Abstract
In peripheral blood mononuclear cells, syncytium-inducing (SI) human immunodeficiency virus type 1 (HIV-1) infected and depleted all CD4+ T cells, including naive T cells. Non-SI HIV-1 infected and depleted only the CCR5-expressing T-cell subset. This may explain the accelerated CD4 cell loss after SI conversion in vivo.
Infection with human immunodeficiency virus type 1 (HIV-1) is generally established by preferentially macrophage-tropic, slowly replicating, non-syncytium-inducing (NSI) variants (27, 32) that use β-chemokine receptor 5 (CCR5) as a coreceptor (1, 11, 13). In 50% of cases, disease progression is associated with the emergence of SI variants which at least use α chemokine receptor 4 (CXCR4) (8, 12, 15). SI conversion precedes a more rapid CD4 cell decline and an accelerated progression to AIDS (9, 20), for which the mechanism is not completely understood. Naive CD4+ T cells express CXCR4 but not CCR5 (5), which makes them targets for SI-HIV-1 infection in vivo (4, 23, 31). Infection and death of these naive CD4 T cells may directly interfere with T-cell renewal (4). In addition, SI HIV-1 variants are generally considered to be more cytopathic than NSI HIV-1 variants (16, 17, 24, 28), although high cytopathicity of NSI HIV-1 variants has also been reported (14, 22, 33).
In this study, we analyzed the in vitro cytopathic effects of closely related NSI and SI HIV-1 variants with different coreceptor usage on different T-cell subsets in peripheral blood mononuclear cells (PBMC) in vitro.
Pooled PBMC from 10 healthy blood donors, selected for a CCR5 wild-type genotype, were isolated from buffy-coat cells using Ficoll density centrifugation. For phytohemagglutinin (PHA) stimulation, cells were grown in medium (Iscove's modified Dulbecco's medium [IMDM] supplemented with 10% fetal calf serum, penicillin [100 U/ml], and streptomycin [100 μg/ml]) with PHA (1 μg/ml) for 2 days.
PHA-stimulated PBMC (PHA-peripheral blood lymphocytes) were then depleted for CD8+ cells using a magnetic cell sorting (MACS) system (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions. The cell fraction depleted for CD8+ cells was collected and resuspended to a concentration of 106 cells/ml in IMDM supplemented with recombinant human interleukin-2 (rIL2) (Proleukin; Chiron Benelux BV, Amsterdam, The Netherlands) (20 U/ml) and Polybrene (5 μg/ml).
Subsequently, inoculation was performed with 1,000 50% tissue culture infective doses (the multiplicity of infection ranged from 0.006 to 0.0013) of primary biological HIV-1 clones of ACH 208, from a participant in the Amsterdam Cohort Studies. These HIV-1 biological clones were previously obtained by coculture of limiting dilutions of PBMC from patients and PHA-stimulated PBMC from healthy donors (21, 27). PBMC from ACH 208 were obtained 20 months after the diagnosis of AIDS, which was 74 months postseroconversion. We used one NSI variant (208∗4ac12), one CXCR4-restricted SI variant (208∗4c8), and one CCR5- and/or CXCR4-using SI variant (208∗4ac10). Virus stocks were grown on PHA-stimulated PBMC. For mock infections, we used the supernatants of uninfected PBMC cultures.
Changes in the distribution of T-cell subsets present in PHA-stimulated PBMC that had been inoculated in vitro with NSI or SI HIV-1 were studied on a FACSCalibur (Becton Dickinson, San Jose, Calif.) after staining for CD4 (peridinin chlorophyll protein [PerCP]; Becton Dickinson), CCR5 (fluorescein isothiocyanate [FITC], 2D7; PharMingen, San Diego, Calif.), and CXCR4 (phycoerythrin [PE], 12G5; PharMingen). Lymphocytes were gated according to their relative positions in the forward and sideward scatter plot. Cells were further gated for CD4 expression and for the membrane markers that defined other subsets.
Increased expression of CXCR4 and CCR5 on CD4 T cells was first observed in PBMC from healthy individuals after 2 days of PHA stimulation, which was just before inoculation. The major cell population present was either CD4+ CXCR4+ CCR5− or CD4+ CXCR4+ CCR5+. The two other cell populations (CD4+ CXCR4− CCR5+ or/and CD4+ CXCR4− CCR5− subsets) were seen from day 4 after inoculation onward as minor populations (approximately 1 to 3 and 1 to 10% of gated CD4 lymphocytes, respectively) (data not shown).
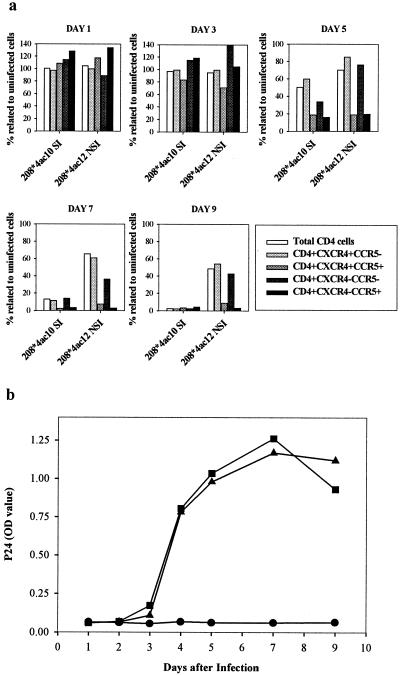
Infection with SI HIV-1 induced a depletion of the total CD4 T-cell subset, which was complete at day 9 after inoculation. In the NSI-HIV-1-infected cultures, CD4 cell depletion was more moderate, with 50% depletion of total CD4 T cells compared to the number still present in the uninfected control at day 9 (Fig. 1a).
FIG. 1.
Longitudinal analysis of the distribution of CD4 T-cell subsets, defined by HIV-1 coreceptor expression in PHA PBMC cultures inoculated with 208∗4ac10 (SI HIV-1) or 208∗4ac12 (NSI HIV-1) (a) and replication kinetics of the viruses (b). Cells were isolated at sequential time points after infection and stained with MAbs against CD4, CXCR4, and CCR5. CD4 T-cell subsets are depicted as percentages of total cells relative to numbers in uninfected controls. Viral replication kinetics of both viruses were assessed by measuring p24 Gag antigen in the culture supernatant. ●, uninfected control: ▴, 208∗4ac10 (R5/X4-SI) infection; ■, 208∗4ac12 (R5-NSI) infection. OD, optical density. Graphs are representative of three independently performed experiments.
Both the SI- and the NSI-HIV-1-infected cultures showed reductions in their numbers of CD4+ CXCR4+ CCR5+ and CD4+ CXCR4− CCR5+ cells compared to the numbers in the uninfected control, which were first seen at day 5 after inoculation (Fig. 1a). In the SI-HIV-1-infected culture, the depletion of both the CD4+ CXCR4− CCR5− and the CD4+ CXCR4+ CCR5− subset was also evident at day 5 after inoculation, although to a much lesser extent. In the NSI-HIV-1-infected culture, an almost complete depletion of the CCR5+ cells had occurred by day 7 whereas the CXCR4+ subset remained relatively unaffected compared to that of the uninfected control. Identical observations were made with PBMC that had been stimulated with immobilized CD3 and CD28 monoclonal antibodies (MAbs) (data not shown). In parallel cultures, the kinetics of virus production was monitored in the supernatants using an in-house p24 antigen capture enzyme-linked immunosorbent assay (29). Virus production was first detected at day 4 after inoculation. The kinetics and the maximum levels of virus production were the same in NSI- and SI-HIV-1-infected cultures (Fig. 1b).
Next, we wanted to analyze whether, within the T-cell population expressing CCR5 and/or CXCR4, different CD4+ T-cell subsets were depleted with different kinetics. For this purpose, the cells were stained with MAbs directed against CD4, CCR5, CXCR4, CD45RO (allophycocyanin [APC]; Becton Dickinson), CD27 (biotin; CLB, Amsterdam, The Netherlands), and streptavidin (PE; Molecular Probes, Eugene, Oreg.) at different time points after inoculation with NSI or SI HIV-1.
Based on the expression of CD45RO and CD27 on CD4 subsets, we distinguished a CD27− memory subset (CD4+ CD27− CD45RO+), a CD27+ memory subset (CD4+ CD27+ CD45RO+), and a naive T-cell subset (CD4+ CD27+ CD45RO−) (2, 10). Upon infection with the SI HIV-1 variant, we observed a rapid depletion of the CD27− memory subset, which was complete at day 7 after inoculation. Depletion of the CD27+ memory subset occurred with slower kinetics and was complete at day 9 after inoculation. The naive subset remained intact until day 5 after inoculation but was also completely depleted at day 9 (data not shown).
Inoculation with the NSI HIV-1 variants resulted in a 50% depletion of the CD4 cell population. The CD27+ memory and CD27− memory T-cell subsets were most affected, a result in agreement with the high proportion of CCR5+ T cells in the memory T-cell subsets. The CD4+ CCR5+ T lymphocytes were almost completely depleted at day 7. This depletion included both the CD27− CCR5+ memory and the CD27+ CCR5+ memory T-cell subsets. CCR5 expression on naive CD4 T cells was very low (data not shown).
To establish whether the subsets that were depleted upon inoculation of the cell culture with HIV were indeed productively infected, we stained for the presence of intracellular p24 Gag antigen (FITC, KC57; Coulter, Miami, Fla.). We then also included the CXCR4-restricted SI HIV-1 variant 208∗4c8 from the same patient.
Cells were first stained for membrane markers, fixed with paraformaldehyde, permeabilized with a permeabilizing solution (Becton Dickinson), and then stained for intracellular p24 Gag antigen at days 2, 5, 7, and 9 after inoculation. To distinguish between input virus and newly produced p24 antigen, we incubated control cultures with zidovudine (AZT) to prevent productive infection. At all time points, supernatants of AZT-treated cell cultures remained negative for p24 Gag as measured by enzyme-linked immunosorbent assay (data not shown). In agreement with these results, less than 1% of the AZT-treated CD4 lymphocytes stained positive for intracellular p24 (data not shown).
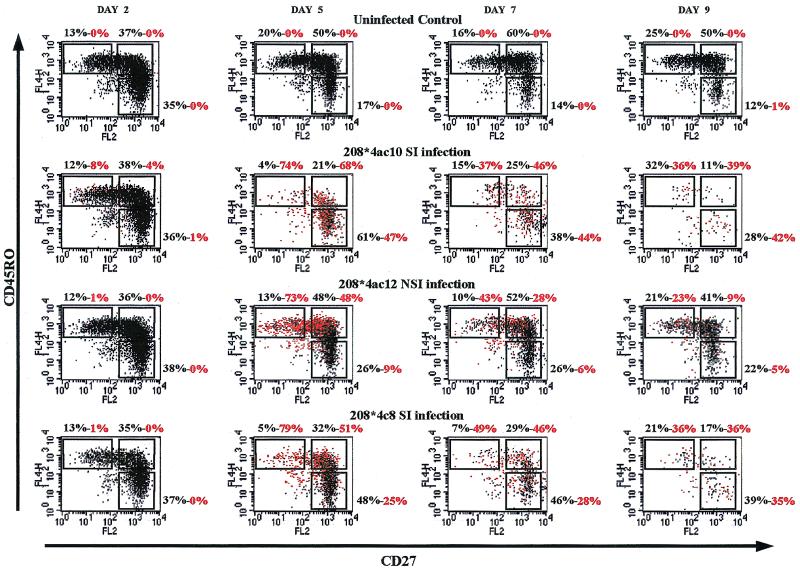
The cultures infected with the R5 NSI HIV-1 variant showed intracellular staining for p24 Gag antigen in the memory CD4+ T-cell subsets. After infection with the X4 and R5/X4 SI HIV-1 variants, the presence of p24 Gag antigen in the CD27− memory and in the CD27+ CD45RO− naive CD4 T-cell subsets could be demonstrated by intracellular staining and fluorescence-activated cell sorter (FACS) analysis (Fig. 2). These findings are in good correspondence with the expression of CXCR4 on almost all CD4 cells and the expression of CCR5 mainly on the memory CD4 T cells. The SI-HIV-1 infection of CXCR4-expressing naive T cells is in agreement with the fact that SI HIV-1, but not NSI HIV-1, can be isolated from naive T cells from SI-HIV-1-infected individuals, as was reported recently (4, 31). Furthermore, irrespective the virus used for inoculation, the CD27− memory T cells contained the highest percentage of productively infected cells at days 2 and 5 after infection. This T-cell subset was the subset depleted most rapidly.
FIG. 2.
Productive infection as demonstrated by the presence of intracellular p24 Gag antigen in CD4+ T-cell subsets after inoculation with SI or NSI HIV-1 variants. FACScan dot plot images of cells stained at different time points after inoculation with MAbs against CD4, intracellular p24, CD27, and CD45RO are shown. Red dots represent p24-positive cells. (Top graphs) uninfected control; (upper middle graphs) SI-HIV-1 (208∗4ac10)-inoculated cultures; (lower middle graphs) NSI-HIV-1 (208∗4ac12)-inoculated cultures; (bottom graphs) SI-HIV-1 (208∗4c8)-inoculated cultures. Percentages are depicted above each graph; the first value represents the percentage of cells of gated CD4 T cells within the region, and the second (red) value represents the percentage of p24-positive cells within the region.
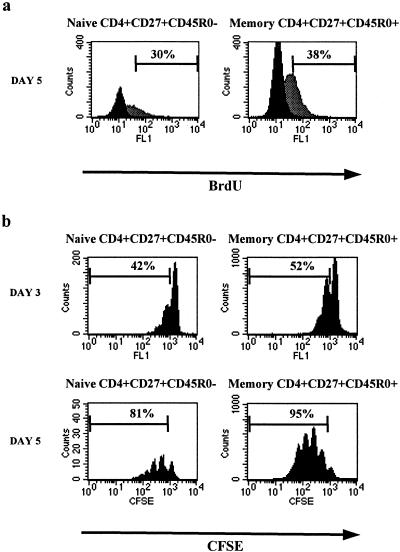
The presence of intracellular p24 Gag antigen in the so-called naive T cells may point to productive HIV-1 infection. As a certain cellular activation state is required to support HIV-1 infection (6, 7, 34, 35), it has long been questioned whether naive cells can be infected with HIV-1 while keeping their naive phenotype. We therefore wanted to determine whether, in our culture system, the naive CD4 T-cell subset (CD4+ CD27+ CD45RO−) proliferated upon PHA stimulation, without losing the naive phenotype. For this purpose, cells were PHA-stimulated for 2 days, CD8 depleted, and incubated in medium containing rIL-2, Polybrene, and bromodeoxyuridine (BrdU, 20 μM; Sigma), a uridine analog that incorporates into DNA in place of thymidine. After 24 h, unadsorbed BrdU was washed away and cells were cultured in medium for another 2 days. Cells were then stained for membrane markers against CD4, CD27, and CD45RO; fixed and permeabilized with 2% paraformaldehyde and 1% Tween 20; and treated with DNase (20 U/ml, RQ1; Promega Corp., Madison, Wis.) supplemented with MgCl2 for 30 min at 37°C. Incorporated BrdU was subsequently visualized with anti-BrdU (FITC; Becton Dickinson). At day 5 after the start of PHA stimulation, about 30% of the cells in the naive cell population had incorporated BrdU indicative of proliferation. In the CD27+ memory cell population, 38% of the cells had incorporated BrdU (Fig. 3a).
FIG. 3.
FACS analysis of the cell division status of CD4+ CD27+ CD45RO− and CD4+ CD27+ CD45RO+ T cells within PHA-stimulated PBMC. (a) BrdU incorporation in naive and memory T cells. Gray and black histograms represent cells incubated with and without BrdU, respectively. Naive CD27+ CD45RO− and memory CD27+ CD45RO+ CD4+ T cells were gated. Percentages of BrdU positive cells are indicated within each histogram. (b) CFSE labeling of naive and memory T cells 3 and 5 days after initiation of PHA stimulation. The dilution of the CFSE label within each subset is shown. Percentages indicate the proportion of cells within each subset that have divided at least once.
The division histories of CD4+ CD27+ CD45RO+ (memory) and CD4+ CD27+ CD45RO− (naive) T-cell populations were also analyzed using the intracytoplasmic fluorescent dye 5- (and 6-)carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes). CFSE is equally distributed over daughter cells upon cell division and is discretely diluted in subsequent cell division rounds. We first positively selected for CD4 and CD45RA from PBMC with a MACS multisort kit and labeled the cells with CFSE for 10 min at 37°C (final concentration, 6 μM) before stimulating these with PHA. Cells were subsequently stained with MAbs against CD4, CD27, and CD45RO. We could indeed observe a dilution of CFSE over daughter cell populations in both the memory and the naive T-cell subsets in PHA-stimulated cultures (Fig. 3b). At day 3, about 42% of the naive subset had undergone cell division, which increased to 81% at day 5. In the CD27+ memory subset, the percentages of cells that had divided were 52% at day 3 and 95% at day 5. A cytokine-induced proliferation of naive cells without conversion to a memory cell phenotype has also been described by Unutmaz et al. (30). Moreover, a relatively high expression of Ki67 in naive T cells during the late stage of HIV-1 infection has been observed (19, 25).
We here show that target cells for HIV-1 are defined by the expression of the appropriate coreceptors, which has also been observed in the SCID-hu Thy/Liv mouse model. In that model, infection with SI HIV-1 resulted in strong depletion of thymocytes that expressed CXCR4 whereas infection with NSI HIV-1 had no or little effect, a finding which agrees with the very low number of CCR5-expressing cells in the thymus (3). Moreover, coreceptor expression-dependent cell killing has also been described for HIV-2 and SIV (26). Our findings are also in agreement with the observations by Grivel and Margolis (18), who observed in lymphoid tissue cultures in vitro depletion of CCR5-expressing cells upon NSI infection and depletion of all CD4+ T cells upon SI infection, irrespective of cellular coreceptor expression. One of their explanations for the SI-HIV-1-mediated depletion of CXCR4-negative cells was that SI HIV-1 could perhaps utilize CXCR4 at very low expression levels, even below the limit of detection of FACS analysis.
Indeed, as we performed intracellular staining for p24 Gag antigen, we were able to demonstrate that cells productively infected with HIV were more readily depleted than nonproductively infected cells. Since inoculation with SI HIV-1 resulted in a productive infection and also in the depletion of CXCR4-negative subsets, it may very well be that a low expression of CXCR4 is sufficient to support HIV-1 infection. Alternatively, the cells may have had detectable CXCR4 expression at the moment of inoculation, which was subsequently down regulated. In addition, aspecific cell depletion may have occurred as a consequence of an increased amount of toxic factors and cell debris due to SI-HIV-1-mediated cell depletion.
In addition, we have shown that NSI and SI HIV-1 are equally cytopathic for their respective target cells and, finally, that naive T cells can proliferate and that these cells are indeed susceptible to SI-HIV-1 infection. Our data suggest that, although differential cytopathicity of NSI and SI HIV-1 may be excluded as an explanation for the accelerated CD4 cell decline after the emergence of SI HIV-1 variants, the underlying mechanism may still be multifactorial. Indeed, SI-HIV-1 infection of naive T cells may directly interfere with T-cell renewal. In addition, the much broader expression of CXCR4, also within the memory T-cell pool, provides a much larger SI-HIV-1 target cell population. Consequently, despite the equal levels of cytopathicity of NSI and SI HIV-1, the impact of SI-HIV-1 infection on the depletion of CD4 cells is much larger.
Acknowledgments
We thank Ester Zagwijn for technical support; Mette Hazenberg, Sigrid Otto, and Dörte Hamann for very helpful discussions; and Ronald van Rij and Frank Miedema for critical reading of the manuscript.
Financial support for this study was provided by NWO (grant no. 901-02-214).
This study was performed as part of the Amsterdam Cohort Studies, a collaboration between the Academic Medical Center, the Municipal Health Service, and the CLB in Amsterdam, The Netherlands.
REFERENCES
- 1.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 2.Baars P A, Maurice M M, Rep M, Hooibrink B, Van Lier R A W. Heterogeneity of the circulating human CD4+ T-cell population: further evidence that the CD4+ CD45RA− CD27− T-cell subset contains specialized primed cells. J Immunol. 1995;154:17–25. [PubMed] [Google Scholar]
- 3.Berkowitz R D, Alexander S, Bare C, Linquist-Stepps V, Bogan M, Moreno M E, Gibson L, Wieder E D, Kosek J, Stoddart C L, McCune J M. CCR5- and CXCR4-utilizing strains of human immunodeficiency virus type 1 exhibit differential tropism and pathogenesis in vivo. J Virol. 1998;72:10108–10117. doi: 10.1128/jvi.72.12.10108-10117.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaak H, Van 't Wout A B, Brouwer M, Hooibrink B, Hovenkamp E, Schuitemaker H. In vivo HIV-1 infection of CD45RA+CD4+ T cells is established primarily by syncytium-inducing variants and correlates with the rate of CD4+ T cell decline. Proc Natl Acad Sci USA. 2000;97:1269–1274. doi: 10.1073/pnas.97.3.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bleul C C, Wu L, Hoxie J A, Springer T A, Mackay C R. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci USA. 1997;94:1925–1930. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bukrinsky M I, Sharova N, Dempsey M P, Stanwick T L, Bukrinskaya A G, Haggerty S, Stevenson M. Active nuclear import of human immunodeficiency virus type 1 preintegration complexes. Proc Natl Acad Sci USA. 1992;89:6580–6584. doi: 10.1073/pnas.89.14.6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bukrinsky M I, Stanwick T L, Dempsey M P, Stevenson M. Quiescent T lymphocytes as an inducible virus reservoir in HIV-1 infection. Science. 1991;254:423–427. doi: 10.1126/science.1925601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. The β-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 9.Connor R I, Mohri H, Cao Y, Ho D D. Increased viral burden and cytopathicity correlate temporally with CD4+ T-lymphocyte decline and clinical progression in human immunodeficiency virus type 1-infected individuals. J Virol. 1993;67:1772–1777. doi: 10.1128/jvi.67.4.1772-1777.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Jong R, Brouwer M, Hooibrink B, van der Pouw-Kraan T, Miedema F, Van Lier R A W. The CD27− subset of peripheral blood memory CD4+ lymphocytes contains functionally differentiated T lymphocytes that develop by persistent antigenic stimulation in vivo. Eur J Immunol. 1992;22:993–999. doi: 10.1002/eji.1830220418. [DOI] [PubMed] [Google Scholar]
- 11.Deng H K, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Suttons R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of the major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 12.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. A dual-tropic primary HIV-1 isolate that uses fusin and the β-chemokine receptors CKR-5, CKR-3 and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 13.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 14.Fais S, Lapenta C, Santini S M, Spada M, Parlato S, Logozzi M, Rizza P, Belardelli F. Human immunodeficiency virus type 1 strains R5 and X4 induce different pathogenic effects in hu-PBL-SCID mice, depending on the state of activation/differentiation of human target cells at the time of primary infection. J Virol. 1999;73:6453–6459. doi: 10.1128/jvi.73.8.6453-6459.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 16.Fouchier R A M, Meyaard L, Brouwer M, Hovenkamp E, Schuitemaker H. Broader tropism and higher cytopathicity for CD4+ T cells of a syncytium-inducing compared to a non-syncytium-inducing HIV-1 isolate as a mechanism for accelerated CD4+ T cell decline in vivo. Virology. 1996;219:87–95. doi: 10.1006/viro.1996.0225. [DOI] [PubMed] [Google Scholar]
- 17.Glushakova S, Grivel J-C, Fitzgerald W, Sylwester A, Zimmerberg J, Margolis L B. Evidence for the HIV-1 phenotype switch as a causal factor in acquired immunodeficiency. Nat Med. 1998;4:346–349. doi: 10.1038/nm0398-346. [DOI] [PubMed] [Google Scholar]
- 18.Grivel J-C, Margolis D B. CCR5- and CXCR4-tropic HIV-1 are equally cytopathic for their T-cell targets in human lymphoid tissue. Nat Med. 1999;5:344–346. doi: 10.1038/6565. [DOI] [PubMed] [Google Scholar]
- 19.Hazenberg M D, Cohen Stuart J W T, Otto S A, Borleffs J C C, Boucher C A, De Boer R J, Miedema F, Hamann D. T cell division in human immunodeficiency virus (HIV-1)-infection is mainly due to immune activation: a longitudinal analysis in patients before and during highly active antiretroviral therapy (HAART) Blood. 2000;95:249–255. [PubMed] [Google Scholar]
- 20.Koot M, Keet I P M, Vos A H V, De Goede R E Y, Roos M T L, Coutinho R A, Miedema F, Schellekens P T A, Tersmette M. Prognostic value of HIV-1 syncytium-inducing phenotype for rate of CD4+ cell depletion and progression to AIDS. Ann Intern Med. 1993;118:681–688. doi: 10.7326/0003-4819-118-9-199305010-00004. [DOI] [PubMed] [Google Scholar]
- 21.Koot M, Van 't Wout A B, Kootstra N A, De Goede R E Y, Tersmette M, Schuitemaker H. Relation between changes in cellular load, evolution of viral phenotype, and the clonal composition of virus populations in the course of human immunodeficiency virus type 1 infection. J Infect Dis. 1996;173:349–354. doi: 10.1093/infdis/173.2.349. [DOI] [PubMed] [Google Scholar]
- 22.Mosier D E, Gulizia R J, MacIsaac P D, Torbett B E, Levy J A. Rapid loss of CD4+ T cells in human-PBL-SCID mice by noncytopathic HIV isolates. Science. 1993;260:689–692. doi: 10.1126/science.8097595. [DOI] [PubMed] [Google Scholar]
- 23.Ostrowski M A, Chun T-W, Justement S J, Motola I, Spinelli M A, Adelsberger J, Ehler L A, Mizell S B, Hallahan C W, Fauci A S. Both memory and CD45RA+/CD62L+ naive CD4+ T cells are infected in human immunodeficiency virus type 1-infected individuals. J Virol. 1999;73:6430–6435. doi: 10.1128/jvi.73.8.6430-6435.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Picchio G R, Gulizia R J, Wehrly K, Chesebro B, Mosier D E. The cell tropism of human immunodeficiency virus type 1 determines the kinetics of plasma viremia in SCID mice reconstituted with human peripheral blood leukocytes. J Virol. 1998;72:2002–2009. doi: 10.1128/jvi.72.3.2002-2009.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sachsenberg N, Perelson A S, Yerly S, Schokmel G A, Leduc D, Hirschel B, Perrin L. Turnover of CD4+ and CD8+ T lymphocytes in HIV-1 infection as measured by Ki-67 antigen. J Exp Med. 1998;187:1295–1303. doi: 10.1084/jem.187.8.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schramm B, Penn M L, Palacios E H, Grant R M, Kirchhoff F, Goldsmith M A. Cytopathicity of human immunodeficiency virus type 2 (HIV-2) in human lymphoid tissue is coreceptor dependent and comparable to that of HIV-1. J Virol. 2000;74:9594–9600. doi: 10.1128/jvi.74.20.9594-9600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schuitemaker H, Koot M, Kootstra N A, Dercksen M W, de Goede R E Y, van Steenwijk R P, Lange J M A, Eeftink Schattenkerk J K M, Miedema F, Tersmette M. Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: progression of disease is associated with a shift from monocytotropic to T-cell-tropic virus populations. J Virol. 1992;66:1354–1360. doi: 10.1128/jvi.66.3.1354-1360.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tersmette M, Lange J M A, De Goede R E Y, De Wolf F, Eeftink-Schattenkerk J K M, Schellekens P T A, Coutinho R A, Huisman J G, Goudsmit J, Miedema F. Association between biological properties of human immunodeficiency virus variants and risk for AIDS and AIDS mortality. Lancet. 1989;i:983–985. doi: 10.1016/s0140-6736(89)92628-7. [DOI] [PubMed] [Google Scholar]
- 29.Tersmette M, Winkel I N, Groenink M, Gruters R A, Spence P, Saman E, van der Groen G, Miedema F, Huisman J G. Detection and subtyping of HIV-1 isolates with a panel of characterized monoclonal antibodies to HIV-p24 gag. Virology. 1989;171:149–155. doi: 10.1016/0042-6822(89)90521-7. [DOI] [PubMed] [Google Scholar]
- 30.Unutmaz D, Pileri P, Abrignani S. Antigen-independent activation of naive and memory resting T cells by a cytokine combination. J Exp Med. 1994;180:1159–1164. doi: 10.1084/jem.180.3.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Rij R P, Blaak H, Visser J A, Brouwer M, Rientsma R, Broersen S, De Roda Husman A M, Schuitemaker H. Differential coreceptor expression allows for independent evolution of non-syncytium-inducing and syncytium-inducing HIV-1. J Clin Investig. 2000;160:1039–1052. doi: 10.1172/JCI7953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van 't Wout A B, Kootstra N A, Mulder-Kampinga G A, Albrecht-van Lent N, Scherpbier H J, Veenstra J, Boer K, Coutinho R A, Miedema F, Schuitemaker H. Macrophage-tropic variants initiate human immunodeficiency virus type 1 infection after sexual, parenteral and vertical transmission. J Clin Investig. 1994;94:2060–2067. doi: 10.1172/JCI117560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu X, McLane M F, Ratner L, O'Brien W, Collman R, Essex M, Lee T-H. Killing of primary CD4+ T cells by non-syncytium-inducing macrophage-tropic human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1994;91:10237–10241. doi: 10.1073/pnas.91.21.10237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zack J A, Arrigo S J, Weitsman S R, Go A S, Haislip A, Chen I S Y. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990;61:213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- 35.Zack J A, Haislip A M, Krogstad P, Chen I S Y. Incompletely reverse-transcribed human immunodeficiency virus type 1 genomes in quiescent cells can function as intermediates in the retroviral life cycle. J Virol. 1992;66:1717–1725. doi: 10.1128/jvi.66.3.1717-1725.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]