Abstract
Ischemic stroke is a major global public health concern that lacks effective treatment options. A significant challenge lies in delivering therapeutic agents to the brain due to the restrictive nature of the blood-brain barrier (BBB). The BBB's selectivity hampers the delivery of therapeutically relevant quantities of agents to the brain, resulting in a lack of FDA-approved pharmacotherapies for stroke. In this article, we review therapeutic agents that have been evaluated in clinical trials or are currently undergoing clinical trials. Subsequently, we survey strategies for synthesizing and engineering nanoparticles (NPs) for drug delivery to the ischemic brain. We then provide insights into the potential clinical translation of nanomedicine, offering a perspective on its transformative role in advancing stroke treatment strategies. In summary, existing literature suggests that drug delivery represents a major barrier for clinical translation of stroke pharmacotherapies. While nanotechnology has shown significant promise in addressing this challenge, further advancements aimed at improving delivery efficiency and simplifying formulations are necessary for successful clinical translation.
Keywords: Ischemic stroke, Nanotechnology, Blood-brain barrier, Clinical translation
Graphical abstract
Highlights
-
•
Ischemic stroke is a major global public health concern with no effective treatment options.
-
•
Inadequate drug delivery represents a significant hurdle for the clinical translation of pharmacological therapies.
-
•
Nanotechnology presents a promising approach to overcoming this drug delivery challenge.
1. Introduction
Stroke is a significant global public health issue accounting for one in every 20 deaths [1]. Stroke can be classified as ischemic, resulting from an artery blockage, or hemorrhagic, stemming from a ruptured vessel. Ischemic stroke represents the more prevalent form, constituting around 87 % of stroke cases [2]. As the population ages, the burden of ischemic stroke is predicted to rise [3]. Despite its prevalence, there are no FDA-approved pharmacotherapies for this disease.
At present, the only approved therapeutic approaches for the clinical management of stroke include intravenous tissue-type plasminogen activator (tPA) administration and mechanical thrombectomy, both designed for reperfusion [[4], [5], [6]]. These approaches are inherently limited in that, in addition to increasing the risk of inducing cerebral hemorrhage and secondary damage to ischemic tissue, the therapeutic window for intravenous thrombolysis is narrow [7]. Considering this time constraint and the reality that there still are no optimized reperfusion or neuroprotective strategies, the current therapies extend their benefits to only a fraction of stroke patients due to eligibility constraints.
From a pathophysiological perspective, ischemic stroke manifests as an acute deprivation of oxygen and nutrients, culminating in local acidosis, activation of the Na+/H+ exchange pathway, and a rapid surge in reactive oxygen species (ROS) [8,9]. The distorted activation of ion exchangers such as the Na+/H+ exchange pathway contributes to cellular swelling, cerebral edema, and the release of excitatory neurotransmitters, particularly glutamate. This cascade triggers excitotoxicity, initiating irreversible neuronal cell death [10] (summary of these processes seen in Fig. 1A). The dramatically increased ROS production ultimately overwhelms endogenous antioxidant mechanisms, inducing substantial toxicity to the cells within the neurovascular unit (NVU) [11]. In the subacute phase, inflammation initiates and is the principal driver of cell death within the ischemic brain [9]. This intricate sequence of events leads to the irreversible damage of the ischemic core, juxtaposed with the potentially salvageable hypoperfused tissue surrounding this core, known as the penumbra. An overview of the time-dependent factors of the described ischemic injury pathology can be seen in Fig. 1B. From a clinical perspective, the penumbra represents the pivotal target for managing ischemic stroke.
Fig. 1.
An overview of the pathophysiological processes and its time course after ischemic stroke. (A) After cerebral ischemia, the resulting hypoxia triggers the activation of microglia, astrocytes, pericytes, and peripheral infiltrating cells near the ischemic site. This cascade leads to the generation of ROS, inflammatory factors, and chemokines, and, consequently, structural transformations within endothelial cells (ECs) and tight junctions (TJs). The intricate interplay between these components simultaneously contributes to the disruption and repair of the BBB. (B) The 2 weeks following ischemic insult, termed the acute phase, excitotoxicity, and oxidative stress significantly contribute to brain damage. Transitioning into the subsequent subacute phase, which can extend up to 6 months, inflammation plays a dualistic role, manifesting as both detrimental and beneficial, depending on the context. Created with BioRender.com.
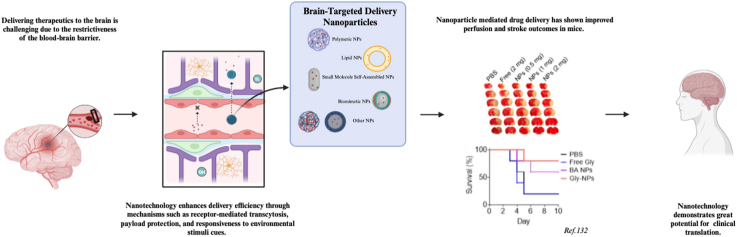
Due to its unique pathophysiological mechanisms, efficacious management of ischemic stroke may necessitate the formulation of multimodal therapeutic strategies aimed at precisely targeting the penumbra across distinct stages of the disease, with the imperative of timely intervention. Unfortunately, the efficient delivery of therapeutic agents to the brain poses a formidable challenge in ischemic stroke treatment, primarily due to the highly selective nature of the blood-brain barrier (BBB). Notwithstanding the potential for partial BBB compromise following ischemic insult, this barrier effectively prevents the delivery of a pharmacological dosage of most therapeutics to the brain. In this article, we undertake an in-depth review of the therapeutic agents that have been or are currently being evaluated in clinical trials, following the advancements in the development of nanotechnology approaches for drug delivery to the ischemic brain.
2. BBB as the major hurdle for stroke drug delivery
2.1. BBB and its normal physiological functions
The BBB, a selective barrier that protects the microenvironment of the central nervous system (CNS) from the peripheral circulatory system, is composed of endothelial cells (ECs), pericytes, and astrocytes. ECs line the lumen of the BBB and are situated on the apical surface of blood vessels. Unlike in other organs, ECs in the brain lack transport mechanisms and don't allow the passage of large molecules. The membrane of ECs contains various ATP-binding cassette (ABC) transporters, including P-glycoprotein (P-gp), multidrug resistance-associated proteins (MRPs), and breast cancer resistance proteins (BCRRs), that transport selected substances that leak through ECs back into the bloodstream. Tight junctions (TJs) between ECs are formed by proteins like claudins, occludin, and junctional adhesion molecules (JAMs). These are connected to actin by scaffolding proteins known as zonula occludens (ZO) −1/2/3. Astrocytes, covering more than 90 % of the capillary walls and situated on the BBB's basal luminal membrane, provide additional support to endothelial capillaries. Astrocytes influence the BBB by regulating EC proliferation, differentiation, and the synthesis of TJs. Pericytes, positioned between ECs and astrocytes and mostly within the basement membrane, also play an important role. Pericytes contain specialized proteins like alpha-smooth muscle actin (α-SMA), tropomyosin, and myosin, endowing them with contractile ability to control BBB permeability [12]. An overview of the key components that define the BBB is also illustrated in Fig. 1A.
Due to its distinct physical and cellular makeup, the BBB effectively limits the passage of most substances from the blood to the brain through both paracellular and transcellular transport mechanisms. The BBB's selectivity permits only lipid-soluble small molecules under 400 Da, which are not substrates to the ABC transporters, to cross. While this specialized barrier staunchly prevents the infiltration of pathogens and harmful compounds from the peripheral circulatory system into the CNS, it equally hinders the entry of most therapeutic agents into the brain. Consequently, addressing diseases in the brain becomes exceptionally challenging. The selectively permeable nature of the BBB stands as the primary hurdle in delivering drugs to the CNS.
2.2. BBB compromised by an ischemic insult
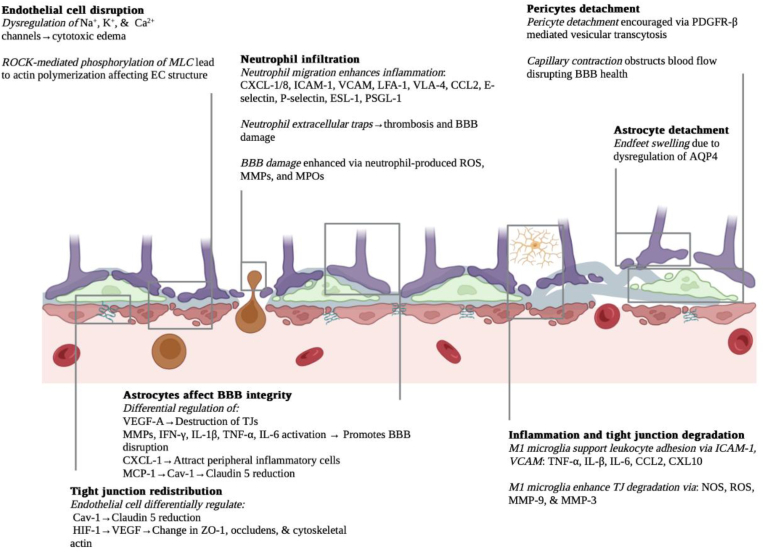
Following an ischemic insult, the BBB experiences a partial disruption. In murine stroke models, investigations demonstrate that this post-stroke BBB breakdown is a reflection of intricate physiological alterations occurring at both the cellular and molecular levels [13,14]. Following an ischemic insult, the impacted regions of the brain are subjected to oxygen-glucose deprivation (OGD), triggering abnormal molecular responses across various cell types. Notably, major significant molecular responses include: 1) Activation of the ROCK/MLC pathway, resulting in the phosphorylation of myosin light chain (MLC) and actin polymerization within endothelial cells (ECs) [15]; 2) Expression and activation of primary and secondary active transporters, including those found naturally in brain cells like aquaporin-4 (AQP4) [16], as well as those that emerge de novo in the brain post-stroke, such as the SUR1-TRPM4 channel [17], leading to cerebral edema [17]; 3) Cytosolic translocation of Caveolin-1 (Cav-1), subsequently resulting in the redistribution and endocytosis of Claudin-5 [18]; 4) Activation of the hypoxia-inducible factor (HIF) pathway, triggering the expression and activation of vascular endothelial growth factor (VEGF) and leading to the reduction of TJ proteins [19]; 5) Astrocytic expression of PDGF-β and other factors resulting in capillary contraction, hindrance of blood flow, and vesicular transcytosis [20]; 6) Generation of matrix metalloproteinases (MMPs) and adhesion molecules such as ICAM-1 and VCAM, contributing to the degradation of TJs and recruitment of peripheral leukocytes [21]; and 7) Release of cytokines, MMPs, and DNA, giving rise to the formation of neutrophil extracellular traps (NETs) and subsequent damage to the BBB [22,23] (Fig. 2).
Fig. 2.
Cellular and molecular mechanisms of BBB disruption after an ischemic stroke. Stroke onset initiates a heterogeneous cascade of cellular and molecular signaling pathways which result in alterations of cell structures, cell death, vasogenic and cytotoxic edema, inflammation, tight junction redistribution, and degradation, characterizing BBB disruption. Created with BioRender.com.
The timeline of BBB disruption can be largely described through a biphasic pattern. One study suggested that the most pronounced BBB damage occurs at 8 h and 120 h following cerebral ischemia [24], while another study indicated that the BBB opened significantly 1.5–2 min after ischemia-reperfusion (IR), closed for 1–4 h, and then reopened after 22 h [25]. This variance between these studies likely stems from a difference in the animal models used and methodologies employed to assess BBB permeability. In human patients, it was observed that BBB compromise manifests within the initial 90 h after symptom onset. BBB permeability, quantified as the ratio of infarct permeability to contralateral permeability, was calculated to reach its peak around 6–48 h [26]. The average time from ischemia onset to the detection of BBB disruption in human patients is approximately 12.9 h [27,28]. The initiation and duration of BBB leakage due to secondary injury can span from 3 days post-stroke and may remain detectable for up to 6 months in rodents and 12 months in patients [29]. The compromised BBB is beneficial, in that it enables the infiltration of peripheral immune cells to facilitate debris clearance and wound healing, and can be seen as an opportunity to deliver therapeutics to the brain [30]. However, significantly increased BBB permeability can also be harmful, leading to neuronal dysfunction, heightened intracranial pressure, and immune-mediated damage to neurons.
3. Pharmacotherapy for ischemic stroke
Here we focus on reviewing pharmacotherapeutic agents that are designed to promote post-stroke neurological recovery. These agents are developed to mainly target detrimental factors that contribute to secondary injury, including cerebral edema, excitotoxicity, oxidative stress, and inflammation [31]. Up to the present time, over 114 clinical trials, involving 49 pharmacotherapeutic agents, have been conducted. Among them, 21 agents have undergone assessment in phase 2/3 clinical trials (Table 1). Unfortunately, the collective outcomes have proven to be less than satisfactory, with only a handful of agents exhibiting promising clinical potential.
Table 1.
Phase 2/3 clinical trial explored pharmacotherapies for ischemic stroke.
| Registration number | Phase | Therapeutic | Description/Mechanism | Results | Year |
|---|---|---|---|---|---|
| ALIAS (NCT00235495) | 3 | Albumin | An endogenous plasma protein with important physiochemical properties, has commonly been regarded as an alternative hemodiluting agent to dextran | No clinical benefit of 25 % albumin given within 5 h after AIS | 2013 [32] |
| AXIS 2 (NCT00927836) | 2b | G-CSF | A potent neuronal growth factor with multimodal antiapoptotic, arteriogenic, and neurogenic properties | G-CSF did not provide any significant benefit with respect to either clinical outcome or imaging biomarkers | 2013 [33] |
| PIMSS (ACTRN12612000237886) | 2 | Minocycline | A semisynthetic tetracycline Neuroprotection |
Intravenous minocycline was safe but not efficacious | 2013 [34] |
| CERE-LYSE-1 (NCT00840671) | 3 | Cerebrolysin | A low-molecular-weight neuropeptide and free amino acid of porcine origin; Neuroprotective and neurotrophic properties |
The combination of Cerebrolysin with tPA is safe for treatment of acute ischemic stroke but did not improve outcome at day 90 | 2013 [35] |
| URICO-ICTUS (NCT00860366) | 2b/3 | Uric acid | A small molecule that has antioxidant and neuroprotectant activities | The addition of uric acid to thrombolytic therapy did not increase the proportion of patients who achieved excellent outcome after stroke compared with placebo, but it did not lead to any safety concerns | 2014 [36] |
| NCT02002390 | 2 | Fingolimod | A sphingosine analog that acts on sphingosine-1-phosphate receptors; Immunology modulator | Combination therapy of fingolimod and alteplase was well tolerated, attenuated reperfusion injury, and improved clinical outcomes in patients with acute ischemic stroke | 2015 [37] |
| ISRCTN71371114 | 2 | Erythropoietin | Anti-ischemic and anti-apoptotic properties, promotion of neovascularization, mobilization of endothelial progenitor cells, and enhancement of angiogenesis | Erythropoietin therapy significantly improved long-term neurological outcomes in patients after AIS | 2015 [38] |
| GAMES-RP (NCT01794182) | 2 | Glyburide (RP-1127) | A SUR1-TRPM4 channel antagonist that reduces cerebral edema | Intravenous glyburide was well tolerated in patients with large hemispheric stroke at risk for cerebral edema. There was no difference in the composite primary outcome | 2016 [39] |
| FAST-MAG (NCT00059332) | 3 | Magnesium | Vasodilatory and neuroprotective & glioprotective effects | Magnesium is not biologically neuroprotective in acute stroke | 2017 [40] |
| MASTERS (NCT01436487) | 2 | Multipotent adult progenitor cells | A bone marrow-derived, allogeneic, cell therapy product modulates the immune system | Administration of multipotent adult progenitor cells was safe in patients with AIS. No significant improvement was observed at 90 days in neurological outcomes | 2017 [41] |
| EPICA (NCT02793687) | 3 | MEXIDOL | A succinate salt that has antioxidant and antihypoxant and suppresses excitotoxicity | Use of Mexidol in the acute and early recovery phases of AIS is recommended | 2018 [42] |
| ACTION II (NCT02730455) | 2 | Natalizumab | An α-4 integrin-targeting immunomodulation antibody that inhibits transmigration of leukocytes across the vascular endothelium | Natalizumab administered ≤24 h after AIS did not improve patient outcomes | 2020 [43] |
| RESTORE BRAIN(NCT02877615) | 2 | S44819 | A GABA α5 antagonist improves neurological recovery | There was no evidence that S44819 improved clinical outcome in patients after AIS | 2020 [44] |
| ESCAPE-NA1 (NCT02930018) | 3 | Nerinetide | Neuroprotection A PSD95-NR2B inhibitory peptide decreases excitotoxicity |
Nerinetide did not improve the proportion of patients achieving good clinical outcomes after endovascular thrombectomy. Among patients who were not treated with alteplase, they observed a treatment effect | 2020 [45] |
| NCT02430350 | 3 | Compound Edaravone Injection | A novel neuroprotective agent with synergistic effects of antioxidant and anti-inflammatory | When edaravone dexborneol versus edaravone was administered within 48 h after AIS, 90-day good functional outcomes favored the edaravone dexborneol group, especially in female patients | 2021 [46] |
| CHARM (NCT02864953) | 3 | Glyburide (BIIB093) | Anti-edema A SUR1-TRPM4 channel antagonist reduces cerebral edema |
CHARM will include 680 participants across 22 countries | Recently Completed |
| MASTERS-2 (NCT03545607) | 3 | MultiStem | Neuroprotection/immunomodulation Adult-derived multipotent adult progenitor cells to provide neuroprotection, immunomodulation, and (less likely) cell replacement effects |
300 participants; Single intravenous infusion 18–36 h after AIS | Ongoing |
| PMZ-1620 (NCT04047563) | 3 | Sovateltide (PMZ-1620) | Neurological recovery An endothelin-B receptor agonist augments the activity of neuronal progenitor cells |
110 participants; Efficacy of sovateltide (PMZ-1620) in patients of AIS | Ongoing |
| NCT03639922 | 3 | Imatinib | Reduces intracerebral hemorrhage and edema | 1260 participants; Imatinib in acute ischemic stroke | Ongoing |
| NCT04904341 | 3 | Cerebrolysin | A low-molecular-weight neuropeptide and free amino acid of porcine origin; Neuroprotective and neurotrophic properties |
50 participants; Efficacy of cerebrolysin treatment as an add-on therapy to mechanical thrombectomy in AIS | Ongoing |
AIS, acute ischemic stroke; ALIAS, Albumin in Acute Ischemic Stroke Trial; AXIS 2, AX200 for the Treatment of Ischemic Stroke; G-CSF, Granulocyte colony-stimulating factor; PIMSS, Perth IV Minocycline Stroke Study; CERE-LYSE-1, Combined Treatment With Alteplase (Rt-PA) and Cerebrolysin in Acute Ischemic Hemispheric Stroke; Urico-Ictus, Efficacy Study of Combined Treatment With Uric Acid and rtPA in Acute Ischemic Stroke; FAST-MAG, Field Administration of Stroke Therapy-Magnesium Trial; MASTERS, Study to Examine the Effects of MultiStem in Ischemic Stroke; MEXIDOL, ethylmethylhydroxypyridine succinate; ACTION II, Safety and Efficacy of Intravenous Natalizumab in Acute Ischemic Stroke; RESTORE BRAIN, Efficacy and Safety Trial With S 44819 After Recent Ischemic Cerebral Event; ESCAPE-NA1, Safety and Efficacy of Nerinetide (NA-1) in Subjects Undergoing Endovascular Thrombectomy for Stroke; CHARM, Phase 3 Study to Evaluate the Efficacy and Safety of Intravenous BIIB093 (Glyburide) for Severe Cerebral Edema Following Large Hemispheric Infarction; MASTERS-2, MultiStem® Administration for Stroke Treatment and Enhanced Recovery Study; PMZ-1620, Efficacy of Sovateltide (PMZ-1620) in Patients of Acute Ischemic Stroke.
3.1. Glibenclamide
Cerebral edema commonly develops in patients with moderate to severe stroke, denoted by an NIH Stroke Scale (NIHSS) score exceeding 4, accounting for around half of the U.S. stroke cases [47,48]. The progression of cerebral edema in stroke patients unfolds through three stages [49,50]. In the initial stage, cytotoxic edema emerges shortly after stroke onset. This process involves cellular swelling caused by an influx of osmolytes, primarily Na+ and Cl–, guiding the movement of water from interstitial spaces into cells. This phenomenon takes place across all brain cell types. Following cytotoxic edema, the second stage sees the formation of ionic edema. This extracellular edema occurs while the BBB remains intact [51]. The transendothelial Na + gradient from cytotoxic edema drives the extravasation of osmolytes and water, propelled by potential energy. Hours after the ischemic insult, the final stage involves partial disruption of the BBB. Transendothelial channels allow protein and water passage from the vascular to the interstitial compartment, culminating in vasogenic edema.
At the molecular level, primary and secondary active transporters drive cytotoxic and ionic edema, including those found in brain cells, such as aquaporin-4 (AQP4), and those emerging de novo after stroke, like the SUR1-TRPM4 channel [17]. The SUR1-TRPM4 channel, typically not expressed or expressed minimally in a normal brain, becomes active when intracellular ATP is depleted following ischemic stroke. Under cerebral ischemia and hypoxia conditions, the SUR1-TRPM4 channel's expression surges across all cells in the NVU, including capillary endothelial cells, neurons, astrocytes, and oligodendrocytes [[52], [53], [54], [55]]. Abnormal SUR1 expression leads to sustained sodium influx, subsequent water influx, oncotic cell swelling, and cell death, resulting in space-occupying edema and even hemorrhagic transformation [51,53,56]. The SUR1-TRPM4 channel's activity can be effectively blocked by glyburide (INN: glibenclamide), a sulfonylurea diabetes medication that binds to SUR1 with subnanomolar affinity [57,58]. Animal studies showed that glyburide administration reduces edema, lesion volume, and mortality, and enhances neurological outcomes [53,[59], [60], [61], [62], [63]]. These pre-clinical studies led to clinical trials, where the glyburide formulation was designated as RP-1127. Initially assessed in the GAMES-PILOT study with 10 stroke patients [64,65], it was further evaluated in the GAMES-RP phase II clinical trial with 86 patients (18–80 years old) experiencing acute large-area AIS (within <10 h, average 9 h) [39]. At the 90-day follow-up, the glibenclamide group displayed no distinct primary endpoint differences compared to the placebo group. Subsequent analysis revealed reduced brain midline shift and a trend toward improved survival, implying reduced mass effect and water uptake [39,66]. These promising findings led to FDA approval for the recently completed phase III clinical trial, CHARM (NCT02864953) [67].
3.2. NA1
One of the primary mechanisms of neuronal injury following stroke is NMDAR-dependent excitotoxicity, which is induced by the binding of PSD-95 with NMDARs and nNOS at excitatory synapses [68,69]. It was shown that protection of neurons against NMDAR-mediated excitotoxicity could be achieved through disruption of the interaction of NMDARs with PSD-95 using the last nine amino acids of the carboxyl tail of GluN2B (NR2B9c) [68]. Since NR2B9c cannot penetrate the cell membrane, therapeutic use of NR2B9c requires fusion with TAT, a cell penetration peptide. The resulting Tat-NR2B9c peptide was evaluated in rodent and nonhuman primate models and demonstrated a significant reduction in infarct volume and improvements in neurological outcomes [68,70]. The promising pre-clinical studies led to serial clinical trials, in which Tat-NR2B9c was rebranded as NA1 45, 71. Results of the phase II clinical trial ENACT found that treatment with NA1 effectively reduced the number and volume of iatrogenic embolization strokes in patients with endovascular aneurysm repair [71]. These findings led to a multi-center, double-blind, randomized phase III clinical trial, ESCAPE-NA1, which was reportedly completed in 2020 [45]. Although the overall results of the study were neutral, NA1 treatment was found to be associated with improved 90-day clinical outcomes (59.3 % vs 49.8 %, RR 1.18, 95 % CI 1.10 to 1.38).
Further analysis showed that tPA degrades NA1, making NA1 in tPA-treated patients ineffective. To overcome this limitation, a new generation of plasmin-resistant NA1 formed by substituting cleavage-prone amino acids from their l-to their d-enantiomeric form was developed [72]. Currently, the new plasmin-resistant NA1 is being tested in human patients.
3.3. Uric acid (UA)
In response to the need for neuroprotective strategies alongside mechanical thrombectomy, the National Institutes of Health (NIH) recently launched the Stroke Preclinical Assessment Network (SPAN). This initiative was designed to evaluate selected pharmacotherapies for the treatment of ischemic stroke. In a recently concluded phase 1 pilot study, uric acid (UA) emerged as a particularly promising neuroprotective candidate based on its performance in rodent models [73]. UA, a product of purine metabolism, is typically quickly degraded by hepatic uricase to allantoin in most mammals. However, due to the nonfunctional uricase gene in humans [74], UA levels tend to be higher in humans than in most mammals.
UA acts as a robust endogenous antioxidant and scavenger of free radicals. Notably, its levels can surge during periods of high oxidative stress, such as in the case of ischemic stroke. Earlier experiments conducted on rats found that in ischemic conditions, adenosine triphosphate (ATP) degradation led to the generation of adenine and xanthine. This, in turn, triggered an upswing in xanthine oxidase production, resulting in increased UA generation and elevated oxidant formation [75].
In preclinical studies, UA has demonstrated a capacity to enhance stroke outcomes in various stroke models derived from hyperglycemic, female, and male mice [76,77]. It was also found that treatment with UA is synergistic with rtPA infusion [78]. There is evidence that UA has a limited ability to cross the BBB, as intravenous administration of UA led to increased plasma levels within 10 min, whereas brain tissue UA levels remained unaffected [79,80].
In the clinical domain, a study by Chamorro and colleagues reported a 12 % increase in the odds of a favorable clinical outcome for each milligram per deciliter of serum uric acid added [81], while another study suggested that low UA concentrations are modestly associated with positive short-term outcomes [82]. A pilot clinical study found that intravenous UA administration alongside rtPA infusion within 3 h of symptom onset was safe and that the treatment reduced the level of activated MMP-9 [83]. The Phase 2 URICO-ICTUS trial, involving 421 AIS patients treated with rtPA within 4.5 h of symptom onset, confirmed the safety of a single 90-min infusion of 1 g UA administration. Although there was an overall nonsignificant 6 % rise in the rate of favorable outcomes at follow-up, significant effects of UA therapy were found in various subgroups including women, hyperglycemic patients, and those treated with mechanical thrombectomy [84].
3.4. Minocycline
With its high lipophilicity, minocycline can penetrate the BBB. The compound's neuroprotective effects primarily stem from its capacity to inhibit microglial activation. This capacity, in turn, reduces T cell migration, cellular apoptosis, free radical generation, and inflammatory responses [85,86]. Moreover, minocycline exhibits a significant ability to restrain the production of MMP-9 [87], a pivotal factor implicated in the transformation of intracranial hemorrhage associated with thrombolytic therapy. Consequently, minocycline holds the potential for diminishing tissue damage and hemorrhage transformation. Supported by a Dose-Finding Study [88], minocycline is safe and compatible with tPA infusion. While the effectiveness of minocycline in treating acute ischemic stroke (AIS) was demonstrated in two distinct clinical trials [89,90], uncertainties persist regarding its capacity to significantly curtail mortality and disability post-AIS [34].
3.5. Other therapeutic agents
Numerous other therapeutic agents hold potential promise for the treatment of ischemic stroke. These include fasudil, a Rho-associated kinase (ROCK) inhibitor, tocilizumab (Actemra), an immunosuppressive agent, fingolimod (Gilenya, FTY720), an S1P analogue, and NEP1-40, a Nogo receptor (NgR) antagonist peptide. Among them, fasudil, tocilizumab, and fingolimod were characterized in the recently completed SPAN phase 1 pilot study.
Fasudil is a non-specific inhibitor of Rho kinases (ROCK), which are well-characterized therapeutic targets for ischemic stroke [91]. In rodent studies, Fasudil demonstrated compatibility with tPA as well as the potential to prevent MMP-9-related hemorrhagic transformation and reduce cerebral infarct size while improving neurological outcomes [[92], [93], [94], [95]]. Fasudil was tested in a placebo-controlled double-blind trial for its safety and efficacy. Fasudil was administered within 48 h of ischemic stroke onset and results revealed fasudil is safe for human use and that treatment with fasudil significantly improves clinical outcomes [96].
Veliparib is an inhibitor of poly (ADP-ribose) polymerase (PARP), which plays a pivotal role in the repair of DNA damage by catalyzing the transfer of an ADP ribose unit from NAD + to specific target proteins, including histones and transcription factors [97]. When PARP becomes overly active, it disrupts mitochondrial homeostasis by depleting NAD + reserves. This depletion leads to an escalation in the levels of reactive oxygen and nitrogen species, as well as a surge in intracellular Ca2+ concentrations. Several studies have demonstrated that PARP inhibitors can prevent the activation of microglia and enhance neuronal survival [98,99]. However, the exploration of veliparib's application for treatment of ischemic stroke remains limited.
Tocilizumab is a humanized monoclonal anti-interleukin-6 receptor antibody and used as an immunosuppressive medication for treatment of rheumatoid arthritis and systemic juvenile idiopathic arthritis. As cerebral ischemia causes time-dependent recruitment and activation of inflammatory cells, inhibition of the inflammatory response has the potential to reduce ischemic size and improve neurological outcomes. Experimental studies in murine stroke models showed that treatment with tocilizumab lowered the immune system's response to stroke and that the neuroprotective dose of tocilizumab was different between males and females [100,101].
Fingolimod functions as an agonist for sphingosine 1-phosphate (S1P) receptors and has demonstrated neuroprotective effects in various animal stroke models [102,103]. Mechanistically, fingolimod acts by impeding the migration of lymphocytes during the reperfusion phase. This action leads to the attenuation of inflammation within cerebral blood vessels and neural tissue, fostering improved blood circulation and evident neuroprotective outcomes. Fingolimod has been evaluated in two small-scale trials, where it significantly reduced infarct size and ameliorated neurological deficits in stroke patients [37,104]. Furthermore, fingolimod was characterized in a randomized and blinded clinical study involving 23 stroke patients who received either tissue plasminogen activator (tPA) or a combination of tPA and fingolimod within a time frame of 4.5–6 h after stroke onset. Results showed that the combination therapy group displayed not only superior clinical enhancements compared to the tPA-alone group but also notable improvements in anterograde reperfusion and retrograde collateral blood flow [105].
NEP1-40 is a Nogo receptor (NgR) antagonist peptide, which can inhibit the effect of Nogo-A and enhance short-term neurosynaptic plasticity and neurological function after cerebral ischemia [106]. Nogo-A has the effect of resisting synapse reconstruction after CNS injury. NgR1 is the receptor of Nogo-A. Inhibiting NgR1 can promote axon regeneration. Similar to NR2B9c, NEP1-40 is also a peptide drug. Therefore, they possess similar issues with ensuring efficient delivery.
4. Nanotechnology as a potential solution to address the challenges of effective drug delivery
As surmised, the therapeutic window for many effective pharmacotherapies is within the first 12 h of stroke onset yet, this presents a challenge as the mean time from the onset of ischemia to observation of BBB disruption is around 12.9 h [27,28,107]. In this period and beyond, the injured BBB is still highly selective and can be accompanied by reperfusion injury, tissue no-reflow, poor collateral circulation, hemorrhage transformation, impaired cerebrovascular auto-regulation function, and large hypoperfusion volume, all factors that impact clinical prognosis and treatment design [108]. As such, the BBB remains a major barrier to the development of pharmacotherapies that effectively treat stroke and might account for why some pharmacotherapeutic agents showed limited efficacy in the clinic. For example, it was well characterized that glyburide has a limited ability to penetrate the brain [109,110]. Using PET/CT and labeling glyburide with a radiotracer, we demonstrated there was no significant difference in glyburide uptake between the ischemic and the contralateral hemispheres in rats bearing large stroke, suggesting that the penetrability of glyburide is not enhanced in the ischemic brain [109]. To overcome the drug delivery hurdle, emerging nanotechnology approaches hold great promise [[111], [112], [113], [114]] as nanoparticles (NPs) can potentially be engineered through various mechanisms to enhance drug accumulation in the ischemic brain tissue [[115], [116], [117], [118]]. Employing NPs has additional advantages in that they provide physical protection to cargo agents that otherwise are insoluble, unstable, toxic, or substrates to ATP-binding cassette (ABC) transporters on the BBB.
4.1. Applications of NPs for drug delivery to the ischemic brain
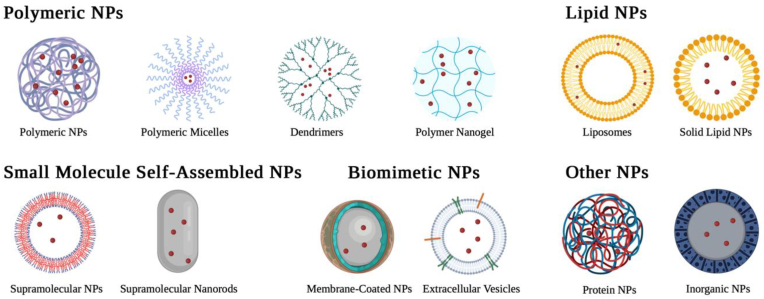
NPs can be classified based on their main compositions. Though not all types are extensively covered in this review, Fig. 3 exemplifies the structures of many NPs types and subtypes that are studied for ischemic stroke treatment. Please note this list is not exhaustive. To date, various types of NPs, including polymeric NPs, lipid NPs, small-molecule self-assembled NPs, protein NPs, metal NPs, and extracellular vesicles, have been explored for drug delivery to the ischemic brain.
Fig. 3.
Schematic representation of the types of NPs that can potentially be engineered for the treatment of ischemic stroke. Created with BioRender.com.
4.1.1. Polymeric NPs
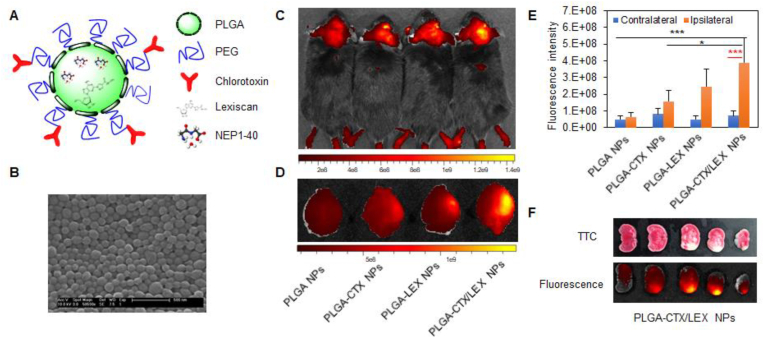
Polymeric NPs, mainly including solid NPs, micellular NPs, and dendrimer NPs, refer to those NPs consisting of polymeric materials, which are formed by covalent attachment of a set of small molecule monomers. This class of NPs often has excellent biocompatibility and sustained-release characteristics, making them suitable for various drug delivery applications [106,113,[119], [120], [121]]. In a recent study using poly(lactic-co-glycolic acid) (PLGA) as the starting material, one of the most often used polymers in the biomedical field, we synthesized and engineered solid polymeric NPs for autocatalytic brain targeting through surface conjugation of chlorotoxin and internal encapsulation of lexiscan. We showed that the resulting NPs were capable of efficiently penetrating the ischemic brain and delivering peptide NEP1-40 for effective treatment of ischemic stroke [106] (Fig. 4). Through a similar approach, Lu and colleagues synthesized micellular NPs using a ROS-scavenging polymer and showed that the NPs could be utilized for efficient delivery of rapamycin to the ischemic brain for stroke treatment [122].
Fig. 4.
Targeted drug delivery to ischemic stroke via chlorotoxin-anchored, lexiscan-loaded nanoparticles improved drug delivery capabilities. (A) Schematic diagram of a NEP1-40-loaded PLGA-CTX/LEX NP. (B) Representative image of NEP1-40-loaded PLGA-CTX/LEX NPs as captured by scanning electron microscope. Scale bar: 500 nm. (C) Representative image of NPs in the brains of live animals. (D) Representative image of NPs in the excised brains. (E) Semi-quantification of NPs in the excised brains. (F) Representative images of brain slices prepared from mice receiving 2 treatments of unlabeled PLGA-CTX/LEX NPs prior to the final administration of IR780-loaded PLGA-CTX/LEX NPs. Reproduced from Ref. [106].
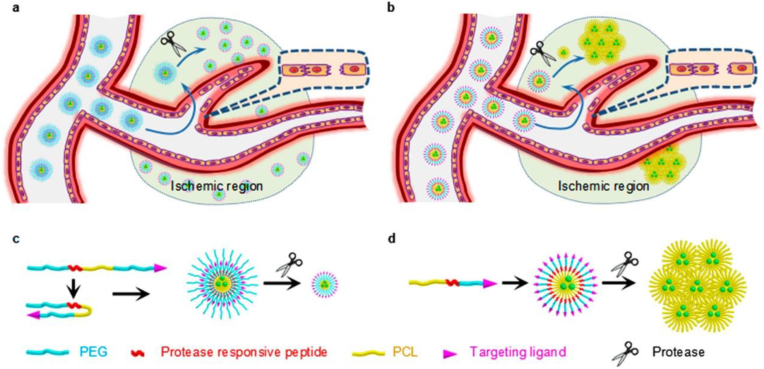
Unlike solid NPs, which maintain their structures during delivery, micellular NPs have a relatively unstable structure and could be engineered to respond to external stimuli by changing size. We recently synthesized polycaprolactone (PCL)-derived micellular NPs, which respond to thrombin, a protease preferentially accumulated in the ischemic microenvironment, by expanding or shrinking in size. The approach to engineering size-changing NPs will be discussed in section 4.2.2. We found that the micellular NPs were able to efficiently penetrate the ischemic brain and deliver the glyburide payload to achieve effective treatment of ischemic stroke [123].
Other types of polymeric NPs, including dendrimers, polymeric nanogels, and melanin NPs, have also been explored for drug delivery to the ischemic brain. In one study, Li et al. employed dendrimers for drug delivery to the ischemic brain [124]. Compared to polymeric solid NPs and micellular NPs, dendrimers have a unique advantage in their small size and uniform dispersion [125]. The authors engineered dendrimers for brain targeting through surface conjugation of an EC-targeting peptide COG1410. They showed that the resulting NPs penetrated the brain with high efficiency, and when salvianolic acid A, an antioxidant, was conjugated, the NPs effectively promoted stroke recovery [124]. In another study, Liu and colleagues explored NPs consisting of melanin, a biological polymer known to have scavenging activities, as an antioxidant therapeutic for stroke treatment and found that the intraventricular administration of these NPs significantly reduced infarct volume [126].
4.1.2. Lipid NPs (LNPs)
LNPs are nanoformulations mainly consisting of lipid materials. Liposomes, an early generation LNP, possess a unique vesicular structure formed by a lipid bilayer in the shape of a hollow sphere. The double-layer structure enables liposomes to be loaded with either hydrophilic or hydrophobic drugs. Unlike liposomes, the latest LNP formulations possess a liposome-like structure but do not necessarily have a continuous bilayer.
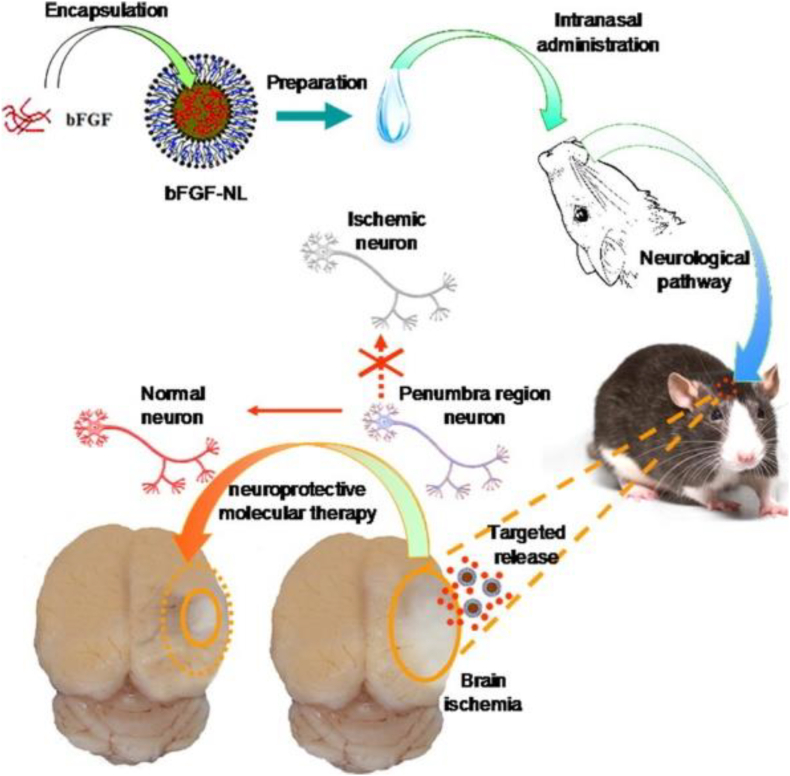
LNPs, particularly liposomes, are the earliest nanomedicine delivery platform to have successfully advanced to clinical applications. LNPs have been explored for drug delivery in cases of stroke. Several studies reported that liposomes could be employed for the encapsulation of hemoglobin to deliver oxygen to ischemic brain tissue, leading to accelerated stroke recovery [[127], [128], [129]]. In another study, Zhao and colleagues reported the use of liposomes to deliver basic fibroblast growth factor (bFGF) to the brain and demonstrated that intranasal administration of bFGF-loaded liposomes efficiently bypassed the BBB, effectively reduced infarct volume, and promoted functional recovery [130] (Fig. 5).
Fig. 5.
Scheme of intranasal administration of bFGF-nanoliposomes (bFGF-NL) for targeted therapy of IR damage in a rodent stroke model resulting in reduced infarct volume. Reproduced from Ref. [130].
4.1.3. Small molecule-assembled nanoparticles
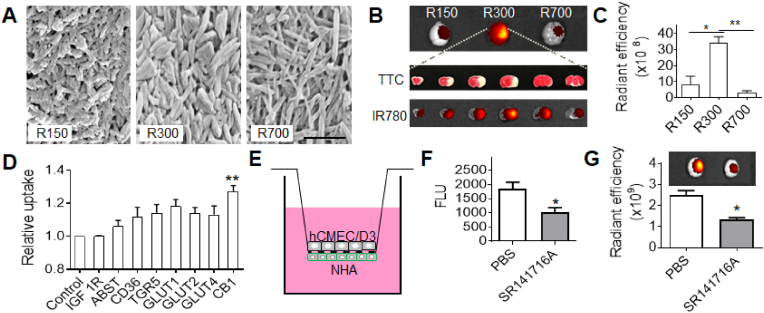
Recently, we and others identified a group of small molecules, which bear ring structures and are capable of self-assembly into spherical and rod-shaped NPs [[131], [132], [133], [134], [135]]. Many of these molecules are unique in that they possess pharmacological activities and are derived from natural herbal materials known to be effective for stroke treatment [133]. As such, NPs consisting of these small molecules could be potentially employed not only for drug delivery to the ischemic brain but also as a therapeutic agent for stroke treatment. In a recent study [133], we studied betulinic acid (BA) and found it formed rod-shaped NPs of different sizes and shapes, including lengths of ∼156 nm and diameter of ∼45 nm (156(l) x 45(d)), 315(l) x 60(d), and 730(l) x 35(d) that were designated as R150, R300, and R700, respectively (Fig. 6A). We characterized all of these NPs for drug delivery to the ischemic brain and found that, after intravenous administration, R300 demonstrated the greatest efficiency (Fig. 6B and C). We showed that the excellent brain penetration capabilities of R300 can be attributed not only to their unique size and shape, but also their interaction with cannabinoid receptor 1 (CB1), which is highly expressed in ischemic brain tissue after stroke, and pre-treatment with SR141716A, a CB1 receptor blocker, reduced their brain penetrating efficiency (Fig. 6D–G). We further characterized the use of BA NPs as a drug carrier for the delivery of glyburide and found that glyburide-loaded BA NPs limit the risk of hypoglycemia induced by glyburide while achieving anti-edema and antioxidant combinatory therapeutic benefits greater than either glyburide or BA NPs alone. In another recent study, we converted the BA chemistry to BAM and conjugated AMD3100 on the surface (A-BAM NPs) for targeted delivery of NA1 to acidic ischemic tissue [135]. Recall, that in the recently completed ESCAPE clinical trial, NA1 failed to demonstrate therapeutic benefits when tPA was co-administered [45]. We found that intravenous administration of NA1-loaded A-BAM NPs can not only effectively improve the treatment of stroke, but also enable NA1 therapy to be compatible with tPA infusion.
Fig. 6.
BA NPs for drug delivery to the ischemic brain. (A) Representative SEM images of BA NPs. BA forms rod-shaped NPs in different sizes and shapes, designated as R150, R300, and R700, respectively. Scale bar: 500 nm. (B,C) Representative images (B) and semi-quantification (C) of BA NPs in the brains isolated from MCAO mice received the indicated treatment. (D) Flow cytometry analysis of the uptake of BA NPs in cells that were engineered to overexpress the indicated surface molecules. (E) Schematic diagram of in vitro BBB transcytosis assay. (F) In vitro analysis of the inhibitory effect of SR141716A on NP transcytosis. (G) Representative images (upper panel) and semi-quantification (bottom panel) of IR780-loaded BA NPs in the brains isolated from MCAO mice with and without pre-treatment of SR141716A. Reproduced from Ref. [133].
4.1.4. Inorganic nanoparticles
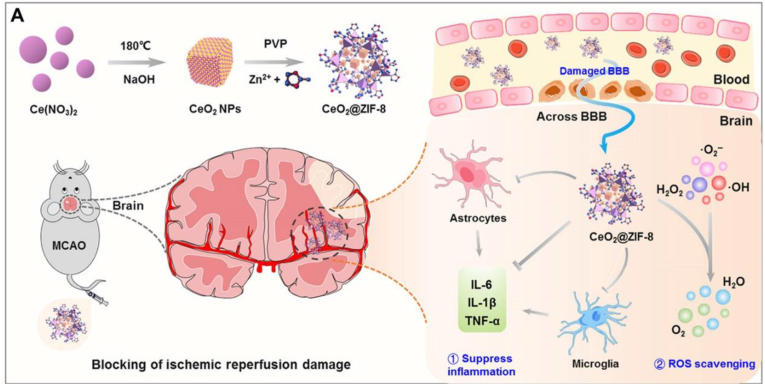
Inorganic NPs refer to those NPs derived from inorganic materials, such as metals, semiconductors, and carbon. Compared to organic NPs, most inorganic NPs have a higher degree of stability and better-controlled tunability in size and shape. Some of inorganic NPs possess unique biological, chemical, electrical, and magnetic properties, making them attractive for specific biological applications. Among various inorganic NPs, NPs consisting of ceria have been extensively explored for treatment of ischemic stroke. Mechanistically, cerium ion at the surface of NPs can shift between the reduced (Ce3+) and the oxidized (Ce4+) forms through binding with oxygen, allowing ceria NPs to have both superoxide dismutase-mimetic and catalase-mimetic activities. Ceria NPs can protect cells from two major ROS, superoxide anion and hydrogen peroxide [136]. Kim and colleagues showed that intravenous administration of PEGylated ceria NPs reduced infarct volume in a rat stroke model [137]. Bao and colleagues further engineered PEGylated ceria NPs through surface conjugation of Angiopep-2 and demonstrated that the resulting NPs penetrated the ischemic brain with a high efficiency. After intravenous administration, the NPs effectively improved stroke recovery and their efficacy was further improved through loading with edaravone [138]. To overcome the major challenges associated with ceria NPs for clinical translation, including short blood circulation time, aggregation, and uncontrollable catalytic reaction, the authors developed an strategy for in situ synthesis of bioactive zeolitic imidazolate framework-8–capped ceria nanoparticles (CeO2@ZIF-8 NPs) [139] (Fig. 7). They showed that the resulting nanosystem exhibited enhanced catalytic and antioxidative activities, increased the blood circulation time, and improved BBB penetration and accumulation in the brain. As a result, treatment with CeO2@ZIF-8 NPs notably decreased neuronal damage and suppressed inflammation and immune response.
Fig. 7.
Schematic illustration for in situ synthetic approach of CeO2@ZIF-8 nanotherapeutics and its neuroprotective application mechanisms against reperfusion-induced injury in AIS. Reproduced from Ref. [139].
Other than CeO2 NPs, manganous tetroxide NPs (Mn3O4 NPs) have also been explored for stroke treatment. Compared to CeO2 NPs, Mn3O4 NPs were shown to have greater antioxidase activities (SOD, catalase) [140,141]. Shi and colleagues developed an engineered nanosponge, Mn3O4@nanoerythrocyte-T7, and demonstrated that the nanosponge could remodel the ischemic microenvironment through self-adapted oxygen regulating and free radical scavenging, effectively improving post-stroke recovery [142]. More, recently, Wang et al. employed upconversion nanoparticles (UCNPs) to drive a nanophotosynthesis biosystem for local generation of oxygen and demonstrated this combination efficiently enhanced angiogenesis, reduced infarction, and facilitated brain tissue repair in a stroke mouse model [143].
4.1.5. Others
There are several other types of NPs, such as protein NPs, nanoconjugates, and biomimetic NPs, which have been also evaluated for drug delivery to the ischemic brain for stroke treatment.
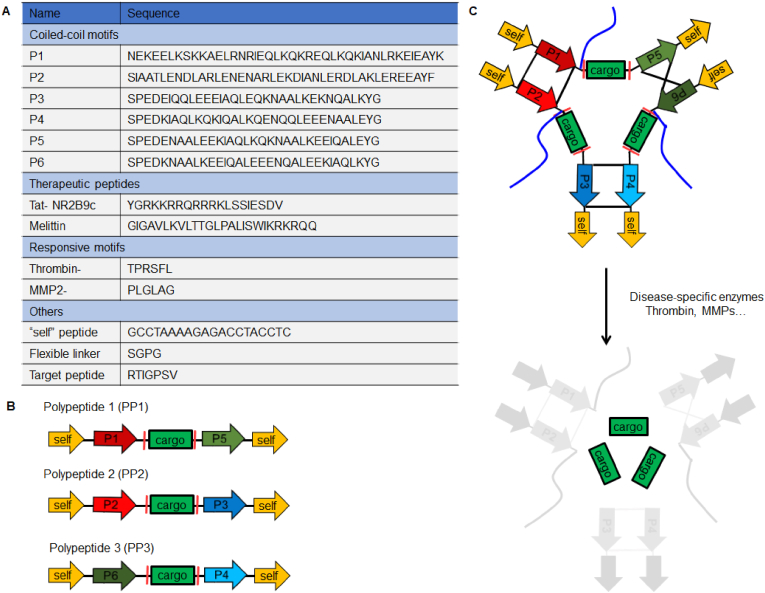
We recently designed and synthesized thrombin-activatable protein nanoparticles (APNPs) for the targeted delivery of NR2B9C, a neuroprotective peptide, to the ischemic brain (Fig. 8) [144]. The NPs were formed through the self-assembly of three recombinant polypeptides based on pairwise coiled-coil dimerization and further engineered for enhanced blood circulation and improved brain penetration through PEGylation and surface display of a transferrin receptor (TfR)-targeting peptide. Upon reaching the ischemic microenvironment, APNPs were cleaved by thrombin to release payload NR2B9C, which, in turn, effectively improve stroke recovery [144].
Fig. 8.
Design of APNPs. (A) Function and sequences of modular peptides used in the study. (B) Schematic of polypeptides containing functional modular motifs. (C) Formation and activation of APNPs. Reproduced from Ref. [144].
A variety of nanoconjugates have been developed and tested in murine stroke models. Adenosine is a nucleoside known to have potentially significant beneficial activity for many neurological disorders. Unfortunately, this compound is unsuitable for clinical translation because it is limited by its short plasma half-life and poor ability to penetrate the BBB. Gaudin and colleagues demonstrated that an adenosine bioconjugation with lipid squalene formed NPs, demonstrating prolonged circulation and neuroprotection in a mouse stroke model [145]. In another study, Zhang and colleagues reported an approach to selectively target inflammatory neutrophils using NPs composed of DOX-BSA conjugate [146]. They showed that treatment with these NPs inhibited neutrophil transmigration and decreased post-stroke brain damage [146].
More recently, various biomimetic vesicles have been developed for drug delivery to the ischemic brain. Among them, extracellular vesicles (EVs), which are cell-secreted nanoscale vesicles with subcellular structures, are particularly attractive as they often have great stability and long blood circulation time. Exosomes often carry microRNA and other cargo from the parent cells, making themselves alone a neurorestorative therapeutic. Applications of exosomes for the treatment of stroke have been reviewed in a recently published article [147]. However, clinical translation of exosomes has been limited by their low yield and purity as well as concerns about genetic materials within the vesicles. To overcome these limitations, other types of biomimetic vesicles, such as cell membrane-derived nanovesicles [148] and the fusion of cell membrane with liposomes [149], have been developed and shown great promise for drug delivery to stroke.
4.2. Engineering NPs for targeted drug delivery to the ischemic brain
Nanotechnology has the major advantage of manipulating the engineering of NPs for enhanced drug delivery to the ischemic brain. Various approaches, including surface functionalization, stimuli-responsiveness, and biomimicry, have been explored.
4.2.1. Surface functionalization for passive or active targeting
PEGylation through surface coating with polyethylene glycol (PEG) is a commonly used approach to passively improve the delivery of NPs to the ischemic brain [[127], [128], [129],144,145]. In addition to reducing protein adsorption, PEGylation alters the composition of the protein corona to prevent non-specific cellular uptake [150]. As a result, PEGylation helps NPs to evade the mononuclear phagocyte system (MPS), resulting in prolonged blood circulation and increased potential to penetrate the compromised BBB.
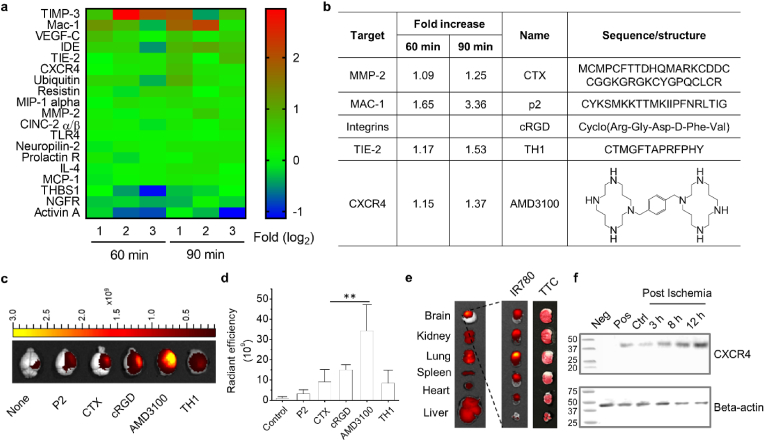
However, passive targeting through PEGylation has limited efficiency, as the brain penetrability of PEGylated NPs solely depends on the degree of BBB disruption. Improving drug delivery to the ischemic brain could be potentially achieved through active targeting strategies via surface conjugation of ligands, which can interact with receptors or “receptor like” molecules highly expressed in the BBB, such as transferrin receptors (TfR) [151], or molecules enriched in the ischemic microenvironment, such as MMP-2 106 and fibrin fibrils [152]. To identify molecular targets which are preferentially accumulated in the ischemic brain tissue, we recently profiled proteins in both the ischemic brain and normal brain using an antibody array, through which we identified 19 proteins that had significantly different levels of expression in the ischemic hemisphere, compared to those in the contralateral control. Further characterization identified CXCR4 as the most prominent target, which is highly expressed in the ischemic brain shortly after the onset of stroke and can be targeted by a well-characterized antagonist, AMD3100. We showed that surface conjugation of AMD3100 enhanced the accumulation of NPs in the brain by over 30-fold (Fig. 9a,b) [123]. In another study, we reported the development of multifunctional AMD3100-conjugated, shrinkable poly (2,2′-thiodiethylene 3,3′-thiodipropionate) (PTT) NPs (ASPTT NPs) which are synthesized using a ROS-reactive PTT polymer and conjugated with AMD3100 [153]. This work showed that ASPTT NPs are capable of efficient encapsulation and delivery of glyburide to achieve anti-edema and antioxidant combination therapy, resulting in effective stroke treatment. Additional active targeting strategies were summarized in Table 2.
Fig. 9.
Identification and selection of ligands for targeted delivery of nanoparticles to stroke. (a) Heat diagram of proteins that are differentially enriched in the ischemic brain and in the control normal brain (∗P < 0.05). (b) Candidate ligands that bind to the selected proteins. (c) Representative images and (d) semi-quantification of nanoparticles in the brains of MCAO mice received treatments with the indicated ligand-conjugated nanoparticles. (e) Representative images of brain slices with TTC staining (left) and fluorescence imaging (right). (f) Western Blot confirmed that the level of CXCR4 was significantly elevated in the ischemic brain. Negative control: 293T cell lysate. Positive control: U87 flank tumor lysate. Control: normal brain tissue. Reproduced from Ref. [123].
Table 2.
Summary of ligands on nanoparticles for ischemic stroke.
| Nanocarriers | Ligands | Targets | Cargo | Animal model | Application | Ref. |
|---|---|---|---|---|---|---|
| AMD3100-conjugated, size-shrinkable NPs (ASNPs) | AMD3100 | CXCR4 | Glibenclamide | Mice MCAO |
High efficiency in penetrating the ischemic brain and low toxicity | [123] |
| Liposome (T7&SHp-P-LPs/ZL006) | T7 peptide and SHp | TfR and homed to ischemic brain tissue | ZL006 | Rats MCAO |
Penetrating the BBB and targeting the ischemic area | [154] |
| T7-conjugated PEGylated liposomes (T7-P-LPs) | HAIYPRH (T7) peptide | TfR | ZL006 | Rats MCAO |
Mediate the transport of nanocarriers across the BBB | [155] |
| Combining magnetic Fe3O4 NPs and RGD-modified dendrimers | RGD tripeptide | GPIIb/IIIa receptor expressed at the surface of the activated platelets | Nattokinase | Rats FeCl3-induced thrombosis |
Targeted delivery to enhance the efficacy of site-specific thrombolytic treatment | [156] |
| Angiopep-2-PGP-STA-PEG-PAMAM NPs | Angiopep-2; PGP |
Low density LRP1; CXCR2 |
STA | Rats, mice; MCAO |
Dual-targeting delivery system; Improve their therapeutic effect against AIS | [157] |
| Anti-HSP72 vectorized liposomes | Anti-HSP72 | HSP72 | Citicoline | Rats MCAO |
Targeted delivery to the peri-infarct region | [158] |
| tPA-loaded, cRGD-coated, PEGylated liposomes | Cyclic RGD | Activated platelets | tPA | Facilitate selective delivery and effective release of tPA at the site of thrombus | [159] | |
| PLGA-CTX/LEX NPs | CTX | MMP-2 | NEP1-40 | Mice MCAO |
The NPs efficiently and specifically accumulated in the brain's ischemic microenvironment | [106] |
| Fas ligand - conjugated PLNs loaded with NBP | Fas ligand antibody | Recruiting microglia | dl-NBP | Mice MCAO |
The NPs specifically accumulated on microglia cells in ischemic region | [160] |
| Perfluorocarbon NPs | Anti-fibrin antibody and urokinase | Fibrin fibrils | Anti-fibrin antibody and urokinase | Dogs Femoral artery thrombosis |
The fibrin-targeted urokinase NPs could evolve into an alternative to r-tPA for use in acute ischemic stroke victims | [152] |
NPs, nanoparticles; MCAO, middle cerebral artery occlusion; TfR, Transferrin receptor; PEG, polyethylene glycol; RGD, arginine–glycine–aspartic; SHp, stroke homing peptide; LRP1, lipoprotein receptor-related protein 1; AIS, acute ischemic stroke; STA, scutellarin; PGP, N-acetylated proline-glycine-proline CTX, chlorotoxin; LEX, lexiscan; PLNs, PEGylated lipid nanoparticles; dl-NBP, 3-n-Butylphthalide.
4.2.2. Stimuli-responsiveness
Despite great progress over many decades, accumulating evidence shows that active targeting is significantly complicated by the complex physiological system and limited efficiency for clinical translation [161]. Further improved targeted drug delivery requires engineering NPs to be “intelligent” and responsive to disease microenvironments by changing their physical or chemical structures [162,163]. To explore intelligent responsiveness as a novel approach to enhancing the delivery of NPs to the ischemic brain, we synthesized PCL-PEG based micellular NPs, which contain a thrombin-cleavable peptide that either expanded or shrank in size in response to thrombin cleavage (Fig. 10). We found that both the size expandable and shrinkable NPs penetrated the brain with greater efficiency than unresponsive NPs. Compared to the expandable nanoparticles, the size shrinkable nanoparticles were more efficient [123].
Fig. 10.
Schematic diagrams of the intelligent responsiveness of (a, c) shrinkable and (b, d) expandable micellar nanoparticles in response to proteases enriched in the ischemic microenvironment. Reproduced from Ref. [123].
In addition to biological cues, intelligent NPs can also be designed to respond to physical or chemical stimuli in the ischemic brain. For instance, it is known that the blood vessels in the brain narrow due to thrombus formation leading to accelerated blood flow and higher fluid shear stress. This unique physical characteristic may provide an opportunity for targeted drug delivery. In a study by Korin and colleagues, a shear stress-targeting strategy was tested by synthesizing microscale aggregates of NPs, which break up into nanoscale components when exposed to abnormally high fluid shear stress. They found that after intravenous administration these shear-activated nanotherapeutics preferentially released payload tPA in the clotted area, inducing rapid clot dissolution and restoration of normal flow dynamics [164]. Similar thrombolytic approaches could be also employed for the treatment of ischemic stroke [165].
4.2.3. Biomimicry through surface coating with cell membranes
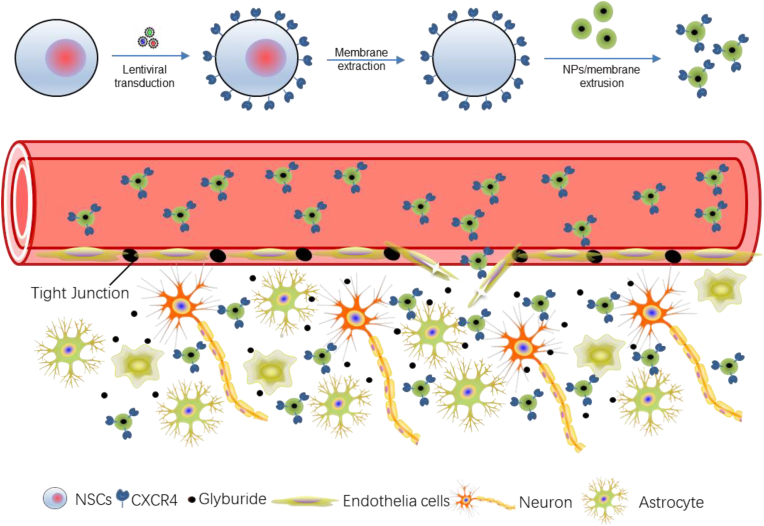
Biomimicry, which is often achieved through surface coating with cell membranes, allows for hijacking the biological interactions cells have with the host. This technique has recently emerged as a promising approach to engineering NPs for drug delivery to the brain. To date, various cell-based therapies have been explored for the treatment of ischemic stroke. Among them, neural stem cells (NSCs) are particularly attractive because they express various chemokine factors, such as CXCR4, and thus can efficiently migrate to the injured area [166]. To take advantage of the unique tropism of NSCs, we synthesized PLGA NPs and engineered them for targeted delivery to the ischemic brain through surface coating with the membrane of NSCs. We found that the membrane-coated NPs efficiently accumulated in the ischemic brain tissue after intravenous administration and the efficiency could be further improved using CXCR4-overexpressing membrane. We demonstrated that the resulting CXCR4-overexpressing NSC membrane-coated NPs enabled targeted delivery of glyburide to the brain for effective stroke treatment (Fig. 11) [167]. Similarly, Li and colleagues synthesized platelet membrane-coated magnetic nanoparticles and found that the resulting NPs inherited the natural properties of the platelet membrane and efficiently accumulated in ischemic stroke lesions after intravenous administration [168]. Lv and colleagues developed dextran NPs for drug delivery to the ischemic brain. These NPs possesses a surface coating of membrane isolated from red blood cell (RBC) which were shown to help prolong blood circulation life [169].
Fig. 11.
Schematic diagram of targeted delivery of membrane-coated NPs to the ischemic brain through the surface coating of the membrane of CXCR4-overexpressing NSCs. NSCs are engineered through lentiviral transduction to overexpress CXCR4. Reproduced from Ref. [167].
5. Perspectives and conclusion
Designing effective treatments through employing NP technology as drug delivery vehicles equally requires consideration of the physiochemical characteristics in additions to the payload. Improving targeted delivery to the ischemic brain could be potentially achieved by tuning these physical characteristics of NPs such as size, shape, and charge. NPs with a diameter smaller than 6 nm are subjected to rapid elimination by the kidneys while those larger than 50 nm are vulnerable to clearance by the reticuloendothelial system (RES) [170,171]. Compared to large-size NPs, NPs of smaller size have greater tissue penetration ability [172]. We recently showed that compared to non-responsive NPs, which maintained a diameter of ∼120 nm, thrombin-responsive NPs, which were ∼200 nm in the circulatory system but shrank into 80 nm upon reaching thrombin-enriched ischemic brain tissue, demonstrated significantly greater stroke targeting efficiency [123]. The marked enhanced efficiency could be partially attributed to the greater ability of small NPs to penetrate the brain. Like size, the shape of the NPs also plays an important role in drug delivery. Compared to nanospheres, rod-shaped NPs were shown to have greater interactions with brain ECs and demonstrate a higher efficiency in brain penetration [173]. Among various sizes of rod-shaped NPs, we found that NPs of 315(l) x 60(d) have greater brain penetrability than both short (156(l) x 45(d)) or longer 730(l) x 35(d) NPs, suggesting that both the size and shape must be considered when designing optimal NPs for drug delivery to the brain (Fig. 7) [133]. In addition, the surface charge could also be a key parameter that determines the biodistribution of NPs. For example, Campos-Martorell and colleagues studied the impact of charge of liposomal NPs in an ischemic stroke rat model and found that neutral and negatively charged, but not positively charged NPs were able to penetrate the brain 90 min after intravenous administration [174].
Targeted delivery of NPs may also be achieved through external cues, such as magnetic force. It was reported that delivery of l-arginine to the ischemic brain was successfully improved through co-encapsulation with magnetic NPs and guidance by an external magnetic field. A similar approach could be employed to enhance the delivery of tPA for improved thrombolytic efficiency [175,176].
Currently, the standard care for ischemic stroke involves intravenous thrombolytic therapy and intravascular therapy, which, unfortunately, do not benefit most patients due to their narrow therapeutic windows. There are no FDA-approved pharmacotherapies that target improving post-stroke recovery. While further improved understanding of the biology of stroke is warranted, the lack of approved pharmacotherapies may not be solely due to the lack of therapeutic targets. In fact, numerous agents targeting a range of therapeutic targets have been tested in various clinical trials, including over 20 drugs tested in phase 2/3 clinical trials (Table 1). Alas, most of them failed to significantly improve clinical outcomes. The failure could potentially be attributed to the fact that most therapeutic agents cannot efficiently penetrate the brain. Owed to the great versatility in of NP engineering for brain targeting, adaptation to environmental stimuli, and BBB penetration, emerging nanotechnology has demonstrated great promise to overcome the drug delivery barrier (Table 2). However, the employment of NPs has major limitations in that it not only increases the complexity of therapeutic agents but also often leads to non-specific accumulation in organs associated with the reticuloendothelial system, mainly including the liver and the lung. As a result, the difficulty in GMP manufacture and quality control and concerns regarding off-target effects could prevent the clinical translation of NP-based stroke therapeutics. Development of the next generation of NPs with simple structures but potential for “intelligent” multifunctionality is greatly needed.
Ethics approval and consent to participate
Not applicable. This work is a review of existing published works.
CRediT authorship contribution statement
Bin Peng: Writing – review & editing, Writing – original draft, Conceptualization. Farrah S. Mohammed: Writing – review & editing, Writing – original draft, Conceptualization. Xiangjun Tang: Conceptualization. Jia Liu: Writing – original draft. Kevin N. Sheth: Writing – review & editing. Jiangbing Zhou: Writing – review & editing, Writing – original draft, Resources, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare no competing financial interests.
Acknowledgements
This work was partially supported by NIH Grant NS110721 (JZ, KNS).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Bin Peng, Email: binpeng85@126.com.
Farrah S. Mohammed, Email: Farrah.mohammed@yale.edu.
Jia Liu, Email: jia.liu.jl3777@yale.edu.
Kevin N. Sheth, Email: kevin.sheth@yale.edu.
Jiangbing Zhou, Email: jiangbing.zhou@yale.edu.
References
- 1.Feigin V.L., Forouzanfar M.H., Krishnamurthi R., Mensah G.A., Connor M., Bennett D.A., et al. Global and regional burden of stroke during 1990-2010: findings from the global burden of disease study 2010. Lancet. 2014;383:245–255. doi: 10.1016/s0140-6736(13)61953-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin E.J., Blaha M.J., Chiuve S.E., Cushman M., Das S.R., Deo R., et al. Heart disease and stroke statistics-2017 update: a report from the american heart association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collaborators G.B.D.N. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019;18:459–480. doi: 10.1016/S1474-4422(18)30499-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwamm L.H., Ali S.F., Reeves M.J., Smith E.E., Saver J.L., Messe S., et al. Temporal trends in patient characteristics and treatment with intravenous thrombolysis among acute ischemic stroke patients at get with the guidelines-stroke hospitals. Circ. Cardiovas. Qual. Outcome. 2013;6:543–549. doi: 10.1161/CIRCOUTCOMES.111.000303. [DOI] [PubMed] [Google Scholar]
- 5.Powers W.J. Acute ischemic stroke. N. Engl. J. Med. 2020;383:252–260. doi: 10.1056/NEJMcp1917030. [DOI] [PubMed] [Google Scholar]
- 6.Mendelson S.J., Prabhakaran S. Diagnosis and management of transient ischemic attack and acute ischemic stroke: a review. JAMA. 2021;325:1088–1098. doi: 10.1001/jama.2020.26867. [DOI] [PubMed] [Google Scholar]
- 7.Campbell B.C.V., Ma H., Ringleb P.A., Parsons M.W., Churilov L., Bendszus M., et al. Extending thrombolysis to 4.5-9 h and wake-up stroke using perfusion imaging: a systematic review and meta-analysis of individual patient data. Lancet. 2019;394:139–147. doi: 10.1016/S0140-6736(19)31053-0. [DOI] [PubMed] [Google Scholar]
- 8.Wu L.-J., Wu G., Sharif M.R.A., Baker A., Jia Y., Fahey F.H., et al. The voltage-gated proton channel hv1 enhances brain damage from ischemic stroke. Nat. Neurosci. 2012;15:565–573. doi: 10.1038/nn.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chouchani E.T., Pell V.R., Gaude E., Aksentijević D., Sundier S.Y., Robb E.L., et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ros. Nature. 2014;515:431–435. doi: 10.1038/nature13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andrabi S.A., Kang H.C., Haince J.-F., Lee Y.-I., Zhang J., Chi Z., et al. Iduna protects the brain from glutamate excitotoxicity and stroke by interfering with poly (adp-ribose) polymer-induced cell death. Nat. Med. 2011;17:692–699. doi: 10.1038/nm.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun M.S., Jin H., Sun X., Huang S., Zhang F.L., Guo Z.N., et al. Free radical damage in ischemia-reperfusion injury: an obstacle in acute ischemic stroke after revascularization therapy. Oxid. Med. Cell. Longev. 2018;2018 doi: 10.1155/2018/3804979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao Y., Chen Z.L., Norris E.H., Strickland S. Astrocytic laminin regulates pericyte differentiation and maintains blood brain barrier integrity. Nat. Commun. 2014;5:3413. doi: 10.1038/ncomms4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiao H., Wang Z., Liu Y., Wang P., Xue Y. Specific role of tight junction proteins claudin-5, occludin, and zo-1 of the blood-brain barrier in a focal cerebral ischemic insult. J. Mol. Neurosci. 2011;44:130–139. doi: 10.1007/s12031-011-9496-4. [DOI] [PubMed] [Google Scholar]
- 14.Kuntz M., Mysiorek C., Petrault O., Petrault M., Uzbekov R., Bordet R., et al. Stroke-induced brain parenchymal injury drives blood-brain barrier early leakage kinetics: a combined in vivo/in vitro study. J. Cerebr. Blood Flow Metabol. : Offic. J. Int. Soc. Cerebr. Blood Flow Metabol. 2014;34:95–107. doi: 10.1038/jcbfm.2013.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi Y., Zhang L., Pu H., Mao L., Hu X., Jiang X., et al. Rapid endothelial cytoskeletal reorganization enables early blood-brain barrier disruption and long-term ischaemic reperfusion brain injury. Nat. Commun. 2016;7 doi: 10.1038/ncomms10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitchen P., Salman M.M., Halsey A.M., Clarke-Bland C., MacDonald J.A., Ishida H., et al. Targeting aquaporin-4 subcellular localization to treat central nervous system edema. Cell. 2020;181:784–799 e719. doi: 10.1016/j.cell.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo Z.W., Ovcjak A., Wong R., Yang B.X., Feng Z.P., Sun H.S. Drug development in targeting ion channels for brain edema. Acta Pharmacol. Sin. 2020;41:1272–1288. doi: 10.1038/s41401-020-00503-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blochet C., Buscemi L., Clement T., Gehri S., Badaut J., Hirt L. Involvement of caveolin-1 in neurovascular unit remodeling after stroke: effects on neovascularization and astrogliosis. J. Cerebr. Blood Flow Metabol. 2020;40:163–176. doi: 10.1177/0271678X18806893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madan E., Parker T.M., Pelham C.J., Palma A.M., Peixoto M.L., Nagane M., et al. Hif-transcribed p53 chaperones hif-1α. Nucleic Acids Res. 2019;47:10212–10234. doi: 10.1093/nar/gkz766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Armulik A., Genove G., Mae M., Nisancioglu M.H., Wallgard E., Niaudet C., et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 21.Szalay G., Martinecz B., Lénárt N., Környei Z., Orsolits B., Judák L., et al. Microglia protect against brain injury and their selective elimination dysregulates neuronal network activity after stroke. Nat. Commun. 2016;7:1–13. doi: 10.1038/ncomms11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jickling G.C., Liu D., Ander B.P., Stamova B., Zhan X., Sharp F.R. Targeting neutrophils in ischemic stroke: translational insights from experimental studies. J. Cerebr. Blood Flow Metabol. 2015;35:888–901. doi: 10.1038/jcbfm.2015.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang L., Yu H., Yang X., Zhu Y., Bai X., Wang R., et al. Neutrophil extracellular traps released by neutrophils impair revascularization and vascular remodeling after stroke. Nat. Commun. 2020;11:2488. doi: 10.1038/s41467-020-16191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu P., Zhang R., Liu D., Wang J., Yuan C., Zhao X., et al. Time-course investigation of blood-brain barrier permeability and tight junction protein changes in a rat model of permanent focal ischemia. J. Physiol. Sci. 2018;68:121–127. doi: 10.1007/s12576-016-0516-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang Z.G., Xue D., Karbalai H., Buchan A.M., Preston E. Biphasic opening of the blood-brain barrier following transient focal ischemia: effects of hypothermia. Can. J. Neurol. Sci. 1999;26:298–304. doi: 10.1017/s0317167100000421. [DOI] [PubMed] [Google Scholar]
- 26.Merali Z., Huang K., Mikulis D., Silver F., Kassner A. Evolution of blood-brain-barrier permeability after acute ischemic stroke. PLoS One. 2017;12 doi: 10.1371/journal.pone.0171558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Latour L.L., Kang D.W., Ezzeddine M.A., Chalela J.A., Warach S. Early blood-brain barrier disruption in human focal brain ischemia. Ann. Neurol. 2004;56:468–477. doi: 10.1002/ana.20199. [DOI] [PubMed] [Google Scholar]
- 28.Warach S., Latour L.L. Evidence of reperfusion injury, exacerbated by thrombolytic therapy, in human focal brain ischemia using a novel imaging marker of early blood-brain barrier disruption. Stroke; J. Cereb. Circ. 2004;35:2659–2661. doi: 10.1161/01.STR.0000144051.32131.09. [DOI] [PubMed] [Google Scholar]
- 29.Weber R.Z., Grönnert L., Mulders G., Maurer M.A., Tackenberg C., Schwab M.E., et al. Characterization of the blood brain barrier disruption in the photothrombotic stroke model. Front. Physiol. 2020;11 doi: 10.3389/fphys.2020.586226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bang O.Y., Saver J.L., Alger J.R., Shah S.H., Buck B.H., Starkman S., et al. Patterns and predictors of blood-brain barrier permeability derangements in acute ischemic stroke. Stroke. 2009;40:454–461. doi: 10.1161/STROKEAHA.108.522847. [DOI] [PubMed] [Google Scholar]
- 31.Iadecola C., Anrather J. Stroke research at a crossroad: asking the brain for directions. Nat. Neurosci. 2011;14:1363–1368. doi: 10.1038/nn.2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ginsberg M.D., Palesch Y.Y., Hill M.D., Martin R.H., Moy C.S., Barsan W.G., et al. High-dose albumin treatment for acute ischaemic stroke (alias) part 2: a randomised, double-blind, phase 3, placebo-controlled trial. Lancet Neurol. 2013;12:1049–1058. doi: 10.1016/S1474-4422(13)70223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ringelstein E.B., Thijs V., Norrving B., Chamorro A., Aichner F., Grond M., et al. Granulocyte colony–stimulating factor in patients with acute ischemic stroke: results of the ax200 for ischemic stroke trial. Stroke. 2013;44:2681–2687. doi: 10.1161/STROKEAHA.113.001531. [DOI] [PubMed] [Google Scholar]
- 34.Kohler E., Prentice D.A., Bates T.R., Hankey G.J., Claxton A., van Heerden J., et al. Intravenous minocycline in acute stroke: a randomized, controlled pilot study and meta-analysis. Stroke. 2013;44:2493–2499. doi: 10.1161/STROKEAHA.113.000780. [DOI] [PubMed] [Google Scholar]
- 35.Lang W., Stadler C.H., Poljakovic Z., Fleet D., Group L.S. A prospective, randomized, placebo-controlled, double-blind trial about safety and efficacy of combined treatment with alteplase (rt-pa) and cerebrolysin in acute ischaemic hemispheric stroke. Int. J. Stroke. 2013;8:95–104. doi: 10.1111/j.1747-4949.2012.00901.x. [DOI] [PubMed] [Google Scholar]
- 36.Chamorro Á., Amaro S., Castellanos M., Segura T., Arenillas J., Martí-Fábregas J., et al. Safety and efficacy of uric acid in patients with acute stroke (urico-ictus): a randomised, double-blind phase 2b/3 trial. Lancet Neurol. 2014;13:453–460. doi: 10.1016/S1474-4422(14)70054-7. [DOI] [PubMed] [Google Scholar]
- 37.Zhu Z., Fu Y., Tian D., Sun N., Han W., Chang G., et al. Combination of the immune modulator fingolimod with alteplase in acute ischemic stroke: a pilot trial. Circulation. 2015;132:1104–1112. doi: 10.1161/CIRCULATIONAHA.115.016371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsai T.-H., Lu C.-H., Wallace C.G., Chang W.-N., Chen S.-F., Huang C.-R., et al. Erythropoietin improves long-term neurological outcome in acute ischemic stroke patients: a randomized, prospective, placebo-controlled clinical trial. Crit. Care. 2015;19:1–9. doi: 10.1186/s13054-015-0761-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sheth K.N., Elm J.J., Molyneaux B.J., Hinson H., Beslow L.A., Sze G.K., et al. Safety and efficacy of intravenous glyburide on brain swelling after large hemispheric infarction (games-rp): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2016;15:1160–1169. doi: 10.1016/S1474-4422(16)30196-X. [DOI] [PubMed] [Google Scholar]
- 40.Shkirkova K., Starkman S., Sanossian N., Eckstein M., Stratton S., Pratt F., et al. Paramedic initiation of neuroprotective agent infusions: successful achievement of target blood levels and attained level effect on clinical outcomes in the fast-mag pivotal trial (field administration of stroke therapy–magnesium) Stroke. 2017;48:1901–1907. doi: 10.1161/STROKEAHA.116.015664. [DOI] [PubMed] [Google Scholar]
- 41.Hess D.C., Wechsler L.R., Clark W.M., Savitz S.I., Ford G.A., Chiu D., et al. Safety and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (masters): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2017;16:360–368. doi: 10.1016/S1474-4422(17)30046-7. [DOI] [PubMed] [Google Scholar]
- 42.Stakhovskaya L., Shamalov N., Khasanova D., Mel’nikova E., Agaf’ina A., Golikov K., et al. Results of a randomized, double-blind, multicenter, placebo-controlled, parallel-group study of the efficacy and safety of mexidol in prolonged sequential therapy of patients in the acute and early recovery stages of hemispheric stroke (the epica study) Neurosci. Behav. Physiol. 2018;48:929–938. [Google Scholar]
- 43.Elkind M.S., Veltkamp R., Montaner J., Johnston S.C., Singhal A.B., Becker K., et al. Natalizumab in acute ischemic stroke (action ii): a randomized, placebo-controlled trial. Neurol. 2020;95:e1091–e1104. doi: 10.1212/WNL.0000000000010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chabriat H., Bassetti C.L., Marx U., Audoli-Inthavong M.-L., Sors A., Lambert E., et al. Safety and efficacy of gabaa α5 antagonist s44819 in patients with ischaemic stroke: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2020;19:226–233. doi: 10.1016/S1474-4422(20)30004-1. [DOI] [PubMed] [Google Scholar]
- 45.Hill M.D., Goyal M., Menon B.K., Nogueira R.G., McTaggart R.A., Demchuk A.M., et al. Efficacy and safety of nerinetide for the treatment of acute ischaemic stroke (escape-na1): a multicentre, double-blind, randomised controlled trial. Lancet. 2020;395:878–887. doi: 10.1016/S0140-6736(20)30258-0. [DOI] [PubMed] [Google Scholar]
- 46.Xu J., Wang A., Meng X., Yalkun G., Xu A., Gao Z., et al. Edaravone dexborneol versus edaravone alone for the treatment of acute ischemic stroke: a phase iii, randomized, double-blind, comparative trial. Stroke. 2021;52:772–780. doi: 10.1161/STROKEAHA.120.031197. [DOI] [PubMed] [Google Scholar]
- 47.Reeves M., Khoury J., Alwell K., Moomaw C., Flaherty M., Woo D., et al. Distribution of national institutes of health stroke scale in the cincinnati/northern Kentucky stroke study. Stroke. 2013;44:3211–3213. doi: 10.1161/STROKEAHA.113.002881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Battey T.W., Karki M., Singhal A.B., Wu O., Sadaghiani S., Campbell B.C., et al. Brain edema predicts outcome after nonlacunar ischemic stroke. Stroke; J. Cereb. Circ. 2014;45:3643–3648. doi: 10.1161/STROKEAHA.114.006884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stokum J.A., Gerzanich V., Simard J.M. Molecular pathophysiology of cerebral edema. J. Cerebr. Blood Flow Metabol. 2016;36:513–538. doi: 10.1177/0271678X15617172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen S., Shao L., Ma L. Cerebral edema formation after stroke: emphasis on blood-brain barrier and the lymphatic drainage system of the brain. Front. Cell. Neurosci. 2021;15 doi: 10.3389/fncel.2021.716825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simard J.M., Kent T.A., Chen M., Tarasov K.V., Gerzanich V. Brain oedema in focal ischaemia: molecular pathophysiology and theoretical implications. Lancet Neurol. 2007;6:258–268. doi: 10.1016/S1474-4422(07)70055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Woo S.K., Kwon M.S., Geng Z., Chen Z., Ivanov A., Bhatta S., et al. Sequential activation of hypoxia-inducible factor 1 and specificity protein 1 is required for hypoxia-induced transcriptional stimulation of abcc8. J. Cerebr. Blood Flow Metabol. : Offic. J. Int. Soc. Cerebr. Blood Flow Metabol. 2012;32:525–536. doi: 10.1038/jcbfm.2011.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simard J.M., Chen M., Tarasov K.V., Bhatta S., Ivanova S., Melnitchenko L., et al. Newly expressed sur1-regulated nc(ca-atp) channel mediates cerebral edema after ischemic stroke. Nat. Med. 2006;12:433–440. doi: 10.1038/nm1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kunte H., Busch M.A., Trostdorf K., Vollnberg B., Harms L., Mehta R.I., et al. Hemorrhagic transformation of ischemic stroke in diabetics on sulfonylureas. Ann. Neurol. 2012;72:799–806. doi: 10.1002/ana.23680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mehta R.I., Ivanova S., Tosun C., Castellani R.J., Gerzanich V., Simard J.M. Sulfonylurea receptor 1 expression in human cerebral infarcts. J. Neuropathol. Exp. Neurol. 2013;72:871–883. doi: 10.1097/NEN.0b013e3182a32e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen M., Simard J.M. Cell swelling and a nonselective cation channel regulated by internal ca2+ and atp in native reactive astrocytes from adult rat brain. J. Neurosci. Offic. J. Soc. Neurosci. 2001;21:6512–6521. doi: 10.1523/JNEUROSCI.21-17-06512.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simard J.M., Woo S.K., Schwartzbauer G.T., Gerzanich V. Sulfonylurea receptor 1 in central nervous system injury: a focused review. J. Cerebr. Blood Flow Metabol. Offic. J. Int. Soc. Cerebr. Blood Flow Metabol. 2012;32:1699–1717. doi: 10.1038/jcbfm.2012.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sheth K.N., Taylor Kimberly W., Elm J.J., Kent T.A., Yoo A.J., Thomalla G., et al. Exploratory analysis of glyburide as a novel therapy for preventing brain swelling. Neurocritical Care. 2014;21:43–51. doi: 10.1007/s12028-014-9970-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Simard J.M., Yurovsky V., Tsymbalyuk N., Melnichenko L., Ivanova S., Gerzanich V. Protective effect of delayed treatment with low-dose glibenclamide in three models of ischemic stroke. Stroke. 2009;40:604–609. doi: 10.1161/STROKEAHA.108.522409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ortega F.J., Gimeno-Bayon J., Espinosa-Parrilla J.F., Carrasco J.L., Batlle M., Pugliese M., et al. Atp-dependent potassium channel blockade strengthens microglial neuroprotection after hypoxia-ischemia in rats. Exp. Neurol. 2012;235:282–296. doi: 10.1016/j.expneurol.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 61.Simard J.M., Tsymbalyuk N., Tsymbalyuk O., Ivanova S., Yurovsky V., Gerzanich V. Glibenclamide is superior to decompressive craniectomy in a rat model of malignant stroke. Stroke; J. Cereb. Circ. 2010;41:531–537. doi: 10.1161/STROKEAHA.109.572644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Simard J.M., Woo S.K., Tsymbalyuk N., Voloshyn O., Yurovsky V., Ivanova S., et al. Glibenclamide-10-h treatment window in a clinically relevant model of stroke. Transl Stroke Res. 2012;3:286–295. doi: 10.1007/s12975-012-0149-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Simard J.M., Geng Z., Silver F.L., Sheth K.N., Kimberly W.T., Stern B.J., et al. Does inhibiting sur1 complement rt-pa in cerebral ischemia? Ann. N. Y. Acad. Sci. 2012;1268:95–107. doi: 10.1111/j.1749-6632.2012.06705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sheth K.N., Kimberly W.T., Elm J.J., Kent T.A., Mandava P., Yoo A.J., et al. Pilot study of intravenous glyburide in patients with a large ischemic stroke. Stroke; J. Cereb. Circ. 2014;45:281–283. doi: 10.1161/STROKEAHA.113.003352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kimberly W.T., Battey T.W., Pham L., Wu O., Yoo A.J., Furie K.L., et al. Glyburide is associated with attenuated vasogenic edema in stroke patients. Neurocrit Care. 2014;20:193–201. doi: 10.1007/s12028-013-9917-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vorasayan P., Bevers M.B., Beslow L.A., Sze G., Molyneaux B.J., Hinson H.E., et al. Intravenous glibenclamide reduces lesional water uptake in large hemispheric infarction. Stroke; J. Cereb. Circ. 2019;50:3021–3027. doi: 10.1161/STROKEAHA.119.026036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stokum J.A., Gerzanich V., Sheth K.N., Kimberly W.T., Simard J.M. Emerging pharmacological treatments for cerebral edema: evidence from clinical studies. Annu. Rev. Pharmacol. Toxicol. 2020;60:291–309. doi: 10.1146/annurev-pharmtox-010919-023429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aarts M., Liu Y., Liu L., Besshoh S., Arundine M., Gurd J.W., et al. Treatment of ischemic brain damage by perturbing nmda receptor- psd-95 protein interactions. Science. 2002;298:846–850. doi: 10.1126/science.1072873. [DOI] [PubMed] [Google Scholar]
- 69.Zhou L., Li F., Xu H.B., Luo C.X., Wu H.Y., Zhu M.M., et al. Treatment of cerebral ischemia by disrupting ischemia-induced interaction of nnos with psd-95. Nat. Med. 2010;16:1439–1443. doi: 10.1038/nm.2245. [DOI] [PubMed] [Google Scholar]
- 70.Cook D.J., Teves L., Tymianski M. A translational paradigm for the preclinical evaluation of the stroke neuroprotectant tat-nr2b9c in gyrencephalic nonhuman primates. Sci. Transl. Med. 2012;4:154ra133. doi: 10.1126/scitranslmed.3003824. [DOI] [PubMed] [Google Scholar]
- 71.Hill M.D., Martin R.H., Mikulis D., Wong J.H., Silver F.L., Terbrugge K.G., et al. Safety and efficacy of na-1 in patients with iatrogenic stroke after endovascular aneurysm repair (enact): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2012;11:942–950. doi: 10.1016/S1474-4422(12)70225-9. [DOI] [PubMed] [Google Scholar]
- 72.Mayor-Nunez D., Ji Z., Sun X., Teves L., Garman J.D., Tymianski M. Plasmin-resistant psd-95 inhibitors resolve effect-modifying drug-drug interactions between alteplase and nerinetide in acute stroke. Sci. Transl. Med. 2021;13 doi: 10.1126/scitranslmed.abb1498. [DOI] [PubMed] [Google Scholar]
- 73.https://spannetwork.Org/uric_acid, access on August/22/2023.
- 74.Amaro S., Planas A.M., Chamorro Á. Uric acid administration in patients with acute stroke: a novel approach to neuroprotection. Expert Rev. Neurother. 2008;8:259–270. doi: 10.1586/14737175.8.2.259. [DOI] [PubMed] [Google Scholar]
- 75.Uemura Y., Miller J.M., Matson W., Beal M. Neurochemical analysis of focal ischemia in rats. Stroke. 1991;22:1548–1553. doi: 10.1161/01.str.22.12.1548. [DOI] [PubMed] [Google Scholar]
- 76.Justicia C., Salas-Perdomo A., Pérez-de-Puig I., Deddens L.H., van Tilborg G.A., Castellví C., et al. Uric acid is protective after cerebral ischemia/reperfusion in hyperglycemic mice. Transl. Stroke Res. 2017;8:294–305. doi: 10.1007/s12975-016-0515-1. [DOI] [PubMed] [Google Scholar]
- 77.Dhanesha N., Vázquez-Rosa E., Cintrón-Pérez C.J., Thedens D., Kort A.J., Chuong V., et al. Treatment with uric acid reduces infarct and improves neurologic function in female mice after transient cerebral ischemia. J. Stroke Cerebrovasc. Dis. 2018;27:1412–1416. doi: 10.1016/j.jstrokecerebrovasdis.2017.12.043. [DOI] [PubMed] [Google Scholar]
- 78.Romanos E., Planas A.M., Amaro S., Chamorro A. Uric acid reduces brain damage and improves the benefits of rt-pa in a rat model of thromboembolic stroke. J. Cerebr. Blood Flow Metabol. 2007;27:14–20. doi: 10.1038/sj.jcbfm.9600312. [DOI] [PubMed] [Google Scholar]
- 79.Jiménez-Xarrié E., Pérez B., Dantas A.P., Puertas-Umbert L., Martí-Fabregas J., Chamorro Á., et al. Uric acid treatment after stroke prevents long-term middle cerebral artery remodelling and attenuates brain damage in spontaneously hypertensive rats. Transl. Stroke Res. 2020;11:1332–1347. doi: 10.1007/s12975-018-0661-8. [DOI] [PubMed] [Google Scholar]
- 80.Amaro S., Jimenez-Altayo F., Chamorro A. Uric acid therapy for vasculoprotection in acute ischemic stroke. Brain Circ. 2019;5:55–61. doi: 10.4103/bc.bc_1_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chamorro A., Obach V., Cervera A., Revilla M., Deulofeu R., Aponte J.H. Prognostic significance of uric acid serum concentration in patients with acute ischemic stroke. Stroke. 2002;33:1048–1052. doi: 10.1161/hs0402.105927. [DOI] [PubMed] [Google Scholar]
- 82.Chiquete E., Ruiz-Sandoval J.L., Murillo-Bonilla L.M., Arauz A., Orozco-Valera D.R., Ochoa-Guzmán A., et al. Serum uric acid and outcome after acute ischemic stroke: premier study. Cerebrovasc. Dis. 2013;35:168–174. doi: 10.1159/000346603. [DOI] [PubMed] [Google Scholar]
- 83.Amaro S., Soy D., Obach Vc, Cervera A., Planas A.M., Chamorro A. A pilot study of dual treatment with recombinant tissue plasminogen activator and uric acid in acute ischemic stroke. Stroke. 2007;38:2173–2175. doi: 10.1161/STROKEAHA.106.480699. [DOI] [PubMed] [Google Scholar]
- 84.Chamorro A., Amaro S., Castellanos M., Gomis M., Urra X., Blasco J., et al. Uric acid therapy improves the outcomes of stroke patients treated with intravenous tissue plasminogen activator and mechanical thrombectomy. Int. J. Stroke. 2017;12:377–382. doi: 10.1177/1747493016684354. [DOI] [PubMed] [Google Scholar]
- 85.Chen M., Ona V.O., Li M., Ferrante R.J., Fink K.B., Zhu S., et al. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of huntington disease. Nat Med. 2000;6:797–801. doi: 10.1038/77528. [DOI] [PubMed] [Google Scholar]
- 86.Zhu S., Stavrovskaya I.G., Drozda M., Kim B.Y., Ona V., Li M., et al. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature. 2002;417:74–78. doi: 10.1038/417074a. [DOI] [PubMed] [Google Scholar]
- 87.Switzer J.A., Hess D.C., Ergul A., Waller J.L., Machado L.S., Portik-Dobos V., et al. Matrix metalloproteinase-9 in an exploratory trial of intravenous minocycline for acute ischemic stroke. Stroke. 2011;42:2633–2635. doi: 10.1161/STROKEAHA.111.618215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fagan S.C., Waller J.L., Nichols F.T., Edwards D.J., Pettigrew L.C., Clark W.M., et al. Minocycline to improve neurologic outcome in stroke (minos): a dose-finding study. Stroke. 2010;41:2283–2287. doi: 10.1161/STROKEAHA.110.582601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lampl Y., Boaz M., Gilad R., Lorberboym M., Dabby R., Rapoport A., et al. Minocycline treatment in acute stroke: an open-label, evaluator-blinded study. Neurol. 2007;69:1404–1410. doi: 10.1212/01.wnl.0000277487.04281.db. [DOI] [PubMed] [Google Scholar]
- 90.Padma Srivastava MV., Bhasin A., Bhatia R., Garg A., Gaikwad S., Prasad K., et al. Efficacy of minocycline in acute ischemic stroke: a single-blinded, placebo-controlled trial. Neurol. India. 2012;60:23–28. doi: 10.4103/0028-3886.93584. [DOI] [PubMed] [Google Scholar]
- 91.Vesterinen H.M., Currie G.L., Carter S., Mee S., Watzlawick R., Egan K.J., et al. Systematic review and stratified meta-analysis of the efficacy of rhoa and rho kinase inhibitors in animal models of ischaemic stroke. Syst. Rev. 2013;2:1–9. doi: 10.1186/2046-4053-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ishiguro M., Kawasaki K., Suzuki Y., Ishizuka F., Mishiro K., Egashira Y., et al. A rho kinase (rock) inhibitor, fasudil, prevents matrix metalloproteinase-9-related hemorrhagic transformation in mice treated with tissue plasminogen activator. Neuroscience. 2012;220:302–312. doi: 10.1016/j.neuroscience.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 93.Li Q., Huang X.J., He W., Ding J., Jia J.T., Fu G., et al. Neuroprotective potential of fasudil mesylate in brain ischemia-reperfusion injury of rats. Cell. Mol. Neurobiol. 2009;29:169–180. doi: 10.1007/s10571-008-9308-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fukuta T., Yanagida Y., Asai T., Oku N. Co-administration of liposomal fasudil and tissue plasminogen activator ameliorated ischemic brain damage in occlusion model rats prepared by photochemically induced thrombosis. Biochem. Biophys. Res. Commun. 2018;495:873–877. doi: 10.1016/j.bbrc.2017.11.107. [DOI] [PubMed] [Google Scholar]
- 95.Satoh S., Utsunomiya T., Tsurui K., Kobayashi T., Ikegaki I., Sasaki Y., et al. Pharmacological profile of hydroxy fasudil as a selective rho kinase inhibitor on ischemic brain damage. Life Sci. 2001;69:1441–1453. doi: 10.1016/s0024-3205(01)01229-2. [DOI] [PubMed] [Google Scholar]
- 96.Shibuya M., Hirai S., Seto M., Satoh S-i, Ohtomo E., Group F.I.S.S. Effects of fasudil in acute ischemic stroke: results of a prospective placebo-controlled double-blind trial. Journal of the neurological sciences. 2005;238:31–39. doi: 10.1016/j.jns.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 97.Jian Z., Liu R., Zhu X., Smerin D., Zhong Y., Gu L., et al. The involvement and therapy target of immune cells after ischemic stroke. Front. Immunol. 2019;10:2167. doi: 10.3389/fimmu.2019.02167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Takahashi K., Greenberg J.H., Jackson P., Maclin K., Zhang J. Neuroprotective effects of inhibiting poly (adp-ribose) synthetase on focal cerebral ischemia in rats. J. Cerebr. Blood Flow Metabol. 1997;17:1137–1142. doi: 10.1097/00004647-199711000-00001. [DOI] [PubMed] [Google Scholar]
- 99.Hamby A.M., Suh S.W., Kauppinen T.M., Swanson R.A. Use of a poly (adp-ribose) polymerase inhibitor to suppress inflammation and neuronal death after cerebral ischemia-reperfusion. Stroke. 2007;38:632–636. doi: 10.1161/01.STR.0000250742.61241.79. [DOI] [PubMed] [Google Scholar]
- 100.Hudobenko J., Verma R., McCullough L. Abstract tp270: interleukin-6 receptor inhibition with tocilizumab ameliorates ischemic stroke damage in mice. Stroke. 2017;48 ATP270-ATP270. [Google Scholar]
- 101.Hudobenko J., Chauhan A., McCullough L. Amelioration of ischemic stroke damage through inhibition of interleukin-6 signaling with tocilizumab requires sex specific dosing. Stroke. 2019;50 A128-A128. [Google Scholar]
- 102.Wang Z.F., Kawabori M., Houkin K. Fty720 (fingolimod) ameliorates brain injury through multiple mechanisms and is a strong candidate for stroke treatment. Curr. Med. Chem. 2020;27:2979–2993. doi: 10.2174/0929867326666190308133732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu J., Zhang C., Tao W., Liu M. Systematic review and meta-analysis of the efficacy of sphingosine-1-phosphate (s1p) receptor agonist fty720 (fingolimod) in animal models of stroke. Int. J. Neurosci. 2013;123:163–169. doi: 10.3109/00207454.2012.749255. [DOI] [PubMed] [Google Scholar]
- 104.Fu Y., Zhang N., Ren L., Yan Y., Sun N., Li Y.J., et al. Impact of an immune modulator fingolimod on acute ischemic stroke. Proc. Natl. Acad. Sci. U. S. A. 2014;111:18315–18320. doi: 10.1073/pnas.1416166111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tian D.C., Shi K., Zhu Z., Yao J., Yang X., Su L., et al. Fingolimod enhances the efficacy of delayed alteplase administration in acute ischemic stroke by promoting anterograde reperfusion and retrograde collateral flow. Ann. Neurol. 2018;84:717–728. doi: 10.1002/ana.25352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Han L., Cai Q., Tian D., Kong D.K., Gou X., Chen Z., et al. Targeted drug delivery to ischemic stroke via chlorotoxin-anchored, lexiscan-loaded nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2016;12:1833–1842. doi: 10.1016/j.nano.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kaplan B., Brint S., Tanabe J., Jacewicz M., Wang X.J., Pulsinelli W. Temporal thresholds for neocortical infarction in rats subjected to reversible focal cerebral ischemia. Stroke; J. Cereb. Circ. 1991;22:1032–1039. doi: 10.1161/01.str.22.8.1032. [DOI] [PubMed] [Google Scholar]
- 108.Meinel T.R., Lerch C., Fischer U., Beyeler M., Mujanovic A., Kurmann C., et al. Multivariable prediction model for futile recanalization therapies in patients with acute ischemic stroke. Neurol. 2022;99:e1009–e1018. doi: 10.1212/WNL.0000000000200815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tournier N., Saba W., Cisternino S., Peyronneau M.-A., Damont A., Goutal S., et al. Effects of selected oatp and/or abc transporter inhibitors on the brain and whole-body distribution of glyburide. AAPS J. 2013;15:1082–1090. doi: 10.1208/s12248-013-9514-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lahmann C., Kramer H.B., Ashcroft F.M. Systemic administration of glibenclamide fails to achieve therapeutic levels in the brain and cerebrospinal fluid of rodents. PLoS One. 2015;10 doi: 10.1371/journal.pone.0134476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Deeken J.F., Loscher W. The blood-brain barrier and cancer: transporters, treatment, and trojan horses. Clin. Cancer Res. : Offic. J. Am. Assoc. Cancer Res. 2007;13:1663–1674. doi: 10.1158/1078-0432.CCR-06-2854. [DOI] [PubMed] [Google Scholar]
- 112.Dominguez A., Suarez-Merino B., Goni-de-Cerio F. Nanoparticles and blood-brain barrier: the key to central nervous system diseases. J. Nanosci. Nanotechnol. 2014;14:766–779. doi: 10.1166/jnn.2014.9119. [DOI] [PubMed] [Google Scholar]
- 113.Patel T., Zhou J., Piepmeier J.M., Saltzman W.M. Polymeric nanoparticles for drug delivery to the central nervous system. Adv. Drug Deliv. Rev. 2012;64:701–705. doi: 10.1016/j.addr.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhou J., Atsina K.B., Himes B.T., Strohbehn G.W., Saltzman W.M. Novel delivery strategies for glioblastoma. Cancer J. 2012;18:89–99. doi: 10.1097/PPO.0b013e318244d8ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Qiao R., Jia Q., Huwel S., Xia R., Liu T., Gao F., et al. Receptor-mediated delivery of magnetic nanoparticles across the blood-brain barrier. ACS Nano. 2012;6:3304–3310. doi: 10.1021/nn300240p. [DOI] [PubMed] [Google Scholar]
- 116.Li J., Guo Y., Kuang Y., An S., Ma H., Jiang C. Choline transporter-targeting and co-delivery system for glioma therapy. Biomaterials. 2013;34:9142–9148. doi: 10.1016/j.biomaterials.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 117.Liu L., Venkatraman S.S., Yang Y.Y., Guo K., Lu J., He B., et al. Polymeric micelles anchored with tat for delivery of antibiotics across the blood-brain barrier. Biopolymers. 2008;90:617–623. doi: 10.1002/bip.20998. [DOI] [PubMed] [Google Scholar]
- 118.Veiseh O., Sun C., Fang C., Bhattarai N., Gunn J., Kievit F., et al. Specific targeting of brain tumors with an optical/magnetic resonance imaging nanoprobe across the blood-brain barrier. Cancer Res. 2009;69:6200–6207. doi: 10.1158/0008-5472.CAN-09-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhou J., Liu J., Cheng C.J., Patel T.R., Weller C.E., Piepmeier J.M., et al. Biodegradable poly(amine-co-ester) terpolymers for targeted gene delivery. Nat. Mater. 2011;11:82–90. doi: 10.1038/nmat3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhou J., Patel T.R., Sirianni R.W., Strohbehn G., Zheng M.Q., Duong N., et al. Highly penetrative, drug-loaded nanocarriers improve treatment of glioblastoma. Proc. Natl. Acad. Sci. U. S. A. 2013;110:11751–11756. doi: 10.1073/pnas.1304504110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhou J., Patel T.R., Fu M., Bertram J.P., Saltzman W.M. Octa-functional plga nanoparticles for targeted and efficient sirna delivery to tumors. Biomaterials. 2012;33:583–591. doi: 10.1016/j.biomaterials.2011.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lu Y., Li C., Chen Q., Liu P., Guo Q., Zhang Y., et al. Microthrombus‐targeting micelles for neurovascular remodeling and enhanced microcirculatory perfusion in acute ischemic stroke. Adv. Mater. 2019;31 doi: 10.1002/adma.201808361. [DOI] [PubMed] [Google Scholar]
- 123.Guo X., Deng G., Liu J., Zou P., Du F., Liu F., et al. Thrombin-responsive, brain-targeting nanoparticles for improved stroke therapy. ACS Nano. 2018;12:8723–8732. doi: 10.1021/acsnano.8b04787. [DOI] [PubMed] [Google Scholar]
- 124.Li Y., Zhang X., Qi Z., Guo X., Liu X., Shi W., et al. The enhanced protective effects of salvianic acid a: a functionalized nanoparticles against ischemic stroke through increasing the permeability of the blood-brain barrier. Nano Res. 2020;13:2791–2802. [Google Scholar]
- 125.Khandare J., Calderón M., Dagia N.M., Haag R. Multifunctional dendritic polymers in nanomedicine: opportunities and challenges. Chem. Soc. Rev. 2012;41:2824–2848. doi: 10.1039/c1cs15242d. [DOI] [PubMed] [Google Scholar]
- 126.Liu Y., Ai K., Ji X., Askhatova D., Du R., Lu L., et al. Comprehensive insights into the multi-antioxidative mechanisms of melanin nanoparticles and their application to protect brain from injury in ischemic stroke. J. Am. Chem. Soc. 2017;139:856–862. doi: 10.1021/jacs.6b11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kawaguchi A.T., Fukumoto D., Haida M., Ogata Y., Yamano M., Tsukada H. Liposome-encapsulated hemoglobin reduces the size of cerebral infarction in the rat: evaluation with photochemically induced thrombosis of the middle cerebral artery. Stroke. 2007;38:1626–1632. doi: 10.1161/STROKEAHA.106.467290. [DOI] [PubMed] [Google Scholar]
- 128.Kaneda S., Ishizuka T., Sekiguchi A., Morimoto K., Kasukawa H. Efficacy of liposome‐encapsulated hemoglobin in a rat model of cerebral ischemia. Artif. Organs. 2014;38:650–655. doi: 10.1111/aor.12358. [DOI] [PubMed] [Google Scholar]
- 129.Kawaguchi A.T., Haida M., Yamano M., Fukumoto D., Ogata Y., Tsukada H. Liposome-encapsulated hemoglobin ameliorates ischemic stroke in nonhuman primates: an acute study. J. Pharmacol. Exp. Therapeut. 2010;332:429–436. doi: 10.1124/jpet.109.160051. [DOI] [PubMed] [Google Scholar]
- 130.Zhao Y.-Z., Lin M., Lin Q., Yang W., Yu X.-C., Tian F.-R., et al. Intranasal delivery of bfgf with nanoliposomes enhances in vivo neuroprotection and neural injury recovery in a rodent stroke model. J. Contr. Release. 2016;224:165–175. doi: 10.1016/j.jconrel.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 131.Yang X., Ma C., Chen Z., Liu J., Liu F., Xie R., et al. Single small molecule-assembled nanoparticles mediate efficient oral drug delivery. Nano Res. 2019;12:2468–2476. doi: 10.1007/s12274-019-2470-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bao Y., Zhang S., Chen Z., Chen A.T., Ma J., Deng G., et al. Synergistic chemotherapy for breast cancer and breast cancer brain metastases via paclitaxel-loaded oleanolic acid nanoparticles. Mol. Pharm. 2020;17:1343–1351. doi: 10.1021/acs.molpharmaceut.0c00044. [DOI] [PubMed] [Google Scholar]
- 133.Deng G., Ma C., Zhao H., Zhang S., Liu J., Liu F., et al. Anti-edema and antioxidant combination therapy for ischemic stroke via glyburide-loaded betulinic acid nanoparticles. Theranostics. 2019;9:6991. doi: 10.7150/thno.35791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Xie Y., Ma C., Yang X., Wang J., Long G., Zhou J. Phytonanomaterials as therapeutic agents and drug delivery carriers. Adv. Drug Deliv. Rev. 2021;176 doi: 10.1016/j.addr.2021.113868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang S., Peng B., Chen Z., Yu J., Deng G., Bao Y., et al. Brain-targeting, acid-responsive antioxidant nanoparticles for stroke treatment and drug delivery. Bioact. Mater. 2022;16:57–65. doi: 10.1016/j.bioactmat.2022.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wu J., Wang X., Wang Q., Lou Z., Li S., Zhu Y., et al. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes (ii) Chem. Soc. Rev. 2019;48:1004–1076. doi: 10.1039/c8cs00457a. [DOI] [PubMed] [Google Scholar]
- 137.Kim C.K., Kim T., Choi I.-Y., Soh M., Kim D., Kim Y.-J., et al. Ceria nanoparticles that can protect against ischemic stroke. Angew. Chem. Int. Ed. 2012;51:11039–11043. doi: 10.1002/anie.201203780. [DOI] [PubMed] [Google Scholar]
- 138.Bao Q., Hu P., Xu Y., Cheng T., Wei C., Pan L., et al. Simultaneous blood–brain barrier crossing and protection for stroke treatment based on edaravone-loaded ceria nanoparticles. ACS Nano. 2018;12:6794–6805. doi: 10.1021/acsnano.8b01994. [DOI] [PubMed] [Google Scholar]
- 139.He L., Huang G., Liu H., Sang C., Liu X., Chen T. Highly bioactive zeolitic imidazolate framework-8–capped nanotherapeutics for efficient reversal of reperfusion-induced injury in ischemic stroke. Sci. Adv. 2020;6 doi: 10.1126/sciadv.aay9751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yao J., Cheng Y., Zhou M., Zhao S., Lin S., Wang X., et al. Ros scavenging mn 3 o 4 nanozymes for in vivo anti-inflammation. Chem. Sci. 2018;9:2927–2933. doi: 10.1039/c7sc05476a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Huang Y., Liu Z., Liu C., Ju E., Zhang Y., Ren J., et al. Self‐assembly of multi‐nanozymes to mimic an intracellular antioxidant defense system. Angew. Chem. 2016;128:6758–6762. doi: 10.1002/anie.201600868. [DOI] [PubMed] [Google Scholar]
- 142.Shi J., Yu W., Xu L., Yin N., Liu W., Zhang K., et al. Bioinspired nanosponge for salvaging ischemic stroke via free radical scavenging and self-adapted oxygen regulating. Nano Lett. 2019;20:780–789. doi: 10.1021/acs.nanolett.9b04974. [DOI] [PubMed] [Google Scholar]
- 143.Wang J., Su Q., Lv Q., Cai B., Xiaohalati X., Wang G., et al. Oxygen-generating cyanobacteria powered by upconversion-nanoparticles-converted near-infrared light for ischemic stroke treatment. Nano Lett. 2021;21(11):4654–4665. doi: 10.1021/acs.nanolett.1c00719. [DOI] [PubMed] [Google Scholar]
- 144.Yu X., Gou X., Wu P., Han L., Tian D., Du F., et al. Activatable protein nanoparticles for targeted delivery of therapeutic peptides. Adv. Mater. 2018;30 doi: 10.1002/adma.201705383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gaudin A., Yemisci M., Eroglu H., Lepetre-Mouelhi S., Turkoglu O.F., Dönmez-Demir B., et al. Squalenoyl adenosine nanoparticles provide neuroprotection after stroke and spinal cord injury. Nat. Nanotechnol. 2014;9:1054–1062. doi: 10.1038/nnano.2014.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhang C.Y., Dong X., Gao J., Lin W., Liu Z., Wang Z. Nanoparticle-induced neutrophil apoptosis increases survival in sepsis and alleviates neurological damage in stroke. Sci. Adv. 2019;5 doi: 10.1126/sciadv.aax7964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhang Z.G., Buller B., Chopp M. Exosomes—beyond stem cells for restorative therapy in stroke and neurological injury. Nat. Rev. Neurol. 2019;15:193–203. doi: 10.1038/s41582-018-0126-4. [DOI] [PubMed] [Google Scholar]
- 148.Dong X., Gao J., Zhang C.Y., Hayworth C., Frank M., Wang Z. Neutrophil membrane-derived nanovesicles alleviate inflammation to protect mouse brain injury from ischemic stroke. ACS Nano. 2019;13:1272–1283. doi: 10.1021/acsnano.8b06572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wu H., Jiang X., Li Y., Zhou Y., Zhang T., Zhi P., et al. Engineering stem cell derived biomimetic vesicles for versatility and effective targeted delivery. Adv. Funct. Mater. 2020;30 [Google Scholar]
- 150.Schottler S., Becker G., Winzen S., Steinbach T., Mohr K., Landfester K., et al. Protein adsorption is required for stealth effect of poly(ethylene glycol)- and poly(phosphoester)-coated nanocarriers. Nat. Nanotechnol. 2016;11:372–377. doi: 10.1038/nnano.2015.330. [DOI] [PubMed] [Google Scholar]
- 151.Yemisci M., Caban S., Gursoy-Ozdemir Y., Lule S., Novoa-Carballal R., Riguera R., et al. Systemically administered brain-targeted nanoparticles transport peptides across the blood-brain barrier and provide neuroprotection. J Cerebr Blood F Met. 2015;35:469–475. doi: 10.1038/jcbfm.2014.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Marsh J.N., Hu G., Scott M.J., Zhang H.Y., Goette M.J., Gaffney P.J., et al. A fibrin-specific thrombolytic nanomedicine approach to acute ischemic stroke. Nanomedicine. 2011;6:605–615. doi: 10.2217/nnm.11.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wu H., Peng B., Mohammed F.S., Gao X., Qin Z., Sheth K.N., et al. Brain targeting, antioxidant polymeric nanoparticles for stroke drug delivery and therapy. Small. 2022 doi: 10.1002/smll.202107126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhao Y., Jiang Y., Lv W., Wang Z., Lv L., Wang B., et al. Dual targeted nanocarrier for brain ischemic stroke treatment. J. Contr. Release. 2016;233:64–71. doi: 10.1016/j.jconrel.2016.04.038. [DOI] [PubMed] [Google Scholar]
- 155.Wang Z., Zhao Y., Jiang Y., Lv W., Wu L., Wang B., et al. Enhanced anti-ischemic stroke of zl006 by t7-conjugated pegylated liposomes drug delivery system. Sci. Rep. 2015;5:1–15. doi: 10.1038/srep12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhang S.-F., Lü S., Yang J., Huang M., Liu Y., Liu M. Synthesis of multiarm peptide dendrimers for dual targeted thrombolysis. ACS Macro Lett. 2020;9:238–244. doi: 10.1021/acsmacrolett.0c00054. [DOI] [PubMed] [Google Scholar]
- 157.Dang Y., An C., Li Y., Han D., Liu X., Zhang F., et al. Neutrophil-mediated and low density lipoprotein receptor-mediated dual-targeting nanoformulation enhances brain accumulation of scutellarin and exerts neuroprotective effects against ischemic stroke. RSC Adv. 2019;9:1299–1318. doi: 10.1039/c8ra06688d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Agulla J., Brea D., Campos F., Sobrino T., Argibay B., Al-Soufi W., et al. In vivo theranostics at the peri-infarct region in cerebral ischemia. Theranostics. 2014;4:90. doi: 10.7150/thno.7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Huang Y., Yu L., Ren J., Gu B., Longstaff C., Hughes A.D., et al. An activated-platelet-sensitive nanocarrier enables targeted delivery of tissue plasminogen activator for effective thrombolytic therapy. J. Contr. Release. 2019;300:1–12. doi: 10.1016/j.jconrel.2019.02.033. [DOI] [PubMed] [Google Scholar]
- 160.Lu Y-m, Huang J-y, Wang H., Lou X-f, Liao M-h, Hong L-j, et al. Targeted therapy of brain ischaemia using fas ligand antibody conjugated peg-lipid nanoparticles. Biomaterials. 2014;35:530–537. doi: 10.1016/j.biomaterials.2013.09.093. [DOI] [PubMed] [Google Scholar]
- 161.Wilhelm S., Tavares A.J., Dai Q., Ohta S., Audet J., Dvorak H.F., et al. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016;1 [Google Scholar]
- 162.Albanese A., Tang P.S., Chan W.C. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012;14:1–16. doi: 10.1146/annurev-bioeng-071811-150124. [DOI] [PubMed] [Google Scholar]
- 163.Lu Y., Aimetti A.A., Langer R., Gu Z. Bioresponsive materials. Nat. Rev. Mater. 2016;2:1–17. [Google Scholar]
- 164.Korin N., Kanapathipillai M., Matthews B.D., Crescente M., Brill A., Mammoto T., et al. Shear-activated nanotherapeutics for drug targeting to obstructed blood vessels. Science. 2012;337:738–742. doi: 10.1126/science.1217815. [DOI] [PubMed] [Google Scholar]
- 165.Korin N., Gounis M.J., Wakhloo A.K., Ingber D.E. Targeted drug delivery to flow-obstructed blood vessels using mechanically activated nanotherapeutics. JAMA Neurol. 2015;72:119–122. doi: 10.1001/jamaneurol.2014.2886. [DOI] [PubMed] [Google Scholar]
- 166.Sinden J.D., Hicks C., Stroemer P., Vishnubhatla I., Corteling R. Human neural stem cell therapy for chronic ischemic stroke: charting progress from laboratory to patients. Stem Cells Dev. 2017;26:933–947. doi: 10.1089/scd.2017.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ma J., Zhang S., Liu J., Liu F., Du F., Li M., et al. Targeted drug delivery to stroke via chemotactic recruitment of nanoparticles coated with membrane of engineered neural stem cells. Small. 2019;15 doi: 10.1002/smll.201902011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Li M., Li J., Chen J., Liu Y., Cheng X., Yang F., et al. Platelet membrane biomimetic magnetic nanocarriers for targeted delivery and in situ generation of nitric oxide in early ischemic stroke. ACS Nano. 2020;14:2024–2035. doi: 10.1021/acsnano.9b08587. [DOI] [PubMed] [Google Scholar]
- 169.Lv W., Xu J., Wang X., Li X., Xu Q., Xin H. Bioengineered boronic ester modified dextran polymer nanoparticles as reactive oxygen species responsive nanocarrier for ischemic stroke treatment. ACS Nano. 2018;12:5417–5426. doi: 10.1021/acsnano.8b00477. [DOI] [PubMed] [Google Scholar]
- 170.Du B., Jiang X., Das A., Zhou Q., Yu M., Jin R., et al. Glomerular barrier behaves as an atomically precise bandpass filter in a sub-nanometre regime. Nat. Nanotechnol. 2017;12:1096–1102. doi: 10.1038/nnano.2017.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Blanco E., Shen H., Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015;33:941–951. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ohta S., Kikuchi E., Ishijima A., Azuma T., Sakuma I., Ito T. Investigating the optimum size of nanoparticles for their delivery into the brain assisted by focused ultrasound-induced blood-brain barrier opening. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-75253-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Da Silva-Candal A., Brown T., Krishnan V., Lopez-Loureiro I., Ávila-Gómez P., Pusuluri A., et al. Shape effect in active targeting of nanoparticles to inflamed cerebral endothelium under static and flow conditions. J. Contr. Release. 2019;309:94–105. doi: 10.1016/j.jconrel.2019.07.026. [DOI] [PubMed] [Google Scholar]
- 174.Campos-Martorell M., Cano-Sarabia M., Simats A., Hernandez-Guillamon M., Rosell A., Maspoch D., et al. Charge effect of a liposomal delivery system encapsulating simvastatin to treat experimental ischemic stroke in rats. Int. J. Nanomed. 2016;11:3035–3048. doi: 10.2147/IJN.S107292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Cheng R., Huang W., Huang L., Yang B., Mao L., Jin K., et al. Acceleration of tissue plasminogen activator-mediated thrombolysis by magnetically powered nanomotors. ACS Nano. 2014;8:7746–7754. doi: 10.1021/nn5029955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hu J., Huang S., Zhu L., Huang W., Zhao Y., Jin K., et al. Tissue plasminogen activator-porous magnetic microrods for targeted thrombolytic therapy after ischemic stroke. ACS Appl. Mater. Interfaces. 2018;10:32988–32997. doi: 10.1021/acsami.8b09423. [DOI] [PubMed] [Google Scholar]