Abstract
Glucagon‐like peptide‐1 (GLP‐1)‐based therapies, effective in treating obesity and type 2 diabetes, hold potential for reducing alcohol‐seeking behaviour. However, the understanding of how alcohol consumption affects endogenous GLP‐1 responses—important for understanding GLP‐1‐based therapies' potential in addressing alcohol misuse—is limited, given the absence of placebo‐controlled studies examining these effects. This study aimed to determine the acute effects of alcohol ingestion on GLP‐1 and other peptides and evaluate whether metabolic surgery, which increases GLP‐1 responses, blood alcohol concentrations (BAC) and alcohol misuse risk, influences this effect. Additionally, we assessed the acute effects of alcohol on plasma glucose and insulin concentrations. Using a placebo‐controlled crossover study, we examined hormonal and glucose responses after oral alcohol consumption (0.5 g/kg of fat‐free mass) versus placebo drinks in 18 women who underwent metabolic surgery <5 years ago and in 14 non‐operated controls (equivalent in age, body mass index [BMI], race and alcohol consumption patterns). Women had a mean (SD) age of 41 (10) years and a BMI of 33 (5) kg/m2. Compared with the control group, the surgery group exhibited a higher peak BAC (0.99 [0.20] g/L vs. 0.75 [0.16] g/L; P < 0.005). Alcohol decreased GLP‐1 by 34% (95% CI, 16%–52%) in both groups and decreased ghrelin more in the control (27%) than in the surgery group (13%). Alcohol modestly decreased plasma glucose and transiently increased insulin secretion in both groups (P < 0.05). However, alcohol lowered blood glucose concentrations to the hypoglycaemic range in 28% of the women in the surgery group versus none in the control group. These findings provide compelling evidence that acute alcohol consumption decreases GLP‐1, a satiation signal, elucidating alcohol's ‘apéritif’ effect. This study also highlights the potential increase in alcohol‐related hypoglycaemic effects after metabolic surgery.
Keywords: bariatric surgery, ethanol, gut peptides, hypoglycaemia, weight‐loss surgery
There is considerable interest in GLP‐1‐based therapies, highly effective for weight loss and type 2 diabetes, as potential treatments for reducing alcohol consumption. However, it is unclear how alcohol consumption affects endogenous GLP‐1, which was the focus of this study. In addition, we determined whether metabolic surgery, which enhances GLP‐1 responses and alcohol risk, influences this effect. We found that alcohol reduces endogenous GLP‐1 levels in women, irrespective of surgical status, and that metabolic surgery may exacerbate alcohol‐related hypoglycaemic effects.
1. INTRODUCTION
There is a growing interest in innovative approaches to treating alcohol use disorder (AUD), including a recent focus on gut‐brain peptides that regulate both homeostatic hunger and reward processing and motivation for food‐seeking. 1 , 2 , 3 , 4 One such peptide is glucagon‐like peptide‐1 (GLP‐1), an anorectic incretin hormone derived from intestinal preproglucagon‐containing cells and neurons in the nucleus tractus solitarii. 5 Indeed, GLP‐1 receptor agonists are among the most efficacious treatments for managing obesity and type 2 diabetes, and growing evidence suggests that those GLP‐1 receptor agonists show promise in reducing alcohol consumption and seeking behaviour in animal models. 1 , 2 , 3 , 4 , 6 , 7 Although clinical data are limited, a Danish pharmacoepidemiologic study found that GLP‐1‐based therapies for diabetes management were associated with reduced alcohol‐related events within the initial 3 months of prescription compared to the corresponding 3‐month period pre‐treatment. 8 Additionally, results from a randomized, double‐blind, placebo‐controlled clinical trial involving treatment‐seeking patients with AUD indicate that exenatide, a first‐generation GLP‐1 receptor agonist, reduced heavy drinking days and overall alcohol intake in a sub‐group of patients with AUD and obesity. 9 Finally, a recent study revealed that 12‐week treatment with dulaglutide, another GLP‐1 receptor agonist, reduced weekly alcohol intake in patients treated for smoking cessation. 10 However, these results 9 , 10 are in need of replication, given that they were based on secondary analysis.
To better determine the potential of GLP‐1‐based therapies for AUD, it is also important to understand how alcohol consumption affects endogenous GLP‐1 responses. Although there are limited human data on alcohol's impact on GLP‐1, recent secondary analyses of studies with non‐treatment‐seeking AUD people suggest that acute alcohol exposure reduces plasma GLP‐1 concentrations. 11 However, given the nature of this initial work (secondary analyses in studies designed to address different a priori outcomes), essential controls were not included, such as comparing alcohol to a placebo condition or considering concurrent food intake, which could confound alcohol's effects on GLP‐1 responses.
The primary focus of this study was to assess the acute effects of alcohol, compared to placebo, on plasma GLP‐1 concentrations. Recognizing GLP‐1's fundamental role in glucose regulation, the potential hypoglycaemic effects of alcohol and its crosstalk with other gut–brain peptides, we also aimed to determine the acute effects of oral alcohol intake on plasma glucose, C‐peptide, insulin and ghrelin concentrations. The rationale for measuring ghrelin is that, like GLP‐1, ghrelin plays a role in food and alcohol reward 1 and is affected by metabolic surgery. Additionally, we aimed to determine whether metabolic surgeries, such as Roux‐en‐Y‐gastric bypass (RYGB) and sleeve gastrectomy (SG), which enhance GLP‐1 responses 12 and alcohol misuse risk, 13 , 14 , 15 , 16 , 17 , 18 influence these effects. Although it appears contradictory that surgeries increasing GLP‐1 endogenous responses also raise AUD risk, it is noteworthy that the AUD risk typically does not increase until 2 years post‐surgery. In contrast, 40%–50% of patients with high‐risk alcohol use before surgery effortlessly reduce their harmful alcohol intake within the first year post‐procedure. 19 , 20 Therefore, metabolic surgery provides a valuable experimental model to further assess the acute effect of alcohol ingestion on hormonal responses. Accordingly, using a cross‐sectional study design, we compared hormonal and glucose responses to acute alcohol consumption in participants who underwent metabolic surgery versus non‐operated controls. We hypothesized that compared to consuming a placebo beverage, consuming a moderate dose of alcohol would lead to decreased plasma GLP‐1, ghrelin and glucose concentrations in both groups. However, because of surgery‐induced alterations in alcohol pharmacokinetics that can double peak blood alcohol concentration (BAC) compared to when drinking the same amount before surgery, 21 , 22 , 23 we hypothesized that participants in the surgery group would be more likely to experience alcohol‐induced hypoglycaemia than those in the control group.
2. METHODS
2.1. Participants
This study was part of a larger one on bariatric surgeries' effects on alcohol pharmacokinetics and pharmacodynamics. 21 , 22 , 23 The parent study included women who were planning to undergo bariatric surgery as well as women who underwent bariatric surgery in the last 5 years and a non‐operated control group. The study focused exclusively on women because they represent the vast majority of patients undergoing bariatric surgery, 24 and sex can affect alcohol pharmacokinetics. 25 During the initial screening, women were excluded if they were abstaining from drinking alcohol or reported consuming more than seven standard drinks per week (or more than four standard drinks per drinking occasion, considering one standard drink contains approximately 14 g of pure alcohol). The rationale for excluding risky drinking is that we aimed to study bariatric surgeries' effects on alcohol pharmacokinetics and pharmacodynamics in participants who were not already at high risk for AUD. They were also excluded if they were younger than 21 years of age or older than 64; were pregnant or breastfeeding; had anaemia, gastritis, colitis, Crohn's disease, malabsorptive diseases, inflammatory diseases, liver disease (i.e., aspartate aminotransferase or alanine aminotransferase >2 times the upper reference range of normal or abnormal bilirubin), kidney disease (i.e. out of normal range plasma sodium, creatinine and BUN), stroke or severe organ dysfunction or cancer less than 5 years ago; had a diagnosis of alcohol abuse or dependence or current regular use of drugs with potential for misuse (based on an interview with the Semi‐Structured Assessment for Genetics of Alcoholism [SSAGA] 26 that refers to DSM‐IV diagnostic terminology); were taking medications that could affect alcohol metabolism; were currently smoking cigarettes or quitted smoking less than 2 months ago; or had a body weight >450 lbs (because of a weight limit on the dual‐energy x‐ray absorptiometry [DXA] machine). Additional eligibility criteria for the control group were having no history of gastric surgery and being equivalent in age, race, body mass index (BMI) and alcohol intake to those in the surgery group. A total of 82 participants underwent screening for eligibility in this parent study. Of these, 50 were excluded from further analysis in this data set for reasons detailed in the flow diagram (Figure 1). Data on alcohol pharmacokinetics from a subsample of these subjects have been reported previously. 21 , 22 , 23
FIGURE 1.
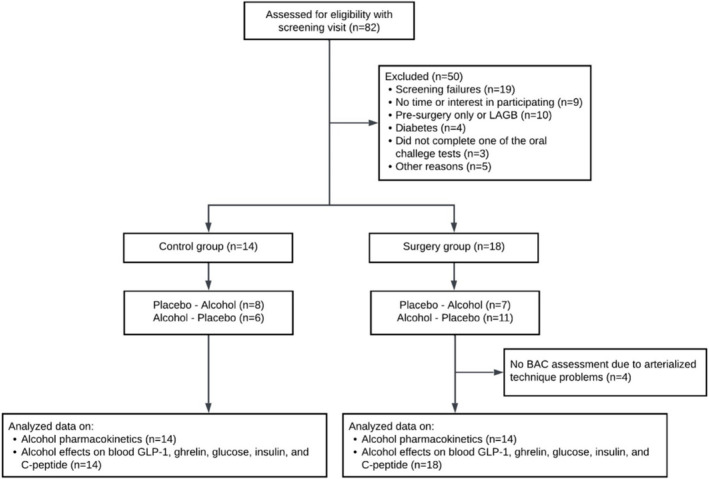
Flow diagram of study participants.
2.2. Study approval
The University of Illinois at Urbana‐Champaign (UIUC) and the Carle Foundation Hospital (CFH) Institutional Review Boards approved the study protocol; all participants provided written informed consent before participation, and guidelines from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) on ‘Administering Alcohol in Human Studies’ were followed.
2.3. Screening visit
All potential participants completed an in‐person screening evaluation. We performed a detailed medical, medication and socio‐demographic history, including a review of pre‐surgery medical records, routine blood tests and a urine pregnancy test to confirm non‐pregnancy. Participants were also asked to complete a series of standardized questionnaires, including the Alcohol module of the SSAGA. 26 Body fat‐free mass (FFM) was assessed using DXA (Horizon® DXA system, Hologic Inc., MA) and/or air displacement plethysmograph (BODPOD; COSMED USA Inc., IL) to calculate alcohol dose based on FFM. 27 To ensure that differences in the dose of alcohol calculated with FFM estimated from Bod Pod and DXA were acceptable, we measured FFM for 26 participants using both methods. Although Bod Pod consistently overestimated FFM (the mean difference between the two methods was 5.0 ± 0.5 kg), this resulted in only a 3 mL difference in the alcohol dose calculated by DXA. Because this volume of alcohol is the same amount that we use to spray on the placebo drink as a flavour mask, we considered the discrepancy between methods in calculating FFM acceptable for this purpose.
2.4. Oral alcohol challenge test
Participants were evaluated in a private room in CFH or UIUC over two visits approximately 2 weeks apart (the average days between visits was 14 ± 2 days). Before each challenge test began, we rechecked non‐pregnancy status with a urine pregnancy test. After abstaining from alcohol for at least 3 days, participants arrived at approximately 8:00 AM after an overnight fast and remained fasted during the entire testing procedure. Approximately 30 min after arrival, an intravenous line attached to a three‐way stopcock for blood collection was inserted into a hand vein. After acclimatization, arterialized heated‐hand venous blood samples were obtained before and at various times after consuming 0.5 g of alcohol per kilogram of FFM mixed with a non‐caloric fruity juice (alcohol condition) or a non‐alcohol version of the same drink (placebo condition) on visit one and the alternative drink during visit two. The alcohol drink was prepared as a 20% vol/vol solution of 190‐proof ethanol mixed with a fruit‐flavoured juice (Kool‐Aid, Kraft Heinz Company, Chicago, IL) sweetened with Splenda (Heartland Consumer Products, Carmel, IN). 21 The drinks were provided in two equally divided aliquots, each consumed within two consecutive 5‐min periods (i.e. 10 min total), and the rim of the cups containing all drinks was sprayed with 2 mL of alcohol to serve as a smell and flavour mask, as previously described. 21
2.5. Biochemical measurements
Blood samples were collected in chilled EDTA tubes containing a protease inhibitor cocktail (Millipore, Billerica, MA). These samples were placed on ice and centrifuged at 4°C, and the plasma was immediately aliquoted in four different tubes and stored at −80°C for subsequent analyses. Plasma active GLP‐1 (here referred to as GLP‐1) and active ghrelin (‘active’ is the term used by the manufacturer in reference to acyl‐ghrelin 28 and here referred to as ghrelin) were measured by radioimmunoassay (Millipore RIA kits, Billerica MA). For GLP‐1, samples were extracted with 95% ethanol, dried under nitrogen, rehydrated with sample hydrating solution and then assayed per the kit instructions. For ghrelin, samples were acidified with 1 N HCl before analysis and then analysed per kit instructions. Insulin and C‐peptide were measured by electrochemiluminescence using Roche Elecsys kits on the Roche Cobas e 601 module for immunoassay tests (Roche Diagnostics, Indianapolis, IN). All hormones were measured at the Core laboratory at Washington University in St. Louis. Plasma glucose concentrations were measured at the bedside using a biochemistry analyser (YSI 2300 STAT plus; Yellow Spring Instrument Co., Yellow Springs, OH). BAC was determined by headspace gas chromatography in our laboratory, as described. 21 The total areas under the curve (AUC) for alcohol, GLP‐1, ghrelin, glucose, insulin and C‐peptide were calculated using the trapezoid method. 29
2.6. Classical alcohol pharmacokinetic measures
We used BAC data to determine peak, time‐to‐peak and AUC. We also determined the disappearance rate of alcohol (β60), the total amount of alcohol eliminated per hour (b60) and the alcohol elimination rate (R) as previously described. 30 The disappearance rate of alcohol (β60) was estimated for each participant from the slope of the linear least‐squares regression lines within the apparent linear portion of the descending limb of the BAC versus time curve. To exclude the upper distribution phase and lower first‐order elimination phase of the apparent lineal portion of the curve, the first value was taken 0.5 h after the peak BAC, and all subsequent readings ≥0.20 g/L were used. The total amount of alcohol eliminated per hour (b60) was calculated as follows: b60 = β60 × TBW/Bw, with total body water (TBW), TBW = [0.1069 × height (cm)] + [0.2466 × weight (kg)] − 2.097, and Bw = 0.80. This standardized anthropometric equation estimates TBW for women with a precision of ±9%–11%. 31 Alcohol elimination rate (R) was expressed as the amount of alcohol eliminated per kilogram of the body per hour (R = b60/weight).
2.7. Statistical analysis
This study was part of a larger investigation into the effects of bariatric surgeries on alcohol (NCT02766322). Our power calculations relied on primary endpoints—alcohol pharmacokinetics (e.g. peak BAC) and subjective effects. We used mean and SD differences from the literature 32 and our preliminary data. Preliminary results showed that post‐RYGB women had a peak BAC of 1.10 g/L, post‐SG women 0.86 g/L and controls 0.67 g/L (similar to pre‐surgery). With 16 individuals per group, we had 80% power to detect these differences in peak BAC. Sample sizes like this are also typical for studies examining alcohol's hormonal effects. 33 , 34 , 35
The differences in the hormonal and metabolic responses to alcohol consumption between surgery and control groups were evaluated using general linear mixed models (PROC MIXED). Condition (placebo and alcohol), time and group (control and surgery), as well as all interactions, were included in the model and treated as fixed effects, and the subject was included as a random effect. We originally also included order of the visit as a between factor, that is, alcohol‐placebo versus placebo‐alcohol to investigate whether the order in which they were assessed influenced the outcome variables. Because the order of the visits did not interact with condition or group for any of the outcome variables, this factor was excluded from further analysis. Considering that differences in the risk of developing AUD varied with times since surgery, we explored whether acute effects of alcohol on these responses differed in women who underwent surgery <2 years ago (n = 14) versus >2 years ago (n = 4). Results were similar for both subgroups; therefore, all women who underwent surgery <5 years ago were included in the analysis. When applicable, significant interactions were further analysed using Fisher's least significance difference tests. Differences between the two groups' clinical characteristics and alcohol‐related variables were compared using a two‐sample independent t‐test or Mann–Whitney U test when data were not normally distributed. We used SAS version 9.4 (SAS Institute Inc. Cary, NC, USA) for statistical analysis. We adjusted for multiple testing using the false discovery rate (FDR; Benjamini–Hochberg) 36 and a P‐value ≤ 0.028 determined statistical significance.
2.8. Missing data
The study design included 640 plasma samples (20 samples per woman: 10 for the alcohol and 10 for the non‐alcohol placebo condition, ×32 women). Due to technical difficulties with the IV line, we missed one plasma sample from three participants (two on the placebo and one on the alcohol condition). We replaced the missing values (3 out of 640) with the value from the closest time point to the missing point.
3. RESULTS
3.1. Characteristics of study participants
Thirty‐two women completed the study. Eighteen of these women underwent metabolic surgery (14 SG and 4 RYGB), hereafter referred to as surgery group, and 14 were in the non‐operated control group, hereafter referred to as control group. The study cohort characteristics are shown in Table 1. As expected, women in the surgery group had higher fasting plasma GLP‐1 concentrations (mean difference 9.4 pmol/L, 95% CI, 1.9–16.9 pmol/L), lower fasting plasma ghrelin (mean difference −40.3 pg/mL, 95% CI, −73.5 to −7.0 pg/mL), glucose (mean difference −0.40 mmol/L,95% CI, −0.65 to −0.16 mmol/L) and insulin (mean difference: −37.7 pmol/L, 95% CI, −63.6 to −11.7 pmol/L) concentrations and were more insulin sensitive than the control group (mean difference in HOMA‐IR2−0.7, 95% CI, −1.2 to −0.2). Groups were similar in their frequency of alcohol consumption per month, but compared to the control group, those in the surgery group tended to consume fewer alcohol drinks per drinking day (mean [SD], 2.0 [1.0] vs. 1.0 [1.0]; P = 0.06), reached a higher peak BAC (mean difference 0.25 g/L; 95% CI, 0.10–0.39 g/L) sooner (mean difference −0.2 h, 95% CI, −0.3 to −0.1 h) and, therefore, tended to exhibit a greater alcohol bioavailability (i.e. greater BAC AUC) after consuming the same dose of alcohol during the study visit (Table 1).
TABLE 1.
Characteristics of study participants and alcohol‐related variables.
| Characteristic | Control group | Surgery group | P value |
|---|---|---|---|
| No. of participants | 14 | 18 | NA |
| Age, mean (SD), yr | 40.8 (9.1) | 40.5 (10.5) | 0.93 |
| Race | |||
| White, n (%) | 13 (93) | 17 (94) | 0.69 |
| Black/African American, n (%) | 0 (0) | 1 (6) | |
| Asian/Asian American, n (%) | 1 (7) | 0 (0) | |
| Weight, mean (SD), kg | 91.4 (19.2) | 88.2 (12.4) | 0.57 |
| BMI, mean (SD), kg/m2 | 33.3 (5.5) | 32.7 (4.7) | 0.75 |
| FFM, mean (SD), kg | 49.0 (8.7) | 49.2 (5.6) | 0.92 |
| Time from surgery, mean (SD), yr | NA | 1.5 (0.9) | NA |
| Fasting plasma concentrations | |||
| Glucose, mean (SD), mmol/L | 5.1 (0.3) | 4.7 (0.3) | 0.002 |
| Insulin, median (IQR), pmol/L | 85.9 (53.5) | 37.3 (30.2) | 0.004 |
| C‐peptide, mean (SD), nmol/L | 0.9 (0.3) | 0.8 (0.2) | 0.20 |
| GLP‐1, median (IQR), pmol/L | 5.5 (4.6) | 13.0 (11.8) | 0.01 |
| Ghrelin, mean (SD), pg/mL | 128.4 (32.7) | 88.1 (58.0) | 0.03 |
| HOMA‐IR2, median (IQR) | 1.6 (0.9) | 0.7 (0.6) | 0.004 |
| Alcohol‐related variables | |||
| Age | |||
| Onset of alcohol drinking, mean (SD), yr | 16.4 (2.8) | 16.1 (4.2) | 0.81 |
| Regular drinking began, median (IQR), yr | 19.0 (4.0) | 19.5 (3.0) | 0.78 |
| Drinking over the past 6 months | |||
| No. of drinking days per month, median (IQR) | 2.1 (2.0) | 1.7 (1.3) | 0.19 |
| No. of alcohol drinks per drinking day, median (IQR) | 2.0 (1.0) | 1.0 (1.0) | 0.06 |
| Classical alcohol pharmacokinetics | |||
| No. of participants | 14 | 14 a | NA |
| Peak BAC, mean (SD), g/L | 0.75 (0.16) | 0.99 (0.20) | 0.002 |
| Time to reach peak BAC, mean (SD), h b | 0.5 (0.2) | 0.3 (0.1) | <0.001 |
| Area under the BAC time curve, mean (SD), g/L × h0–3 | 1.04 (0.21) | 1.20 (0.22) | 0.06 |
| Alcohol elimination measures | |||
| Disappearance rate, β60, mean (SD), g/L × h0–3 | 0.19 (0.04) | 0.17 (0.05) | 0.35 |
| Total eliminated, b60, mean (SD), g/h | 8.9 (2.3) | 7.8 (2.1) | 0.20 |
| Elimination rate, R, mean (SD), g/kg of body weight /h | 0.10 (0.02) | 0.09 (0.03) | 0.45 |
| No. of standard drinks given on alcohol challenge test, mean (SD) | 1.7 (0.3) | 1.7 (0.2) | 0.89 |
Note: Conversions to SI units have been made in this table as follows: glucose mg/dL × 0.0555 = mmol/L, insulin μIU/mL × 6.945 = pmol/L, C‐peptide ng/mL × 0.331 = nmol/L. Values in bold indicate significant differences between groups.
Abbreviations: BAC, blood alcohol concentration; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); FFM, fat‐free mass; HOMA‐IR2, Homeostatic Model Assessment‐Insulin Resistance 2; NA, not applicable.
Pharmacokinetics data from four participants was excluded due to problems with the arterialized technique.
From the time of the first sip of alcohol drink consumed over 10 min.
3.2. Effects of the oral alcohol challenge test on plasma GLP‐1 concentrations
Overall, the surgery group had higher GLP‐1 concentrations than the control group, and compared with the placebo drink, the alcohol drink reduced GLP‐1 concentrations in both groups (Figure 2 and Table 2). Because of the different GLP‐1 baseline values between groups, we calculated the % alcohol‐associated decrease in total GLP‐1 AUC for each participant with respect to their placebo day (i.e. delta difference; Figure 2D). Alcohol similarly reduced GLP‐1 AUC versus placebo by ~34% in both groups (mean difference in delta GLP‐1 AUC between groups = 9.1 pmol/L × h(0–3), 95% CI, −5.2–23.4 pmol/L × h(0–3); P = 0.19).
FIGURE 2.
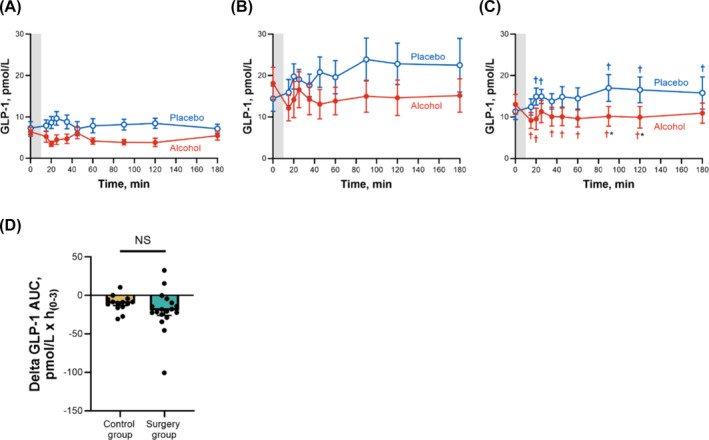
Differences in plasma GLP‐1 concentrations for control and surgery groups after ingestion of alcohol and placebo drinks. Effect of ingesting an alcohol drink (0.5 g/kg fat‐free mass; ~1.7 standard drinks; red closed symbols) compared with drinking a non‐alcohol version of the same drink sprayed with 2 ml of alcohol (placebo; blue open symbols) over 10 min (grey bar) on plasma GLP‐1 (A–C) for the control group (A; n = 14), for the surgery group (B; n = 18) and for both groups averaged (C; n = 32). Data in D represent the difference in AUCs within conditions (alcohol minus placebo) for each participant between groups for plasma GLP‐1. Plasma GLP‐1 concentrations were analysed using separate general linear mixed model (PROC MIXED) analyses. Condition (placebo and alcohol), time and group (control and surgery), as well as all interactions, were included in the model and treated as fixed effects, and subject was included as random effect. Post hoc Fisher's least significant difference was used when interactions were statistically significant. Main effect of group: F (1,30) = 11.62, P = 0.002; time × condition: F (9,270) = 2.72, P = 0.005 (C); time × condition × group: F (9,270) = 1.77, P = 0.07 (A and B, no post hoc shown because no significant triple interaction). Individual differences in GLP‐1 AUCs between alcohol and placebo conditions (delta GLP‐1 AUC) were analysed using a two‐sample independent t‐test. t (23) = 1.32, P = 0.20. Data are presented as mean values ± SEM, and D includes individual data points within each group. *Signifies difference from placebo at P < 0.05. †Signifies difference from baseline value within each condition (blue symbol for placebo and red symbol for alcohol) at P < 0.05.
TABLE 2.
Total AUCs for GLP‐1, ghrelin, glucose, insulin, and C‐peptide and differences between groups (control and surgery) and within conditions (alcohol and placebo drinks).
| Control group | Surgery group | Difference between control and surgery group (mean, 95% CI) | Difference within alcohol and placebo drink (mean, 95% CI) | |||
|---|---|---|---|---|---|---|
| AUC placebo (mean ± SEM) | AUC alcohol (mean ± SEM) | AUC placebo (mean ± SEM) | AUC alcohol (mean ± SEM) | |||
| GLP‐1 total AUC, pmol/L × h(0–3) | 24.2 ± 3.1 | 13.8 ± 2.4 | 63.4 ± 13.0 | 43.9 ± 11.1 | −34.7 (−59.6, −9.8) | −14.9 (−22.7, −7.2) |
| Ghrelin total AUC, pg/mL × h(0–3) | 411.6 ± 32.8* | 300.3 ± 12.0 | 260.6 ± 34.2 | 226.6 ± 23.8 | 112.3 (36.4, 188.3) | −72.6 (−102.2, −43.0) |
| Glucose total AUC, mmol/L × h(0–3) | 14.7 ± 0.2 | 14.0 ± 0.3 | 13.8 ± 0.2 | 13.3 ± 0.2 | 0.8 (0.1, 1.4) | −0.6 (−0.8, −0.4) |
| Insulin total AUC, pmol/L × h(0–3) | 242.4 ± 34.7 | 263.4 ± 33.1 | 140.4 ± 19.7 | 131.3 ± 15.7 | 117.1 (39.8, 194.3) | 5.9 (−14.0, 25.9) |
| C‐peptide total AUC, nmol/L × h(0–3) | 2.5 ± 0.2 | 2.5 ± 0.2 | 2.2 ± 0.2 | 2.0 ± 0.2 | 0.3 (−0.2, 0.9) | −0.1 (−0.2, 0.1) |
Note: Conversions to SI units have been made in Table 2 as follows: glucose mg/dL × 0.0555 = mmol/L, insulin μIU/mL × 6.945 = pmol/L, C‐peptide ng/mL × 0.331 = nmol/L. AUCs were analysed using separate general linear mixed model (PROC MIXED) analyses. Condition (placebo and alcohol), group (control and surgery) and their interaction were included in the model and treated as fixed effects, and the subject was included as a random effect. Post hoc Fisher's least significant difference was used when interaction was statistically significant. GLP‐1: Group, F (1,30) = 6.68, P = 0.01 condition, F (1,30) = 15.45, P = 0.0005; group × condition, F (1,30) = 1.44, P = 0.24, group × condition; ghrelin, F (1,30) = 9.13, P = 0.005, group; F (1,30) = 25.09, P < 0.0001, condition; F (1,30) = 7.11, P = 0.01; glucose, group, F (1,30) = 5.43, P = 0.03 (this main effect did not pass FDR); condition, F (1,30) = 30.68, P < 0.0001; group × condition, F (1,30) = 1.13, P = 0.30; insulin, F (1,30) = 11.41, P = 0.002, group; F (1,30) = 0.37, P = 0.55, condition; F (1,30) = 2.37, P = 0.13, group × condition; C‐peptide, F (1,30) = 1.94, P = 0.17, group; F (1,30) = 1.31, P = 0.26, condition. F (1,30) = 1.21, P = 0.28, group × condition.
Abbreviation: AUC, total area under the curve.
Signifies difference from all ghrelin means at P < 0.05.
3.3. Effects of the oral alcohol challenge test on plasma ghrelin concentrations
In contrast to GLP‐1, the surgery group had lower overall ghrelin concentrations than the control group. Compared with the placebo drink, the alcohol drink reduced ghrelin concentrations in both groups (Figure 3 and Table 2). However, the reduction in ghrelin AUC was smaller in the surgery than in the control group (mean difference in delta ghrelin AUC between groups = −77.3 pg/mL × h(0–3), 95% CI, −142.3 to −12.3 pg/mL × h(0–3); P = 0.02; Figure 3). Compared with placebo, alcohol reduced ghrelin by 27% (95% CI, 12%–42%) in the control group and by 13% (95% CI, 2%–24%) in the surgery group.
FIGURE 3.
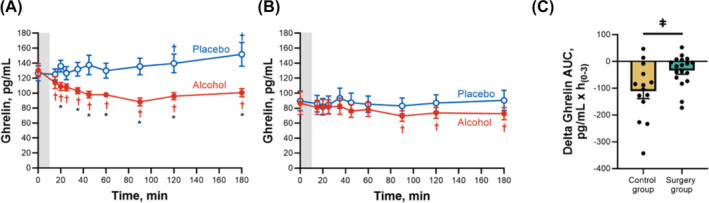
Differences in plasma ghrelin concentrations for control and surgery groups after ingestion of alcohol and placebo drinks. Effect of ingesting an alcohol drink (0.5 g/kg fat‐free mass; ~1.7 standard drinks; red closed symbols) compared with drinking a non‐alcohol version of the same drink sprayed with 2 mL of alcohol (placebo; blue open symbols) over 10 min (grey bar) on plasma ghrelin (A,B) for the control group (A; n = 14) and the surgery group (B; n = 18). Data in C represent the difference in AUCs within conditions (alcohol minus placebo) for each participant between groups for plasma ghrelin. Plasma ghrelin concentrations were analysed using separate general linear mixed model (PROC MIXED) analyses. Condition (placebo and alcohol), time and group (control and surgery), as well as all interactions, were included in the model and treated as fixed effects, and subject was included as random effect. Post hoc Fisher's least significant difference was used when interactions were statistically significant. Time × condition × group F (9,270) = 2.85, P = 0.003. Individual differences in ghrelin AUCs within alcohol and placebo conditions (delta ghrelin AUC) were analysed using a two‐sample independent t‐test. t (19) = − 2.49, P = 0.02. Data are presented as mean values ±SEM, and C includes individual data points within each group. *Signifies difference from placebo at P < 0.05. †Signifies difference from baseline value within each condition (blue symbol for placebo and red symbol for alcohol) at P < 0.05. ǂSignifies a difference between groups at P < 0.05.
3.4. Effects of the oral alcohol challenge test on plasma glucose, insulin and C‐peptide concentrations
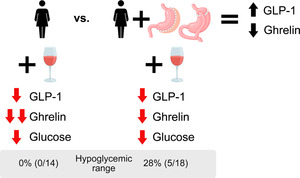
On average, compared with the control group, the surgery group had lower glucose and insulin concentrations across time and smaller glucose and insulin AUCs (Figure 4 and Table 2). C‐peptide concentrations were also lower in the surgery than in the control group, but only transiently (25–45 min) (Figure 4), and C‐peptide AUCs were similar between groups (Table 2). Compared with placebo, alcohol ingestion decreased plasma glucose in both groups (Figure 4). However, alcohol ingestion had a biphasic effect on glucose in the surgery group, manifested by an early increase and subsequent decrease in plasma glucose concentrations (Figure 4). All in all, alcohol slightly reduced the glucose AUC similarly in both groups (mean difference in glucose AUC between placebo and alcohol drink for both groups averaged = −0.59 mmol/L × h(0–3), 95% CI, −0.81 to −0.37 mmol/L × h(0–3); Table 2 and Figure 4C). However, 28% (5/18) of the women in the surgery group but none (0/14) in the control group reached plasma glucose in the hypoglycaemic range (≤3.9 mmol/L) 3 hours post‐alcohol consumption (Figure S1). The effect was specific to alcohol because none of the women in either group reached plasma glucose concentrations ≤3.9 mmol/L after consumption of the placebo drink (Figure S1). Of note, none of the participants required a medical intervention because of their hypoglycaemic levels.
FIGURE 4.
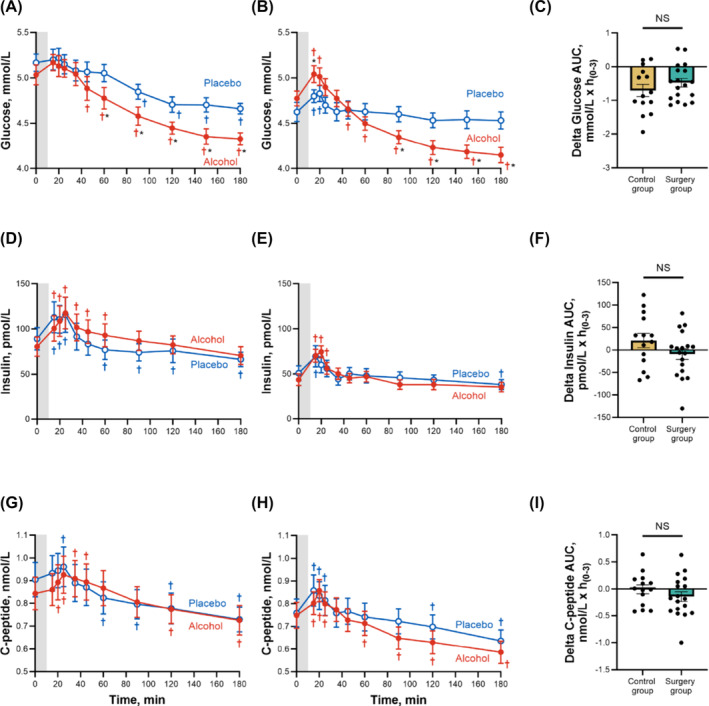
Differences in plasma glucose, insulin and C‐peptide concentrations for control and surgery groups after ingestion of alcohol and placebo drinks. Effect of ingesting an alcoholic drink (0.5 g/kg fat‐free mass; ~1.7 standard drinks; red closed symbols) compared with drinking a non‐alcoholic version of the same drink sprayed with 2 mL of alcohol (placebo; blue open symbols) over 10 min (grey bar) on plasma glucose (A,B), insulin (D,E) and C‐peptide (G,H) for the control group (left panel; n = 14) and the surgery group (middle panel; n = 18). Data in C, F and I (right panel) represent the difference in AUCs within conditions (alcohol minus placebo) for each participant between groups for plasma glucose I, insulin (F) and C‐peptide (I). Plasma glucose, insulin and C‐peptide concentrations were analysed using separate general linear mixed model (PROC MIXED) analyses. Condition (placebo and alcohol), time and group (control and surgery), as well as all interactions, were included in the model and treated as fixed effects, and subject was included as random effect. Post hoc Fisher's least significant difference was used when interactions were statistically significant. Glucose: time × condition × group, F (10,300) = 2.32, P = 0.01; insulin: time × condition × group, F (9,270) = 4.59, P < 0.0001; C‐peptide: time × condition × group, F (9,270) = 6.29, P < 0.0001. Individual differences in glucose, insulin and C‐peptide AUCs within alcohol and placebo conditions (delta glucose AUC, delta insulin AUC and delta C‐peptide AUC) were analysed using separate two‐sample independent t‐tests. Glucose: t (30) = − 1.07, P = 0.29; insulin: t (30) = 1.54, P = 0.13; C‐peptide: t (30) = 1.07, P = 0.29. Data are presented as mean values ± SEM, and C, F and I include individual data points within each group. *Signifies difference from placebo at P < 0.05. †Signifies difference from baseline value within each condition (blue symbol for placebo and red symbol for alcohol) at P < 0.05. Conversions to SI units are as follows: glucose mg/dL × 0.0555 = glucose in mmol/L, insulin μIU/mL × 6.945 = insulin in pmol/L, C‐peptide ng/mL × 0.331 = C‐peptide in nmol/L.
The consumption of a drink (either alcohol or placebo) triggered a small but significant transient increase in insulin and C‐peptide in both groups (Figure 4). However, alcohol caused a more sustained increase in insulin and C‐peptide than placebo, and both insulin and C‐peptide concentrations returned to baseline levels sooner in the surgery than in the control group (Figure 4). Compared to placebo, alcohol did not significantly change total insulin or C‐peptide AUCs in either group (Table 2).
4. DISCUSSION
There is a high interest in GLP‐1‐based therapies, currently a powerhouse for weight loss and type 2 diabetes treatment, for their potential to decrease alcohol consumption. 1 , 2 , 3 , 4 However, to better evaluate the suitability of GLP‐1‐based therapies for AUD, it is also essential to understand the relationship between alcohol exposure and the GLP‐1 system, including how alcohol, a potential hypoglycaemic agent, affects endogenous GLP‐1 responses. This study provides compelling evidence that alcohol consumption decreases endogenous GLP‐1 in women who drink moderately, regardless of whether they have undergone metabolic surgery. Not only do our findings confirm previous results from non‐treatment‐seeking AUD patients, 11 but they also significantly extend and provide novel information by (1) using a placebo‐control design, which allows us to differentiate the effects of alcohol consumption on GLP‐1 (and on other peptides) from endocrine responses that may naturally vary over time, and (2) comparing people with versus without history of metabolic surgery.
Despite women in the surgery group achieving a higher peak BAC when consuming the same alcohol dose as women in the control group, both groups experienced a similar reduction of ~34% in plasma GLP‐1 in response to alcohol. Although we did not test different doses of alcohol in this study, it is possible that the alcohol dose tested in the control group was already sufficient to reach a plateau effect in reducing GLP‐1 concentrations; hence, the higher alcohol exposure in the surgery group did not lead to further reductions in the peptide levels.
The effect of alcohol on endogenous GLP‐1 was unclear before our current study, as the few previous studies that had investigated such an effect have had limitations on their study design, and their conflicting results have further added to the uncertainty. 11 , 37 , 38 For example, one study showed a reduction in endogenous GLP‐1 after both oral and intravenous alcohol administration, 11 while the other reported no change. 38 Notably, in the former study, 11 alcohol exposure was confounded with food consumption, which affects GLP‐1 responses, and none of these two studies included a placebo control. Our data emphasize that the decline in GLP‐1 induced by alcohol is only apparent when comparing GLP‐1 concentrations on the alcohol consumption day with that on the placebo day. GLP‐1 differences from baseline levels within the alcohol condition were subtle, suggesting that acute alcohol consumption decreases the tonic release of GLP‐1 during fasting.
In addition to GLP‐1, we also measured ghrelin levels. Here, alcohol had a more pronounced effect on lowering ghrelin concentrations in the control group compared to the surgery group. This observed disparity in ghrelin response might be attributed to floor effects, as women in the surgery group, as expected, had lower basal ghrelin concentrations (along with elevated GLP‐1 levels) than women in the control group due to surgical alterations in the gastrointestinal system.
Our findings on ghrelin were expected, as several studies have documented that acute alcohol exposure lowers plasma ghrelin concentrations in people with or without AUD. 33 , 34 , 35 , 37 , 39 , 40 , 41 Although it has been hypothesized that this effect may be mediated, at least in part, by alcohol calories, the exact mechanisms of how alcohol acutely lowers ghrelin levels are unclear. Indeed, a recent set of ex vivo, rodent and human studies confirm alcohol's acute effects in reducing plasma ghrelin concentrations but not in proportion to alcohol's caloric value or through direct interaction with ghrelin‐secreting gastric mucosal cells of its receptor. 42
It is widely recognized that alcohol consumption before a meal, and even during a meal, leads to increased food intake in the short term, a phenomenon known as the apéritif's effect. 39 , 43 , 44 Despite its well‐known occurrence, the mechanism underlying such an effect has remained elusive. Our findings suggest that one of the potential mechanisms for the apéritif's effect is attributable to decreased satiation resulting from alcohol's reduction of endogenous GLP‐1. In fact, previous research indicates that alcohol ingestion before a meal does not increase self‐reported hunger but results in delayed satiation. 43 , 44 Supporting this theory, findings from clinical studies that focused on the effects of alcohol consumption on hormonal responses to mixed meals suggest that alcohol suppresses or delays the secretion of incretins early in the postprandial phase in both participants with type 2 diabetes 45 and healthy individuals. 46 Additionally, a neuroimaging study using intravenous alcohol before a meal confirmed the apéritif effect and revealed heightened hypothalamic responses to food aromas compared to non‐food aromas. The hypothalamus, crucial for feeding behaviour, has a high GLP‐1 receptor expression. 47
Despite decreasing GLP‐1, alcohol transiently and modestly increased insulin secretion and decreased plasma total glucose AUC in both groups. However, consistent with earlier findings in our laboratory, when studying women who underwent RYGB, 48 we observed a distinct effect of alcohol consumption post‐surgery, characterized by an initial rise followed by a subsequent decrease in plasma glucose concentration in this predominantly post‐SG women sample. The mechanism underlying the transient increase in plasma glucose observed after women drank alcohol post‐surgery is unknown. However, it might be caused by the impact of the sharp peak BAC achieved after surgery on liver metabolism. Supporting this hypothesis, a recent study in men whose BAC resembled those of our surgery group (they reached a BAC of ~1.0 g/L within 15 min of receiving alcohol as an intragastric bolus infusion) also found that plasma glucose initially increased by ~0.4 mmol/L above baseline followed by a gradual decline. 38 While acute alcohol consumption is typically associated with hypoglycaemic effects through decreased gluconeogenesis, 49 data from rodent models revealed that when directly infused into the liver, alcohol increases plasma glucose by decreased glycogenesis and increased glycogenolysis. 50 , 51 Our current study lacked glucose tracers; however, a previous study from our laboratory that did include glucose tracers revealed a biphasic effect of alcohol on plasma glucose of women assessed post‐RYGB surgery that was characterized by an initial increase followed by a decrease in endogenous glucose production rate. 48 Suggesting that metabolic surgery increases the risk of experiencing alcohol‐induced hypoglycaemia, 28% of women in the surgery group experienced plasma glucose in the hypoglycaemic range following alcohol consumption, contrasting with none in the control group.
The reason why women in the surgery group were at higher risk for alcohol‐induced hypoglycaemia, despite returning sooner toward insulin and C‐peptide baseline concentrations after ingesting alcohol than those in the control group, is unclear. However, we hypothesized that surgery‐associated improvements in insulin sensitivity 12 combined with the inhibitory effects of alcohol on gluconeogenesis 49 may partially account for it. Supporting this notion, although the groups were matched in sex, age, BMI and body composition, women in the surgery group exhibited lower HOMA‐IR2 than those in the control group, indicating they were less insulin resistant (i.e. more insulin sensitive).
A limitation of this study is the exclusion of men. Given that the vast majority (~80%) of bariatric patients are women, 24 we focused on women to control for sex‐related variations in alcohol pharmacokinetics. 25 Nevertheless, the previous study, which showed reduced plasma GLP‐1 associated with alcohol exposure in non‐treatment‐seeking patients with AUD, primarily involved men, 11 suggesting that our findings may generalize to other populations. However, additional limitations should be considered, as our findings may not extrapolate to groups of younger age, lower BMI, heavier patterns of alcohol consumption or chronic versus acute effects of alcohol consumption.
In conclusion, this study demonstrates that acute alcohol consumption reduces plasma GLP‐1 concentrations in women irrespective of metabolic surgery status. Furthermore, this study aligns with findings from several studies that show that despite alcohol's appetite‐stimulating effects, it acutely reduces plasma ghrelin and suggests that such apéritif's effect may result from impaired satiation. Additionally, our findings highlight an increased risk for alcohol‐associated hypoglycaemia post‐metabolic surgery that is not driven by GLP‐1 or an insulinogenic effect.
AUTHOR CONTRIBUTIONS
Conceptualization and study design: Marta Yanina Pepino. Conduction of experiment: Neda Seyedsadjadi and Danisa Nieto. Analysis of the data: Mariel Molina‐Castro, Neda Seyedsadjadi and Marta Yanina Pepino. Writing—original draft: Mariel Molina‐Castro and Marta Yanina Pepino. Writing—review and editing: Mariel Molina‐Castro, Neda Seyedsadjadi, Lorenzo Leggio, Blair Rowitz, Danisa Nieto and Marta Yanina Pepino.
CONFLICT OF INTEREST STATEMENT
All authors declare no conflicts of interest. Lorenzo Leggio is a federal employee at the NIH, and he is supported jointly by NIDA and NIAAA intramural research programs.
Supporting information
Figure S1. Individual plasma glucose concentrations for each group (control and surgery) and each condition (placebo and alcohol).
ACKNOWLEDGEMENTS
We acknowledge the valuable expert technical assistance of Rafael Troconis and Molly Black and the supportive assistance with study coordination of Christine Canfield. We also thank M. Belen Acevedo for helping with participant recruitment and data collection, and we are grateful to the study participants.
Molina‐Castro M, Seyedsadjadi N, Nieto D, Leggio L, Rowitz B, Pepino MY. The glucagon‐like peptide‐1 and other endocrine responses to alcohol ingestion in women with versus without metabolic surgery. Addiction Biology. 2024;29(10):e13441. doi: 10.1111/adb.13441
Funding information This study was supported, in part, by the National Institutes of Health (NIH) grant AA024103.
DATA AVAILABILITY STATEMENT
Some or all datasets generated during and/or analysed during the current study are not publicly available but are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Tufvesson‐Alm M, Shevchouk OT, Jerlhag E. Insight into the role of the gut‐brain axis in alcohol‐related responses: emphasis on GLP‐1, amylin, and ghrelin. Front Psych. 2023;13. doi: 10.3389/fpsyt.2022.1092828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Suchankova P, Yan J, Schwandt ML, et al. The glucagon‐like peptide‐1 receptor as a potential treatment target in alcohol use disorder: evidence from human genetic association studies and a mouse model of alcohol dependence. Transl Psychiatry. 2015;5(6):e583. doi: 10.1038/tp.2015.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Klausen MK, Thomsen M, Wortwein G, Fink‐Jensen A. The role of glucagon‐like peptide 1 (GLP‐1) in addictive disorders. Br J Pharmacol. 2022;179(4):625‐641. doi: 10.1111/bph.15677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vallöf D, MacCioni P, Colombo G, et al. The glucagon‐like peptide 1 receptor agonist liraglutide attenuates the reinforcing properties of alcohol in rodents. Addict Biol. 2016;21(2):422‐437. doi: 10.1111/adb.12295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Van Bloemendaal L, IJzerman RG, Ten Kulve JS, et al. GLP‐1 receptor activation modulates appetite‐ and reward‐related brain areas in humans. Diabetes. 2014;63(12):4186‐4196. doi: 10.2337/db14-0849 [DOI] [PubMed] [Google Scholar]
- 6. Chuong V, Farokhnia M, Khom S, et al. The glucagon‐like peptide‐1 (GLP‐1) analogue semaglutide reduces alcohol drinking and modulates central GABA neurotransmission. JCI Insight. 2023;8(12):e170671. doi: 10.1172/jci.insight.170671DS1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aranäs C, Edvardsson CE, Shevchouk OT, et al. Semaglutide reduces alcohol intake and relapse‐like drinking in male and female rats. EBioMedicine. 2023;93:104642. doi: 10.1016/j.ebiom.2023.104642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wium‐Andersen IK, Wium‐Andersen MK, Fink‐Jensen A, Rungby J, Jørgensen MB, Osler M. Use of GLP‐1 receptor agonists and subsequent risk of alcohol‐related events. A nationwide register‐based cohort and self‐controlled case series study. Basic Clin Pharmacol Toxicol. 2022;131(5):372‐379. doi: 10.1111/bcpt.13776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Klausen MK, Jensen ME, Møller M, et al. Exenatide once weekly for alcohol use disorder investigated in a randomized, placebo‐controlled clinical trial. JCI Insight. 2022;7(19):e159863. doi: 10.1172/jci.insight.159863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Probst L, Monnerat S, Vogt DR, et al. Effects of dulaglutide on alcohol consumption during smoking cessation. JCI Insight. 2023;8(22):e170419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Farokhnia M, Browning BD, Crozier ME, Sun H, Akhlaghi F, Leggio L. The glucagon‐like peptide‐1 system is modulated by acute and chronic alcohol exposure: findings from human laboratory experiments and a post‐mortem brain study. Addict Biol. 2022;27(5):e13211. doi: 10.1111/adb.13211 [DOI] [PubMed] [Google Scholar]
- 12. Hutch CR, Sandoval D. The role of GLP‐1 in the metabolic success of bariatric surgery. Endocrinology. 2017;158(12):4139‐4151. doi: 10.1210/en.2017-00564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. King WC, Chen JY, Mitchell JE, et al. Prevalence of alcohol use disorders before and after bariatric surgery. Jama. 2012;307(23):2516‐2525. doi: 10.1001/jama.2012.6147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Östlund MP, Backman O, Marsk R, et al. Increased admission for alcohol dependence after gastric bypass surgery compared with restrictive bariatric surgery. JAMA Surg. 2013;148(4):374‐377. doi: 10.1001/jamasurg.2013.700 [DOI] [PubMed] [Google Scholar]
- 15. Ibrahim N, Alameddine M, Brennan J, Sessine M, Holliday C, Ghaferi AA. New onset alcohol use disorder following bariatric surgery. Surg Endosc. 2019;33(8):2521‐2530. doi: 10.1007/s00464-018-6545-x [DOI] [PubMed] [Google Scholar]
- 16. Maciejewski ML, Smith VA, Berkowitz TSZ, et al. Association of bariatric surgical procedures with changes in unhealthy alcohol use among US veterans. JAMA Netw Open. 2020;3(12):e2028117. doi: 10.1001/jamanetworkopen.2020.28117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Conason A, Teixeira J, Hsu CH, Puma L, Knafo D, Geliebter A. Substance use following bariatric weight loss surgery. JAMA Surg. 2013;148(2):145‐150. doi: 10.1001/2013.jamasurg.265 [DOI] [PubMed] [Google Scholar]
- 18. Kenkre JS, Gesell S, Keller A, Milani RM, Scholtz S, Barley EA. Alcohol misuse post metabolic and bariatric surgery: a systematic review of longer‐term studies with focus on new onset alcohol use disorder and differences between surgery types. Curr Obes Rep. 2024;13(3):596‐616. doi: 10.1007/s13679-024-00577-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wee CC, Mukamal KJ, Huskey KW, et al. High risk alcohol use after weight loss surgery. Surg Obes Relat Dis. 2014;10(3):508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Davis JF, Schurdak JD, Magrisso IJ, et al. Gastric bypass surgery attenuates ethanol consumption in ethanol‐preferring rats. Biol Psychiatry. 2012;72(5):354‐360. doi: 10.1016/j.biopsych.2012.01.035 [DOI] [PubMed] [Google Scholar]
- 21. Acevedo MB, Eagon JC, Bartholow BD, Klein S, Bucholz KK, Pepino MY. Sleeve gastrectomy surgery: when 2 alcoholic drinks are converted to 4. Surg Obes Relat Dis. 2018;14(3):277‐283. doi: 10.1016/j.soard.2017.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Acevedo MB, Teran‐Garcia M, Bucholz KK, et al. Alcohol sensitivity in women after undergoing bariatric surgery: a cross‐sectional study. Surg Obes Relat Dis. 2020;16(4):536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Seyedsadjadi N, Acevedo MB, Alfaro R, et al. Site of alcohol first‐pass metabolism among women. JAMA Netw Open. 2022;5(3):e223711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fuchs HF, Broderick RC, Harnsberger CR, et al. Benefits of bariatric surgery do not reach obese men. J Laparoendosc Adv Surg Tech a. 2015;25(3):196‐201. doi: 10.1089/lap.2014.0639 [DOI] [PubMed] [Google Scholar]
- 25. Baraona E, Abittan CS, Dohmen K, et al. Gender differences in pharmacokinetics of alcohol. Alcohol Clin Exp Res. 2001;25(4):502‐507. doi: 10.1111/j.1530-0277.2001.tb02242.x [DOI] [PubMed] [Google Scholar]
- 26. Bucholz KK, Cadoret R, Cloninger CR, et al. A new, semi‐structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55(2):149‐158. doi: 10.15288/jsa.1994.55.149 [DOI] [PubMed] [Google Scholar]
- 27. Seyedsadjadi N, Ramchandani VA, Plawecki MH, et al. Fat‐free mass accounts for most of the variance in alcohol elimination rate in women. Alcohol Clin Exp Res. 2023;47(5):848‐855. doi: 10.1111/acer.15047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Perelló M, Dickson SL, Zigman JM, Leggio L. Toward a consensus nomenclature for ghrelin, its non‐acylated form, liver expressed antimicrobial peptide 2 and growth hormone secretagogue receptor. J Neuroendocrinol. 2023;35(1):e13224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Allison DB, Paultre F, Maggio C, Mezzitis N, Pi‐Sunyer FX. The use of areas under curves in diabetes research. Diabetes Care. 1995;18(2):245‐250. doi: 10.2337/diacare.18.2.245 [DOI] [PubMed] [Google Scholar]
- 30. Pepino MY, Steinmeyer AL, Mennella JA. Lactational state modifies alcohol pharmacokinetics in women. Alcohol Clin Exp Res. 2007;31(6):909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Watson PE, Watson ID, Batt RD. Total body water volumes for adult males and females estimated from simple anthropometric measurements. Am J Clin Nutr. 1980;33(1):27‐39. doi: 10.1093/ajcn/33.1.27 [DOI] [PubMed] [Google Scholar]
- 32. Klockhoff H, Näslund I, Jones AW. Faster absorption of ethanol and higher peak concentration in women after gastric bypass surgery. Br J Clin Pharmacol. 2002;54(6):587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Calissendorff J, Danielsson O, Brismar K, Röjdmark S. Alcohol ingestion does not affect serum levels of peptide YY but decreases both total and octanoylated ghrelin levels in healthy subjects. Metabolism. 2006;55(12):1625‐1629. doi: 10.1016/j.metabol.2006.08.003 [DOI] [PubMed] [Google Scholar]
- 34. Calissendorff J, Danielson O, Brismar K, Röjdmark S. Inhibitory effect of alcohol on ghrelin secretion in normal man. Eur J Endocrinol. 2005;152(5):743‐747. doi: 10.1530/eje.1.01905 [DOI] [PubMed] [Google Scholar]
- 35. Zimmermann US, Buchmann A, Steffin B, Dieterle C, Uhr M. Alcohol administration acutely inhibits ghrelin secretion in an experiment involving psychosocial stress. Addict Biol. 2007;12(1):17‐21. doi: 10.1111/j.1369-1600.2006.00026.x [DOI] [PubMed] [Google Scholar]
- 36. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B Methodol. 1995;57(1):289‐300. doi: 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 37. Calissendorff J, Gustafsson T, Holst JJ, Brismar K, Röjdmark S. Alcohol intake and its effect on some appetite‐regulating hormones in man: influence of gastroprotection with sucralfate. Endocr Res. 2012;37(3):154‐162. doi: 10.3109/07435800.2012.662662 [DOI] [PubMed] [Google Scholar]
- 38. Lanng AR, Gasbjerg LS, Bergmann NC, et al. Gluco‐metabolic effects of oral and intravenous alcohol administration in men. Endocr Connect. 2019;8(10):1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Eiler WJA, Džemidžic M, Case KR, et al. The apéritif effect: Alcohol's effects on the brain's response to food aromas in women. Obesity (Silver Spring). 2015;23(7):1386‐1393. doi: 10.1002/oby.21109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Leggio L, Schwandt ML, Oot EN, Dias AA, Ramchandani VA. Fasting‐induced increase in plasma ghrelin is blunted by intravenous alcohol administration: a within‐subject placebo‐controlled study. Psychoneuroendocrinology. 2013;38(12):3085‐3091. doi: 10.1016/j.psyneuen.2013.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ralevski E, Horvath TL, Shanabrough M, Hayden R, Newcomb J, Petrakis I. Ghrelin is Supressed by intravenous alcohol and is related to stimulant and sedative effects of alcohol. Alcohol Alcohol. 2017;52(4):431‐438. doi: 10.1093/alcalc/agx022 [DOI] [PubMed] [Google Scholar]
- 42. Deschaine SL, Farokhnia M, Gregory‐Flores A, et al. A closer look at alcohol‐induced changes in the ghrelin system: novel insights from preclinical and clinical data. Addict Biol. 2022;27(1):e13033. doi: 10.1111/adb.13033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yeomans MR. Effects of alcohol on food and energy intake in human subjects: evidence for passive and active over‐consumption of energy. Br J Nutr. 2004;92 Suppl 1(S1):S31‐S34. doi: 10.1079/BJN20041139 [DOI] [PubMed] [Google Scholar]
- 44. Westerterp‐Plantenga MS, Verwegen CRT. The appetizing effect of an apéritif in overweight and normal‐weight humans. Am J Clin Nutr. 1999;69(2):205‐212. doi: 10.1093/ajcn/69.2.205 [DOI] [PubMed] [Google Scholar]
- 45. Dalgaard M, Thomsen C, Rasmussen BM, Holst JJ, Hermansen K. Ethanol with a mixed meal decreases the incretin levels early postprandially and increases postprandial lipemia in type 2 diabetic patients. Metabolism. 2004;53(1):77‐83. doi: 10.1016/j.metabol.2003.08.011 [DOI] [PubMed] [Google Scholar]
- 46. Raben A, Agerholm‐Larsen L, Flint A, Holst JJ, Astrup A. Meals with similar energy densities but rich in protein, fat, carbohydrate, or alcohol have different effects on energy expenditure and substrate metabolism but not on appetite and energy intake. Am J Clin Nutr. 2003;77(1):91‐100. doi: 10.1093/ajcn/77.1.91 [DOI] [PubMed] [Google Scholar]
- 47. Alvarez E, Martínez MD, Roncero I, et al. The expression of GLP‐1 receptor mRNA and protein allows the effect of GLP‐1 on glucose metabolism in the human hypothalamus and brainstem. J Neurochem. 2005;92(4):798‐806. doi: 10.1111/j.1471-4159.2004.02914.x [DOI] [PubMed] [Google Scholar]
- 48. Acevedo MB, Ferrando R, Patterson BW, Eagon JC, Klein S, Pepino MY. Effect of alcohol ingestion on plasma glucose kinetics after roux‐en‐Y gastric bypass surgery. Surg Obes Relat Dis. 2019;15(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Siler SQ, Neese RA, Christiansen MP, Hellerstein MK. The inhibition of gluconeogenesis following alcohol in humans. Am J Physiol. 1998;275(5):E897‐E907. [DOI] [PubMed] [Google Scholar]
- 50. Mokuda O, Tanaka H, Hayashi T, Ooka H, Okazaki R, Sakamoto Y. Ethanol stimulates glycogenolysis and inhibits both glycogenesis via gluconeogenesis and from exogenous glucose in perfused rat liver. Ann Nutr Metab. 2004;48(4):276‐280. doi: 10.1159/000080463 [DOI] [PubMed] [Google Scholar]
- 51. Kubota M, Virkamäki A, Yki‐Järvinen H. Ethanol stimulates glycogenolysis in livers from fed rats. Proc Soc Exp Biol Med. 1992;201(1):114‐118. doi: 10.3181/00379727-201-43488 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Individual plasma glucose concentrations for each group (control and surgery) and each condition (placebo and alcohol).
Data Availability Statement
Some or all datasets generated during and/or analysed during the current study are not publicly available but are available from the corresponding author upon reasonable request.


