Abstract
Background
Cyclin-dependent kinase 4/6 inhibitor (CDK4/6i) and everolimus (EVE) are effective for patients with hormone receptor–positive (HR+), human epidermal growth factor receptor 2-negative (HER2-) metastatic breast cancer (MBC). However, the efficacy of different sequences of CDK4/6i and EVE are largely unknown. The study aimed to explore the efficacy of different sequences in China.
Methods
146 patients with HR+/HER2- MBC who received both CDK4/6i and EVE in salvage setting were collected. Objective response rate (ORR), clinical benefit rate (CBR), progression-free survival (PFS), and overall survival (OS) were investigated.
Results
56 patients received CDK4/6i prior to EVE (Group A), 90 patients received CDK4/6i subsequent to EVE (Group B). The median PFS of CDK4/6i and EVE in Group A vs Group B were 8.4m and 2.5m vs 4.6m and 6.1m respectively. The total PFS of first-line and second-line endocrine therapy were not different between Group A and Group B [13.1m vs 17.7m (P = 0.330, HR = 0.738, 95%CI: 0.399–1.365)]. The 5y OS of patients in Group A or Group B were 62.0 % vs 57.4 %, P = 0.569.
Conclusions
We found that no matter CDK4/6i or EVE was used first, the survival were not significantly different between Group A and Group B. Both can be clinical options.
Keywords: Therapeutic sequence, CDK4/6 inhibitor, Everolimus, Survival outcome
1. Background
Hormone receptor–positive (HR+), human epidermal growth factor receptor 2-negative (HER2-) breast cancer (BC) constitutes approximately 70 % among all types of BC, which has a better prognosis [1]. Endocrine therapy (ET) is a pivotal clinical option for HR + patients even after metastasis develops [2]. However, resistance to ET has been increasingly developed that emphasizes effective therapeutic strategies for the control of HR + metastatic breast cancer (MBC).
The mammalian target of rapamycin (mTOR) inhibitor everolimus (EVE) has been approved in patients with HR + MBC. Patients with HR + MBC suffering from endocrine resistance could still derive benefits from the addition of EVE, regardless of the menopausal status [[3], [4], [5], [6], [7]]. In BOLERO-2 trial, the addition of EVE to exemestane improved progression-free survival (PFS) form 3.2 months–7.8 months (P < 0.001) in postmenopausal patients with HR+/HER2- MBC [7]. Based on the results from BOLERO-2, EVE became the first approved targeted therapy in HR + MBC. Our team investigated the role of EVE in premenopausal HR+/HER2- MBC patients who were resistant to ET, EVE plus letrozole achieved a better clinical benefit (72.7 % vs 47.5 %, P = 0.004) and PFS (19.4 months vs 12.9 months, P = 0.008) compared to letrozole alone [4]. Though EVE was the first target agent approved for advanced HR+/HER2-breast cancer, with the success of CDK4/6i, it is now recommended mainly in subsequent line after CDK4/6i progression with limited data.
CDK4/6i combined with endocrine therapy is now the standard first-line (1L) treatment in HR+/HER2- MBC patients. Compared to chemotherapy, CDK4/6i combined with endocrine therapy showed comparable efficacy even in some breast caner patients with visceral crisis. Giuliano et al. [8] compared endocrine therapy to chemotherapy in HR+/HER2-metastatic breast cancer, concluding that endocrine therapy should be preferred as the first-line treatment, due to its favorable efficacy-to-toxicity ratio. The approved CDK4/6 inhibitors include palbociclib, abemaciclib, ribociclib. A series of PALOMA [[9], [10], [11]], MONARCH [12,13] studies have proved PFS benefits of palbociclib, and abemaciclib. MONALEESA trials demonstrated not only PFS but also OS benefits of ribociclib even for patients experienced progression following ET [14,15]. Schettini et al. [16] conducted a comprehensive systematic review and meta-analysis of CDK4/6 inhibitor-based treatments, demonstrating significant overall survival benefits across various subgroups of metastatic HR+/HER2-breast cancer patients. Although current clinical practice suggests the sequence of CDK4/6i first and then EVE, data was quite limited for patients with HR+/HER2– MBC receiving EVE after progressing on CDK4/6i [[17], [18], [19], [20]]. Data about EVE followed by CDK4/6i is even less, as PALOMA and MONARCH trials excluded patients previously treated with EVE. Rare studies have ever compared the survival of EVE followed by CDK4/6i or vice versa. As EVE was approved in China prior to CDK4/6 inhibitors, we performed a multicenter real-world study on patients with HR+/HER2- MBC used both CDK4/6i and EVE, attempting to shed light on the treatment sequence of CDK4/6i and EVE.
2. Materials and methods
2.1. Study design and data collection
Data was collected from patients treated in three cancer centers in China (National Cancer Center, Chinese PLA General Hospital, Peking University Cancer Hospital & Institute during January 2014 to November 2022.
Eligible patients were identified according to the following criteria:(1) Female patients. (2) Estrogen receptor (ER) or progesterone receptor (PR) positive and HER2 negative. The status of ER and PR were determined by immunohistochemical (IHC) staining in line with the ASCO/CAP guideline. HER2 status was evaluated by IHC and fluorescence in situ hybridization (FISH) based on the ASCO guidelines and current scoring system. HER2 negative was defined as IHC scoring 0, HER2 low was IHC scoring 1+ or 2+ with negative FISH assay. (3) Metastasis was confirmed radiologically or histologically. (4) Patients were recorded to use both CDK4/6i- and EVE-based regimens in advanced setting. (5) Patients must have complete timeline of using CDK4/6i and EVE.
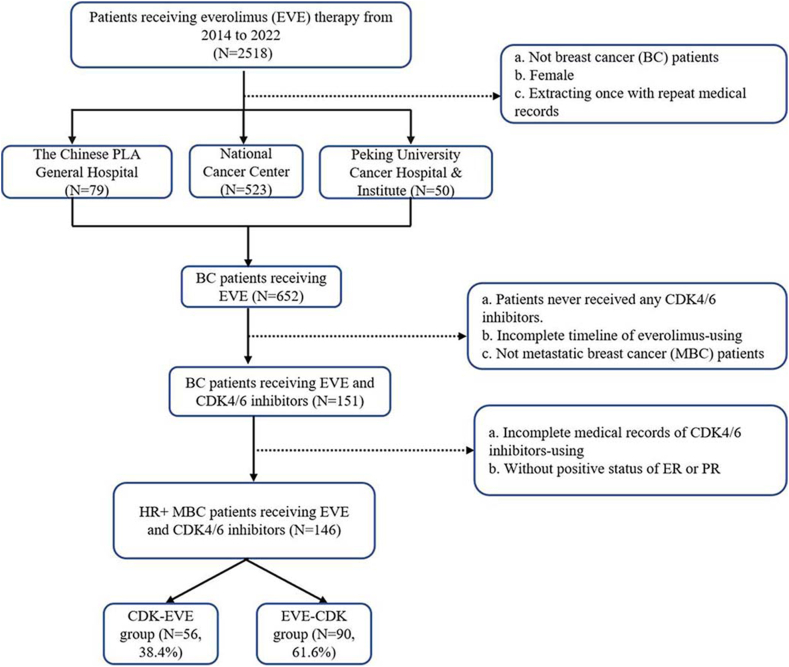
From 2014 to 2022, 2518 cancer patients who used EVE were collected from three cancer centers in China. 652 female patients diagnosed with BC were filtered out. Among those 652 BC patients, 501 patients were excluded given that they never received CDK4/6i therapy or had incomplete medical records of EVE administration. 151 patients receiving both CDK4/6i and EVE therapy in salvage setting remained. With 5 patients excluded due to incomplete documentation of CDK4/6i using, 146 HR+/HER2- MBC patients with complete documentation of CDK4/6i and EVE-based treatment were enrolled in our study. Patients were divided into two groups. Group A included patients received CDK4/6i followed by EVE. Group B included patients received EVE followed by CDK4/6i. We also defined two subsets. Subsets A included patients received CDK4/6i as 1L ET and EVE as 2L ET. Subsets B included patients received EVE as 1L ET and CDK4/6i as 2L ET. The flowchart of patients screening is presented as Fig. 1.
Fig. 1.
Flowchart of patient screening in the study.
2.2. Evaluation
2.2.1. Patients
Objective response rate (ORR) and clinical benefit rate (CBR), PFS and OS were used as indicators of drug efficacy. The PFS of patients was set as the primary endpoint, while ORR, CBR and OS were the secondary endpoints in the study.
Tumor response was assessed as complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD) based on Response Evaluation Criteria in Solid Tumors (RECIST) 1.1. Response evaluation was conducted mainly by computed tomography (CT), magnetic Resonance Imaging (MRI), and physical examination. ORR was defined as the percentage of patients with CR or PR on the corresponding regimens. CBR was defined as the rate of patients with CR or PR or SD more than 6 months.
PFS was defined as the time from the beginning of CDK4/6i or EVE treatment to confirmed progression or death. OS was defined as the interval from the initiation of CDK4/6i or EVE treatment whichever first to death from any cause. We performed further analysis of patients in Subset A and Subset B. PFStotal was defined as PFS of 1L ET plus PFS of 2L ET for patients in Subset A and Subset B.
Primary endocrine resistance is defined as relapse while on the first 2 years of adjuvant endocrine therapy (ET), or PD within the first 6 months of first-line ET for advanced breast cancer (ABC) while on ET. Secondary endocrine resistance is defined as relapse while on adjuvant ET after the first 2 years, or relapse within 12 months of completing adjuvant ET, or PD ≥ 6 months after initiating ET for ABC while on ET [21]. First line/Second line endocrine therapy is defined as the first or second endocrine treatment in the metastatic setting irrespective of chemotherapy and endocrine resistance.
2.3. Statistical analysis
The demographic and clinicopathological characteristics comprised age, menopausal status, TNM stage, type of metastasis, number of metastatic sites, endocrine therapy sensitivity. Clinicopathological characteristics at the time of initiation of CDK4/6i or EVE treatment were collected and compared using the Chi-square test or the Fisher's exact test.
PFS and OS were estimated via the Kaplan–Meier method. Statistical difference was calculated by the log-rank test. Hazard ratio (HR) and 95 % confidential interval (95%CI) were obtained via univariate Cox analysis. P value less than 0.05 was considered as statistical significance. SPSS software version 22.0 was applied to conduct statistical analysis.
3. Results
3.1. Baseline characteristics
The median age at enrollment was 52 years. 60.3 % of patients were postmenopausal. 16.4 % had a family history of malignancies and 5.5 % with a family history of BC. More than 80 % of patients were with BMI ≤25 kg/m2. Pathologically, the main histological type was invasive ductal carcinoma (IDC) (75.3 %). Over three quarter of patients (78.0 %) had at least two sites of metastases, with bone being the most common site (64.4 %). 56 patients were in Group A (CDK-EVE), and 90 patients were in Group B (EVE-CDK). When comparing the clinical characteristics between the two groups, more patients in Group A had liver (P = 0.042) and visceral metastasis (P = 0.056). The median endocrine treatment line of CDK4/6i and EVE was 3 (1–10) and 2 (1–7). Details are presented in Table 1.
Table 1.
Baseline characteristics of patients.
| Characteristics | Total(n = 146) n (%) | CDK-EVE(n = 56) n (%) | EVE-CDK(n = 90) n (%) | P value |
|---|---|---|---|---|
| Age, years, median (min, max) | 52.0(24.0, 79.0) | 52.0(31.0, 79.0) | 50.0(24.0, 74.0) | 0.386 |
| <65 years | 127(87.0 %) | 47(83.9 %) | 80(88.9 %) | |
| ≥65 years | 19(13.0 %) | 9(16.1 %) | 10(11.1 %) | |
| BMI, Kg/m2, median (min, max) | 23.2(15.6,38.8) | 0.580 | ||
| >25 | 24(16.4 %) | 8(14.3 %) | 16(17.8 %) | |
| ≤25 | 122(83.6 %) | 48(85.7 %) | 74(82.2 %) | |
| Menopausal status | 0.932 | |||
| Premenopausal or perimenopausal | 58(39.7 %) | 22(39.3 %) | 36(40.0 %) | |
| Postmenopausal | 88(60.3 %) | 34(60.7 %) | 54(60.0 %) | |
| Pathology | 0.267 | |||
| Invasive ductal carcinoma | 110(75.3 %) | 45(80.4 %) | 65(72.2 %) | |
| others | 36(24.7 %) | 11(19.6 %) | 25(27.8 %) | |
| Disease-free interval | 0.300 | |||
| Median, months(95%CI) | 57.8(50.4–65.2) | 53.0(41.1–64.9) | 59.9(52.7–67.1) | |
| Endocrine resistance status | 0.342 | |||
| Endocrine sensitivity | 59(40.4 %) | 24(42.9 %) | 35(38.9 %) | |
| Primary endocrine resistance | 20(13.7 %) | 10(17.9 %) | 10(11.1 %) | |
| Secondary endocrine resistance | 67(45.9 %) | 22(39.2 %) | 45(50.0 %) | |
| Metastatic sites at enrollment | ||||
| Lung | 75(51.4 %) | 28(50.0 %) | 47(52.2 %) | 0.794 |
| Liver | 81(55.5 %) | 37(66.1 %) | 44(48.9 %) | 0.042 |
| Bone | 94(64.4 %) | 38(67.9 %) | 56(62.2 %) | 0.489 |
| Brain | 10(6.8 %) | 4(7.1 %) | 6(6.7 %) | 1.000 |
| Bone-only metastasis | 13(8.9 %) | 2(3.6 %) | 11(12.2 %) | 0.132 |
| Non-visceral metastasis | 27(18.5 %) | 6(10.7 %) | 21(23.3 %) | 0.056 |
| Number of metastatic sites at enrollment | 0.510 | |||
| 1 | 32(22.0 %) | 12(21.4 %) | 20(22.2 %) | |
| 2 | 57(39.0 %) | 19(33.9 %) | 38(42.2 %) | |
| ≥3 | 57(39.0 %) | 25(44.7 %) | 32(35.6 %) | |
| Previous chemotherapy in metastatic setting at enrollment | 0.558 | |||
| 0 line | 63(43.2 %) | 27(48.2 %) | 36(40.0 %) | |
| 1 line | 40(27.4 %) | 12(21.4 %) | 28(31.2 %) | |
| 2 lines | 23(15.8 %) | 10(17.9 %) | 13(14.4 %) | |
| ≥3lines | 20(13.6 %) | 7(12.5 %) | 13(14.4 %) |
Abbreviations: CDK, Cyclin-dependent kinase 4/6 inhibitors; EVE, Everolimus; ER: Estrogen receptor; PR: Progesterone receptor; 1L: First-line treatment; 2L: Second-line treatment; 3L: Third-line treatment.
The most frequently prescribed CDK4/6i was palbociclib, with 121 (82.9 %) patients in the study receiving palbociclib-based treatment. 15, 5 and 5 patients received abemaciclib, ribociclib and dalpiciclib, respectively. Among the 146 patients enrolled, 19.9 % (29/146) of patients received CDK4/6i as 1L ET, 29.5 % (43/146) as 2L, 25.3 % (37/146) as 3L. In this study, 19.2 % (28/146) of patients received EVE as 1L treatment, 41.8 % (61/146) as 2L, 27.4 % (40/146) as 3L.
26 patients received CDK4/6i as first line endocrine therapy and EVE as second line endocrine therapy (Subset A). 21 patients received EVE as first line endocrine therapy and CDK4/6i as second line endocrine therapy (Subset B).
3.2. Responses
For all the 146 patients enrolled, the ORR of CDK4/6i was significantly lower than ORR of EVE, 13.0 % (19/146) vs 22.6 % (33/146), P = 0.032. The CBR of EVE and CDK4/6i were not significantly different, 50.7 % (74/146) vs 47.3 % (69/146), P = 0.558.
The response of CDK4/6i was better when it was used in earlier line treatment. The ORR and CBR of CDK4/6i were significantly higher in Group A than in Group B (ORR: 21.4 % vs 7.8 %, P = 0.017; CBR: 61.5 % vs 43.3 %, P = 0.024). The CBR of EVE was also significantly higher in Group B than in Group A (60.0 % vs 26.8 %, P < 0.001). However, the ORR of EVE between the two groups was numerically but not significantly different (27.8 % vs 14.2 %, P = 0.058).
The ORR and CBR of patients received CDK4/6i or EVE as first line treatments were not significantly different (ORR: 26.9 % vs 33.3 %, P = 0.633, HR = 1.357, 95%CI: 0.387–4.759; CBR: 65.4 % vs 71.4 %, P = 0.659, HR = 1.324, 95%CI: 0.381–4.595). The ORR and CBR of patients received CDK4/6i and EVE as 2L ET were also not significantly different (ORR: 19.2 % vs 14.3 %, P = 0.655, HR = 0.700, 95%CI: 0.147–3.343; CBR: 38.5 % vs 38.1.0 %, P = 0.980, HR = 0.985, 95%CI: 0.302–3.215). Details are presented in Table 2.
Table 2.
Response of patients stratified by therapeutic sequence.
| Characteristics | CDK-EVE(n = 56) n (%) | EVE-CDK(n = 90) n (%) | P value |
|---|---|---|---|
|
CDK4/6i-efficacy | |||
| Best response1 | |||
| PR | 12(21.4 %) | 7(7.8 %) | |
| SD | 30(53.6 %) | 47(52.2 %) | |
| PD | 14(25.0 %) | 36(40.0 %) | |
| Objective response rate | 12(21.4 %) | 7(7.8 %) | 0.017 |
| Clinical benefit rate |
35(61.5 %) |
39(43.3 %) |
0.024 |
|
Everolimus-efficacy | |||
| Best response1 | |||
| PR | 8(14.2 %) | 25(27.8 %) | |
| SD | 22(39.3 %) | 40(44.4 %) | |
| PD | 26(46.5 %) | 25(27.8 %) | |
| Objective response rate | 8(14.2 %) | 25(27.8 %) | 0.058 |
| Clinical benefit rate | 15(26.8 %) | 54(60.0 %) | <0.001 |
Abbreviations: CDK, Cyclin-dependent kinase 4/6 inhibitors; EVE, Everolimus; PR: Partial response; SD: Stable disease; PD: Progression disease; HR, Hazard ratios; CI, Confdence interval; Ref, Reference.
4. Survivals
4.1. Progression free survival
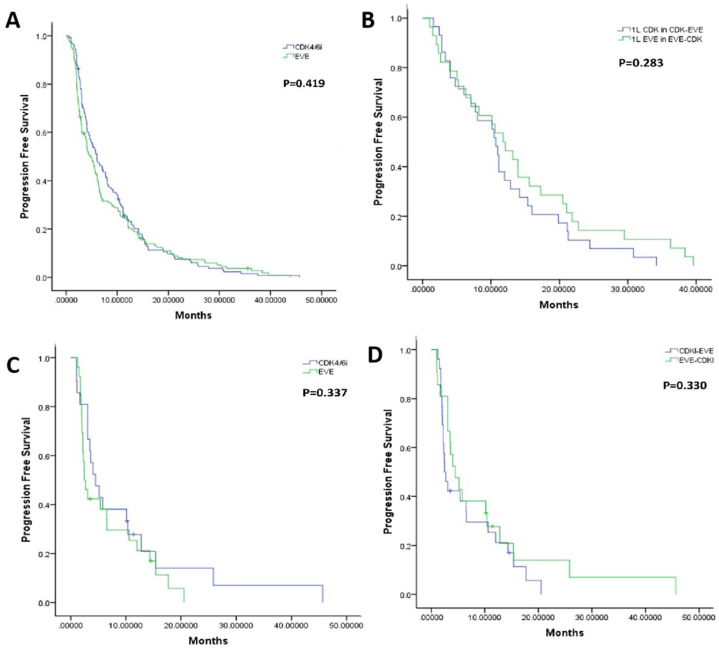
For all the 146 patients enrolled, the PFS for CDK4/6i and EVE were 5.9m and 4.3m, respectively, P = 0.419 (Fig. 2A). The median PFS for CDK4/6i and EVE in group A was 8.4 months and 2.5 months respectively while the median PFS for EVE and CDK4/6i in group B was 6.1 months and 4.6 months respectively.
Fig. 2.
A. Progression free survival of CDK4/6i and EVE for all 146 patients enrolled.
B. Progression free survival of patients receiving CDK4/6i and EVE as 1L ET.
C. Progression free survival of patients receiving EVE and CDK4/6i as 2L ET after progression of CDK4/6i and EVE treatment.
D. Progression free survival (PFStotal) of patients received 1L CDK4/6i followed by 2L EVE treatment or vice versa.
The median PFS of patients received CDK4/6i or EVE as first line endocrine treatment was not different, 10.7m vs 11.8m, respectively (P = 0.283, HR = 0.746, 95%CI: 0.435–1.278) (Fig. 2B). The PFS of EVE or CDK4/6i as second line treatment was 2.4m vs 4.5m (P = 0.337, HR = 0.738, 95%CI: 0.394–1.381) (Fig. 2C).
For patients in Subset A and Subset B, the PFStotal was not different, 13.1m vs 17.7m (P = 0.330, HR = 0.738, 95%CI: 0.399–1.365) (Fig. 2D).
4.2. Overall survival
For all the 146 patients enrolled, the estimated 5y OS for CDK4/6i or EVE was 52.1 % and 55.7 %, respectively (P = 0.227) (Fig. 3A). The 5y OS of patients in Group A and Group B were 62.0 % vs 57.4 %, P = 0.569 (Fig. 3B). For patients in Subset A or Subset B, the OS were also not significantly different (73.7 % vs 49.4 %, P = 0.196, HR = 2.013, 95%CI: 0.698–5.804) (Fig. 3C).
Fig. 3.
A. Overall survival of CDK4/6i and EVE for all 146 patients enrolled.
B. Overall survival of patients in CDK-EVE and EVE-CDK groups.
C. Overall survival of patients received 1L CDK4/6i followed by 2L EVE treatment or vice versa.
5. Discussion
CDK4/6i-based therapy has now secured its position as the standard treatment for first- and second-line patients if CDK4/6i was not applied in the previous line. It basically lies on several influential and prospective clinical trials, involving PALOMA, MONALEESA and MONARCH series. In first-line setting, PFS was significantly improved by adding CDK4/6i to endocrine therapy [10,22]. And Ribociclib plus ET showed significantly longer OS than ET alone as in pre-/perimenopausal or postmenopausal patients [14,15]. In second-line setting, data from POLOMA-3 [11], MONARH-2 [12] and MONALEESA-3 [23] suggested that PFS and OS of CDK4/6i combined with ET were about 10m and 2y. The standard treatment after patients progressed on CDK4/6i has not been established. mTORi [19,24] and PI3Ki [25] were supposed to be effective with limited data. Some retrospective studies reported results for mTORi following CDK4/6i. Data from these trials indicated that the median PFS of mTORi after 1L CDK4/6i was 3–6 months [19,24]. In our study, 56 patients received EVE after progression of CDK4/6i, the mPFS of EVE was only 2.6m. Among the 56 patients, 26 patients received 1–7 lines of chemotherapy before EVE which might contribute to the worse PFS.
Actually, the concept of combining target agent and hormonal therapy to overcome endocrine resistance was first established by mTOR inhibitor. Everolimus was the first targeted therapy to obtain its Marketing Authorization in China. Results from BOLERO [7] and MIRACLE [4] indicated that mPFS of EVE plus endocrine therapy was significantly higher than endocrine therapy alone. Only a few trials had reported the efficacy of CDK4/6i after mTORi. Dhakal et al. [26] performed a small retrospective study of 23 patients treated with palbociclib after prior treatment with everolimus. 95 % of patients enrolled were treated with ≥3 lines of ET. They reported an ORR of 0 % and a median PFS of 2.9 months. In Rusquec's research, for patients who previously received a median of 5 (1–14) lines of treatment including EVE, the mPFS was 5.8m [27]. Other studies that included larger numbers of patients have reported median PFS of approximately 5–6 months [28]. 90 patients received CDK4/6i after EVE in our research, the PFS of CDK4/6i was 4.6m which supported the previously reported results.
Till now, no clinical trial has directly compared the survival of patients receiving CDK4/6i or EVE as first or second-line treatment. In first-line setting, the PFS of CDK4/6i was about 20–30m, but most patients enrolled were endocrine-sensitive. In PALOMA-1, 17.6 % of patients enrolled were with DFI≤12m (defined as the time from the end of adjuvant therapy to disease progression) [10]. In Phase III study PALOMA-2, about 21.9 % of patients enrolled were with DFI≤12m [22]. MONARCH-3 [29] and MONALEESA-3 [15] first-line treatment cohort enrolled no patients with DFI≤12m. In MONALEESA-7 trial, most patients (70 %) were endocrine sensitive [14]. First-line treatment clinical trials of EVE enrolled more endocrine-resistant patients and the PFS was shorter. In BOLERO-2 first-line treatment cohort [30] and BOLERO-5 [31], patients enrolled were those who got recurrence or metastasis during or within 12 months after the end of adjuvant treatment. The PFS were only 11.5 and 7.4m in EVE group. MIRACLE research of our team reported better PFS [4], the mPFS was 19.4m in everolimus plus letrozole group vs 12.9m in letrozole group (hazard ratio, 0.64; 95 % CI: 0.46–0.89; P = 0.008). In Miracle trial, 88.1 % patients were endocrine-resistance, 57.4 % patients with visceral metastasis. When EVE was used in more endocrine-sensitive patients as first-line treatment, the mPFS was even longer. In BOLERO-4 trial [32], nonsteroidal aromatase inhibitors must have been completed 1 or more years before enrollment. The median PFS was 22.0m for everolimus plus exemestane in BOLERO-4 which was similar to the PFS of CDK4/6i from PALOMA tirals. In second-line setting, our team previously compared the benefits and safety of agents including CDK4/6 inhibitors, PI3K/mTOR inhibitors, and HDAC inhibitors by conducting network meta-analysis. We found that both mTOR inhibitor and CDK4/6 inhibitor-based regimens demonstrated superior clinical efficacy and comparable safety profiles as second-line treatment in patients with HR+/HER2- MBC [33].
From data mentioned above, we found that with the more precious definition of endocrine resistance and with better management of EVE's side effect, the PFS of EVE in first or second line setting was more close to that of CDK4/6i during the latest years. In this study, we got similar results. 59.6 % patients in our research were endocrine-resistance and more than half received prior treatment before enrollment, the median PFS of patients received CDK4/6i or EVE as first or second line endocrine treatment were not different.
Rare studies had compared the results from different treatment sequence of CDK and EVE. Kim et al. compared CDK4/6i-EVE and EVE-CDK4/6i in Korea patients [34]. It was a single-center retrospective trial. Since most survival outcomes were not significantly different between the two groups, the researchers recommended CDK4/6i- and EVE-based treatments can be valid options in circumstances where the other treatment had been already given. Actually, in SONIA study [35], CDK4/6i used in first line treatment didn't show better PFS/OS or QOL compared with in second line. In our research, the PFStotal was not significantly different between Subset A and Subset B, which supported the results form Kim's trial and SONIA trial. Taken all these together, we assume that although these target agents greatly improve the outcome of HR+/HER2- ABC patients, the sequence of target agents may not matter. Although both SONIA trial and our study might not be practice changing, it showed alternative way of patients management and broken fixed thinking patterns.
The PFS of CDK and EVE in our study was much shorter than those previously reported. The reasons included that patients enrolled in our study was younger. The median age of patients was 52y, while most trials previously reported enrolled postmenopausal patients. Secondly, in PALOMA or BOLERO trials about half patients were with visceral metastasis, while in our study more than 80 % patients had visceral metastasis. Thirdly, about 60 % patients in our study were endocrine-resistant as mentioned above. Besides, in our study, more patients in CDK-EVE group has liver and visceral metastasis and most patients received palbociclib as CDK4/6i. As we know, some trails with palbociclib showed no survival benefit. In a recent study by Roncato et al. [36], the impact of body mass index (BMI) on the pharmacokinetics and clinical outcomes of palbociclib was investigated, revealing that lower BMI may be associated with reduced drug exposure and potentially worse clinical outcomes. These findings suggest that BMI could be an important factor to consider in dose adjustment of palbociclib for optimizing treatment efficacy. In our study, more than 80 % of patients were with BMI ≤25 kg/m2 which might influence the survival data. These factors mentioned above may contribute to the shorter PFS in our study.
There are some limitations of our study. Firstly, the sample size of our study was small which may affect the statistical power and results explanation of the study. And we were unable to stratify patients based on specific CDK4/6i, as there were too few patients received abemaciclib, ribociclib and dalpiciclib to allow for adequate subgroup analysis. We are now collecting more patients for more in-depth subgroup analyses and to define the accurate patient who is suitable for special treatment sequence. Secondly, our results were derived from retrospective medical records. The records might be not standardized. This factor can lead to several biases including selection of patients and the choice of treatment. In addition, since these medications are oral, it can be difficult to accurately assess adverse effects, patients’ compliance, dosage and so on. Thirdly, we defined 1L or 2L ET irrespective of chemotherapy and endocrine resistance. Additionally, this study was conducted in China, it should be careful to extend these conclusions to other populations as we know genetic factors and healthcare systems might influence the results. Considering the limitations mentioned above, the progress of Artificial Intelligence [37] and precise treatment in HR + HER2-breast cancer, it is necessary to design a large, randomized, prospective and more comprehensive trial to address the unanswered questions.
6. Conclusion
Based on multicenter and real-world data, we first reported that the sequence of CDK4/6i and EVE did not impact the survival outcome of patients. When CDK4/6i was not feasible in the front line, EVE can be a reasonable choice for some patients.
Funding
This study was funded by CAMS Innovation Fund for Medical Sciences (CIFMS) 2023-I2M-C&T-B-077, Major project of Medical Oncology Key Foundation of Cancer Hospital Chinese Academy of Medical Sciences (CICAMS-MOMP202203) and Wu Jieping Medical Foundation Clinical Research Special Fund (320.6750.17191).
Availability of data and materials
Supporting data are available by request to the corresponding author in accordance with regulations from NCBR and SweLiv.
Ethics approval and consent to participate
Patients provided written informed consent. The trial was conducted in accordance with the Declaration of Helsinki and the protocol was approved by the independent ethics committees and institutional review boards of the participating centers.
Consent for publication
Not applicable.
CRediT authorship contribution statement
Yuqian Liao: Writing – original draft, Software, Methodology, Investigation, Formal analysis. Yujing Tan: Writing – original draft, Resources, Methodology, Investigation, Formal analysis, Data curation. Yipeng Li: Writing – review & editing, Visualization, Software, Data curation. Fei Ma: Writing – review & editing, Validation, Supervision, Project administration, Data curation, Conceptualization. Jiayu Wang: Writing – review & editing, Supervision, Project administration, Methodology, Data curation. Pin Zhang: Writing – review & editing, Supervision, Resources, Project administration. Qing Li: Writing – review & editing, Validation, Supervision, Resources, Methodology, Data curation. Qiao Li: Resources, Project administration, Methodology, Formal analysis, Data curation. Yang Luo: Visualization, Resources, Project administration, Formal analysis, Data curation. Bo Lan: Visualization, Software, Resources, Methodology, Formal analysis, Data curation. Shanshan Chen: Validation, Resources, Project administration, Methodology, Investigation. Binghe Xu: Writing – review & editing, Validation, Supervision, Conceptualization. Hanfang Jiang: Writing – review & editing, Supervision, Resources, Project administration, Methodology, Investigation, Conceptualization. Weihong Zhao: Writing – review & editing, Validation, Supervision, Resources, Project administration, Funding acquisition, Conceptualization. Ying Fan: Writing – review & editing, Supervision, Resources, Funding acquisition, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Hanfang Jiang, Email: hfjiangcn@163.com.
Weihong Zhao, Email: zhaowh0818@163.com.
Ying Fan, Email: fanying@cicams.ac.cn.
References
- 1.Prat A., et al. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast. 2015;24(Suppl 2):S26–S35. doi: 10.1016/j.breast.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Waks A.G., Winer E.P. Breast cancer treatment: a review. JAMA. 2019;321(3):288–300. doi: 10.1001/jama.2018.19323. [DOI] [PubMed] [Google Scholar]
- 3.Bachelot T., et al. Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer with prior exposure to aromatase inhibitors: a GINECO study. J. Clin. Oncol. 2012;30(22):2718–2724. doi: 10.1200/JCO.2011.39.0708. [DOI] [PubMed] [Google Scholar]
- 4.Fan Y., et al. Effectiveness of adding everolimus to the first-line treatment of advanced breast cancer in premenopausal women who experienced disease progression while receiving selective estrogen receptor modulators: a phase 2 randomized clinical trial. JAMA Oncol. 2021;7(10) doi: 10.1001/jamaoncol.2021.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmid P., et al. Fulvestrant plus vistusertib vs fulvestrant plus everolimus vs fulvestrant alone for women with hormone receptor-positive metastatic breast cancer: the MANTA phase 2 randomized clinical trial. JAMA Oncol. 2019;5(11):1556–1564. doi: 10.1001/jamaoncol.2019.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piccart M., et al. Everolimus plus exemestane for hormone-receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: overall survival results from BOLERO-2†. Ann. Oncol. 2014;25(12):2357–2362. doi: 10.1093/annonc/mdu456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yardley D.A., et al. Everolimus plus exemestane in postmenopausal patients with HR(+) breast cancer: BOLERO-2 final progression-free survival analysis. Adv. Ther. 2013;30(10):870–884. doi: 10.1007/s12325-013-0060-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giuliano M., et al. Endocrine treatment versus chemotherapy in postmenopausal women with hormone receptor-positive, HER2-negative, metastatic breast cancer: a systematic review and network meta-analysis. Lancet Oncol. 2019;20(10):1360–1369. doi: 10.1016/S1470-2045(19)30420-6. [DOI] [PubMed] [Google Scholar]
- 9.Finn R.S., et al. Palbociclib and letrozole in advanced breast cancer. N. Engl. J. Med. 2016;375(20):1925–1936. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 10.Finn R.S., et al. Overall survival results from the randomized phase 2 study of palbociclib in combination with letrozole versus letrozole alone for first-line treatment of ER+/HER2- advanced breast cancer (PALOMA-1, TRIO-18) Breast Cancer Res. Treat. 2020;183(2):419–428. doi: 10.1007/s10549-020-05755-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cristofanilli M., et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17(4):425–439. doi: 10.1016/S1470-2045(15)00613-0. [DOI] [PubMed] [Google Scholar]
- 12.Sledge G.W., Jr., et al. Monarch 2: abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J. Clin. Oncol. 2017;35(25):2875–2884. doi: 10.1200/JCO.2017.73.7585. [DOI] [PubMed] [Google Scholar]
- 13.Sledge G.W., Jr., et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy-MONARCH 2: a randomized clinical trial. JAMA Oncol. 2020;6(1):116–124. doi: 10.1001/jamaoncol.2019.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu Y.S., et al. Updated overall survival of ribociclib plus endocrine therapy versus endocrine therapy alone in pre- and perimenopausal patients with hr+/HER2- advanced breast cancer in MONALEESA-7: a phase III randomized clinical trial. Clin. Cancer Res. 2022;28(5):851–859. doi: 10.1158/1078-0432.CCR-21-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neven P., et al. Updated overall survival from the MONALEESA-3 trial in postmenopausal women with HR+/HER2- advanced breast cancer receiving first-line ribociclib plus fulvestrant. Breast Cancer Res. 2023;25(1):103. doi: 10.1186/s13058-023-01701-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schettini F., et al. Overall survival of CDK4/6-inhibitor-based treatments in clinically relevant subgroups of metastatic breast cancer: systematic review and meta-analysis. J Natl Cancer Inst. 2020;112(11):1089–1097. doi: 10.1093/jnci/djaa071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xi J., Ma C.X. Sequencing endocrine therapy for metastatic breast cancer: what do we do after disease progression on a CDK4/6 inhibitor? Curr. Oncol. Rep. 2020;22(6):57. doi: 10.1007/s11912-020-00917-8. [DOI] [PubMed] [Google Scholar]
- 18.Li Y., et al. A multicenter analysis of treatment patterns and clinical outcomes of subsequent therapies after progression on palbociclib in HR+/HER2- metastatic breast cancer. Ther Adv Med Oncol. 2021;13 doi: 10.1177/17588359211022890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choong G.M., et al. Clinical management of metastatic hormone receptor-positive, HER2-negative breast cancer (MBC) after CDK 4/6 inhibitors: a retrospective single-institution study. Breast Cancer Res. Treat. 2022;196(1):229–237. doi: 10.1007/s10549-022-06713-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dhakal A., et al. Outcome of everolimus-based therapy in hormone-receptor-positive metastatic breast cancer patients after progression on palbociclib. Breast Cancer. 2020;14 doi: 10.1177/1178223420944864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cardoso F., et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5) Ann. Oncol. 2020;31(12):1623–1649. doi: 10.1016/j.annonc.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gelmon K., et al. Efficacy and safety of palbociclib in patients with estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with preexisting conditions: a post hoc analysis of PALOMA-2. Breast. 2021;59:321–326. doi: 10.1016/j.breast.2021.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slamon D.J., et al. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N. Engl. J. Med. 2020;382(6):514–524. doi: 10.1056/NEJMoa1911149. [DOI] [PubMed] [Google Scholar]
- 24.Sawaki M., et al. Real-world treatment patterns of subsequent therapy after palbociclib in patients with advanced breast cancer in Japan. Breast. 2023;70:1–7. doi: 10.1016/j.breast.2023.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rugo H.S., et al. Alpelisib plus fulvestrant in PIK3CA-mutated, hormone receptor-positive advanced breast cancer after a CDK4/6 inhibitor (BYLieve): one cohort of a phase 2, multicentre, open-label, non-comparative study. Lancet Oncol. 2021;22(4):489–498. doi: 10.1016/S1470-2045(21)00034-6. [DOI] [PubMed] [Google Scholar]
- 26.Dhakal A., et al. Efficacy of palbociclib combinations in hormone receptor-positive metastatic breast cancer patients after prior everolimus treatment. Clin. Breast Cancer. 2018;18(6):e1401–e1405. doi: 10.1016/j.clbc.2018.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.du Rusquec P., et al. Efficacy of palbociclib plus fulvestrant after everolimus in hormone receptor-positive metastatic breast cancer. Breast Cancer Res. Treat. 2018;168(2):559–566. doi: 10.1007/s10549-017-4623-8. [DOI] [PubMed] [Google Scholar]
- 28.KovaČ A., et al. Efficacy and safety of selective cyclin-dependent kinases 4/6 inhibitors in hormone-receptor-positive, HER2-negative advanced breast cancer - results from a real-world setting. Cancer Treat Res Commun. 2020;25 doi: 10.1016/j.ctarc.2020.100201. [DOI] [PubMed] [Google Scholar]
- 29.Goetz M.P., et al. Monarch 3: abemaciclib as initial therapy for advanced breast cancer. J. Clin. Oncol. 2017;35(32):3638–3646. doi: 10.1200/JCO.2017.75.6155. [DOI] [PubMed] [Google Scholar]
- 30.Beck J.T., et al. Everolimus plus exemestane as first-line therapy in HR⁺, HER2⁻ advanced breast cancer in BOLERO-2. Breast Cancer Res. Treat. 2014;143(3):459–467. doi: 10.1007/s10549-013-2814-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shao Z., et al. BOLERO-5: a phase II study of everolimus and exemestane combination in Chinese post-menopausal women with ER+/HER2- advanced breast cancer. Ann. Oncol. 2021;32(S5):S463. doi: 10.1007/s12672-024-01027-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Royce M., et al. Everolimus plus endocrine therapy for postmenopausal women with estrogen receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: a clinical trial. JAMA Oncol. 2018;4(7):977–984. doi: 10.1001/jamaoncol.2018.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ji D., et al. CDK4/6 inhibitors, PI3K/mTOR inhibitors, and HDAC inhibitors as second-line treatments for hormone receptor-positive, HER2-negative advanced breast cancer: a network meta-analysis. BMC Cancer. 2023;23(1):805. doi: 10.1186/s12885-023-11290-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeong H., et al. Comparison of the effectiveness and clinical outcome of everolimus followed by CDK4/6 inhibitors with the opposite treatment sequence in hormone receptor-positive, HER2-negative metastatic breast cancer. Cancer Res Treat. 2022;54(2):469–477. doi: 10.4143/crt.2021.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sonke G.S., et al. Primary outcome analysis of the phase 3 SONIA trial (BOOG 2017-03) on selecting the optimal position of cyclin-dependent kinases 4 and 6 (CDK4/6) inhibitors for patients with hormone receptor-positive (HR+), HER2-negative (HER2-) advanced breast cancer (ABC) J. Clin. Oncol. 2023;41(17_suppl) LBA1000-LBA1000. [Google Scholar]
- 36.Roncato R., et al. Clinical impact of body mass index on palbociclib treatment outcomes and effect on exposure. Biomed. Pharmacother. 2023;164 doi: 10.1016/j.biopha.2023.114906. [DOI] [PubMed] [Google Scholar]
- 37.Doshi R., et al. Artificial intelligence's significance in diseases with malignant tumours. Mesopotamian Journal of Artificial Intelligence in Healthcare. 2023;2023(2023):35–39. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Supporting data are available by request to the corresponding author in accordance with regulations from NCBR and SweLiv.