Abstract
A crucial and time-consuming stage in aerogel production is the solvent exchange process for alcogel formation. This process involves multiple steps, exposing the hydrogel to ethanol solutions with increasing concentration until the equilibrium in each step. Currently, the determination of contact time between phases (hydrogel and liquid solution) is either arbitrary or based on prior studies. However, considering the unique physicochemical characteristics of each system, as well as the solid-liquid interactions and the liquid diffusion within the matrix, the required time may vary. Monitoring this step can lead to a reduction in the time needed for alcogel production and the optimization of the entire process. The refractive index serves as a tool to assess ethanol concentration in the liquid solution over time, providing immediate information about the status of the solvent exchange. Alongside, differential scanning calorimetry can be employed to evaluate ethanol content in the alcogel (solid phase), confirming the attainment of equilibrium between phases.
-
•
This research introduces a technique for monitoring solvent exchange.
-
•
Refractive index measurement of the liquid solvent offers immediate concentration information into the status of the solvent exchange.
-
•
Differential scanning calorimetry is applicable for measuring the ethanol content within the alcogel and validating refractive index findings.
Keywords: Solvent exchange, Ethanol, Alcogel, Refractive index, Differential scanning calorimetry, Aerogel
Method name: Validation of alcogel formation
Graphical abstract
Specifications table
| Subject area: | Materials Science |
| More specific subject area: | Alcogel formation |
| Name of your method: | Validation of alcogel formation |
| Name and reference of original method: | Bento, C.S.A., Agostinho, B., Teixeira, A., Reis, M.S., Sousa, H.C.D., Braga, M.E.M., 2024. Alcogel formation analysis: An important step for scCO2 aerogel production. J. Supercrit. Fluids, 211, 106,321 10.1016/j.supflu.2024.106321 |
| Resource availability: | Refractometer; Different Scanning Calorimetry |
Background
The synthesis of aerogels comprises three processing stages: gelation for the formation of a hydrogel, solvent exchange to obtain an alcogel, and the final stage of supercritical carbon dioxide (scCO2) drying, to form an aerogel [1]. The solvent exchange phase is the most time-consuming step of the process, primarily due to the volumetric reduction that typically occurs in this step [2]. The new solvent may have a different affinity to the polymer or a different solubility, which may lead to shrinkage. Therefore, to avoid this, the solvent exchange is performed incrementally in multiple steps wherein the ethanolic concentration is increased at each step until the equilibrium. This approach serves to maintain a small driving force and preserve the structural integrity of the solid matrix (hydrogel).
In each step, the hydrogel is exposed to a water/ethanol solution, starting with a low concentration of ethanol, replacing the solvent within the hydrogel (water) with ethanol. Once the equilibrium between phases is reached, the ethanol concentration in the contacting solution is gradually increased. This iterative process continues until a complete solvent exchange occurs, forming an alcogel with an internal ethanol concentration of 100 % [3]. However, no control is done in this process, which difficult the scCO2 drying process. Normally, the contact time between the alcogel and the liquid solution is extended to guarantee that the equilibrium is reached. The selection of contact time is arbitrary or relies on previous studies, but given the distinct nature of each system, the necessary time may differ.
Nevertheless, to reduce the time required for solvent exchange and optimize the process, it becomes imperative to monitor the ethanol content in both the liquid solvent and the alcogel throughout the process. An ongoing measurement not only streamlines the duration of each step but also enhances the efficiency of the alcogel formation process. Achieving this can be done by conducting refractive index measurements on the liquid solution and assessing water content in the alcogel through differential scanning calorimetry [4,5].
Method details
Sample collection and preparation:
The process of hydrogel formation begins with the careful selection of polymers, which can be either natural or synthetic, depending on the desired properties of the final material. In this particular instance, biopolymers were used for method validation. Solutions of the selected polymers should be prepared using an adequate solvent for dissolution. Once dissolved, the polymeric solutions can be mixed. Crosslinkers or other additional components can be added if required. The resulting mixture is then placed onto a mold to shape the hydrogel, with the specific chosen based on its intended application. For method validation, cylindric hydrogels with 1 cm of diameter and 2 cm of height were used. Ageing can be necessary in certain cases to achieve the desired characteristics.
Once the hydrogel is formed, solvent exchange can be performed. Ethanol aqueous solutions of different concentrations should be prepared using anhydrous ethanol, e.g. 20 %, 40 %, 70 %, 100 % (v/v). Solvent exchange can be performed either at ambient pressure and temperature or using high-pressure. For ambient pressure the hydrogel is immersed in the ethanol aqueous solutions, increasing the concentration once equilibrium is reached. For high-pressure solvent exchange, the hydrogel is placed inside a high-pressure cell and the ethanol aqueous solution is pumped into the cell, replacing the solution as equilibrium is attained. In this case, a continuous flow of solvent is being pumped inside the cell, and the solvent mixture passes through the sample changing the solvent composition [10].
For each ethanol concentration, it is anticipated that an equilibrium will be achieved between the alcogel and the liquid solution. Over time, it becomes necessary to increase the concentration of ethanol in the liquid solution to ensure a complete solvent exchange.
Although this method is proposed for rapid measurement, to determine when this concentration should be adjusted or when the solvent exchange is complete, it is advisable to collect samples of both the liquid solution and the solid (alcogel), for analysis. It is important to immediately condition them, to prevent ethanol evaporation. In cases where immediate processing of measurements is not feasible, it is recommended to freeze the samples.
For liquid sample storage, a glass container with a lid is recommended. Please ensure that the container is properly cleaned and contains no residues that could affect the measurement.
Additional preparation is necessary for the analysis of solid alcogel samples. A segment of the alcogel, preferably obtained from the middle section, should be enclosed within an aluminium crucible with a lid for thermal analysis.The shape and chemical composition of the sample may influence the diffusion of solvents and mass transfer, affecting this way the solvent exchange process [6]. Taking this into account, segments from different sections (perpendicular and radial) can be selected along the sample to guarantee the complete diffusion and solvent exchange. Once again, if an immediate analysis is not possible, freezing is advised.
Refractive Index measurement
The determination of ethanol concentration in the liquid solution is achievable through the use of the Refractive Index (RI). This method is commonly employed for assessing solute concentrations in aqueous solutions, such as the ethanolic solutions employed in the solvent exchange process [5].
The first step in assessing ethanol content in each solution involves establishing a calibration curve to correlate ethanol concentration (% v/v) with the measured refractive index. To construct this curve, aqueous solutions with known ethanol concentrations (% v/v) should be prepared, and their refractive indices should be recorded [7]. Table 1 illustrates an example of refractive indices measured at 25 °C for aqueous ethanol solutions with increasing concentration, while Fig. 1 depicts the resulting calibration curve based on these outcomes. The refractometer from ATAGO, model RX-5000α was used for the measurements.
Table 1.
Ethanol concentration and respective refractive index at 25 °C.
| [EtOH] (% v/v) | RI | [EtOH] (% v/v) | RI | [EtOH] (% v/v) | RI |
|---|---|---|---|---|---|
| 0 | 1.33252 | 45 | 1.35527 | 84 | 1.36293 |
| 5 | 1.33721 | 50 | 1.35778 | 86 | 1.36299 |
| 10 | 1.34252 | 55 | 1.35799 | 88 | 1.36298 |
| 15 | 1.34576 | 60 | 1.35901 | 90 | 1.36293 |
| 20 | 1.34916 | 65 | 1.35924 | 92 | 1.36285 |
| 25 | 1.35074 | 70 | 1.36100 | 94 | 1.36276 |
| 30 | 1.35236 | 75 | 1.36272 | 96 | 1.36271 |
| 35 | 1.35251 | 80 | 1.36283 | 98 | 1.36259 |
| 40 | 1.35428 | 82 | 1.36294 | 100 | 1.35927 |
Fig. 1.
Ethanol concentration of liquid solutions (% v/v) vs. refractive index at 25 °C.
Once the calibration curve is obtained it is possible to now determine the ethanol concentration in each of the liquid samples collected. Based on the determined calibration curve, linear regression should be applied to determine an Eq. (1) that correlates ethanol concentration and the measured refractive index.
| (1) |
Three equations to correlate the ethanol concentration with the measured index were determined and the correlation coefficients are presented in Table 2. The selection of which equation to use should be based on the initial ethanol concentration of the ethanolic solution to obtain a more accurate measurement.
Table 2.
Ethanol concentration and refractive index correlation coefficients.
| [EtOH]initial (% v/v) | A | b | R2 |
|---|---|---|---|
| 0 – 20 % | 0.0008 | 1..3331 | 0.99 |
| 20 −90 % | 0.0002 | 1.3463 | 0.98 |
| 90 −100 % | −0.0003 | 1.3891 | 0.52 |
Differential scanning calorimetry analysis
Regarding the alcogel, the quantification of ethanol concentration can be accomplished through the application of differential scanning calorimetry (DSC) [4,8]. DSC is commonly employed to assess the thermal properties of materials, establishing a relationship between temperature and a specific property. It is the only method that can be employed to determine the enthalpy associated with a particular process. In the context of aerogels, DSC can be employed to ascertain the enthalpy of fusion of water present in their structure, facilitating the determination of water content in alcogels.
To execute this process, the aluminium crucible containing the sample for analysis should undergo DSC analysis. Initially, the sample needs to be frozen at −80 °C. Subsequently, a heating ramp of 10 °C/min must be applied until reaching at least 50 °C. Given the distinct freezing/melting points of water (0 °C) and ethanol (−114.5 °C), it becomes feasible to selectively freeze the water component in the sample while maintaining ethanol in a liquid state. Consequently, the thermogram obtained will show a thermal event occurring around 0 °C, signifying the melting of water. The enthalpy associated with this peak corresponds to the enthalpy necessary for either the melting or freezing of water. The enthalpy values for freezing and melting are identical but they exhibit opposite signals. Considering the known freezing enthalpy of pure water (ΔH_fH2O), recognized as 333.5 J.g-1 [3], it becomes feasible to ascertain the percentage of freezable water within the alcogel. This result allows for the extrapolation of ethanol concentration using Eqs. (2)–(4).
| (2) |
| (3) |
| (4) |
In the equations, represents the enthalpy of the peak at ∼0 °C, is the density of the sample and is the density of water at 25 °C, 0.997 g.cm−3. The density of the sample should be predetermined using a valid technique, for example helium pycnometry.
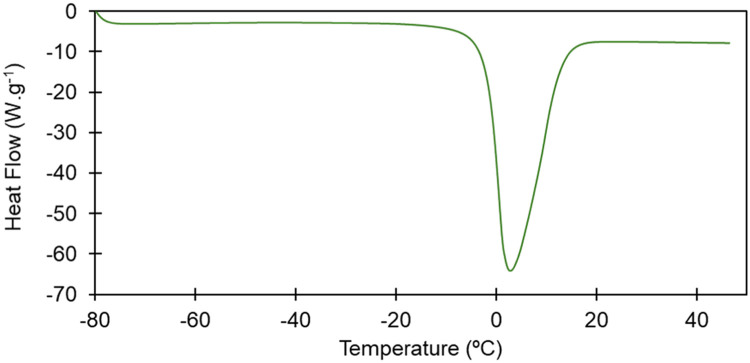
In Fig. 2, it is possible to observe an example of a thermogram of an alginate-gelatin aerogel sample containing 10 % ethanol, obtained using the equipment TA Instruments Q100 DSC, with 50 mL.min−1 nitrogen flow rate. The peak observed corresponds to the melting of water present, after being frozen to −80 °C.
Fig. 2.
Thermogram of an alginate-gelatin aerogel.
The authors tested using the DSC method for analysis of the liquid solution. A correlation between the enthalpy of freezing and the ethanol concentration was established, with similar findings to those obtained through refractive index measurement.
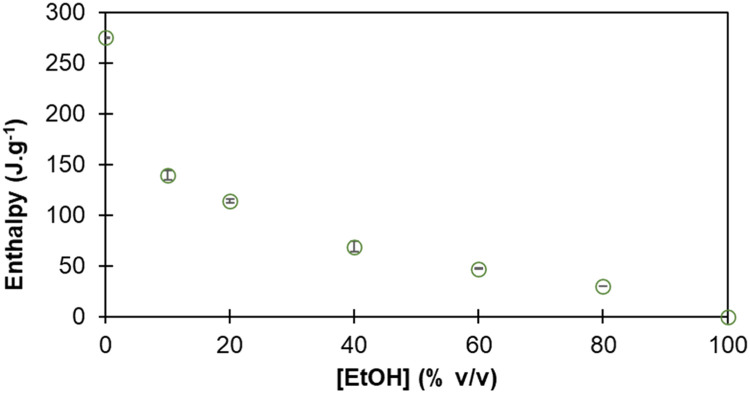
Fig. 3 illustrates a calibration curve depicting the correlation between the enthalpy of freezing and the ethanol concentration (% v/v). Solutions with predetermined ethanol concentrations were prepared to construct this curve. Notably, the enthalpy of freezing exhibits a decrease with increasing ethanol concentration. Additionally, it was observed that the freezing point of the liquid samples changed with the increase in ethanol concentration and became lower [9].
Fig. 3.
Ethanol concentration in liquid solutions (%v/v) vs. enthalpy of freezing (J.g−1).
The correlation coefficients obtained are presented in Table 3. The choice of which equation to employ should be determined by the initial ethanol concentration of the ethanolic solution. Be aware that these equations are only valid for liquid samples.
Table 3.
Ethanol concentration and enthalpy correlation coefficients.
| [EtOH]initial (% v/v) | A | b | R2 |
|---|---|---|---|
| 0 – 20 % | −1.3388 | 132.34 | 0.86 |
| 20 −100 % | −8.065 | 257.08 | 0.97 |
Method validation
The method outlined is specifically designed for ethanol/water solutions. The presence of other solvents within the mixture could potentially influence the results, making them unreliable. This does not mean that the method cannot be adapted to other organic solvent/water mixtures.
While different equipment or analysis conditions may be used, it is imperative to establish a calibration curve for each system, as the solvent exchange may be affected by the hydrogel's shape and composition or by the solvent conditions applied (e.g. continuous or static flow, pressure, temperature). Detection limits are determined based on the resulting calibration curve.
Samples of alginate-gelatin (5 %, m m-1, each component) hydrogel in a ratio of 1/1 (v/v) [10] were used as examples of the system to evaluate the method.
For method evaluation, the accuracy, precision and reproducibility of the results were taken into account. To accomplish this, the method was applied during the alcogel production process, considering the same operating conditions (pressure, temperature, time, ethanol volume) or varying them. Over 100 experiments were conducted in total. Samples of the liquid solvent and the alcogel were collected throughout the solvent exchange procedure, confirming a consistent increase in the ethanolic content and demonstrating reproducibility of results (Table 4). The standard deviation (σ) in all cases did not exceed 5 %, being considered acceptable. In most cases, the variance (σ2) obtained was low, indicating a minimal spread of data values relative to the mean (µ).
Table 4.
Ethanol concentration measured using the proposed method, considering different processing conditions and alcogel systems.
| Theoretical |
Measured [10,11] |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| [EtOH] (% v/v) | [EtOH]liquid solvent (% v/v) |
[EtOH]alcogel (% v/v) |
||||||||
| n | x | µ | σ | σ2 | x | µ | σ | σ2 | ||
| System A Alginate5 %-Gelatin5 % (1/1, v/v) |
1 | 9.7 | 9.1 | 7.463 | 1.486 | 2.207 | 4.088 | 6.166 | 3.632 | 13.195 |
| 2 | 6.2 | 4.049 | ||||||||
| 3 | 7.09 | 10.360 | ||||||||
| 1 | 19.4 | 10.437 | 10.725 | 0.424 | 0.180 | 12.413 | 9.701 | 4.244 | 18.015 | |
| 2 | 11.212 | 11.881 | ||||||||
| 3 | 10.525 | 4.810 | ||||||||
| 1 | 38.9 | 24.025 | 20.733 | 2.921 | 8.533 | 39.024 | 40.304 | 1.524 | 2.321 | |
| 2 | 18.450 | 41.989 | ||||||||
| 3 | 19.725 | 39.898 | ||||||||
| 1 | 58.8 | 59.075 | 59.875 | 0.728 | 0.531 | 57.309 | 58.500 | 1.933 | 3.736 | |
| 2 | 59.075 | 57.461 | ||||||||
| 3 | 60.501 | 60.703 | ||||||||
| 1 | 78.9 | 77.375 | 80.758 | 2.948 | 8.691 | 78.160 | 78.427 | 0.038 | 0.147 | |
| 2 | 82.125 | 78.867 | ||||||||
| 3 | 82.775 | 78.255 | ||||||||
| 1 | 99.3 | 88.95 | 87.6 | 1.176 | 1.382 | 88.656 | 89.180 | 2.465 | 6.078 | |
| 2 | 87.05 | 87.018 | ||||||||
| 3 | 86.8 | 91.865 | ||||||||
| System B Chitosan3 %- Tripolyphosphate2,5 % (1/20, v/v) |
1 | 99.3 | 96.833 | Undetermined | ||||||
| 2 | 99.3 | 95.833 | 96.199 | 0.551 | 0.303 | |||||
| 3 | 99.3 | 95.933 | ||||||||
n – number of measurements; x – value measured; µ – mean value; σ – standard deviation; σ2 – variance.
Accuracy assessment involved analysing samples with known theoretical ethanolic contents, determined based on the expected concentration upon reaching equilibrium. The results demonstrated close agreement with the theoretical values (Table 4), particularly for higher ethanol concentrations, ranging from 40 to 80 % %(v/v), where an accuracy level higher than 96 % could be attained. However, at low concentrations, the accuracy level was not as satisfactory. This discrepancy may be due to the high concentration differentials noted at the earlier stages of the solvent exchange. To address this issue, it is recommended, that at the beginning of the solvent exchange, the increase of ethanol concentration is done gradually and steadily, using more intermediate concentrations. Also, it is noteworthy that the accuracy of the results ranging from 80 to 100 % may be compromised due to the azeotropic of the ethanol/water mixture, which is a challenge for any kind of analytical measurement. These conclusions can be observed in Fig. 4.
Fig. 4.
Correlation between theoretical ethanol content and measured ethanol content on the liquid solvent and the alcogel (solid) along the solvent exchange.
Furthermore, the method was also tested for different alcogel systems, including alginate5 %-gelatine5 % (1/1, v/v) [10] and chitosan3 %-tripolyphophate2,5 % (1/20, v/v) [11], demonstrating efficiency and reproducibility in both cases and proving its robustness (Table 4). Since the equilibrium conditions will be different for different alcogel compositions, the equilibrium between liquid and solid must be studied for each kind of sample.
The application of this method for other scenarios remains uncertain. For instance, measuring the solvent exchange in a flow presents a challenge due to factors such as the flow rate, the turbulence and the interaction between the solvent and the material. However, as mentioned previously, the solvent exchange could be performed under high-pressure conditions and in such cases, a continuous flow of solvent occurs, entering the cell at one end and exiting at the opposite end, where the ethanol content is measured. This opens up the possibility to expand further the application of this method, considering that calibration curves are always predetermined for each system and each processing condition.
Conclusions
This method introduces a straightforward solution for monitoring the solvent exchange process in aerogel production. By actively monitoring the process it is possible to reduce the required processing time, thereby optimizing the entire procedure. Refractive index measurement emerges as a method that demands no special preparation and can be conveniently employed throughout the solvent exchange. The sample can be collected and immediately measured, providing real-time insights into the status of the solvent exchange. Given the inherent variations in each system, calibration is recommended in all cases.
Conversely, differential scanning calorimetry requires a certain level of preparation. Consequently, differential scanning calorimetry analysis is suggested as a complementary technique to validate the results obtained through refractive index measurement.
Limitations
Not applicable.
Ethics statements
Not applicable.
CRediT authorship contribution statement
Cristiana S.A. Bento: Writing – original draft, Validation, Methodology. Hermínio C. de Sousa: Writing – review & editing, Supervision. Mara E.M. Braga: Conceptualization, Writing – review & editing, Methodology.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was financially supported by Fundação para a Ciência e Tecnologia (FCT), Portugal, through the project STERILAEROGEL – Green method to prepare sterilised biopolymer-based aerogel (POCI-01–0145-FEDER-032625) and Strategic Projects 10.54499/UIDB/00102/2020 and Programmatic Project 10.54499/UIDP/00102/2020 of the CERES. C. S. A. Bento acknowledges for PhD grant 10.54499/UI/BD/151008/2021.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.mex.2024.102960.
Appendix. Supplementary materials
Data availability
Data will be made available on request.
References
- 1.Muhammad A., Lee D., Shin Y., Park J. Recent progress in polysaccharide aerogels: their synthesis, application, and future outlook. Polymers (Basel) 2021;13 doi: 10.3390/polym13081347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Subrahmanyam R., Gurikov P., Dieringer P., Sun M., Smirnova I. On the road to biopolymer aerogels—Dealing with the solvent. Gels. 2015;1:291–313. doi: 10.3390/gels1020291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Subrahmanyam R., Gurikov P., Meissner I., Smirnova I. Preparation of biopolymer aerogels using green solvents. J. Vis. Exp. 2016:3–7. doi: 10.3791/54116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gnanaprakasam Thankam F., Muthu J. Influence of plasma protein-hydrogel interaction moderated by absorption of water on long-term cell viability in amphiphilic biosynthetic hydrogels. RSC Adv. 2013;3:24509–24520. doi: 10.1039/c3ra43710h. [DOI] [Google Scholar]
- 5.Owuama C.I., Ododo J.C. Refractometric determination of ethanol concentration. Food Chem. 1993;48:415–417. doi: 10.1016/0308-8146(93)90327-C. [DOI] [Google Scholar]
- 6.Subrahmanyam R., Gurikov P., Dieringer P., Sun M., Smirnova I. On the road to biopolymer aerogels—Dealing with the solvent. Gels. 2015;1:291–313. doi: 10.3390/gels1020291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Refractometer. pl Refractive index of ethanol solutions. ChemBuddy. 2022 http://www.refractometer.pl/refraction-datasheet-ethanol (accessed January 12, 2024) [Google Scholar]
- 8.Yang P., Mather P.T. Thermal analysis to determine various forms of water present in hydrogels. TA Instrum. 2014:1–4. http://molbiol.ru/forums/index.php?act=Attach&type=post&id=220252 [Google Scholar]
- 9.Zheng F., Li Z. Proceedings of the 20th International Conference on Solid-State Sensors, Actuators Microsystems Eurosensors XXXIII. 2019. A disposable array chip using temperature-responsive color change to record temperature history in terminal cold chain transportation; pp. 1941–1944. [Google Scholar]
- 10.Bento C.S.A., Agostinho B., Teixeira A., Reis M.S., de Sousa H.C., Braga M.E.M. Alcogel formation analysis: an important step for scCO2 aerogel production. J. Supercrit. Fluids. 2024;211 doi: 10.1016/j.supflu.2024.106321. [DOI] [Google Scholar]
- 11.Bento C.S.A., Ruivo J.P., Sousa H.C.d., Dias A.M.A., Braga M.E.M. Chitosan-TPP based aerogels for tissue engineering applications. Proceedings of the VI Iberoamerican Conference on Supercritical Fluids (Prosciba 2023); Cordoba; 2023. Paper presented in. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.