Abstract
Short-chain enoyl-CoA hydratase, encoded by ECHS1, plays a major role in the valine catabolic pathway and mitochondrial fatty acid β-oxidation. Deficiency of this enzyme causes Leigh syndrome. Herein, we report a case of ECHS1-related Leigh syndrome with a prominent ketone body spectrum on magnetic resonance spectroscopy during acute exacerbation. A 6-month-old boy with mild motor developmental delay presented with disturbances of consciousness and hypercapnia without prior infection or feeding failure. Upon admission, investigations revealed prominent ketosis and elevated 2,3-dihydroxy-2-methylbutyric acid excretion. Brain magnetic resonance imaging revealed symmetrical T2 prolongation with restricted diffusion in the basal ganglia. Magnetic resonance spectroscopy showed a prominent ketone body spectrum in the cerebral white matter, and prominent ketone bodies, elevated lactate and markedly decreased N-acetylaspartate levels in the basal ganglia. Genetic analysis identified compound heterozygous variants of ECHS1. Short-chain enoyl-CoA hydratase deficiency is a disease for which a valine-restricted diet is reported to be beneficial, and early diagnosis is desirable. Severe ketosis and the ketone body magnetic resonance spectroscopy spectrum during acute exacerbation may aid in the diagnosis of this disease.
Keywords: ECHS1, Ketosis, Magnetic resonance spectroscopy, Leigh syndrome, Metabolic encephalopathies
Introduction
Short-chain enoyl-CoA hydratase (SCEH), encoded by ECHS1 (MIM*602292), plays a major role in valine catabolism and mitochondrial fatty acid β-oxidation. SCEH deficiency caused by ECHS1 variants leads to Leigh syndrome with acute episodes of encephalopathy, neurological deterioration, severe developmental delay, movement disorders, and epilepsy [1,2]. Leigh syndrome is typically associated with defective oxidative phosphorylation due to mitochondrial or nuclear gene mutations, and its clinical manifestations differ from those of other fatty acid β-oxidation disorders [3,4]. Although hyperintensities on T2-weighted images in the bilateral basal ganglia and elevated lactate levels on magnetic resonance spectroscopy (MRS) are common in SCEH deficiency, as in Leigh syndrome [1], specific radiologic findings have not been reported. In SCEH deficiency, as accumulation of toxic intermediates from valine catabolism induces neurodegeneration, some patients benefit from a valine-restricted diet, and prompt diagnosis is desirable to initiate effective early treatment [5,6]. Herein, we report a case of SCEH deficiency with acute episodes of encephalopathy and neurological deterioration, in which MRS revealed a prominent ketone body spectrum that may be key for diagnosis.
Case presentation
A 6-month-old boy, born to healthy, nonconsanguineous parents with no reported perinatal problems, was hospitalized with disturbed consciousness and hypercapnia without prior infection or feeding failure. This patient was intubated and required ventilatory management for 5 days after admission. The patient's weight (6.8 kg, -1.4SD), height (65 cm, -1.2SD), and head circumference (44 cm, +0.5SD) at 6 months were within the normal range. Mild motor developmental delays had been observed during infancy. The patient had held his head up at 4 months while not yet rolling over and sitting up independently.
Biochemical analyses upon admission indicated prominent ketosis (blood pH 6.9, total ketone bodies, 15,200 µmol/L) and slightly elevated serum lactate levels (24 mg/dL) without hypoglycemia (blood glucose, 77 mg/dL). Urinary organic acid profiling revealed elevated excretion of 2,3-dihydroxy-2-methylbutyric acid. Although the newborn hearing test results were normal, the auditory brainstem response after disease onset showed severe bilateral sensorineural hearing loss. No hepatomegaly, cardiomyopathy, or ocular abnormalities, such as optic atrophy or corneal clouding, were observed.
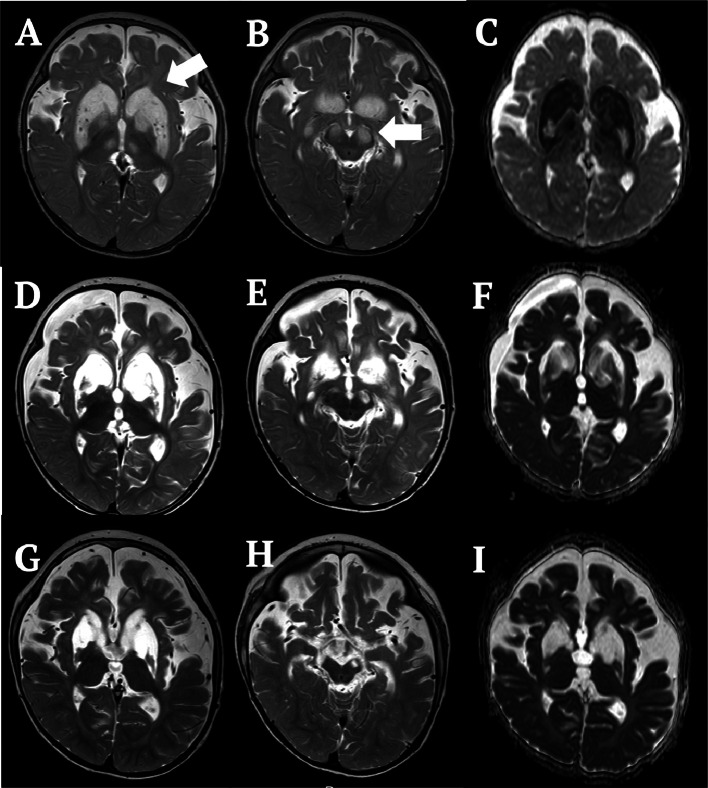
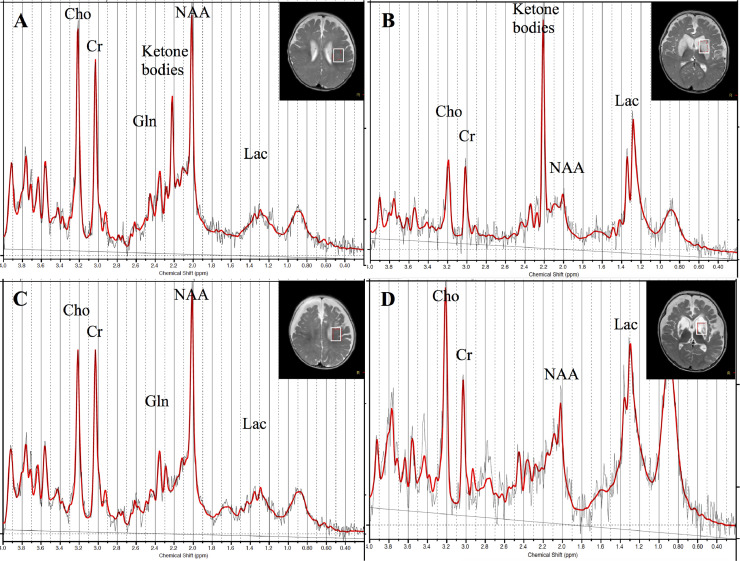
Brain magnetic resonance imaging (MRI) on the third day of admission revealed symmetrical T2 prolongation with restricted diffusion in the caudate nucleus, putamen, globus pallidus, medial thalamus, and substantia nigra (Figs. 1A-C). MRS (point resolved spectroscopy, repetition time/echo time/number of excitations = 5,000/30/32, volume of interest = 15 × 15 × 20 mm3) spectra from the white matter were quantitatively analyzed with LCModel. MRS spectra from the basal ganglia were semi-quantitatively analyzed as ratios with creatine (Cr) to prevent underestimation of the metabolite concentration shown on LCModel due to high proton and prolonged T2 values. Values deviating from mean ± 2SD for age-matched controls were considered abnormal. Brain MRS on the same day revealed a peak at 2.2 ppm in the cerebral white matter (1.37 mM; nil in controls) (Fig. 2A) and in the basal ganglia (ketone bodies/Cr 1.25; nil in controls) (Fig. 2B), indicating the presence of ketone bodies. By this time, serum pH and ketone bodies (200 µmol/L) had normalized. Elevation of glutamine (5.53 mM; age-matched control, 2.10 ± 0.28 mM) and mild reduction of N-acetylaspartate (NAA) (4.12 mM; age-matched control, 6.60 ± 0.50 mM) levels in the white matter (Fig. 2A), and marked elevation of lactate/Cr (2.26; nil in controls) and a decrease in NAA/Cr (0.33; age-matched control, 1.06 ± 0.07) levels in the basal ganglia (Fig. 2B) were also observed. Follow-up brain MRI and MRS on the 11th day of admission showed the disappearance of diffusion restriction and the ketone body peak in the white matter and basal ganglia (Figs. 2C and D). MRI on the 26th day showed progression of cystic degeneration of the basal ganglia and cerebral atrophy (Figs. 1D and F). The findings at 1 year and 9 months after the onset did not change significantly (Figs. 1G and I).
Fig. 1.
Brain MRI findings of the patient. (A-C) Brain MRI at 6 months of age on the third day of admission (A, B: T2-weighted image, C: ADC) reveals symmetrical T2 signal prolongation with restricted diffusion in the caudate nucleus, putamen, globus pallidus, medial thalamus, and substantia nigra (white arrow). (D-F) Brain MRI on the 26th day of admission (D, E: T2-weighted image, F: ADC) shows progression of liquid degeneration of the basal ganglia and cerebral atrophy. (G-I) Brain MRI at 1 year and 9 months after onset (G, H: T2-weighted image, I: ADC) shows severe liquid degeneration of the basal ganglia and cerebral atrophy.
MRI, magnetic resonance imaging; ADC, apparent diffusion coefficient.
Fig. 2.
Brain MRS findings of the patient. (A and B) Brain MRS on the third day of admission (A: left white matter; B: left basal ganglia) revealed a peak at 2.2 ppm, which was assumed to be the ketone body spectrum. Elevation of glutamine level, mild reduction of NAA level in the white matter, marked elevation of lactate level, and a decrease in NAA level in the basal ganglia were also observed. (C and D) MRS on the 11th day of admission (C: left white matter, D: left basal ganglia) showing disappearance of the ketone body peak in the white matter and basal ganglia.
MRS, magnetic resonance spectroscopy; NAA, N-acetylaspartate; Cho, choline; Cr, creatine; Gln, glutamine; Lac, lactate.
Mitochondrial respiratory chain enzymatic activity in skin fibroblasts on the 35th day was normal; however, the oxygen consumption rate, measured using a Seahorse XF96, showed a significant reduction in oxidative metabolism. Maximum respiration rates were low—58% in presence of glucose (cutoff, 71.6%) and 65% in the presence of galactose—compared with those in the control. Exome sequencing and sequencing chromatograms identified compound heterozygous variants in NM_004092.4 (ECHS1), c.833C>T: p.Ala278Val and c.489G>A: p.Pro163=, leading to a diagnosis of ECHS-1 related Leigh syndrome. Parents were carriers of 1 variant.
Upon admission, the tentative diagnosis of acute encephalopathy led to vitamin supplementation (B1, C, E, biotin, carnitine, and CoQ10). The MRI findings and oxygen consumption rate measurements indicated mitochondrial encephalopathy. A low-protein diet was initiated on the 10th day, but no neurological improvement was observed. Development regressed; at the age of 2 years; the patient was unable to hold his head up, was bedridden, and tube-fed, although he was able to follow objects and smile. Nystagmus was also observed. Based on the genetic analysis, a valine-restricted diet was initiated at the age of 2 years and 2 months, and its effectiveness is currently being verified.
Written informed consent was obtained from the guardians for their participation in the study and publication of images. Genetic analyses were only used for clinical diagnosis and did not require approval from the ethics committee of our institution.
Discussion
The patient presented with Leigh syndrome, marked ketosis, and characteristic MRS findings of ketone bodies in the brain even after normalization of blood ketone body levels during acute exacerbation. The MRS findings of this patient are considered to reflect severe ketosis, and severe ketosis has been reported in cases with acute episodes of ECHS1-related Leigh syndrome. Leigh syndrome, caused by variants in other genes, was not linked to severe ketosis or changes in the ketone body MRS spectrum. Therefore, MRS can be useful for diagnosing ECHS1-related diseases, even when blood ketone body levels are normalized.
Patients with acute episodes of encephalopathy and neurological deterioration in SCEH deficiency sometimes present with marked hyperketonemia, similar to our findings [7,8]. One of the causes of hyperketonemia in SCEH deficiency is presumed to be impaired ATP production, possibly through several mechanisms. The first mechanism is impaired mitochondrial fatty acid β-oxidation in SCEH deficiency [9]. Second, inadequate conversion of methacrylyl-CoA into 3-OH-isobutyryl-CoA,and acryloyl-CoA into 3-OH-propionyl-CoA, caused by the disruption of key SCEH activity, results in accumulation of toxic precursors [1]. Intramitochondrial methacrylyl-CoA reacts with mitochondrial enzymes containing cysteine residues, including pyruvate dehydrogenase complex and respiratory chain enzymes, and reduces their activities and ATP production [10,11]. A study using ECHS1 “knockout” cells demonstrated reduced mitochondrial oxidoreductase activity and mitochondrial oxygen consumption rates [9]. Third, accumulation of methacrylyl-CoA depletes mitochondrial pools of thioredoxin and glutathione, which are involved in mitochondrial redox reactions [11]. It, however, remains unclear whether hyperketonemia in patients with SCEH deficiency results solely from impaired ATP production, because other genetic variants that cause Leigh syndrome may also impair ATP production without hyperketonemia. Additionally, since ketone bodies are produced from acetyl-CoA, impaired β-oxidation is expected to decrease the production of acetyl-CoA and ketone bodies. Therefore, the mechanism underlying ketosis during SCEH deficiency remains unclear. Blood glucose (4.3 mM) multiplied by the total ketone body (15.2 mM) in our case was 65, and the ratio of free fatty acid to total ketone bodies was less than 0.3 [12], indicating impaired ketone body utilization, which requires further investigation.
MRS is often useful for the diagnosis of numerous inborn errors of metabolism and neurodegenerative disorders, including amino acid metabolism disorders, urea cycle disorders, organic acid disorders, lysosomal disorders, peroxisomal disorders, mitochondrial disorders, fatty acid oxidation disorders, lactic acidosis disorders, leukodystrophies, lipid metabolism disorders, and so on [13]. Additionally, MRS is also effective as a predictor of outcomes for perinatal hypoxic–ischemic encephalopathy [14] and acute excitotoxic encephalopathy [15], as well as for the differentiation of brain tumors [16]. Moreover, MRS can serve as a biomarker for evaluating treatment effectiveness in metabolic diseases, and it also provides valuable insights into the pathophysiology of various disorders to support the development of new therapies [17]. MRS is an effective noninvasive method for examining various brain metabolites and a valuable diagnostic tool for pediatric metabolic and neurological disorders.
In conclusion, SCEH deficiency requires early diagnosis. Severe ketosis and ketone body MRS spectrum during acute exacerbation may help diagnose SCEH deficiency early.
Patient consent
Written informed consent was obtained from the guardians for their participation in the study and publication of images.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments: This study was supported in part by grants from Japan Agency for Medical Research and Development (AMED) (JP23ek0109637), JSPS KAKENHI Grants (JP23K07192), Grants-in-Aid for Research on Measures for Intractable Diseases (24FC1010, 24FC1008) from the Ministry of Health, Labor, and Welfare, Japan, to J.T., and the Practical Research Project for Rare/Intractable Diseases (JP22ek0109468) from AMED, to K.M. We express our deep gratitude to the guardians of the patients who provided permission to publish this manuscript. Written informed consent was obtained from the guardians for participation in the study and publication of images. We would like to thank Editage (www.editage.com) for the English language editing. Genetic analyses in our patient are for clinical diagnosis only, and our institution's ethics committee's approval is not required.
References
- 1.Marti-Sanchez L, Baide-Mairena H, Marcé-Grau A, Pons R, Skouma A, López-Laso E, et al. Delineating the neurological phenotype in children with defects in the ECHS1 or HIBCH gene. J Inherit Metab Dis. 2020;44:401–414. doi: 10.1002/jimd.12288. [DOI] [PubMed] [Google Scholar]
- 2.Muntean C, Tripon F, Bogliș A, Bănescu C. Pathogenic biallelic mutations in ECHS1 in a case with short-chain enoyl-CoA hydratase (SCEH) deficiency-case report and literature review. Int J Environ Res Public Health. 2022;19:2088. doi: 10.3390/ijerph19042088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharpe A, McKenzie M. Mitochondrial fatty acid oxidation disorders associated with short-chain enoyl-CoA hydratase (ECHS1) deficiency. Cells. 2018;7:46. doi: 10.3390/cells7060046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burgin HJ, McKenzie M. Understanding the role of OXPHOS dysfunction in the pathogenesis of ECHS1 deficiency. FEBS Lett. 2020;594:590–610. doi: 10.1002/1873-3468.13735. [DOI] [PubMed] [Google Scholar]
- 5.Sato-Shirai I, Ogawa E, Arisaka A, Osaka H, Murayama K, Kuwajima M, et al. Valine-restricted diet for patients with ECHS1 deficiency: divergent clinical outcomes in two Japanese siblings. Brain Dev. 2021;43:308–313. doi: 10.1016/j.braindev.2020.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Abdenur JE, Sowa M, Simon M, Steenari M, Skaar J, Eftekharian S, et al. Medical nutrition therapy in patients with HIBCH and ECHS1 defects: clinical and biochemical response to low valine diet. Mol Genet Metab Rep. 2020;24:100617. doi: 10.1016/j.ymgmr.2020.100617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uesugi M, Mori J, Fukuhara S, Fujii N, Omae T, Sasai H, et al. Short-chain enoyl-CoA hydratase deficiency causes prominent ketoacidosis with normal plasma lactate levels: a case report. Mol Genet Metab Rep. 2020;25:100672. doi: 10.1016/j.ymgmr.2020.100672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simon MT, Eftekharian SS, Ferdinandusse S, Tang S, Naseri T, Reupena MS, et al. ECHS1 disease in two unrelated families of Samoan descent: common variant-rare disorder. Am J Med Genet A. 2021;185:157–167. doi: 10.1002/ajmg.a.61936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burgin H, Sharpe AJ, Nie S, Ziemann M, Crameri JJ, Stojanovski D, et al. Loss of mitochondrial fatty acid β-oxidation protein short-chain Enoyl-CoA hydratase disrupts oxidative phosphorylation protein complex stability and function. FEBS J. 2023;290:225–246. doi: 10.1111/febs.16595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamada K, Naiki M, Hoshino S, Kitaura Y, Kondo Y, Nomura N, et al. Clinical and biochemical characterization of 3-hydroxyisobutyryl-CoA hydrolase (HIBCH) deficiency that causes Leigh-like disease and ketoacidosis. Mol Genet Metab Rep. 2014;1:455–460. doi: 10.1016/j.ymgmr.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferdinandusse S, Waterham HR, Heales SJ, Brown GK, Hargreaves IP, Taanman JW, et al. HIBCH mutations can cause Leigh-like disease with combined deficiency of multiple mitochondrial respiratory chain enzymes and pyruvate dehydrogenase. Orphanet J Rare Dis. 2013;8:188. doi: 10.1186/1750-1172-8-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukao T, Mitchell G, Sass JO, Hori T, Orii K, Aoyama Y. Ketone body metabolism and its defects. J Inherit Metab Dis. 2014;37:541–551. doi: 10.1007/s10545-014-9704-9. [DOI] [PubMed] [Google Scholar]
- 13.Lai LM, Gropman AL, Whitehead MT. MR neuroimaging in pediatric inborn errors of metabolism. Diagnostics (Basel) 2022;12:861. doi: 10.3390/diagnostics12040861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thayyil S, Chandrasekaran M, Taylor A, Bainbridge A, Cady EB, Chong WK, et al. Cerebral magnetic resonance biomarkers in neonatal encephalopathy: a meta-analysis. Pediatrics. 2010;125:e382–e395. doi: 10.1542/peds.2009-1046. [DOI] [PubMed] [Google Scholar]
- 15.Takanashi JI, Murofushi Y, Hirai N, Sano K, Matsuo E, Saito K, et al. Prognostic value of MR spectroscopy in patients with acute excitotoxic encephalopathy. J Neurol Sci. 2020;408:116636. doi: 10.1016/j.jns.2019.116636. [DOI] [PubMed] [Google Scholar]
- 16.Shiroishi MS, Panigrahy A, Moore KR, Nelson MD, Jr., Gilles FH, Gonzalez-Gomez I, et al. Combined MRI and MRS improves pre-therapeutic diagnoses of pediatric brain tumors over MRI alone. Neuroradiology. 2015;57:951–956. doi: 10.1007/s00234-015-1553-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aida N. 1H-MR spectroscopy of the early developmental brain, neonatal encephalopathies, and neurometabolic disorders. Magn Reson Med Sci. 2022;21:9–28. doi: 10.2463/mrms.rev.2021-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]