Abstract
Excessive peripheral microvascular constriction during acute psychological stress reflects similar changes in coronary blood flow and is a predictor of adverse cardiovascular outcomes. Among individuals with coronary artery disease (CAD), we sought to determine if genetic factors contribute to the degree of microvascular constriction during mental stress. A total of 580 stable CAD individuals from two prospective cohort studies underwent mental stress testing. Digital pulse wave amplitude was continuously measured and the stress/rest (sPAT) ratio of pulse wave amplitude was calculated. Race stratified genome-wide association studies (GWAS) of sPAT-ratio were conducted using linear regression of additive genetic models. A trans-ethnic meta-analysis integrated the four sets of GWAS results. Participants were followed for the outcome of recurrent cardiovascular events (myocardial infarction, heart failure, revascularization, and CV death) for a median of 5 years. We used Wei-Lin-Weissfeld (WLW) model to assess the association between sPAT-ratio with recurrent events. Mean age was 63 ± 9. We identified three SNPs in linkage disequilibrium, closely related to chr7:111,666,943 T > C (rs6466396) that were associated with sPAT-ratio (p = 6.68E-09). Participants homozygous for the T allele had 80% higher risk of incident adverse events (HR 1.8, 95% CI, 1.4–2.2). Also, participants with a lower sPAT-ratio (< median) had a higher adverse event rate, hazard ratio (HR) = 1.3, [95%confidence interval (CI), 1.1–1.6]. However, adjustment for the genotypes did not substantially alter the impact of sPAT ratio on adverse outcome rate. In conclusion, we have identified a genetic basis for stress-induced vasomotion. The 3 linked variants modulate vasoconstriction during mental stress may have a prognostic importance.
Subject terms: Cardiovascular biology, Medical research
Introduction
Acute psychological stress is an emerging global risk factor for cardiovascular disease1,2. Abnormal coronary stress vasomotor responses play a critical role in promoting myocardial ischemia during psychological stress and adverse cardiac events in certain individuals with coronary artery disease3,4. Peripheral microvascular vasoconstriction as measured by peripheral arterial tonometry (PAT)-derived changes in digital pulse amplitude during mental stress, is a potential marker for systemic microvascular reactivity to mental stress and cardiac vulnerability to future adverse cardiac events5–10. Simultaneous measurements in the coronary and digital circulations have shown that peripheral vasoconstriction during mental stress is closely correlatesd with coronary vasomotion11. Recurrent epicardial vasoconstriction with acute mental stress may precipitate plaque disruption and ultimately lead to acute coronary events12–15.
Peripheral microvascular constriction is associated with mental stress-induced myocardial ischemia (MSIMI), a transient myocardial ischemic response to acute mental stress that predicts adverse cardiovascular events16. Endothelial dysfunction and microvascular disease are potential mechanisms underlying the association between stress and adverse cardiovascular events17,18. However, the genetic role of microvascular constriction and its possible interaction with psychosocial distress has not yet been studied. Identifying genetic polymorphisms that mediate stress-induced vasoconstriction can provide further insight into the mechanisms underlying the prognosis of stress-related cardiovascular disease. We hypothesized that genetic variation moderates the association between psychosocial distress and stress-induced vasoconstriction. We investigated whether any genetic variants (SNPs) in the entire genome are associated with mental stress-induced microvascular constriction. We also investigated whether the risk allele mediated the association between mental stress-induced microvascular constriction and adverse cardiovascular events.
Results
Study sample
Individuals with coronary artery disease (CAD) were enrolled in the MIMS2 study (Myocardial Infarction and Mental Stress 2) and the Mental Stress Ischemia Mechanisms and Prognosis Study (MIPS) were included19,20 Patients were then divided into two separate sub-cohorts based on racial composition (MIMS2 White, MIMS2 Black, MIPS White and MIPS Black). The MIMS2 study enrolled patients with a documented history of myocardial infarction (MI) in the previous 8 months who were admitted to Emory-affiliated hospitals between 2012 and 2015 in Atlanta, Georgia. Participants were 18–61 years of age at the time of screening20. The diagnosis of MI (type 1) was verified by a medical record review based on elevated troponin levels in addition to symptoms of ischemia, ECG changes, or other evidence of myocardial necrosis21.
The MIPS study, as described previously, enrolled patients with stable CAD from Emory University-affiliated hospitals and clinics between July 2009 and July 201422 Patients were defined as having CAD if any of the following criteria were met: (1) abnormal coronary angiography or intravascular ultrasound findings demonstrating atherosclerosis with at least luminal irregularities, (2) previous coronary revascularization, (3) prior myocardial infarction (MI), or (4) positive nuclear or exercise stress testing. The baseline characteristics of the participants enrolled in the two studies are presented in Table 1. Patients enrolled in the MIMS2 study were younger and included more African American and female participants. Due to the larger sample size, the MIPS White subcohort was used as the primary cohort. In this cohort, the mean age (SD) was 65 years9 with 84% male participants.
Table 1.
Baseline characteristics of the study participants by race and cohort.
| N | White | Black | ||
|---|---|---|---|---|
| MIPS | MIMS2 | MIPS | MIMS2 | |
| 337 | 42 | 111 | 90 | |
| Age (years), mean (SD) | 65 (9) | 50 (8.7) | 60(8) | 50 (7) |
| Male, % | 84 | 54 | 66 | 38 |
| BMI, (kg/m2), mean (SD) | 29 (5) | 29 (5) | 31 (5) | 33 (8) |
| Dyslipidemia, % | 83 | 63 | 86 | 65 |
| Ever smoking, % | 59 | 38 | 67 | 55 |
| Diabetes, % | 30 | 15 | 43 | 35 |
| Depression, % | 11 | 26 | 14 | 35 |
| Beta Blockers Use, % | 70 | 45 | 85 | 69 |
| CAD severity (stenosis ≥ 50%), % | 79 | 90 | 74 | 85 |
| Prior myocardial infarction, % | 33 | 100 | 31 | 100 |
| Heart failure, % | 20 | 2.4 | 35 | 9 |
| sPAT-ratio, mean (SD) | 0.71(0.31) | 0.82 (0.60) | 0.71 (0.38) | 0.84 (0.54) |
| Ejection Fraction, mean (SD) | 55 (13) | 51.8 (9.2) | 54(16) | 52.5 (12.4) |
BMI, body mass index; CAD, coronary artery disease; sPAT-ratio, stress-rest peripheral arterial tonometry, SD, standard deviation.
* PAT Effect due to SNP (p value).
Genome-wide association analysis
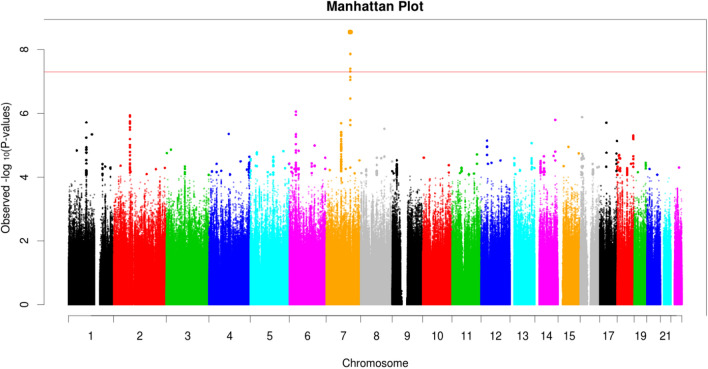
The overall GWAS results for the MIPS White cohort are summarized in Fig. 1 and S1, with minimal genomic inflation (λGC = 1.04). We found one locus, including three variants in linkage disequilibrium, to be associated with the sPAT-ratio (stress-induced vasoconstriction), with a genome-wide significance of P < 5 × 10−8. The lead SNP was the chr7:111,666,943 variant (T allele) with a frequency of 46% in this cohort was associated with a lower sPAT-ratio (beta − 0.14; 95% CI − 0.19, − 0.10; p = 2.81E-09). The other two variants showed similar effects (Table 2). An incremental vasoconstrictive effect (lower sPAT-ratio) was observed with two alleles of Chr7:111,666,943 compared to one or zero allele (Fig. 2).
Figure 1.
Manhattan Plot for the GWAS of Stress-Induced Peripheral Vasoconstriction (sPAT-ratio) in the MIPS White cohort (p values for each SNP are plotted according to their chromosome location on the X-axis, with Y-axis indicating –log10 of p-values; the red line indicates the genome-wide significance threshold of 5X10−8).
Table 2.
Variants associated with stress induced vasoconstriction among the MIPS white population.
| Variant | Ref allele | Risk allele | Effect allele frequency | Beta | Lower CI | Higher CI | p value |
|---|---|---|---|---|---|---|---|
| chr7:111,666,943 (rs6466396) | C | T | 46% | − 0.14 | − 0.19 | − 0.10 | 2.81E-09 |
| chr7:111,668,622 (rs876170) | T | G | 48% | − 0.14 | − 0.18 | − 0.09 | 1.35E-08 |
| chr7:111,668,623 (rs876169) | T | G | 48% | − 0.14 | − 0.18 | − 0.09 | 1.37E-08 |
CI, confidence limit; MIPS, Mental Stress Ischemia Mechanisms and Prognosis Study.
Figure 2.
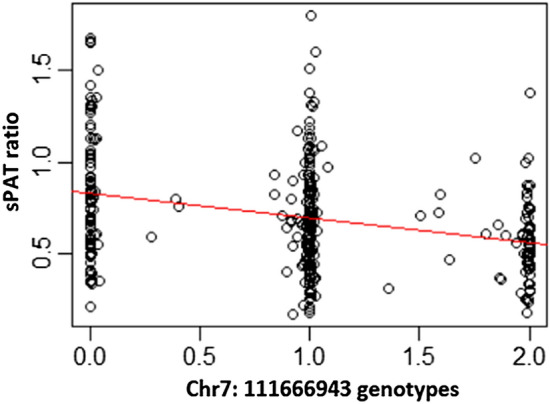
Association Between the chr7:111,666,943 Variants and Stress-induced Peripheral Vasoconstriction Measured as sPAT Ratio.
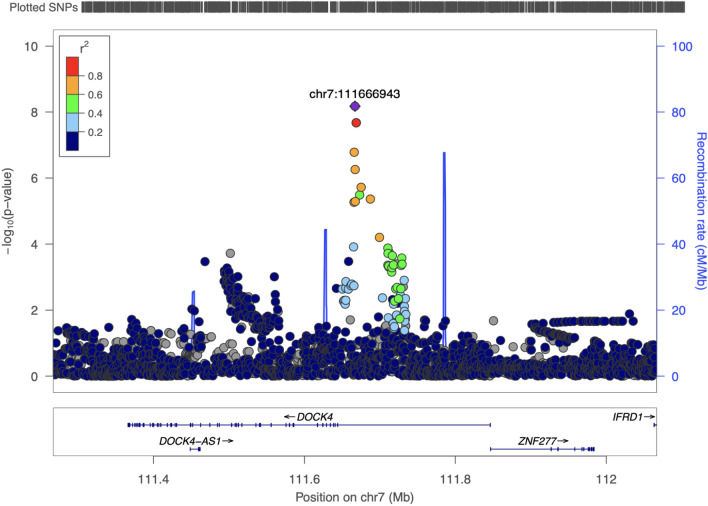
DOCK4 (dedicator of cytokinesis 4) was the nearest functional locus (Fig. 3). In a meta-analysis integrating the four cohorts, the results were consistent for the lead SNP chr7:111,666,943, with the T allele associated with a lower sPAT ratio (beta − 0.13; 95% CI − 0.17, − 0.09; p = 6.68E-09). The other two variants within this locus had similar effects (Table S1).
Figure 3.
Regional Plot for the Significant Locus in the GWAS for Stress-Induced Peripheral Vasoconstriction (sPAT-ratio) in the MIPS White cohort.
Association between genome-wide significant genotypes, sPAT-ratio and adverse cardiovascular events
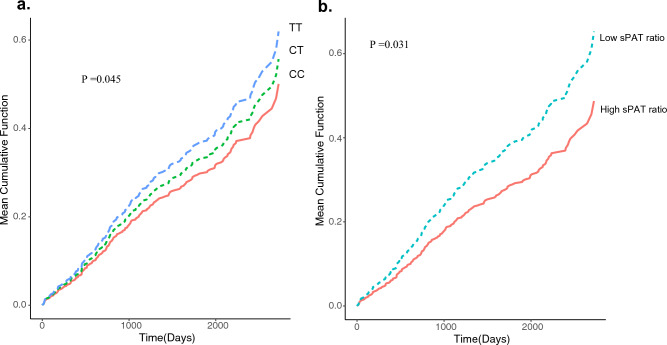
Over a 5-year follow-up period, 358 first and recurrent events for the composite endpoint of cardiovascular death, MI, revascularization, or hospitalization for heart failure were ascertained. Having an increment of one allele of Chr7:111,666,943 was associated with a 40% greater risk for the study endpoint [hazard ratio [HR] = 1.35, 95%confidence interval [95% CI, 1.16–1.56] (Table 3 and Fig. 4a). However, after adjusting for demographic and clinical risk variables, genotype was not associated with greater risk of events (Table S2). Given that the three loci are highly correlated, we presented the results for Chr7:111,666,943 only.
Table 3.
Association between effect allele (T) on chr7:111,666,943 (rs6466396) locus and adverse cardiovascular events (N = 573).
| N events | HR 95%CI | p value | |
|---|---|---|---|
| Model 1† | 358 | 1.35 (1.16–1.56) | < 0.001 |
| Model 2‡ | 358 | 1.31 (1.12–1.52) | < 0.001 |
| Model 3§ | 358 | 1.02 (0.86–1.20) | 0.086 |
CI, confidence limit; HR, hazard ratio.
Adverse events = revascularization, heart failure hospitalization, myocardial infarction and cardiovascular death.
† Hazard ratio for recurrent adverse events per 1 increment of effect allele.
†Model 1: Unadjusted.
‡Model 2: Model 1 + sPAT-ratio.
§ Model 3: Model 2 + demographics (age, sex and face) and clinical risk factors (dyslipidemia, hypertension, diabetes, body mass index, ejection fraction and beta blockers use.
Figure 4.
Cumulative incidence of adverse cardiovascular outcomes stratified by the (a) number of risk alleles (T) (0 vs. 1 vs. 2) (b) sPAT-ratio ≤ median vs. > median. The p-values were generated from Grays cumulative incidence function homogeneity test. CC is a wild-type polymorphism; CT is a heterozygous and TT is the risk homozygous polymorphism.
Compared with participants with higher sPAT-ratio (≥ median), those with low sPAT-ratio (< median) had a greater adverse event rate, hazard ratio [HR] = 1.34, [95%confidence interval (CI), 1.09–1.64] (Table 4 and Fig. 4b). After adjustment for demographic and clinical risk variables, the association remained significant (HR = 1.2 [95% CI, 1.0–1.5]). Further adjustment for the risk genotypes did not alter the association of the sPAT-ratio with the outcomes.
Table 4.
Association between stress induced vasoconstriction (sPAT-ratio < median vs > = median) and adverse cardiovascular events (N = 573).
| N events | HR 95%CI† | p value | |
|---|---|---|---|
| Model 1† | 358 | 1.34 (1.09–1.64) | 0.0055 |
| Model 2‡ | 358 | 1.26 (1.02–1.55) | 0.032 |
| Model 3§ | 358 | 1.23 (0.99–1.54) | 0.059 |
CI, confidence limit; HR, hazard ratio.
*Adjusted for demographics (age, sex and face) and clinical risk factors (dyslipidemia, hypertension, diabetes, body mass index, ejection fraction and beta blockers use).
Adverse events = revascularization, heart failure hospitalization, myocardial infarction and cardiovascular death.
† Hazard ratio for low sPAT-ratio versus higher sPAT-ratio.
†Model 1: Unadjusted.
‡Model 2: Model 1 + genotype.
§ Model 3: Model 2 + demographics (age, sex and face) and clinical risk factors (dyslipidemia, hypertension, diabetes, body mass index, ejection fraction and beta blockers use).
Discussion
In a large cohort of patients with stable CAD, we performed a GWAS to study the genetic determinants of stress-induced vasomotion, measured as sPAT-ratio. We detected three novel susceptibility loci on chromosome 7q31.1, gene cluster which modulate vascular reactivity in response to acute psychological stress. Moreover, these loci are associated with a risk of adverse cardiovascular events. However, this genetic risk is not independent of demographic and clinical risk factors. We confirmed that greater stress-induced vasoconstriction, measured as a lower sPAT-ratio, is associated with a greater risk of adverse recurrent cardiovascular events, independent of all clinical risk factors, during a longer follow-up period10. However, adjusting for individual’s genotype did not substantially alter this association. This relationship further advances our understanding of the linkage between acute psychological stress, vasoreactivity, and genetics in patients with CAD.
Genetic susceptibility to acute psychological stress has previously been investigated. Polymorphisms in the β1-adrenergic receptor (ADRB1) and β2-adrenergic receptor (ADRB2) genes alter the effects of epinephrine on cardiac and vascular physiology and have been associated with cardiovascular disease23. An association between a variant of the ADRB1 gene (rs1801252: substitution of the major allele adenine by guanine) and metal stress-induced ischemia has been reported, but there was no adjustment for multiple testing and population stratification23. Although the exact mechanisms are unknown, our findings suggest that the prognostic role of these variants is likely mediated by traditional risk factors. Further studies are required to understand the interactions between cardiovascular risk factors and these variants.
The three susceptibility loci in chromosome 7q31.1 are near the DOCK4 gene that encodes a family of proteins that provide a docking platform for the assembly of multimolecular signaling complexes involving atypical Rho guanine nucleotide exchange factors for Rac and/or Cdc42 GTPases24. The DOCK protein family plays a role in vascular smooth muscle cell (VSMC) migration through bone morphogenic protein 4 and miRNA 2125, in the morphogenesis of blood vessels26, and neural and synaptic development27. Future studies should investigate whether the risk allele is associated with a higher number or greater reactivity of VSMCs to stressful stimuli. The DOCK protein family is also involved in LDL uptake by endothelial cells, leading to atherosclerosis28. However, the exact role of these variants in atherosclerosis remains unknown. Finally, our findings have clinical implications. Laboratory-provoked mental stress may reasonably approximate the responses to stress in daily life29,30. An increased vasoconstrictor response to mental stress may also be a manifestation of the interaction between this genetic variant and the autonomic response of brain circuits to psychological stress. Therefore, it is necessary to study the incremental value of this variant to understand CAD secondary risk prediction models.
Our study had several strengths and limitations. Although we previously found that lower sPAT-ratio is associated with adverse cardiovascular events over the near term in a smaller cohort, this study did not include a cohort of healthy individuals10. Thus, our findings cannot be generalized to individuals without CAD. Moreover, our present study included a longer follow-up duration and a larger study population, and the results remained consistent with the findings in the shorter follow-up period. Furthermore, we found that although genetic factors are implicated in vasoreactivity to mental stress, the association between the sPAT-ratio and adverse events remained independent of genetic factors. Other limitations include the limited sample size and lack of evidence for the direct mechanistic effect of this variant on cardiovascular prognosis. Our research addresses an understudied high-risk population with unmet needs for secondary prevention.
In conclusion, we identified a genetic locus on chromosome 7 that modulates stress-induced vasomotion. Three linked variants at this locus, chr7:111,666,943C > T (rs6466396), chr7: 111, 665,941 T > G (rs876170), and chr7: 111,668,623 T > G (rs876169), are associated with greater vasoconstriction during mental stress, which in turn is associated with recurrent cardiovascular events, and thus may indicate a genetic effect on long-term cardiovascular risk. These findings require further validation using larger GWAS studies. Given that our phenotype was unique, correlated phenotypes should be investigated in larger cohorts to better understand this mechanistic pathway. Additionally, the clinical value of these genetic variants should be investigated in individuals with CAD to better understand the role of stress-induced cardiovascular disease. Furthermore, the prognostic value of this variant should be investigated in healthy individuals as well as in others with established cardiovascular disease to better understand the role of this variant in cardiovascular disease pathogenesis.
Methods
Study population
Patients with coronary artery disease (CAD) enrolled in the MIMS2 study (Myocardial Infarction and Mental Stress 2) and the Mental Stress Ischemia Mechanisms and Prognosis Study (MIPS) were included19,20. Patients were then divided into two separate sub-cohorts based on racial composition (MIMS2 White, MIMS2 Black, MIPS White, and MIPS Black). The MIMS2 study enrolled patients with a documented history of myocardial infarction (MI) in the previous 8 months who were admitted to Emory-affiliated hospitals between 2012 and 2015 in Atlanta, Georgia. The patients were 18–61 years of age at the time of screening20. The diagnosis of MI (type 1) was verified by a medical record review based on elevated troponin levels in addition to symptoms of ischemia, ECG changes, or other evidence of myocardial necrosis21.
As described previously, the MIPS study enrolled patients with stable CAD from Emory University-affiliated hospitals and clinics between July 2009 and July 201422. Patients were defined as having CAD if any of the following criteria were met: (1) abnormal coronary angiography or intravascular ultrasound findings demonstrating atherosclerosis with at least luminal irregularities, (2) previous coronary revascularization, (3) prior myocardial infarction (MI), or 4) positive nuclear or exercise stress testing. All methods were performed in accordance with relevant guidelines and regulations. Both study protocols were approved by the Emory University Institutional Review Board and all participants provided written informed consent at the time of enrollment. We included MIMS2 control participants in this study to help understand the left atrial response to mental stress in healthy subjects.
Mental stress test
The details of the study protocol have been previously described and published10. Briefly, the testing procedure was performed in the morning after a 12-h fast, and any anti-anginal medications (beta blockers, calcium channel blockers, and long-acting nitrates), xanthine derivatives, and caffeine-containing products were withheld for 24 h prior to the mental stress testing procedure to reduce potential interference with their vasoactive properties. The subjects first spent a 30-min rest period in a temperature-controlled (21–23 °C), quiet, and dimly lit room. Vital signs were measured, and a standardized mental stress protocol was performed. Specifically, the participants were asked to imagine a stressful situation in which a close relative was mistreated in a nursing home. They were subsequently asked to prepare a statement for two minutes and were given the following three minutes to present it in front of an evaluative audience wearing white coats. All mental stress testing procedures were conducted by trained and experienced staff to ensure standardization of stress-provoking elements. Throughout the protocol, hemodynamic parameters, including blood pressure and heart rate, were recorded using an IntelliSense Professional Digital Blood Pressure Monitor (HEM-907EL; OMRON, Japan). The cuff was positioned over the upper arm of the contralateral side of the PAT device, and each hemodynamic parameter was measured every 5 min during the rest period, every 1 min during the mental stress period, and every 5 min during the recovery period. Rate-pressure product (RPP) was calculated as systolic blood pressure heart rate, and the change in RPP was quantified as the percentage difference between the maximum value during speech and the minimum resting value at the baseline.
Measurement of peripheral microvascular constriction
The PAT (Itamar-Medical, Israel) device was used to measure digital arterial pulse wave amplitude continuously during rest and the mental stress test, as previously described.13 Briefly, PAT uses a modified form of plethysmography to measure pulsatile blood volume changes. The probe was applied to the index finger of the contralateral arm to blood pressure measurements. The PAT probe applies a constant subdiastolic pressure over the distal-two thirds of the finger to prevent distal venous blood stasis, unload arterial wall tension, and stabilize the probe to reduce noise. Consequently, the changes in pulsatile volume only reflect changes in digital arterial blood perfusion. The device was also connected via thin tubing to an isolated volume reservoir to buffer within the probe itself. Pulsatile pressure changes from the probe are registered from a pressure transducer and then fed into specialized software that filters, amplifies, stores, and analyzes the signal in an operator-independent manner. The baseline pulse wave amplitude during rest was determined by averaging the last 3 min of the recording that preceded the mental stress test. The pulse wave amplitude during the mental stress test was determined visually as the area of maximum vasoconstriction during the speaking period, with a duration of 30 s to 2 min. The sPAT ratio during mental stress was calculated as the ratio of the pulse wave amplitude during mental stress to the resting pulse wave amplitude, such that a ratio < 1 signifies peripheral arterial vasoconstriction during mental stress.
Genotyping
Genomic DNA (gDNA) was extracted from blood or saliva samples and quantified using the PicoGreen assay, standardized to 50 ng/mL, and processed following the standard Illumina protocol including hybridization, incubation, and scanning.
For all four cohorts, genotyping was performed using Illumina’s Multi-Ethnic Genotyping Array (MEGA) platform and imputed to the 1000 Genome Phase 3 reference panel. Imputation was performed using the Michigan Imputation Server31. Only autosomal SNPs with imputation quality > 0.5 were retained for analysis. Further quality control procedures included removing SNPs with call rate < 0.95, individual call rate < 0.90, minor allele frequency (MAF) < 0.05, and Hardy–Weinberg equilibrium (HWE) p-value < 1e-4. The total number of SNPs in the GWAS was 6,752,496 for MIPS white, 9,170,066 for MIPS black, 6,690,990 for MIMS2 white, and 9,223,266 for MIMS2 black.
Genome-wide association analysis
Genome-wide association analysis was conducted in the discovery cohort of MIPS white participants. All genotyped and imputed variants were tested additive genetic model. A linear regression model was utilized, with adjustments for age, sex, and top 10 principal components calculated from genotypes to control for population structure. Similar analyses were also performed in the remaining three cohorts: MIPS black, MIMS2 white, and MIMS2 black. Then, the four GWAS result summaries were integrated using the inverse variance weighted fixed effects model.
GWAS analysis was conducted using RVTEST32 and a meta-analysis of individual study results was conducted using METAL33. Regional plots for the top associations were plotted using the web-based tool LocusZoom34. Variants with p-value < 5 × 10−8 were considered statistically significant.
Follow-up and study outcomes
The participants were prospectively followed up for a median of 5 years. Follow-up data were collected through patient contacts, medical record reviews, and querying the Social Security Death Index. Because CAD is characterized by recurrent nonfatal cardiovascular events, we considered both the first and recurrent events for a more accurate reflection of the burden of disease35. All events were adjudicated by cardiologists who were blinded to other study data. The study endpoint was a composite of events, including cardiovascular death, MI, revascularization, or hospitalization for heart failure. Cardiovascular death was defined as death attributable to an ischemic cardiovascular cause (fatal MI), cardiac arrhythmia, or heart failure.
Statistical analysis
Given the similarity of protocols, we pooled the two cohorts using an individual patient data meta-analysis approach with random effects to preserve clustering within the studies36. We examined the association between the sPAT-ratio and adverse cardiovascular events as binary variables (< median vs ≥ median). As we were interested in the overall burden of events over time, we used the Wei-Lin-Weissfeld (WLW) model for recurrent events37, which allows a separate underlying hazard for each event. Using the WLW model, we derived hazard ratios (HR) and 95% confidence intervals (CI) for the association between the sPAT-ratio and adverse outcomes before and after adjustment for demographic variables, including age, sex, race (black vs. Non-black), and cardiovascular risk factors and medical history, including BMI, hypertension, dyslipidemia, ejection fraction, diabetes, and history of smoking (ever vs. never). Furthermore, we examined the effects of adding genotype to the models. Finally, we plotted the mean cumulative function curves for the study endpoints in patients by the number of effect allele and the sPAT-ratio (by median). Data analysis was conducted using the SAS software (version 9.4 [SAS Institute Inc., Cary, NC]). Statistical significance was set at pvalue s < 0.05.
Supplementary Information
Author contributions
Z.A wrote the main manuscript. C.L. contributed to the main analysis. J.H.K, M. H. and A.A contributed to the data collection. P.R. and A.J. critically revised the manuscript. J.D.B. and V.V. contributed to the study design and critically revised the manuscript. Y.V.S and A.A.Q oversaw data collection, analysis and critically revised the manuscript.
Funding
This work was supported by the NIH, through the following grants: P01 HL101398, R01 HL109413, R01HL109413-02S1, R01HL125246, K24HL077506, K24 MH076955, UL1TR000454, KL2TR000455, K23HL127251, F32HL151163, UL1TR002378, TL1TR002382 and T32HL130025.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available to protect privacy but are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-54566-z.
References
- 1.Dimsdale, J. E. Psychological stress and cardiovascular disease. J. Am. Coll. Cardiol.51, 1237–1246 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steptoe, A. & Kivimäki, M. Stress and cardiovascular disease. Nat. Rev. Cardiol.9, 360 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Steptoe, A. & Brydon, L. Emotional triggering of cardiac events. Neurosci. Biobehav. Rev.33, 63–70 (2009). [DOI] [PubMed] [Google Scholar]
- 4.Arri, S. S., Ryan, M., Redwood, S. R. & Marber, M. S. Mental stress-induced myocardial ischaemia. Heart102, 472–480 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Goor, D. A. et al. Peripheral arterial tonometry: A diagnostic method for detection of myocardial ischemia induced during mental stress tests: A pilot study. Clinical Cardiology27, 137–141 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burg, M. M. et al. Non-invasive detection of risk for emotion provoked myocardial ischemia. Psychosomatic Med.71, 14–20 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hassan, M. et al. Usefulness of peripheral arterial tonometry in the detection of mental stress-induced myocardial ischemia. Clin. Cardiol.32, E1–E6 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramadan, R. et al. Myocardial ischemia during mental stress: Role of coronary artery disease burden and vasomotion. J. Am. Heart Assoc.2, e000321 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hammadah, M. et al. Hemodynamic, catecholamine, vasomotor and vascular responses: Determinants of myocardial ischemia during mental stress. Int. J. Cardiol.243, 47–53 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim, J. H. et al. Peripheral vasoconstriction during mental stress and adverse cardiovascular outcomes in patients with coronary artery disease. Circ. Res.125, 874–883 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hammadah, M. et al. Coronary and peripheral vasomotor responses to mental stress. J. Am. Heart Assoc.7, e008532 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muller, J. E., Abela, G. S., Nesto, R. W. & Tofler, G. H. Triggers, acute risk factors and vulnerable plaques: The lexicon of a new frontier. J. Am. College Cardiol.23, 809–813 (1994). [DOI] [PubMed] [Google Scholar]
- 13.Tofler Geoffrey, H. & Muller, J. E. Triggering of acute cardiovascular disease and potential preventive strategies. Circulation114, 1863–1872 (2006). [DOI] [PubMed] [Google Scholar]
- 14.Fuster, V., Fayad, Z. A. & Badimon, J. J. J. T. L. Acute coronary syndromes: Biology. Lancet353, s5–s9 (1999). [DOI] [PubMed] [Google Scholar]
- 15.Maseri, A. et al. Coronary vasospasm as a possible cause of myocardial infarction. A conclusion derived from the study of “preinfarction” angina. New England J. Med.299, 1271–7 (1978). [DOI] [PubMed] [Google Scholar]
- 16.Wei, J. et al. Meta-analysis of mental stress-induced myocardial ischemia and subsequent cardiac events in patients with coronary artery disease. Am. J. Cardiol.114, 187–192 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steptoe, A. & Kivimaki, M. Stress and cardiovascular disease. Nat. Rev. Cardiol.9, 360–370 (2012). [DOI] [PubMed] [Google Scholar]
- 18.Ramadan, R. et al. Myocardial ischemia during mental stress: Role of coronary artery disease burden and vasomotion. J. Am. Heart Assoc.2, e000321 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hammadah, M. et al. Al the mental stress ischemia prognosis study: Objectives, study design, and prevalence of inducible ischemia. Psychosom Med.79, 311–317 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaccarino, V. et al. Mental stress-induced-myocardial ischemia in young patients with recent myocardial infarction: Sex differences and mechanisms. Circulation137, 794–805 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thygesen, K. et al. Third universal definition of myocardial infarction. Eur. Heart J.33, 2551–2567 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Vaccarino, V. et al. Sex differences in mental stress-induced myocardial ischemia in patients with coronary heart disease. J. Am. Heart Assoc.5(9), e003630 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hassan, M. et al. Association of beta1-adrenergic receptor genetic polymorphism with mental stress-induced myocardial ischemia in patients with coronary artery disease. Arch. Int. Med.168, 763–770 (2008). [DOI] [PubMed] [Google Scholar]
- 24.Shi, L. Dock protein family in brain development and neurological disease. Commun. Integr. Biol.6, e26839 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang, H. et al. Bone morphogenetic protein 4 promotes vascular smooth muscle contractility by activating microRNA-21 (miR-21), which down-regulates expression of family of dedicator of cytokinesis (DOCK) proteins. J. Biol. Chem.287, 3976–3986 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abraham, S. et al. A Rac/Cdc42 exchange factor complex promotes formation of lateral filopodia and blood vessel lumen morphogenesis. Nat. Commun.6, 7286 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang, M. et al. Two autism/dyslexia linked variations of DOCK4 disrupt the gene function on Rac1/Rap1 activation, neurite outgrowth, and synapse development. Front Cell Neurosci.13, 577 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernandez-Ruiz, I. Active LDL trafficking drives atherosclerosis. Nat. Rev. Cardiol.16, 384 (2019). [DOI] [PubMed] [Google Scholar]
- 29.Blumenthal, J. A. et al. Mental stress-induced ischemia in the laboratory and ambulatory ischemia during daily life: Association and hemodynamic features. Circulation92, 2102–2108 (1995). [DOI] [PubMed] [Google Scholar]
- 30.Gottdiener, J. S. et al. Induction of silent myocardial ischemia with mental stress testing: Relation to the triggers of ischemia during daily life activities to ischemic functional severity. J. Am. College Cardiol.24, 1645–1651 (1994). [DOI] [PubMed] [Google Scholar]
- 31.Das, S. et al. Next-generation genotype imputation service and methods. Nat. Genet.48, 1284–1287 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhan, X., Hu, Y., Li, B., Abecasis, G. R. & Liu, D. J. RVTESTS: an efficient and comprehensive tool for rare variant association analysis using sequence data. Bioinformatics (Oxford, England)32, 1423–1426 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Willer, C. J., Li, Y. & Abecasis, G. R. METAL: Fast and efficient meta-analysis of genomewide association scans. Bioinformatics26, 2190–2191 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pruim, R. J. et al. LocusZoom: Regional visualization of genome-wide association scan results. Bioinformatics (Oxford, England)26, 2336–2337 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Solomon, S. D. & Pfeffer, M. A. The future of clinical trials in cardiovascular medicine. Circulation133, 2662–2670 (2016). [DOI] [PubMed] [Google Scholar]
- 36.de Jong, V. M. T. et al. Individual participant data meta-analysis of intervention studies with time-to-event outcomes: A review of the methodology and an applied example. Res. Synth. Methods11, 148–168 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Therneau, T. M., Grambsch, P. M. The Cox Model, in Modeling Survival Data: Extending the Cox Model. Statistics for Biology and Health. Springer, New York, NY. (2000).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available to protect privacy but are available from the corresponding author on reasonable request.