaling pathways identified in the publication (column D). DAPs underwent IPA analysis to confirm existing and/or predict new significant signaling pathways affected in myopia (column E), with a p-value of < 0.05 for all pathways.
| (A) Animal model | (B) Proteomics technique | (C) Identified DAPs | (D) Signaling Pathways identified in publication | (E)b The top categories of the most significant pathways identified in myopic eyes after IPA analysis |
|---|---|---|---|---|
| (1) Retina, Pigmented guinea pig, Started treatment from 4 days of age, 4D lenses on one eye for 8 days71 | Fluorescence 2D-GE | 8 proteins were identified by MS: β-actin, enolase 1, cytosolic malate dehydrogenase, protein-l-isoaspartate (D-aspartate) O-methyltransferase, PKM2 protein, X-linked eukaryotic translation initiation factor 1A, Ras-related protein Rab-11B, ACP1 protein | No signaling pathways were speculated. (Specific biological functions of identified, differentially expressed protein spots are described one by one.) |
Biosynthesis: more energy-producing oxidation reactions Cellular immune response: more signaling pathways involved, including crosstalk between immune cells Neurotransmitter signaling: actin-based motility Intercellular and second messenger signaling: EIF2 signaling, RHOA signaling Growth factor signaling: FDGF and VEGF signaling |
| (2) Retina, Chick, 10D lenses on right eyes and + 10D lenses on left eyes for 3 and 7 days, respectively72 | nano LC–ESI–MS/MS | PRDX6, GSTM2, APOA-I, VIM, IRBP, PKM, GAPDH, ENO1, LDHA | No signaling pathways were speculated. Specific function, and involved biological process of identified, differentially expressed proteins, are described one by one |
Biosynthesis: Heparan Sulfate, Dermatan Sulfate, Chondroitin Sulfate Intercellular and second messenger signaling: lipid metabolites involved in immune regulation, such as Eicosanoid Signaling, Apelin Adipocyte Signaling Pathway Cellular stress and injury: hypoxia and HIF1α signaling pathway |
| (3) Retina, Pigmented guinea pig, Started treatment at 4 days of age, 5D lenses on one eye for 4 days36 | Discovery: SWATH-MS; Protein validation: Multiple Reaction Monitoring assay; IPA |
3202 nonredundant proteins were identified at 1% FDR Levels of 58 proteins were significantly changed after LIM treatment SLC6A6 and PTGES2 were validated |
Using IPA, “lipid metabolism” was found as the main function associated with the differentially expressed proteins |
Biosynthesis: inflammation relevant fatty acid metabolism Cellular immune response: PTGES2 involved pathway Neurotransmitters signaling: actin-based motility Intercellular and second messenger signaling: calcium signaling |
| (4) Retina, Albino guinea pig, Started treatment at 5 days of age, FDM for 4 weeks22 | 2D-GE; validation: MALDI-TOF | β-soluble NSF attachment protein; Phosphoglycerate mutase 1; VIM; Septin-6; Fascin 1; Collapsin response mediator protein-2A (Dihydropyrimidinase- Related Protein 2 DRP2); Succinate dehydrogenase complex subunit A; Preproalbumin | No signaling pathways were speculated. Reported proteins’ functions one by one, according to GeneOntology; |
Biosynthesis: carbohydrate metabolism and biosynthesis of its regulator Cellular immune response: more signaling pathways involved, including nitric oxide pathway Nuclear receptor signaling: LXR/RXR and FXR/RXR activation Apoptosis: 14-3-3-mediated signaling and tight junction signaling Organismal growth and development: through post-transcriptional changes |
| (5) Retina, Pigmented guinea pig, Started treatment at 21 days of age, FDM for 4 weeks73 |
First stage: fractionated iTRAQ, coupled with nano-LC–MS/MS Second stage: label-free SWATH-MS Alpha-synuclein was verified using immunohisto-chemistry and confocal imaging |
29 confident proteins at 1% FDR, comprising 12 up-regulated and 17 down-regulated proteins The most significantly regulated proteins were closely connected to Alpha-synuclein |
Bioinformatics analysis using IPA and STRING databases identified signaling: EIF2 signaling; glycolysis; and dopamine secretion |
Biosynthesis: phosphatidylglycerol biosynthesis II, CDP-diacylglycerol biosynthesis I, D-myo-inositol (1,4,5)-trisphosphate biosynthesis Cellular stress and injury: nucleotide excision repair pathway Cellular growth, proliferation and development of transcriptional and translational regulation: assembly of RNA polymerase II complex, mTOR signaling, regulation of elF4 and p70S6K signaling, sumoylation pathway, EIF2 signaling |
| (6) Combined retina, RPE and choroid, Tilapia, Started treatment at 3 months of age, FDM for 4 weeks31 |
2D-GE and MS A total of 18 protein spots separated |
Annexin A5, gelsolin, and chaperonin-containing TCP-1 (CCT) are downregulated | No signaling pathways were speculated |
Transport: calcium transport I Cellular growth, proliferation and development: bone remodeling and development and activation of osteoclasts (possibly in the modulation of immune responses)34 Neurotransmitters and other nervous system signaling: Actin-based Motility Intercellular and second messenger signaling: integrin signaling |
| (7) Sclera, Pigmented guinea pigs, Started treatment at 2 weeks of age, FDM for 4 weeks74 |
iTRAQ labeling combined with LC − MS/MS GO and IPA were used to identify the proteins’ functions and networks involved |
A total of 2,579 unique proteins with < 1% FDR were identified Levels of 56 proteins were increased, and those of 84 proteins were decreased, in FDM eyes Validated proteins: RhoA, myosin IIB, ACTIN 3, RAP1A |
44 of 140 proteins differing in amount were involved in cellular movement and cellular assembly and organization Relevant pathways (GO BP): cellular process (GO: 0009987), single-organism process (GO: 0044699), metabolic process (GO: 0008152) |
64 significantly different signaling pathways were identified and relevant to: Cellular immune response: activated Cellular stress and injury: activated Cytokine signaling: activated Biosynthesis: mainly cholesterol biosynthesis, the differences between myopic and control eyes are significant but the direction of change (activation vs. inhibition) was not reliably predictable by IPA analysis |
bThe top categories of the most significant pathways identified in myopic eyes after IPA analysis are listed in column E.
In general, for a given tissue, specific signaling pathways were frequently identified in multiple studies (Table 2, Column E). For example, in the first 6 studies summarized in the Table, similar pathways were identified in the retina. Moreover, the number of affected signaling pathways under each category (Table 2, column E) tended to increase with prolonged myopia induction, with more pathways upregulated than downregulated; for example, the cellular immune response pathway in the fourth entry (row 4; form-deprivation myopia (FDM) for 4 weeks)22 has more pathways involved (and specifically upregulated) than the third entry (row 3; minus-lens-induced myopia (LIM) for 4 days)36.
Analysis of data from atropine-induced inhibition of lens-induced myopia in mice identified five closely interlinked signaling pathways, including the inflammation-related S100 family
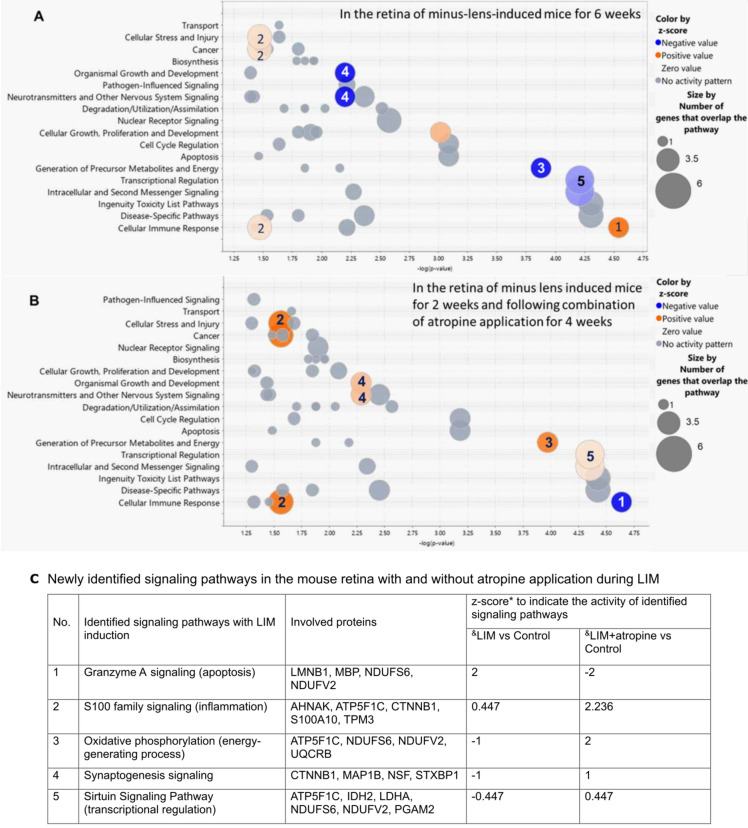
Barathi et al. performed a comprehensive proteomics analysis using a mouse model with minus-lens-induced myopia (LIM), and using MetaCore from GeneGo for assessing biological processes and relevant biomarkers37. This study compared data for LIM or LIM plus atropine treatment with data for normal controls, to elucidate the effect of atropine eye drops on myopia development. Proteomics data published in this study—from the retinas of LIM-induced mouse eyes, with or without the application of atropine eye drops, and from normal control eyes after equal treatment duration—were extracted and the analysis with the QIAGEN IPA software repeated with an updated database. Several previously unrecognized, significantly-affected retinal signaling pathways involved in atropine-induced inhibition of LIM were identified (Fig. 1). A z-score was included to enhance the stringency of analysis, which indicated whether the affected the signaling pathways were activated or inhibited by topical atropine-induced inhibition of myopiagenesis. By comparing the bubble charts for data from LIM and normal control mouse eyes (Fig. 1A), atropine eye drops were found to reverse the activity of five of the signaling pathways that were significantly affected during myopia induction (Fig. 1B). The five signaling pathways identified are: granzyme A (GzmA), S100 family, oxidative phosphorylation, synaptogenesis, and sirtuin; these will be further discussed in the following section.
Figure 1.
Retinal signaling pathways in mice significantly affected by (A) imposed negative defocus (LIM vs control), and (B) the same, combined with application of atropine eye drops for 4 weeks after minus-lens induction for 2 weeks (atropine-treated LIM vs LIM), with (C) the respective z-scores of each newly identified signaling pathway in both groups. The x-axis represents statistical significance, and the y-axis represents pathway categories. Each bubble represents a signaling pathway, and the numbers inside the bubbles indicate the same signaling pathways in A and B. The colors of the bubbles indicate their relative effects on the signaling pathways that were significantly affected by the experimental treatment—from low activation (pink) to high activation (orange); from low inhibition (light blue) to high inhibition (dark blue); and no net activity (gray).
IPA analysis revealed that net activity (protein content) in the five newly-identified myopia-associated signaling pathways was altered by the topical application of atropine eye drops in the mouse LIM model (Fig. 1C). GzmA signaling was activated during LIM and inhibited with atropine application. The S100 family signaling pathway was activated in LIM eyes, both with and without atropine, but was significantly more activated with atropine. The oxidative phosphorylation (OXPHOS), synaptogenesis, and sirtuin pathways were inhibited during LIM and activated with atropine application.
Analysis of tree shew data during recovery from minus-lens induced myopia identifies signaling pathways that are altered in this process
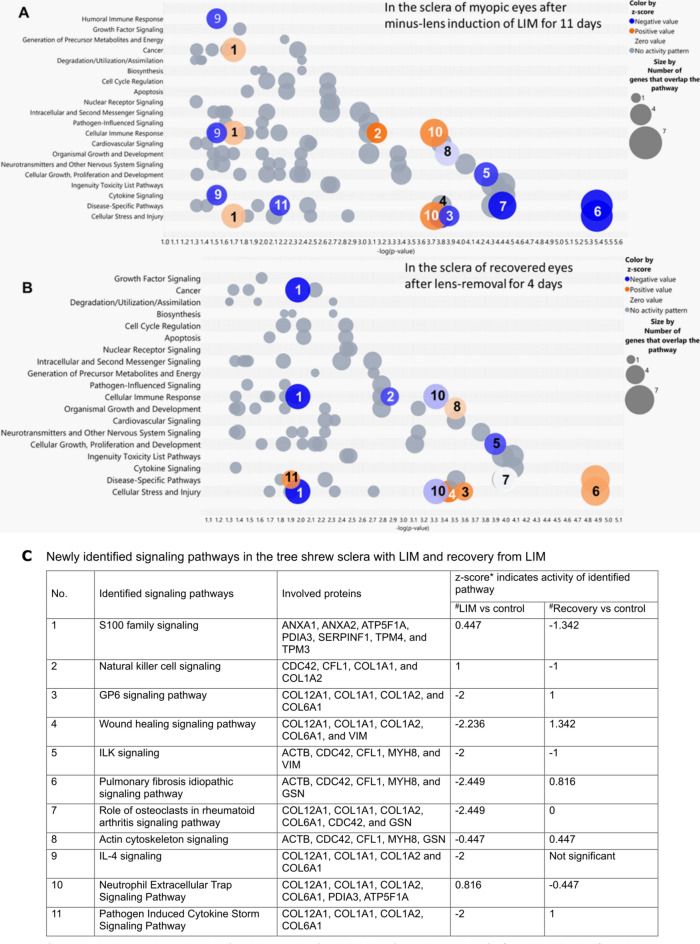
Frost and Norton comprehensively investigated the scleral proteins in tree shrews by mass spectrometry after LIM induction and recovery38. Biological functions or processes of differentially expressed proteins were summarized in their original publication, using UniProt (www.uniprot.org) and Panther. IPA was used to reanalyze the fold change of proteins that were previously identified to be differentially expressed in treated and contralateral eyes (found in Tables 1 and 2 of the original publication)38, to gain a better understanding of the signaling pathways involved. Interestingly, 11 signaling pathways among those affected by minus-lens treatment in tree shrew (Fig. 2A) were reversed after 4 days of recovery without lenses (Fig. 2B), as listed in Fig. 2C. Eleven signaling pathways were found to have opposite changes in activity during LIM and recovery. The S100 family and the natural killer (NK) cell and neutrophil extracellular trap signaling pathways in the sclera were activated during myopia induction, but significantly less during recovery. Multiple signaling pathways were less active in myopic eyes than in recovering eyes; these included GP6, wound healing, pulmonary fibrosis idiopathic, actin cytoskeleton, and pathogen-induced cytokine storm signaling pathways—among which GP6, wound healing, IL-4 and pathogen-induced cytokine storm signaling pathways share many of the same proteins. The “role of osteoclasts in rheumatoid arthritis” (RA) and interleukin-4 (IL-4) signaling pathways were significantly inhibited in myopic eyes and returned to normal in recovering eyes. Integrin-linked kinase (ILK) signaling was inhibited in myopic eyes, and this inhibition was reduced in recovering eyes.
Figure 2.
Signaling pathways significantly affected in the sclera of tree shrew: (A) after 11 days of imposed minus-defocus (LIM vs contralateral eyes), and (B) after 4 days of recovery from minus-lens treatment (treated eyes vs contralateral eyes), with (C) the respective z-score of each newly identified signaling pathway of both groups. The x-axis represents statistical significance, and the y-axis represents pathway categories. Each bubble represents a signaling pathway, and the numbers inside the bubbles indicate the same signaling pathways in (A) and (B). The colors of the bubbles indicate their relative effects on the signaling pathways that were significantly affected by the experimental treatment—from low activation (pink) to high activation (orange); from low inhibition (light blue) to high inhibition (dark blue); and no net activity (gray).
Discussion
By means of novel analyses of merged, publicly-available databases, cellular immune response and cytokine signaling pathways were found to be commonly affected in both human myopia and experimental animal myopia. Data from human studies indicated that nuclear receptor signaling pathways were significantly affected23,26–28, and that immune-system activity was significantly higher than normal25,26,30 in HM and PM eyes. In particular, the intraocular classical pathway and alternative pathway of the complement system were partially activated39; these increases could be, at least in part, the result of structural damage to the excessively enlarged eye. Data from animal studies demonstrated that a wider range of biological processes, including those related to inflammation such as the LXR/RXR (function as a critical signaling node linking inflammation and immune cell function)40 and S100 families (known mediators of inflammation)41, were increased in myopia, and that atropine37 and normal vision42 could reverse these increases in the affected signaling pathways (Figs. 1C, 2C).
While humans are the most relevant model for studying human diseases, they are not sufficient for fully understanding myopia. Early stages of myopia, crucial for identifying causal factors and therapeutic targets, cannot be extensively studied in humans since pathologic changes occur in less accessible, posterior ocular tissues (e.g. sclera, choroid and retina) and surgical human tissue samples tend to be available predominately from eye with more advanced, late-staged disease that warrant intervention. Thus, proteomic studies have also focused on animal models.
Preventing myopia requires understanding the early changes in its formation, identifying causal factors, and finding therapeutic targets for early intervention. This has led researchers to use animal models, which allow detailed study from the initiation to late stages of myopia. Animal studies have greatly enhanced our knowledge by identifying novel myopia-associated proteins and signaling pathways across the retina, choroid, and sclera. These processes, previously overlooked, may also be involved in human myopia onset. Leveraging on the wealth of knowledge from both human and animal models allows for further understanding of myopia development, that may ultimately lead to better clinical outcomes and more effective myopia control.
As reported above, atropine treatment causes further activation of the S100 family signaling pathway in the retina (Fig. 1C). The S100 family of proteins has a dual role in inflammation. They amplify inflammatory signals but can also resolve inflammation and promote tissue repair41. Since chronic inflammation worsens myopia43, upregulating S100 signaling may mitigate inflammation, promote tissue repair, and help control myopia development.
The induction of high myopia leads to retinal thinning and degeneration44,45, which is consistent with the activation of GzmA signaling, given that GzmA activates cellular caspase-independent apoptosis46 and modulates inflammation47. Reduction of retinal GzmA levels (indicative of reduced inflammation) was found after atropine application48, possibly attributable to the fact that atropine reduced TNF-α levels (as was reported in a mouse inflammation model)49.
Similarly, an increased activity in S100 signaling during LIM and atropine application might reflect increases in apoptosis, inflammation and energy metabolism50. The inhibition of oxidative phosphorylation (OXPHOS) during LIM, and the activation of OXPHOS after atropine application, suggest that atropine might have a role in the recovery of normal retinal functions and the inhibition of myopia progression by increasing energy production in choroidal capillaries, which increases energy supply to the RPE and photoreceptors51. This might be possible if atropine could enhance glucose production and utilization in rat blood via systemic administration52. Also, as the application of 1% atropine eyedrops increased choroidal thickness in human eyes53, it is reasonable to suppose that the effect of atropine could also reach the choroid through the thinner sclera of the globally smaller mouse eye. The finding that synaptogenesis signaling is involved in myopia supports the main finding of Barathi et al.37 that GABAergic signaling—a component of the synaptogenesis signaling pathway—is associated with the anti-myopic effects of atropine in mouse eyes; these authors found that the retinal content of GABA transporter 1 (GAT-1) was decreased in atropine-treated eyes. Additionally, IPA analysis implicated sirtuin signaling in myopization; this is consistent with the findings of Karouta et al.54, who reported that regulation of gene transcription is involved in myopia development (since sirtuins are major regulators of transcription via histones and regulatory proteins55 and also play a role in modulating inflammation)56.
In the sclera, with the exception of actin cytoskeleton signaling, all signaling pathways that were newly identified in the IPA analysis are known to be involved in inflammation and its associated processes. In four of these signaling pathways (GP6, wound healing, IL-4, and pathogen-induced cytokine storm), the majority of the proteins involved were collagen-related: COL12A1, COL1A1, COL1A2 and COL6A1. These proteins are known to be involved in multiple, potentially myopia-related functions—such as immune response signaling, inflammatory cell death, and myofibroblast transdifferentiation. Specifically, these functions would be essential for connective-tissue remodelling during normal and pathological wound healing57—and are known to have altered expression and be associated with myopia development in guinea pig retina58 and mouse sclera59. The fact that these particular proteins are associated with both the immune response/inflammation and myopia development, this is consistent with the notion that inflammation could play a role in myopia pathogenesis. In three signaling pathways, ILK, Pulmonary Fibrosis idiopathic and actin cytoskeleton signaling, four common proteins were implicated: ACTB, CDC42, CFL1, and MYH8. These cytoskeletal proteins are known to play a role in cell cycle regulation, motility and structure, which are key processes underlying myofibroblast transdifferentiation that occurs during remodeling of scleral ECM during myopic axial elongation59. This further indicates that the inflammation-related proteins are involved in myopia development, although it remains uncertain whether they drive myopia or are part of the response to it.
In summary, inflammation and immune responses in both retina and sclera have been identified as associated with myopia. The effects of atropine on the mouse retina included upregulation of pathways related to inflammation, oxidative phosphorylation, synaptogenesis, and transcriptional regulation, as well as downregulation in pathways related to apoptosis. The pathways involved in the tree shrew sclera are commonly found associated with inflammation-related pathways (activated in response to infection or mechanical damage, for example). Therefore, it is possible that their activation in myopia is due to the significant changes of pathways relevant to myofibroblast transdifferentiation, which generally comprises rapid responses in injured tissues and part of the wound healing process60.
The notion that inflammation is related to myopia was suggested more than a century ago61. Human studies have shown modulation of cellular immune responses and nuclear receptor signaling pathways in ocular fluids in HM and PM, which might be in reaction to the structural disruption experienced by the eye due to excessive stretching of the fundal tissues. This consistent with accumulated evidence suggesting that nuclear receptors are actively involved in immune responses in damaged tissue, in the eye as in other organs62. In animal model studies, the long-term induction of myopia resulted in alterations of signaling pathways similar to those identified in human studies—mainly on those related to immune responses. In contrast, the short-term induction of myopia affected mainly inflammation-related signaling (via inflammation-related proteins). This is consistent with reports that high incidence of myopia progression is observed in patients with chronic inflammatory conditions43, and other conditions such as juvenile chronic arthritis in humans63 and osteoarthritis in guinea pigs64. Given that chondrogenesis is common in the sclera of non-mammalian vertebrates (especially birds and fish) and the human sclera has the chondrogenic potential65 the sclera and joint cartilage might be common targets of inflammation in rheumatoid arthritis. These prior reports are consistent with the findings that many of the scleral pathways altered in myopia, are related to inflammation.
The correlation between myopia and intraocular inflammation has been reviewed comprehensively elsewhere66, but a direct, causal relationship between inflammation and myopia has yet to be demonstrated experimentally. However, in a study of form-deprived Syrian hamster sclerae, myopia progression worsened in hamsters treated with the inflammatory stimulators lipopolysaccharide and peptidoglycan, but slowed when treated with the immunosuppressive agent cyclosporine A43. This suggests that myopia might be ameliorated by reducing inflammation with local application of anti-inflammatory compounds. Vascular endothelial growth factor (VEGF) is well known for its role in neovascular age-related macular degeneration (AMD) and myopic choroidal neovascularization in pathologic myopia eyes67,68. Proangiogenic and antiangiogenic proteins were differentially expressed in the vitreous humor of myopic foveoschisis patients, with and without intravitreal anti-VEGF therapy69. These findings are consistent with the potential role of inflammation in pathologic myopia, especially at the more severe stages as VEGF is a mediator of both angiogenesis and inflammation66.
The present analysis of two publicly-available proteomic databases highlights the intimate association of the innate immune system with myopia development—detailing specific signaling pathways that may be involved in both innate immunity and myopia development, such as GzmA and S100 family signaling in the retina and activated myofibroblast trans-differentiation in the sclera (in response to inflammatory signals in the retina). These findings suggest that atropine and normal vision might mitigate myopia progression by reversing the activity of these signaling pathways.
There are several limitations to this study. Although human studies provide the most accurate representation of human diseases and their mechanisms70, human ocular tissue samples are not readily harvestable, because of technical and ethical limitations. Also, the findings in human studies must be considered cautiously, since the eyes in control groups were not always completely healthy, but often had other pathologies that called for pars plana vitrectomy. All these indicate that the increased immune-system activity in HM and PM eyes could be, at least, in part, the result of pathological changes in the ocular structure—instead of, or in addition to, the cause of ocular pathologies. Changes in the proteome detected in later stages of myopia progression might be the consequences, rather than causes, of myopia progression. Moreover, due to ethical considerations, human tissue samples tend to be available predominately from eyes with more advanced, late-staged disease. Finally, proteins found to be associated with specific functional pathway(s), via pathway analysis, might subserve other functions in the particular tissues and conditions involved in HM and PM.
In conclusion, comparative profiling of the proteome—for the identification of proteins and quantification of protein expression, between ocular tissues in myopic and control groups—helps to better understand the molecular mechanisms involved in the pathogenesis of myopia. It can also generate essential data for developing better treatment strategies, by identifying biomarkers that are critical in the myopization process.
Shorter-term myopia induction were reported to elicit changes in levels of proteins associated with energy biosynthesis pathways, which could modify immune response—whereas prolonged myopia induction could affect levels of proteins in nuclear receptor signaling pathways. The application of atropine tends to promote energy-generating processes that would enhance neuronal networking in the retina; in contrast, normal vision inhibits the innate inflammatory response, as manifested by increased myofibroblast-to-fibroblast transdifferentiation in the sclera—and this would retard myopia progression.
Myopia onset and development could be initiated by changes in energy metabolism in the retina caused by changes of cell–cell signaling in retinal neural networks, followed by activation of inflammatory signaling pathways in the choroid, ultimately leading to changes in protein synthesis and transdifferentiation of cells in the sclera. Since inflammation-related proteins associated with high myopia are also significantly involved in the innate immune system, an abnormal innate immune system could predispose subjects to myopia development. A new perspective in myopia research that focuses on the relationship between myopia and inflammation might first determine what is causal and what is effect, and thereby identify, conclusively, the key biological mechanisms responsible for myopia development and prevention, thereby facilitating the development of more effective and targeted therapeutic agents. While proteomics may not necessarily provide direct clinical guidance, it can help guide future research directions that may lead to better clinical outcomes in diagnosis, prognosis, and therapeutics.
Methods
A broad, thorough literature review (approach detailed below) was performed to identify myopia studies that utilized proteomics analysis (of either human serum, eye tissue and/or eye fluid, or animal ocular tissue samples) to inform thinking about, and possibly to validate identification of, the underlying molecular mechanism. The carefully-selected data sets (see Study Selection section below) from 16 original studies were reanalysed to obtain novel insights into myopia onset and pathologic myopia development at the protein level.
Primary search strategy and eligibility criteria
Articles were identified predominantly from PubMed, using a search strategy consisting of combining the following Medical Subject Headings (MeSH) and keywords: “myopia”, “human”, “pathologic myopia”, “animal model”, “signaling pathway”, “form-deprivation myopia”, “lens-induced myopia”, “inflammation”, “proteomics” and “quantitative proteomics”. English-language articles that were published up to October 2022 were searched and the results imported to EndNote X9.3.3 (Bld 13966), to exclude duplicates and irrelevant references and to manage the remaining records. The titles and abstracts of all articles were screened for relevance in each phase of the review (screening, eligibility, and inclusion). The references cited in these retrieved articles were also reviewed to identify articles not captured by the initial search. Two reviewers (LQJ and YSD) independently assessed the quality of the studies, and disagreements were resolved by discussion to arrive at a consensus.
Eligibility criteria included the following: original research articles concerning either human or animal myopia studies (specifically, form-deprivation myopia or lens-induced myopia), reporting untargeted proteomics analysis (with or without protein validation) relevant to the onset and development of myopia, on data from serum, ocular fluid, or tissue samples collected from myopic eyes (free from myopia-related pathologic changes). Review articles, case reports and conference abstracts were excluded; otherwise, no restrictions were imposed on the study design, in an attempt to achieve as broad an overview of the available literature as possible. In the search for untargeted proteomics in myopia, studies that were found to be only confirmatory in nature or described complications of myopia were excluded.
Study selection and data extraction
From 742 search results that were initially identified, 37 non-English and 10 duplicate articles were excluded. A further 569 articles were excluded as they did not evaluate untargeted proteomics analysis in myopia. Of the remaining 126 articles, 87 were excluded because they were not original research papers. In the end, 39 articles that evaluated proteomics analyses in human myopia and animal models, in early stages of disease and pathogenesis in myopia, were included in the systematic review. Of the 39 articles included in the present review, 16 made proteomics data publicly-available and were therefore eligible for IPA analysis.
Pre-designed data extraction forms were used to gather information on the following: first author’s name, year of publication, study design, sample size, serum and ocular fluids from human studies and ocular tissue samples from animal studies, proteomics technique used, proteins identified, signaling pathway category, and study findings. Key proteins and signaling pathways data were collected from untargeted proteomics studies to include information for all proteins and signaling pathways. The analysis was restricted to identifying signaling pathways affected in the myopic retina and sclera, as no proteomic studies of solely isolated choroidal tissue were found. Although other ocular tissues such as the cornea and lens might also be affected, for practical purposes this study focused on signaling pathways occurring in the posterior segment of the eye.
IPA analysis
The names of the key proteins extracted from selected articles were uploaded to QIAGEN IPA software (March 2023 database update), and canonical pathway analysis was performed. The names of individual proteins identified from human studies were uploaded to identify the most strongly involved signaling pathways, while groups of proteins were uploaded to ascertain the predominant categories of the identified signaling pathways. The top 3–5 functional categories derived from the most relevant signaling pathways identified were adopted for further investigation. The determination of “most relevant” pathway categories was based on an attempt to encompass the widest variety of biological functions, in order to maximize the potential for uncovering new perspectives in myopia research.
Acknowledgements
We thank Prof. William K. Stell for his critical and inspirational comments on the manuscript and kind editing of this manuscript.
Abbreviations
- 2D-GE
Two-dimensional gel electrophoresis
- ACP1
Acid phosphatase 1
- ACTB
Actin beta
- AH
Aqueous humor
- AL
Axial length
- AMD
Age-related macular degeneration
- APO B-100
Apolipoprotein B-100
- APOA-I
Apolipoprotein A-I
- APOA-II
Apolipoprotein A-II
- APOA-IV
Apolipoprotein A-IV
- APOH
Apolipoprotein H
- CCT
Chaperonin-containing TCP-1
- CDC42
Cell division cycle 42
- CDP
Cytidine diphosphate
- CFL1
Cytokine-like factor-1
- COL1A1
Collagen type I alpha 1 chain
- COL1A2
Collagen type I alpha 2 chain
- COL12A1
Collagen type XII alpha 1 chain
- COL6A1
Collagen type VI alpha 1 chain
- CST3
Cystatin C
- D
Diopter
- DAP
Differentially abundant protein
- DBP
Vitamin D binding protein
- DKK3
Dickkofp-related protein 3
- ELISA
Enzyme-linked immunosorbent assay
- EIF2
Eukaryotic initiation factor-2
- elF4
E74 like ETS transcription factor 4
- ENO1
Enolase 1
- FDGF
Fibroblast-derived growth factor
- FDM
Form-deprivation myopia
- FDR
False discovery rate
- FXR/RXR
Farnesoid X receptor/retinoid X receptor
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- GAT-1
GABA transporter 1
- GO
Gene ontology
- GP6
Glycoprotein VI platelet
- GSN
Gelsolin
- GSTM2
Glutathione S-transferase Mu 2
- GzmA
Granzyme A
- HIF1 α
Hypoxia-inducible factor 1
- HM
High myopia
- HP
Haptoglobin
- HP/HPr
Haptoglobin/haptoglobin-related protein
- HPX
Hemopexin
- HRG
Histidine-rich glycoprotein
- IL-4
Interleukin-4
- ILK
Integrin-linked kinase
- IPA
Ingenuity pathway analysis
- IRBP
Interphotoreceptor retinoid-binding protein
- iTRAQ
Fractionated isobaric tags for a relative and absolute quantification
- LC–MS
Liquid chromatography-mass spectrometry
- LC–MS/MS
Liquid chromatography-tandem mass spectrometry
- LDHA
Lactate dehydrogenase A
- LIM
Lens-induced myopia
- LTQ
Linear trap quadrupole
- LXR/RXR
Liver X receptor/retinoid X receptor
- MALDI-TOF
Matrix-assisted laser desorption/ionization coupled to time-of-flight
- MeSH
Medical subject headings
- MMD
Myopic macular degeneration
- MS
Mass spectrometry
- MTM
Myopic traction maculopathy
- mTOR
Mammalian target of rapamycin
- MYH8
Myosin heavy chain 8
- NK
Natural killer cell
- OXPHOS
Oxidative phosphorylation pathway
- p70S6K
P70 S6 kinase
- PKM
Pyruvate kinase M1/2
- PKM2
Pyruvate kinase M2
- PLG
Plasminogen
- PM
Pathologic myopia
- PPAR
Peroxisome proliferator-activated receptor
- PRDX6
Peroxiredoxin-6
- PTGES2
Prostaglandin E synthase 2
- RA
Rheumatoid arthritis
- Rab-11B
Ras-related protein Rab-11B
- RHOA
Ras homolog family member A
- RNA
Ribonucleic acid
- RPE
Retinal pigment epithelium
- S100A10
S100 calcium binding protein A10
- SE
Spherical equivalent
- SLC6A6
Solute carrier family 6 member 6
- STRING
Search tool for the retrieval of interacting genes/proteins
- SWATH-MS
Sequential window acquisition of all theoretical mass spectra
- TCP-1
T-complex protein 1
- TR/RXR
Thyroid hormone receptor/retinoid X receptor
- TTR
Transthyretin
- VEGF
Vascular endothelial growth factor
- VH
Vitreous humor
- VIM
Vimentin
- WB
Western blot
Author contributions
L.J., L.Z., V.A.B., and Q.V.H. supervised the whole project and provided funding. L.J., J.H.Z.K., S.S., Y.S.D. and Q.V.H. wrote the manuscript. L.J., S.S., Y.S.D., Z.W., and X.C. performed literature searching and bioinformatics analysis.
Funding
BMRC/IAF-ICP/JJVC_2019(VAB), SNEC/HREF/2018/0618-8 (LJ), SingHealth Duke-NUS Academic Medicine Research Grant (AM-SU048-2020 to LJ) and the SERI Lee-Foundation Pilot Grant R1756/79/2020 (LF0620-6 to LJ), National Medical Research Council (MOH-000531-00 (QVH) and MOH-001103-00(QVH)), the SERI-Lee Foundation (LF0621-1 (QVH)), the Lee Foundation (TLF1021-3 (QVH) and TLF 0322-8 (QVH)) and the SingHealth Foundation-SNEC (R1499/82/2017 (QVH)) in Singapore. InnoHK initiative and the Hong Kong Special Administrative Region Government (LZ), and Hong Kong Polytechnic University grants (P0044266 and P0043882 to LZ).
Data availability
The data that support the findings of this study are available on request from the corresponding author (Q.V.H).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Liqin Jiang and James H. Z. Koh.
These authors jointly supervised this work: Veluchamy Amutha Barathi and Quan V. Hoang.
Contributor Information
Veluchamy Amutha Barathi, Email: amutha.b.veluchamy@seri.com.sg.
Quan V. Hoang, Email: donny.hoang@duke-nus.edu.sg
References
- 1.Flitcroft, D. I. et al. IMI—Defining and classifying myopia: A proposed set of standards for clinical and epidemiologic studies. Investig. Ophthalmol. Vis. Sci.60(3), M20–M30 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Impact of Myopia and High Myopia (University of New South Wales, 2015).
- 3.Ej, T. The Optical Elements of the Refractive Power of the Eye (Hoeber Press, 1940). [Google Scholar]
- 4.Saw, S. M., Gazzard, G., Shih-Yen, E. C. & Chua, W. H. Myopia and associated pathological complications. Ophthal. Physiol. Opt.25(5), 381–391 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Morgan, I. G., Ohno-Matsui, K. & Saw, S. M. Myopia. Lancet.379(9827), 1739–1748 (2012). [DOI] [PubMed] [Google Scholar]
- 6.Pan, C. W., Ramamurthy, D. & Saw, S. M. Worldwide prevalence and risk factors for myopia. Ophthal. Physiol. Opt.32(1), 3–16 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Verhoeven, V. J. et al. Visual consequences of refractive errors in the general population. Ophthalmology.122(1), 101–109 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Flitcroft, D. I. The complex interactions of retinal, optical and environmental factors in myopia aetiology. Prog. Retin. Eye Res.31(6), 622–660 (2012). [DOI] [PubMed] [Google Scholar]
- 9.Leske, M. C., Chylack, L. T. Jr. & Wu, S. Y. The lens opacities case-control study. Risk factors for cataract. Arch. Ophthalmol.109(2), 244–251 (1991). [DOI] [PubMed] [Google Scholar]
- 10.Chihara, E. et al. Severe myopia as a risk factor for progressive visual field loss in primary open-angle glaucoma. Ophthalmologica.211(2), 66–71 (1997). [DOI] [PubMed] [Google Scholar]
- 11.Jacobi, F. K. & Hessemer, V. Pseudophakic retinal detachment in high axial myopia. J. Cataract. Refract. Surg.23(7), 1095–1102 (1997). [DOI] [PubMed] [Google Scholar]
- 12.Ueta, T. et al. Pathologic myopia: An overview of the current understanding and interventions. Glob. Health Med.2(3), 151–155 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohno-Matsui, K. et al. IMI pathologic myopia. Investig. Ophthalmol. Vis. Sci.62(5), 5 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen, B. J., Lam, T. C., Liu, L. Q. & To, C. H. Post-translational modifications and their applications in eye research (review). Mol. Med. Rep.15(6), 3923–3935 (2017). [DOI] [PubMed] [Google Scholar]
- 15.Zhang, Z., Wu, S., Stenoien, D. L. & Pasa-Tolic, L. High-throughput proteomics. Annu. Rev. Anal. Chem. (Palo Alto Calif).7, 427–454 (2014). [DOI] [PubMed] [Google Scholar]
- 16.Keerthikumar, S. An introduction to proteome bioinformatics. Methods Mol. Biol.1549, 1–3 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Lam, T. C., Chun, R. K., Li, K. K. & To, C. H. Application of proteomic technology in eye research: A mini review. Clin. Exp. Optom.91(1), 23–33 (2008). [DOI] [PubMed] [Google Scholar]
- 18.Skeie, J. M., Roybal, C. N. & Mahajan, V. B. Proteomic insight into the molecular function of the vitreous. PLoS One.10(5), e0127567 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lam, T. C., Li, K. K., Lo, S. C., Guggenheim, J. A. & To, C. H. A chick retinal proteome database and differential retinal protein expressions during early ocular development. J. Proteome Res.5(4), 771–784 (2006). [DOI] [PubMed] [Google Scholar]
- 20.Husband, S. S. T. Evolution of the avian visual system. Avian visual cognition [On-line]. IV (2001).
- 21.Lam, T. C., Li, K.-K., Lo, S. C. L., Guggenheim, J. A. & To, C. H. Application of fluorescence difference gel electrophoresis technology in searching for protein biomarkers in chick myopia. J. Proteome Res.6(11), 4135–4149 (2007). [DOI] [PubMed] [Google Scholar]
- 22.Wu, Y. et al. Differential retinal protein expressions during form deprivation myopia in albino guinea pigs. Curr. Proteom.11, 37–47 (2014). [Google Scholar]
- 23.Shao, J., Xin, Y., Li, R. & Fan, Y. Vitreous and serum levels of transthyretin (TTR) in high myopia patients are correlated with ocular pathologies. Clin. Biochem.44(8–9), 681–685 (2011). [DOI] [PubMed] [Google Scholar]
- 24.Shao, J. et al. Proteomics analysis of serum biomarkers in patients with pathological myopia. Zhonghua Yan Ke Za Zhi.48(3), 246–252 (2012). [PubMed] [Google Scholar]
- 25.Wei, Q. et al. Pathological myopia-induced antioxidative proteins in the vitreous humor. Ann. Transl. Med.8(5), 193 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duan, X. et al. Proteomic analysis of aqueous humor from patients with myopia. Mol. Vis.14, 370–377 (2008). [PMC free article] [PubMed] [Google Scholar]
- 27.Tsai, C. Y. et al. Proteomic analysis of exosomes derived from the aqueous humor of myopia patients. Int. J. Med. Sci.18(9), 2023–2029 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xue, M. et al. Proteomic analysis of aqueous humor in patients with pathologic myopia. J. Proteom.234, 104088 (2021). [DOI] [PubMed] [Google Scholar]
- 29.Zhang, X. & Young, H. A. PPAR and immune system—What do we know?. Int. Immunopharmacol.2(8), 1029–1044 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wen, K. et al. The plasminogen protein is associated with high myopia as revealed by the iTRAQ-based proteomic analysis of the aqueous humor. Sci. Rep.11(1), 8789 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jostrup, R. et al. Identification of myopia-related marker proteins in tilapia retinal, RPE, and choroidal tissue following induced form deprivation. Curr. Eye Res.34(11), 966–975 (2009). [DOI] [PubMed] [Google Scholar]
- 32.Hou, J., Ikeda, S., Mori, K., et al. The orientation of choroidal macrophage polarization significantly influences the development of myopia in murine models. bioRxiv. 2023:2023.2006.2012.544445.
- 33.Harre, U. et al. Moonlighting osteoclasts as undertakers of apoptotic cells. Autoimmunity.45(8), 612–619 (2012). [DOI] [PubMed] [Google Scholar]
- 34.Madel, M. B. et al. Immune function and diversity of osteoclasts in normal and pathological conditions. Front. Immunol.10, 1408 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirayama, D., Iida, T., Nakase, H. The phagocytic function of macrophage-enforcing innate immunity and tissue homeostasis. Int. J. Mol. Sci.19(1) (2017). [DOI] [PMC free article] [PubMed]
- 36.Bian, J., Sze, Y. H., Tse, D. Y., et al. SWATH based quantitative proteomics reveals significant lipid metabolism in early myopic Guinea Pig Retina. Int. J. Mol. Sci.22(9) (2021). [DOI] [PMC free article] [PubMed]
- 37.Barathi, V. A. et al. Involvement of GABA transporters in atropine-treated myopic retina as revealed by iTRAQ quantitative proteomics. J. Proteome Res.13(11), 4647–4658 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frost, M. R. & Norton, T. T. Alterations in protein expression in tree shrew sclera during development of lens-induced myopia and recovery. Investig. Ophthalmol. Vis. Sci.53(1), 322–336 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeng, L. et al. Intraocular complement activation is related to retinal vascular and neuronal degeneration in myopic retinopathy. Front. Cell Neurosci.17, 1187400 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schulman, I. G. Liver X receptors link lipid metabolism and inflammation. FEBS Lett.591(19), 2978–2991 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sreejit, G. et al. S100 family proteins in inflammation and beyond. Adv. Clin. Chem.98, 173–231 (2020). [DOI] [PubMed] [Google Scholar]
- 42.Frost, M. R. & Norton, T. T. Differential protein expression in tree shrew sclera during development of lens-induced myopia and recovery. Mol. Vis.13, 1580–1588 (2007). [PMC free article] [PubMed] [Google Scholar]
- 43.Lin, H. J. et al. Role of chronic inflammation in myopia progression: Clinical evidence and experimental validation. EBioMedicine.10, 269–281 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duan, M. M., Liu, H. & Zhong, Y. L. Effect of shape deprivation on retinal thickness in myopic mice using an OCT method. Front. Neurosci.17, 1156990 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matalia, J., Anegondi, N. S., Veeboy, L. & Roy, A. S. Age and myopia associated optical coherence tomography of retina and choroid in pediatric eyes. Indian J. Ophthalmol.66(1), 77–82 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chowdhury, D. & Lieberman, J. Death by a thousand cuts: Granzyme pathways of programmed cell death. Annu. Rev. Immunol.26, 389–420 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Daalen, K. R., Reijneveld, J. F. & Bovenschen, N. Modulation of inflammation by extracellular granzyme A. Front. Immunol.11, 931 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garzon-Tituana, M. et al. Granzyme A inhibition reduces inflammation and increases survival during abdominal sepsis. Theranostics.11(8), 3781–3795 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fuentes, J. M., Fulton, W. B., Nino, D., Talamini, M. A. & Maio, A. D. Atropine treatment modifies LPS-induced inflammatory response and increases survival. Inflamm. Res.57(3), 111–117 (2008). [DOI] [PubMed] [Google Scholar]
- 50.Gonzalez, L. L., Garrie, K. & Turner, M. D. Role of S100 proteins in health and disease. Biochim. Biophys. Acta Mol. Cell Res.1867(6), 118677 (2020). [DOI] [PubMed] [Google Scholar]
- 51.Mulfaul, K. et al. The essential role of the choriocapillaris in vision: Novel insights from imaging and molecular biology. Annu. Rev. Vis. Sci.8, 33–52 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Durkot, M., Martinez, O., Pease, V., Francesconi, R. & Hubbard, R. Atropine: Effects on glucose metabolism. Aviat. Space Environ. Med.61(5), 424–429 (1990). [PubMed] [Google Scholar]
- 53.Ye, L. et al. Effects of atropine treatment on choroidal thickness in myopic children. Investig. Ophthalmol. Vis. Sci.61(14), 15 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karouta, C. et al. Transcriptome-based insights into gene networks controlling myopia prevention. FASEB J.35(9), e21846 (2021). [DOI] [PubMed] [Google Scholar]
- 55.Feige, J. N. & Auwerx, J. Transcriptional targets of sirtuins in the coordination of mammalian physiology. Curr. Opin. Cell Biol.20(3), 303–309 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang, Y. et al. Regulation of SIRT1 and its roles in inflammation. Front. Immunol.13, 831168 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tomasek, J. J., Gabbiani, G., Hinz, B., Chaponnier, C. & Brown, R. A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol.3(5), 349–363 (2002). [DOI] [PubMed] [Google Scholar]
- 58.Yang, Y. et al. Intravitreal brimonidine inhibits form-deprivation myopia in guinea pigs. Eye Vis. (Lond).8(1), 27 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu, H. et al. Scleral hypoxia is a target for myopia control. Proc. Natl. Acad. Sci. U. S. A.115(30), E7091–E7100 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mann, J. et al. Regulation of myofibroblast transdifferentiation by DNA methylation and MeCP2: Implications for wound healing and fibrogenesis. Cell Death Differ.14(2), 275–285 (2007). [DOI] [PubMed] [Google Scholar]
- 61.Fox, L. A Practical Treatise on Ophthalmology (D. Appleton and company, 1910).
- 62.Zhao, L. et al. Immunoregulatory functions of nuclear receptors: Mechanisms and therapeutic implications. Trends Endocrinol. Metab.31(2), 93–106 (2020). [DOI] [PubMed] [Google Scholar]
- 63.Fledelius, H., Zak, M. & Pedersen, F. K. Refraction in juvenile chronic arthritis: A long-term follow-up study, with emphasis on myopia. Acta Ophthalmol. Scand.79(3), 237–239 (2001). [DOI] [PubMed] [Google Scholar]
- 64.Huebner, J. L., Seifer, D. R. & Kraus, V. B. A longitudinal analysis of serum cytokines in the Hartley guinea pig model of osteoarthritis. Osteoarthr. Cartil.15(3), 354–356 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seko, Y. et al. Human sclera maintains common characteristics with cartilage throughout evolution. PLoS One.3(11), e3709 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Herbort, C. P., Papadia, M. & Neri, P. Myopia and inflammation. J. Ophthal. Vis. Res.6(4), 270–283 (2011). [PMC free article] [PubMed] [Google Scholar]
- 67.Ruiz-Moreno, J. M. et al. Intravitreal anti-vascular endothelial growth factor therapy for choroidal neovascularization secondary to pathologic myopia: Six years outcome. Retina.35(12), 2450–2456 (2015). [DOI] [PubMed] [Google Scholar]
- 68.Ceklic, L., Munk, M. R., Wolf-Schnurrbusch, U., Gekkieva, M. & Wolf, S. Visual acuity outcomes of ranibizumab treatment in pathologic myopic eyes with macular retinoschisis and choroidal neovascularization. Retina.37(4), 687–693 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wei, Q. et al. Vitreous proteomics provides new insights into antivascular endothelial growth factor therapy for pathologic myopia choroid neovascularization. J. Interferon Cytokine Res.39(12), 786–796 (2019). [DOI] [PubMed] [Google Scholar]
- 70.Saeidnia, S., Manayi, A. & Abdollahi, M. From in vitro experiments to in vivo and clinical studies; Pros and Cons. Curr. Drug Discov. Technol.12(4), 218–224 (2015). [DOI] [PubMed] [Google Scholar]
- 71.Wu, Y. et al. Early quantitative profiling of differential retinal protein expression in lens-induced myopia in guinea pig using fluorescence difference two-dimensional gel electrophoresis. Mol. Med. Rep.17(4), 5571–5580 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yu, F. J. et al. Alteration of retinal metabolism and oxidative stress may implicate myopic eye growth: Evidence from discovery and targeted proteomics in an animal model. J. Proteom.221, 103684 (2020). [DOI] [PubMed] [Google Scholar]
- 73.Zhu, Y. et al. Alteration of EIF2 signaling, glycolysis, and dopamine secretion in form-deprived myopia in response to 1% atropine treatment: Evidence from interactive iTRAQ-MS and SWATH-MS proteomics using a guinea pig model. Front. Pharmacol.13, 814814 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yuan, Y., Zhu, C., Liu, M. & Ke, B. Comparative proteome analysis of form-deprivation myopia in sclera with iTRAQ-based quantitative proteomics. Mol. Vis.27, 494–505 (2021). [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author (Q.V.H).